Abstract
The extracellular matrix (ECM) is a major component of the biomechanical environment with which cells interact, and it plays important roles in both normal development and disease progression. Mechanical and biochemical factors alter the biomechanical properties of tissues by driving cellular remodeling of the ECM. This review provides an overview of the structural, compositional, and mechanical properties of the ECM that instruct cell behaviors. Case studies are reviewed that highlight mechanotransduction in the context of two distinct tissues: tendons and the heart. Although these two tissues demonstrate differences in relative cell–ECM composition and mechanical environment, they share similar mechanisms underlying ECM dysfunction and cell mechanotransduction. Together, these topics provide a framework for a fundamental understanding of the ECM and how it may vary across normal and diseased tissues in response to mechanical and biochemical cues. This article is part of a Special Issue entitled: Mechanobiology.
Keywords: Mechanotransduction, Cytoskeleton, Biomechanics, Cell mechanics, Tendinopathy, Diastolic dysfunction
1. Introduction
1.1. Mechanical properties of the extracellular matrix instruct cell behaviors
The local microenvironment of cells plays important roles in both normal development and disease progression. A major component of the biomechanical environment of cells is the dense meshwork of fibrous proteins and other biopolymers called the extracellular matrix (ECM). This intricate biomaterial provides structural support for many tissues in vivo and provides cells with mechanical cues that modulate both cell morphology and physiology. Numerous cell types possess ATP-driven molecular machinery that applies forces and responds to the ECM.
Cell responses to the ECM are driven by intrinsic properties that include adhesive affinity, matrix stiffness, fiber alignment, and matrix density [1]. In mature tissues, cell adhesion to the ECM is primarily, but not exclusively, mediated by integrins, which are transmembrane heterodimeric receptors that interact with a range of ECM components and cluster into different kinds of adhesion sites with an array of intra-cellular components [2–4]. Integrins interact with the force-producing actin cytoskeleton, where changes in force alter the assembly of adhesion complexes and activate adhesion-mediated cell signaling, such as RhoA-induced actin stress fiber formation (Fig. 1) [5,6]. Integrin type and density determine the cell adhesion affinity for, and mechanical sensing of, the ECM [3,7,8]. The most commonly studied ECM mechanical properties are substrate stiffness (a structural property) and elastic modulus (a material property) (Table 1) [4,5,9]. Cells sense substrate stiffness primarily via integrin-dependent actomyosin contraction (Fig. 1). Human tissues have various collagen compositions and a wide range of moduli varying from 100 Pa in brain tissue to over 1 MPa in bone (Fig. 2A, B) [10,11]. Substrate stiffness has been shown to guide stem cell lineage specification in vitro and affect proliferation, motility, contractility, and many other cell functions by changing both acute signaling and transcriptional programs [12–14]. Fiber orientation in the matrix is also critical for various cell behaviors, including stem cell differentiation [15], cell alignment [16–18], and migration [19].
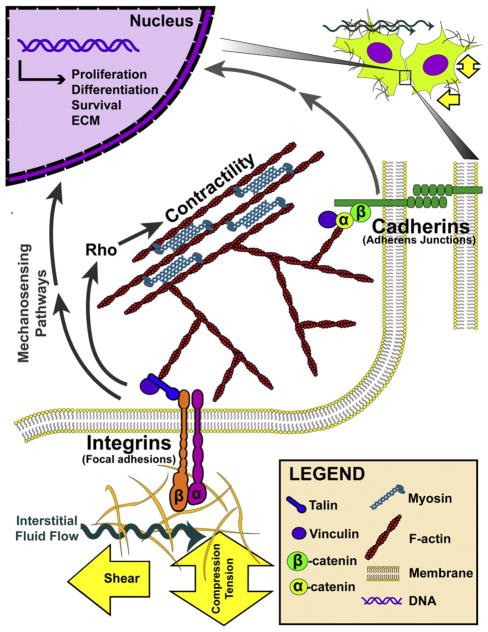
Fig. 1.
Model of cell–ECM and cell-cell mechanotransduction. Cells sense ECM stiffness and external loading by balancing the force between the actomyosin machinery and integrin adhesions. Important molecules involved in the generation of cytoskeletal tension include F-actin, myosin, and the Rho signaling cascade. It is noted that this illustration highlights only the basic subunits of these adhesion complexes, and that these components come together into higher order assemblies in vivo.
Table 1.
Engineering terms and definitions.
| Term | Definition |
|---|---|
| Stress (σ) | Force (F) applied over an area (A), either perpendicular (e.g., pulling (tensile) and pushing (compressive)) or parallel (shear) to the material's axis; σ= F/A |
| Ultimate stress | Maximum stress before material failure |
| Strain (ε) | Unitless parameter quantifying the extent of deformation after application of stress. Strains are also categorized as tensile, compressive, or shear. |
| Principal strain | Maximum or minimum strain along the direction of zero shear strains |
| Strain stiffening | Increase in elastic modulus with applied strain |
| Stiffness (k) | Rigidity of a material defined as force (F) per resultant displacement (δ) (structural property); k = F/δ |
| Elasticity | Material property characterizing the ability of a deformed material to recover completely to its original state when the force is removed |
| Elastic modulus (E) | Resistance of a material to elastic deformation, measured by the ratio of stress and strain (material property); For linearly elastic materials, E = σ/ε |
| Viscoelastic | Combination of viscous and elastic mechanical properties. Viscosity refers to the resistance of fluid to flow, measured by the ratio of stress to the rate of strain or flow rate. |
| Poroelastic | Property of a porous material consisting of an elastic solid matrix and viscous fluid in which mechanical relaxations arise from fluid flow through the matrix. |
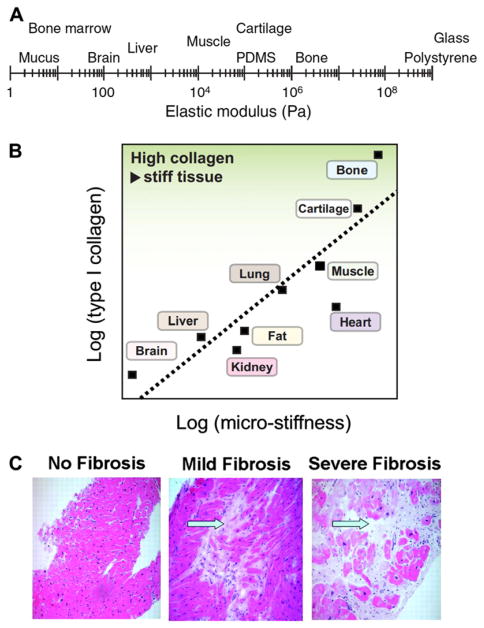
Fig. 2.
Tissue stiffness and its relationship to collagen content. (A) Tissues exhibit a wide range of stiffnesses as measured by the elastic modulus. (B) Tissue stiffness relates to the quantity of type I collagen. As the most prevalent protein in many tissues, collagen modulates mechanical properties of tissue. (C) Hematoxylin and eosin staining shows increasing severity of cardiac fibrosis with elevated collagen content (arrows) between muscle fibers (red), leading to tissue stiffening. Panel A: Reproduced with permission from Janmey and Miller [11]. Panel B: Reproduced with permission from Swift and Discher [10]. Panel C: Reproduced with permission from Weidemann et al. [44].
In cell-dense tissues such as the heart, cells are bound together by adherens junctions, and mechanotransduction occurs via calcium-dependent transmembrane proteins (termed cadherins) (Fig. 1) [20, 21]. Much like integrins, cadherins form complex adhesions, connect to the actin cytoskeleton via numerous proteins including catenins, and participate in various intracellular signaling pathways [22,23]. Thus, both cell–ECM and cell–cell contacts play important roles in the detection of tissue mechanical properties.
1.2. Effects of internal and external mechanical stimuli on cell responses
Cell- and environment-generated mechanical loads on the ECM can induce a variety of cell responses (Fig. 1). The types of stimuli include tensile, compressive, and shear stresses and strains (Table 1). Transformation of internal and external mechanical cues into cellular responses occurs via collagen fiber kinematics [24], focal adhesions (macromolecular assemblies of integrins and proteoglycans) [25], and cell–cell contacts [20,22, 23,18]. An internal source of ECM loading is cell traction force. Cells achieve tensional homeostasis with their ECM by balancing traction forces with matrix stiffness [26–28]. Maintenance of tensional homeostasis plays an important role in the regulation of critical cell functions. For example, during wound healing, fibroblasts reorganize the collagen matrix by exerting traction forces [18]. The increased stresses within the ECM cause the cells to generate a stiffer matrix by producing collagen, become more contractile in response to the increased stiffness, and differentiate into myofibroblasts that propagate this feedback loop and ultimately contract the wound [29]. Normally, this positive feedback is countered by other signals that limit the extent of contraction and matrix deposition, but when left unchecked can lead to scar formation and other tissue defects. Forces transferred through the ECM have a wide range of magnitudes. Although macroscale orthopaedic tissues may experience up to 3500 N of loading [30,31], cell–ECM force interactions are nearly 12 orders of magnitude smaller (nN) (e.g., [32]), and subcellular interactions between myosin and actin crossbridges can balance forces of less than 1 pN [33].
Cells remodel the ECM in response to external mechanical signals as well as the biomechanical properties of the matrix [34–40]. Through tensional homeostasis, cells demonstrate reduced cell-mediated contraction with increased externally applied loads [26]. External stress- and strain-induced changes in cell behavior have been extensively studied in tissue injury. For example, acute mechanical compression of articular cartilage enhances chondrocyte proliferation and decreases proteoglycan synthesis [35]. Also, the production of various ECM components by cardiac fibroblasts in response to cyclic loads is implicated in pathological fibrosis of the heart [36]. Fluid flow through the ECM can also significantly impact cell behaviors [37–40]. For example, interstitial fluid flow has been demonstrated to regulate fibroblast alignment and lymphatic and vascular endothelial functions in three-dimensional cell cultures [39–41].
1.3. Mechanotransduction in the context of diseases and injury
Alterations in ECM composition, stiffness, and loading environment affect cell behaviors, which feed back to ECM dysregulation and disease progression. For example, in pulmonary and cardiac fibrosis, enhanced myofibroblast proliferation and collagen production increase tissue stiffness (Fig. 2C) [42–44]. In addition, abnormal mechanical stimulation can aberrantly activate signaling pathways, such as TGF-β signaling associated with osteoarthritis [45] and β-catenin signaling in cancer progression (Table 2) [46]. In light of these examples, it is important to understand the underlying mechanism of mechanotransduction in order to target ECM or cell mechanosensing to ameliorate the disease condition.
Table 2.
Medical terms and definitions.
| Term | Definition |
|---|---|
| Angiogenesis | Formation of new blood vessels from existing ones |
| Arterial and venous tissue | Tissues pertaining to arteries and veins |
| Artery and veins | Blood vessels that carry blood away from and towards the heart, respectively |
| Ejection fraction | The fraction of total blood pumped from the heart with each heartbeat, typically measured by echocardiogram |
| Electrophysiology | Study of the electrical characteristics of cells and tissues |
| Infarction | Tissue death caused by a lack of oxygen supply (hypoxia) |
| Ischemic cardiomyopathy | Inferior ability of the heart to pump blood due to poor oxygen supply to the myocardium |
| Myocardium | Muscular tissue of the heart |
| Myocardial/cardiac or pulmonary fibrosis | Abnormal scarring of the muscular heart and lung tissues, respectively |
| Osteoarthritis | Degenerative musculoskeletal joint disease affecting articular cartilage and bone |
| Overuse | High repetitive loading such as labor-intensive work that may result in injury |
| Pressure overload | Pathological state of cardiac muscle whereby the heart contracts while experiencing excessive stress |
| Rotator cuff | Group of muscles and tendons that stabilize the shoulder joint. These include the supraspinatus, infraspinatus, teres minor, and subscapularis. |
1.4. Overview for the rest of the paper
This review highlights the effects of ECM function and dysfunction on cellular responses in different tissues. Specifically, the remainder of this review examines the musculoskeletal and cardiovascular organ systems, with a focus on the tendon and the heart ECM. Although these two tissues demonstrate differences in relative cell–ECM composition and mechanical environment, they share similar mechanisms underlying ECM dysfunction and cell mechanotransduction. In each case study, we discuss the techniques and models used to investigate cell and ECM responses to injury and disease at tissue, cellular, and molecular levels.
2. Case study 1: the extracellular matrix in the tendon
2.1. Structure–function relationships in tendon ECM
Tendons connect and transmit forces from muscle to bone [47] and are composed of tenocytes (tendon fibroblasts) and tendon-derived stem cells (TSCs) [48]. These cells are embedded in a heterogeneous matrix of collagens (types I, II, V, IX, and X) [49,50], proteoglycans, elastin, fibronectin, and fluid (70% wet weight) [51,52]. Together, these molecules form a hierarchical network from the macro(“fascicle”) to nano- (“fibril”) scales (Fig. 3A) [53]. While collagen is generally thought to be the main mechanical element in tendons, removal of non-collagenous molecules, such as elastin or glycosaminoglycans, reduces the whole tendon mechanical response, emphasizing their role in development, homeostasis, and load transfer [54–56].
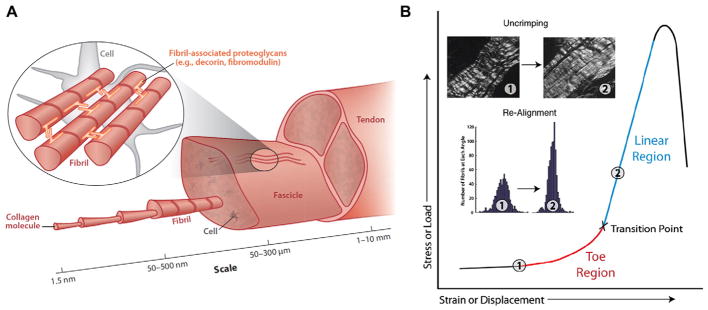
Fig. 3.
Tendon hierarchical structure and a typical stress-strain curve. (A) Tendons contain tenocytes (tendon fibroblasts) embedded in a hierarchical matrix of collagenous and noncollagenous components. Tendons are composed of fascicles, fibers, and fibrils that form from collagen molecules. (B) During mechanical loading, tendons exhibit nonlinearity in their stress strain curve, containing a toe region prior to a transition to a linear region. With increases in stress and strain, collagen fibers uncrimp and re-align. Panels A and B: Reproduced with permission from Connizzo et al. [201].
Tendons exhibit enormous variation in material properties as they anatomically originate from muscle (compliant material) and insert into bone (stiff material). This variation in tendon stiffness changes based on anatomical location and species [57], as well as the amount of strain applied [58]. Disorganized ECM becomes more aligned and less wavy (termed “crimp”) [59] with mechanical loading, giving way to a distinct “toe-region” or strain stiffening mechanism (Table 1; Fig. 3B). In addition, tendons are viscoelastic and poroelastic (Table 1), which results in rate- and time-dependent properties and fluid flow within the ECM [60], similar to other biological tissues (e.g., cartilage, bone, liver, heart, and lung).
During normal human motion, the stresses and strains that tendons experience vary based on anatomical position and activity level. Although many tendons operate in the toe-region of tissue's stress–strain curve where resistance to deformation begins to increase (less than 5% of their load until rupture) [61], higher load bearing tendons, such as the Achilles, can experience forces nearly 70% of their maximum load and stress before rupture (~3500 N or ~55 MPa) [30,31]. The primary direction of loading in tendons is tensile, yet compressive, biaxial, and shear stresses may be present [62,63]. The wide variation in mechanical properties and loading environments across tendons emphasizes their dynamic role in the musculoskeletal system and the complexity of research necessary to understand their basic mechanisms of homeostasis and injury.
2.2. Effects of external and internal mechanical stresses on the ECM and cell response in tendinopathy
Maintenance of mechanical, structural, and compositional properties is heavily influenced by mechanical loading and biochemical factors. Aberrant mechanical loading [64–68] can cause pathological changes resulting in tendinopathy, a degenerative condition characterized by an imbalance between degradation and synthesis of the ECM. For example, rotator cuff (Table 2) tendinopathy affects approximately 4–6% of the population between the ages of 25–64, with a much higher percentage in laborers (19%) [64] and athletes, such as elite swimmers (69%) [69, 70]. Tendinopathy can affect several tendons, especially those highly load bearing including the Achilles [53], patellar [71], and rotator cuff [72]. Individuals with tendinopathy present with tendon thickening and increased vascularization, as evaluated with ultrasound [73]. While the pathogenesis of tendinopathy is poorly understood, it is suggested that aberrant mechanical stimuli may drive tenocytes and TSCs towards pathologic changes [48,74]. Although biochemical mechanisms driving tendinopathy may be present, we focus on the mechanical mechanisms at play from whole tendon biomechanics down to the ECM, cellular, and sub-cellular levels.
2.2.1. The ECM and cell response to in vivo loading in tendinopathy
The effect of in vivo joint loading on the ECM and the corresponding cell response in the tendon has been evaluated in both humans and animal models. External loading may generate interstitial pressures surrounding the tendon, fluid shift, and alterations in blood flow that activate mechanotransductive pathways [75]. Human studies have assessed changes in tendon stiffness using ultrasound [76,77], biochemical changes via tissue biopsy (e.g., collagen content and crosslinking) [78], or serum levels (e.g., TNF-α, IL-10, and CTGF) [79]. Without loading, tendon structural organization and dynamic elastic and viscous properties decreased [80], which may have been caused by increased matrix degradation [81] and increased expression of inflammatory cytokines [82]. In contrast, a single session of activity modulated the expression of ECM proteins and upstream cell signaling markers [83]. A model of repetitive loading increased tensile modulus (52%), stress to rupture (69%) [84], and tendon thickness (~9–20%) [85], which may be due to increased collagen synthesis [84,86]. However, it is noted that some studies have demonstrated no differences in tendon material properties following various bouts of moderate, repetitive loading [87,88], suggesting that the specific moderate-load protocols that create an adaptive response are not fully defined.
Excessive loading promoted tendon matrix synthesis through increased growth factor production, proliferation of TSCs, and expression of type I collagen, as well as cartilage and bone phenotypes [89]. Histopathological characterization of tendinopathy in humans demonstrated altered collagen content, decreased fiber organization, aberrant ECM deposition (calcification, ossification, lipid accumulation), and accumulation of proteoglycans between degenerated collagen fibers (i.e., mucoid degeneration) [90]. Rodent models of shoulder overuse (Table 2) induced similar tendinopathic conditions [91]. Specifically, overuse loading in the rat supraspinatus tendon increased inflammation, angiogenesis, the production of cartilage markers and proteoglycans, and type III/I collagen ratio (Table 2) [92–94]. Similarly, high-cycle fatigue loading produced a degenerative, microstructural damage response [95]. In addition to overuse, abnormal loading, such as disuse, compression or shear from contact with neighboring structures, or change in loading direction caused by injury can initiate a pathologic response and contribute to the development of tendinopathy [96]. For example, tendon impingement is a leading cause of rotator cuff tendinopathy. Additionally, rotator cuff tears can cause a force imbalance in the shoulder joint, which results in tendinopathy in adjacent rotator cuff tendons [97–99]. Taken together, these data suggest that overuse and abnormal loading may disrupt the homeostatic balance between synthesis and degradation, creating an overall catabolic response and development of disease.
2.2.2. The ECM and cell response to ex vivo tissue-level loading in tendinopathy
While in vivo models of tendinopathy provide the most clinically relevant information about the disease, these models have a limited ability to evaluate how the mechanical loads are transmitted at a smaller scale, and therefore their mechanistic effects on the cells and ECM. Thus, alternative methods apply tensile loads to ex vivo tendon explants to preserve the native architecture of the ECM, while also providing more controlled experimental conditions than are possible in vivo. Experiments on non-living tendon explants have investigated structural and mechanical alterations that occur to the ECM with repeated induction of micro-damage via overloading and fatigue loading models. When tendons are subjected to high dynamic loading, fatigue damage accumulation occurs [100] in concert with alterations in crimp properties [101]. Repeated subrupture loading results in collagen fibril kinking [102], which can affect cell morphology and matrix degradation [103]. These mechanisms of microdamage accumulation may be tendon type [104] and age specific [105].
To further investigate the effects of tissue-level loading on biological response, in vitro bioreactor model systems are used. Typically, fascicles derived from tail [106–109], flexor [110], extensor [111], or patellar tendons [112,113] are cultured in standard media conditions under various amounts of applied load, duration, and frequency. In vitro stress deprivation flattened and elongated fibroblasts, decreased cell number, and decreased tensile modulus [110]. Although low and moderate loading had no effect on water content [110] or glycosaminoglycan production [109], higher loading increased glycosaminoglycan content [114], lowered mechanical strength, and caused the release of pro-inflammatory cytokines and vasodilators, such as prostaglandin E2 (PGE2) and nitric oxide (NO) [115]. Certain loading regimes may promote optimal mechanical properties [113], potentially through collagen synthesis [109,111] as the molecular mechanisms switch from a catabolic to anabolic response [106]. Such mechanisms could depend on the frequency and duration of the loading [112].
Taken together, these studies suggest that ex vivo loading of tendon explants provides a controlled method for evaluating the specific response of tendon to loading, thus removing confounding variables present in whole tissue model systems. Many of the same mechanical and biological mechanisms are conserved, demonstrating the validity of these models.
2.2.3. ECM and cell response to cellular-level loading in tendinopathy
Loading applied directly to cells in vitro can provide more direct information on the cell level response to mechanical stimuli. Scaffolds seeded with cells under cyclic strains have been used to investigate cell behavior in response to loading. Tenocytes isolated from patellar and Achilles tendons subjected to physiological strain levels upregulated only tenocyte markers (type I collagen and tenomodulin), whereas higher levels of strain upregulated biomarkers found in cartilage [89]. Cyclically stretched human patellar tendon fibroblasts responded with a load-dependent increase in inflammatory cytokines, which could reduce cell proliferation and collagen synthesis and lead to the development of tendinopathy [116,117]. Over-tensioning TSCs caused osteogenic differentiation and upregulation of BMP-2 [118,119]. This response was regulated through the mechanosensitive activation of RhoA, which plays a role in cell proliferation, differentiation, and adhesion formation [119]. Microfluidics and modeling approaches [120,121] have provided further insight into the response of cells under fluid shear stresses. Fluid shear stresses have been implicated in gene expression changes in degradation [122], collagen remodeling [123], anti-fibrosis [124], ecto-ATPase activity [125], NO production [126], and calcium signaling [127] in the tendon. In addition to the application of fluid shear stresses to modulate cell behavior, biochemical cues activated by mechanical stimulation [128,129] might also drive phenotypic behaviors. In particular, the effect of cyclic strain has been shown to mirror that of TGF-β stimulation [130]. Primary tendon fibroblasts treated with TGF-β demonstrated increased expression of miRNAs that regulate cell proliferation, ECM synthesis, and scleraxis (a tendon marker) [83].
Observed changes in gene expression, cytokine release, and nontenogenic differentiation following high loading may be due to upstream mechanosensing in the cytoskeleton. Application of strains on cells can create an adaptive response to the cytoskeleton and adherens junctions [131]. Specifically, N-cadherin and vinculin levels increased in strained cultures, and cells organized their actin into stress fibers along the axis of principal strains (Table 1) [131]. In addition, tenocyte communication via gap junctions may be altered with loading [132,133]. For example, when tenocytes were subjected to cyclic strain, their gap junctions became disrupted and apoptosis was induced [134]. Cell–matrix adhesions allow tenocytes to sense and respond to their mechanical environment while also allowing them to act on the ECM through actomyosin mediated contractile forces [135]. Altering the tensile forces on tendons can elicit changes in integrin receptors, as well as in downstream ECM proteins [135]. Specifically, de-tensioning tenocytes in vitro caused a decrease in collagen binding integrin α11β 1, which is associated with the organization of collagen in the ECM, and an increase in collagen binding integrin α2β 1 and fibronectin integrin receptor α5β 1, which are associated with the transmission of cytoskeletal forces through collagen fibrils causing contraction and adhesion strength, respectively [135]. Additionally, de-tensioning decreased expression of tenomodulin and Mohawk homeobox, which are associated with tendon differentiation, as well as decreased collagens, decorin, and matrix organization and increased pro-inflammatory markers [135]. These results demonstrate that mechanical loading alters mechanosensitive proteins and therefore plays a large role in the maintenance of a normal tendon phenotype as well as the development of pathology.
2.3. Summary and future work
Since the primary function of tendons is to transmit forces from muscle to bone, its ability to adapt and respond to loads is essential to prevent injury. Previous work has shown the sensitivity of the tendon to alterations in mechanical stimuli (Fig. 4). Establishing changes in gene and protein levels following various mechanical protocols is necessary to confirm that models accurately represent the human condition. Once these model systems have been optimized, a more mechanistic evaluation of alterations in the cell and ECM that elicit tendinopathic responses is necessary.
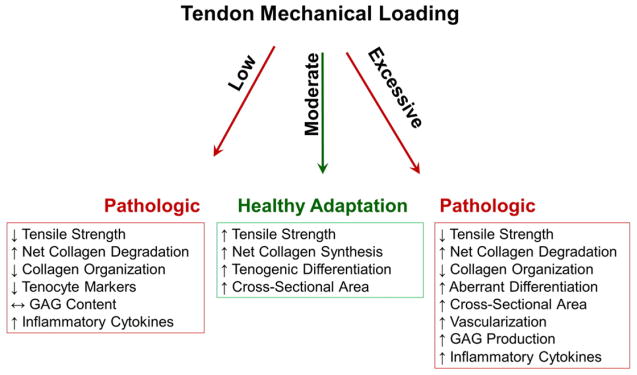
Fig. 4.
Various pathways that may result following mechanical loading in tendon. Low loading decreased tensile strength [80,82,110], collagen organization [80,110], and tenocyte markers [135]; net collagen degradation and inflammatory cytokines increased [82,135], and GAG content was unchanged [109]. Moderate loading increased tensile strength [84], net collagen synthesis [83,84,86,109,111], tenogenic differentiation [89], and cross-sectional area [84,85]. Knowledge regarding collagen organization, vascularization, GAG production, and inflammatory cytokines with moderate loading remains limited. Excessive loading decreased tensile strength [77,115] and collagen organization [91,95], and increased net collagen degradation [78,90], aberrant differentiation [89,92–94,118,119], cross-sectional area [73], vascularization [73,91,115], GAG production [114], and inflammatory cytokines [79,115].
Animal models can help to elucidate the underlying in vivo mechanisms of tendinopathy in humans, but they have limitations. Although bioreactor studies may overcome some limitations, they likely oversimplify true in vivo biological complexity. Additional knowledge may be gained from other, genetically-tractable model systems that focus on cell–ECM interactions. Drosophila tendon cells have adopted a compact microtubule [136] and F-actin [137] array as cytoskeletal structures to withstand high mechanical loads, and may be used to study the muscle–tendon junction. In addition, zebrafish craniofacial tendons, which connect cartilage and muscle, contain parallel arrays of collagen fibrils, suggesting that they are structurally similar to mammalian tendons. These tendons are derived from neural crest cells, specified by muscle-induced expression of tendon-differentiation markers, and upregulate tenomodulin and type I collagen, as in mammals [138]. Therefore, zebrafish may provide an additional model system for elucidating mechanisms of tendinopathy.
3. Case study 2: the extracellular matrix in the heart
3.1. Structure–function relationships in the heart ECM
The heart is a muscular pump that circulates blood throughout the body composed of four major chambers (two atria and two ventricles), each containing several tissue compartments. First, the parenchyma is composed of specialized cardiac muscle cells called cardiomyocytes. These cells are further subdivided into atrial, ventricular, and conductive system cardiomyocytes. Cardiomyocytes are terminally differentiated, non-proliferating, excitable cells, which generate electrical signals that induce a coordinated contractile behavior allowing the heart to eject blood into the systemic and pulmonary circulations. The coronary vasculature represents a second tissue compartment that comprises arterial and venous tissue (Table 2) and oxygenates and facilitates removal of waste products. The cardiomyocytes and coronary vessels are tethered to an ECM comprising the endomysium, perimysium, and epimysium, which surround the myofibers and coronary vessels. The main component of the heart ECM is fibrillar type I collagen, with types III and V contributing 10–15% and <5%, respectively [139]; proteoglycans and glycoproteins are also present. Cardiac fibroblasts reside in the ECM and form the largest population of cells in the heart (two-thirds) whereas cardiomyocytes occupy two-thirds of the total tissue volume [140]. Further, these fibroblasts mediate a constant homeostatic state of synthesis and degradation of ECM.
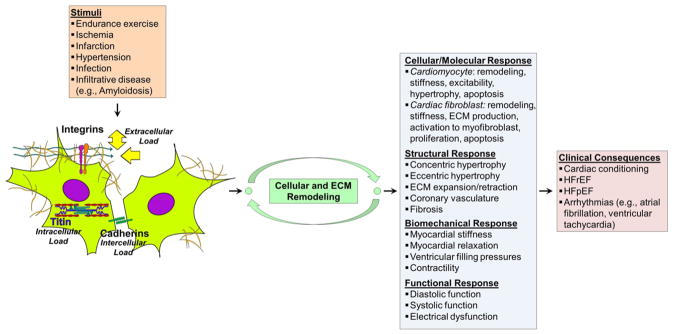
During pumping, the heart undergoes continuous cycles of systole and diastole. Systole involves muscular contraction and the ejection of blood into the systemic and pulmonary circulations, whereas diastole involves relaxation and filling of the left and right ventricles (LV, RV) [141]. The heart ECM contributes to contractility, compliance, relaxation, and electrophysiology (Table 2). During stress states (e.g., hypoxia/infarction and pressure overload), fibroblasts adopt a phenotypic change into alpha smooth muscle actin- (α-SMA) positive myofibroblasts (activated fibroblasts able to promote ECM overexpansion) (Table 2). The interactions among the cardiomyocytes, fibroblasts, coronary vasculature, and ECM provide the structure necessary for mediating biomechanical cross talk, mechanotransduction, and the development of cardiac stress, stretch, and stiffness (Fig. 5) [139,142].
Fig. 5.
Feedback mechanisms of loading on cell–ECM, cell–cell, and intracellular proteins that regulate cytoskeletal architecture, remodeling, and functional response. Myocardial remodeling represents changes in the cell (fibroblasts and cardiomyocyte) and ECM compartments of the heart in response to physiologic (e.g., endurance exercise) and pathologic (e.g., ischemia, infarction, infection, infiltration, and hypertension) stimuli. This leads to changes in cardiac biomechanics (stiffness), electrophysiology, and function (systole and diastole). Adverse myocardial remodeling represents a major mechanism and endpoint leading to the development of HF. HFrEF — Heart Failure with Reduced Ejection Fraction, HFpEF — Heart Failure with Preserved Ejection Fraction.
3.2. Introduction to heart failure pathophysiology
Abnormalities in heart biomechanics cause several common and highly morbid cardiovascular diseases including heart failure (HF), which is associated with 50% mortality at 5 years following diagnosis [143]. Aberrant changes in the cellular and ECM compartments of the myocardium (Table 2) lead to increases in tissue and cellular stiffness and wall stress [142,144–148]. These changes induce systolic and/or diastolic dysfunction, which has been strongly associated with the development of HF [149,150].
HF is a pathophysiological state mediated by myocardial (systolic and diastolic dysfunction) and extramyocardial (e.g. vascular stiffness, endothelial dysfunction, skeletal muscle metabolic derangements) abnormalities that either (1) undermine the ability of the heart to pump sufficient blood to meet the body's metabolic demands, or (2) allow it to meet these demands only when ventricular filling pressures are significantly elevated as a result of increased chamber stiffness and slowed active relaxation [141,151,152]. Two major subtypes of the HF syndrome are HF with reduced ejection fraction (HFrEF) (i.e., systolic dysfunction) and HF with preserved ejection fraction (HFpEF) (i.e., diastolic dysfunction) (Table 2) [153]. Although therapies targeting systolic dysfunction have improved the outcomes of many subjects with HFrEF [143,154], no therapeutic interventions in the HFpEF population have improved clinical outcomes. Furthermore, diastolic dysfunction is usually present in patients with HFrEF, and subclinical abnormalities in systolic function (detected non-invasively through assessment of systolic strain) are often present in patients with HFpEF.
3.3. Effects of HF on ECM remodeling and biomechanics
Abnormal diastolic biomechanics play a central role in the pathophysiology of HF. Severity of abnormalities correlates with worsening clinical outcomes. Furthermore, even the presence of abnormal diastolic biomechanics in asymptomatic individuals associates with a higher risk of developing HF, underscoring the importance of biomechanics in heart function [143,152,155–160].
Although these echocardiography-based studies introduced the concepts of abnormal diastolic biomechanics (e.g., slowed relaxation, increased stiffness, increased filling pressures), the mechanistic basis for these abnormalities (in humans) remained elusive until the advent of magnetic resonance imaging (MRI) to noninvasively characterize cardiac tissue properties in humans. In vivo cardiac MRI measures of myocardial fibrosis (Table 2) have demonstrated that expansion of the ECM compartment is associated with increased risk of sudden cardiac death, HF, mortality, and preclinical myocardial dysfunction [161–163]. Furthermore, increased fibrosis in HFpEF patients correlates with worse clinical outcomes [164]. In vivo measures of both focal and diffuse myocardial fibrosis have been strongly associated with major cardiac disease states and clinical events [165]. Remodeling in both the ECM and the cardiomyocyte myocardial compartments including fibrosis, deposition of advanced glycosylation end products, cardiomyocyte hypertrophy, myocardial thickening, and increased stiffness has been confirmed in myocardial tissue from humans with HFpEF and HFrEF [166–168].
Studies examining tissue-level rheology have also demonstrated increased stiffness of myocardial tissue in pathological stress states. Increased elastic modulus of infarcted versus non-infarcted tissue and the border zone myocardium were found in a rat model of ischemic cardiomyopathy (Table 2). Increased stiffness was also observed in the peri-infarct zone in a mouse infarction model [169]. The extension of adverse biomechanical remodeling beyond the infarct zone is well known to be central to the development of HF. Furthermore, tissue rheological changes occur in the presence of increased fibrosis and correlate to in vivo measures of systolic and diastolic dysfunction [170].
Changes in the cardiomyocyte compartments, in addition to the ECM, can also lead to abnormal global cardiac biomechanics, function (systolic and diastolic), and HF. Reduced cardiac strain assessed with echocardiography correlated with cardiomyocyte stiffening, disorganization of T-tubule structure, and abnormalities in intracellular calcium handling in a hypertensive rat model [171]. Interestingly, adverse cardiomyocyte remodeling preceded increased ECM stiffness in the transition from hypertensive heart disease to HF. This demonstrates that one source of increased stiffness may originate from the cardio-myocyte tissue compartment.
In summary, these strong associations of diastolic dysfunction, ECM, and cardiomyocyte remodeling with cardiovascular events and death in humans with HF underscore the importance of biomechanical derangements in human cardiovascular diseases. The potential molecular mechanisms mediating increased cardiomyocyte stiffness are addressed in the next section.
3.4. Cellular and molecular mechanisms underlying cardiac mechanotransduction and mechanics
The molecular composition of the ECM impacts the stiffness of cardiomyocytes, as demonstrated in in vitro cultures of cardiomyocytes on different matrices [172]. Integrins, hyaluronic acid content, and matrix stiffness are suggested to be tightly regulated for normal development of structure and function of cardiomyocytes, highlighting the importance of ECM remodeling and regulation in development [173]. The importance of cell–matrix interactions in the heart and of integrin-mediated matrix stiffness sensing by cardiomyocytes has also been demonstrated. Integrin-mediated (i.e., fibronectin–cardiomyocyte) interactions are critical for mediating normal ventricular contraction and relaxation [174]. The costamere, which couples the sarcomere (basic unit of muscle) with the sarcolemma (cell membrane of a striated muscle fiber cell), contains integrins that connect to the Z-disk (line forming sarcomere boundaries) and cytoskeleton via proteins including talin, vinculin, desmin, FAK, and α-actinin [175]. This forms the cardiomyocyte focal adhesion and mediates mechanotransduction between the cardiomyocyte and its surrounding matrix. In one study, fibronectin–integrin adhesion force and adhesion probability increased during isolated cardiomyocyte contraction and decreased during relaxation [174]. Specific cardiac integrins (e.g., α5-β 1, αV-β 3) are essential for the heart's response to stress states and overall survival [176]. Integrin β 1 and FAK mediate mechanotransduction in the heart in response to mechanical stretch. Integrin β 1 also mediates the phosphorylation of important intracellular MAP kinase signaling pathways including phosphorylation of ERK, p38, and JNK following mechanical stretch of neonatal rat ventricular myocytes. Inhibiting FAK activity reduced resting and stretch-induced phosphorylation of ERK and integrin β 1 was required for stretch-induced phosphorylation of FAK [177]. Integrin β 1 may also be involved in age-related myocardial stiffening and diastolic diameter decrease [178].
Cell–cell interactions through cadherins have also been shown to be important in the response of cardiomyocytes to changes in their mechanical environment (Fig. 5). Matrices coated with N-cadherin elicited changes in myofibrillar organization, myocyte shape, and cell stiffness, demonstrating the importance of cell–cell mediated force perception via N-cadherin [173].
Mechanosensing molecules downstream from integrins and cadherins in cardiac tissue (including FAK, vinculin, and catenins) also play a role in cardiac remodeling, biomechanics, function, and mechanotransduction. Mechanical stretching of cardiomyocytes increased phosphorylation of FAK, migration of FAK from the perinuclear region of neonatal rat ventricular myocytes to the myofilaments, and activation of the ANF gene [179]. Cyclical mechanical stretch- induced FAK phosphorylation in rat cardiac fibroblasts and resulted in mTOR-dependent proliferation and activation of these fibroblasts into myofibroblasts [180]. This fibroblast activation to myofibroblast is believed to play a central role in adverse cardiac ECM remodeling in response to stress states resulting in HF [139]. FAK signaling in response to mechanical pulsatile stretch is also present in cardiomyocytes and involves MAPK and SPAK signaling [181]. Cardiac-specific FAK knockout mice developed significant hypertrophy, increased LV mass, increased myocardial fibrosis, increased markers of fibrosis, and increased collagen expression in response to vasoconstrictor or transaortic constriction, two pressure overload models [182]. Furthermore, isolation of cardiomyocytes from these mice revealed disorganized myofibrils and decreased phosphorylation of p130Cas (docking protein involved in tyrosine-kinase signaling in cell adhesion) and paxillin (protein contributing to a focal adhesion), implicating these additional known mechanosensing proteins in this process. The importance of FAK in the cardiac remodeling response has also been demonstrated in a model of pressure overload [183]. An important role for vinculin in cardiac tissue and cardiomyocytes was demonstrated in cardiac-specific vinculin knockout mice whereby loss of vinculin caused abnormal myocardial biomechanics resulting in the development of HF [184]. Additional molecules, such as β-catenin, mediate the hypertrophic response of cardiomyocytes and the activation response of cardiac fibroblasts to myofibroblasts during post-infarct healing [185].
As mentioned above, a major source of increased stiffness in the heart, in addition to the ECM compartment, is the cardiomyocyte compartment. Studies demonstrated that the large intracellular cardio-myocyte protein, titin, mediated diastolic biomechanics and acted as a source of increased stiffness in HF (Fig. 5). Passive force occurs in the myocardium during diastole and increases as the LV chamber expands and fills [186]. Titin, located in the sarcomere of the cardiomyocyte, is a major contributor to passive force and stiffness of the cardiomyocyte during diastole [187]. Titin exists in various isoforms based on its phosphorylation status, and myocardium co-expresses varying amounts of both isoforms. Cardiomyocytes from pigs expressing higher levels of the N2BA isoform were less stiff compared to cardiomyocytes from rats and mice that expressed higher levels of the N2B isoform [188]. Increased expression of the stiffer titin isoform has been demonstrated in cardiomyocytes from failing myocardium [189]. Furthermore, increased expression of a stiffer titin isoform has been demonstrated in human HFpEF, suggesting that a component of the increased diastolic stiffness seen in HFpEF may be attributed to isoform expression shifts favoring the increased expression of the stiffer titin isoform by cardiomyocytes [166,190]. Therefore, therapeutic strategies targeting abnormal cardiac biomechanics to treat HF may need to target both ECM and cardiomyocyte tissue compartments.
Changes in the biomechanics and composition of myocardial tissue have also been demonstrated during development whereby the myocardial tissue and ECM become stiffer from the embryonic to neonatal period through to adult periods. Optimum matrix stiffness is required for neonatal cardiomyocytes to develop into structurally, functionally, and biomechanically normal adult cardiomyocytes, underscoring the importance of changes in cardiac biomechanics for normal cardiovascular development [191–193].
3.5. Summary and future work
Therapies demonstrated to improve systolic function have improved the outcomes of many subjects with HFrEF [143]. Unfortunately, no therapeutic interventions in the HFpEF population have demonstrated improved clinical outcomes [154]. Therefore, there is an urgent need to further characterize the cardiac and extracardiac phenotypes of this syndrome, identify and target novel risk factors, and propel the discovery of new therapeutic interventions.
Since myocardial infarction results in scar formation at the infarct site and deleterious remodeling in the infarct border zones and at distant sites, prevention and attenuation of these mechanisms are of clinical interest. To overcome these defects, valuable lessons may be learned in the zebrafish and newt since these animals regrow cardiac tissues with no scar tissue [42,43]. Injection of human mesenchymal stem cells into the acutely ischemic myocardium attenuated fibrosis, improved cardiac biomechanics, and reduced stiffness [170]. Furthermore, Drosophila may prove to be a powerful model for investigating the molecular basis of age-related cardiomyopathy. LV diastolic dysfunction is associated with aging [143], and senescent fruit flies experience decreased diastolic diameters, impaired myocardial relaxation, and increased myocardial stiffness, as measured in vivo by atomic force microscopy [178,194]. Such a model is genetically tractable, amenable to in vivo stiffness measurements, and recapitulates phenotypes observed in higher mammals [195]. This phenotype is dependent on integrin-linked kinase and β-integrin, implicating cell–ECM adhesion as a novel therapeutic target [178].
4. Conclusions and future directions
This review integrates the essential mechanisms of mechanotransduction underlying cell response to ECM dysfunction in the context of injury and disease by examining two specific cases, tendinopathy and HF. Tenocytes, TSCs, cardiomyocytes, and cardiac fibroblasts all sense ECM stiffness, one of the most profound and well-studied mechanical properties influencing cell behavior, using integrin-based adhesion complexes that couple the ECM to the actin cytoskeleton.
Although the tendon and the heart are both soft tissues, they demonstrate distinct composition, structure, and function in both normal and diseased states. Tenocytes are present in lower cell densities in the tendon than cardiomyocytes in the heart. In HF, the stiffening of the heart tissue is a result of the production of fibrotic ECM by myofibroblasts as well as the stiffening of cardiomyocytes themselves in response to an altered microenvironment. Although the ECM in the tendon also adapts, changes in tenocyte biomechanics during disease progression remain unknown. The diseased state in tendinopathy is decreased stiffening of the ECM, whereas the diseased state in HF is increased stiffening.
Excessive loading from repetitive overuse activity causes TSCs to differentiate into non-tenocyte lineages which produce aberrant ECM components, further altering the mechanical environment and perpetuating the degeneration of native tendon properties. In the case of cardiac tissue, increased ECM stiffness causes fibroblasts to differentiate into myofibroblasts, which produce more ECM components and stiffen the local environment. Although the ECMs in the diseased tendon and heart are reorganized in dramatically different ways in response to their respective sources of disease onset, cells in these two tissues respond to changes in the same ECM mechanical properties using similar mechanotransduction machinery.
Despite this wealth of knowledge that highlights the central interaction between the ECM and cell mechanotransduction, the current treatments ameliorating dysfunctional ECM and cell mechanosensing are limited. In addition to continued work using human and animal model systems, additional knowledge may be gained from newt and zebrafish models that undergo unique ECM remodeling to regenerate heart and limb tissues without scarring [196–200]. Given that altered matrix stiffness often underlies tendinopathy and HF, understanding how this remodeling supports regeneration biochemically and mechanically may lead to novel therapeutic strategies for targeting the dysfunctional ECM.
Acknowledgments
We thank Dr. Richard Assoian and Dr. Rebecca Wells for discussion. Additionally, we acknowledge our funding sources: GAANN P200A120246, T32 GM-07229, T32-HL007843, NIH (U01EB016633 and R01EB017753), and the NSF Graduate Research Fellowship Program.
Footnotes
This article is part of a Special Issue entitled: Mechanobiology.
Disclosures
All authors contributed equally to the preparation of this manuscript.
Conflicts of interest
The authors have no conflicts of interest to report.
References
- 1.Provenzano PP, Inman DR, Eliceiri KW, Keely PJ. Matrix density-induced mechanoregulation of breast cell phenotype, signaling and gene expression through a FAK–ERK linkage. Oncogene. 2009;28:4326–4343. doi: 10.1038/onc.2009.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwartz MA. Integrins and extracellular matrix in mechanotransduction. Cold Spring Harb Perspect Biol. 2010;2:a005066. doi: 10.1101/cshperspect.a005066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Millon-Fremillon A, Bouvard D, Grichine A, Manet-Dupe S, Block MR, Albiges-Rizo C. Cell adaptive response to extracellular matrix density is controlled by ICAP-1-dependent beta1-integrin affinity. J Cell Biol. 2008;180:427–441. doi: 10.1083/jcb.200707142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore SW, Roca-Cusachs P, Sheetz MP. Stretchy proteins on stretchy substrates: the important elements of integrin-mediated rigidity sensing. Dev Cell. 2010;19:194–206. doi: 10.1016/j.devcel.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoffman BD, Grashoff C, Schwartz MA. Dynamic molecular processes mediate cellular mechanotransduction. Nature. 2011;475:316–323. doi: 10.1038/nature10316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bershadsky AD, Balaban NQ, Geiger B. Adhesion-dependent cell mechanosensitivity. Annu Rev Cell Dev Biol. 2003;19:677–695. doi: 10.1146/annurev.cellbio.19.111301.153011. [DOI] [PubMed] [Google Scholar]
- 7.Koo LY, Irvine DJ, Mayes AM, Lauffenburger DA, Griffith LG. Co-regulation of cell adhesion by nanoscale RGD organization and mechanical stimulus. J Cell Sci. 2002;115:1423–1433. doi: 10.1242/jcs.115.7.1423. [DOI] [PubMed] [Google Scholar]
- 8.Maheshwari G, Brown G, Lauffenburger DA, Wells A, Griffith LG. Cell adhesion and motility depend on nanoscale RGD clustering. J Cell Sci. 2000;113(Pt 10):1677–1686. doi: 10.1242/jcs.113.10.1677. [DOI] [PubMed] [Google Scholar]
- 9.Ricca BL, Venugopalan G, Fletcher DA. To pull or be pulled: parsing the multiple modes of mechanotransduction. Curr Opin Cell Biol. 2013;25:558–564. doi: 10.1016/j.ceb.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swift J, Discher DE. The nuclear lamina is mechano-responsive to ECM elasticity in mature tissue. J Cell Sci. 2014;127:3005–3015. doi: 10.1242/jcs.149203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janmey PA, Miller RT. Mechanisms of mechanical signaling in development and disease. J Cell Sci. 2011;124:9–18. doi: 10.1242/jcs.071001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wen JH, Vincent LG, Fuhrmann A, Choi YS, Hribar KC, Taylor-Weiner H, Chen S, Engler AJ. Interplay of matrix stiffness and protein tethering in stem cell differentiation. Nat Mater. 2014;13:979–987. doi: 10.1038/nmat4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wells RG. The role of matrix stiffness in regulating cell behavior. Hepatology. 2008;47:1394–1400. doi: 10.1002/hep.22193. [DOI] [PubMed] [Google Scholar]
- 14.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 15.Subramony SD, Dargis BR, Castillo M, Azeloglu EU, Tracey MS, Su A, Lu HH. The guidance of stem cell differentiation by substrate alignment and mechanical stimulation. Biomaterials. 2013;34:1942–1953. doi: 10.1016/j.biomaterials.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keller R, Shook D, Skoglund P. The forces that shape embryos: physical aspects of convergent extension by cell intercalation. Phys Biol. 2008;5:015007. doi: 10.1088/1478-3975/5/1/015007. [DOI] [PubMed] [Google Scholar]
- 17.Horne-Badovinac S. The Drosophila egg chamber—a new spin on how tissues elongate. Integr Comp Biol. 2014;54:667–676. doi: 10.1093/icb/icu067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barocas VH, Tranquillo RT. An anisotropic biphasic theory of tissue-equivalent mechanics: the interplay among cell traction, fibrillar network deformation, fibril alignment, and cell contact guidance. J Biomech Eng. 1997;119:137–145. doi: 10.1115/1.2796072. [DOI] [PubMed] [Google Scholar]
- 19.Perris R, Perissinotto D. Role of the extracellular matrix during neural crest cell migration. Mech Dev. 2000;95:3–21. doi: 10.1016/s0925-4773(00)00365-8. [DOI] [PubMed] [Google Scholar]
- 20.Leckband DE, le Duc Q, Wang N, de Rooij J. Mechanotransduction at cadherin-mediated adhesions. Curr Opin Cell Biol. 2011;23:523–530. doi: 10.1016/j.ceb.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 21.Ladoux B, Anon E, Lambert M, Rabodzey A, Hersen P, Buguin A, Silberzan P, Mege RM. Strength dependence of cadherin-mediated adhesions. Biophys J. 2010;98:534–542. doi: 10.1016/j.bpj.2009.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwartz MA, DeSimone DW. Cell adhesion receptors in mechanotransduction. Curr Opin Cell Biol. 2008;20:551–556. doi: 10.1016/j.ceb.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen CS, Tan J, Tien J. Mechanotransduction at cell–matrix and cell–cell contacts. Annu Rev Biomed Eng. 2004;6:275–302. doi: 10.1146/annurev.bioeng.6.040803.140040. [DOI] [PubMed] [Google Scholar]
- 24.Sander EA, Stylianopoulos T, Tranquillo RT, Barocas VH. Image-based multiscale modeling predicts tissue-level and network-level fiber reorganization in stretched cell-compacted collagen gels. Proc Natl Acad Sci U S A. 2009;106:17675–17680. doi: 10.1073/pnas.0903716106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim SH, Turnbull J, Guimond S. Extracellular matrix and cell signalling: the dynamic cooperation of integrin, proteoglycan and growth factor receptor. J Endocrinol. 2011;209:139–151. doi: 10.1530/JOE-10-0377. [DOI] [PubMed] [Google Scholar]
- 26.Brown RA, Prajapati R, McGrouther DA, Yannas IV, Eastwood M. Tensional homeostasis in dermal fibroblasts: mechanical responses to mechanical loading in three-dimensional substrates. J Cell Physiol. 1998;175:323–332. doi: 10.1002/(SICI)1097-4652(199806)175:3<323::AID-JCP10>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 27.Zemel A, Rehfeldt F, Brown AE, Discher DE, Safran SA. Optimal matrix rigidity for stress fiber polarization in stem cells. Nat Phys. 2010;6:468–473. doi: 10.1038/nphys1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 29.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 30.Fukashiro S, Komi PV, Jarvinen M, Miyashita M. In vivo Achilles tendon loading during jumping in humans. Eur J Appl Physiol Occup Physiol. 1995;71:453–458. doi: 10.1007/BF00635880. [DOI] [PubMed] [Google Scholar]
- 31.Wren TA, Yerby SA, Beaupre GS, Carter DR. Mechanical properties of the human Achilles tendon. Clin Biomech (Bristol, Avon) 2001;16:245–251. doi: 10.1016/s0268-0033(00)00089-9. [DOI] [PubMed] [Google Scholar]
- 32.Grashoff C, Hoffman BD, Brenner MD, Zhou R, Parsons M, Yang MT, McLean MA, Sligar SG, Chen CS, Ha T, Schwartz MA. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature. 2010;466:263–266. doi: 10.1038/nature09198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.VanBuren P, Work SS, Warshaw DM. Enhanced force generation by smooth muscle myosin in vitro. Proc Natl Acad Sci U S A. 1994;91:202–205. doi: 10.1073/pnas.91.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chiquet M, Renedo AS, Huber F, Fluck M. How do fibroblasts translate mechanical signals into changes in extracellular matrix production? Matrix Biol. 2003;22:73–80. doi: 10.1016/s0945-053x(03)00004-0. [DOI] [PubMed] [Google Scholar]
- 35.Ryan JA, Eisner EA, DuRaine G, You Z, Reddi AH. Mechanical compression of articular cartilage induces chondrocyte proliferation and inhibits proteoglycan synthesis by activation of the ERK pathway: implications for tissue engineering and regenerative medicine. J Tissue Eng Regen Med. 2009;3:107–116. doi: 10.1002/term.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.MacKenna D, Summerour SR, Villarreal FJ. Role of mechanical factors in modulating cardiac fibroblast function and extracellular matrix synthesis. Cardiovasc Res. 2000;46:257–263. doi: 10.1016/s0008-6363(00)00030-4. [DOI] [PubMed] [Google Scholar]
- 37.Nesbitt WS, Westein E, Tovar-Lopez FJ, Tolouei E, Mitchell A, Fu J, Carberry J, Fouras A, Jackson SP. A shear gradient-dependent platelet aggregation mechanism drives thrombus formation. Nat Med. 2009;15:665–673. doi: 10.1038/nm.1955. [DOI] [PubMed] [Google Scholar]
- 38.Weinbaum S, Duan Y, Thi MM, You L. An integrative review of mechanotransduction in endothelial, epithelial (renal) and dendritic cells (osteocytes) Cell Mol Bioeng. 2011;4:510–537. doi: 10.1007/s12195-011-0179-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miteva DO, Rutkowski JM, Dixon JB, Kilarski W, Shields JD, Swartz MA. Transmural flow modulates cell and fluid transport functions of lymphatic endothelium. Circ Res. 2010;106:920–931. doi: 10.1161/CIRCRESAHA.109.207274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ng CP, Swartz MA. Fibroblast alignment under interstitial fluid flow using a novel 3-D tissue culture model. Am J Physiol Heart Circ Physiol. 2003;284:H1771–H1777. doi: 10.1152/ajpheart.01008.2002. [DOI] [PubMed] [Google Scholar]
- 41.Galie PA, Nguyen DH, Choi CK, Cohen DM, Janmey PA, Chen CS. Fluid shear stress threshold regulates angiogenic sprouting. Proc Natl Acad Sci U S A. 2014;111:7968–7973. doi: 10.1073/pnas.1310842111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oga T, Matsuoka T, Yao C, Nonomura K, Kitaoka S, Sakata D, Kita Y, Tanizawa K, Taguchi Y, Chin K, Mishima M, Shimizu T, Narumiya S. Prostaglandin F(2alpha) receptor signaling facilitates bleomycin-induced pulmonary fibrosis independently of transforming growth factor-beta. Nat Med. 2009;15:1426–1430. doi: 10.1038/nm.2066. [DOI] [PubMed] [Google Scholar]
- 43.Kasner M, Westermann D, Lopez B, Gaub R, Escher F, Kuhl U, Schultheiss HP, Tschope C. Diastolic tissue Doppler indexes correlate with the degree of collagen expression and cross-linking in heart failure and normal ejection fraction. J Am Coll Cardiol. 2011;57:977–985. doi: 10.1016/j.jacc.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 44.Weidemann F, Herrmann S, Stork S, Niemann M, Frantz S, Lange V, Beer M, Gattenlohner S, Voelker W, Ertl G, Strotmann JM. Impact of myocardial fibrosis in patients with symptomatic severe aortic stenosis. Circulation. 2009;120:577–584. doi: 10.1161/CIRCULATIONAHA.108.847772. [DOI] [PubMed] [Google Scholar]
- 45.Zhen G, Wen C, Jia X, Li Y, Crane JL, Mears SC, Askin FB, Frassica FJ, Chang W, Yao J, Carrino JA, Cosgarea A, Artemov D, Chen Q, Zhao Z, Zhou X, Riley L, Sponseller P, Wan M, Lu WW, Cao X. Inhibition of TGF-beta signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat Med. 2013;19:704–712. doi: 10.1038/nm.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Whitehead J, Vignjevic D, Futterer C, Beaurepaire E, Robine S, Farge E. Mechanical factors activate beta-catenin-dependent oncogene expression in APC mouse colon. HFSP J. 2008;2:286–294. doi: 10.2976/1.2955566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Freedman BR, Gordon JA, Soslowsky LJ. The Achilles tendon: fundamental properties and mechanisms governing healing. Muscles Ligaments Tendons J. 2014;4:245–255. [PMC free article] [PubMed] [Google Scholar]
- 48.Bi Y, Ehirchiou D, Kilts TM, Inkson CA, Embree MC, Sonoyama W, Li L, Leet AI, Seo BM, Zhang L, Shi S, Young MF. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13:1219–1227. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- 49.Fukuta S, Oyama M, Kavalkovich K, Fu FH, Niyibizi C. Identification of types II, IX and X collagens at the insertion site of the bovine Achilles tendon. Matrix Biol. 1998;17:65–73. doi: 10.1016/s0945-053x(98)90125-1. [DOI] [PubMed] [Google Scholar]
- 50.Sun M, Connizzo BK, Adams SM, Freedman BR, Wenstrup RJ, Soslowsky LJ, Birk DE. Targeted deletion of collagen V in tendons and ligaments results in a classic Ehlers–Danlos syndrome joint phenotype. Am J Pathol. 2015;185(5):1436–1447. doi: 10.1016/j.ajpath.2015.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buckley MR, Sarver JJ, Freedman BR, Soslowsky LJ. The dynamics of collagen uncrimping and lateral contraction in tendon and the effect of ionic concentration. J Biomech. 2013;46(13):2242–2249. doi: 10.1016/j.jbiomech.2013.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ethier C, Simmons C. Introductory biomechanics: from cells to organisms. Cambridge Texts in Biomedical Engineering. 2007 [Google Scholar]
- 53.Longo UG, Ronga M, Maffulli N. Achilles tendinopathy. Sports Med Arthrosc. 2009;17:112–126. doi: 10.1097/JSA.0b013e3181a3d625. [DOI] [PubMed] [Google Scholar]
- 54.Henninger HB, Underwood CJ, Romney SJ, Davis GL, Weiss JA. Effect of elastin digestion on the quasi-static tensile response of medial collateral ligament. J Orthop Res. 2013;31:1226–1233. doi: 10.1002/jor.22352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rigozzi S, Muller R, Snedeker JG. Local strain measurement reveals a varied regional dependence of tensile tendon mechanics on glycosaminoglycan content. J Biomech. 2009;42:1547–1552. doi: 10.1016/j.jbiomech.2009.03.031. [DOI] [PubMed] [Google Scholar]
- 56.Ahmadzadeh H, Connizzo BK, Freedman BR, Soslowsky LJ, Shenoy VB. Determining the contribution of glycosaminoglycans to tendon mechanical properties with a modified shear-lag model. J Biomech. 2013 Sep 27;46(14):2497–2503. doi: 10.1016/j.jbiomech.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.LaCroix AS, Duenwald-Kuehl SE, Lakes RS, Vanderby R., Jr Relationship between tendon stiffness and failure: a metaanalysis. J Appl Physiol. 2013;115:43–51. doi: 10.1152/japplphysiol.01449.2012. 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Woo SL. Mechanical properties of tendons and ligaments. I. Quasi-static and nonlinear viscoelastic properties. Biorheology. 1982;19:385–396. doi: 10.3233/bir-1982-19301. [DOI] [PubMed] [Google Scholar]
- 59.Miller KS, Connizzo BK, Feeney E, Soslowsky LJ. Characterizing local collagen fiber re-alignment and crimp behavior throughout mechanical testing in a mature mouse supraspinatus tendon model. J Biomech. 2012;45:2061–2065. doi: 10.1016/j.jbiomech.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yin L, Elliott DM. A biphasic and transversely isotropic mechanical model for tendon: application to mouse tail fascicles in uniaxial tension. J Biomech. 2004;37:907–916. doi: 10.1016/j.jbiomech.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 61.Maganaris CN, Paul JP. In vivo human tendon mechanical properties. J Physiol. 1999;521(Pt 1):307–313. doi: 10.1111/j.1469-7793.1999.00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fang F, Sawhney AS, Lake SP. Different regions of bovine deep digital flexor tendon exhibit distinct elastic, but not viscous, mechanical properties under both compression and shear loading. J Biomech. 2014;47:2869–2877. doi: 10.1016/j.jbiomech.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 63.Szczesny SE, Peloquin JM, Cortes DH, Kadlowec JA, Soslowsky LJ, Elliott DM. Biaxial tensile testing and constitutive modeling of human supraspinatus tendon. J Biomech Eng. 2012;134:021004. doi: 10.1115/1.4005852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Forde MS, Punnett L, Wegman DH. Prevalence of musculoskeletal disorders in union ironworkers. J Occup Environ Hyg. 2005;2:203–212. doi: 10.1080/15459620590929635. [DOI] [PubMed] [Google Scholar]
- 65.Jobe FW, Kvitne RS, Giangarra CE. Shoulder pain in the overhand or throwing athlete, The relationship of anterior instability and rotator cuff impingement. Orthop Rev. 1989;18:963–975. [PubMed] [Google Scholar]
- 66.McMaster WC, Troup J. A survey of interfering shoulder pain in United States competitive swimmers. Am J Sports Med. 1993;21:67–70. doi: 10.1177/036354659302100112. [DOI] [PubMed] [Google Scholar]
- 67.Renstrom P, Johnson RJ. Overuse injuries in sports. A review. Sports Med. 1985;2:316–333. doi: 10.2165/00007256-198502050-00002. [DOI] [PubMed] [Google Scholar]
- 68.Sommerich CM, McGlothlin JD, Marras WS. Occupational risk factors associated with soft tissue disorders of the shoulder: a review of recent investigations in the literature. Ergonomics. 1993;36:697–717. doi: 10.1080/00140139308967931. [DOI] [PubMed] [Google Scholar]
- 69.Sein ML, Walton J, Linklater J, Appleyard R, Kirkbride B, Kuah D, Murrell GA. Shoulder pain in elite swimmers: primarily due to swim-volume-induced supraspinatus tendinopathy. Br J Sports Med. 2010;44:105–113. doi: 10.1136/bjsm.2008.047282. [DOI] [PubMed] [Google Scholar]
- 70.Bureau of Labor Statistics, 1988. Aug, 1990. Occupational injuries and illnesses in the United States by industry, Bulletin No. 2368. [Google Scholar]
- 71.Rudavsky A, Cook J. Physiotherapy management of patellar tendinopathy (jumper's knee) J Physiother. 2014;60:122–129. doi: 10.1016/j.jphys.2014.06.022. [DOI] [PubMed] [Google Scholar]
- 72.Factor D, Dale B. Current concepts of rotator cuff tendinopathy. Int J Sports Phys Ther. 2014;9:274–288. [PMC free article] [PubMed] [Google Scholar]
- 73.Malliaras P, Chan O, Simran G, Martinez de Albornoz P, Morrissey D, Maffulli N. Doppler ultrasound signal in Achilles tendinopathy reduces immediately after activity. Int J Sports Med. 2012;33:480–484. doi: 10.1055/s-0032-1304636. [DOI] [PubMed] [Google Scholar]
- 74.Zhang J, Wang JH. BMP-2 mediates PGE(2)-induced reduction of proliferation and osteogenic differentiation of human tendon stem cells. J Orthop Res. 2012;30:47–52. doi: 10.1002/jor.21485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Langberg H, Skovgaard D, Karamouzis M, Bulow J, Kjaer M. Metabolism and inflammatory mediators in the peritendinous space measured by microdialysis during intermittent isometric exercise in humans. J Physiol. 1999;515(Pt 3):919–927. doi: 10.1111/j.1469-7793.1999.919ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Farris DJ, Trewartha G, McGuigan MP, Lichtwark GA. Differential strain patterns of the human Achilles tendon determined in vivo with freehand three-dimensional ultrasound imaging. J Exp Biol. 2013;216:594–600. doi: 10.1242/jeb.077131. [DOI] [PubMed] [Google Scholar]
- 77.Lichtwark GA, Cresswell AG, Newsham-West RJ. Effects of running on human Achilles tendon length–tension properties in the free and gastrocnemius components. J Exp Biol. 2013;216(Pt 23):4388–4394. doi: 10.1242/jeb.094219. [DOI] [PubMed] [Google Scholar]
- 78.Hansen P, Kovanen V, Holmich P, Krogsgaard M, Hansson P, Dahl M, Hald M, Aagaard P, Kjaer M, Magnusson SP. Micromechanical properties and collagen composition of ruptured human Achilles tendon. Am J Sports Med. 2013;41:437–443. doi: 10.1177/0363546512470617. [DOI] [PubMed] [Google Scholar]
- 79.Barbe MF, Gallagher S, Popoff SN. Serum biomarkers as predictors of stage of work-related musculoskeletal disorders. J Am Acad Orthop Surg. 2013;21:644–646. doi: 10.5435/JAAOS-21-10-644. [DOI] [PubMed] [Google Scholar]
- 80.Ikoma K, Kido M, Nagae M, Ikeda T, Shirai T, Ueshima K, Arai Y, Oda R, Fujiwara H, Kubo T. Effects of stress-shielding on the dynamic viscoelasticity and ordering of the collagen fibers in rabbit Achilles tendon. J Orthop Res. 2013;(11):1708–1712. doi: 10.1002/jor.22424. [DOI] [PubMed] [Google Scholar]
- 81.Thornton GM, Shao X, Chung M, Sciore P, Boorman RS, Hart DA, Lo IK. Changes in mechanical loading lead to tendon specific alterations in MMP and TIMP expression: influence of stress deprivation and intermittent cyclic hydrostatic compression on rat supraspinatus and Achilles tendons. Br J Sports Med. 2010;44:698–703. doi: 10.1136/bjsm.2008.050575. [DOI] [PubMed] [Google Scholar]
- 82.Uchida H, Tohyama H, Nagashima K, Ohba Y, Matsumoto H, Toyama Y, Yasuda K. Stress deprivation simultaneously induces over-expression of interleukin-1beta, tumor necrosis factor-alpha, and transforming growth factor-beta in fibroblasts and mechanical deterioration of the tissue in the patellar tendon. J Biomech. 2005;38:791–798. doi: 10.1016/j.jbiomech.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 83.Mendias CL, Gumucio JP, Lynch EB. Mechanical loading and TGF-beta change the expression of multiple miRNAs in tendon fibroblasts. J Appl Physiol. 2012;113:56–62. doi: 10.1152/japplphysiol.00301.2012. 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Heinemeier KM, Skovgaard D, Bayer ML, Qvortrup K, Kjaer A, Kjaer M, Magnusson SP, Kongsgaard M. Uphill running improves rat Achilles tendon tissue mechanical properties and alters gene expression without inducing pathological changes. J Appl Physiol. 2012;113:827–836. doi: 10.1152/japplphysiol.00401.2012. 1985. [DOI] [PubMed] [Google Scholar]
- 85.Cho NS, Hwang JH, Lee YT, Chae SW. Tendinosis-like histologic and molecular changes of the Achilles tendon to repetitive stress: a pilot study in rats. Clin Orthop Relat Res. 2011;469:3172–3180. doi: 10.1007/s11999-011-2000-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Moerch L, Pingel J, Boesen M, Kjaer M, Langberg H. The effect of acute exercise on collagen turnover in human tendons: influence of prior immobilization period. Eur J Appl Physiol. 2013;113:449–455. doi: 10.1007/s00421-012-2450-5. [DOI] [PubMed] [Google Scholar]
- 87.Kongsgaard M, Qvortrup K, Larsen J, Aagaard P, Doessing S, Hansen P, Kjaer M, Magnusson SP. Fibril morphology and tendon mechanical properties in patellar tendinopathy: effects of heavy slow resistance training. Am J Sports Med. 2010;38:749–756. doi: 10.1177/0363546509350915. [DOI] [PubMed] [Google Scholar]
- 88.Foure A, Nordez A, Cornu C. Plyometric training effects on Achilles tendon stiffness and dissipative properties. J Appl Physiol. 2010;109:849–854. doi: 10.1152/japplphysiol.01150.2009. 1985. [DOI] [PubMed] [Google Scholar]
- 89.Zhang J, Wang JH. The effects of mechanical loading on tendons —an in vivo and in vitro model study. PLoS One. 2013;8:e71740. doi: 10.1371/journal.pone.0071740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kannus P, Jozsa L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J Bone Joint Surg Am. 1991;73:1507–1525. [PubMed] [Google Scholar]
- 91.Soslowsky LJ, Thomopoulos S, Tun S, Flanagan CL, Keefer CC, Mastaw J, Carpenter JE. Neer Award 1999. Overuse activity injures the supraspinatus tendon in an animal model: a histologic and biomechanical study. J Shoulder Elb Surg. 2000;9:79–84. [PubMed] [Google Scholar]
- 92.Archambault JM, Jelinsky SA, Lake SP, Hill AA, Glaser DL, Soslowsky LJ. Rat supraspinatus tendon expresses cartilage markers with overuse. J Orthop Res. 2007;25:617–624. doi: 10.1002/jor.20347. [DOI] [PubMed] [Google Scholar]
- 93.Perry SM, McIlhenny SE, Hoffman MC, Soslowsky LJ. Inflammatory and angiogenic mRNA levels are altered in a supraspinatus tendon overuse animal model. J Shoulder Elb Surg. 2005;14:79S–83S. doi: 10.1016/j.jse.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 94.Attia M, Scott A, Duchesnay A, Carpentier G, Soslowsky LJ, Huynh MB, Van Kuppevelt TH, Gossard C, Courty J, Tassoni MC, Martelly I. Alterations of overused supraspinatus tendon: a possible role of glycosaminoglycans and HARP/pleiotrophin in early tendon pathology. J Orthop Res. 2012;30:61–71. doi: 10.1002/jor.21479. [DOI] [PubMed] [Google Scholar]
- 95.Sun HB, Andarawis-Puri N, Li Y, Fung DT, Lee JY, Wang VM, Basta-Pljakic J, Leong DJ, Sereysky JB, Ros SJ, Klug RA, Braman J, Schaffler MB, Jepsen KJ, Flatow EL. Cycle-dependent matrix remodeling gene expression response in fatigue-loaded rat patellar tendons. J Orthop Res. 2010;28:1380–1386. doi: 10.1002/jor.21132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Soslowsky LJ, Carpenter JE, DeBano CM, Banerji I, Moalli MR. Development and use of an animal model for investigations on rotator cuff disease. J Shoulder Elb Surg. 1996;5:383–392. doi: 10.1016/s1058-2746(96)80070-x. [DOI] [PubMed] [Google Scholar]
- 97.Lakemeier S, Reichelt JJ, Timmesfeld N, Fuchs-Winkelmann S, Paletta JR, Schofer MD. The relevance of long head biceps degeneration in the presence of rotator cuff tears. BMC Musculoskelet Disord. 2010;11:191. doi: 10.1186/1471-2474-11-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Peltz CD, Dourte LM, Kuntz AF, Sarver JJ, Kim SY, Williams GR, Soslowsky LJ. The effect of postoperative passive motion on rotator cuff healing in a rat model. J Bone Joint Surg Am. 2009;91:2421–2429. doi: 10.2106/JBJS.H.01121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Reuther KE, Thomas SJ, Tucker JJ, Sarver JJ, Gray CF, Rooney SI, Glaser DL, Soslowsky LJ. Disruption of the anterior-posterior rotator cuff force balance alters joint function and leads to joint damage in a rat model. J Orthop Res. 2014;32:638–644. doi: 10.1002/jor.22586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang XT, Ker RF, Alexander RM. Fatigue rupture of wallaby tail tendons. J Exp Biol. 1995;198:847–852. doi: 10.1242/jeb.198.3.847. [DOI] [PubMed] [Google Scholar]
- 101.Freedman BR, Zuskov A, Sarver JJ, Buckley MR, Soslowsky LJ. Evaluating changes in tendon crimp with fatigue loading as an ex vivo structural assessment of tendon damage. J Orthop Res. 2015 doi: 10.1002/jor.22875. http://dx.doi.org/10.1002/jor.22875 (In Press) [DOI] [PMC free article] [PubMed]
- 102.Veres SP, Harrison JM, Lee JM. Repeated subrupture overload causes progression of nanoscaled discrete plasticity damage in tendon collagen fibrils. J Orthop Res. 2013;31:731–737. doi: 10.1002/jor.22292. [DOI] [PubMed] [Google Scholar]
- 103.Veres SP, Brennan-Pierce EP, Lee JM. Macrophage-like U937 cells recognize collagen fibrils with strain-induced discrete plasticity damage. J Biomed Mater Res A. 2015;103:397–408. doi: 10.1002/jbm.a.35156. [DOI] [PubMed] [Google Scholar]
- 104.Shepherd JH, Legerlotz K, Demirci T, Klemt C, Riley GP, Screen HR. Functionally distinct tendon fascicles exhibit different creep and stress relaxation behaviour. Proc Inst Mech Eng H. 2014;228:49–59. doi: 10.1177/0954411913509977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Thorpe CT, Riley GP, Birch HL, Clegg PD, Screen HR. Fascicles from energy-storing tendons show an age-specific response to cyclic fatigue loading. J R Soc Interface. 2014;11:20131058. doi: 10.1098/rsif.2013.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Maeda E, Fleischmann C, Mein CA, Shelton JC, Bader DL, Lee DA. Functional analysis of tenocytes gene expression in tendon fascicles subjected to cyclic tensile strain. Connect Tissue Res. 2010;51:434–444. doi: 10.3109/03008201003597056. [DOI] [PubMed] [Google Scholar]
- 107.Maeda E, Shelton JC, Bader DL, Lee DA. Time dependence of cyclic tensile strain on collagen production in tendon fascicles. Biochem Biophys Res Commun. 2007;362:399–404. doi: 10.1016/j.bbrc.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 108.Maeda E, Shelton JC, Bader DL, Lee DA. Differential regulation of gene expression in isolated tendon fascicles exposed to cyclic tensile strain in vitro. J Appl Physiol. 2009;106:506–512. doi: 10.1152/japplphysiol.90981.2008. 1985. [DOI] [PubMed] [Google Scholar]
- 109.Screen HR, Shelton JC, Bader DL, Lee DA. Cyclic tensile strain upregulates collagen synthesis in isolated tendon fascicles. Biochem Biophys Res Commun. 2005;336:424–429. doi: 10.1016/j.bbrc.2005.08.102. [DOI] [PubMed] [Google Scholar]
- 110.Hannafin JA, Arnoczky SP, Hoonjan A, Torzilli PA. Effect of stress deprivation and cyclic tensile loading on the material and morphologic properties of canine flexor digitorum profundus tendon: an in vitro study. J Orthop Res. 1995;13:907–914. doi: 10.1002/jor.1100130615. [DOI] [PubMed] [Google Scholar]
- 111.Legerlotz K, Jones GC, Screen HR, Riley GP. Cyclic loading of tendon fascicles using a novel fatigue loading system increases interleukin-6 expression by tenocytes. Scand J Med Sci Sports. 2013;23:31–37. doi: 10.1111/j.1600-0838.2011.01410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yamamoto E, Kogawa D, Tokura S, Hayashi K. Effects of the frequency and duration of cyclic stress on the mechanical properties of cultured collagen fascicles from the rabbit patellar tendon. J Biomech Eng. 2005;127:1168–1175. doi: 10.1115/1.2073587. [DOI] [PubMed] [Google Scholar]
- 113.Yamamoto E, Tokura S, Hayashi K. Effects of cyclic stress on the mechanical properties of cultured collagen fascicles from the rabbit patellar tendon. J Biomech Eng. 2003;125:893–901. doi: 10.1115/1.1634286. [DOI] [PubMed] [Google Scholar]
- 114.Devkota AC, Tsuzaki M, Almekinders LC, Banes AJ, Weinhold PS. Distributing a fixed amount of cyclic loading to tendon explants over longer periods induces greater cellular and mechanical responses. J Orthop Res. 2007;25:1078–1086. doi: 10.1002/jor.20389. [DOI] [PubMed] [Google Scholar]
- 115.Flick J, Devkota A, Tsuzaki M, Almekinders L, Weinhold P. Cyclic loading alters biomechanical properties and secretion of PGE2 and NO from tendon explants. Clin Biomech (Bristol, Avon) 2006;21:99–106. doi: 10.1016/j.clinbiomech.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 116.Wang JH, Jia F, Yang G, Yang S, Campbell BH, Stone D, Woo SL. Cyclic mechanical stretching of human tendon fibroblasts increases the production of prostaglandin E2 and levels of cyclooxygenase expression: a novel in vitro model study. Connect Tissue Res. 2003;44:128–133. doi: 10.1080/03008200390223909. [DOI] [PubMed] [Google Scholar]
- 117.Cilli F, Khan M, Fu F, Wang JH. Prostaglandin E2 affects proliferation and collagen synthesis by human patellar tendon fibroblasts. Clin J Sport Med. 2004;14:232–236. doi: 10.1097/00042752-200407000-00006. [DOI] [PubMed] [Google Scholar]
- 118.Rui YF, Lui PP, Ni M, Chan LS, Lee YW, Chan KM. Mechanical loading increased BMP-2 expression which promoted osteogenic differentiation of tendon-derived stem cells. J Orthop Res. 2011;29:390–396. doi: 10.1002/jor.21218. [DOI] [PubMed] [Google Scholar]
- 119.Shi Y, Fu Y, Tong W, Geng Y, Lui PP, Tang T, Zhang X, Dai K. Uniaxial mechanical tension promoted osteogenic differentiation of rat tendon-derived stem cells (rTDSCs) via the Wnt5a–RhoA pathway. J Cell Biochem. 2012;113:3133–3142. doi: 10.1002/jcb.24190. [DOI] [PubMed] [Google Scholar]
- 120.Chen CT, Malkus DS, Vanderby R., Jr A fiber matrix model for interstitial fluid flow and permeability in ligaments and tendons. Biorheology. 1998;35:103–118. doi: 10.1016/S0006-355X(99)80001-8. [DOI] [PubMed] [Google Scholar]
- 121.Lavagnino M, Arnoczky SP, Kepich E, Caballero O, Haut RC. A finite element model predicts the mechanotransduction response of tendon cells to cyclic tensile loading. Biomech Model Mechanobiol. 2008;7:405–416. doi: 10.1007/s10237-007-0104-z. [DOI] [PubMed] [Google Scholar]
- 122.Archambault JM, Hart DA, Herzog W. Response of rabbit Achilles tendon to chronic repetitive loading. Connect Tissue Res. 2001;42:13–23. doi: 10.3109/03008200109014245. [DOI] [PubMed] [Google Scholar]
- 123.Maeda T, Sakabe T, Sunaga A, Sakai K, Rivera AL, Keene DR, Sasaki T, Stavnezer E, Iannotti J, Schweitzer R, Ilic D, Baskaran H, Sakai T. Conversion of mechanical force into TGF-beta-mediated biochemical signals. Curr Biol. 2011;21:933–941. doi: 10.1016/j.cub.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fong KD, Trindade MC, Wang Z, Nacamuli RP, Pham H, Fang TD, Song HM, Smith RL, Longaker MT, Chang J. Microarray analysis of mechanical shear effects on flexor tendon cells. Plast Reconstr Surg. 2005;116:1393–1404. doi: 10.1097/01.prs.0000182345.86453.4f. discussion 1405–1396. [DOI] [PubMed] [Google Scholar]
- 125.Tsuzaki M, Bynum D, Almekinders L, Faber J, Banes AJ. Mechanical loading stimulates ecto-ATPase activity in human tendon cells. J Cell Biochem. 2005;96:117–125. doi: 10.1002/jcb.20491. [DOI] [PubMed] [Google Scholar]
- 126.van Griensven M, Zeichen J, Skutek M, Barkhausen T, Krettek C, Bosch U. Cyclic mechanical strain induces NO production in human patellar tendon fibroblasts—a possible role for remodelling and pathological transformation. Exp Toxicol Pathol. 2003;54:335–338. doi: 10.1078/0940-2993-00268. [DOI] [PubMed] [Google Scholar]
- 127.Maeda E, Hagiwara Y, Wang JH, Ohashi T. A new experimental system for simultaneous application of cyclic tensile strain and fluid shear stress to tenocytes in vitro. Biomed Microdevices. 2013;15:1067–1075. doi: 10.1007/s10544-013-9798-0. [DOI] [PubMed] [Google Scholar]
- 128.Buscemi L, Ramonet D, Klingberg F, Formey A, Smith-Clerc J, Meister JJ, Hinz B. The single-molecule mechanics of the latent TGF-beta1 complex. Curr Biol. 2011;21:2046–2054. doi: 10.1016/j.cub.2011.11.037. [DOI] [PubMed] [Google Scholar]
- 129.Wells RG, Discher DE. Matrix elasticity, cytoskeletal tension, and TGF-beta: the insoluble and soluble meet. Sci Signal. 2008;1:e13. doi: 10.1126/stke.110pe13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jones ER, Jones GC, Legerlotz K, Riley GP. Cyclical strain modulates metalloprotease and matrix gene expression in human tenocytes via activation of TGFbeta. Biochim Biophys Acta. 2013;1833:2596–2607. doi: 10.1016/j.bbamcr.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ralphs JR, Waggett AD, Benjamin M. Actin stress fibres and cell–cell adhesion molecules in tendons: organisation in vivo and response to mechanical loading of tendon cells in vitro. Matrix Biol. 2002;21:67–74. doi: 10.1016/s0945-053x(01)00179-2. [DOI] [PubMed] [Google Scholar]
- 132.Maeda E, Ye S, Wang W, Bader DL, Knight MM, Lee DA. Gap junction permeability between tenocytes within tendon fascicles is suppressed by tensile loading. Biomech Model Mechanobiol. 2012;11:439–447. doi: 10.1007/s10237-011-0323-1. [DOI] [PubMed] [Google Scholar]
- 133.Waggett AD, Benjamin M, Ralphs JR. Connexin 32 and 43 gap junctions differentially modulate tenocyte response to cyclic mechanical load. Eur J Cell Biol. 2006;85:1145–1154. doi: 10.1016/j.ejcb.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 134.Qi J, Chi L, Bynum D, Banes AJ. Gap junctions in IL-1beta-mediated cell survival response to strain. J Appl Physiol. 2011;110:1425–1431. doi: 10.1152/japplphysiol.00477.2010. 1985. [DOI] [PubMed] [Google Scholar]
- 135.Bayer ML, Schjerling P, Herchenhan A, Zeltz C, Heinemeier KM, Christensen L, Krogsgaard M, Gullberg D, Kjaer M. Release of tensile strain on engineered human tendon tissue disturbs cell adhesions, changes matrix architecture, and induces an inflammatory phenotype. PLoS One. 2014;9:e86078. doi: 10.1371/journal.pone.0086078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Subramanian A, Prokop A, Yamamoto M, Sugimura K, Uemura T, Betschinger J, Knoblich JA, Volk T. Shortstop recruits EB1/APC1 and promotes microtubule assembly at the muscle–tendon junction. Curr Biol. 2003;13:1086–1095. doi: 10.1016/s0960-9822(03)00416-0. [DOI] [PubMed] [Google Scholar]
- 137.Alves-Silva J, Hahn I, Huber O, Mende M, Reissaus A, Prokop A. Prominent actin fiber arrays in Drosophila tendon cells represent architectural elements different from stress fibers. Mol Biol Cell. 2008;19:4287–4297. doi: 10.1091/mbc.E08-02-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chen JW, Galloway JL. The development of zebrafish tendon and ligament progenitors. Development. 2014;141:2035–2045. doi: 10.1242/dev.104067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Weber KT, Sun Y, Bhattacharya SK, Ahokas RA, Gerling IC. Myofibroblast-mediated mechanisms of pathological remodelling of the heart. Nat Rev Cardiol. 2012;10:15–26. doi: 10.1038/nrcardio.2012.158. [DOI] [PubMed] [Google Scholar]
- 140.Fan D, Takawale A, Lee J, Kassiri Z. Cardiac fibroblasts, fibrosis and extracellular matrix remodeling in heart disease. Fibrogenesis Tissue Repair. 2012;5:15. doi: 10.1186/1755-1536-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Oh JK, Park SJ, Nagueh SF. Established and novel clinical applications of diastolic function assessment by echocardiography. Circ Cardiovasc Imaging. 2011;4:444–455. doi: 10.1161/CIRCIMAGING.110.961623. [DOI] [PubMed] [Google Scholar]
- 142.Su M-YM, Lin L-Y, Tseng Y-HE, Chang C-C, Wu C-K, Lin J-L, Tseng W-YI. CMR-verified diffuse myocardial fibrosis is associated with diastolic dysfunction in HFpEF. J Am Coll Cardiol Img. 2014;7:991–997. doi: 10.1016/j.jcmg.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 143.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 144.Borbely A. Cardiomyocyte stiffness in diastolic heart failure. Circulation. 2005;111:774–781. doi: 10.1161/01.CIR.0000155257.33485.6D. [DOI] [PubMed] [Google Scholar]
- 145.van Heerebeek L. Myocardial structure and function differ in systolic and diastolic heart failure. Circulation. 2006;113:1966–1973. doi: 10.1161/CIRCULATIONAHA.105.587519. [DOI] [PubMed] [Google Scholar]
- 146.Ellims AH, Shaw JA, Stub D, Iles LM, Hare JL, Slavin GS, Kaye DM, Taylor AJ. Diffuse myocardial fibrosis evaluated by post-contrast T1 mapping correlates with left ventricular stiffness. J Am Coll Cardiol. 2014;63:1112–1118. doi: 10.1016/j.jacc.2013.10.084. [DOI] [PubMed] [Google Scholar]
- 147.Donekal S, Venkatesh BA, Liu YC, Liu CY, Yoneyama K, Wu CO, Nacif M, Gomes AS, Hundley WG, Bluemke DA, Lima JAC. Interstitial Fibrosis, left ventricular remodeling, and myocardial mechanical behavior in a population-based multiethnic cohort: the Multi-Ethnic Study of Atherosclerosis (MESA) study. Circ Cardiovasc Imaging. 2014;7:292–302. doi: 10.1161/CIRCIMAGING.113.001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Iwanaga Y, Nishi I, Furuichi S, Noguchi T, Sase K, Kihara Y, Goto Y, Nonogi H. B-type natriuretic peptide strongly reflects diastolic wall stress in patients with chronic heart failure: comparison between systolic and diastolic heart failure. J Am Coll Cardiol. 2006;47:742–748. doi: 10.1016/j.jacc.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 149.Heusch G, Libby P, Gersh B, Yellon D, Bohm M, Lopaschuk G, Opie L. Cardiovascular remodelling in coronary artery disease and heart failure. Lancet. 2014;383:1933–1943. doi: 10.1016/S0140-6736(14)60107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Burchfield JS, Xie M, Hill JA. Pathological ventricular remodeling: mechanisms: part 1 of 2. Circulation. 2013;128:388–400. doi: 10.1161/CIRCULATIONAHA.113.001878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ouzounian M, Lee DS, Liu PP. Diastolic heart failure: mechanisms and controversies. Nat Clin Pract Cardiovasc Med. 2008;5:375–386. doi: 10.1038/ncpcardio1245. [DOI] [PubMed] [Google Scholar]
- 152.Braunwald E, Bonow RO. Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine. 9. Saunders; Philadelphia: 2012. [Google Scholar]
- 153.Senni M, Paulus WJ, Gavazzi A, Fraser AG, Diez J, Solomon SD, Smiseth OA, Guazzi M, Lam CS, Maggioni AP, Tschope C, Metra M, Hummel SL, Edelmann F, Ambrosio G, Stewart Coats AJ, Filippatos GS, Gheorghiade M, Anker SD, Levy D, Pfeffer MA, Stough WG, Pieske BM. New strategies for heart failure with preserved ejection fraction: the importance of targeted therapies for heart failure phenotypes. Eur Heart J. 2014;35:2797–2815. doi: 10.1093/eurheartj/ehu204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, LeWinter MM, Rouleau JL, Bull DA, Mann DL, Deswal A, Stevenson LW, Givertz MM, Ofili EO, O'Connor CM, Felker GM, Goldsmith SR, Bart BA, McNulty SE, Ibarra JC, Lin G, Oh JK, Patel MR, Kim RJ, Tracy RP, Velazquez EJ, Anstrom KJ, Hernandez AF, Mascette AM, Braunwald E, Trial R. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. Jama. 2013;309:1268–1277. doi: 10.1001/jama.2013.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. Jama. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 156.Daneshvar D, Wei J, Tolstrup K, Thomson LE, Shufelt C, Merz CN. Diastolic dysfunction: improved understanding using emerging imaging techniques. Am Heart J. 2010;160:394–404. doi: 10.1016/j.ahj.2010.06.040. [DOI] [PubMed] [Google Scholar]
- 157.Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J. 2011;32:670–679. doi: 10.1093/eurheartj/ehq426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wan SH, Vogel MW, Chen HH. Pre-clinical diastolic dysfunction. J Am Coll Cardiol. 2014;63:407–416. doi: 10.1016/j.jacc.2013.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kane GC, Karon BL, Mahoney DW, Redfield MM, Roger VL, Burnett JC, Jr, Jacobsen SJ, Rodeheffer RJ. Progression of left ventricular diastolic dysfunction and risk of heart failure. Jama. 2011;306:856–863. doi: 10.1001/jama.2011.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ohtani T, Mohammed SF, Yamamoto K, Dunlay SM, Weston SA, Sakata Y, Rodeheffer RJ, Roger VL, Redfield MM. Diastolic stiffness as assessed by diastolic wall strain is associated with adverse remodelling and poor outcomes in heart failure with preserved ejection fraction. Eur Heart J. 2012;33(14):1742–1749. doi: 10.1093/eurheartj/ehs135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gulati A, Jabbour A, Ismail TF, Guha K, Khwaja J, Raza S, Morarji K, Brown TD, Ismail NA, Dweck MR, Di Pietro E, Roughton M, Wage R, Daryani Y, O'Hanlon R, Sheppard MN, Alpendurada F, Lyon AR, Cook SA, Cowie MR, Assomull RG, Pennell DJ, Prasad SK. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. Jama. 2013;309:896–908. doi: 10.1001/jama.2013.1363. [DOI] [PubMed] [Google Scholar]
- 162.Wong TC, Piehler K, Meier CG, Testa SM, Klock AM, Aneizi AA, Shakesprere J, Kellman P, Shroff SG, Schwartzman DS, Mulukutla SR, Simon MA, Schelbert EB. Association between extracellular matrix expansion quantified by cardiovascular magnetic resonance and short-term mortality. Circulation. 2012;126:1206–1216. doi: 10.1161/CIRCULATIONAHA.111.089409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ng AC, Auger D, Delgado V, van Elderen SG, Bertini M, Siebelink HM, van der Geest RJ, Bonetti C, van der Velde ET, de Roos A, Smit JW, Leung DY, Bax JJ, Lamb HJ. Association between diffuse myocardial fibrosis by cardiac magnetic resonance contrast-enhanced T(1) mapping and subclinical myocardial dysfunction in diabetic patients: a pilot study. Circ Cardiovasc Imaging. 2012;5:51–59. doi: 10.1161/CIRCIMAGING.111.965608. [DOI] [PubMed] [Google Scholar]
- 164.Mascherbauer J, Marzluf BA, Tufaro C, Pfaffenberger S, Graf A, Wexberg P, Panzenbock A, Jakowitsch J, Bangert C, Laimer D, Schreiber C, Karakus G, Hulsmann M, Pacher R, Lang IM, Maurer G, Bonderman D. Cardiac magnetic resonance postcontrast T1 time is associated with outcome in patients with heart failure and preserved ejection fraction. Circ Cardiovasc Imaging. 2013;6:1056–1065. doi: 10.1161/CIRCIMAGING.113.000633. [DOI] [PubMed] [Google Scholar]
- 165.Ambale-Venkatesh B, Lima JA. Cardiac MRI: a central prognostic tool in myocardial fibrosis. Nat Rev Cardiol. 2014;12(1):18–29. doi: 10.1038/nrcardio.2014.159. [DOI] [PubMed] [Google Scholar]
- 166.van Heerebeek L, Franssen CP, Hamdani N, Verheugt FW, Somsen GA, Paulus WJ. Molecular and cellular basis for diastolic dysfunction. Curr Heart Fail Rep. 2012;9:293–302. doi: 10.1007/s11897-012-0109-5. [DOI] [PubMed] [Google Scholar]
- 167.van Heerebeek L, Borbely A, Niessen HW, Bronzwaer JG, van der Velden J, Stienen GJ, Linke WA, Laarman GJ, Paulus WJ. Myocardial structure and function differ in systolic and diastolic heart failure. Circulation. 2006;113:1966–1973. doi: 10.1161/CIRCULATIONAHA.105.587519. [DOI] [PubMed] [Google Scholar]
- 168.van Heerebeek L, Hamdani N, Handoko ML, Falcao-Pires I, Musters RJ, Kupreishvili K, Ijsselmuiden AJ, Schalkwijk CG, Bronzwaer JG, Diamant M, Borbely A, van der Velden J, Stienen GJ, Laarman GJ, Niessen HW, Paulus WJ. Diastolic stiffness of the failing diabetic heart: importance of fibrosis, advanced glycation end products, and myocyte resting tension. Circulation. 2008;117:43–51. doi: 10.1161/CIRCULATIONAHA.107.728550. [DOI] [PubMed] [Google Scholar]
- 169.Hiesinger W, Brukman MJ, McCormick RC, Fitzpatrick JR, Frederick JR, Yang EC, Muenzer JR, Marotta NA, Berry MF, Atluri P, Woo YJ. Myocardial tissue elastic properties determined by atomic force microscopy after stromal cell-derived factor 1α angiogenic therapy for acute myocardial infarction in a murine model. J Thorac Cardiovasc Surg. 2012;143:962–966. doi: 10.1016/j.jtcvs.2011.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Berry MF, Engler AJ, Woo YJ, Pirolli TJ, Bish LT, Jayasankar V, Morine KJ, Gardner TJ, Discher DE, Sweeney HL. Mesenchymal stem cell injection after myocardial infarction improves myocardial compliance. Am J Physiol Heart Circ Physiol. 2006;290:H2196–H2203. doi: 10.1152/ajpheart.01017.2005. [DOI] [PubMed] [Google Scholar]
- 171.Shah SJ, Aistrup GL, Gupta DK, O'Toole MJ, Nahhas AF, Schuster D, Chirayil N, Bassi N, Ramakrishna S, Beussink L, Misener S, Kane B, Wang D, Randolph B, Ito A, Wu M, Akintilo L, Mongkolrattanothai T, Reddy M, Kumar M, Arora R, Ng J, Wasserstrom JA. Ultrastructural and cellular basis for the development of abnormal myocardial mechanics during the transition from hypertension to heart failure. Am J Physiol Heart Circ Physiol. 2014;306:H88–H100. doi: 10.1152/ajpheart.00642.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Deitch S, Gao BZ, Dean D. Effect of matrix on cardiomyocyte viscoelastic properties in 2D culture. Mol Cell Biomech. 2012;9:227–249. [PMC free article] [PubMed] [Google Scholar]
- 173.Chopra A, Lin V, McCollough A, Atzet S, Prestwich GD, Wechsler AS, Murray ME, Oake SA, Kresh JY, Janmey PA. Reprogramming cardiomyocyte mechanosensing by crosstalk between integrins and hyaluronic acid receptors. J Biomech. 2012;45:824–831. doi: 10.1016/j.jbiomech.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wu X, Sun Z, Foskett A, Trzeciakowski JP, Meininger GA, Muthuchamy M. Cardiomyocyte contractile status is associated with differences in fibronectin and integrin interactions. Am J Physiol Heart Circ Physiol. 2010;298:H2071–H2081. doi: 10.1152/ajpheart.01156.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.McCain ML, Parker KK. Mechanotransduction: the role of mechanical stress, myocyte shape, and cytoskeletal architecture on cardiac function. Pflugers Arch. 2011;462:89–104. doi: 10.1007/s00424-011-0951-4. [DOI] [PubMed] [Google Scholar]
- 176.Harston RK, Kuppuswamy D. Integrins are the necessary links to hypertrophic growth in cardiomyocytes. J Signal Transduct. 2011;2011:521742. doi: 10.1155/2011/521742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lal H, Verma SK, Smith M, Guleria RS, Lu G, Foster DM, Dostal DE. Stretch-induced MAP kinase activation in cardiac myocytes: differential regulation through beta1-integrin and focal adhesion kinase. J Mol Cell Cardiol. 2007;43:137–147. doi: 10.1016/j.yjmcc.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Nishimura M, Kumsta C, Kaushik G, Diop SB, Ding Y, Bisharat-Kernizan J, Catan H, Cammarato A, Ross RS, Engler AJ, Bodmer R, Hansen M, Ocorr K. A dual role for integrin-linked kinase and beta1-integrin in modulating cardiac aging. Aging Cell. 2014;13:431–440. doi: 10.1111/acel.12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Torsoni AS, Constancio SS, Nadruz W, Jr, Hanks SK, Franchini KG. Focal adhesion kinase is activated and mediates the early hypertrophic response to stretch in cardiac myocytes. Circ Res. 2003;93:140–147. doi: 10.1161/01.RES.0000081595.25297.1B. [DOI] [PubMed] [Google Scholar]
- 180.Dalla Costa AP, Clemente CF, Carvalho HF, Carvalheira JB, Nadruz W, Jr, Franchini KG. FAK mediates the activation of cardiac fibroblasts induced by mechanical stress through regulation of the mTOR complex. Cardiovasc Res. 2010;86:421–431. doi: 10.1093/cvr/cvp416. [DOI] [PubMed] [Google Scholar]
- 181.Seko Y, Takahashi N, Tobe K, Kadowaki T, Yazaki Y. Pulsatile stretch activates mitogen-activated protein kinase (MAPK) family members and focal adhesion kinase (p125(FAK)) in cultured rat cardiac myocytes. Biochem Biophys Res Commun. 1999;259:8–14. doi: 10.1006/bbrc.1999.0720. [DOI] [PubMed] [Google Scholar]
- 182.Peng X, Kraus MS, Wei H, Shen T-L, Pariaut R, Alcaraz A, Ji G, Cheng L, Yang Q, Kotlikoff MI, Chen J, Chien K, Gu H, Guan J-L. Inactivation of focal adhesion kinase in cardiomyocytes promotes eccentric cardiac hypertrophy and fibrosis in mice. J Clin Invest. 2006;116:217–227. doi: 10.1172/JCI24497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.DiMichele LA, Doherty JT, Rojas M, Beggs HE, Reichardt LF, Mack CP, Taylor JM. Myocyte-restricted focal adhesion kinase deletion attenuates pressure overload-induced hypertrophy. Circ Res. 2006;99(6):636–645. doi: 10.1161/01.RES.0000240498.44752.d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Tangney JR, Chuang JS, Janssen MS, Krishnamurthy A, Liao P, Hoshijima M, Wu X, Meininger GA, Muthuchamy M, Zemljic-Harpf A, Ross RS, Frank LR, McCulloch AD, Omens JH. Novel role for vinculin in ventricular myocyte mechanics and dysfunction. Biophys J. 2013;104:1623–1633. doi: 10.1016/j.bpj.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Hahn JY, Cho HJ, Bae JW, Yuk HS, Kim KI, Park KW, Koo BK, Chae IH, Shin CS, Oh BH, Choi YS, Park YB, Kim HS. Beta-catenin overexpression reduces myocardial infarct size through differential effects on cardiomyocytes and cardiac fibroblasts. J Biol Chem. 2006;281:30979–30989. doi: 10.1074/jbc.M603916200. [DOI] [PubMed] [Google Scholar]
- 186.Sharma K, Kass DA. Heart failure with preserved ejection fraction: mechanisms, clinical features, and therapies. Circ Res. 2014;115(1):79–96. doi: 10.1161/CIRCRESAHA.115.302922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lewinter MM, Granzier HL. Titin is a major human disease gene. Circulation. 2013;127:938–944. doi: 10.1161/CIRCULATIONAHA.112.139717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Cazorla O, Freiburg A, Helmes M, Centner T, McNabb M, Wu Y, Trombitás K, Labeit S, Granzier H. Differential expression of cardiac titin isoforms and modulation of cellular stiffness. Circ Res. 2000;86:59–67. doi: 10.1161/01.res.86.1.59. [DOI] [PubMed] [Google Scholar]
- 189.Krüger M, Linke WA. Titin-based mechanical signalling in normal and failing myocardium. J Mol Cell Cardiol. 2009;46:490–498. doi: 10.1016/j.yjmcc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 190.Hamdani N, Bishu KG, von Frieling-Salewsky M, Redfield MM, Linke WA. Deranged myofllament phosphorylation and function in experimental heart failure with preserved ejection fraction. Cardiovasc Res. 2013;97:464–471. doi: 10.1093/cvr/cvs353. [DOI] [PubMed] [Google Scholar]
- 191.Jacot JG, Martin JC, Hunt DL. Mechanobiology of cardiomyocyte development. J Biomech. 2010;43:93–98. doi: 10.1016/j.jbiomech.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Majkut S, Dingal PC, Discher DE. Stress sensitivity and mechanotransduction during heart development. Curr Biol. 2014;24:R495–R501. doi: 10.1016/j.cub.2014.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Majkut S, Idema T, Swift J, Krieger C, Liu A, Discher DE. Heart-specific stiffening in early embryos parallels matrix and myosin expression to optimize beating. Curr Biol. 2013;23:2434–2439. doi: 10.1016/j.cub.2013.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kaushik G, Zambon AC, Fuhrmann A, Bernstein SI, Bodmer R, Engler AJ, Cammarato A. Measuring passive myocardial stiffness in Drosophila melanogaster to investigate diastolic dysfunction. J Cell Mol Med. 2012;16:1656–1662. doi: 10.1111/j.1582-4934.2011.01517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Nishimura M, Ocorr K, Bodmer R, Cartry J. Drosophila as a model to study cardiac aging. Exp Gerontol. 2011;46:326–330. doi: 10.1016/j.exger.2010.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Mercer SE, Odelberg SJ, Simon HG. A dynamic spatiotemporal extracellular matrix facilitates epicardial-mediated vertebrate heart regeneration. Dev Biol. 2013;382:457–469. doi: 10.1016/j.ydbio.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Wang J, Karra R, Dickson AL, Poss KD. Fibronectin is deposited by injury-activated epicardial cells and is necessary for zebrafish heart regeneration. Dev Biol. 2013;382:427–435. doi: 10.1016/j.ydbio.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Calve S, Odelberg SJ, Simon HG. A transitional extracellular matrix instructs cell behavior during muscle regeneration. Dev Biol. 2010;344:259–271. doi: 10.1016/j.ydbio.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Brockes JP, Kumar A. Plasticity and reprogramming of differentiated cells in amphibian regeneration. Nat Rev Mol Cell Biol. 2002;3:566–574. doi: 10.1038/nrm881. [DOI] [PubMed] [Google Scholar]
- 200.Gemberling M, Bailey TJ, Hyde DR, Poss KD. The zebrafish as a model for complex tissue regeneration. Trends Genet. 2013;29:611–620. doi: 10.1016/j.tig.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Connizzo BK, Yannascoli SM, Soslowsky LJ. Structure–function relationships of postnatal tendon development: a parallel to healing. Matrix Biol. 2013;32:106–116. doi: 10.1016/j.matbio.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]