Abstract
Purpose
The clinical outcome of multiple myeloma (MM) is heterogeneous. A simple and reliable tool is needed to stratify patients with MM. We combined the International Staging System (ISS) with chromosomal abnormalities (CA) detected by interphase fluorescent in situ hybridization after CD138 plasma cell purification and serum lactate dehydrogenase (LDH) to evaluate their prognostic value in newly diagnosed MM (NDMM).
Patients and Methods
Clinical and laboratory data from 4,445 patients with NDMM enrolled onto 11 international trials were pooled together. The K-adaptive partitioning algorithm was used to define the most appropriate subgroups with homogeneous survival.
Results
ISS, CA, and LDH data were simultaneously available in 3,060 of 4,445 patients. We defined the following three groups: revised ISS (R-ISS) I (n = 871), including ISS stage I (serum β2-microglobulin level < 3.5 mg/L and serum albumin level ≥ 3.5 g/dL), no high-risk CA [del(17p) and/or t(4;14) and/or t(14;16)], and normal LDH level (less than the upper limit of normal range); R-ISS III (n = 295), including ISS stage III (serum β2-microglobulin level > 5.5 mg/L) and high-risk CA or high LDH level; and R-ISS II (n = 1,894), including all the other possible combinations. At a median follow-up of 46 months, the 5-year OS rate was 82% in the R-ISS I, 62% in the R-ISS II, and 40% in the R-ISS III groups; the 5-year PFS rates were 55%, 36%, and 24%, respectively.
Conclusion
The R-ISS is a simple and powerful prognostic staging system, and we recommend its use in future clinical studies to stratify patients with NDMM effectively with respect to the relative risk to their survival.
INTRODUCTION
Multiple myeloma (MM) is a heterogeneous disease, with survival duration ranging from a few months to more than 10 years. New, simple, and more robust biomarkers are needed to better dissect different disease categories within MM associated with different outcomes.1
The International Staging System (ISS) is a simple risk stratification algorithm based on two parameters; high serum β2-microglobulin level reflects high tumor mass and reduced renal function, and low serum albumin in MM is mainly caused by inflammatory cytokines such as interleukin-6 secreted by the myeloma microenvironment. The ISS score, defined in 2005, identifies three patient groups with different prognoses; the median overall survival (OS) was 62 months in the ISS stage I, 44 months in the ISS stage II, and 29 months in the ISS stage III groups (P < .001).2
Chromosomal abnormalities (CA) detected by interphase fluorescent in situ hybridization (iFISH) are a key element to define the biologic features of MM.3 In newly diagnosed MM (NDMM), standard-risk disease is characterized by the absence of del(17p), translocation t(4;14)(p16;q32), or translocation t(14;16)(q32;q23) and is associated with a median OS of 50.5 months, whereas high-risk disease is characterized by the presence of at least one of the previously mentioned abnormalities and is associated with a median OS of 24.5 months (P < .001).4
Serum lactate dehydrogenase (LDH) is another relevant biomarker in MM. LDH level above the upper limit of normal denotes an increased disease aggressiveness and suggests high proliferation rate and/or the presence of tumor mass, in particular extramedullary and extraosseous disease.5–9 Studies performed before the availability of novel agents showed that high LDH levels were associated with shorter OS.5 Also in the era of novel agents, LDH confirmed its impact on survival.6
To improve the predictive value of these risk factors evaluated per se, different combinations have been tested. Several studies that combined ISS stage and CA [mainly translocation t(4;14) and del(17p)] identified the following three risk categories: a low-risk group with a 5-year OS rate of a 60% to 70%; an intermediate-risk group with a 5-year OS rate of 40% to 60%; and a high-risk group with a 5-year OS rate of 15% to 40%.10–12 Another study that combined ISS, CA, and LDH data defined four risk categories. In the very low–risk category, the 2-year OS rate was 93%; by contrast, in the very high–risk category, the 2-year OS rate was 55%, (Appendix Table A1, online only).13
These data strongly suggest the need for combining these prognostic factors to better stratify patients into homogeneous survival subgroups. Most of the data currently available were obtained in young patients who received autologous stem-cell transplantation (ASCT), with limited evaluation in elderly patients and patients ineligible for high-dose therapy. Here, we propose a simple model including ISS, CA, and LDH data to define subgroups of patients with different prognosis to better allow stratifications on clinical trials and data comparisons across studies. We assessed this new algorithm, the revised ISS (R-ISS), in 3,060 young and elderly patients with NDMM.
PATIENTS AND METHODS
Patients
A total of 4,445 patients with NDMM were enrolled onto 11 international, multicenter clinical trials, from 2005 to 2012 (Appendix Table A2, online only). The results of these trials were previously reported (ClinicalTrials.gov identifiers: NCT01346787, NCTC00551928, NCT01091831, NCT01093196, NCT01190787, NCT01063179, NCT01134484, NCT00461747, NCT00200681; Eudract: 2005-004714-32; Netherlands Trial Register: NTR213).14–24 Patients gave written informed consent before entering the source trials, which were performed in accordance with the Declaration of Helsinki.
All patients received new drugs (immunomodulatory agents [IMIDs] or proteasome inhibitors [PIs]) in association with conventional chemotherapy as up-front treatment or incorporated into pretransplantation induction or post-transplantation maintenance strategies, except for the patients enrolled onto the Intergroupe Français du Myélome 2005-01 trial who were randomly assigned to vincristine, doxorubicin, and dexamethasone (VAD) induction and to VAD plus dexamethasone, cyclophosphamide, etoposide, and cisplatin before ASCT (Appendix Table A3, online only).
Baseline data collected included the following: age, sex, ISS stage, CA detected by iFISH, and serum LDH level. Data about ISS stage, CA by FISH, and serum LDH were simultaneously available in 3,060 of 4,445 patients. The primary end point was OS, defined as the time from start of treatment until death as a result of any cause or until the last date the patient was known to be alive. The secondary end point was progression-free survival (PFS), defined as the time from start of treatment until progression or death as a result of any cause or until the last date the patient was known to be progression free.
ISS, CA, and LDH Analyses
ISS stage I was defined as serum β2-microglobulin level less than 3.5 mg/L and serum albumin level ≥ 3.5 g/dL. ISS stage II included all patients with neither stage I nor stage III disease. ISS stage III was defined as serum β2-microglobulin level ≥ 5.5 mg/L, irrespective of serum albumin level.2
Bone marrow plasma cells (BMPCs) for iFISH analyses were enriched using anti-CD138–coated magnetic MicroBeads and AutoMACS Pro Separator (Miltenyi Biotech, San Diego, CA) following manufacturer's instructions. BMPCs were then fixed in Carnoy's solution and stored at −20°C. Slides for iFISH were prepared using probes purchased from Cytocell (Cambridge, United Kingdom), Kreatech (Buffalo Grove, IL), and Vysis (Des Plaines, IL), according to manufacturer's instructions. The routine panel included baseline evaluation of del(13), del(17p), and immunoglobulin H translocations. Nuclei were analyzed using a fluorescent light microscope. One hundred to 200 BMPC nuclei from each sample were scored. Del(17p), translocation t(4;14), and translocation t(14;16) detected by iFISH were considered high-risk CA. Patients were considered positive for a given CA when it was present in a percentage higher than the cutoff threshold, defined by each local laboratory. Details of interlaboratory variability are reported in the Appendix (online only).
Serum LDH level was recorded at baseline and classified as normal or high according to the local laboratory definition of normal range. High LDH was defined as a serum level greater than the upper limit of normal range; normal LDH was defined as a serum level less than the upper limit of normal (Table 1).
Table 1.
Standard Risk Factors for MM and the R-ISS
| Prognostic Factor | Criteria |
|---|---|
| ISS stage | |
| I | Serum β2-microglobulin < 3.5 mg/L, serum albumin ≥ 3.5 g/dL |
| II | Not ISS stage I or III |
| III | Serum β2-microglobulin ≥ 5.5 mg/L |
| CA by iFISH | |
| High risk | Presence of del(17p) and/or translocation t(4;14) and/or translocation t(14;16) |
| Standard risk | No high-risk CA |
| LDH | |
| Normal | Serum LDH < the upper limit of normal |
| High | Serum LDH > the upper limit of normal |
| A new model for risk stratification for MM | |
| R-ISS stage | |
| I | ISS stage I and standard-risk CA by iFISH and normal LDH |
| II | Not R-ISS stage I or III |
| III | ISS stage III and either high-risk CA by iFISH or high LDH |
Abbreviations: CA, chromosomal abnormalities; iFISH, interphase fluorescent in situ hybridization; ISS, International Staging System; LDH, lactate dehydrogenase; MM, multiple myeloma; R-ISS, revised International Staging System.
Statistical Analysis
An explorative analysis of the 3,060 patients for whom ISS, CA, and LDH data were simultaneously available was conducted. K-adaptive partitioning,25 dedicated to censored survival data (minimax-based partitioning rule by log-rank test), was used for ISS/CA/LDH grouping; this routine gave an optimal number of three subgroups (R-ISS I, II, and III). The OS and PFS curves were estimated using the Kaplan-Meier method and compared using the log-rank test. OS and PFS were then analyzed through the Cox proportional hazards model, comparing the following risk factors by the Wald test: age at diagnosis (≤ v > 65 years), sex (male v female), iFISH (high-risk v standard-risk CA), LDH (high v normal), ISS stage (II v I and III v I), and R-ISS grouping as defined by the recursive partitioning procedure. The effects of the baseline features (age, sex, and R-ISS) were also assessed by the multivariable Cox model; as in the univariable analysis, the R-ISS stage was treated as a time-dependent variable. Subgroup analyses of PFS and OS were performed to confirm the effect of R-ISS in different subgroups of patients (ie, in patients older and younger than 65 years of age and in patients receiving or not receiving ASCT, PI, or IMIDs). Patients characteristics were tested using the Fisher's exact test for categorical variables and the Mann-Whitney U test for continuous variables. All reported P values were two-sided, at the conventional 5% significance level. Data were analyzed as of December 2014 by R 3.0.1 package kaps (www.r-project.org) and IBM SPSS 21.0.0 (Chicago, IL).
RESULTS
Patient Characteristics and Treatments
Median age was 62 years (range, 18 to 91 years); 65% of patients were ≤ 65 years old, and 35% were older than age 65 years. In the overall population of 4,445 patients, 60% received ASCT (most of these patients were ≤ 65 years old), 44% received PIs, and 66% received IMIDs; only 5% of patients did not receive any novel agent up front (Table 2). Patient characteristics and treatments of the overall population were similar when compared with the 3,060 patients with ISS, CA, and LDH data available. Of note, of 1,171 patients with ISS stage I, 26% had high-risk CA and/or high LDH levels. Of 715 patients with ISS stage III, 57% had standard-risk CA and normal LDH levels (Table 3).
Table 2.
Patient Characteristics and Treatments
| Characteristic | No. of Patients (%) |
|
|---|---|---|
| Total (N = 4,445) | Evaluable (n = 3,060) | |
| Age, years | 2,872 (65) | 2,080 (68) |
| ≤ 65 | 1,573 (35) | 980 (32) |
| > 65 | ||
| Sex | ||
| Male | 2,393 (54) | 1,652 (54) |
| Female | 2,050 (46) | 1,408 (46) |
| Missing | 2 | — |
| HR-CA by iFISH | ||
| No | 2,718 (61) | 2,337 (76) |
| Yes | 851 (19) | 723 (24) |
| Missing | 876 (20) | — |
| ISS stage | ||
| I | 1,615 (36) | 1,171 (38) |
| II | 1,630 (37) | 1,174 (38) |
| III | 987 (22) | 715 (24) |
| Missing | 213 (5) | — |
| LDH level | ||
| Normal | 3,443 (77) | 2,653 (87) |
| High | 530 (12) | 407 (13) |
| Missing | 472 (11) | — |
| Treatment | ||
| ASCT | 2,666 (60) | 1,998 (65) |
| No ASCT | 1,779 (40) | 1,062 (35) |
| Proteasome inhibitors | 1,971 (44)* | 1,345 (44)† |
| Immunomodulatory agents | 2,954 (66)* | 2,045 (66)† |
| No new drugs | 242 (5) | 180 (6) |
Abbreviations: ASCT, autologous stem-cell transplantation; HR-CA, high-risk chromosomal abnormalities [defined by the presence of del(17p), translocation t(4;14), or translocation t(14;16)]; iFISH, interphase fluorescent in situ hybridization; ISS, International Staging System; LDH, lactate dehydrogenase.
Seven hundred twenty-two patients received immunomodulatory agents and proteasome inhibitors and were counted twice.
Five hundred ten patients received immunomodulatory agents and proteasome inhibitors and were counted twice.
Table 3.
ISS, CA, and LDH Distribution in Patients Included in R-ISS Analysis (n = 3,060)
| No. of Patients (%) |
|||
|---|---|---|---|
| ISS Stage I (n = 1,171) | ISS Stage II (n = 1,174) | ISS Stage III (n = 715) | |
| No other risk factors | 863 (74) | 763 (65) | 406 (57) |
| Plus high-risk CA | 200 (17) | 262 (22) | 159 (22) |
| Plus high LDH level | 84 (7) | 114 (10) | 107 (15) |
| Plus high-risk CA and high LDH level | 24 (2) | 35 (3) | 43 (6) |
Abbreviations: ISS: International Staging System, CA: chromosomal abnormalities, LDH: lactate dehydrogenase; R-ISS, revised International Staging System.
Individual ISS, CA, and LDH
The individual role of each predictor was initially evaluated in the total population of 4,445 patients. One thousand six hundred fifteen patients (36%) had ISS stage I, 1,630 (37%) had ISS stage II, and 987 (22%) had ISS stage III; 213 patients (5%) had missing data. At a median follow-up of 46 months, the 5-year OS rate was 77% for ISS stage I, 62% for ISS stage II, and 47% for ISS stage III (P < .001); the 5-year PFS rate was 49% for ISS stage I, 36% for ISS stage II, and 30% for ISS stage III (P < .001).
Two thousand seven hundred eighteen patients (61%) had standard-risk CA, 851 patients (19%) had high-risk CA, and 876 patients (20%) had missing data. The 5-year OS rate was 69% in the standard-risk group and 50% in the high-risk group (P < .001), whereas the 5-year PFS rates were 45% and 24% (P < .001), respectively.
Three thousand four hundred forty-three patients (77%) had a normal LDH level, 530 patients (12%) had a high LDH level, and 472 patients (11%) had missing data. The 5-year OS rate was 68% for patients with normal LDH and 47% for patients with high LDH (P < .001), whereas the 5-year PFS rates were 42% and 31% (P = .004), respectively.
Combined ISS, CA, and LDH
The K-adaptive partitioning was performed in 3,060 patients with ISS, CA, and LDH data. The following three ISS/CA/LDH groups were identified (Table 1): 871 patients (28%) with R-ISS stage I, (ISS stage I, no high-risk CA, and normal LDH); 295 patients (10%) with R-ISS stage III (ISS stage III plus high-risk CA or high LDH); and 1,894 patients (62%) with R-ISS stage II (all the other ISS/CA/LDH combinations).
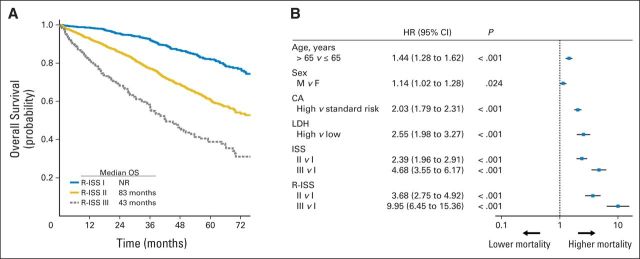
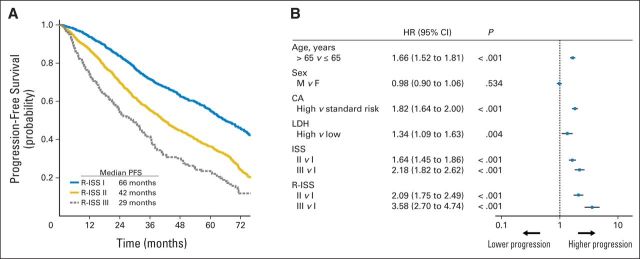
At a median follow-up of 46 months, the 5-year OS was 82% for R-ISS stage I, 62% for R-ISS stage II, and 40% for R-ISS stage III, with a median OS time of not reached for R-ISS stage I, 83 months for R-ISS stage II, and 43 months for R-ISS stage III (Fig 1). The 5-year PFS rate was 55% for R-ISS stage I, 36% for R-ISS stage II, and 24% for R-ISS stage III, with a median PFS time of 66 months for R-ISS stage I, 42 months for R-ISS stage II, and 29 months for R-ISS stage III (Fig 2).
Fig 1.
(A) Overall survival (OS) in patients with multiple myeloma stratified by revised International Staging System (R-ISS) algorithm. Median OS was not reached for patients included in R-ISS stage I, whereas it was 83 months for R-ISS stage II and 43 months for R-ISS stage III. (B) Univariable analysis of OS. CA, chromosomal abnormalities; F, female; HR, hazard ratio; LDH, lactate dehydrogenase; M, male; NR, not reached.
Fig 2.
(A) Progression-free survival (PFS) in patients with multiple myeloma stratified by revised International Staging System (R-ISS) algorithm. Median PFS was 66 months for patients with R-ISS stage I, 42 months for patients with R-ISS stage II, and 29 months for patients with R-ISS stage III. (B) Univariable analysis of PFS. CA, chromosomal abnormalities; F, female; HR, hazard ratio; LDH, lactate dehydrogenase; M, male; NR, not reached.
In the univariable Cox analysis, the risk of death was increased for ISS stage II versus I (hazard ratio [HR], 2.39) and stage III versus I (HR, 4.68). Similarly, the risk of death was higher for high-risk CA versus standard-risk CA (HR, 2.03), as well as for high LDH versus normal LDH (HR, 2.55). By applying the R-ISS, the mortality risk was considerably increased for R-ISS stage II versus I (HR, 3.68), as well as for R-ISS stage III versus I (HR, 9.95; Fig 1). The risk of progression was higher for ISS stage II versus I (HR, 1.64) and stage III versus I (HR, 2.18). Similarly, the risk of progression was increased for high-risk CA versus standard-risk CA (HR, 1.82), as well as for high LDH versus normal LDH (HR, 1.34). The risk of progression was higher for R-ISS stage II versus I (HR, 2.09), as well as for R-ISS stage III versus I (HR, 3.58; Fig 2).
In the multivariable Cox model for OS, including age, sex, and R-ISS, the risk of death was increased for age more than 65 years (HR, 1.32; 95% CI, 1.14 to 1.52; P < .001), male sex (HR, 1.16; 95% CI, 1.02 to 1.33; P = .029), R-ISS stage II versus I (HR, 3.59; 95% CI, 2.68 to 4.80; P < .001), and R-ISS stage III versus I (HR, 9.64; 95% CI, 6.24 to 14.88; P < .001). In the multivariable Cox model for PFS, the risk of progression was higher for age greater than 65 years (HR, 1.57; 95% CI, 1.42 to 1.75; P < .001), R-ISS stage II versus I (HR, 1.99; 95% CI, 1.61 to 2.37; P < .001), and R-ISS stage III versus I (HR, 3.37; 95% CI, 2.54 to 4.56; P < .001).
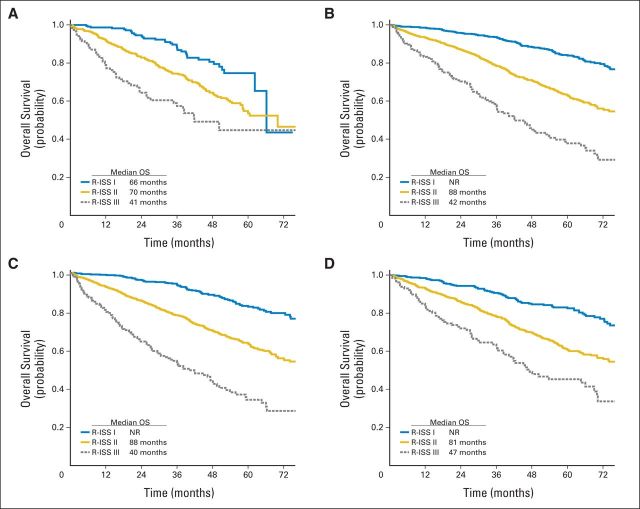
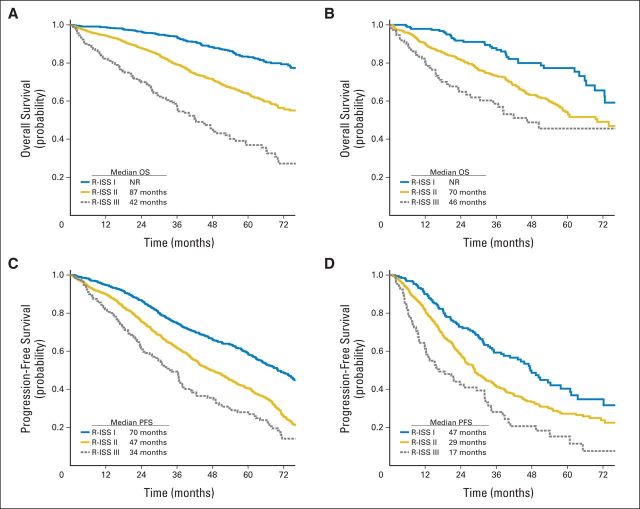
Subgroup analyses for PFS and OS were also performed. The R-ISS staging system confirms its prognostic role in patients younger and older than 65 years of age (Appendix Fig A1, online only) as well as in patients who did and who did not receive ASCT, PIs, and IMIDs (Fig 3).
Fig 3.
Revised International Staging System (R-ISS) and overall survival (OS) by type of treatment. (A) OS in regimens non–transplantation-based regimens. (B) OS in transplantation-based regimens. (C) OS in immunomodulatory-based regimens. (D) OS in proteasome inhibitor–based regimens. NR, not reached.
DISCUSSION
In this study, the most common prognostic tools (ISS stage, CA, and serum LDH level) were combined to define a simple, reliable, and pragmatic risk stratification of patients with NDMM. The R-ISS allowed a clear identification of the following three survival patterns through a simplified rule: R-ISS stage I includes ISS stage I, no high-risk CA, and normal LDH; R-ISS stage III includes ISS stage III with high-risk CA and/or high LDH levels; R-ISS stage II includes all the remaining conditions. Patients with R-ISS stage I, II, and III had 5-year OS rates of 82%, 62%, and 40%, respectively.
We initially evaluated the prognostic power of each single component of the R-ISS. The impact of ISS was confirmed for both OS (ISS stage III v I: HR, 4.68) and PFS (ISS stage III v I: HR, 2.18). As for CA, the presence of del(17p), translocation t(4;14), or translocation t(14;16) commonly identifies high-risk patients with poor outcome,3,4 and our study confirmed their prognostic impact for both OS (HR, 2.03) and PFS (HR, 1.82). Consistent with previous studies, we found that high serum LDH level predicted worse OS (HR, 2.55) and PFS (HR, 1.34).
Subsequently, we developed the R-ISS algorithm that combines these three prognostic features. Our major aim was to create a simple and easily applicable model that combined validated and reliable disease-related prognostic factors. The combination of three different prognostic tools in the R-ISS allows a better evaluation of patient prognosis; approximately 26% of patients would have been wrongly allocated to a good-prognosis group if we had considered only one of these three factors.
In the univariable Cox analyses, we found that ISS stage III, high-risk CA, and elevated serum LDH were associated with a significantly poorer OS (HR from 2.03 to 4.68). Their combination in the R-ISS improved the stratification and the impact on OS (HR from 3.68 to 9.95). The prognostic impact of R-ISS on OS was confirmed independently of age and therapy.
To define the three R-ISS stages. we did not use the classical recursive partitioning, which is a multivariable statistical method based on a binary tree representation, because it can easily manage categorical but not continuous variables.26 Conversely, we adopted K-partitioning, a new technique that can manage censored survival data. K-partitioning provides minimal-based partitioning rules by log-rank test and finds an optimal set of cutoff points, and the number of groups does not need to be established in advance.25
Previous studies have combined different prognostic tools (Appendix Table A1).10–13 Compared with other studies, the R-ISS has been developed in a larger sample size of 3,060 patients, including both young and elderly patients. In the other studies, the majority of patients were in the low-risk group (42% to 58%)10–13; in our analysis, 62% of patients were in the intermediate-risk group, whereas 28% were in the low-risk group and 10% were in the high-risk group. This distribution may explain the slightly better survival dissection among the different groups in our study, with HR for OS ranging from 3.68 to 9.95. We based our staging system on validated, simple, easily applicable, and reliable disease-related prognostic factors. There are novel, alternative approaches, such as gene expression profiling and single nucleotide polymorphisms, currently used for a detailed molecular analysis of MM. Yet, studies including gene expression profiling and single nucleotide polymorphism analyses showed inconsistent results, and further validation to achieve a more general consensus is still necessary.27–29 In addition, these techniques are quite complex and expensive, which limits their routine use.
In our study, 95% of patients received novel agents. Although this was not the objective of our analysis, our results showed an improvement in OS with novel therapies across prognostic subgroups. In the original ISS study including patients worldwide treated from 1981 to 2002, with minimal exposure to any novel agents, the median OS was 62 months for patients with ISS stage I, 44 months for patients with ISS stage II, and 29 months for patients with ISS stage III. In our analysis, the median OS was not reached for patients with ISS stage I, 87 months for patients with ISS stage II, and 56 months in patients ISS stage III.2
Our study has some limitations. We included only patients enrolled onto experimental trials (65% < 65 years old). We excluded some patients from the final analysis as a result of the lack of baseline data; in addition, information about chromosome 1 abnormalities was not collected in all trials, and thus we could not include this prognostic parameter. There was no interlaboratory standardization of FISH analysis, and heterogeneous cutoff levels for LDH were used in the different trials. In our model, we did not include host-related prognostic factors such as age, performance status, and comorbidities, which still play an important role in defining patient prognosis. The relatively short median follow-up time is another limitation. Furthermore, we used a single-step modeling approach, without splitting the series into training and validation autonomous sets. The lack of a real validation sample can be a limitation of the analysis. Nevertheless, both the large sample size and the homogeneity of the main clinical characteristics were expected to minimize the risk of data overfitting.
Future analyses will try to overcome such limitations and will validate this new risk stratification in population-based studies, including a higher proportion of elderly patients. The European Myeloma Network will also focus on FISH standardization in MM, and future efforts will be directed to combine both disease-related and patient-related factors to develop a more comprehensive prognostic evaluation.
In conclusion, there is a clear need for a better differentiation of patients with MM. MM can no longer be considered a single disease, but a mix of different disease entities. Today, new treatments are available, and survival of patients with MM has significantly improved. In clinical practice, a better definition of MM subgroups is essential to provide more effective personalized therapies. The R-ISS staging system is a new risk stratification algorithm with an improved prognostic power compared with the individual ISS, CA, and LDH parameters. It includes simple, reliable, and widely used prognostic markers, and it allows the identification of three different MM entities with clearly different outcomes.
Acknowledgment
We thank Lisa Paik and Diana Wang from the International Myeloma Foundation, and the data managers Antonella Fiorillo and Jessica Mastrovito and the editorial assistant Giorgio Schirripa from the Torino site, for their assistance in collecting data and forms, coordinating the authors, and formatting and submitting the article.
Appendix
Fluorescent in situ hybridization analyses were performed in a few European laboratories. Despite interlaboratory variability, all the analyses were performed on purified plasma cells obtained with immunomagnetic techniques, and the analyses of p53 deletion, t(4,14), and t(14;16) were commonly included in each multiple myeloma panel and tested using commercial probes. Of note, however, the cutoff levels were not identical, ranging from 8% to 20% for numerical aberrations and from 10% to 15% for immunoglobulin H translocations.
International Myeloma Working Group
Niels Abildgaard, Syddansk Universitet, Odense, Denmark
Rafat Abonour, Indiana University School of Medicine, Indianapolis, IN
Melissa Alsina, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL
Kenneth C. Anderson, Dana-Farber Cancer Institute, Boston, MA
Michel Attal, Purpan Hospital, Toulouse, France
Hervé Avet-Loiseau, University of Toulouse, Toulouse, France
Ashraf Badros, University of Maryland, Baltimore, MD
Bart Barlogie, Myeloma Institutes for Research Therapy, University of Arkansas for Medical Sciences, Little Rock, AR
Régis Bataille, Institute de Biologie, Nantes, France
Meral Beksaç, Ankara University, Ankara, Turkey
Andrew Belch, University of Alberta, Edmonton, Alberta, Canada
Dina Ben-Yehuda, Hadassah University Hospital, Hadassah, Israel
Bill Bensinger, Fred Hutchinson Cancer Center, Seattle, WA
P. Leif Bergsagel, Mayo Clinic Scottsdale, Scottsdale, AZ
Joan Bladé, Hospital Clinica, Barcelona, Spain
Mario Boccadoro, University of Torino, Torino, Italy
Jo Caers, Centre Hospitalier Universitaire de Liège, Liège, Belgium
Michele Cavo, Universita di Bologna, Bologna, Italy
Asher Chanan-Khan, Mayo Clinic, Jacksonville, FL
Wen Ming Chen, Beijing Chaoyang Hospital, Beijing, China
Marta Chesi, Mayo Clinic Scottsdale, Scottsdale, AZ
J. Anthony Child, Leeds General Hospital, Leeds, United Kingdom
Chor Sang Chim, Department of Medicine, Queen Mary Hospital, Hong Kong
Wee-Joo Chng, National University Health System, Singapore
Ray Comenzo, Tufts Medical School, Boston, MA
John Crowley, Cancer Research and Biostatistics, Seattle, WA
William Dalton, H. Lee Moffitt, Tampa, FL
Faith Davies, Royal Marsden Hospital, London, United Kingdom
Javier de la Rubia, Hospital Universitario La Fe, Valencia, Spain
Cármino de Souza, Univeridade de Campinas, Caminas, Brazil
Michel Delforge, University Hospital Gasthuisberg, Leuven, Belgium
Meletios Dimopoulos, University of Athens School of Medicine, Athens, Greece
Angela Dispenzieri, Mayo Clinic, Rochester, MN
Johannes Drach, University of Vienna, Vienna, Austria
Matthew Drake, Mayo Clinic Rochester, Rochester, MN
Juan Du, Changzhen Hospital, Shanghai, China
Brian G.M. Durie, Cedars-Sinai Samuel Oschin Cancer Center, Los Angeles, CA
Hermann Einsele, Universitätsklinik Würzburg, Würzburg, Germany
Theirry Facon, Centre Hospitalier Regional Universitaire de Lille, Lille, France
Dorotea Fantl, Socieded Argentinade Hematolgia, Buenos Aires, Argentina
Jean-Paul Fermand, Hopital Saint-Louis, Paris, France
Carlos Fernández de Larrea, Hospital Clínic de Barcelona, Barcelona, Spain
Rafael Fonseca, Mayo Clinic Scottsdale, Scottsdale, AZ
Gösta Gahrton, Karolinska Institute for Medicine, Huddinge, Sweden
Ramón García-Sanz, University Hospital of Salamanca, Salamanca, Spain
Laurent Garderet, Hopital Saint Antoine, Paris, France
Christina Gasparetto, Duke University Medical Center, Durham, NC
Morie Gertz, Mayo Clinic, Rochester, MN
Irene Ghobrial, Dana-Farber Cancer Institute, Boston, MA
John Gibson, Royal Prince Alfred Hospital, Sydney, Australia
Peter Gimsing, University of Copenhagen, Copenhagen, Denmark
Sergio Giralt, Memorial Sloan-Kettering Cancer Center, New York, NY
Hartmut Goldschmidt, University Hospital Heidelberg, Heidelberg, Germany
Jingli Gu, The First Hospital, Sun Yat-Sen University, Guangdong, China
Roman Hajek, University Hospital Ostrava and School of Medicine OU, Ostrava, Czech Republic
Izhar Hardan, Tel Aviv University, Tel Aviv, Israel
Parameswaran Hari, Medical College of Wisconsin, Milwaukee, WI
Hiroyuki Hata, Kumamoto University Hospital, Kumamoto, Japan
Yutaka Hattori, Keio University School of Medicine, Tokyo, Japan
Tom Heffner, Emory University, Atlanta, GA
Jens Hillengass, University of Heidelberg, Heidelberg, Germany
Joy Ho, Royal Prince Alfred Hospital, Sydney, New South Wales, Australia
Antje Hoering, Cancer Research and Biostatistics, Seattle, WA
Jian Hou, Shanghai Chang Zheng Hospital, Shanghai, China
Jeffrey Huang, National Taiwan University Hospital, Taiwan
Vania Hungria, Clinica San Germano, Sao Paolo, Brazil
Shinsuke Ida, Nagoya City University Medical School, Nagoya, Japan
Peter Jacobs, Constantiaberg Medi-Clinic, Plumstead, South Africa
Sundar Jagannath, Mt. Sinai Cancer Institute, New York, NY
Andrzej J. Jakubowiak, University of Chicago, Chicago, IL
Hans Johnsen, Aalborg Hospital Science and Innovation Center, Aalborg, Denmark
Douglas Joshua, Royal Prince Alfred Hospital, Sydney, New South Wales, Australia
Artur Jurczyszyn, University Hospital, Cracow, Poland
Efstathios Kastritis, University of Athens, Athens, Greece
Jonathan Kaufman, Emory Clinic, Atlanta, GA
Michio Kawano, Yamaguchi University, Ube, Japan
Neha Korde, National Institutes of Health, Bethesda, MD
Eva Kovacs, Cancer Immunology Research-Life, Birsfelden, Switzerland
Amrita Krishnan, City of Hope, Duarte, CA
Sigurdur Kristinsson, Karolinska University Hospital and Karolinska Institutet, Stockholm, Sweden
Nicolaus Kröger, University Hospital Hamburg, Hamburg, Germany
Shaji Kumar, Department of Hematology, Mayo Clinic, MN
Robert A. Kyle, Department of Laboratory Medicine and Pathology, Mayo Clinic, MN
Chara Kyriacou, Northwick Park Hospital, London, United Kingdom
Martha Lacy, Mayo Clinic Rochester, Rochester, MN
Juan José Lahuerta, Grupo Español di Mieloma, Hospital Universitario 12 de Octubre, Madrid, Spain
Ola Landgren, Memorial Sloan-Kettering Cancer Center, New York, NY
Alessandra LaRocca, Divisione Universitaria di Ematologia, Torino, Itay
Jacob Laubach, Dana-Farber Cancer Institute, Boston, MA
Fernando Leal da Costa, Instituto Portugues De Oncologia, Lisbon, Portugal
Jae-Hoon Lee, Gachon University Gil Hospital, Incheon, Korea
Merav Leiba, Sheba Medical Center, Tel Hashomer, Israel
Xavier LeLeu, Hospital Huriez, Centre Hospitalier Regionale et Universitaire Lille, Lille, France
Suzanne Lentzsch, Columbia University, New York, NY
Nelson Leung, Mayo Clinic Rochester, Rochester, MN
Henk Lokhorst, University Medical Center Utrecht, Utrecht, the Netherlands
Sagar Lonial, Emory University Medical School, Atlanta, GA
Jin Lu, Peoples Hospital, Beijing University, Beijing, China
Heinz Ludwig, Wilhelminenspital Der Stat Wien, Vienna, Austria
Anuj Mahindra, University of California, San Francisco, San Francisco, CA
Angelo Maiolino, Rua Fonte da Saudade, Rio de Janeiro, Brazil
María-Marivi Mateos, University Hospital of Salamanca-IBSAL, IBMCC (USAL-CSIC), Salamanca, Spain
Amitabha Mazumder, New York University Comprehensive Cancer Center, New York, NY
Philip McCarthy, Roswell Park Cancer Center, Buffalo, NY
Jayesh Mehta, Northwestern University, Chicago, IL
Ulf-Henrik Mellqvist, Sahlgrenska University Hospital, Gothenburg, Sweden
Giampaolo Merlini, University of Pavia, Pavia, Italy
Joseph Mikhael, Mayo Clinic Arizona, Scottsdale, AZ
Philippe Moreau, University Hospital, Nantes, France
Gareth Morgan, Royal Marsden Hospital, London, United Kingdom
Nikhil Munshi, Dana-Farber Cancer Institute, Boston, MA
Hareth Nahi, Karolinska University Hospital, Stockholm, Sweden
Weerasak Nawarawong, Chiang Mai University, Chiang Mai, Thailand
Ruben Niesvizky, Weill Cornell Medical College, New York, NY
Amara Nouel, Hospital Rutz y Paez, Bolivar, Venezuela
Yana Novis, Hospital Sírio Libanês, Bela Vista, Brazil
Enrique Ocio, University Hospital of Salamanca-IBSAL, IBMCC (USAL-CSIC), Salamanca, Spain
Alberto Orfao, University Hospital of Salamanca-IBSAL, IBMCC (USAL-CSIC), Salamanca, Spain
Robert Orlowski, The University of Texas MD Anderson Cancer Center, Houston, TX
Bruno Paiva, Clinica Universitaria de Navarra, CIMA, Pamplona, Spain
Antonio Palumbo, University of Torino, Torino, Italy
Santiago Pavlovsky, Fundaleu, Buenos Aires, Argentina
Linda Pilarski, University of Alberta, Edmonton, Alberta, Canada
Raymond Powles, Parkside Cancer Centre, London, United Kingdom
Noopur Raje, Massachusetts General Hospital, Boston, MA
S. Vincent Rajkumar, Mayo Clinic, Rochester, MN
Donna Reece, Princess Margaret Hospital, Toronto, Ontario, Canada
Anthony Reiman, Saint John Regional Hospital, Saint John, New Brunswick, Canada
Paul G. Richardson, Dana-Farber Cancer Institute, Boston, MA
Angelina Rodríguez Morales, Bonco Metro Politano de Sangre, Caracas, Venezuela
Kenneth R. Romeril, Wellington Hospital, Wellington, New Zealand
David Roodman, Indiana University, Indianapolis, IN
Laura Rosiñol, Hospital Clinic, Barcelona, Spain
Murielle Rousseau, University of Toulouse, Toulouse, France
Stephen Russell, Mayo Clinic, Rochester, MN
Jesús San Miguel, Clinica Universitaria de Navarra, CIMA, Pamplona, Spain
Rik Schots, Universitair Ziekenhuis Brussel, Brussels, Belgium
Sabina Sevcikova, Masaryk University, Brno, Czech Republic
Orhan Sezer, Memorial Sisli Hastanesi, Istanbul, Turkey
Jatin J. Shah, The University of Texas MD Anderson Cancer Institute, Houston, TX
Kazuyuki Shimizu, Tokai Central Hospital, Kakamigahara, Japan
Chaim Shustik, McGill University, Montreal, Quebec, Canada
David Siegel, Hackensack University Medical Center, Hackensack, NJ
Seema Singhal, Northwestern University, Chicago, IL
Pieter Sonneveld, Erasmus MC, Rotterdam, the Netherlands
Andrew Spencer, The Alfred Hospital, Melbourne, Australia
Edward Stadtmauer, University of Pennsylvania, Philadelphia, PA
Keith Stewart, Mayo Clinic Arizona, Scottsdale, AZ
Daryl Tan, Singapore General Hospital, Singapore
Evangelos Terpos, University of Athens School of Medicine, Athens, Greece
Patrizia Tosi, Italian Cooperative Group, Istituto di Ematologia Seragnoli, Bologna, Italy
Guido Tricot, University of Iowa Hospital and Clinics, Iowa City, IA
Ingemar Turesson, SKANE University Hospital, Malmo, Sweden
Saad Usmani, Levine Cancer Institute/Carolinas Healthcare System, Charlotte, NC
Ben Van Camp, Vrije Universiteit Brussels, Brussels, Belgium
Brian Van Ness, University of Minnesota, Minneapolis, MN
Ivan Van Riet, Brussels Vrije University, Brussels, Belgium
Isabelle Vande Broek, Vrije Universiteit Brussels, Brussels, Belgium
Karin Vanderkerken, Vrije University Brussels, Brussels, Belgium
Robert Vescio, Cedars-Sinai Cancer Center, Los Angeles, CA
David Vesole, Hackensack University Medical Center, Hackensack, NJ
Ravi Vij, Washington University School of Medicine, St Louis, MO
Peter Voorhees, University of North Carolina, Chapel Hill, NC
Anders Waage, University Hospital, Trondheim, Norway
Michael Wang, The University of Texas MD Anderson, Houston, TX
Donna Weber, The University of Texas MD Anderson, Houston, TX
Jan Westin, Sahlgrenska University Hospital, Gothenburg, Sweden
Keith Wheatley, University of Birmingham, Birmingham, United Kingdom
Elena Zamagni, University of Bologna, Bologna, Italy
Jeffrey Zonder, Karmanos Cancer Institute, Detroit, MI
Sonja Zweegman, VU University Medical Center, Amsterdam, the Netherlands.
Table A1.
Previous Studies Assessing Combinations of Prognostic Tools
| Risk Category | OS Rate (%) |
|---|---|
| ISS+CA10 in ASCT-eligible patients with NDMM | |
| Favorable ISS stage I and no t(4;14) or del(17p13) | 72 at 60 months |
| Intermediate, not favorable or poor categories | 62 at 60 months |
| Poor ISS stage II/III and t(4;14) or del(17p13) | 41 at 60 months |
| ISS+CA11 in ASCT-eligible and -ineligible patients with NDMM | |
| Favorable ISS stage I/II and no t(4;14), t(14,16), +1q21, del(13), or del(17) | 50 at 68 months |
| Intermediate ISS stage I and > 1 CA, or ISS stage II and 1 CA, or ISS stage III and ≤ 1 CA | 50 at 41 months |
| Ultra-high-risk ISS stage II/III and > 1 CA | 50 at 19 months |
| ISS+CA12 in ASCT-eligible and -ineligible patients with NDMM | |
| Favorable ISS stage I/II and no t(4;14) or del(17) | 71 at 48 months |
| Intermediate ISS stage III and no t(4;14) or del(17), or ISS stage I and t(4;14) or del(17) | 45 at 48 months |
| Poor ISS stage II/III and t(4,14) or del(17p) | 33 at 48 months |
| ISS+CA+LDH13 in ASCT-eligible patients with NDMM | |
| Score 0, no adverse factors of the other categories | 93 at 24 months |
| Score 1, only 1 adverse factor of categories 2 and 3 | 85 at 24 months |
| Score 2, high LDH, ISS stage III, no t(4,14) or del(17p) | 67 at 24 months |
| Score 3, t(4,14) and/or del(17p), and ISS stage III and/or high LDH | 55 at 24 months |
Abbreviations: ASCT, autologous stem-cell transplantation; CA, chromosomal abnormalities; ISS, International Staging System; LDH, lactate dehydrogenase; NDMM, newly diagnosed multiple myeloma; OS, overall survival.
Table A2.
Patient Demographic and Clinical Characteristics in the 11 Studies Included in the Analysis
| Characteristic | No. of Patients (%) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| IST-CAR-50614 | EMN 0115 | MM-EMN-44116 | MM-RV-PI-20917 | GIMEMA-MM-05-0518 | GIMEMA-MM-03-0519 | MMY206920 | HOVON-65/GMMG-HD421 | VTD v TD22 | GEM05MENOS6523 | IFM 2005-0124 | |
| Sex | |||||||||||
| Male | 27 (47) | 322 (49) | 196 (50) | 219 (54) | 53 (52) | 252 (49) | 78 (51) | 500 (60) | 273 (57) | 207 (53) | 266 (55) |
| Female | 31 (53) | 338 (51) | 193 (50) | 183 (46) | 49 (48) | 259 (51) | 74 (49) | 327 (40) | 201 (42) | 179 (47) | 216 (45) |
| Missing | — | 2 | — | — | — | — | — | — | — | — | — |
| CA by iFISH | |||||||||||
| del(17) | 8 (14) | 7 (11) | 29 (7) | 42 (14) | 12 (15) | 55 (11) | 19 (12) | 65 (8) | 33 (7) | 19 (5) | 46 (9) |
| t(4;14) | 9 (16) | 63 (9) | 41 (10) | 42 (14) | 16 (20) | 59 (11) | 9 (6) | 70 (8) | 87 (18) | 42 (10) | 30 (6) |
| t(14;16) | 1 (2) | 16 (2) | 19 (5) | 14 (3) | 4 (6) | 15 (3) | 5 (3) | NA | NA | 10 (2) | NA |
| Missing | 7(12) | 129 (19) | 150 (38) | 111 (27) | 28 (27) | 135 (26) | 25 (16) | 166 (20) | 33 (7) | 62 (16) | 16 (3) |
| ISS stage | |||||||||||
| I | 16 (27) | 183 (28) | 172 (44) | 192 (48) | 48 (47) | 115 (23) | 41 (27) | 288 (35) | 214 (45) | 147 (38) | 199 (41) |
| II | 19 (33) | 299 (45) | 155 (40) | 115 (29) | 30 (29) | 188 (37) | 44 (29) | 274 (33) | 183 (38) | 160 (42) | 163 (34) |
| III | 23 (40) | 178 (27) | 62 (16) | 95 (23) | 12 (12) | 104 (20) | 67 (44) | 188 (23) | 77 (16) | 75 (20) | 106 (22) |
| Missing | — | 2 | — | — | 12 (12) | 104 (20) | — | 77 (9) | — | 4 | 14 (3) |
| LDH level | |||||||||||
| Normal | 35 (61) | 471 (71) | 311 (80) | 361 (90) | 77 (75) | 373 (73) | 78 (51) | 660 (80) | 404 (85) | 326 (84) | 347 (72) |
| High | 5 (8) | 55 (8) | 24 (6) | 40 (10) | 9 (9) | 51 (10) | 17 (11) | 144 (17) | 42 (9) | 60 (16) | 83 (17) |
| Missing | 18 (31) | 136 (21) | 54 (14) | 1 | 16 (16) | 87 (17) | 57 (38) | 23 (3) | 28 (6) | - | 52 (11) |
Abbreviations: CA, chromosomal abnormalities; iFISH, interphase fluorescent in situ hybridization; ISS, International Staging System; LDH, lactate dehydrogenase; NA, not available; TD, thalidomide plus dexamethasone; VTD, bortezomib with thalidomide plus dexamethasone.
Table A3.
Treatment Regimens in the Source Studies
| Trial | Regimens and Doses | No. of Patients | Age (years) |
|---|---|---|---|
| IST-CAR-50614 | C: carfilzomib IV 20 mg/m2 per day on days 1 and 2 of cycle 1 followed by 36 mg/m2 per day on days 8, 9, 15, and 16 and then for all subsequent cycles C: cyclophosphamide PO 300 mg/m2 per day on days 1, 8, and 15 D: dexamethasone PO 40 mg per day on days 1, 8, 15, and 22 (nine 28-days cycles followed by maintenance with carfilzomib alone until PD) |
58 | ≥ 65 |
| EMN 0115 | |||
| Arm A | R: lenalidomide PO 25 mg per day for 21 days D: dexamethasone PO 40 mg per day on days 1, 8, 15, and 22 or 20 mg in patients > 75 years old |
222 | ≥ 65 |
| Arm B | M: melphalan PO 0.18 mg/kg or 0.13 mg/kg in patients > 75 years old on days 1-4 P: prednisone PO 1.5 mg/kg per day on days 1-4 R: lenalidomide PO 10 mg per day for 21 days |
218 | |
| Arm C | C: cyclophosphamide PO 50 mg per day for 21 days or 50 mg every other day in patients > 75 years old P: prednisone PO 25 mg every other day R: lenalidomide PO 25 mg per day for 21 days (nine 28-day cycles followed by maintenance with lenalidomide or lenalidomide and prednisone) |
222 | |
| RV-MM-EMN-44116 | |||
| Arm A | C: cyclophosphamide PO 300 mg/m2 per day on days 1, 8, and 15 R: lenalidomide PO 25 mg per day for 21 days D: dexamethasone PO 40 mg per day on days 1, 8, 15, and 22 (six 28-day cycles followed by maintenance with lenalidomide or lenalidomide and prednisone) |
194 | ≤ 65 |
| Arm B | 2 doses of melphalan IV 200 mg/m2 followed by stem-cell support (followed by maintenance with lenalidomide or lenalidomide and prednisone) | 195 | |
| MM-RV-PI-20917 | |||
| Arm A | M: melphalan PO 0.18 mg/kg per day on days 1-4 P: prednisone PO 2 mg/kg per day on days 1-4 R: lenalidomide PO 10 mg per day for 21 days (six 28-days cycles followed by maintenance with lenalidomide or no maintenance) |
202 | |
| Arm B | 2 doses of melphalan IV 200 mg/m2 followed by stem-cell support (followed by maintenance with lenalidomide or no maintenance) | 200 | ≤ 65 |
| GIMEMA-MM-05-0518 | P: bortezomib IV 1.3 mg per day on days 1, 4, 8, and 11 A: pegylated liposomal doxorubicin IV 30 mg/m2 on day 4 D: dexamethasone on days 1-4, 8-11, and 15-18 in cycle 1 and on days 1-4 in cycles 2-4 2 doses of melphalan IV 100 mg/m2 followed by consolidation with lenalidomide 25 mg per day for 21 days + prednisone 50 mg every other day followed by maintenance with lenalidomide 10 mg per day for 21 days until PD |
102 | ≤ 75 |
| GIMEMA-MM-03-0519 | |||
| Arm A | V: bortezomib IV 1.3 mg per day on days 1, 8, 15, and 22 M: melphalan PO 9 mg/m2 per day on days 1-4 or 2 mg every other day P: prednisone PO 60 mg/m2 per day on days 1-4 |
257 | ≥ 65 |
| Arm B | V: bortezomib IV 1.3 mg/m2 per day on days 1, 8, 15, and 22 M: melphalan PO 9 mg/m2 per day on days 1-4 P: prednisone os 60 mg/m2 per day on days 1-4 T: thalidomide PO 50 mg (only in VMPT arm, nine 28-day cycles followed by maintenance with bortezomib and thalidomide until PD) |
254 | |
| MMY206920 | |||
| Group 1 | V: bortezomib SC 1.3 mg/m2 per day on days 1, 8, 15, and 22 P: prednisone PO 50 mg every other day |
51 | |
| Group 2 | C: cyclophosphamide PO 50 mg every other day V: bortezomib SC 1.3 mg/m2 per day on days 1, 8, 15, and 22 P: prednisone PO 50 mg every other day |
51 | |
| Group 3 | V: bortezomib SC 1.3 mg per day on days 1, 8, 15, and 22 M: melphalan PO 2 mg every other day P: prednisone PO 50 mg every other day (nine 28-days cycles followed by maintenance with bortezomib until PD) |
50 | |
| HOVON-65/GMMG-HD421 | |||
| Arm A | P: bortezomib IV 1.3 mg per day on days 1, 4, 8, and 11 A: doxorubicin IV 9 mg/m2 per day on days 1-4 D: dexamethasone PO 50 mg per day on days 1-4, 9-12, and 17-20 (three 28-day cycles, followed by one or two doses of melphalan 200 mg/m2 and stem-cell support, followed by maintenance with IV bortezomib 1.3 mg/m2 once every 2 weeks for 2 years) |
413 | ≤ 65 |
| Arm B | V: vincristine IV 0.4 mg d 1-4 A: doxorubicin IV 9 mg/mq d 1-4 D: dexamethasone os 50 mg d 1-4, 9-12, 17-20 (three 28-day cycles followed by one or two doses of melphalan 200 mg/m2 and stem-cell support, followed by maintenance with thalidomide 50 mg per day for 2 years) |
414 | |
| VTD v TD22 | |||
| Arm A | V: bortezomib IV 1.3 mg per day on days 1, 4, 8, and 11 T: thalidomide PO 100 mg daily for the first 14 days and 200 mg daily thereafter D: dexamethasone PO 40 mg per day on days 1, 2, 4, 5, 8, 9, 11, and 12 (three 21-day cycles, followed by two doses of melphalan IV 200 mg/m2 and stem-cell support, followed by consolidation with two cycles of VTD) |
236 | ≤ 65 |
| Arm B | T: thalidomide PO 100 mg daily for the first 14 days and 200 mg daily thereafter D: dexamethasone PO 40 mg per day on days 1, 2, 4, 5, 8, 9, 11, and 12 (three 21-day cycles, followed by two doses of melphalan 200 mg/m2 and stem-cell support, followed by consolidation with two cycles of TD) |
238 | |
| GEM05MENOS6523* | |||
| Arm A | V: vincristine IV 0.03 mg/kg (upper limit, 2 mg) on day 1 B: carmustine 0.5 mg/kg IV on day 1 M: melphalan 0.25 mg/kg PO per day on days 1- 4 C: cyclophosphamide 10 mg/kg IV on day 1 P: prednisone 1 mg/kg per day on days 1-4, 0.5 mg/kg per day on days 5-8, and 0.25 mg/kg per day on days 9-12 V: vincristine 1 mg IV on day 1 B: carmustine 30 mg/m2 IV on day 1 A: doxorubicin 40 mg/m2 IV on day 1 D: dexamethasone 40 mg PO per day on days 1-4, 9-12, and 17-20 (four 35-day alternating cycles followed by two bortezomib cycles on days 1, 4, 8, and 11, followed by one or two doses of melphalan 200 mg/m2 and stem-cell support) |
129 | ≤ 65 |
| Arm B | T: thalidomide PO 200 mg daily (with escalating doses from 50 to 100 to 200 mg) D: dexamethasone PO 40 mg per day on days 1-4 and 9-12 (six 4-week cycles, followed by one or two doses of melphalan 200 mg/m2 and stem-cell support) |
127 | |
| Arm C | V: bortezomib IV 1.3 mg/m2 per day on days 1, 4, 8, and 11 T: thalidomide PO 200 mg daily (with escalating doses from 50 to 100 to 200 mg) D: dexamethasone PO 40 mg per day on days 1-4 and 9-12 (six 4-week cycles, followed by one or two doses of melphalan 200 mg/m2 and stem cell support |
130 | |
| IFM 2005-0124 | |||
| Arm A1 | V: vincristine 0.4 mg IV per day on days 1-4 (all cycles), 9-12, and 17-20 (cycles 1 and 2) A: doxorubicin 9 mg/m2 IV per day on days 1-4 (all cycles), 9-12, and 17-20 (cycles 1 and 2) D: dexamethasone 40 mg PO per day on days 1-4 (all cycles), 9-12, and 17-20 (cycles 1 and 2) (four 4-week cycles, followed by one or two doses of melphalan 200 mg/m2 and stem-cell support) |
121 | ≤ 65 |
| Arm A2 | VAD (four 4-week cycles) plus: D: dexamethasone PO 40 mg per day on days 1-4 C: cyclophosphamide IV 400 mg/m2 on day 14 E: etoposide IV 40 mg/m2 per day on days 1-4 P: cisplatin IV 15 mg/m2 per day on days 1-4 (two 4-week cycles, followed by one or two doses of melphalan 200 mg/m2 and stem-cell support) |
121 | |
| Arm B1 | V: bortezomib IV 1.3 mg/m2 per day on days 1, 4, 8, and 11 D: dexamethasone PO 40 mg per day on days 1-4 (all cycles), 9-12, and 17-20 (cycles 1 and 2) (four 3-week cycles, followed by one or two doses of melphalan 200 mg/m2 and stem-cell support) |
121 | |
| Arm B2 | VD (four 3-week cycles) + DCEP (two 4-week cycles) followed by one or two doses of melphalan 200 mg/m2 and stem-cell support | 119 |
Abbreviations: IV, intravenous; PD, progression disease; PO, oral; SC, subcutaneous; TD, thalidomide plus dexamethasone; VTD, bortezomib with thalidomide plus dexamethasone.
Four patients, randomized in the study, were excluded from the analysis since they did not meet the eligibility criteria.20
Fig A1.
Revised International Staging System (R-ISS) and survival by age. (A) Overall survival (OS) in patients ≤ 65 years of age. (B) OS in patients older than age 65 years. (C) Progression-free survival (PFS) in patients ≤ 65 years old. (D) PFS in patients older than age 65 years. NR, not reached.
Footnotes
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Antonio Palumbo, Roberto Passera, Shaji Kumar, Brian G.M. Durie, Pieter Sonneveld, Jésus San Miguel, S. Vincent Rajkumar, Philippe Moreau
Collection and assembly of data: Antonio Palumbo, Stefania Oliva, Simona Caltagirone, Sara Bringhen, Francesca Gay
Data analysis and interpretation: Antonio Palumbo, Hervé Avet-Loiseau, Stefania Oliva, Henk M. Lokhorst, Hartmut Goldschmidt, Laura Rosinol, Paul Richardson, Simona Caltagirone, Juan José Lahuerta, Thierry Facon, Sara Bringhen, Francesca Gay, Michel Attal, Roberto Passera, Andrew Spencer, Massimo Offidani, Shaji Kumar, Pellegrino Musto, Sagar Lonial, Maria T. Petrucci, Robert Z. Orlowski, Elena Zamagni, Gareth Morgan, Meletios A. Dimopoulos, Brian G.M. Durie, Kenneth C. Anderson, Pieter Sonneveld, Jésus San Miguel, Michele Cavo, S. Vincent Rajkumar, Philippe Moreau
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Revised International Staging System for Multiple Myeloma: A Report From International Myeloma Working Group
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Antonio Palumbo
Honoraria: Amgen, Novartis, Bristol-Myers Squibb, Genmab, Janssen-Cilag, Millennium, Onyx, Sanofi
Consulting or Advisory Role: Amgen, Bristol-Myers Squibb, Genmab, Celgene, Janssen-Cilag, Millennium, Onyx
Hervé Avet-Loiseau
Consulting or Advisory Role: Celgene, Janssen, Amgen, Sanofi
Research Funding: Celgene (Inst), Sanofi (Inst)
Stefania Oliva
No relationship to disclose
Henk M. Lokhorst
Research Funding: Janssen (Inst), Genmab (Inst)
Expert Testimony: Janssen, Genmab
Travel, Accommodations, Expenses: Genmab, Janssen, Roche
Hartmut Goldschmidt
Consulting or Advisory Role: Janssen-Cilag (Inst), Celgene (Inst), Novartis (Inst), Amgen (Inst), Millennium (Inst), Bristol-Meyers Squibb (Inst)
Speakers' Bureau: Celgene, Janssen-Cilag, Novartis (I), Chugai Pharma, Amgen, Millennium
Research Funding: Celgene (Inst), Janssen-Cilag (Inst), Chugai Pharma (Inst), Novartis (Inst), Bristol-Meyers Squibb (Inst), Millennium (Inst)
Laura Rosinol
Honoraria: Celgene, Janssen-Cilag
Paul Richardson
Consulting or Advisory Role: Celgene, Takeda, Johnson & Johnson
Simona Caltagirone
No relationship to disclose
Juan José Lahuerta
Honoraria: Celgene, Janssen-Cilag
Thierry Facon
Consulting or Advisory Role: Celgene, Janssen-Cilag, Millennium, Novartis, Amgen, Bristol-Meyers Squibb
Speakers' Bureau: Celgene, Janssen-Cilag
Sara Bringhen
Honoraria: Celgene, Janssen-Cilag, Novartis
Consulting or Advisory Role: Merck Sharp & Dohme
Francesca Gay
Honoraria: Celgene, Janssen-Cilag
Consulting or Advisory Role: Celgene, Sanofi
Michel Attal
No relationship to disclose
Roberto Passera
Travel, Accommodations, Expenses: Celgene, Roche
Andrew Spencer
Honoraria: Celgene, Janssen-Cilag, Novartis, Bristol-Meyers Squibb, Takeda
Research Funding: Onyx (Inst)
Massimo Offidani
No relationship to disclose
Shaji Kumar
Consulting or Advisory Role: Celgene (Inst), Onyx (Inst), Millennium (Inst), Sanofi (Inst), Janssen Oncology (Inst)
Research Funding: Celgene (Inst), Onyx (Inst), Novartis (Inst), Millennium (Inst), Sanofi (Inst), Janssen Oncology (Inst)
Pellegrino Musto
Honoraria: Celgene, Janssen, Novartis, Sanofi, Amgen, Roche
Sagar Lonial
Consulting or Advisory Role: Millennium Takeda, Celgene, Bristol-Myers Squibb, Janssen Oncology, Onyx, Novartis, Sanofi
Research Funding: Millennium (Inst), Bristol-Meyers Squibb (Inst), Janssen Oncology (Inst), Celgene (Inst)
Maria T. Petrucci
Honoraria: Janssen-Cilag, Sanofi, Mundipharma, Celgene
Robert Z. Orlowski
Consulting or Advisory Role: Array BioPharma, Bristol-Myers Squibb, Celgene, Janssen Pharmaceuticals, Millennium, Onyx, Spectrum Pharmaceuticals, FORMA Therapeutics, Genentech, Novartis
Research Funding: Array BioPharma, Bristol-Myers Squibb, Celgene, Millennium, Onyx, Spectrum Pharmaceuticals
Elena Zamagni
Honoraria: Janssen-Cilag, Celgene, Amgen
Gareth Morgan
No relationship to disclose
Meletios A. Dimopoulos
Honoraria: Celgene, Janssen-Cilag, Onyx
Brian G.M. Durie
No relationship to disclose
Kenneth C. Anderson
Stock or Other Ownership: OncoPep, Acetylon Pharmaceuticals
Consulting or Advisory Role: Gilead Sciences, Celgene, Millennium, sanofi-aventis, Bristol-Myers Squibb
Pieter Sonneveld
Consulting or Advisory Role: Celgene, Janssen-Cilag, Amgen, Takeda, Karyopharm Therapeutics, Novartis
Research Funding: Celgene (Inst), Janssen (Inst), Onyx (Inst), Karyopharm (Inst)
Jésus San Miguel
Consulting or Advisory Role: Millennium, Celgene, Novartis, Onyx, Janssen-Cilag, Bristol-Meyers Squibb, MSD
Michele Cavo
Honoraria: Janssen-Cilag, Celgene, Millennium, Amgen, Onyx, Bristol-Myers Squibb
Consulting or Advisory Role: Janssen-Cilag, Celgene, Millennium, Amgen
S. Vincent Rajkumar
No relationship to disclose
Philippe Moreau
Honoraria: Celgene, Janssen-Cilag, Takeda, Novartis, Amgen
Consulting or Advisory Role: Celgene, Janssen-Cilag, Takeda
REFERENCES
- 1.Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364:1046–1060. doi: 10.1056/NEJMra1011442. [DOI] [PubMed] [Google Scholar]
- 2.Greipp PR, San Miguel J, Durie BG, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23:3412–3420. doi: 10.1200/JCO.2005.04.242. [DOI] [PubMed] [Google Scholar]
- 3.Ross FM, Avet-Loiseau H, Ameye G, et al. Report from the European Myeloma Network on interphase FISH in multiple myeloma and related disorders. Haematologica. 2012;97:1272–1277. doi: 10.3324/haematol.2011.056176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fonseca R, Bergsagel PL, Drach J, et al. International Myeloma Working Group molecular classification of multiple myeloma: Spotlight review. Leukemia. 2009;23:2210–2221. doi: 10.1038/leu.2009.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dimopoulos MA, Barlogie B, Smith TL, et al. High serum lactate dehydrogenase level as a marker for drug resistence and short survival in multiple myeloma. Ann Intern Med. 1991;115:931–935. doi: 10.7326/0003-4819-115-12-931. [DOI] [PubMed] [Google Scholar]
- 6.Terpos E, Katodritou E, Roussou M, et al. High serum lactate dehydrogenase adds prognostic value to the international staging system even in the era of novel agents. Eur J Haematol. 2010;85:114–119. doi: 10.1111/j.1600-0609.2010.01466.x. [DOI] [PubMed] [Google Scholar]
- 7.Anagnostopoulos A, Gika D, Symeonidis A, et al. Multiple myeloma in elderly patients: Prognostic factors and outcome. Eur J Haematol. 2005;75:370–375. doi: 10.1111/j.1600-0609.2005.00532.x. [DOI] [PubMed] [Google Scholar]
- 8.Barlogie B, Bolejack V, Schell M, et al. Prognostic factor analysis of myeloma survival with intergroup trial S9321 (INT 0141): Examining whether different variables govern different time segments of survival. Ann Hematol. 2011;90:423–428. doi: 10.1007/s00277-010-1130-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barlogie B, Smallwood L, Smith T, et al. High serum levels of lactic dehydrogenase identify a high-grade lymphoma-like myeloma. Ann Intern Med. 1989;110:521–525. doi: 10.7326/0003-4819-110-7-521. [DOI] [PubMed] [Google Scholar]
- 10.Neben K, Jauch A, Bertsch U, et al. Combining information regarding chromosomal aberrations t(4;14) and del (17p13) with the International Staging System classification allows stratification of myeloma patients undergoing autologous stem cell transplantation. Haematologica. 2010;95:1150–1157. doi: 10.3324/haematol.2009.016436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyd KD, Ross FM, Chiecchio L, et al. A novel prognostic model in myeloma based on co-segregating adverse FISH lesions and the ISS: Analysis of patients treated in the MRC Myeloma IX trial. Leukemia. 2012;26:349–355. doi: 10.1038/leu.2011.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Avet-Loiseau H, Durie BG, Cavo M, et al. Combining fluorescent in situ hybridization data with ISS staging improves risk assessment in myeloma: An International Myeloma Working Group collaborative project. Leukemia. 2013;27:711–717. doi: 10.1038/leu.2012.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moreau P, Cavo M, Sonneveld P, et al. Combination of international scoring system 3, high lactate dehydrogenase, and t(4;14) and/or del(17p) identifies patients with multiple myeloma (MM) treated with front-line autologous stem cell transplantation at high-risk of early MM progression-related death. J Clin Oncol. 2014;32:2173–2180. doi: 10.1200/JCO.2013.53.0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bringhen S, Petrucci MT, Larocca A, et al. Carfilzomib, cyclophosphamide, and dexamethasone in patients with newly diagnosed multiple myeloma: A multicenter, phase 2 study. Blood. 2014;124:63–69. doi: 10.1182/blood-2014-03-563759. [DOI] [PubMed] [Google Scholar]
- 15.Magarotto V, Bringhen S, Musto P, et al. Doublet vs triplet lenalidomide-containing regimens in newly diagnosed myeloma patients, younger or older than 75 years: Subgroup analysis of a phase III study. Blood. 2014;124:2110. (abstr) [Google Scholar]
- 16.Palumbo A, Gay F, Spencer A, et al. A phase III study of ASCT vs cyclophosphamide-lenalidomide-dexamethasone and lenalidomide-prednisone maintenance vs lenalidomide alone in newly diagnosed myeloma patients. Blood. 2013;122:763. (abstr) [Google Scholar]
- 17.Palumbo A, Cavallo F, Gay F. Autologous transplantation and maintenance therapy in multiple myeloma. N Engl J Med. 2014;371:895–905. doi: 10.1056/NEJMoa1402888. [DOI] [PubMed] [Google Scholar]
- 18.Gay F, Magarotto V, Crippa C, et al. Bortezomib induction, reduced-intensity transplantation and lenalidomide consolidation-maintenance for myeloma: Updated results. Blood. 2013;122:1376–1383. doi: 10.1182/blood-2013-02-483073. [DOI] [PubMed] [Google Scholar]
- 19.Palumbo A, Bringhen S, Larocca A, et al. Bortezomib-melphalan-prednisone-thalidomide followed by maintenance with bortezomib-thalidomide compared with bortezomib-melphalan-prednisone for initial treatment of multiple myeloma: Updated follow-up and improved survival. J Clin Oncol. 2014;32:634–640. doi: 10.1200/JCO.2013.52.0023. [DOI] [PubMed] [Google Scholar]
- 20.Larocca A, Cavallo F, Magarotto V, et al. Reduced dose-intensity subcutaneous bortezomib plus prednisone (VP) or plus cyclophosphamide (VCP) or plus melphalan (VMP) for newly diagnosed multiple myeloma patients older than 75 years of age. Blood. 2013;122:539. (abstr) [Google Scholar]
- 21.Sonneveld P, Schmidt-Wolf IG, van der Holt B, et al. Bortezomib induction and maintenance treatment in patients with newly diagnosed multiple myeloma: Results of the randomized phase III HOVON-65/ GMMG-HD4 trial. J Clin Oncol. 2012;30:2946–2955. doi: 10.1200/JCO.2011.39.6820. [DOI] [PubMed] [Google Scholar]
- 22.Cavo M, Tacchetti P, Patriarca F, et al. Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before, and consolidation therapy after, double autologous stem-cell transplantation in newly diagnosed multiple myeloma: A randomised phase 3 study. Lancet. 2010;376:2075–2085. doi: 10.1016/S0140-6736(10)61424-9. [DOI] [PubMed] [Google Scholar]
- 23.Rosiñol L, Oriol A, Teruel AI, et al. Superiority of bortezomib, thalidomide, and dexamethasone (VTD) as induction pre transplantation therapy in multiple myeloma: A randomized phase 3 PETHEMA/GEM study. Blood. 2012;120:1589–1596. doi: 10.1182/blood-2012-02-408922. [DOI] [PubMed] [Google Scholar]
- 24.Harousseau JL, Attal M, Avet-Loiseau H, et al. Bortezomib plus dexamethasone is superior to vincristine plus doxorubicin plus dexamethasone as induction treatment prior to autologous stem-cell transplantation in newly diagnosed multiple-myeloma: Results of the IFM 2005-01 phase III trial. J Clin Oncol. 2010;28:4621–4629. doi: 10.1200/JCO.2009.27.9158. [DOI] [PubMed] [Google Scholar]
- 25.Eo S-H, Hong S-M, Cho HY. K-adaptive partitioning for survival data: The Kaps add-on package for R. J Stat Software. (in press) [Google Scholar]
- 26.Breiman Leo. Classification and Regression Trees. Boca Raton, FL: Chapman & Hall/CRC; 1984. [Google Scholar]
- 27.Shaughnessy JD, Jr, Zhan F, Burington BE, et al. A validated gene expression model of high-risk multiple myeloma is defined by deregulated expression of genes mapping to chromosome 1. Blood. 2007;109:2276–2284. doi: 10.1182/blood-2006-07-038430. [DOI] [PubMed] [Google Scholar]
- 28.Kuiper R, van Vliet M, de Knegt Y, et al. Comparison of conventional, FISH and GEP prognostic factors in multiple myeloma: Introducing a novel risk stratification. Blood. 2013;122:3092. (abstr) [Google Scholar]
- 29.López-Corral L, Sarasquete ME, Beà S, et al. SNP-based mapping arrays reveal high genomic complexity in monoclonal gammopathies, from MGUS to myeloma status. Leukemia. 2012;26:2521–2529. doi: 10.1038/leu.2012.128. [DOI] [PubMed] [Google Scholar]