Abstract
BACKGROUND
MicroRNAs (miRNAs) are small, noncoding RNAs that play an important role in regulating various biological processes through their interaction with cellular messenger RNAs. Extracellular miRNAs in serum, plasma, saliva, and urine have recently been shown to be associated with various pathological conditions including cancer.
METHODS
With the goal of assessing the distribution of miRNAs and demonstrating the potential use of miRNAs as biomarkers, we examined the presence of miRNAs in 12 human body fluids and urine samples from women in different stages of pregnancy or patients with different urothelial cancers. Using quantitative PCR, we conducted a global survey of the miRNA distribution in these fluids.
RESULTS
miRNAs were present in all fluids tested and showed distinct compositions in different fluid types. Several of the highly abundant miRNAs in these fluids were common among multiple fluid types, and some of the miRNAs were enriched in specific fluids. We also observed distinct miRNA patterns in the urine samples obtained from individuals with different physiopathological conditions.
CONCLUSIONS
MicroRNAs are ubiquitous in all the body fluid types tested. Fluid type–specific miRNAs may have functional roles associated with the surrounding tissues. In addition, the changes in miRNA spectra observed in the urine samples from patients with different urothelial conditions demonstrates the potential for using concentrations of specific miRNAs in body fluids as biomarkers for detecting and monitoring various physiopathological conditions.
MicroRNAs (miRNAs)5 are small, 19- to 23-nucleotide-long, single-stranded RNA molecules. It has been shown that miRNAs can affect the stability of messenger RNA (mRNA) and in some cases influence protein synthesis through partial sequence complementation with their interacting mRNA targets (1, 2). The specific regulation and biological function of miRNAs is largely unknown. The network of interactions is probably as complicated as other biomolecular networks in the body. Unlike most classes of biomolecules, however, there are far fewer known miRNA species. Like mRNAs, some miRNAs also show restricted tissue distribution; for example, miR-122 is highly enriched in liver, whereas miR-124 is preferentially expressed in neurological tissues (3, 4). It has been shown that changes in the spectrum of cellular miRNAs correlate with various physiopathological conditions, including differentiation, inflammation, diabetes, and several types of cancers (5–11). Currently, there are 706 known miRNAs for humans and 547 for mice (miRBase release 13.0), and they do not have known postprocessing modifications, which makes their composition much less complex than that of other biomolecules. Recently, some of the miRNAs previously identified in cells and tissues have also been found in extracellular fluids such as plasma, serum, saliva, and urine (12–15). The level and composition of these extracellular miRNAs again show changes that correlate well with diseases or injurious conditions (14–19). These observations suggest that extracellular miRNAs can be used as informative biomarkers to assess and monitor the body’s physiopathological status.
The ideal biomarker must be accessible using noninvasive protocols, inexpensive to quantify, specific to the disease of interest, translatable from model systems to humans, and a reliable early indication of disease before clinical symptoms appear. Biomarkers that can be used to stratify disease and assess response to therapeutics are also medically valuable. Although most current biomarkers are protein based, challenges for developing new protein-based biomarkers include the complexity of protein composition in most biological samples (especially blood), the assorted posttranslational modifications of proteins, the low abundance of many proteins of interest in serum and plasma, and the difficulty of reliably developing suitable high-affinity capture agents. These intricacies make the discovery and development of additional protein-based biomarkers with the proper diagnostic specificity and sensitivity an expensive, time-consuming, and difficult task. Detecting specific miRNA species, while not trivial, is generally much easier. Synthetic complimentary oligonucleotides can deliver sufficient detection specificity in most cases, and PCR or other DNA amplification methods can be used to improve the detection limit. Lower complexity, no known postprocessing modifications, simple detection and amplification methods, tissue-restricted expression profiles, and sequence conservation between humans and model organisms make extracellular miRNAs ideal candidates for noninvasive biomarkers to reflect and study various physiopathological conditions in the body.
To better understand the distribution of miRNA in body fluids, we examined the spectrum of miRNA in 12 fluids: plasma, saliva, tears, urine, amniotic fluid, colostrum, breast milk, bronchial lavage, cerebrospinal fluid, peritoneal fluid, pleural fluid, and seminal fluid from normal individuals. As a proof of principle, we also examined several urine samples from individuals with a variety of urothelial conditions, including pregnancy, renal cancer, and bladder cancer.
Materials and Methods
SAMPLE COLLECTION, PREPARATION, AND RNA ISOLATION
We obtained body fluid samples from 5 healthy individual donors (except for colostrum from just 1 donor) from Bioreclamation Inc., and pregnancy samples, with 1 sample from 1 donor for each trimester, from Innovative Research. Normal control urine and urine from patients with urothelial cancers were collected by 1 of the authors (K.H.H.). We deidentified and collected all of the samples with the proper approval by institutional review boards. Samples were centrifuged at 1000g for 10 min to pellet cellular debris. We discarded the upper layer of fat from the breast milk and colostrum samples before using the supernatant for RNA extraction. We extracted total RNA from 300 μL fluids using the miRNeasy kit (Qiagen) as described (18).
We assessed the extracted RNA for quality and quantity using an Agilent 2100 Bioanalyzer and Nano-Drop 1000 spectrophotometer (Thermo Scientific). For the bioanalyzer, the RNA 6000 Pico chip was used for quantification and an initial quality measurement, followed by the Small RNA chip to gain a more detailed view of RNAs in the 6- to 150-nucleotide size range.
miRNA MEASUREMENT
We performed quantitative real-time PCR (qPCR) according to manufacturer’s instructions (Qiagen) to profile the miRNA distribution in body fluid samples. In brief, 5 μL total RNA was collected and pooled from the samples from the same fluid type, and the cDNA was produced using the miScript Reverse Transcription kit (Qiagen). We used the Matrix Hydra eDrop (Thermo Scientific) to mix the cDNA sample and the qPCR master reagent [Human miScript Assay 384 set v10.1 (Qiagen)] to reduce pipetting error. Any wells with multiple melting-temperature values were excluded from further analysis. We also used individual Human miScript Assays to validate the 384 miRNA qPCR set. Data were analyzed using SDS Enterprise Database 2.3 (Applied Biosystems) and normalized with global mean instead of specific miRNA or noncoding RNA signals.
Results
The body fluids tested can be divided into 2 different types. The first are those that can be obtained without any invasive means: breast milk, colostrum, saliva, seminal fluid, tears, and urine. The other type of body fluids—amniotic fluid, cerebrospinal fluid, plasma, pleural fluid, and peritoneal fluid—are maintained within the body to serve as transportation systems to deliver nutrients or remove waste or as lubricants/protective barriers between organs. Invasive procedures are required to acquire these fluids. Among the body fluids tested, amniotic fluid, breast milk, colostrum, and seminal fluid are unique to 1 sex.
LARGE RNA CONCENTRATION VARIATION AMONG DIFFERENT BODY FLUIDS
Excluding colostrum, we had 5 independent samples from each of the 12 body fluid types from healthy donors. The amount of RNA extracted from the different types of body fluids ranged from 113 to 48 240 μg/L (Table 1), based on the estimation from the Agilent 2100 Bioanalyzer. In general, cerebrospinal fluid, tears, and urine contained less RNA than breast milk and seminal fluid. Like total RNA, the concentration of RNA in the miRNA size range (10–40 nucleotides) also exhibited large variations. Although this is the fraction that contained miRNA, this fraction also probably contained large amounts of degraded RNA molecules. Therefore, the true miRNA concentration was difficult to estimate from the body fluid samples.
Table 1.
Concentration of RNA isolated from different body fluids.a
| Sample | Median total RNA concentration, μg/L (interquartile range) | Number of detectable miRNAs |
|---|---|---|
| Amniotic fluid | 570 (354) | 359 |
| Breast milk | 47 240 (73 180) | 429 |
| Bronchial lavage | 1128 (886) | 260 |
| Cerebrospinal fluid | 111 (66) | 212 |
| Colostrum | 585 (NA) | 386 |
| Peritoneal fluid | 775 (345) | 397 |
| Plasma | 308 (104) | 349 |
| Pleural fluid | 470 (190) | 210 |
| Saliva | 1945 (2495) | 458 |
| Seminal fluid | 17770 (7673) | 436 |
| Tears | 564 (631) | 320 |
| Urine | 94 (129) | 204 |
As estimated by the Agilent 2100 Bioanalyzer using the RNA 6000 Pico Total RNA chip, median concentration across all 5 samples except colostrum. The number of detected miRNAs in each body fluid is based on the number of miRNA species with a level of >80% of the global mean.
BODY FLUIDS SHARE A LARGE NUMBER OF miRNAS
We performed a survey of the miRNA composition of the body fluids using the Human miScript Assay panel from Qiagen, which included assays for 714 different human miRNA species. The number of detectable miRNAs from the qPCR results ranged from 210 miRNAs in pleural fluid to 458 miRNAs in saliva, based on the number of miRNAs that had concentrations >80% of the global mean among the samples from the same body fluid type. The individual miRNA concentrations from different fluid types are listed in Supplemental Table 1, which accompanies the online version of this article at www.clinchem.org/content/vol56/issue11. Saliva, breast milk, and seminal fluid had a higher number of detectable miRNA species, whereas urine, cerebrospinal fluid, and pleural fluid had far fewer. A total of 600 different miRNAs could be detected in at least 1 body fluid type, and 61 of these miRNA species were detectable in all body fluid types (see online Supplemental Table 1). The 20 miRNAs with the highest concentrations for each body fluid type, based on qPCR assays, are shown in Table 2. Some of the highly abundant miRNA species were shared among different fluid types, which suggested a common function or origin of these miRNAs; miR-509-5p, miR-515-3p, and miR-335* were among the most abundant miRNAs in most of the body fluid samples tested. Based on the list of detectable miRNAs, several miRNAs were uniquely present in specific body fluid types, such as miR-224 in plasma, miR-637 in tears, miR-193b in breast milk, and miR-508-5p in seminal fluid. Plasma had the highest number of unique miRNA species, followed by saliva. Urine, bronchial lavage, and pleural fluid had no unique miRNA species in the samples we tested (Table 2).
Table 2.
The top twenty most abundant miRNAs from each fluid type.a
| Plasma | Tears | Urine | Breast milk | Seminal fluid | Saliva | Amniotic fluid | Bronchial lavage | Cerebrospinal fluid | Pleural fluid | Peritoneal fluid | Colostrum |
|---|---|---|---|---|---|---|---|---|---|---|---|
| miR-335* | miR-335* | miR-515-3p | miR-335* | miR-518e | miR-335* | miR-518e | miR-515-3p | miR-335* | miR-515-3p | miR-515-3p | miR-509-5p |
| miR-325 | miR-515-3p | miR-335* | miR-26a-2* | miR-590-3p | miR-892a | miR-335* | miR-335* | miR-515-3p | miR-892a | miR-892a | miR-181d |
| miR-377* | miR-137 | miR-892a | miR-181d | miR-588b | miR-515-3p | miR-302cb | miR-509-5p | miR-892a | miR-223* | miR-518e | miR-335* |
| miR-586b | miR-509-5p | miR-509-5p | miR-509-5p | miR-873 | miR-545* | miR-515-3p | miR-616* | miR-873 | miR-616* | miR-134 | miR-518e |
| miR-518e | miR-590-3p | miR-223* | miR-524-5p | miR-590-5p | miR-27ab | miR-452b | miR-545* | miR-509-5p | miR-873 | miR-509-5p | miR-515-5p |
| let-7i | miR-873 | miR-873 | miR-137 | miR-197b | miR-518e | miR-892a | miR-302d | miR-223* | miR-335* | miR-223* | miR-223* |
| miR-539 | miR-892a | miR-302d | miR-26a-1* | miR-218 | miR-509-5p | miR-671-5p | miR-223* | miR-302d | miR-302d | miR-515-5p | miR-671-5p |
| miR-616* | miR-223* | miR-616* | miR-595b | miR-515-5p | miR-492b | miR-515-5p | miR-25* | miR-616* | miR-134 | miR-616* | miR-873 |
| miR-302d | miR-302d | miR-134 | miR-580 | miR-137 | miR-483-5p | miR-137 | miR-134 | miR-483-5p | miR-509-5p | miR-302d | miR-483-5p |
| miR-589 | miR-590-5p | miR-923 | miR-130a | miR-335* | miR-923 | miR-593*b | miR-483-5p | miR-134 | miR-377* | miR-873 | miR-186b |
| miR-556-3p | miR-195b | miR-483-5p | miR-515-3p | miR-617b | miR-873 | miR-590-3p | miR-923 | miR-518e | miR-589 | miR-483-5p | miR-515-3p |
| miR-151-3p | miR-130a | miR-101* | miR-513cb | miR-410 | miR-92b | miR-873 | miR-518e | miR-923 | miR-518e | miR-923 | miR-616* |
| miR-548b-3p | miR-675b | miR-325 | miR-671-5p | miR-524-5p | miR-302d | miR-410 | miR-548b-3p | miR-377* | miR-556-3p | miR-374ab | miR-134 |
| miR-192b | miR-29bb | miR-589 | miR-490-5pb | miR-20bb | let-7ab | miR-509-5p | miR-1225-3pb | miR-589 | miR-652b | miR-598 | miR-892a |
| miR-151-5p | miR-616* | miR-556-3p | miR-367b | miR-181d | miR-580 | miR-548d-5pb | miR-325 | miR-556-3p | miR-590-5p | miR-548b-3p | miR-590-5p |
| miR-598 | miR-410 | miR-545* | miR-181bb | miR-1b | miR-616* | miR-223* | miR-589 | miR-652 | miR-923 | miR-1238b | miR-590-3p |
| miR-187b | miR-101* | miR-377* | miR-598 | miR-671-5p | miR-134 | miR-616* | miR-92b | miR-498 | miR-371-3p | miR-92b | miR-425b |
| miR-873 | miR-134 | let-7i | miR-515-5p | miR-509-5p | miR-25* | miR-148b*b | miR-371–3p | miR-767–3pb | miR-890 | miR-498 | miR-454b |
| miR-218 | miR-487b | miR-890 | miR-578b | miR-515-3p | let-7i | miR-590-5p | miR-324–3pb | miR-505 | miR-498 | miR-937b | miR-101* |
| miR-923 | miR-483-5p | miR-505 | miR-487b | miR-892a | miR-410 | miR-302d | miR-885–5pb | miR-151–5p | miR-483-5p | miR-377* | miR-132b |
The miRNA species are arranged in descending order with the highest concentration as the top value.
miRNA species that occur in only 1 fluid in this list.
BODY FLUID TYPES SHARE SIMILAR miRNA PROFILES
Unsupervised hierarchical clustering (HCL) analysis of the common “expressed” miRNAs (concentration >80% of the global mean) revealed 2 major groups (Fig. 1). The miRNA spectrum in plasma was different from that of most of the other body fluids, indicating an extensive “filtering” process separating the plasma from other body fluid types, biases caused by the differential uptake or release of miRNAs from different circulating cell types that come in contact with the blood, or other processes not yet understood. The reproduction-related body fluids breast milk, seminal fluid, and amniotic fluid all clustered together with bronchial lavage, peritoneal fluid, saliva, and tears. Interestingly, tears contained a large number of miRNAs, which may suggest an important role for miRNAs in maintaining or regulating the normal function of the eyes. Urine, cerebrospinal fluid, and pleural fluid were the 3 fluid types containing the least amount of detectable miRNA (Table 1) and were clustered together.
Fig. 1. The body fluid types can be grouped into 2 major clusters based on the profile of commonly expressed miRNAs (unsupervised hierarchical clustering).
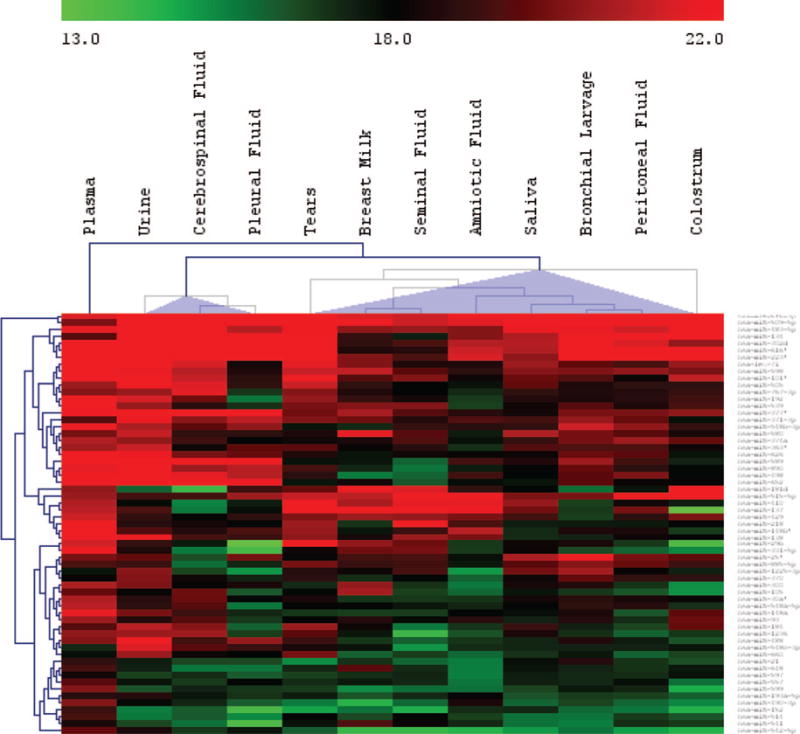
Plasma is separate from the 2 major clusters.
URINE miRNA SPECTRA SHOWED PROMISE AS DIAGNOSTIC TOOLS FOR UROTHELIAL CONDITIONS
As a proof of principle for using the miRNAs in body fluids as biomarkers, we inspected the miRNA spectra of urine samples from 3 patients with upper urinary tract urothelial cancer and from 3 patients with bladder urothelial cancer. In addition, we also included urine samples from 3 normal individuals and 1 sample each from women in each trimester of pregnancy. The number of detectable miRNA species ranged from 230 to 336 (see complete list of detectable miRNA species in online Supplemental Table 2). There were generally a higher number of detectable miRNA species in the samples from patients with urothelial cancers. We also observed an increase in the number of detectable miRNA species in the first trimester of pregnancy, with 336 detectable miRNA species in first-trimester sample vs a mean of 241 among the normal urine samples. This may be caused by normal physiological changes during pregnancy.
Several miRNA species, such as miR-105*, miR-449-3p, and miR-514, were observed only in samples other than the normal controls (Fig. 2A). Other miRNAs, such as miR-620 and miR-125a-3p, were observed only in samples from cancer patients. miR-200a and miR-891b were not observed in samples from patients with bladder urothelial cancers, whereas miR-1 and miR-449b were not observed in samples from upper urothelial cancer patients. Among the commonly expressed miRNA species, the concentrations for miR-1236, miR-374a, and miR-767-3p were higher in cancer samples, whereas the concentrations for miR-509-5p were lower in cancer samples (Fig. 2B). The complete list of miRNA species can be found in online Supplemental Table 2. The differences in the urine miRNA spectra among various urothelial conditions suggest the possibility that changes in the miRNA composition in body fluids can be used to identify physiopathological conditions.
Fig. 2. miRNA distribution in various urine samples.
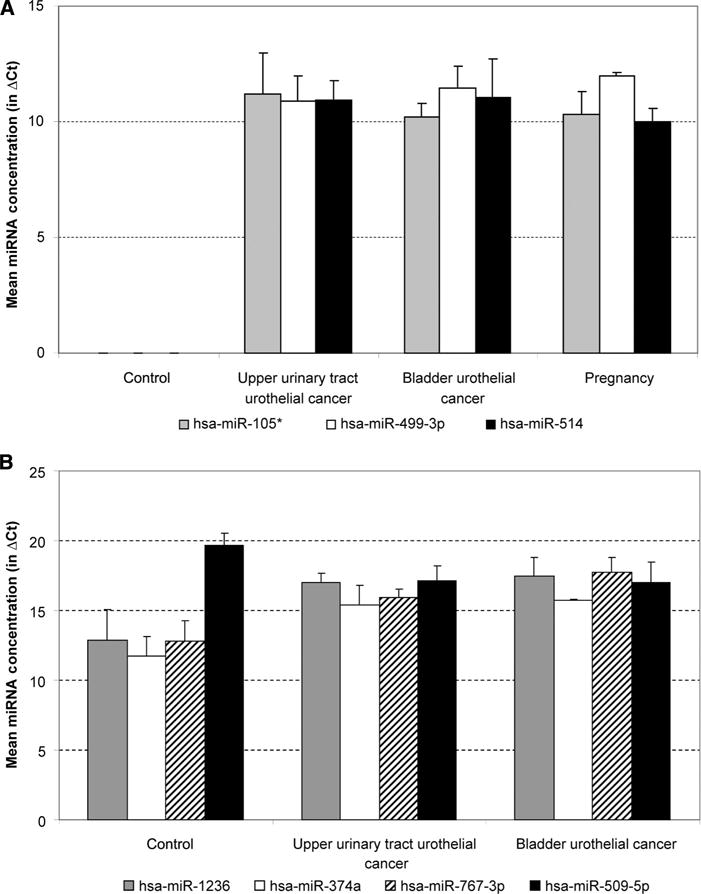
(A), Examples of miRNAs detectable only in urine samples other than the control samples. (B), miRNAs that show differences between the urothelial cancers. ΔCt, difference in cycle threshold values.
Discussion
In this study, we investigated the distribution of miRNA in 12 body fluids. Total RNA concentrations varied among different body fluids tested. Part of these RNAs may come from cells or cell debris; however, we did not observe any ribosomal RNA bands from bioanalyzer images. The large interfluid RNA concentration differences could be caused either by real physiological differences or by procedural artifacts. Blood contamination and hemolysis are the 2 most likely artifacts for fluid types that are acquired by invasive means, such as cerebrospinal fluid, bronchial lavage, peritoneal fluid, and pleural fluid. However, the miRNA clustering analysis grouped these fluids with saliva and urine, which makes the likelihood of substantial blood contamination low.
One of the challenges in analyzing extracellular body fluid miRNA is data normalization. For cellular miRNAs, the concentrations of several noncoding small nuclear RNAs, such as U6 and SNORD 44, have been used for sample-to-sample normalization, like the use of housekeeping genes in mRNA analysis. Global normalization procedures, such as using global mean, are normally used for extracellular miRNA measurement because there are no known suitable gene or miRNA candidates for normalization purposes. Creatinine is a product of normal muscle metabolism and is produced and removed through the kidney at a relatively constant rate. The amount of urine creatinine has been used to normalize urine concentrations and other substances measured in urine, such as proteins. Thus, it would be of value to explore the possibility of using blood and urine creatinine concentrations to normalize plasma and urine miRNA measurements.
One of the most interesting observations was the diverse, high concentration of miRNAs in tears. The variation in concentrations of specific proteins such as interleukin-6 have been identified and investigated in tears, and have been suggested as biomarkers for Sjögren’s syndrome, ocular rosacea, and glaucoma (20–24). This finding, in combination with the large number of miRNAs in tears, provides the potential for miRNA-based biomarkers that may help to offer an early, noninvasive diagnosis for glaucoma, age-related macular degeneration, and malignancies.
Among the 20 most abundant miRNAs present in all or most of the fluid types (Table 2), miR-335*, miR-509-5p, and miR-515-3p were found in 11 of the 12 fluids, and miR-873 and miR-616* were found in 10, suggesting a common origin for all of the fluid types in the body or a common functional role for these miRNAs.
With the exception of tears and urine, several miRNAs were enriched in select fluid types (Table 3). Plasma contained the highest number of “enriched” or “specific” miRNA species, whereas urine, pleural fluid, and bronchial lavage had none. A pairwise comparison (Jaccard similarity coefficient, results listed in online Supplemental Table 3) of the commonly detected miRNA species between the different fluid types showed that plasma shares a large number of miRNAs with saliva, perhaps the result of exchange between the 2 fluids. Plasma and urine shared the fewest number of commonly detectable miRNA species, suggesting that a majority of the circulating miRNAs were either picked up by the kidneys or were destroyed in the urine. Amniotic fluid had a large number of detectable miRNA species (359 miRNAs), but the composition of these miRNAs did not show substantial overlap with the other body fluids tested. This may result from a filtering process by the placenta that reduces the content exchange between amniotic fluid and other body fluids.
Table 3.
The miRNA species that are uniquely detected in each of the different body fluid types.
| Plasma | Tears | Breast milk | Seminal fluid | Saliva | Amniotic fluid | Cerebrospinal fluid | Peritoneal fluid | Colostrum |
|---|---|---|---|---|---|---|---|---|
| miR-224 | miR-637 | miR-193b | miR-508-5p | miR-182* | miR-636 | miR-577 | miR-129* | miR-18a* |
| miR-483-3p | miR-10a | miR-644 | miR-450b-5p | miR-92a-1* | miR-583 | miR-513a-5p | ||
| miR-518f* | miR-28-5p | miR-17 | miR-622 | miR-376b | miR-223 | miR-10b* | ||
| miR-508-3p | miR-924 | miR-380* | miR-141 | miR-26b | miR-627 | miR-192* | ||
| miR-551b | miR-150* | miR-29b-2* | miR-26a | miR-556-5p | miR-29b-1* | miR-193b* | ||
| miR-182 | miR-518c* | miR-340 | miR-145* | miR-593* | miR-130a* | |||
| miR-135a* | miR-217 | miR-135b* | ||||||
| miR-139-3p | miR-381 | |||||||
| miR-801 | miR-96* | |||||||
| miR-369-3p | miR-1228 | |||||||
| miR-519d | miR-431* | |||||||
| miR-299-5p | ||||||||
| miR-373 | ||||||||
| miR-330-5p |
An interesting observation from our data was the distribution of miR-302c, 1 of the most abundant miRNA species in amniotic fluid (Table 2 and Fig. 3). This miRNA is probably involved in maintaining the “stemness” status of cells (25, 26). The high abundance of miR-302c in amniotic fluid suggests that it may be involved in maintaining the development of the embryo. Amniotic fluid, seminal fluid, and breast milk also contain several highly abundant miRNAs that also have high expression in related tissues. For example, miR-452 and miR-26b were highly abundant or uniquely detected in amniotic fluid (Figs. 2 and 3), and both are also highly expressed in the placenta (27). In addition, miR-508 was uniquely detected in seminal fluid (Fig. 3) and is highly specific to the testis, and miR-518c* was uniquely detected in breast milk and is also highly expressed in the placenta (27). It is interesting to note that most of the high correlations between fluid miRNA concentration and tissue specificity involve the reproductive systems, consistent with a unique role for miRNAs in these systems.
Fig. 3. Mean concentration of miR-302c across different fluid types.
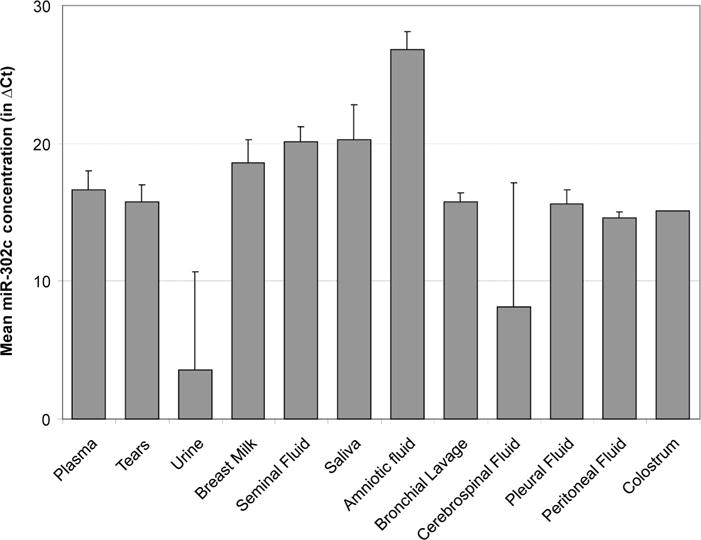
The concentrations were averaged among all samples from the same fluid type. Amniotic fluid shows a higher concentration than the other body fluid types.
Urothelial carcinoma is the most common cancer of the urinary tract. Although upper-tract urothelial carcinoma of the kidney is rare in the US, bladder urothelial carcinoma is quite common, with approximately 67 000 cases every year (28). Current detection of urothelial cancers involves the use of cystoscopy, radiographic imaging, and urine cytology, which are expensive and usually invasive. The protein-based markers nuclear matrix protein 22 (NMP22), complement factor H (CFH), and bladder cancer–specific nuclear matrix protein (BLCA-4) in urine lack the desired diagnostic sensitivity and specificity (20, 21, 29). Recently, changes in the concentrations of 2 specific miRNAs, miR-126 and miR-182, as tumor markers in urine for bladder cancer have been reported (13). We also observed changes in a number of urinary miRNAs among different urothelial cancers, though we could not detect these specific miRNAs in our samples (Fig. 2 and online Supplemental Table 2). This difference may be due to urine concentration, different disease conditions, variations in sample processing, and different miRNA assay platforms. However, caution is called for when interpreting the data. Because miRNA profiling methods measure the concentrations of specific miRNAs at a specific state, dynamically modulated miRNAs may be missed, especially when a limited number of samples are tested.
Our analysis showed that many miRNAs are present in all 12 body fluid types tested, and that the composition and concentrations of miRNAs are measurably different among them. The function of extracellular miRNA is yet to be revealed, but it is likely that at least some of these miRNAs serve as a yet-to-be-characterized cell–cell communication system. Some preliminary evidence for this idea: 1) specific spectra of miRNAs are exported by cells in culture (30) and 2) neurons can pick up mRNA and proteins from adjacent cells through exosomes, small lipid vesicles 30–90 nm in size that are released by cells. The finding that miRNAs are present in exosomes triggered the hypothesis that extracellular miRNA may function in cell–cell communication (27–31). If this hypothesis is true, it will open a new realm of possibilities for both biomarker discovery and therapeutic modalities based on extracellular miRNAs.
Supplementary Material
Acknowledgments
The authors express appreciation to Alton Etheridge for his critical reading of the manuscript and stimulating discussions.
Research Funding: This work was supported by the ISB-University of Luxemburg program, Systems Biology Center grant (GM076547) from the NIH, research contracts from the Battelle Biology and Health Science Initiative (Battelle OP46250), and the Department of Defense (W911SR-07-C-0101 and HDTRA 1–08-C-0023).
Role of Sponsor: The funding organizations played a direct role in the design of study, choice of enrolled patients, review and interpretation of data, or preparation or approval of manuscript.
Footnotes
Nonstandard abbreviations: miRNA, microRNA; mRNA, messenger RNA; qPCR, quantitative real-time PCR; HCL, unsupervised hierarchical clustering; NMP22, nuclear matrix protein 22; CFH, complement factor H; BLCA-4, bladder cancer–specific NMP.
Author Contributions: All authors confirmed they have contributed to the intellectual content of this paper and have met the following 3 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; and (c) final approval of the published article.
Authors’ Disclosures of Potential Conflicts of Interest: Upon manuscript submission, all authors completed the Disclosures of Potential Conflict of Interest form. Potential conflicts of interest:
Employment or Leadership: None declared.
Consultant or Advisory Role: None declared.
Stock Ownership: None declared.
Honoraria: None declared.
Expert Testimony: None declared.
References
- 1.Nelson P, Kiriakidou M, Sharma A, Maniataki E, Mourelatos Z. The microRNA world: small is mighty. Trends Biochem Sci. 2003;28:534–40. doi: 10.1016/j.tibs.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Kuokkanen S, Chen B, Ojalvo L, Benard L, Santoro N, Pollard JW. Genomic profiling of MicroRNAs and messenger RNAs reveals hormonal regulation in microRNA expression in human endometrium. Biol Reprod. 2010;82:791–801. doi: 10.1095/biolreprod.109.081059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mishima T, Mizuguchi Y, Kawahigashi Y, Takizawa T, Takizawa T. RT-PCR-based analysis of microRNA (miR-1 and −124) expression in mouse CNS. Brain Res. 2007;1131:37–43. doi: 10.1016/j.brainres.2006.11.035. [DOI] [PubMed] [Google Scholar]
- 4.Chang J, Nicolas E, Marks D, Sander C, Lerro A, Buendia MA, et al. miR-122, a mammalian liver-specific microRNA, is processed from HCR mRNA and may downregulate the high affinity cationic amino acid transporter CAT-1. RNA Biol. 2004;1:106–13. doi: 10.4161/rna.1.2.1066. [DOI] [PubMed] [Google Scholar]
- 5.Chen XM. MicroRNA signatures in liver diseases. World J Gastroenterol. 2009;15:1665–72. doi: 10.3748/wjg.15.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nimmo RA, Slack FJ. An elegant miRror: microRNAs in stem cells, developmental timing and cancer. Chromosoma. 2009;118:405–18. doi: 10.1007/s00412-009-0210-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi Y, Jin Y. MicroRNA in cell differentiation and development. Sci China C Life Sci. 2009;52:205–11. doi: 10.1007/s11427-009-0040-5. [DOI] [PubMed] [Google Scholar]
- 8.Hebert SS, De Strooper B. Alterations of the microRNA network cause neurodegenerative disease. Trends Neurosci. 2009;32:199–206. doi: 10.1016/j.tins.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Mocellin S, Pasquali S, Pilati P. Oncomirs: from tumor biology to molecularly targeted anticancer strategies. Minirev Med Chem. 2009;9:70–80. doi: 10.2174/138955709787001802. [DOI] [PubMed] [Google Scholar]
- 10.Xiao C, Rajewsky K. MicroRNA control in the immune system: basic principles. Cell. 2009;136:26–36. doi: 10.1016/j.cell.2008.12.027. [DOI] [PubMed] [Google Scholar]
- 11.Sun BK, Tsao H. Small RNAs in development and disease. J Am Acad Dermatol. 2008;59:725–37. doi: 10.1016/j.jaad.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 12.Cortez MA, Calin GA. MicroRNA identification in plasma and serum: a new tool to diagnose and monitor diseases. Exp Opin Biol Ther. 2009;9:703–11. doi: 10.1517/14712590902932889. [DOI] [PubMed] [Google Scholar]
- 13.Hanke M, Hoefig K, Merz H, Feller AC, Kausch I, Jocham D, et al. A robust methodology to study urine microRNA as tumor marker: microRNA-126 and microRNA-182 are related to urinary bladder cancer. Urol Oncol. 2009 Apr 16; doi: 10.1016/j.urolonc.2009.01.027. [Epub ahead of print] as. [DOI] [PubMed] [Google Scholar]
- 14.Zubakov D, Boersma AW, Choi Y, van Kuijk PF, Wiemer EA, Kayser M. MicroRNA markers for forensic body fluid identification obtained from microarray screening and quantitative RT-PCR confirmation. Int J Leg Med. 2010;124:217–26. doi: 10.1007/s00414-009-0402-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park NJ, Zhou H, Elashoff D, Henson BS, Kastratovic DA, Abemayor E, Wong DT. Salivary microRNA: discovery, characterization, and clinical utility for oral cancer detection. Clin Cancer Res. 2009;15:5473–7. doi: 10.1158/1078-0432.CCR-09-0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang Z, Huang D, Ni S, Peng Z, Sheng W, Du X. Plasma microRNAs are promising novel biomarkers for early detection of colorectal cancer. Int J Cancer. 2010;127:118–26. doi: 10.1002/ijc.25007. [DOI] [PubMed] [Google Scholar]
- 17.Rosell R, Wei J, Taron M. Circulating MicroRNA signatures of tumor-derived exosomes for early diagnosis of non-small-cell lung cancer. Clin Lung Cancer. 2009;10:8–9. doi: 10.3816/CLC.2009.n.001. [DOI] [PubMed] [Google Scholar]
- 18.Wang K, Zhang S, Marzolf B, Troisch P, Brightman A, Hu Z, et al. Circulating microRNAs, potential biomarkers for drug-induced liver injury. Proc Natl Acad Sci U S A. 2009;106:4402–7. doi: 10.1073/pnas.0813371106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilad S, Meiri E, Yogev Y, Benjamin S, Lebanony D, Yerushalmi N, et al. Serum microRNAs are promising novel biomarkers. PloS One. 2008;3:e3148. doi: 10.1371/journal.pone.0003148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alvarez A, Lokeshwar VB. Bladder cancer biomarkers: current developments and future implementation. Curr Opinion Urol. 2007;17:341–6. doi: 10.1097/MOU.0b013e3282c8c72b. [DOI] [PubMed] [Google Scholar]
- 21.Kikuchi H. Tumor markers of urinary tract carcinoma [in Japanese] Rinsho Byori. 2004;52:371–80. [PubMed] [Google Scholar]
- 22.Simpson RJ, Lim JW, Moritz RL, Mathivanan S. Exosomes: proteomic insights and diagnostic potential. Exp Rev Proteom. 2009;6:267–83. doi: 10.1586/epr.09.17. [DOI] [PubMed] [Google Scholar]
- 23.Rabinowits G, Gercel-Taylor C, Day JM, Taylor DD, Kloecker GH. Exosomal microRNA: a diagnostic marker for lung cancer. Clin Lung Cancer. 2009;10:42–6. doi: 10.3816/CLC.2009.n.006. [DOI] [PubMed] [Google Scholar]
- 24.Lotvall J, Valadi H. Cell to cell signalling via exosomes through esRNA. Cell Adh Migr. 2007;1:156–8. doi: 10.4161/cam.1.3.5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Card DA, Hebbar PB, Li L, Trotter KW, Komatsu Y, Mishina Y, Archer TK. Oct4/Sox2-regulated miR-302 targets cyclin D1 in human embryonic stem cells. Mol Cell Biol. 2008;28:6426–38. doi: 10.1128/MCB.00359-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zovoilis A, Nolte J, Drusenheimer N, Zechner U, Hada H, Guan K, et al. Multipotent adult germline stem cells and embryonic stem cells have similar microRNA profiles. Mol Hum Reprod. 2008;14:521–9. doi: 10.1093/molehr/gan044. [DOI] [PubMed] [Google Scholar]
- 27.Liang Y, Ridzon D, Wong L, Chen C. Characterization of microRNA expression profiles in normal human tissues. BMC Genom. 2007;8:166. doi: 10.1186/1471-2164-8-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith ND. Management of upper tract urothelial carcinoma. Adv Urol. 2009:492462. doi: 10.1155/2009/492462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nguyen CT, Jones JS. Defining the role of NMP22 in bladder cancer surveillance. World J Urol. 2008;26:51–8. doi: 10.1007/s00345-007-0226-z. [DOI] [PubMed] [Google Scholar]
- 30.Wang K, Zhang S, Weber J, Baxter D, Galas DJ. Export of microRNAs and microRNA-protective protein by mammalian cells. Nucl Acids Res. 2010 Jul 7; doi: 10.1093/nar/gkq601. [Epub ahead of print] as. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–9. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


