Abstract
Objective:
To evaluate T2 relaxation values (T2RVs) of knee joint cartilage after double-bundle anterior cruciate ligament reconstruction (DB-ACLR) in a 6-month follow-up and to correlate changes between T2RVs with meniscal status and clinical findings.
Methods:
27 patients who underwent DB-ACLR and MRI before and 6 months after surgery, and 27 control subjects were enrolled. We compared T2RVs of the control vs pre-operative MR and pre-operative vs post-operative MR using 28 subcompartments, including superficial and deep layers. Correlations between T2RV changes with meniscal status and clinical data were examined.
Results:
The pre-operative T2RV was significantly higher than that of the control group in the medial tibia (posterior-superficial), posterior medial femur (superficial) and posterior lateral femur (superficial and deep). The post-operative T2RV was significantly higher than that of pre-operative T2RV in the posterior medial femur (superficial), medial tibia (anterior-deep and central-deep), lateral femur (anterior-deep, anterior-superficial and central-superficial) and posterior medial femur (deep). Moderate positive correlations between pre-operative and post-operative T2RV changes were found at the posterior medial femur (interval between injury and MR examination, and instability) and posterior lateral femur (Lysholm score).
Conclusion:
Patients with anterior cruciate ligament injury followed by DB-ACLR presented short-term subcompartment T2RV changes at the medial femur, lateral femur and medial tibia. Meniscal status did not affect T2RV; however, clinical findings influenced T2RV at the posterior grooves of the medial and lateral femoral condyles.
Advances in knowledge:
Patients submitted to DB-ACLR presented T2RV changes in both femoral and medial tibial condyles 6 months after the surgery, affecting not just the weight-bearing areas, but also the less-weight-bearing areas.
INTRODUCTION
Anterior cruciate ligament (ACL) rupture is a major risk factor for the development of post-traumatic osteoarthritis (OA).1,2 These changes in articulation may be caused by altered kinematics, particularly if associated with meniscal or chondral lesions.3,4 To avoid early degeneration of the articular cartilage, ACL reconstruction techniques have been commonly employed to restore the anatomy and preserve long-term knee health.5 Both reconstructed and untreated ACL ruptures are associated with an increased risk of degeneration of the articular cartilage; however, surgery is frequently advised to retard OA development, especially among patients involved in sports.2,3,6 Single-bundle reconstruction and double-bundle reconstruction are the current main surgical techniques. The double-bundle reconstruction technique was developed to more closely return to the native anatomy. This technique has become increasingly popular, as studies have shown similar or better results relative to single-bundle reconstruction.5,7–9
Macroscopic alteration of the articular cartilage in patients with post-traumatic OA occurs approximately 2 years after ACL reconstruction.6 However, subtle morphological or compositional alterations can occur much earlier and be detected using compositional MRI techniques.6,10–12 T2 mapping, T1 rho mapping, delayed gadolinium-enhanced MRI and ultrashort echo time (TE)–enhanced T2* can be used to evaluate the status of articular cartilage associated with changed collagen fibre integrity, reduced glycosaminoglycan and increased water content.6,13–18 Among them, T2 mapping is considered one of the most feasible techniques owing to its non-invasiveness and shorter time requirements. It can be easily implemented in most MRI systems with a high magnetic field.19 This method is based on water bound to proteoglycan, which is held in place by the extracellular collagen framework. When this collagen framework breaks down, free water is released, leading to prolonged T2 relaxation values (T2RVs).13
Several studies have described changes in articular cartilage after ACL reconstruction but few have demonstrated early changes in T2 mapping.10,20–24 To our knowledge, no studies have evaluated T2RVs of the articular cartilage of the entire tibiofemoral joint in patients who underwent double-bundle ACL reconstruction (DB-ACLR) at as early as 6 months after the operation. The purposes of this study were to evaluate changes in T2RV after injury and after DB-ACLR and to correlate the changes in T2RV with meniscal status and other clinical information. We hypothesized that the T2RV would increase soon after ACL reconstruction (ACLR) and also that meniscal injuries as well as clinical scores would have positive correlations with higher T2 values.
METHODS AND MATERIALS
Subjects
This retrospective study was approved by Samsung Medical Center institutional review board (IRB No.: 2014-09-011), which waived the need for informed consent. The medical records of 118 patients who underwent ACLR between September 2012 and July 2014 were retrospectively reviewed. The inclusion criteria were as follows: underwent DB-ACLR at our institution, <3 months had passed between ACL injury and surgery, age of 20–40 years, body mass index (BMI) of 20–30 kg m−2, pre-operative and 6-month post-operative MRI included T2 mapping. The exclusion criteria were as follows: previous surgery on the affected knee, history of repeated injuries to the affected knee, suspected underlying inflammatory arthritis and International Cartilage Repair Society Grade III or IV chondral lesions at arthroscopy.25 Our final sample included 27 patients for whom MRI, surgical and clinical data were evaluated.
Lysholm scores, based on a scale used to assess knee function according to the presence of pain, swelling, limp, locking, instability and the ability to climb stairs and squat (range, 0–100), were obtained from all patients before and 6 months after surgery by an orthopaedic surgeon (JHW).26 For graft stability evaluation, the bilateral knee joints were examined using a single-calibrated arthrometer (KT2000™; MEDmetric® Corporation, San Diego, CA) with a constant force of 15, 20 and 30 lbs and manual maximum displacement at about 6 months after the surgery by a physical trainer with 5 years' arthrometry experience at the time of the test. Measurements were calculated with a force of 30 lbs, and the difference between the treated knee and the contralateral uninjured knee was considered the representative value. 27 sequential knee MRI scans, read as “normal” by one of the three musculoskeletal radiologists in our institute, from 27 age- and sex-matched patients with no history of knee injury, no abnormalities on physical examination and mild discomfort that resolved within 1 month after MRI [selected after electronic medical record and MRI review by one musculoskeletal radiologist (YCY)] were included as the control group.
Surgery and post-operative rehabilitation
All patients underwent ACLR surgery performed by an orthopaedic surgeon at our institution (JHW, 10 years' experience in arthroscopic knee surgery). The double-bundle technique used either a hamstring tendon autograft or a posterior tibial tendon allograft. Meniscal status was evaluated during surgery using the probe and was graded according to the intervention: 1 (normal meniscus = no intervention), 2 (stable tear = no intervention), 3 (repairable tear = suture) and 4 (non-repairable tear = partial meniscectomy).
All patients adhered to a strict rehabilitation protocol. In the first 3–4 days, the range of motion of the knee and weight-bearing exercises were performed, while full weight-bearing was allowed after 4 weeks and jogging was encouraged after 3 months if adequate quadriceps recovery was observed. Sports activities were gradually reintroduced, and each patient's desired sporting activity was allowed after 9 months.
MRI protocol
The MRI examinations were performed using a 3.0-T machine (Gyroscan Intera Achieva®, Philips Medical Systems, Best, Netherlands) with a dedicated knee coil (Invivo, Gainesville, FL). Each patient was scanned in a supine position with mild knee flexion. The following MRI sequences were performed: isotropic three-dimensional fat-suppressed (FS) proton density (PD)-weighted turbo spin echo (TSE) sequence in coronal plane, coronal T1 weighted TSE, axial T2 weighted TSE, axial FS PD-weighted TSE, sagittal PD-weighted TSE, sagittal FS PD-weighted TSE and quantitative T2 imaging with a multi-TE technique. The detailed parameters are summarized in Table 1.
Table 1.
MRI parameters
| Parameters | 3D FS PD TSE | Standard 2D TSE MR |
Quantitative T2 imaging | ||||
|---|---|---|---|---|---|---|---|
| Coronal T2W | Axial T2W | Axial PD FS | Sagittal PD | Sagittal PD FS | |||
| TR (ms) | 1800 | 460 | 2670 | 2100 | 6200 | 2140 | 3100 |
| TE (ms) | 35 | 20 | 100 | 30 | 20 | 20 | N* 15 |
| FOV (mm) | 160 × 160 | 160 × 160 | 160 × 160 | 160 × 160 | 160 × 160 | 160 × 160 | 160 × 160 |
| Section thickness (mm) | 0.5 | 4 | 4 | 4 | 1.5 | 4 | 3 |
| Number of section | 250 | 22 | 22 | 22 | 75 | 23 | 20 |
| Flip angle (°) | 90 | 90 | 90 | 90 | 90 | 90 | 90 |
| Number of signal averaging | 1 | 2 | 2 | 1 | 1 | 1 | 1 |
| SENSE factor | RL:2, AP:2 | No | No | No | No | No | 2 |
| Acquisition matrix | 320 × 320 | 516 × 352 | 516 × 352 | 320 × 320 | 320 × 308 | 320 × 308 | 400 × 246 |
| Reconstructed voxel size (mm) | 0.31 × 0.31 × 0.5 | 0.3 × 0.3 × 4 | 0.3 × 0.3 × 4 | 0.31 × 0.31 × 4 | 0.31 × 0.31 × 1.5 | 0.31 × 0.31 × 4 | 0.3 × 0.31 × 3 |
| Echo train length | 46 | 3 | 16 | 8 | 11 | 11 | 6 |
| Bandwidth (Hz) | 639 | 399 | 275.7 | 290.9 | 441.0 | 444.6 | 217.7 |
| Scan time | 6:48 | 3:42 | 4:00 | 2:51 | 5:58 | 2:03 | 6 : 31 |
2D, two-dimensional; 3D, three-dimensional; AP, anterior to posterior; FOV, field of view; FS, fat suppression; PD, proton density, RL, right to left; SENSE, sensitivity encoding; T2W, T2 weighted; TE, echo time; TR, repetition time; TSE, turbo spin echo.
Imaging analysis
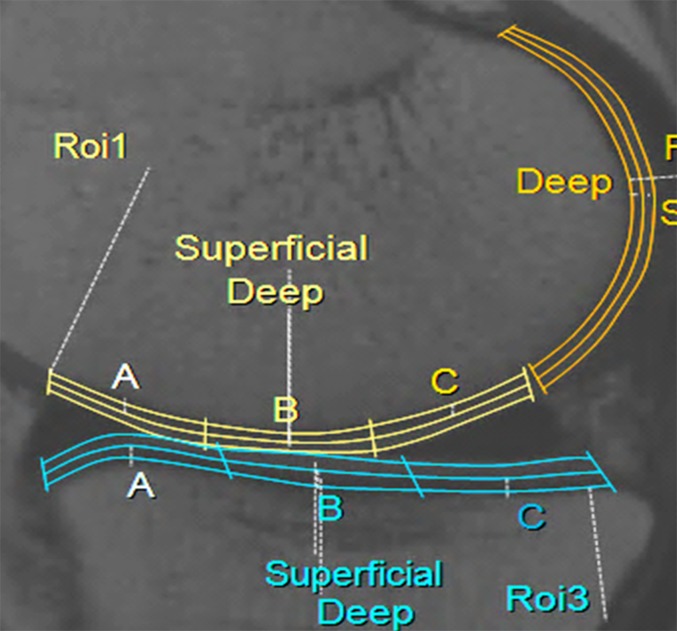
T2 maps of the patients and control group were retrospectively analysed by a musculoskeletal radiologist (RG, 7 years' experience in interpreting knee MRI) and reanalysed by the same reviewer after 4 weeks to assess intraobserver reliability. For calculating the interobserver reliability, the same analysis was independently performed by another musculoskeletal radiologist (YCY, 10 years' experience in interpreting knee MRI). T2RV measurement sequences with a multi-TE technique in the sagittal plane were loaded onto a dedicated software (IntelliSpace Portal; Philips Medical Systems), and the cartilage was segmented semi-automatically. When the software program was started after the multi-echo spin echo sequence was loaded, the anatomical image at an TE of 15 ms was displayed on the left side of the monitor, and the corresponding T2 mapping was simultaneously displayed on the right side. To obtain the T2RV of the articular cartilage, two mid-sagittal consecutive planes for each medial and lateral femorotibial compartment were selected. The observers drew regions of interest around the cartilage surface and the cartilage–bone interface and were careful not to include joint fluid or subchondral bone within them. The cartilage evaluation of the tibiofemoral joint was performed using 28 subcompartments (Figure 1) of four weight-bearing and two less-weight-bearing areas. The 4 weight-bearing areas of the medial and lateral femoral condyles and tibial plateaus were divided into 12 compartments: medial femur–anterior, medial femur–central, medial femur–posterior (MFP), medial tibia–anterior (MTA), medial tibia–central (MTC), medial tibia–posterior (MTP), lateral femur–anterior (LFA), lateral femur–central (LFC), lateral femur–posterior, lateral tibia–anterior, lateral tibia–central and lateral tibia–posterior. The anterior and posterior margins of the weight-bearing compartments were defined by the meniscal margins. The less-weight-bearing area corresponding to the posterior groove of the femoral condyles was named the posterior medial femur (PMF) and posterior lateral femur (PLF). Furthermore, these compartments were automatically further divided into superficial or deep layers using a segmentation tool provided by the software program. The former was oriented to the articular surface, whereas the latter was oriented to the cartilage–bone interface. The average T2RVs of two consecutive slices were considered representative values for each of the 28 subcompartments.
Figure 1.
Examples of cartilage compartments. Weight-bearing areas of the medial femoral condyle (yellow) and medial tibial plateau (blue) are divided into anterior (A), central (B) and posterior (C) compartments and subdivided into superficial and deep layers. Less-weight-bearing areas of posterior medial femur (orange) were divided into superficial and deep layers. ROI, region of interest. For colour image see online.
The T2RVs on pre-operative MRI from the patient and control groups were compared to evaluate the effect of injury and resultant instability on articular cartilage according to the 28 subcompartments. The T2RVs on pre- and post-operative MRI from the patients were compared to evaluate the biochemical changes in articular cartilage after double-bundle reconstruction surgery and rehabilitation in the early post-operative period according to the 28 subcompartments. The T2RV ratio between pre- and post-operative MRI was correlated with the clinical data in terms of injury to MRI interval, injury to surgery interval, surgery to follow-up MRI interval, Lysholm score, residual instability measured by a KT2000 arthrometer and meniscal status evaluated by arthroscopy according to six areas (four weight bearing and two less-weight bearing).
Statistical analysis
For each subcompartment from MRI of the control group and pre-operative MRI of the patient group, T2RV was compared after adjusting for age, sex, knee laterality and BMI by multiple regression. Comparisons between pre- and post-operative T2 values of each subcompartment were performed using a paired t-test. Correlations between T2 values of each area with clinical data in terms of injury to MRI interval, injury to surgery interval, surgery to follow-up MRI interval, Lysholm score, residual instability measured by a KT-2000 arthrometer and meniscal status evaluated by arthroscopy were assessed using Spearman's rank correlation coefficient (rs) and were classified as weak (<0.39), moderate (0.40–0.59) or strong (>0.60). The intra- and interobserver agreement analyses were made using intraclass correlation coefficients and were classified as poor (<0.20), fair (0.21–0.40), moderate (0.41–0.60), good (0.61–0.80) or excellent (>0.81). Statistical significance was considered at p-values <0.05. SAS® 9.4 software (SAS Inc., Cary, NC) was used for the statistical analyses.
RESULTS
A total of 54 subjects (44 males, 10 females) were enrolled in the study. The patient and control groups each included 22 males and 5 females. The average age was 27.59 ± 4.08 years for the control and 28.56 ± 5.44 years for the patient group. The right knee was most affected in both groups, with 14 (51.85%) in the control and 19 (70.37%) in the patient group. Average BMI was 24.38 ± 1.87 kg m−2 for the control and 24.26 ± 2.08 kg m−2 for the patient group.
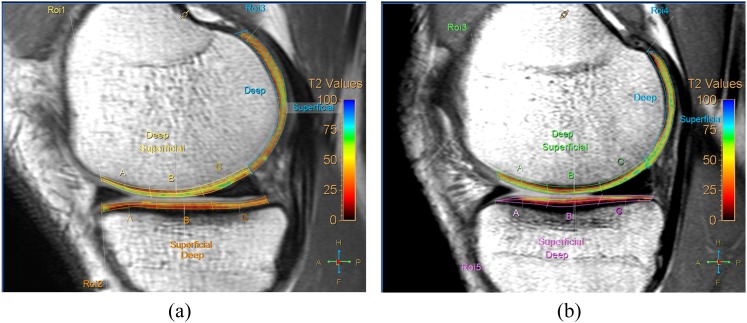
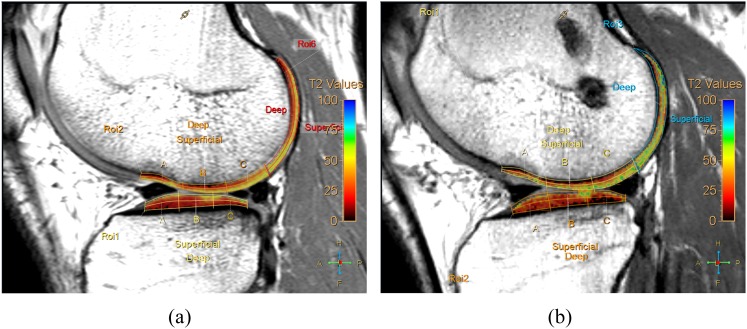
The T2RVs on pre-operative MRI of the patient group tended to be higher than those found in the control group in 20 of 28 subcompartments (Table 2). Among them, T2RVs on pre-operative MRI of the patient group were significantly higher in four subcompartments (superficial MTP, p = 0.0203; superficial PMF, p = 0.0072; deep PLF, p = 0.0051; superficial PLF, p = 0.0002) than those of the control group after the adjustment for age, sex, knee laterality and BMI (Figure 2). The T2RVs on post-operative MRI of the patient group tended to be higher than those on pre-operative MRI of the patient group in 23 of 28 subcompartments (Table 2). Among these, the T2RVs on post-operative MRI of seven subcompartments were significantly higher than those on pre-operative MRI (superficial MFP, p = 0.045; deep MTA, p = 0.0103; deep MTC, p = 0.0038; deep LFA, p = 0.0020; superficial LFA, p = 0.0390; superficial LFC, p = 0.0081; and deep PMF, p = 0.0392) (Figure 3).
Table 2.
Comparison of T2 relaxation values (T2RVs) between control group MR and pre-operative MR, and pre-operative MR and post-operative MR after adjustment of sex, age, laterality and body mass index
| Location |
Control T2RV (ms) |
p-value (between control and pre-operative T2RV) | Pre-operative T2RV (ms) |
p-value (between pre- and post-operative T2RV) | Post-operative T2RV (ms) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Area | Compartment | Mean | SD | Mean | SD | Mean | SD | ||||
| Weight-bearing areas | MF | MFA | Deep | 35.78 | 6.97 | 0.5783 | 34.56 | 6.98 | 0.8076 | 34.93 | 6.13 |
| Superficial | 45.80 | 8.02 | 0.9990 | 46.00 | 5.89 | 0.4520 | 46.85 | 5.08 | |||
| MFC | Deep | 33.67 | 6.25 | 0.3675 | 32.39 | 7.55 | 0.6355 | 33.09 | 6.32 | ||
| Superficial | 45.30 | 6.72 | 0.7995 | 45.81 | 8.65 | 0.5198 | 46.69 | 5.72 | |||
| MFP | Deep | 41.69 | 4.47 | 0.7350 | 42.59 | 5.93 | 0.3707 | 43.80 | 4.79 | ||
| Superficial | 45.12 | 6.85 | 0.0665 | 49.28 | 5.96 | 0.0450 | 51.15 | 6.38 | |||
| MT | MTA | Deep | 30.02 | 3.12 | 0.1100 | 28.20 | 4.93 | 0.0103 | 32.24 | 6.02 | |
| Superficial | 41.85 | 3.12 | 0.4131 | 43.54 | 6.15 | 0.0939 | 46.22 | 5.25 | |||
| MTC | Deep | 28.33 | 4.66 | 0.1420 | 26.17 | 4.87 | 0.0038 | 29.96 | 5.24 | ||
| Superficial | 41.81 | 4.33 | 0.8701 | 42.04 | 4.43 | 0.2062 | 43.72 | 6.05 | |||
| MTP | Deep | 31.17 | 4.97 | 0.2751 | 33.24 | 4.74 | 0.0845 | 35.22 | 6.02 | ||
| Superficial | 38.94 | 4.16 | 0.0072 | 43.74 | 6.19 | 0.4455 | 42.96 | 5.89 | |||
| LF | LFA | Deep | 37.81 | 7.04 | 0.3117 | 36.52 | 8.13 | 0.0020 | 42.52 | 6.77 | |
| Superficial | 44.44 | 5.22 | 0.1170 | 47.54 | 7.25 | 0.0390 | 51.15 | 6.63 | |||
| LFC | Deep | 37.30 | 6.37 | 0.1780 | 35.48 | 7.33 | 0.0081 | 39.91 | 7.46 | ||
| Superficial | 47.69 | 5.07 | 0.9964 | 47.52 | 5.47 | 0.2369 | 49.20 | 6.82 | |||
| LFP | Deep | 43.02 | 4.10 | 0.9051 | 43.56 | 6.21 | 0.2527 | 45.15 | 5.54 | ||
| Superficial | 48.52 | 6.19 | 0.1803 | 50.20 | 4.11 | 0.7790 | 49.83 | 8.09 | |||
| LT | LTA | Deep | 28.09 | 6.06 | 0.1056 | 26.22 | 4.83 | 0.2994 | 27.72 | 5.28 | |
| Superficial | 38.31 | 7.17 | 0.4060 | 41.00 | 6.89 | 0.8546 | 40.65 | 8.60 | |||
| LTC | Deep | 27.28 | 4.60 | 0.8160 | 27.31 | 6.22 | 0.2747 | 29.11 | 5.59 | ||
| Superficial | 40.28 | 3.77 | 0.8266 | 41.72 | 7.16 | 0.9302 | 41.56 | 5.92 | |||
| LTP | Deep | 30.13 | 4.98 | 0.5879 | 31.81 | 6.88 | 0.2282 | 33.87 | 5.31 | ||
| Superficial | 40.26 | 4.56 | 0.0570 | 44.02 | 5.74 | 0.9897 | 44.00 | 4.01 | |||
| Less-weight-bearing areas | PMF |
Deep | 40.90 | 3.02 | 0.3625 | 42.38 | 5.53 | 0.0392 | 44.69 | 4.47 | |
| Superficial | 43.73 | 5.69 | 0.0051 | 48.91 | 6.24 | 0.4796 | 49.87 | 7.43 | |||
| PLF | Deep | 36.36 | 3.21 | 0.0203 | 39.19 | 3.52 | 0.0799 | 40.65 | 4.26 | ||
| Superficial | 40.25 | 3.24 | 0.0002 | 44.41 | 4.79 | 0.0908 | 46.65 | 6.67 | |||
LF, lateral femoral condyle; LFA, lateral femoral anterior; LFC, lateral femoral central; LFP, lateral femoral posterior; LT, lateral tibial plateaus; LTA, lateral tibial anterior; LTC, lateral tibial central; LTP, lateral tibial posterior; MF, medial femoral condyle; MFA, medial femoral anterior; MFC, medial femoral central; MFP, medial femoral posterior; MT, medial tibial plateaus; MTA, medial tibial anterior; MTC, medial tibial central; MTP, medial tibial posterior; PLF, posterior lateral femoral groove; PMF, posterior medial femoral groove; SD, standard deviation.
Bold values indicate statistically significant differences.
Figure 2.
Comparison of T2 relaxation value (T2RV) of knee joint between the control group and pre-operative group. Representative sagittal T2 maps from a 22-year-old male control patient (a) and pre-operative MR from a 24-year-old male patient with anterior cruciate ligament tear (b). T2RV of weight-bearing and less-weight-bearing areas of the medial femoral condyle from the anterior cruciate ligament tear of the patient (b) includes green-coloured pixels, suggestive of higher T2RV, compared with orange- to yellow-coloured pixels in the control patient (a). ROI, region of interest. For colour image see online.
Figure 3.
Comparison of T2 relaxation value (T2RV) of the knee joint between pre-operative and post-operative MR in a patient who underwent double-bundle anterior cruciate ligament reconstruction (DB-ACLR) surgery. Representative sagittal T2 maps of pre-operative MR (a) and post-operative MR 6 months after DB-ACLR surgery in a 26-year-old male. T2RVs of less-weight-bearing areas of and superficial layers of weight-bearing areas of the lateral femoral condyle of post-operative MR (b) include green-coloured pixels, suggestive of higher T2RVs, compared with orange- to yellow-coloured pixels of pre-operative MR (a). ROI, region of interest. For colour image see online.
The correlations between change ratio of pre- and post-operative T2RVs and clinical findings and meniscal status are summarized in Table 3. The average time interval between injury and MRI was 37.7 (±28.0) days; that between injury and surgery was 46.5 (±23.1) days; and that between surgery and follow-up MRI was 179.3 (±15.5) days. The only significant moderate positive correlation was found between the change ratio of pre- and post-operative T2RVs and injury to MRI interval in the less-weight-bearing PMF area (rs = 0.460, p = 0.0159). No other intervals showed a significant correlation to the change ratio of pre- and post-operative T2RVs. The mean post-operative Lysholm score was 88.85 ± 11.54. There was a moderate positive correlation between the change ratio of pre- and post-operative T2RVs and post-operative Lysholm score in the less-weight-bearing PLF area (rs = 0.515, p = 0.0059). The average difference in anterior instability between the injured and uninjured knee measured by the KT2000 arthrometer was 1.61 ± 1.58 mm. There was a moderately positive correlation between the difference in anterior instability between injured and uninjured knees measured by the KT2000 arthrometer and the change ratio of pre- and post-operative T2RVs in the less-weight-bearing PMF areas (rs = 0.435, p = 0.233). 18 medial (66.6%) and 15 (55.5%) lateral menisci were normal at the time of arthroscopic surgery (Grade 1). One medial meniscus (3.7%) and three lateral menisci (11.1%) had stable lesions (Grade 2); seven medial menisci (25.9%) and seven lateral menisci (25.9%) were repaired (Grade 3); and one medial meniscus (3.7%) and two lateral menisci (7.4%) were partially resected (Grade 4). There was no significant correlation between meniscal status and change ratio of pre- and post-operative T2RVs of the six areas.
Table 3.
Correlations of post-operative T2 relaxation value change ratio (T2post-T2pre/T2pre) with clinical data and meniscal status
| Statistical function | Intervals |
Clinical data |
Arthroscopic finding |
||||
|---|---|---|---|---|---|---|---|
| Injury-MR | Injury-Op | Op-MR | Lysholm score | Instability | MM | LM | |
| MF | |||||||
| rs | −0.054 | −0.013 | −0.257 | 0.195 | 0.037 | −0.058 | −0.032 |
| p | 0.7876 | 0.9470 | 0.1952 | 0.3298 | 0.8564 | 0.7745 | 0.8759 |
| MT | |||||||
| rs | 0.095 | 0.021 | 0.073 | 0.230 | 0.192 | 0.152 | 0.057 |
| p | 0.6362 | 0.9181 | 0.7185 | 0.2484 | 0.3384 | 0.4489 | 0.7791 |
| LF | |||||||
| rs | −0.187 | −0.169 | −0.096 | 0.365 | 0.138 | 0.319 | 0.182 |
| p | 0.3495 | 0.3981 | 0.6341 | 0.0614 | 0.4929 | 0.1054 | 0.3634 |
| LT | |||||||
| rs | −0.103 | −0.017 | 0.058 | 0.335 | 0.001 | 0.078 | −0.074 |
| p | 0.6082 | 0.9313 | 0.7748 | 0.0877 | 0.9951 | 0.6987 | 0.7128 |
| PMF | |||||||
| rs | 0.460 | 0.255 | 0.161 | 0.142 | 0.435 | 0.277 | 0.021 |
| p | 0.0159 | 0.1987 | 0.4223 | 0.4791 | 0.0233 | 0.1614 | 0.9184 |
| PLF | |||||||
| rs | 0.064 | −0.119 | 0.261 | 0.515 | −0.014 | 0.138 | 0.144 |
| p | 0.7493 | 0.5531 | 0.1881 | 0.0059 | 0.9443 | 0.4910 | 0.4733 |
LF, lateral femoral condyle; LM, lateral meniscus; LT, lateral tibial plateaus; MF, medial femoral condyle; MM, medial meniscus; MT, medial tibial plateaus; PLF, posterior lateral femoral groove; PMF, posterior medial femoral groove; rs , Spearman's rank correlation coefficient.
Bold values indicate statistically significant differences.
Inter- and intraobserver agreement were analysed using the 28 subcompartment divisions. Both evaluations showed moderate to excellent results as the intra- and interobserver agreement presented means of 0.69 ± 0.16 and 0.74 ± 0.11, respectively (Table 4).
Table 4.
Reliability of T2 relaxation value measurement
| Location |
Intraobserver |
Interobserver |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Area | Compartment | ICC | 95% CI | ICC | 95% CI | ||||
| Weight-bearing areas | MF | MFA | Deep | 0.58 | 0.18 | 0.82 | 0.80 | 0.54 | 0.92 |
| Superficial | 0.83 | 0.61 | 0.93 | 0.84 | 0.63 | 0.94 | |||
| MFC | Deep | 0.57 | 0.16 | 0.81 | 0.81 | 0.58 | 0.93 | ||
| Superficial | 0.69 | 0.36 | 0.87 | 0.85 | 0.65 | 0.94 | |||
| MFP | Deep | 0.92 | 0.79 | 0.97 | 0.85 | 0.66 | 0.94 | ||
| Superficial | 0.85 | 0.65 | 0.94 | 0.90 | 0.75 | 0.96 | |||
| MT | MTA | Deep | 0.75 | 0.45 | 0.90 | 0.67 | 0.32 | 0.86 | |
| Superficial | 0.71 | 0.38 | 0.88 | 0.62 | 0.23 | 0.84 | |||
| MTC | Deep | 0.64 | 0.28 | 0.85 | 0.74 | 0.43 | 0.89 | ||
| Superficial | 0.86 | 0.66 | 0.94 | 0.80 | 0.54 | 0.92 | |||
| MTP | Deep | 0.77 | 0.49 | 0.91 | 0.76 | 0.47 | 0.90 | ||
| Superficial | 0.83 | 0.62 | 0.93 | 0.88 | 0.72 | 0.95 | |||
| LF | LFA | Deep | 0.55 | 0.13 | 0.80 | 0.60 | 0.21 | 0.83 | |
| Superficial | 0.53 | 0.11 | 0.79 | 0.68 | 0.34 | 0.87 | |||
| LFC | Deep | 0.46 | 0.02 | 0.75 | 0.65 | 0.28 | 0.85 | ||
| Superficial | 0.40 | −0.06 | 0.72 | 0.50 | 0.07 | 0.78 | |||
| LFP | Deep | 0.44 | 0.00 | 0.75 | 0.56 | 0.15 | 0.81 | ||
| Superficial | 0.79 | 0.53 | 0.91 | 0.83 | 0.62 | 0.93 | |||
| LT | LTA | Deep | 0.84 | 0.63 | 0.94 | 0.77 | 0.49 | 0.91 | |
| Superficial | 0.62 | 0.24 | 0.84 | 0.63 | 0.25 | 0.84 | |||
| LTC | Deep | 0.82 | 0.59 | 0.93 | 0.69 | 0.34 | 0.87 | ||
| Superficial | 0.55 | 0.14 | 0.80 | 0.48 | 0.05 | 0.77 | |||
| LTP | Deep | 0.60 | 0.21 | 0.83 | 0.80 | 0.56 | 0.92 | ||
| Superficial | 0.41 | −0.04 | 0.73 | 0.73 | 0.42 | 0.89 | |||
| Less-weight-bearing areas | PMF |
Deep | 0.93 | 0.83 | 0.97 | 0.74 | 0.44 | 0.89 | |
| Superficial | 0.94 | 0.86 | 0.98 | 0.94 | 0.85 | 0.98 | |||
| PLF | Deep | 0.89 | 0.74 | 0.96 | 0.80 | 0.54 | 0.92 | ||
| Superficial | 0.76 | 0.48 | 0.90 | 0.82 | 0.59 | 0.93 | |||
CI, confidence interval; ICC, intraclass correlation coefficient; LF, lateral femoral condyle; LFA, lateral femoral anterior; LFC, lateral femoral central; LFP, lateral femoral posterior; LT, lateral tibial plateaus; LTA, lateral tibial anterior; LTC, lateral tibial central; LTP, lateral tibial posterior; MF, medial femoral condyle; MFA, medial femoral anterior; MFC, medial femoral central; MFP, medial femoral posterior; MT, medial tibial plateaus; MTA, medial tibial anterior; MTC, medial tibial central; MTP, medial tibial posterior; PLF, posterior lateral femoral groove; PMF, posterior medial femoral groove.
DISCUSSION
We retrospectively evaluated changes in the T2RVs of articular cartilage that underwent DB-ACLRs in a follow-up period of 6 months and correlated its biochemical changes with meniscal status and clinical findings. We found that the T2RV of articular cartilage on pre-operative MRI was increased in three of four subcompartments of the less-weight-bearing posterior femoral grooves (superficial PMF, deep PLF and superficial PLF) and in a subcompartment of the medial tibial plateau (superficial MTP) compared with that of the control group, whereas the T2RV of the articular cartilage on post-operative MRI was increased in a subcompartment of the medial femoral condyle (superficial MFP), two subcompartments of the medial tibial plateau (deep MTA and deep MTC), three subcompartments of the lateral femoral condyle (deep LFA, superficial LFA, deep LFC) and one subcompartment of the less-weight-bearing posterior medial femoral area (deep PMF) compared with that of pre-operative MRI. The subcompartments that showed significant differences in both comparisons were not overlapped. This finding may be related to a distinct degeneration pattern according to different instability mechanisms, one that occurs in patients with ACL deficiency (without surgery) and another in patients with reconstructed ACL.10,27–32 This emphasizes that the compartments that showed pre- and post-operative differences could be related not just to the initial trauma and instability but to surgery, the rehabilitation protocol or subsequent instability.
Our data showed biochemical changes in the articular cartilage after ACLR in various compartments (medial femoral, medial tibial and lateral femoral). A previous 6-month follow-up study that evaluated cartilage properties after ACLR depicted also changes in the medial compartments; however, in the lateral compartment, just the tibia was affected.20 Studies involving longer follow-up were not very well correlated with short-term follow-up.21,23,24 A study using T1 rho at 18-month follow-up MRI found that changes in T2RV were depicted only in the weight-bearing medial femur.21 Another one, during a 2-year follow-up, depicted initial cartilage changes in the central portion of the medial and anterior portions of the lateral femur.23 Other group emphasized changes in the central region of the weight-bearing portion of the medial compartment and posterior region of the lateral tibia at 1- and 2-year follow-ups.10,24
The T2RVs evaluation of the deep and superficial layers was performed just by a few other studies.10,24,27 In our sample, no preponderance of superficial or deep layer abnormalities was noted. A 2-year follow-up study that only evaluated the medial compartment in post-surgical ACL repair depicted persistent changes only in the deep layers of the central medial femur and posterior medial femur condyles.27 Conversely, a cohort with a 1- and 2-year follow-up period displayed cartilage damage at the superficial layers of the central regions of the medial femur and medial tibia and in the deep layer of the posterior lateral tibia in the first year and persistent cartilage abnormalities in the deep layer of the posterior tibia and superficial layer of the central medial femur condyle in the second year.10,24
The heterogeneous results across studies can be attributed to several factors. First, the follow-up period should be considered. Most previous studies included 1- or 2-year follow-up periods, whereas ours included a 6-month follow-up period. In longer follow-ups, medial compartment abnormalities can be related to an early degree of degeneration due to residual instability, whereas changes in the lateral compartments, some related to the initial trauma mechanism, are no longer observed.10,24,27 Second, a long-term healing process can be observed as demonstrated in a 2-year follow-up study in which cartilage changes in the medial compartment returned to baseline status in patients with intact menisci.27 Third, the rehabilitation protocol and return to sports can interfere with cartilage responses after ACLR. As in the present study, patients are normally discharged from rehabilitation and allowed to participate in sports after 6 months.23 However, in some studies, running or sport activities were allowed in selected patients 4 or 5 months after ACLR.10,20
An interesting result of this study was the elevated T2RVs at the deep layer of the less-weight-bearing posterior groove of the medial femur observed in the pre- and post-surgery groups. The posterior groove of the medial femur was already indicated as a region of maximum cartilage thinning at risk for pre-arthritic changes.33,34 Besides, it has been observed that during gait, patients with a ruptured ACL exhibit anterior tibial shifting that can be reduced by physiological responses, such as weaker quadriceps contraction and stronger hamstring contraction associated with a higher flexion angle.35 This higher flexion is also maintained for a longer time to accomplish a less abrupt weight shift during gait, even in ACL-reconstructed ligaments.32 All of these factors can be related to the observed degenerative changes that affect the posterior groove. The direct effect of arthroscopic surgery itself in terms of potential iatrogenic minor trauma by instruments or artificial joint effusion should be also considered as a possible explanation for the noted changes.
Unlike previous studies, cartilage changes in our sample were not correlated with the adjacent meniscal status.10,27,36 Instead, we observed a correlation between the medial meniscus status and T2RV of the lateral femur. The fact that medial meniscus status correlated with degeneration in the lateral femoral condyle can be related to the gravity of the primary injury or even to a loading environment change.36 Significant correlations between T2RVs and clinical data were observed just at the posterior medial and lateral femur. The moderate positive correlations found in the medial femur for the interval between injury and MRI, as well as for instability, might be attributable to prolonged altered biomechanics in patients sustaining an ACL rupture as indicated in previous studies.21,37 The moderate positive correlation found between Lysholm scores and T2 values suggest that better clinical scores do not reflect intact cartilage and that early degeneration can be found in asymptomatic patients. In a previous study, Lysholm scores were weakly and non-significantly correlated with T2 values.38
Our study had some limitations. First, it was retrospective, which could lead to selection bias. Second, owing to the applied exclusion criteria, the number of patients was small, which limited the study's power. Accordingly, larger-scale research is warranted to confirm our findings. Third, the control group did not include healthy subjects but rather individuals who had “normal” MRI without major complaints. Fourth, the short-term follow-up was not sufficient to observe a possible healing process.
In conclusion, patients submitted to DB-ACLR presented T2RV changes in both femoral and medial tibia condyles 6 months after surgery, affecting not just the weight-bearing areas but also the less-weight-bearing areas. No predilection concerning superficial or deep cartilage abnormalities were noted. Meniscal status did not influence T2RV, whereas instability and Lysholm score were moderately correlated with higher T2RV in the posterior groove of medial and posterior lateral femur, respectively.
Contributor Information
Ramon Gheno, Email: ramon.gheno@ufrgs.br.
Young Cheol Yoon, Email: youngcheol.yoon@gmail.com.
Joon H Wang, Email: mdwang88@gmail.com.
Kyunga Kim, Email: kyunga.j.kim@samsung.com.
Sun-Y Baek, Email: sy.baek@sbri.co.kr.
REFERENCES
- 1.Chu CR, Millis MB, Olson SA. Osteoarthritis: from palliation to prevention: AOA critical issues. J Bone Joint Surg Am 2014; 96: e130. doi: 10.2106/jbjs.m.01209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Potter HG, Jain SK, Ma Y, Black BR, Fung S, Lyman S. Cartilage injury after acute, isolated anterior cruciate ligament tear: immediate and longitudinal effect with clinical/MRI follow-up. Am J Sports Med 2012; 40: 276–85. doi: 10.1177/0363546511423380 [DOI] [PubMed] [Google Scholar]
- 3.Louboutin H, Debarge R, Richou J, Selmi TA, Donell ST, Neyret P, et al. Osteoarthritis in patients with anterior cruciate ligament rupture: a review of risk factors. Knee 2009; 16: 239–44. doi: 10.1016/j.knee.2008.11.004 [DOI] [PubMed] [Google Scholar]
- 4.Oiestad BE, Holm I, Aune AK, Gunderson R, Myklebust G, Engebretsen L, et al. Knee function and prevalence of knee osteoarthritis after anterior cruciate ligament reconstruction: a prospective study with 10 to 15 years of follow-up. Am J Sports Med 2010; 38: 2201–10. doi: 10.1177/0363546510373876 [DOI] [PubMed] [Google Scholar]
- 5.Karlsson J, Irrgang JJ, van Eck CF, Samuelsson K, Mejia HA, Fu FH. Anatomic single- and double-bundle anterior cruciate ligament reconstruction, part 2: clinical application of surgical technique. Am J Sports Med 2011; 39: 2016–26. doi: 10.1177/0363546511402660 [DOI] [PubMed] [Google Scholar]
- 6.Van Ginckel A, Verdonk P, Witvrouw E. Cartilage adaptation after anterior cruciate ligament injury and reconstruction: implications for clinical management and research? A systematic review of longitudinal MRI studies. Osteoarthritis Cartilage 2013; 21: 1009–24. doi: 10.1016/j.joca.2013.04.015 [DOI] [PubMed] [Google Scholar]
- 7.Lorbach O, Kieb M, Domnick C, Herbort M, Weyers I, Raschke M, et al. Biomechanical evaluation of knee kinematics after anatomic single- and anatomic double-bundle ACL reconstructions with medial meniscal repair. Knee Surg Sports Traumatol Arthrosc 2015; 23: 2734–41. doi: 10.1007/s00167-014-3071-9 [DOI] [PubMed] [Google Scholar]
- 8.Sun R, Chen BC, Wang F, Wang XF, Chen JQ. Prospective randomized comparison of knee stability and joint degeneration for double- and single-bundle ACL reconstruction. Knee Surg Sports Traumatol Arthrosc 2015; 23: 1171–8. doi: 10.1007/s00167-014-2934-4 [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Li J, Wang J, Gao S, Zhang Y. Comparison of single-bundle and double-bundle isolated posterior cruciate ligament reconstruction with allograft: a prospective, randomized study. Arthroscopy 2014; 30: 695–700. doi: 10.1016/j.arthro.2014.02.035 [DOI] [PubMed] [Google Scholar]
- 10.Li X, Kuo D, Theologis A, Carballido-Gamio J, Stehling C, Link TM, et al. Cartilage in anterior cruciate ligament-reconstructed knees: MR imaging T1{rho} and T2–initial experience with 1-year follow-up. Radiology 2011; 258: 505–14. doi: 10.1148/radiol.10101006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Theologis AA, Kuo D, Cheng J, Bolbos RI, Carballido-Gamio J, Ma CB, et al. Evaluation of bone bruises and associated cartilage in anterior cruciate ligament-injured and -reconstructed knees using quantitative t(1rho) magnetic resonance imaging: 1-year cohort study. Arthroscopy 2011; 27: 65–76. doi: 10.1016/j.arthro.2010.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baum T, Joseph GB, Karampinos DC, Jungmann PM, Link TM, Bauer JS. Cartilage and meniscal T2 relaxation time as non-invasive biomarker for knee osteoarthritis and cartilage repair procedures. Osteoarthritis Cartilage 2013; 21: 1474–84. doi: 10.1016/j.joca.2013.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller TT. MR imaging of the knee. Sports Med Arthrosc 2009; 17: 56–67. doi: 10.1097/JSA.0b013e3181974353 [DOI] [PubMed] [Google Scholar]
- 14.Hesper T, Hosalkar HS, Bittersohl D, Welsch GH, Krauspe R, Zilkens C, et al. T2* mapping for articular cartilage assessment: principles, current applications, and future prospects. Skeletal Radiol 2014; 43: 1429–45. doi: 10.1007/s00256-014-1852-3 [DOI] [PubMed] [Google Scholar]
- 15.Kijowski R, Chaudhary R. Quantitative magnetic resonance imaging of the articular cartilage of the knee joint. Magn Reson Imaging Clin N Am 2014; 22: 649–69. doi: 10.1016/j.mric.2014.07.005 [DOI] [PubMed] [Google Scholar]
- 16.Hirose J, Nishioka H, Okamoto N, Oniki Y, Nakamura E, Yamashita Y, et al. Articular cartilage lesions increase early cartilage degeneration in knees treated by anterior cruciate ligament reconstruction: T1rho mapping evaluation and 1-year follow-up. Am J Sports Med 2013; 41: 2353–61. doi: 10.1177/0363546513496048 [DOI] [PubMed] [Google Scholar]
- 17.Matzat SJ, van Tiel J, Gold GE, Oei EH. Quantitative MRI techniques of cartilage composition. Quant Imaging Med Surg 2013; 3: 162–74. doi: 10.3978/j.issn.2223-4292.2013.06.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crema MD, Roemer FW, Marra MD, Burstein D, Gold GE, Eckstein F, et al. Articular cartilage in the knee: current MR imaging techniques and applications in clinical practice and research. Radiographics 2011; 31: 37–61. doi: 10.1148/rg.311105084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kijowski R, Blankenbaker DG, Munoz Del Rio A, Baer GS, Graf BK. Evaluation of the articular cartilage of the knee joint: value of adding a T2 mapping sequence to a routine MR imaging protocol. Radiology 2013; 267: 503–13. doi: 10.1148/radiol.12121413 [DOI] [PubMed] [Google Scholar]
- 20.Van Ginckel A, Verdonk P, Victor J, Witvrouw E. Cartilage status in relation to return to sports after anterior cruciate ligament reconstruction. Am J Sports Med 2013; 41: 550–9. doi: 10.1177/0363546512473568 [DOI] [PubMed] [Google Scholar]
- 21.Haughom B, Schairer W, Souza RB, Carpenter D, Ma CB, Li X. Abnormal tibiofemoral kinematics following ACL reconstruction are associated with early cartilage matrix degeneration measured by MRI T1rho. Knee 2012; 19: 482–7. doi: 10.1016/j.knee.2011.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee YS, Jeong YM, Sim JA, Kwak JH, Kim KH, Nam SW, et al. Specific compartmental analysis of cartilage status in double-bundle ACL reconstruction patients: a comparative study using pre- and postoperative MR images. Knee Surg Sports Traumatol Arthrosc 2013; 21: 702–7. doi: 10.1007/s00167-012-2046-y [DOI] [PubMed] [Google Scholar]
- 23.Li H, Tao H, Hua Y, Chen J, Li Y, Chen S. Quantitative magnetic resonance imaging assessment of cartilage status: a comparison between young men with and without anterior cruciate ligament reconstruction. Arthroscopy 2013; 29: 2012–9. doi: 10.1016/j.arthro.2013.09.075 [DOI] [PubMed] [Google Scholar]
- 24.Su F, Hilton JF, Nardo L, Wu S, Liang F, Link TM, et al. Cartilage morphology and T1rho and T2 quantification in ACL-reconstructed knees: a 2-year follow-up. Osteoarthritis Cartilage 2013; 21: 1058–67. doi: 10.1016/j.joca.2013.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alizai H, Roemer FW, Hayashi D, Crema MD, Felson DT, Guermazi A. An update on risk factors for cartilage loss in knee osteoarthritis assessed using MRI-based semiquantitative grading methods. Eur Radiol 2015; 25: 883–93. doi: 10.1007/s00330-014-3464-7 [DOI] [PubMed] [Google Scholar]
- 26.Bengtsson J, Mollborg J, Werner S. A study for testing the sensitivity and reliability of the Lysholm knee scoring scale. Knee Surg Sports Traumatol Arthrosc 1996; 4: 27–31. doi: 10.1007/BF01565994 [DOI] [PubMed] [Google Scholar]
- 27.Chu CR, Williams AA, West RV, Qian Y, Fu FH, Do BH, et al. Quantitative magnetic resonance imaging UTE-T2* mapping of cartilage and meniscus healing after anatomic anterior cruciate ligament reconstruction. Am J Sports Med 2014; 42: 1847–56. doi: 10.1177/0363546514532227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tashman S, Collon D, Anderson K, Kolowich P, Anderst W. Abnormal rotational knee motion during running after anterior cruciate ligament reconstruction. Am J Sports Med 2004; 32: 975–83. doi: 10.1177/0363546503261709 [DOI] [PubMed] [Google Scholar]
- 29.Oberlander KD, Bruggemann GP, Hoher J, Karamanidis K. Knee mechanics during landing in anterior cruciate ligament patients: a longitudinal study from pre- to 12 months post-reconstruction. Clin Biomech (Bristol, Avon) 2014; 29: 512–7. doi: 10.1016/j.clinbiomech.2014.03.014 [DOI] [PubMed] [Google Scholar]
- 30.Li G, Moses JM, Papannagari R, Pathare NP, DeFrate LE, Gill TJ. Anterior cruciate ligament deficiency alters the in vivo motion of the tibiofemoral cartilage contact points in both the anteroposterior and mediolateral directions. J Bone Joint Surg Am 2006; 88: 1826–34. doi: 10.2106/jbjs.e.00539 [DOI] [PubMed] [Google Scholar]
- 31.Carpenter RD, Majumdar S, Ma CB. Magnetic resonance imaging of 3-dimensional in vivo tibiofemoral kinematics in anterior cruciate ligament-reconstructed knees. Arthroscopy 2009; 25: 760–6. doi: 10.1016/j.arthro.2009.01.014 [DOI] [PubMed] [Google Scholar]
- 32.Gao B, Zheng NN. Alterations in three-dimensional joint kinematics of anterior cruciate ligament-deficient and -reconstructed knees during walking. Clin Biomech (Bristol, Avon) 2010; 25: 222–9. doi: 10.1016/j.clinbiomech.2009.11.006 [DOI] [PubMed] [Google Scholar]
- 33.Vignon E. Radiographic issues in imaging the progression of hip and knee osteoarthritis. J Rheumatol Suppl 2004; 70: 36–44. [PubMed] [Google Scholar]
- 34.Hayashi D, Felson DT, Niu J, Hunter DJ, Roemer FW, Aliabadi P, et al. Pre-radiographic osteoarthritic changes are highly prevalent in the medial patella and medial posterior femur in older persons: Framingham OA study. Osteoarthritis Cartilage 2014; 22: 76–83. doi: 10.1016/j.joca.2013.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen CH, Li JS, Hosseini A, Gadikota HR, Gill TJ, Li G. Anteroposterior stability of the knee during the stance phase of gait after anterior cruciate ligament deficiency. Gait Posture 2012; 35: 467–71. doi: 10.1016/j.gaitpost.2011.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Souza RB, Wu SJ, Morse LJ, Subburaj K, Allen CR, Feeley BT. Cartilage MRI relaxation times after arthroscopic partial medial meniscectomy reveal localized degeneration. Knee Surg Sports Traumatol Arthrosc 2015; 23: 188–97. doi: 10.1007/s00167-014-2997-2 [DOI] [PubMed] [Google Scholar]
- 37.Kumar D, Kothari A, Souza RB, Wu S, Benjamin Ma C, Li X. Frontal plane knee mechanics and medial cartilage MR relaxation times in individuals with ACL reconstruction: a pilot study. Knee 2014; 21: 881–5. doi: 10.1016/j.knee.2014.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Welsch GH, Trattnig S, Domayer S, Marlovits S, White LM, Mamisch TC. Multimodal approach in the use of clinical scoring, morphological MRI and biochemical T2-mapping and diffusion-weighted imaging in their ability to assess differences between cartilage repair tissue after microfracture therapy and matrix-associated autologous chondrocyte transplantation: a pilot study. Osteoarthritis Cartilage 2009; 17: 1219–27. doi: 10.1016/j.joca.2009.03.018 [DOI] [PubMed] [Google Scholar]