Abstract
The role of Broca’s area in sentence processing is hotly debated. Prominent hypotheses include that Broca’s area supports sentence comprehension via syntax-specific processes (“syntactic movement” in particular), hierarchical structure building or working memory. In the present fMRI study we adopt a within subject, across task approach using targeted sentence-level contrasts and non-sentential comparison tasks to address these hypotheses regarding the role of Broca’s area in sentence processing. For clarity, we have presented findings as three experiments: (i) Experiment 1 examines selectivity for a particular type of sentence construction, namely those containing syntactic movement. Standard syntactic movement distance effects in Broca’s area were replicated but no difference was found between movement and non-movement sentences in Broca’s area at the group level or consistently in individual subjects. (ii) Experiment 2 examines selectivity for sentences versus non-sentences, to assess claims regarding the role of Broca’s area in hierarchical structure building. Group and individual results differ, but both identify subregions of Broca’s area that are selective for sentence structure. (iii) Experiment 3 assesses whether activations in Broca’s area are selective for sentences when contrasted with simple subvocal articulation. Group results suggest shared resources for sentence processing and articulation in Broca’s area, but individual subject analyses contradict this finding. We conclude that Broca’s area is not selectively involved in processing syntactic movement, but that subregions are selectively responsive to sentence structure. Our findings also reinforce Fedorenko & Kanwishser’s call for the use of more individual subject analyses in functional imaging studies of sentence processing in Broca’s area, as group findings can obscure selective response patterns.
Keywords: Broca’s area, syntax, sentence processing, fMRI, speech comprehension
Introduction
Broca’s area1 has been the major focus of attention in neuroscience research on sentence-level processing since the mid 1970s when it was discovered that Broca’s aphasics have deficits in comprehending syntactically complex sentences despite relatively preserved lexical-semantic comprehension (Caramazza & Zurif, 1976). Since that time, the number of proposals for Broca’s area function has proliferated, ranging from syntactic movement (Grodzinsky & Santi, 2008; Grodzinsky 1986, 2000), mapping from syntax to thematic roles (Linebarger, Schwartz & Saffran, 1983), fast lexical access (Zurif, Swinney & Garrett, 1990), working memory broadly construed (Just et al. 1996; Kaan & Swaab 2002) or articulatory rehearsal specifically (Rogalsky, Matchin & Hickok, 2008; Caplan et al. 2000;), cognitive control (Novick et al. 2005), and hierarchical structure building (Friederici 2009; Makuuchi et al. 2009).
The number of studies targeting sentence processing, the number of conditions under which Broca’s area seems to activate (see above for just a sampling from speech/language), and the relatively large and heterogeneous expanse of this classical language region (Amunts et al. 1999) has resulted in a state-of-affairs such that just about any claim regarding the role of “Broca’s area” in “sentence processing” can find empirical support in the literature. One remedy for this situation is to examine the response properties of Broca’s area under multiple conditions within subjects. Such an approach has recently been reported in which sentence reading was compared with non-word reading, working memory, cognitive control, math, and music tasks (Fedorenko et al. 2011). Using a region of interest approach, Broca’s area (left IFG) showed more activation during sentence reading than any other task. The same was true, however, in a range of other areas including the left pars orbitalis region, left superior frontal gyrus, and the left anterior, middle, and posterior temporal lobe. One problem with this study is that it contrasts semantically and syntactically rich language stimuli with low-level linguistic tasks (non-word reading) or non-linguistic tasks thus capturing processes ranging from lexical access to combinatorial semantics.
Our goal in the present study was to adopt this same within subject, cross task approach but using more targeted sentence-level contrasts and focusing on non-sentential comparison tasks that address specific hypotheses regarding the role of Broca’s area in sentence processing, working memory and hierarchical processing in particular. Our study also differs in that we use auditory rather than written stimuli as the latter may induce articulatory processes (and therefore portions of Broca’s area) more strongly (Daneman & Newsom, 1992; Slowiaczek & Clifton, 1980).
Experiment 1 examines selectivity for a particular type of sentence construction, namely those containing syntactic movement (Grodzinsky, 2008; Santi & Grodzinsky 2007a,b). We contrast long- versus short-movement constructions, which have been shown previously to yield activation in Broca’s area (Santi & Grodzinsky 2007a) and contrast these movement stimuli with non-movement controls, matched for length and semantic load. Experiment 2 examines selectivity for sentences versus non-sentences (scrambled sentences) more broadly. This experiment assesses claims regarding the role Broca’s area in hierarchical structure building (Friederici, 2009), while controlling for word level processes. Finally, Experiment 3 assesses whether activations in Broca’s area are selective for sentences when contrasted with simple subvocal articulation. All of these experimental conditions were run within subject and within session. For clarity, we present them here as three separate experiments.
Experiment 1: Syntactic Movement
Material and Methods
Participants
Fifteen right-handed native English speakers (6 male, 9 female; mean age = 22, range = 18 – 29 years) participated in this study. All participants were free of neurological disease (self-report) and gave informed consent under a protocol approved by the Institutional Review Board of the University of California, Irvine (UCI).
Experimental Design
Our event-related experiment consisted of the subject listening to sentences in which the presence and length of wh- dependencies was manipulated. The speech stimuli were presented over six scanning runs with an average length of 4.5 minutes each. (Note that each scanning run included all of the conditions and tasks described across the three experiments, but only the sentences are discussed here in Experiment 1.) The interstimulus intervals between sentences was randomly selected, ranging from 750 to 1250 ms. The order of presentation of runs was randomized for each participant. Stimulus delivery was performed using Cogent 2000 software (FIL, 2000) for Matlab (Mathworks, Inc.).
Subjects were told that they would hear utterances in English. Subjects were instructed to listen carefully for meaning, as they would have comprehension questions to answer about some of the sentences at the end of each run. At the end of each run, four true/false comprehension questions about four of the sentences were presented. Half of the questions were true; half were false.
Stimuli
Ninety-six sentences were presented to each subject (i.e. 24 sentences of each type). These sentences were adapted from a previously published ERP sentence processing experiment (Phillips et al., 2005), making sure to match the sentential force of experimental and control sentences by turning all declaratives to embedded yes/no interrogatives. Sentences were generated in groups of four, with each group containing the following:
-
No wh-movement, one clause (“short control”):
The townspeople hoped that the cameraman knew whether the mayor would honor the soldiers before the fireworks.
-
wh-movement, one clause (“short movement”):
The townspeople hoped that the cameraman knew which soldiers the mayor would honor () before the fireworks.
-
No wh-movement, two clauses (“long control”):
The cameraman knew whether the townspeople hoped the mayor would honor the soldiers before the fireworks.
-
wh-movement, two clauses (“long movement”):
The cameraman knew which soldiers the townspeople hoped the mayor would honor () before the fireworks.
Short- and long-distance movement sentences contain a wh-dependency, with one or two intervening clauses (and noun phrases), respectively. Short and long control sentences contain an embedded yes/no question, and can act as a control to which the movement sentences with the same embedded question length will be compared. Note that “short” and “long” refer to the length of the embedded question not the overall length of the sentence. The control sentences were designed to control for semantic load: a contrast of the long and short movement sentences may be potentially measuring the brain’s response not only to longer movement distance, but also to greater semantic load as a result of a longer embedded question. The control sentences address this confound in that they contain the same number of clauses in their embedded questions as the movement sentences. In fact, all four variants in each sentence group are matched otherwise in that they have the same number of syllables and have similar lexical content. Each variant was assigned to a different list in a Latin square design, such that each subject would listen to only one of the sentences in each group.
The stimuli were recorded by a phonetically-trained native speaker of American English in a sound-attenuated booth. The materials were down-sampled from 44100 Hz to 22050 Hz. All stimuli were equated for mean RMS power, intensity and controlled for duration across experimental lists. All editing was done on Praat software (Boersma & Weenink, 2011).
fMRI data acquisition and processing
Data were collected on the 3T Phillips Achieva MR scanner at the UCI Research Imaging Center. A high-resolution anatomical image was acquired, in the axial plane, with a three-dimensional spoiled gradient-recalled acquisition pulse sequence for each subject (field of view (FOV) = 240 × 240, repetition time (TR) = 11 ms, voxel size = 1mm × 1mm × 1mm). Functional MRI data were collected using single-shot echo-planar imaging (144 volumes, FOV = 128 × 128, 23 slices, TR = 2 sec, TE = 30 ms, SENSE factor = 1.7, flip angle = 90 degrees, in-plane resolution = 1.875 mm × 1.875 mm, 5mm slice thickness, no gap). An in-house Matlab program was used to reconstruct the high-resolution structural image as well as the echo-planar images. Functional volumes were aligned to the 60th volume in the series using a six-parameter rigid-body model to correct or subject motion (Cox & Jesmanowicz, 1999), and slice-timing correction was performed using Analysis of Functional NeuroImaging (AFNI) software’s 3dtshift command (http://afni.nimh.nih.gov/afni). Each volume then was spatially smoothed (full-width at half maximum = 5 mm) to better accommodate group analysis.
Data analysis
AFNI software (http://afni.nimh.nih.gov/afni) was used to perform analyses on the time course of each voxel’s blood oxygenation level-dependent (BOLD) response for each subject (Cox & Hyde, 1997). Initially, a voxel-wise multiple regression analysis was conducted, with regressors for each sentence type. These regressors were convolved with a hemodynamic response function to create predictor variables for analysis. Motion correction parameters and the grand mean were included as regressors of no interest. An F statistic was calculated for each voxel, and activation maps were created for each subject to identify regions that were more active during each condition, compared to baseline scanner noise. To facilitate group analysis, the functional maps for each subject were transformed into standardized space and resampled into 2 mm × 2 mm × 2 mm voxels (Talairach & Tournoux, 1988) using AFNI’s “@auto_tlrc” program.
To identify possible activity in Broca’s area that is sensitive to syntactic movement, a voxel-wise 2 (movement vs. no-movement) x 2 (long vs. short) Analysis of Variance (ANOVA; using AFNI’s “3dANOVA3”) was performed across data from the 15 subjects to test for main effects of movement and distance, as well as for their interaction. Movement and distance were fixed effects; subjects were considered a random effect. To further explore the ANOVA results and explore the syntactic movement-specific hypothesis, voxel-wise repeated-measures t-tests were computed (using AFNI’s “3dttest++”) to identify voxels across subjects that were significant for long versus short movement, long movement versus long control, and short movement versus short control. To further explore Broca’s area response to syntactic processing, the mean peak amplitudes for the regions of interest (ROIs) identified by the above t-tests were plotted for each sentence type. A relatively liberal threshold of p = 0.005 uncorrected was used throughout these analyses to ensure that potential syntactic movement or distance-specific effects are not overlooked due to thresholding effects.
Results
ANOVA results
The ANOVA yielded a main effect of movement in the left hemisphere STG, MTG, putamen, and thalamus, as well as bilateral postcentral gyrus; a main effect of distance in the left STG, bilateral MTG, bilateral claustrum, and right cingulate gyrus; no significant main effects of movement or distance were observed in Broca’s area (at p = .005 uncorrected; Table 1). The lack of a significant movement main effect suggests that no contiguous part of Broca’s area is responding to syntactic movement per se. However, a significant interaction between movement and distance was found on the anterior boundary of Broca’s area (−42 39 4) (Figure 1; Table 1) along with left middle frontal gyrus, left lingual gyrus, and right precentral gyrus. The significant interaction in Broca’s area is consistent with previous work in that the center of mass of Santi & Grodzinsky’s (2007a) “movement effect” region is a few millimeters from the center of this interaction ROI (Figure 1A). The mean beta values of this interaction ROI for each condition indicate that the interaction effect is driven by greater activation to long versus short movement sentences and the reverse pattern for the long versus short non-movement control sentences. However, the fact that non-movement sentences, particularly the short variants, activated this region at least as well as long movement sentences argues strongly against a movement-specific explanation of the response properties of this region as a whole. (Figure 1B).
Table 1.
Talairach coordinates for all voxel clusters found to be significant at the group level (p< .005 uncorrected) in the ANOVA and t-tests of Experiments 1 & 2.
| Region | x | y | z | |
|---|---|---|---|---|
| main effect: movement | L STG | −41 | 3 | −13 |
| L MTG | −45 | −17 | −15 | |
| L PCG | −60 | −16 | 27 | |
| L putamen | −16 | 3 | −10 | |
| L thalamus | −5 | −29 | 9 | |
| R PCG | 51 | −14 | 24 | |
|
| ||||
| main effect: distance | L STG/MTG | −34 | 7 | −31 |
| R Cingulate Gyrus | 1 | 0 | 36 | |
| L claustrum | −34 | −21 | 4 | |
| R claustrum | 36 | −20 | 4 | |
| R MTG | 47 | −49 | 3 | |
|
| ||||
| interaction: movement x distance | L IFG (PTr) | −42 | 39 | 4 |
| L MFG | −28 | 12 | 44 | |
| L lingual gyrus | −11 | −73 | 5 | |
| R precentral gyrus | 36 | −14 | 33 | |
|
| ||||
| long mvmt > long control | L STG | −44 | −20 | −7 |
| L STG | −43 | −59 | 20 | |
| L MFG | −35 | 12 | 43 | |
|
| ||||
| long mvmt < long control | R postcentral gyrus | 47 | −17 | 45 |
|
| ||||
| short mvmt > short control | L thalamus | −7 | −27 | 7 |
| R thalamus | 9 | −27 | −3 | |
|
| ||||
| short mvmt < short control | L IFG (PTr) | −42 | 39 | 4 |
| L MFG | −43 | 19 | 41 | |
|
| ||||
| long mvmt > short mvmt | L MTG | −58 | −24 | −5 |
| L SMG | −45 | 55 | 32 | |
| L IFG (PTr & PO) | −45 | 17 | 12 | |
| L IFG (PTr) | −43 | 40 | 1 | |
| L MFG | −37 | 13 | 41 | |
|
| ||||
| sentences > scrambled | L STG/MTG | −53 | −7 | −9 |
| L MTG | −51 | −36 | −3 | |
| L MTG | −44 | −62 | 16 | |
| L IFG | −48 | 20 | 11 | |
| L caudate | −6 | 10 | 10 | |
| R STG | 42 | 14 | −24 | |
| R fusiform gyrus | 39 | −65 | −13 | |
|
| ||||
| sentences < scrambled | L SMG | −40 | −51 | 37 |
| L TTG/STG | −45 | −24 | 10 | |
| L MFG | −35 | 49 | 9 | |
| L MFG | −35 | −2 | 51 | |
| L insula | −35 | 6 | −6 | |
| L parahippocampal gyrus | −36 | −28 | −12 | |
| R SMG | 42 | −47 | 35 | |
| R MFG/IFG (PO) | 41 | 7 | 39 | |
| R MFG/IFG (PTr) | 46 | 31 | 23 | |
| R insula | 40 | −10 | −1 | |
| R STG | 58 | −19 | 2 | |
| R SFG | 38 | 48 | 15 | |
L = left hemisphere, R = right hemisphere, STG = superior temporal gyrus, MTG = middle temporal gyrus, IFG = inferior frontal gyrus, PTr = pars triangularis, MFG = middle frontal gyrus, SMG = supramarginal gyrus, PO = pars opercularis, TTG = transverse temporal gyrus,
Figure 1.
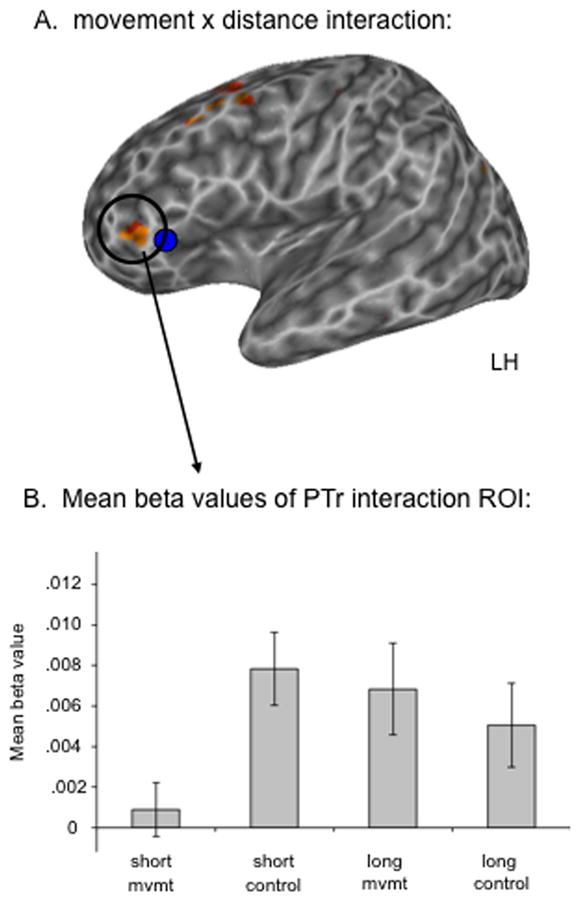
A. Voxels with a significant interaction between movement and distance across subjects; p < .005, uncorrected. The blue sphere is the center of mass of Grodzinsky and Santi’s (2007a) movement effect. B. Mean beta values of the PTr region where a significant movement x distance interaction was found.
Whole brain pair-wise comparisons: movement and distance
Pair-wise comparisons (t-tests) were performed to further explore the effects of movement and distance. First, the activations to sentences containing movement were compared to their respective controls: the contrast between long movement and long control sentences did not identify any voxels in Broca’s area that passed threshold (p < .005). However, this contrast identified clusters in left mid STG/STS (−44 −20 −7), posterior STG/STS (−43 −59 20), and left middle frontal gyrus (MFG; −35 12 43) (Figure 2A). A contrast of the short movement versus short control sentences did find a significant cluster in Broca’s area, the same anterior cluster identified by the ANOVA interaction, as well as an MFG cluster (−43 19 41). However, the direction of the effect was unexpected from a movement-based activation standpoint; both of these voxel clusters had significantly less activation for the short movement sentences than the short control sentences; (Figure 2B). Thus, no portion of Broca’s area (or adjacent regions) was found to respond more to sentences containing syntactic movement relative to non-movement control sentences, suggesting that Broca’s area is not responsive to syntactic movement per se. See Table 1 for a complete list of brain regions identified in each contrast.
Figure 2.
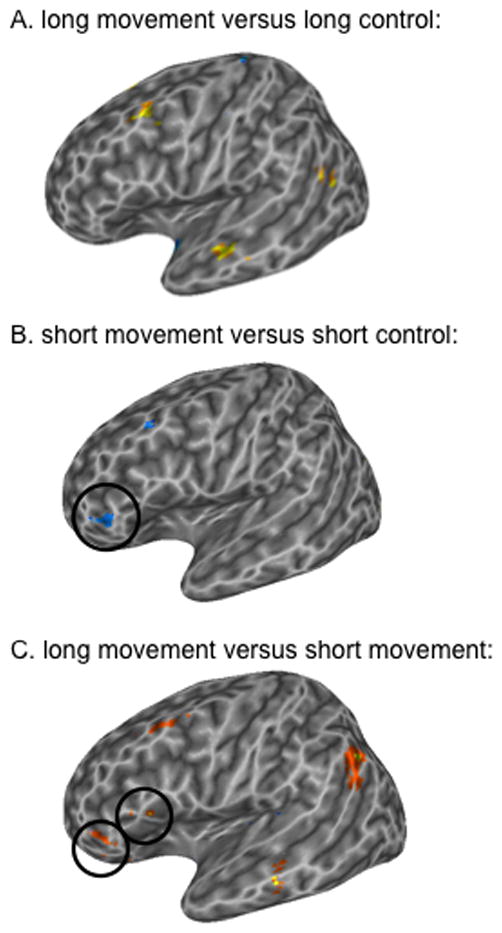
Voxel-wise t-tests for (A.) long movement versus long control sentences, (B.) short movement versus short control sentences, and (C.) long movement versus short movement sentences p < .005 uncorrected. Warm colors indicate greater activation for the first condition listed; cool colors indicate greater activation for the second condition listed. Left hemisphere is shown. Circles encompass significant voxels in Broca’s area.
To explore “classic” movement-distance effects we compared the long movement sentences to the short movement sentences. Two regions in Broca’s area were found to be more responsive to long movement versus short movement at p < .005: an anterior pars triangularis (PTr) cluster (−43 40 1) (very similar to the region found to have the movement x distance interaction), and a PTr/POp cluster (−45 17 12) (Figure 2C; Table 1). However, these ROIs responded equally well to the non-movement control sentences (Figure 3). In addition, a left middle temporal gyrus cluster (−59 −21 −4), a large left supramarginal/angular gyrus cluster (−45 −55 32) and a left middle frontal gyrus cluster (−37 13 41) were found to be more responsive to long movement than to short movement (Figure 2C; 3). These findings suggest that longer syntactic movement increases activation in a frontal-temporal network, not only in Broca’s area.
Figure 3.
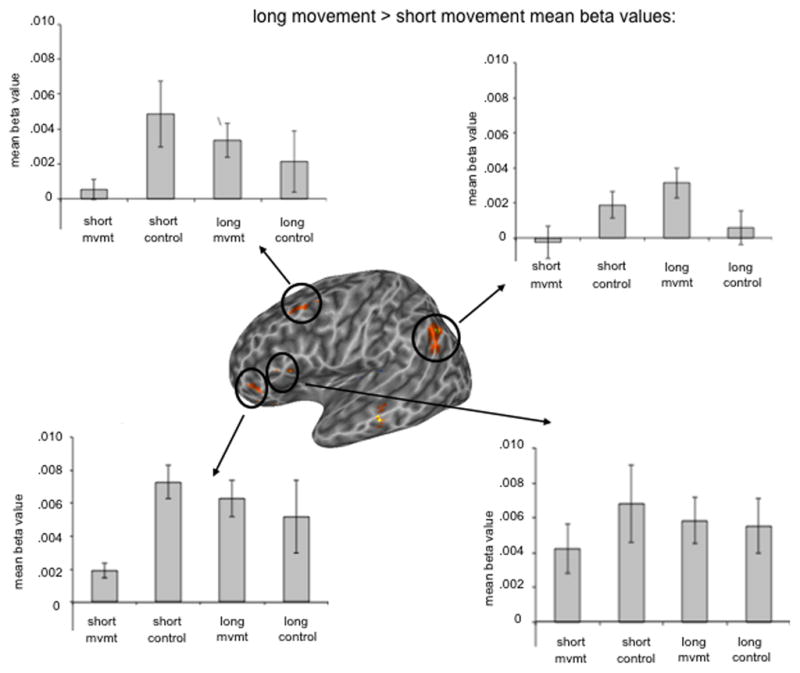
Mean beta values for voxel clusters identified by the long versus short movement voxel-wise t-test, p < .005 uncorrected.
Individual subjects analysis
The analyses described above provide little evidence of selectivity for syntactic movement in Broca’s area. However, previous studies have found significant inter-subject variability in the anatomy within Broca’s area (Amunts, et al. 1999), as well as in the strength and location of activations in Broca’s area in response to sentences (e.g. Fedorenko & Kanwisher 2011; Hickok & Rogalsky 2011; Xiong, et al. 2000). In the present study, it is possible that each subject has a region (or a distributed set of regions) in Broca’s area that is specific to syntactic movement, but that these regions are not in a consistent location across subjects and thus are obscured in the group analysis statistical maps.
Our strategy to explore these possibilities was to first identify voxels anywhere in Broca’s area in individual subjects that are more active for sentences containing syntactic movement than non-movement control sentences. To do so we identified voxels (in native space, no spatial smoothing) in either the pars opercularis or pars triangularis that were more active for sentences containing syntactic movement relative to their non-movement control sentences ([long+short]-[long control+short control]). Six of 15 subjects (40%) had such voxels in the PO (median # of voxels = 9, s.d. = 45.7, range = 2–120) and 8 of 15 (the six subjects with PO voxels plus two additional subjects) had such voxels in the PTr (53%) (median # of voxels = 15.5, s.d. = 23.27, range = 4–64). Thus, the lack of a group-level effect for movement in Broca’s area is not merely due to variability in location of the activation but its robustness across individuals.
Experiment #1 Discussion
The major finding from Experiment 1 was the lack of specificity in the response of Broca’s area to sentences containing syntactic movement. Group analyses provided no evidence for movement specificity and individual subject analyses showed that specificity was only found in approximately half of the subjects (thus explaining the lack of a group effect). Our null results cannot be explained as a simple lack of power as we replicated group-level effects in a standard “localizer” for movement activations in Broca’s area: long-movement sentences activated Broca’s area more than short-movement sentences. But an examination of the beta values from the non-movement conditions in these ROIs showed that non-movement sentences activated these voxels equally well compared to the long movement sentences. Previous research on movement constructions had, for the most part, not controlled the semantic confound of sentential force, which we controlled with our embedded yes/no questions, and so would not have been in a position to reveal the lack of specificity to movement construction. To our knowledge, the only other study to have compared embedded interrogatives with yes/no (such as our control “whether”) questions to embedded interrogatives with wh-movement was Ben Shachar et al. (2004). These authors reported an effect of question type in their LIFG ROI, with wh- questions eliciting overall higher activation compared to yes/no questions. Our results fail to replicate this finding, as there are no clusters in LIFG in which sentences with embedded yes/no questions elicit less activation than sentences with embedded wh- questions (cf. Figure 2A and 2B). In fact, there are clusters that show the exact opposite (Figure 2B), and clusters in which the greatest BOLD response is elicited by sentences in which the yes/no question is the most deeply embedded clause (Figure 3). These results could suggest an interaction between question type and level of embedding, although it is currently unclear what mechanism could explain such a relationship, and thus we must leave this question for future research.
Experiment #2: Hierarchical structure building
If Broca’s area, the pars opercularis in particular, supports sentence processing via its role in hierarchical processing (Friederici, 2009), then this region should be more responsive during processing of structured sentences than during the processing of unstructured sentences (i.e. scrambled sentences). The following experiment tests this idea by examining the BOLD response to sentences and scrambled sentences.
Methods
Subjects listened to 96 word lists randomly presented across the six scanning runs described in experiment 1. The lists were generated by rearranging the words in the sentences (see experiment 1) both within and across items. This rearranging resulted in highly non-sentence-like word lists that were nonetheless matched for lexical content with the sentence stimuli (see below). The word lists also matched the distributions of number of syllables of the sentence stimuli. The word list conditions were read with natural sentence intonation modeled after items in the sentence condition. Subjects were instructed to pay close attention to the word lists and to try to retain as many words from them as possible.
Word list examples:
The during competitors instructor jokes stated new broadcast pastries whether award wedding during.
Impress whether attorney to speculated the the new had the concluded the accident would knew after kitten.
All data processing and analyses are the same as in experiment 1. A voxel-wise repeated-measures t-test (using AFNI’s 3dttest++), compared the hemodynamic response to sentences (combined across all conditions, see experiment 1) versus scrambled sentences to identify brain regions sensitive to hierarchical structure.
Results
For the sentence versus scrambled sentence contrast, the largest clusters of activation (sentences>scrambled sentences) were found in the temporal lobes, in both anterior and posterior sectors (Figure 4). However, a PTr region was also identified (p < .005, uncorrected; circled in Figure 4). No activations were noted in the PO in this contrast.
Figure 4.
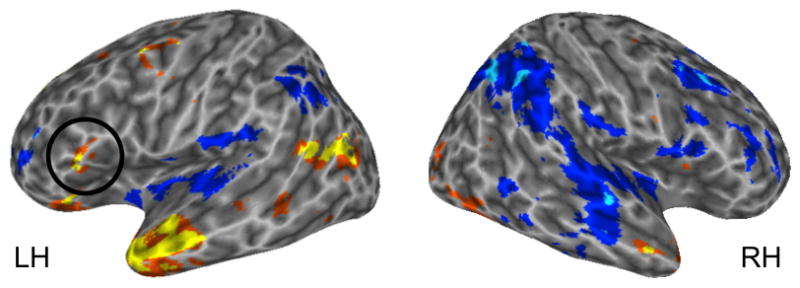
Voxel-wise t-test for sentences versus scrambled sentences, p < .005, uncorrected. Warm colors indicate regions more responsive to sentences; cooler colors indicate regions more responsive to scrambled sentences.
As in experiment 1, it is possible that the group analysis results do not fully capture the response of Broca’s area due to functional and/or anatomical variability within Broca’s area across subjects. Thus, we also identified in each subject (in native space, no smoothing) voxels in Broca’s area that exhibited greater activation for sentences than scrambled sentences (p < .005, uncorrected). Fourteen of the 15 (93%) subjects were found to have significant voxels in both the PO and PTr (PO: median # of voxels = 36.5, sd = 133.0, range = 3–451; PTr: median # of voxels = 30.5, sd = 112.6, range = 2–389); the remaining subject had no activations in either portion of Broca’s area.
Experiment #2 Discussion
Experiment 2 found effects of global sentence structure in Broca’s area. The group analysis found that the pars triangularis responded more during listening to structured sentences compared to scrambled sentences. The individual subject analysis confirmed this effect with 93% of subjects showing this pattern in the PTr. The individual subject analysis also identified a sentence structure effect in the pars opercularis in 93% of subjects, which was not evident in the group analysis. This reinforces the concern raised by Fedorenko and Kanwisher (2011) regarding the potential for group studies to obscure activation patterns when there is functional-anatomic variability in the activation patterns within a region. We further examine the specificity of this sentence effect in Experiment 3 by comparing sentence activations to those elicited by simple articulation. Beyond Broca’s area, this experiment replicates previous work (Humphries et al. 2006; Friederici et al. 2010; Spitsya et al. 2006; Rogalsky et al. 2011b; Rogalsky & Hickok 2009; Mazoyer et al. 1993) showing a large and robust activation in both anterior and posterior temporal lobe regions to sentences compared to scrambled sentences.
Experiment #3: Articulation
There is broad overlap in the network of regions implicated in sentence comprehension (i.e. Broca’s area & posterior temporal/inferior parietal regions) compared to those implicated in articulatory rehearsal and verbal working memory more generally (Awh et al. 1996; Buchsbaum & D’Esposito, 2008; Buchsbaum et al. 2005; Hickok et al. 2003; Smith & Jonides 1997; Smith, Jonides & Koeppe 1996). This raises the question of whether some of the activation attributed to sentence comprehension might be accounted for by working memory, articulatory rehearsal in particular. Experiment 3 examines this possibility by comparing sentence activations to those induced by articulatory rehearsal.
Methods
Each of the six functional scanning runs described in experiment 1 included one 30-second block of subvocal rehearsal, randomly placed within the scan. Subjects were instructed that occasionally the black fixation cross would start flashing between red and blue, at which point participants should start to subvocally rehearse the syllables “ba da ga” until the cross became black again.
The fMRI processing and analysis methods were as described in experiment 1. To test the hypothesis that sentence processing and articulation share resources in Broca’s area, a voxel-wise repeated measures t-test was computed (using AFNI’s “3dttest++”) to identify voxels across subjects that demonstrated significant activation differences for sentences (across all conditions, see Experiment 1) versus articulation.
Results
For the sentence versus articulation group-level contrast, we found extensive activations (sentences>articulation) throughout the temporal lobes bilaterally but only a single voxel that surpassed threshold (p < .005, uncorrected) in Broca’s area, in the PO (Figure 5a). This contrast indicates that there is minimal, if any, selectivity for processing structured sentences in Broca’s area at the group level. Individual subjects analyses, however, tell a different story: In the PO, 15 of 15 (100%) subjects had voxels that exhibited greater activation for sentences than articulation (median = 113 voxels, sd = 126.8, range = 17–518) and in the PTr, 13 of 15 (87%) subjects had voxels that exhibited greater activation for sentences than articulation (median = 134 voxels, sd = 134.7, range = 15–464). These individual subject results indicate that group analyses may be underestimating the response of Broca’s area to sentences above and beyond that elicited by articulation, similar to what was found in Experiment 2.
Figure 5.
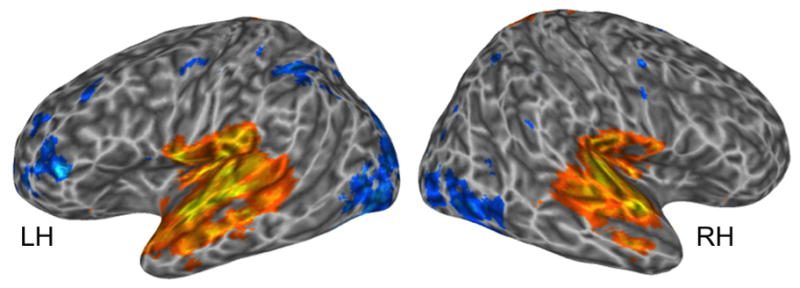
A. Regions across subjects activated more by the sentences (all sentence types combined) versus articulation. Warm colors indicate regions more responsive to sentences; cooler colors indicate regions more responsive to articulation, p < .005, uncorrected.
Relation between movement effects, sentence structure effects, and articulation
Data across the three experiments can be examined together to assess the extent of specificity to both movement constructions and sentence structure compared to unstructured sentences and speech articulation. To assess this we used a split plot analysis in which we used half the data (odd runs) to identify Broca’s area voxels (native space, no spatial smoothing) in individual subjects that showed a movement effect (analysis 1) or that showed a sentence structure effect (analysis 2) and then plotted the amplitude of the response in these voxels across all conditions using the other half of the data (even runs).
Analysis 1: Syntactic movement ROIs
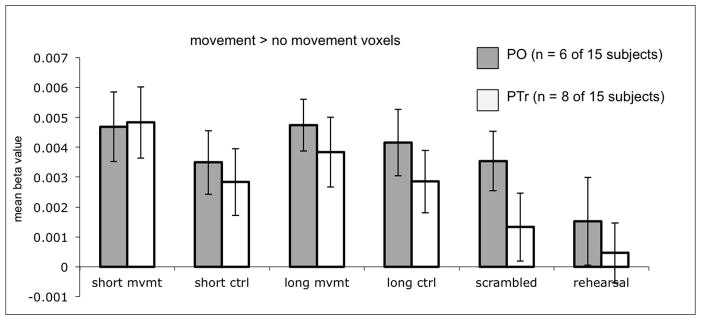
Voxels exhibiting syntactic movement effects were identified by contrasting the movement conditions [long+short movement sentences] with their controls [long+short control sentences] using a threshold of p<0.005. Consistent with the relatively weak effects of movement found in Experiment 1, this process identified PO voxels that were more active for the movement compared to the non-movement conditions in only 6 participants (40%; for the 6 participants, median # of voxels = 7.5, range = 2–103, sd = 41.0) and PTr voxels with the same pattern in 8 participants (53%; for the 8 participants, median # of voxels = 26.5, range = 1–76, sd = 9.35 ). The mean beta values in these voxels during the even runs are presented in Figure 6. Analysis of these values showed that the PO “movement voxels” were equally activated by sentences and scrambled sentences (t = .84, p = .44, two-tailed), but the PTr “movement voxels” were significantly more activated by sentences than scrambled sentences (t = 5.21, p = .001 two-tailed). At best this suggests weak selectivity (present in only 53% of our sample of participants) for movement constructions in the PTr.
Figure 6.
Mean beta values of the voxels in Broca’s area found to be significant for the voxel-wise t-test of movement versus no movement sentences. Voxels were identified in individual subjects by their response in the odd scanning runs (p < .005, uncorrected), and mean beta values were plotted from these voxels during the even scanning runs.
Analysis 2: Sentence structure ROIs
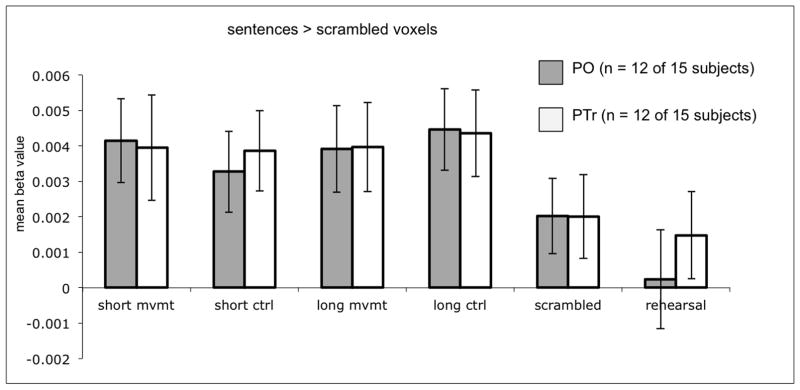
Sentence structure ROIs were identified by contrasting sentence versus scrambled sentence activations. The results are as follows: 12 of the 15 subjects (80%) had such voxels in the PO (for the 12 subjects, median # of voxels = 49.5, sd = 113.5, range = 2–387), and 12 had such voxels in the PTr (80%; for the 12 subjects, median # of voxels = 73.5, sd = 126.0, range = 1–383). Two subjects exhibited neither PO nor PTr sentence > scrambled significant activations. The bar graph of the mean beta values (Figure 7) indicates that these sentence structure ROIs do not respond selectively to syntactic movement; in fact, there are no significant differences across the structured sentence types in these ROIs (e.g. in PO, long movement versus short movement: t = 0.46, p = 0.66; short movement versus short control: t = 1.94, p = .078; long movement versus long control: t = 0.91, p = 0.38; in PTr, long movement versus short movement: t = 0.02, p = 0.99; short movement versus short control: t = 0.19, p = 0.85; long movement versus long control: t = 1.17, p = 0.27) . As expected, structured sentences yielded significantly greater activity than scrambled sentences in these voxels (PO: t = 5.05, p = .0003; PTr: t = 4.88, p = .0005), thus replicating in the even runs the effect found in the odd runs that defined the ROI. The PO sentence structure ROI activations cannot be accounted for by articulation: this ROI is activated significantly activated more for all sentence types than to articulation (t = 3.26, p = .004); this difference does not reach significance in the PTr sentence structure ROI (t = 1.76, p = .11). In summary: the majority of subjects (12/15, 80%) have voxels in Broca’s area that are relatively selective for sentences compared to word lists and compared to articulation in the PO; these “sentence” voxels are not selective for syntactic movement.
Figure 7.
Mean beta values of the voxels in Broca’s area found to be significant for the voxel-wise t-test of all sentences versus scrambled sentences. Voxels were identified in individual subjects by their response in the odd scanning runs (p < .005, uncorrected), and mean beta values were plotted from these voxels during the even scanning runs.
Discussion
The present study investigated the contributions of Broca’s area to sentence comprehension related to syntactic movement, global sentence structure, and speech articulation. Our aim was to assess whether Broca’s area—the pars opercularis and/or pars triangularis—contains subregions that are selective either for syntactic movement or global sentence structure. Group analyses failed to reveal strong evidence of selectivity for sentence processing: (i) sentences containing syntactic movement activated regions of Broca’s area as previously reported, but not more strongly than control sentences without syntactic movement (Experiment 1); similarly (ii) structured sentences activated a region of Broca’s area more than scrambled sentences (Experiment 2), but only a single voxel in Broca’s area was found to be more responsive to sentences that during speech articulation of a list of syllables (Experiment 3). This shows that previous group-level effects in Broca’s area in response to sentences may not reflect syntactic movement or hierarchical structure building, per se, but rather may reflect the contribution of confounding factors such as sentential force or subvocal articulation (e.g., rehearsal).
Individual subject analyses, however, tell a different story, at least for global sentence structure. A large majority of participants (>90%) had voxels in Broca’s area that responded more strongly to structured sentences than unstructured word lists (Experiment 2). Further, a split plot analysis showed that such voxels (identified in a slightly lower fraction of participants, 80% due to reduced power in using only half the dataset) were significantly more active during structured sentence processing than during listening to words lists and during subvocal articulation in the PO but not the PTr.
The individual subject analyses contradict our previous group-level observation that subvocal articulation can explain sentence activations in the pars opercularis (Rogalsky, Matchin & Hickok, 2008). While the present group-level analysis replicated this previous effect (Experiment 3), individual subject data shows that group analyses obscure sentence selective response patterns in Broca’s area. One conclusion we can draw from these observations is that Broca’s area does not serve a unitary function, which one might expect from its complex anatomical structure, connectivity, and the range of tasks that activate it (Amunts et al. 1999; Anwander et al. 2007; Rogalsky & Hickok, 2011). The present study shows, for example, that subportions of the pars opercularis are differentially activated by sentence processing and syllable articulation, two rather different tasks.
The present finding of sentence-selective activation patterns in Broca’s area is consistent with the hypothesis that this region plays a critical role in sentence comprehension. But this conclusion does not necessarily follow. Frontal speech regions are known to activate during speech sound perception (e.g. Wilson et al. 2004), but do not seem to play a significant role in speech recognition (Rogalsky et al. 2011a, Hickok, 2014). It is possible that a similar scenario holds for sentences. Some evidence exists along these lines. One large-scale voxel-based lesion study reports that sentence comprehension deficits are more strongly associated with temporal-parietal lesions than Broca’s area (Thothathiri, Kimberg & Schwartz, 2012) and another corroborated this finding and further found that comprehension of syntactically complex sentences implicated anterior temporal regions (Magnusdottir et al. 2013).
One possible explanation for this pattern of findings—the discrepancy between Broca’s area involvement in sentence processing as revealed by lesion vs. functional imaging studies—is that Broca’s area plays a role in sentence production and that listening to sentences activates this (high-level) “sensorimotor” loop to some extent even though it is not a central node for basic sentence computations during comprehension. We might speculate that sentence production systems serve as a kind of high-level “syntactic working memory” (Fiebach et al. 2005), allowing listeners to mentally rehearse difficult utterances with the assistance of a structured code. Clearly additional work on this and other possibilities is needed.
In summary, the present study reinforces Fedorenko & Kanwisher’s (2011) call for the use of more individual subject analyses in functional imaging studies of sentence processing in Broca’s area. Group studies can completely obscure selective response patterns. Using such individual analyses we corroborate previous work suggesting sentence-selective responses in Broca’s area but failed to find strong evidence for selectivity for syntactic movement constructions. More broadly, just as it is important to look at functional imaging data from multiple analysis perspectives, we need to look at the question of the role of Broca’s area in sentence processing from multiple methodological perspectives (imaging, lesion, etc.). From this broader vantage point, it is clear that we need to look beyond Broca’s for a complete understanding of the neurobiology of sentence processing (Rogalsky et al. 2011b; Dronkers et al. 2004; Wilson & Saygin, 2004; Wilson et al. 2014; Thothathiri et al. 2012; Magnusdottir et al. 2013).
Acknowledgments
This work was supported by NIH NIDCD under grant DC03681 (G. Hickok).
Footnotes
The common definition of Broca’s area, which we use in the present study, is the posterior two-thirds of the inferior frontal gyrus, i.e. the pars triangularis (PTr) and pars opercularis (PO) (Andwander et al. 2007; Brodmann 1909). The PO and PTr roughly correspond to Brodmann areas 45 and 44, respectively, although there is considerable variation (Keller et al. 2009; Amunts et al. 1999).
Contributor Information
Corianne Rogalsky, Email: Corianne.Rogalsky@asu.edu.
Diogo Almeida, Email: diogo@nyu.edu.
Gregory Hickok, Email: greg.hickok@uci.edu.
References
- 1.Amunts K, Schleicher A, Burgel U, Mohlberg H, Uylings HBM, Zillers K. Broca’s region revisited: Cytoarchitecture and intersubject variability. Journal of Comparative Neurology. 1999;412:319–341. doi: 10.1002/(SICI)1096-9861(19990920)412:2<319::AID-CNE10>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 2.Anwander A, Tittgemeyer M, von Cramon DY, Friederici AD, Knosche TR. Connectivity-based parcellation of Broca’s area. Cerebral Cortex. 2007;17:816–825. doi: 10.1093/cercor/bhk034. [DOI] [PubMed] [Google Scholar]
- 3.Awh E, Jonides J, Smith EE, Schumacher EH, Koeppe RA, Katz S. Dissociation of storage and rehearsal in working memory: PET evidence. Psychological Science. 1996;7:25–31. [Google Scholar]
- 4.Boersma P, Weenink D. Praat: Doing phonetics by computer. [Computer Software] Amsterdam: Department of Language and Literature, University of Amsterdam; 2011. Retrieved from http://www.praat.org/ [Google Scholar]
- 5.Brodmann K. Vergleichende Lokalisationslehre der Grobhirnrinde in ihren Prinzipien dargestellt aufgrund des Zellenbaues. Leipzig: Johann Ambrosius Barth Verlag; 1909. [Google Scholar]
- 6.Buchsbaum BR, D’Esposito M. The search for the phonological store: From loop to convolution. Journal of Cognitive Neuroscience. 2008;20:762–778. doi: 10.1162/jocn.2008.20501. [DOI] [PubMed] [Google Scholar]
- 7.Buchsbaum BR, Olsen RK, Koch P, Berman KF. Human dorsal and ventral auditory streams subserve rehearsal-based and echoic processes during verbal working memory. Neuron. 2005;48:687–697. doi: 10.1016/j.neuron.2005.09.029. [DOI] [PubMed] [Google Scholar]
- 8.Caplan D, Alpert N, Waters G, Olivier A. Activation of Broca’s area by syntactic processing under conditions of concurrent articulation. Human Brain Mapping. 2000;9:65–71. doi: 10.1002/(SICI)1097-0193(200002)9:2<65::AID-HBM1>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caramazza A, Zurif EB. Dissociation of algorithmic and heuristic processes in language comprehension: evidence from aphasia. Brain and Language. 1976;3:572–582. doi: 10.1016/0093-934x(76)90048-1. [DOI] [PubMed] [Google Scholar]
- 10.Cox RW, Hyde JS. Software tools for analysis and visualization of fMRI data. NMR in Biomedicine. 1997;10:171–178. doi: 10.1002/(SICI)1099-1492(199706/08)10:4/5<171::AID-NBM453>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 11.Cox RW, Jesmanowicz A. Real-time 3d image registration for functional MRI. Magnetic Resonance in Medicine. 1999;42:1014–1018. doi: 10.1002/(SICI)1522-2594(199912)42:6<1014::AID-MRM4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 12.Daneman M, Newson M. Assessing the importance of subvocalization during normal silent reading. Reading and Writing: An Interdisciplinary Journal. 1992;4:55–77. [Google Scholar]
- 13.Dronkers NF, Wilkins DP, Van Valin RD, Jr, Redfern BB, Jaeger JJ. Lesion analysis of the brain regions involved in language comprehension. Cognition. 2004;92(1–2):145–177. doi: 10.1016/j.cognition.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Fedorenko E, Behr MK, Kanwisher N. Functional specificity for high-level lingustic processing in the human brain. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(39):16428–33. doi: 10.1073/pnas.1112937108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fedorenko E, Kanwisher N. Some regions within Broca’s area do respond more strongly to sentences than to linguistically degraded stimuli: a comment on Rogalsky and Hickok (2011) Journal of Cognitive Neuroscience. 2011;23(10):2632–2635. doi: 10.1162/jocn_a_00044. [DOI] [PubMed] [Google Scholar]
- 16.Fiebach CJ, Schlesewsky M, Lohmann G, von Cramon DY, Friederici AD. Revisiting the role of Broca’s area in sentence processing: Syntactic integration versus syntactic working memory. Human Brain Mapping. 2005;24:79–91. doi: 10.1002/hbm.20070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friederici AD. Pathways to language: Fiber tracts in the human brain. Trends in Cognitive Sciences. 2009;13:175–181. doi: 10.1016/j.tics.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Friederici AD, Kotz SA, Scott SK, Obleser J. Disentangling syntax and intelligibility in auditory language comprehension. Human Brain Mapping. 2010;31:448–457. doi: 10.1002/hbm.20878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grodzinsky Y. Cognitive deficits and the theory of syntax. Brain & Language. 1986;27:135–159. doi: 10.1016/0093-934x(86)90009-x. [DOI] [PubMed] [Google Scholar]
- 20.Grodzinsky Y. The neurology of syntax: language use without Broca’s area. Behavioral and Brain Sciences. 2000;23(1):1–21. doi: 10.1017/s0140525x00002399. [DOI] [PubMed] [Google Scholar]
- 21.Grodzinsky Y. Theoretical perspectives on language deficits. Cambridge, MA: MIT Press; 2008. [Google Scholar]
- 22.Grodzinsky Y, Santi A. The battle for Broca’s region. Trends in Cognitive Sciences. 2008;12(12):474–80. doi: 10.1016/j.tics.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Hickok G, Buchsbaum B, Humphries C, Muftuler T. Auditory-motor interaction revealed by fMRI: Speech, music, and working memory in area Spt. Journal of Cognitive Neuroscience. 2003;15:673–682. doi: 10.1162/089892903322307393. [DOI] [PubMed] [Google Scholar]
- 24.Hickok G, Rogalsky C. What Does Broca’s Area Activation to Sentences Reflect? Journal of Cognitive Neuroscience. 2011;23(10):2629–2631. doi: 10.1162/jocn_a_00044. [DOI] [PubMed] [Google Scholar]
- 25.Humphries C, Binder JR, Medler DA, Liebenthal E. Syntactic and semantic modulation of neural activity during auditory sentence comprehension. Journal of Cognitive Neuroscience. 2006;18:665–679. doi: 10.1162/jocn_a_00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Just MA, Carpenter PA, Keller TA, Eddy WF, Thulbom KR. Brain activation modulated by sentence comprehension. Science. 1996;274:114–116. doi: 10.1126/science.274.5284.114. [DOI] [PubMed] [Google Scholar]
- 27.Kaan E, Swaab TY. The brain circuitry of syntactic comprehension. Trends in Cognitive Sciences. 2002;6:350–356. doi: 10.1016/S1364-6613(02)01947-2. [DOI] [PubMed] [Google Scholar]
- 28.Keller SS, Crow T, Foundas A, Amunts K, Roberts N. Broca’s area: Nomenclature, anatomy, typology and asymmetry. Brain and Language. 2009;109:29–48. doi: 10.1016/j.bandl.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 29.Magnusdottir S, Fillmore P, den Ouden DB, Hjaltason H, Rorden C, Kjartansson O, Bonilha L, Fridriksson J. Damage to left anterior temporal cortex predicts impairment of complex syntactic processing: a lesion-symptom mapping study. Human Brain Mapping. 2013;34(10):2715–2723. doi: 10.1002/hbm.22096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mazoyer BM, Tzourio N, Frak V, Syrota A, Murayama N, Levrier O, et al. The cortical representation of speech. Journal of Cognitive Neuroscience. 1993;5:467–479. doi: 10.1162/jocn.1993.5.4.467. [DOI] [PubMed] [Google Scholar]
- 31.Novick JM, Trueswell JC, Thompson-Schill SL. Cognitive control and parsing: Reexamining the role of Broca’s area in sentence comprehension. Cognitive, Affective & Behavioral Neuroscience. 2005;5:263–281. doi: 10.3758/CABN.5.3.263. [DOI] [PubMed] [Google Scholar]
- 32.Phillips C, Kazanina N, Abada SH. ERP effects of the processing of syntactic long-distance dependencies. Brain Research Cognitive Brain Research. 2005;22(3):407–428. doi: 10.1016/j.cogbrainres.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 33.Rogalsky C, Hickok G. Selective attention to semantic and syntactic features modulates sentence processing networks in anterior temporal cortex. Cerebral Cortex. 2009;19:786–796. doi: 10.1093/cercor/bhn126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rogalsky C, Hickok G. The role of Broca’s area in sentence comprehension. Journal of Cognitive Neuroscience. 2011;23(7):16648–80. doi: 10.1162/jocn.2010.21530. [DOI] [PubMed] [Google Scholar]
- 35.Rogalsky C, Matchin W, Hickok Broca’s area, sentence comprehension, and working memory: an fMRI study. Frontiers in Human Neuroscience. 2008;2(14) doi: 10.3389/neuro.09.014.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rogalsky C, Love T, Driscoll D, Anderson SW, Hickok G. Are mirror neurons the basis of speech perception? Evidence from five cases with damage to the purported human mirror system. Neurocase. 2011a;17(2):178–187. doi: 10.1080/13554794.2010.509318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rogalsky C, Rong F, Saberi K, Hickok G. Functional anatomy of language an music perception: temporal and structural factors investigated using functional magnetic resonance imaging. Journal of Neuroscience. 2011b;31(10):3843–3852. doi: 10.1523/JNEUROSCI.4515-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Santi A, Grodzinsky Y. Working memory and syntax interact in Broca’s area. Neuroimage. 2007a;37(1):8–17. doi: 10.1016/j.neuroimage.2007.04.047. [DOI] [PubMed] [Google Scholar]
- 39.Santi A, Grodzinsky Y. Taxing working memory with syntax: bihemispheric modulations. Human Brain Mapping. 2007b;11:1089–1097. doi: 10.1002/hbm.20329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Slowiaczek ML, Clifton C. Subvocalization and reading for meaning. Journal of Verbal Learning and Verbal Behavior. 1980;19:573–582. [Google Scholar]
- 41.Smith EE, Jonides J. Working memory: A view from neuroimaging. Cognitive Psychology. 1997;33:5–42. doi: 10.1006/cogp.1997.0658. [DOI] [PubMed] [Google Scholar]
- 42.Smith EE, Jonides J, Koeppe RA. Dissociating verbal and spatial working memory using PET. Cerebral Cortex. 1996;6:11–20. doi: 10.1093/cercor/6.1.11. [DOI] [PubMed] [Google Scholar]
- 43.Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical Publishers; 1988. p. 122. [Google Scholar]
- 44.Thothathiri M, Kimberg DY, Schwartz MF. The neural basis of reversible sentence comprehension: evidence from voxel-based lesion symptom mapping in aphasia. Journal of Cognitive Neuroscience. 2012;24(1):212–222. doi: 10.1162/jocn_a_00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilson SM, DeMarco AT, Henry ML, Gesierech B, Babiak M, Mandelli ML, et al. What role does the anterior temporal lobe play in sentence-level processing? Neural correlates of syntactic processing in semantic variant primary progressive aphasia? Journal of Cognitive Neuroscience. 2014;26(5):970–985. doi: 10.1162/jocn_a_00550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilson SM, Saygin AP. Grammaticality judgment in aphasia: deficits are not specific to syntactic structures, aphasic syndromes, or lesion sites. Journal of Cognitive Neuroscience. 2004;16(2):238–252. doi: 10.1162/089892904322984535. [DOI] [PubMed] [Google Scholar]
- 47.Wilson SM, Saygin AP, Sereno MI, Iacoboni M. Listening to speech activates motor areas involved in speech production. Nature Neuroscience. 2004;7(7):7017–702. doi: 10.1038/nn1263. [DOI] [PubMed] [Google Scholar]
- 48.Xiong J, Rao S, Jerabek P, Zamarripa F, Woldorff M, Lancaster J, Fox PT. Intersubject variability in cortical activations during a complex language task. Neuroimage. 2000;12:326–339. doi: 10.1006/nimg.2000.0621. [DOI] [PubMed] [Google Scholar]