Abstract
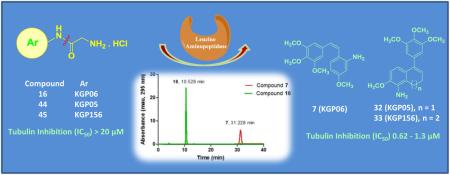
Targeting tumor vasculature represents an intriguing therapeutic strategy in the treatment of cancer. In an effort to discover new vascular disrupting agents with improved water solubility and potentially greater bioavailability, various amino acid prodrug conjugates (AAPCs) of potent amino combretastatin, amino dihydronaphthalene, and amino benzosuberene analogues were synthesized along with their corresponding water-soluble hydrochloride salts. These compounds were evaluated for their ability to inhibit tubulin polymerization and for their cytotoxicity against selected human cancer cell lines. The amino-based parent anticancer agents 7, 8, 32 (also referred to as KGP05) and 33 (also referred to as KGP156) demonstrated potent cytotoxicity (GI50 = 0.11 to 40 nM) across all evaluated cell lines, and they were strong inhibitors of tubulin polymerization (IC50 = 0.62 to 1.5 μM). The various prodrug conjugates and their corresponding salts were investigated for cleavage by the enzyme leucine aminopeptidase (LAP). Four of the glycine water-soluble AAPCs (16, 18, 44 and 45) showed quantitative cleavage by LAP, resulting in the release of the highly cytotoxic parent drug, whereas partial cleavage (<10-90%) was observed for other prodrugs (15, 17, 24, 38 and 39). Eight of the nineteen AAPCs (13-16, 42-45) showed significant cytotoxicity against selected human cancer cell lines. The previously reported CA1-diamine analogue and its corresponding hydrochloride salt (8 and 10, respectively) caused extensive disruption (at a concentration of 1.0 μM) of human umbilical vein endothelial cells growing in a two-dimensional tubular network on matrigel. In addition, compound 10 exhibited pronounced reduction in bioluminescence (greater than 95% compared to saline control) in a tumor bearing (MDA-MB-231-luc) SCID mouse model 2 h post treatment (80 mg/kg), with similar results observed upon treatment (15 mg/kg) with the glycine amino-dihydronaphthalene AAPC (compound 44). Collectively, these results support the further pre-clinical development of the most active members of this structurally diverse collection of water-soluble prodrugs as promising anticancer agents functioning through a mechanism involving vascular disruption.
Keywords: Small-Molecule Synthesis, Inhibitors of Tubulin Polymerization, Vascular Disrupting Agents, Amino Acid Prodrug Salts, Anti-Cancer Agents, Combretastatin Analogues, Benzosuberene Analogues, Dihydronaphthalene Analogues
1. Introduction
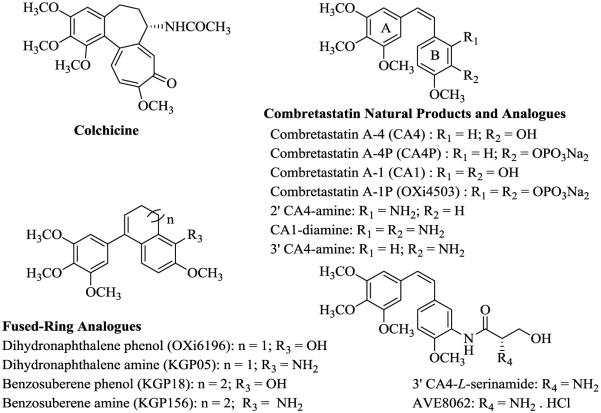
Tumor vasculature is as an attractive target for the treatment of cancer due, in part, to its distinct characteristics, such as rapid and disorganized proliferation of endothelial cells.1–5 Both small-molecules and biologics that specifically interact with tumor vasculature are referred to as vascular targeting agents (VTAs), which are further sub-classified as angiogenesis-inhibiting agents (AIAs) and vascular disrupting agents (VDAs).6,7 AIAs are typically represented by compounds that target vascular endothelial growth factors to prevent the development of new tumor vasculature.7 Conversely, VDAs are comprised of compounds that disrupt and directly damage established tumor vasculature, by affecting rapidly growing endothelial cells, to suppress tumor blood flow. One class of VDAs interacts with the colchicine (Fig. 1) site located on the α,β- heterodimer of tubulin, causing cell retraction, rounding, and ultimately detachment from the aggregated sheet of cells.1,4,8,9
Figure 1.
Colchicine and natural and synthetic combretastatin, dihydronaphthalene, and benzosuberene analogues
The natural products combretastatin A-4 (CA4) and combretastatin A-1 (CA1) (Fig. 1), originally isolated by Pettit and co-workers (Arizona State University) from the South African bush-willow tree, Combretum caffrum Kuntze, are among the colchicine site class of tubulin binding VDAs.10–13 This family of natural products, along with a number of synthetic derivatives and analogues,6,14–17 significantly inhibit microtubule assembly in endothelial cells lining tumor-feeding vasculature, leading to a series of cell signaling events that ultimately result in endothelial cell morphology changes and blood flow reduction.1,7,17 The inhibition of tubulin polymerization results in activation of RhoA, an intracellular coordinator of the cytoskeletal rearrangement of microtubules and actin, and leads to rapid vascular collapse.15,18,19 Administration of these VDAs in amounts significantly lower than their maximum tolerated doses has resulted in tumor necrosis in treated laboratory mice.3,12
The combretastatin water-soluble phosphate prodrugs, combretastatin A-4 phosphate (CA4P also known as ZybrestatTM, Fig. 1) and combretastatin A-1 diphosphate (CA1P also known as OXi4503, Fig. 1) are among a group of VDAs which have demonstrated promising efficacy in human clinical trials.18–27 They undergo enzyme-mediated dephosphorylation and, as their parent compounds (CA4 and CA1), they bind to tubulin and interfere with the tubulin-microtubule protein system. This causes pronounced morphological effects in the tumor vasculature.8,22–28 Pre-clinical and pharmacokinetic evaluations of these combretastatin prodrugs in patients bearing advanced tumors indicate their efficacy as VDAs.22,29–31 Interestingly, CA1P has dual mechanistic capability, functioning as both a VDA and as a cytotoxic agent based on its in vivo mediated conversion to a highly reactive orthoquinone.18,29–31 The relative structural simplicity of the combretastatins has motivated synthetic chemists to develop libraries of structurally inspired analogues through alteration of the A–ring, the B–ring, and the ethylene bridge.6,15,16,32–35
Incorporation of the NH2 substituent within either ring A or ring B in the combretastatin family resulted in new analogues that exhibited important biological activity.15,36,37 In 2006, we reported the initial design and synthesis of the 2' CA4-amine (Fig. 1) and described its potent inhibition of tubulin assembly and its activity as a VDA.15 Later work by others confirmed the potency of this 2' CA4-amine analogue and related compounds.34 Subsequently, our studies showed that the di-amino variant of combretastatin A-1 (CA1-diamine, Fig. 1)16 was strongly cytotoxic against human cancer cell lines (average GI50 = 13.9 nM) and also demonstrated potent activity in regard to inhibition of tubulin assembly (IC50 = 2.8 μM).16 It is fairly common for compounds that interact with tubulin in the low μM range (cell free assay) to demonstrate nM cytotoxicity against human cancer cell lines; a variety of factors are postulated to influence this activity differential.38 The trimethoxyphenyl moiety, the p-methoxyphenyl moiety, the Z-configuration of the two aryl rings, and the optimal 4-5 Å aryl-aryl distance all proved important for enhanced tubulin binding activity of the combretastatin analogues.17,39–41 Inspired, in part, by the SAR studies associated with the combretastatins and their close structural analogues, we were the first to report the synthesis and biological activity of related dihydronaphthalene tubulin-binding agents [for example, OXi6196 and KGP05, Fig. 1],42–44 followed by the discovery of a phenolic-based benzosuberene (KGP18, Fig. 1) analogue and its corresponding amino congener (KGP156, Fig. 1).40,45–48 These compounds have emerged as potential pre-clinical candidates due to their robust in vitro cytotoxicity (sub-nanomolar to picomolar GI50 values) against selected human cancer cell lines and strong tubulin inhibitory activities.45–47 Certain of these dihydronaphthalene analogues have subsequently been reported by another group.49 In our previous work,46 we also demonstrated robust tubule disruption [in a human umbilical vein endothelial cell (HUVEC) tube disruption assay] and cell rounding capability of KGP156, which is one of the parent compounds for several of the prodrugs designed and synthesized in this study.
The issue of limited water solubility associated with these aniline based anti-cancer agents can be addressed through prodrug strategies.50,51 Amino acid prodrugs, like glycine and serine, with shorter hydrocarbon or polar side chains, are reported to be more readily water soluble and more likely to be cleaved because of their structural simplicity.50 Another advantage of using amino acids is that they are capable of undergoing quantitative cleavage by hydrolytic enzymes, likely an aminopeptidase.51,52 The synthesis and biological evaluation of a series of water-soluble amino acid prodrugs of amino-combretastatin were previously reported.15,35,53 A water-soluble serinamide prodrug of 3' CA4-amine (Fig. 1) known as AVE8062 (Ombrabulin, synthesis36 is described in Supplementary data), showed significant promise as a VDA in phase III human clinical trials54,55 and enhanced antitumor activity and decreased toxicity (for normal cells) in both in vitro and in vivo models.36,53,55–57 The parent drug is generated after amide bond cleavage by a hydrolytic enzyme.51,53 Leucine aminopeptidase (LAP)58–60 is one of a widely distributed group of aminopeptidases that exhibit broad specificity61–63 and that catalyzes the hydrolysis of amino acids from the amino terminus of polypeptide chains.64 There are also a number of literature reports of amino acid prodrugs cleaved by LAP.50,53 In humans, LAP is found primarily in the cytosol of liver cells, which makes the serum leucine aminopeptidase a marker of hepatic disorders.65,66 The increase of LAP in human sera can also be diagnostically indicative of a number of cancers such as carcinoma of the pancreas and head and neck cancer.67–69
Inspired by these developments, herein we report the synthesis of eight glycine and serine AAPCs of highly potent amino-bearing structural variants incorporated within the combretastatin, dihydronaphthalene, and benzosuberene molecular scaffolds.42,44,70,71 Furthermore, in order to enhance water solubility (and potentially bioavailability)72 of these newly synthesized AAPCs, their eleven hydrochloride salts were synthesized.51,53 The synthesis of compounds 13, 15, 37, 39, 3' CA4-L-serinamide and AVE8062 were previously reported,15,36,47,53 and they were re-synthesized as a part of our ongoing biological studies. Each of the parent amino-based anticancer agents and their corresponding AAPCs were evaluated for their cytotoxicity against selected human cancer cell lines and for their ability to inhibit tubulin polymerization. In addition, these amino acid prodrug hydrochloride salts and two selected serinamide prodrugs (non-salts) were evaluated for their ability to undergo amide bond cleavage by LAP.53
2. Results and Discussion
2.1 Synthesis
Twenty six compounds (including amino combretastatin, dihydronaphthalene and benzosuberene parent compounds, amino acid prodrugs, and their corresponding water soluble hydrochloride salts) were synthesized for this study. Among these, compounds 7-10, 13, 15,15,16 32,42,43 33, 37, 39,46,47 3' CA4-amine, 3' CA4-L-serinamide and AVE806236,53 were re-synthesized for the purpose of further biological evaluation.
2.1.1. Synthesis of Amino Acid Prodrug Conjugates of Amino Combretastatins
1. Synthesis of Combretastatin Amines
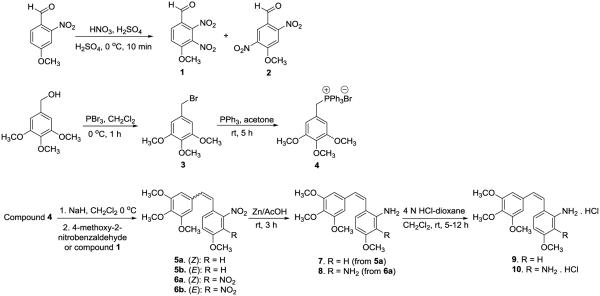
Our previous synthesis of 2' CA4-amine 7 involved a Wittig reaction between (4-methoxy-2-nitrobenzyl)triphenylphosphonium bromide and 3,4,5-trimethoxybenzaldehyde, followed by a reduction of the nitro group to form the corresponding amine using Na2S2O4.15,32 Later, this compound was synthesized and reported by another group as well.34 Switching the Wittig reaction partners to 3,4,5-trimethoxybenzyltriphenylphosphoniumbromide 4 and 4-methoxy-2-dinitrobenzaldehyde (scheme 1), followed by the separation of Z and E-isomers (1:0.4 ratio) and reduction of Z-isomer 5a to amine 6a using zinc35 in acetic acid (AcOH) increased the overall yield for compound 7 about 4-fold over two steps. The synthesis of CA1-diamine (8, Scheme 1) was achieved as reported earlier,16 involving a key Wittig reaction between 3,4,5-trimethoxybenzyltriphenylphosphonium bromide 4 and 4-methoxy-2,3-dinitrobenzaldehyde 1, followed by reduction of the nitro group of Z-isomer 6a to achieve the desired target compound (CA1-diamine 8). Upon treatment with HCl (4 N), these combretastatin-based amines (7 and 8) were converted to their corresponding hydrochloride salts (9 and 10).
Scheme 1.
2. Synthesis of Amino Acid Prodrug Conjugates
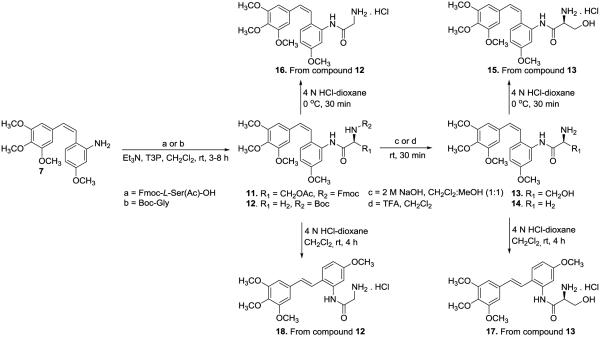
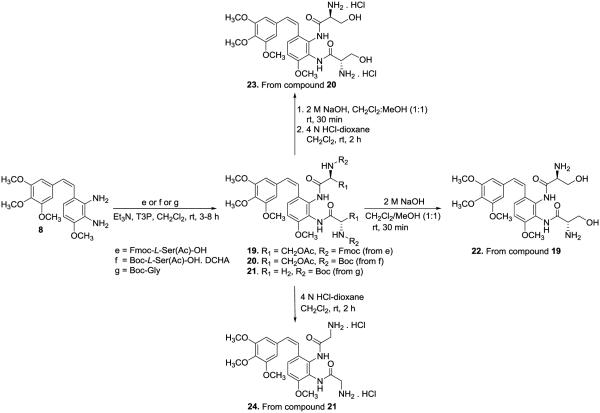
The serine and glycine amino acid prodrug conjugates (13 and 14, Scheme 2) of 2' CA4-amine 7 were prepared by coupling with Fmoc-L-ser(Ac)-OH and Boc-glycine-OH, respectively, in the presence of Et3N and the peptide coupling reagent propylphosphonic anhydride (T3P), followed by deprotection. This procedure is reminiscent of the synthetic strategy employed by Ohsumi and co-workers53 for the synthesis of water-soluble amino acid prodrug salts of 3' CA4-amine56 and our previous studies,15,16 but this modified synthetic route has benefits in terms of higher yield and ease of purification from the use of T3P. Similarly, the serine and glycine amino acid prodrug conjugates (19-21, Scheme 3) of CA1-diamine (8) were synthesized using a standard peptide coupling procedure (Scheme 2).
Scheme 2.
Synthesis of Amino Acid Prodrug Conjugates of 2'-Combretastatin Amines15,53
Scheme 3.
Synthesis of Amino Acid Prodrug Conjugates of Combretastatin Diamine15,16,53
3. Synthesis of Hydrochloride Salts of Amino Acid Prodrug Conjugates
The synthesis of compound 15 is described in our earlier publication.15 Our initial efforts to re-synthesize compound 15 and compound 16 (new compound) at room temperature in the presence of solvent resulted in the complete isomerization from Z to E, affording compounds 17 and 18. These E-isomers were used as a model system to evaluate LAP mediated cleavage of their corresponding amino acid prodrugs. Utilizing solvent free reaction conditions,73 the respective HCl salts of glycine and serine amino acid prodrug conjugates (15 and 16, scheme 2) were synthesized from compounds 12 and 13. The water-soluble hydrochloride salt of the glycine variant of CA1-diamine (24, scheme 3) was obtained using a similar synthetic strategy with only about 10% isomerization to the E-isomer. Our initial attempts to prepare the hydrochloride salt of compound 22 were unsuccessful. However, treatment of compound 20 (scheme 3) with NaOH (2 M) followed by HCl (4 N) yielded the desired CA1-diamine-based water-soluble amino acid prodrug conjugate (23) in a single step.
2.1.2. Synthesis of Amino Acid Prodrug Conjugates of Amino Dihydronaphthalene and Amino Benzosuberene
1. Synthesis of Dihydronaphthalene and Benzosuberene Amines
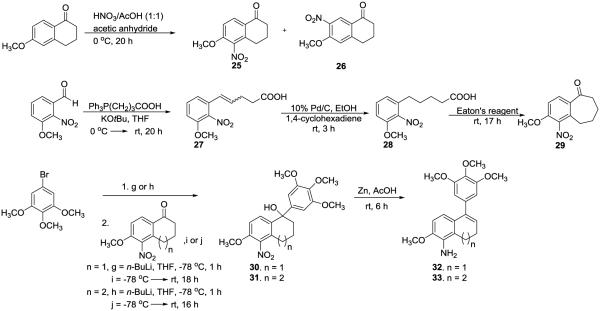
The synthesis of amino dihydronaphthalene (32)42,44 and amino benzosuberene (33),45–47 as previously reported by our group, is illustrated in Scheme 4. Initially, 6-methoxy-1-tetralone was nitrated to form two constitutional isomers, with one isomer being the desired product, 5-nitro-6-methoxy-1-tetralone (25). Compound 32 was obtained by reaction of 5-bromo-1,2,3-trimethoxybenzene with n-butyllithium, followed by the addition of compound 25. The reaction of compound 30 with Zn in the presence of AcOH resulted in reduction of the nitro group, and, following a subsequent condensation reaction, the desired product (32) was obtained in a 53% yield (over these two steps). The synthesis of amino-benzosuberene KGP156 (33), initially reported by us in 2012,46 utilized a sequential Wittig olefination, selective reduction with 1,4-cyclohexadiene, Eaton’s reagent mediated cyclization, 1,2-addition of the appropriately functionalized aryl ring, followed by condensation and reduction.
Scheme 4.
Synthesis of Dihydronaphthalene42,44 and Benzosuberene Amines42,45–47
2. Synthesis of Hydrochloride Salt of Amino Acid Prodrug Conjugates of Amino Dihydronaphthalene and Benzosuberene
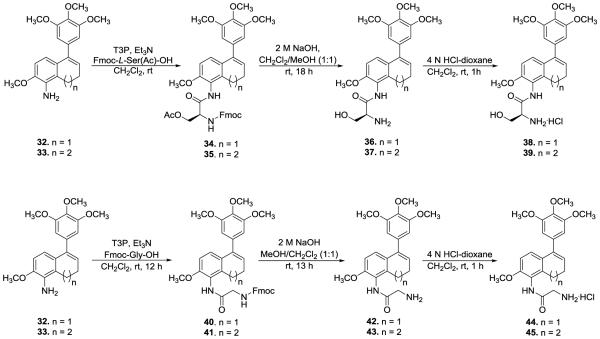
To obtain the desired amino acid prodrugs of amino dihydronaphthalene (32), Fmoc-L-ser(Ac)-OH or Fmoc-gly-OH were reacted utilizing general peptide synthetic methodology. Compound 32 was treated with T3P, Et3N, and the appropriate Fmoc-amino acid to obtain N-Fmoc protected amino acid amides (34) and (40), which upon deprotection resulted in the desired amino acid prodrugs (36) and (42). The AAPCs 37 and 39 (previously reported) were re-synthesized for this study.47 Similarly, the hydrochloride salts (38, 44, and 45) of amino acids (36, 42, and 43, respectively), were obtained upon treatment with a 4 N HCl in dioxane solution as shown in Scheme 5.
Scheme 5.
Synthesis of Amino Acid Prodrug Conjugates of Amino Dihydronaphthalene and Benzosuberene45,47 [Note: A portion of Scheme 5 was reproduced from reference 47 with permission from Elsevier.]
2.2. Biological Evaluation
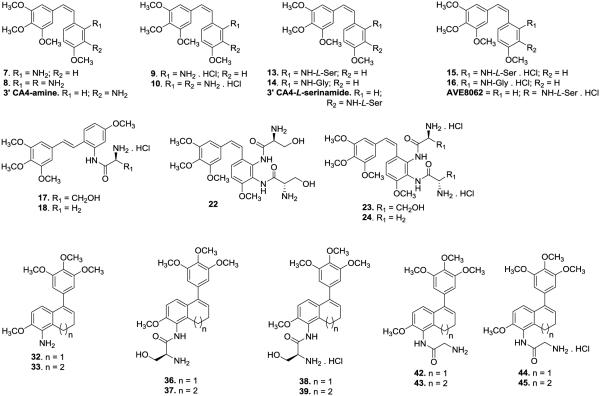
Each of the twenty six compounds synthesized for this study (including parent drugs, amino acid prodrugs, and their corresponding water soluble hydrochloride salts; compiled in Fig. 2) were evaluated for their cytotoxicity against selected human cancer cell lines [SK-OV-3 (ovarian), NCI-H460 (lung), and DU-145 (prostate), Table 1]. Furthermore, these compounds were evaluated biologically for their ability to inhibit tubulin polymerization (cell free assay) and colchicine binding (Table 2). In addition, thirteen water soluble AAPCs (including two as their non-salt forms) were evaluated for enzymatic cleavage by LAP (Table 3 and Fig. 3).37, 42–47 An endothelial tube disruption assay for CA1-diamine (compound 8, Fig. 2) was carried out utilizing HUVECs (Fig. 4). The hydrochloride salts of CA1-diamine (compound 10, Fig. 5) and dihydronaphthalene glycinamide (compound 44, Fig. 2) were initially evaluated for their in vivo ability to disrupt tumor blood flow utilizing dynamic bioluminescence imaging (BLI, Fig. 6 and 7) studies.
Figure 2.
Compilation of parent drugs and their amino-acid prodrug conjugates evaluated in this study
Table 1.
Cytotoxicity data for the amino-based combretastatin, dihydronaphthalene, and benzosuberene analogues against human cancer cell lines [SK-OV-3 (ovarian), NCI-H460 (lung), and DU-145 (prostate)]
| Compound | GI50 (nM) SRB Assaya | ||
|---|---|---|---|
|
| |||
| SK-OV-3 | NCI-H460 | DU-145 | |
| CA1b | 38.4±24.2 | 15.3±15.8 | 32.6±17.3 |
| CA4c | 5.06±0.145 | 5.00±0.359 | 6.02±0.661 |
| 3' CA4-amined | 4.45±0.870 | 4.49±0.894 | 3.30±0.281 |
|
3' CA4-L-
serinamided |
9.02±4.81 | 24.1±2.00 | 16.4±7.29 |
| AVE8062d | 3.96±0.912 | 3.87±0.297 | 3.34±0.261 |
| 7e | 26.6±22.6 | 40.1±17.1 | 33.1±21.4 |
| 8f | 23.5±23.4 | 35.9±21.8 | 39.1±26.5 |
| 9 | 7.35±3.50 | 26.3±16.1 | 28.5±23.2 |
| 10f | 22.8±15.2 | 124±116 | 205±241 |
| 13e | 39.5±21.1 | 33.3±19.0 | 34.7±8.27 |
| 14 | 32.3±6.38 | 53.2±5.23 | 43.6±2.43 |
| 15e | 51.6±6.88 | 31.7±4.87 | 48.4±3.36 |
| 16 | 48.5±8.53 | 29.2±5.67 | 44.7±3.53 |
| 17 | 2290±1170 | 2240±1450 | 4550±2700 |
| 18 | 427±111 (D) | 418±137 (D) | 630±306 (D) |
| 426±94.1 (W) | 380±60.2 (W) | 674±255 (W) | |
| 22 | 25900±2550 | 24400±4420 | 33600±2600 |
| 23 | >86600 | >86600 | >86600 |
| 24 | 39300±13500 (D) | >68500 (D) | >64700 (D) |
| >56400 (W) | >96600 (W) | >90400 (W) | |
| 32g | 0.308±0.402 | 0.290±0.126 | 0.111±0.114 |
| 33h | 0.137±0.0596 | 1.85±1.87 | 2.26±1.58 |
| 36 | 13700±9700 | 31100±9940 | 40600±32300 |
| 37 | 44900±4100 | 45900±1790 | >81300 |
| 38 | 11600±10200 | 18600±7670 | 26000±15700 |
| 39 | 47000±21400 | 53800±864 | 69400±31300 |
| 42i | 13.8±7.54 | 36.5±0.899 | 50.1±4.80 |
| 43 | 195±31.9 | 566±38.2 | 507±188 |
| 44 | 48.5±32.5 | 42.0±3.72 | 58.5±12.0 |
| 45 | 203±29.4 | 567±56.6 | 773±302 |
Table 2.
Inhibition of tubulin polymerization and percent inhibition of colchicine binding
| Compound | Inhibition of Tubulin Polymerization IC50 (±M) ±SD |
Inhibition of Colchicine Binding (% Inhibition±SD) |
|
|---|---|---|---|
|
| |||
| 1 μM | 5 μM | ||
| CA1 | 1.9a | ND | 99.6±0.7b |
| CA4 | 1.2±0.049 | 84±1.1 | 98±0.10 |
| 3' CA4-aminec | 1.2±0.056c | 84±1.8 | 97±1.9 |
| 3' CA4-L-serinamidec | 14±1.1d | ND | ND |
| AVE8062c | 13 ± 0.28d | ND | ND |
| 7 | 1.1±0.057d | 55±3.8 | 88±0.93 |
| 8 | 1.5±0.13e | 55±0.14 | 87±0.19 |
| 9 | 2.5±0.16 | ND | 75±0.90 |
| 10 | 2.3±0.21e | 46±2.3 | 86±3.8 |
| 32f | 0.62±0.019 | 65±3.6 | 92±0.16 |
| 33 | 1.3±0.11g | ND | 82±2.4 |
Table 3.
Activity of LAP toward amino acid prodrug conjugates
| Compound | Prodrug Type | % Cleavagea | Specific Activity of LAP (μM/min/enzyme unit) |
|---|---|---|---|
| 15 | Serinamide | 62% | 1.2 |
| 16 | Glycinamide | 100% | 180 |
| 17 | Serinamide | 90% | Ce |
| 18 | Glycinamide | 100% | Ce |
| 22 | Serinamide | NC | ND |
| 23 | Serinamide | NC | ND |
| 24 | Glycinamide | Cb | ND |
| 38 | Serinamide | Cc, <10% | ND |
| 39 | Serinamide | Cc, <10% | ND |
| 44 | Glycinamide | 100% | 15.5 |
| 45 | Glycinamide | 100% | 0.12 |
|
3' CA4-L-
serinamide |
Serinamide | Cd, 95% | ND |
| AVE8062 | Serinamide | Cd, 95% | ND |
Percentage of cleavage is calculated based on the area ratio of the parent drug and prodrug at a specific wavelength. NC: No cleavage observed using 1.5 units LAP. C: Cleaved (a rate study was not carried out).
, disappearance of the bis-glycinamide prodrug 24 was observed, but less than 50% of the expected final product 8 was detected.
, the serinamide prodrug was cleaved, but less than 10% of the expected product was obtained.
, used as positive controls.52
, the prodrug was readily cleaved, but the absolute rate could not be determined because the parent compound (E-isomer of 2' CA4-amine) was not available since isomerization (Z to E took place as a byproduct during salt formation after installation of the amino acid prodrug).
ND: Not determined
Figure 3.
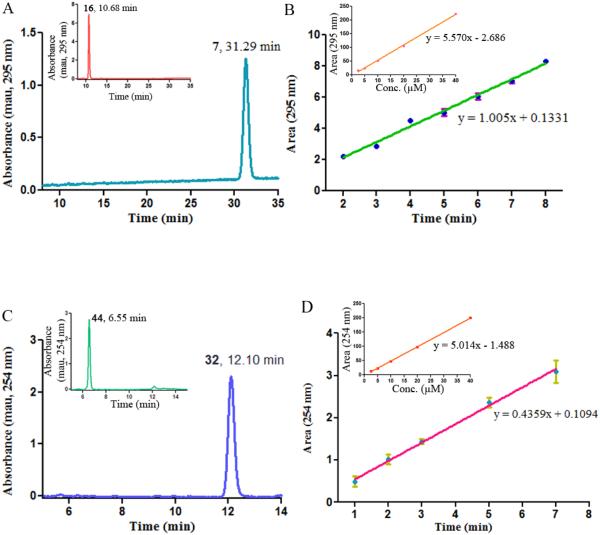
Cleavage of the glycine conjugates of 2' CA4-amine 16 and the amino dihydronaphthalene 44 by LAP. (A) Prodrug 16 was treated with 0.5 units of LAP for 40 h. A single peak corresponding to the product 2' CA4 amine 7 (tR = 31.29 min) was observed (HPLC chromatogram mobile phase: 26% acetonitrile/74% water containing 0.05% TFA). Inset: Control, compound 16 was incubated for 40 h without LAP. (B) Rate study for the cleavage of prodrug 16 to form 2' CA4 amine 7. Inset: Calibration curve for 2' CA4 monoamine 7. (C) Prodrug 44 was treated with 0.05 units of LAP for 2 h. A single peak corresponding to the product amino dihydronaphthalene 32 (tR = 12.10 min) was observed (HPLC chromatogram mobile phase: 28% acetonitrile/72% water containing 0.05% TFA). Inset: Control, compound 44 was incubated for 2 h without LAP. (D) Rate study for the cleavage of prodrug 44 to form amino dihydronaphthalene 32. Inset: Calibration curve for amino dihydronaphthalene 32
Figure 4.
HUVEC Tubule Disruption at Various Inhibitor Concentrations
Figure 5.
Dynamic BLI of Tumor in Response to Treatment with Compound 10.
Figure 6.
Dynamic BLI of Tumor in Response to Treatment with Compound 44.
Figure 7.
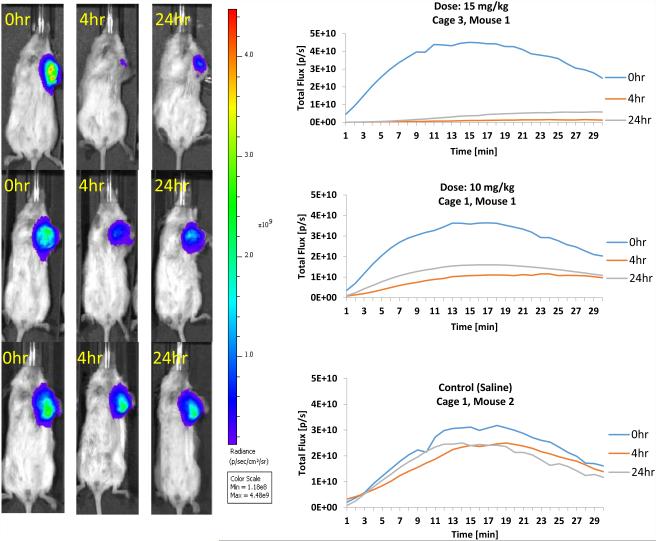
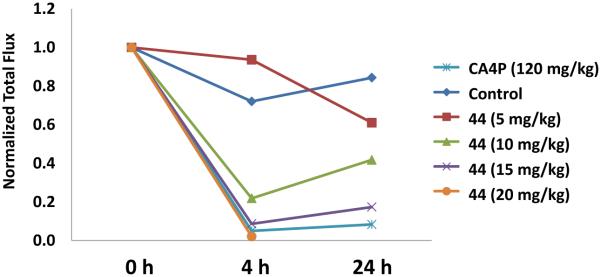
Dose Escalation Data for BLI Evaluation of Compound 44. CA4P and compound 44 (at 10 or 15 mg/kg) each gave significantly less signal at both 4 h and 24 h compared with baseline. Signal from the saline group was significantly reduced at the 4 h time point. At the 4 h time point compound 44 (5 mg/kg) was not significantly different from saline control, but was significantly different (p<0.05) from compound 44 (10 mg/kg) and compound 44 (15 mg/kg). Similarly compound 44 (15 mg/kg) was significantly different from saline and compound 44 (5 mg/kg), but not compound 44 (10 mg/kg). In general it appeared that a dose of 10, but not 5 mg/kg, generated a significantly greater response, as judged by reduced light emission. CA4P was significantly different from saline, compound 44 (5 mg/kg), and compound 44 (10 mg/kg), but not compound 44 (15 mg/kg) or compound 44 (20 mg/kg)
2.2.1. Cytotoxicity, Inhibition of Tubulin Polymerization, and Percent Inhibition of Colchicine Binding
Cytotoxicity studies carried out with all twenty six compounds against selected human cancer cell lines [SK-OV-3 (ovarian), NCI-H460 (lung), and DU-145 (prostate), Table 1] indicated that each of the parent drugs (compounds 7,15 8,16 3242,43 and 3346,47) were highly potent with GI50 values ranging from sub-nanomolar to nanomolar. The amino dihydronaphthalene 32 was found to be especially potent (GI50 = 1-3 nM across all three of the human cancer cell lines evaluated in this study) and was comparable to the clinically relevant agent CA4.35 Both the serinamide and glycinamide AAPCs (compounds 15 and 16, respectively) of the 2' CA4-amine 7 demonstrated significant cytotoxicity against each of the cancer cell lines. When comparing the E-isomers 17 and 18, the glycinamide AAPC 18 was more cytotoxic than the corresponding serinamide AAPC 17. Negligible cytotoxicity was observed for the bis-substituted AAPCs (22-24). The serinamide AAPCs (36-39) of the parent compounds 32 (KGP05) and 33 (KGP156) demonstrated limited cytotoxicity against these cancer cell lines, while the glycinamide analogues (42-45) were more active.
Pronounced inhibition of tubulin polymerization and inhibition of colchicine binding were observed for amino-dihydronaphthalene 32 (assembly IC50 = 0.62 μM and 92% inhibition of colchicine binding at 5 μM, respectively, Table 2). Similarly, the other parent compounds 7, 8 and 33 proved highly potent in these tubulin assays (ranging from 1.1 μM to 1.5 μM and 76% to 88% at 5 μM, respectively). Uniformly, the AAPCs (compounds 13-18, 22-24, 36-39 and 42-45) proved to be inactive (IC50 > 20 μM) as inhibitors of tubulin assembly, which was anticipated in this type of cell free assay.
2.2.2. Enzymatic Assay
Preliminary studies were carried out to determine the ability of LAP to cleave individual amino acid prodrug conjugates (Table 3). The mono-glycinamide prodrugs 16, 18, 44, 45 were quantitatively cleaved to their parent compounds. Treatment of the bis-glycinamide prodrug (24) with LAP (0.12 units) resulted in disappearance of greater than 95% of the prodrug by 25 h, but only 9% of the parent CA1-diamine (compound 8) was formed. Two additional peaks, presumably the mono-glycinamide intermediates, were observed in the HPLC chromatogram. Using 1.5 and 3.7 units of LAP, the amount of CA1 diamine (compound 8) detected increased to 19% and 24% respectively. The mono-serinamide prodrugs of both the Z and E-isomers (15 and 17, respectively) of 2' CA4-amine were effectively cleaved by LAP, as was the mono-serinamide derivative of 3' CA4-amine and its hydrochloride salt (AVE8062), used as positive controls.53 Only a partial release (less than 10%) was achieved with excess LAP (3.6 units, 24 h) for the mono-serinamide analogues of the amino dihydronaphthalene 38 and amino benzosuberene 39 compounds. Treatment of the bis-serinamide prodrug 22 of CA1-diamine or its dihydrochloride salt 23 with a large amount (1.5 units) of LAP did not result in any observable cleavage. For AAPCs that produced more than 50% of the final product upon cleavage with LAP in preliminary experiments, rate studies were conducted. The glycinamide prodrug of the Z-isomer 2' CA4-amine 16 was cleaved at a rate of 180 μM/min/enzyme unit (Fig. 3. A, B), 150 times faster than its corresponding serinamide analogue 15 (1.2 μM/min/enzyme unit). A similar result was observed for the glycinamide prodrug of the E-isomer 2' CA4-amine 18, which was cleaved at a rate more than 100 times faster than its serinamide analogue 17, as determined by the relative rates of cleavage of each prodrug. Differences were also found in the rate of cleavage of the glycinamide prodrugs of amino dihydronaphthalene 44 (15.5 μM/min/enzyme unit, Fig. 3. C, D) and amino benzosuberene 45 (0.12 μM/min/enzyme unit).
Despite a significantly faster cleavage of the glycinamide prodrug 16 versus the serinamide 15 by LAP, the cytotoxicity toward cancer cell lines over a 48 h treatment was very similar for both prodrugs and the parent Z-isomer 2' CA4-amine 7 (Table 1), indicating that release of compound 7 from both prodrugs in the presence of these cancer cell lines was efficient. The slower rate of cleavage for the E-isomer series resulted in a differential cytotoxicity for the serinamide 17 and glycinamide 18 prodrugs. The lack of cleavage by LAP of the bis-serinamide prodrugs 22 and 23 and the very low production of parent CA1-diamine (compound 8) from the bis-glycinamide 24 was reflected in the lack of cytotoxicity for all three compounds (22-24). This may indicate steric hindrance in the interaction of these compounds with LAP. There is a marked difference in the LAP cleavage rates between glycinamide prodrugs (compounds 44 and 45) that was reflected in their differential cytotoxicity in cancer cell lines, and their cytotoxicity was significantly less than that of either of the corresponding parent compounds (KGP05, KGP156, respectively).
Overall, the cancer cell line cytotoxicity mirrored the cleavage by LAP of the amino acid prodrug conjugates. In amino acid amides and peptides, an N-terminal L-serine residue is preferred over glycine, but there is clearly a contribution of binding interactions, steric factors and stereospecificity (L vs. D) in the position adjacent to the N-terminal amino acid that is observed in peptide substrates and in some non-peptidic amino acid conjugates.75,76 For example, Pettit et al. reported that the serinamide prodrug of 3' CA2-amine displayed significantly less activity compared to the glycine derivative in a cancer cell line panel.35
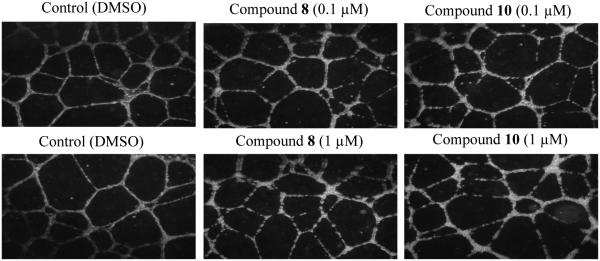
2.2.3. Endothelial Tube Disruption Assay77
Rapidly growing HUVECs can be induced to form a capillary-like network of tubules, which serves as a model for evaluating VDAs. CA1-diamine (compound 8) and its hydrochloride salt (compound 10) demonstrated significant disruption of a capillary-like network of tubules (from HUVECs) at a concentration of 0.1 μM. Our previous work shows a significant tubule disruption and cell rounding effect for compound 33 (KGP156) at 0.1 μM, and this effect was greatly enhanced at a 1 μM concentration.46
2.2.4. Dynamic Bioluminescence Imaging 77
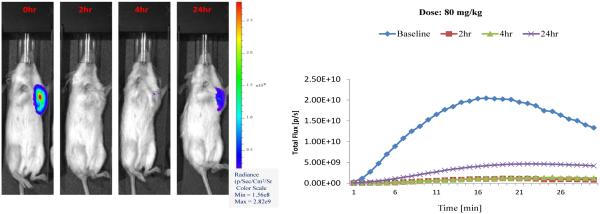
Bioluminescence Imaging (BLI) represents a valuable indirect in vivo technique for the assessment of tumor-specific vascular damage in response to treatment with VDAs.9,78,79 A decrease in light flux emission at various time points post VDA administration presumably results from an inability of the injected luciferin to reach the tumor (grown from luciferase expressing transfected cancer cells) due to VDA-induced damage of the vasculature feeding the tumor. Treatment (Fig. 5) of an MDA-MB-231-luc human breast tumor induced in a SCID mouse model with the diamino-CA1 salt (compound 10) at a dose of 80 mg/kg resulted in approximately 95% reduction in light emission [at 17 min time-point (midpoint)] at 2 h post VDA treatment relative to baseline. This reduction was still maintained (94% decrease) at the 4 h time point (fresh luciferin injection but no further VDA). There was some recovery (79% reduction, compared to baseline) at 24 h, which is typical due to the so-called viable rim.9 The glycinamide dihydronaphthalene AAPC (44) demonstrated (Fig. 6) similarly promising results (97% and 90% decrease in BLI signal at 4 and 24 h, respectively) when administered at a dose of 15 mg/kg, while the signal reduction at a dose of 10 mg/kg was 70% at 4 h and 55% at 24 h. In this experiment, the control mouse (saline administration) demonstrated a 22% decrease in signal at 4 h and 24% reduction at 24 h. These results, which are comparable to the clinically relevant VDA, CA4P (Fig. 7), provide further preliminary evidence suggesting that compounds 10 and 44 function as water-soluble VDAs and that glycinamide 44 is capable of being converted in vivo (presumably through the action of LAP) to its corresponding tubulin-active parent compound KGP05 (compound 32), which then causes the tumor-specific vascular damage.
3. Conclusions
Eleven water-soluble amino acid prodrug conjugates (AAPCs) derived from parent amino combretastatin, dihydronaphthalene, and benzosuberene derivatives (7, 8, 32 and 33) were designed and synthesized. Each of the parent compounds (7, 8, 32 and 33) were potent inhibitors of tubulin assembly binding to the colchicine site and were strongly cytotoxic against human cancer cell lines [SK-OV-3 (ovarian), NCI-H460 (lung), and DU-145 (prostate)]. Without exception, the glycinamide AAPCs based on the dihydronaphthalene and benzosuberene molecular scaffolds demonstrated efficient cleavage by LAP and were superior in this regard to their serinamide counterparts. Cytotoxicity mirrored the relative cleavage of these agents by LAP. The story was slightly different for the AAPCs derived from the combretastatins in that both the glycinamide and serinamde AAPCs (15-18) were efficiently cleaved by LAP for both the 2' and 3' amino variants, but the diamine-CA1 derived glycinamide (24) and serinamide (22 and 23) were resistant to LAP mediated cleavage to regenerate the parent compound (8). CA1-diamine (8) and its corresponding hydrochloride salt (10) caused significant disruption to a network of HUVECs growing on matrigel, and both CA1-diamine salt 10 and dihydronaphthalene glycinamide 44 demonstrated a significant reduction in light emission in a BLI study in SCID mice bearing the luciferase expressing MDA-MB-231-luc human breast cancer cell line induced tumor. These results demonstrate that these compounds can function as water-soluble VDAs.
4. Experimental Section
4.1. Chemistry
General Materials and Methods
AcOH, acetic anhydride, acetonitrile, CH2Cl2, dimethylformamide (DMF), ethanol, methanol, HNO3, H2SO4, and tetrahydrofuran (THF) were used in their anhydrous forms or as obtained from the chemical suppliers. Reactions were performed under N2. Thin-layer chromatography (TLC) plates (precoated glass plates with silica gel 60 F254, 0.25 mm thickness) were used to monitor reactions. Water was used to quench and wash the reaction mixture as appropriate. Purification of intermediates and products was carried out with a Biotage Isolera flash purification system using silica gel (200–400 mesh, 60 Å) or RP-18 pre-packed columns or manually in glass columns. Intermediates and products synthesized during this study were characterized on the basis of their 1H NMR (600 or 500 MHz), 13C NMR (150, 125 or 90 MHz) and 31P NMR (240 MHz) spectroscopic data using a Varian VNMRS 500 MHz or a Bruker DRX 600 MHz or a Bruker DPX 360 MHz instrument. Melting point ranges were determined (single experiment) using a Thomas Hoover capillary melting point apparatus and are uncorrected. Spectral data were recorded in CDCl3, D2O, (CD3)2CO, DMSO-d6, or CD3OD. All chemical shifts are expressed in ppm (δ), coupling constants (J) are presented in Hz, and peak patterns are reported as broad singlet (bs), singlet (s), doublet (d), triplet (t), quartet (q), pentet (p), double doublet (dd), triplet of doublets (td), doublet of triplets (dt) and multiplet (m).
Purity of the final compounds was further analyzed at 25 °C using an Agilent 1200 HPLC system with a diode-array detector (λ = 190–400 nm), a Zorbax XDB-18 HPL column (4.6 mm Å~ 150 mm, 5 μm), and a Zorbax reliance cartridge guard-column; method A: solvent A, acetonitrile, solvent B, 0.1% TFA in H2O; or method B: solvent A, acetonitrile, solvent B, H2O; gradient, 10% A/90% B to 100% A/0% B over 0 to 40 min; post-time 10 min; or method C: solvent A, acetonitrile, solvent B, 0.1% TFA in H2O; isocratic, 10% A/90% B over 0 to 5 min, 10% A/90% B to 100% A/0% B over 5 to 25 min; post-time 5 min, flow rate 1.0 mL/min; injection volume 20 μL; monitored at wavelengths of 210, 254, 230, 280, and 360 nm. Mass spectrometry was carried out under positive or negative electrospray ionization (ESI) using a Thermo Scientific LTQ Orbitrap Discovery instrument. Compound numbering for the combretastatin molecular scaffold followed this protocol: the trimethoxy A ring was numbered 1-6, and the B ring was numbered 1'-6'. The ethylene bridging atoms were numbered as 1a and 1a' respectively for the carbons connected to the A ring and B ring, respectively.
Synthesis of Amino Acid Prodrug Conjugates of Amino Combretastatins
4.1.1. Nitration of aldehyde16
4-Methoxy-2-nitrobenzaldehyde (1.53 g, 8.45 mmol) was dissolved in concentrated H2SO4 (25 mL). To this solution, a pre-cooled mixture of HNO3 and H2SO4 (3.85 mL/2.85mL) was added dropwise. The reaction mixture was stirred for 10 min, and then the resulting solution was added dropwise into ice-water (100 mL). After stirring for 2 h at 0 °C, the solution was filtered, and the solid containing the desired product was rinsed with ice-water (10 mL). Purification by flash column chromatography (40% EtOAc/hexanes) yielded regio-isomers 1 and 2.
4-methoxy-2,3-dinitrobenzaldehyde (1)16
This compound was isolated as a yellow solid (0.879 g, 3.89 mmol, 46%). 1H NMR (500 MHz, CDCl3): δ 9.95 (1H, s), 8.15 (1H, d, J = 8.9 Hz), 7.40 (1H, d, J = 8.9 Hz), 4.09 (3H, s), 13C NMR (125 MHz, CDCl3): δ 184.05, 155.69, 133.11, 128.96, 121.04, 115.92, 114.53, 57.88.
4-methoxy-2,5-dinitrobenzaldehyde (2)16
This compound was isolated as a colorless solid (0.725 g, 3.20 mmol, 38%). 1H NMR (500 MHz, CDCl3): δ 10.30 (1H, s) 8.43 (1H, s), 7.74 (1H, s), 4.15 (3H, s). 13C NMR (125 MHz, CDCl3): δ 184.63, 155.99, 127.12, 123.78, 123.04, 109.63, 57.89.
4.1.2. Synthesis of Wittig salt80
3,4,5-Trimethoxybenzyl bromide (3)80
The mixture of 3,4,5-trimethoxybenzyl alcohol (20.1 g, 101 mmol) and PBr3 (4.80 mL, 50.7 mmol) in anhydrous CH2Cl2 (100 mL) was stirred for 1 h at 0 °C. Water (10 mL) was added, and the mixture was extracted with CH2Cl2 (2 × 100 mL). The combined organic phase was washed with brine, dried over Na2SO4, filtered, and removed by evaporation under reduced pressure. After the recrystallization of the crude solid with EtOAc/hexanes, the bromide 3 (23.6 g, 90.3 mmol, 89%) was obtained as an off-white solid. 1H NMR (500 MHz, CDCl3): δ 6.62 (2H, s) 4.47 (2H, s), 3.87 (6H, s), 3.85 (3H, s). 13C NMR (125 MHz, CDCl3): δ 153.3, 138.2, 133.2, 106.1, 60.9, 56.1, 34.3.
3,4,5-Trimethoxybenzyltriphenylphosphonium bromide (4)80
A mixture of bromide 3 (11.0 g, 42.1 mmol) and PPh3 (12.1 g, 46.3 mmol) in anhydrous acetone (100 mL) was stirred for 5 h. The resulting suspension was filtered through a Buchner funnel, and the solid was washed with acetone (100 mL) followed by hexanes (50 mL) to afford an off-white solid. The solid was dried in vacuo to obtain the phosphonium salt 4 (20.3 g, 38.2 mmol, 92%) as a colorless solid. 1H NMR (600 MHz, CDCl3): δ 7.74 – 7.64 (9H, m), 7.58 – 7.50 (6H, m), 6.43 (2H, d, J=2.6 Hz), 5.29 (2H, d, J=14.1 Hz), 3.70 (3H, d, J=3.4 Hz), 3.43 (6H, d, J=3.7 Hz). 13C
NMR (150 MHz, CDCl3): δ 152.91 (d, J=3.6 Hz), 137.49 d, J=3.7 Hz), 134.84 (d, J=2.9 Hz), 134.58 (d, J=9.8 Hz), 129.99 (d, J=12.5 Hz), 122.44 (d, J=8.9 Hz), 117.76 (d, J=85.7 Hz), 108.76 (d, J=5.2 Hz), 60.83 (d, J=2.3 Hz), 56.16 (s), 30.72 (d, J=46.7 Hz). 31P NMR (240 MHz, CDCl3): δ 23.2.
4.1.3. Procedure for the synthesis of Z- and E-stilbenes (5a and 5b).15,16
At 0 °C, a NaH suspension (0.478 g, 19.9 mmol) in dry CH2Cl2 (50 mL) was stirred for 10 min. The previously prepared solution of 3,4,5-trimethoxybenzylphosphonium bromide (1.73 g, 3.30 mmol) was added dropwise. After stirring for 20 min, 4-methoxy-2-nitrobenzaldehyde (0.501 g, 2.76 mmol) was added. The resulting mixture was stirred for 5 h. At this point, ice-water was added carefully until H2 evolution stopped. Workup of the reaction mixture was carried out by extraction with CH2Cl2 (2 × 25 mL), followed by washing the combined organic layers with water (twice) and brine and finally drying over Na2SO4. The Z-and E-isomers were isolated after flash column chromatography (40% EtOAc/hexanes) and re-crystallization of the Z and E-isomer mixture from EtOAc and hexanes.
(Z)-2-(4'-Methoxy-2'-nitrophenyl)-1-(3,4,5-trimethoxyphenyl)ethene (5a)
This compound was isolated as a yellow solid (0.690 g, 1.99 mmol, 72%). 1H NMR (500 MHz, CDCl3): δ 7.59 (1H, d, J=2.7 Hz), 7.24 (1H, d, J=8.6 Hz), 7.01 (1H, dd, J=8.6, 2.6 Hz), 6.80 (1H, d, J=11.9 Hz), 6.62 (1H, d, J=11.9 Hz), 6.29 (2H, s), 3.87 (3H, s), 3.81 (3H, s), 3.62 (6H, s). 13C NMR (125 MHz, CDCl3): δ 159.0, 152.9, 148.6, 137.4, 133.4, 131.5, 131.2, 125.9, 125.7, 120.0, 108.7, 106.3, 60.9, 55.9, 55.8.
(E)-1,2,3-Trimethoxy-5-(4-methoxy-2-nitrostyryl)benzene (5b)
This compound was isolated as an orange solid (0.239 g, 0.692 mmol, 25%). 1H NMR (CDCl3, 600 MHz): δ 7.67 (1H, d, J=8.8 Hz), 7.47 (1H, d, J=2.7 Hz), 7.44 (1H, d, J=16.0 Hz), 7.16 (1H, dd, J=8.7, 2.7 Hz), 6.92 (1H, d, J=16.0 Hz), 6.74 (2H, s), 3.91 (6H, s), 3.90 (3H, s), 3.87 (3H, s). 13C NMR (150 MHz, CDCl3): δ 159.1, 153.4, 148.3, 138.4, 132.5, 132.2, 129.1, 125.5, 122.8, 120.4, 108.9, 103.9, 61.0, 56.2, 55.9.
4.1.4. Procedure for the synthesis of Z- and E-stilbenes (6a and 6b).16
These compounds were synthesized using the procedure as described for compounds 5a and 5b. Starting from NaH (1.57g, 65.4 mmol), 3,4,5-trimethoxybenzylphosphonium bromide (5.71 g, 1.09 mmol), and 4-methoxy-2,3-dinitrobenzaldehyde (2.24 g, 9.90 mmol) in CH2Cl2 (150 mL), the desired Z and E-isomers were isolated after flash column chromatography (50% EtOAc/hexanes).
(Z)-2-(4'-Methoxy-2',3'-dinitrophenyl)-1-(3,4,5-trimethoxyphenyl)ethane (6a)
This compound was isolated as a yellow solid (0.818 g, 2.09 mmol, 52%). 1H NMR (500 MHz, CDCl3): δ 7.35 (1H, d, J = 8.9 Hz), 7.08 (1H, d, J = 8.9 Hz), 6.77 (1H, d, J = 11.8 Hz), 6.50 (1H, d, J = 11.8 Hz), 6.30 (2H, s), 3.95 (3H, s), 3.83 (3H, s), 3.69 (6H, s). 13C NMR (150 MHz, CDCl3): δ 153.1, 150.9, 143.0, 137.9, 135.2, 134.5, 134.3, 130.7, 124.5, 121.6, 115.9, 106.0, 60.9, 57.4, 56.0.
(E)-2-(4'-Methoxy-2',3'-dinitrophenyl)-1-(3,4,5-trimethoxyphenyl)ethane (6b)
This compound was isolated as an orange solid (0.630 g, 1.61 mmol, 40%). 1H NMR (500 MHz, CDCl3): δ 7.84 (1H, d, J = 9.0 Hz), 7.25 (1H, d, J = 9 Hz), 6.96 (2H, dd, J = 38.2, 16.0 Hz), 6.69 (2H, s), 4.01 (3H, s), 3.91 (6H, s), 3.88 (3H, s). 13C NMR (125 MHz, CDCl3): δ 153.7, 151.0, 142.3, 139.3, 135.0, 134.6, 131.5, 130.0, 124.3, 118.8, 116.3, 104.4, 61.1, 57.5, 56.4.
4.1.5. eneral procedure for the reduction of nitro groups to amines15,16
(Z)-2-(2′-amino-4′-methoxyphenyl)-1-(3,4,5-trimethoxyphenyl)ethene (7)15
Zinc (8.36 g, 128 mmol) was added slowly to a well stirred solution of Z-stilbene (0.884 g, 2.56 mmol) in glacial AcOH (75 mL). The resulting suspension was stirred for 3 h at room temperature. At this point, the reaction mixture was filtered through Celite®, and the Celite® was washed with ethyl acetate. The filtrate was concentrated under reduced pressure. The desired amine was purified by flash column chromatography using a pre-packed 50 g silica column [solvent A: EtOAc; solvent B: hexanes; gradient: 20%A / 80%B (1 CV), 20%A / 80%B → 75%A / 25%B (10 CV), 75%A / 25%B (2 CV); flow rate: 40 mL/min; monitored at 254 and 280 nm] to afford the amine 7 (0.615 g, 1.95 mmol, 76%, melting point range 95-98 °C) as a colorless solid. 1H NMR (500 MHz, CDCl3): δ 7.04 (1H, d, J = 8.4 Hz), 6.52 (2H, s), 6.51 (1H, d, J = 12 Hz), 6.43 (1H, d, J = 11.9 Hz), 6.30 (1H, dd, J = 8.4, 2.5 Hz), 6.26 (1H, d, J = 2.5 Hz), 3.81 (3H, s), 3.76 (3H, s), 3.74 (2H, b), 3.65 (6H, s). 13C NMR (125 MHz, CDCl3): δ 160.1, 152.7, 144.9, 137.3, 132.3, 131.0, 130.6, 125.7, 116.1, 105.8, 104.3, 100.7, 60.8, 55.8, 55.2. HRMS: m/z: obsd 316.1544 [M+H]+, calcd for C18H22NO4+, 316.1543. HPLC (Method B): 13.153 min.
(Z)-2-(2′,3′-Diamino-4′-methoxyphenyl)-1-(3,4,5-trimethoxyphenyl)ethene (8)16
This compound was synthesized using the procedure as described for compound 7. Starting from zinc (13.4 g, 205 mmol) and Z-stilbene (0.797 g, 2.04 mmol) in glacial AcOH (72 mL), the desired product 8 (0.403 g, 1.22 mmol, 60%) was obtained as a brown oil. 1H NMR (500 MHz, CDCl3): δ 6.66 (1H, d, J=8.4 Hz), 6.52 (1H, d, J=12 Hz), 6.50 (1H, d, J=12 Hz), 6.48 (2H, s), 6.39 (1H, d, J=8.4 Hz), 3.83 (3H, s), 3.81 (3H, s), 3.62 (6H, s), 3.49 (4H, bs). 13C NMR (125 MHz, CDCl3): δ 152.68, 147.72, 137.31, 133.00, 132.22, 131.26, 126.14, 123.31, 119.53, 117.99, 105.90, 102.23, 60.84, 55.82, 55.73. HRMS: m/z: obsd 331.1656 [M+H]+, calcd for C18H23N2O4+, 331.1658. HPLC (Method B): 11.603 min.
4.1.6. Preparation of hydrochloride salts of amino combretastatin analogues15,16
(Z)-2-(2'-Methoxy-4'-aminophenyl)-1-(3,4,5-trimethoxyphenyl)ethene hydrochloride (9)15
To a solution of 2' CA4-amine 7 (0.0550 g, 0.175 mmol) in anhydrous CH2Cl2 (8 mL) was added a HCl solution (4 N in dioxane, 3 equiv.) at 0 °C. The reaction was monitored by TLC and stirred for 12 h. After evaporating the solvents under reduced pressure, the final hydrochloride salt 9 (0.0440 g, 0.125 mmol, 72%, melting point range 143-146 °C) was obtained as a green solid after recrystallization with ethanol/diethyl ether solution. 1H NMR (500 MHz, CD3OD): δ 7.27 (1H, d, J=8.3 Hz), 6.97 – 6.91 (2H, m), 6.78 (1H, d, J=11.9 Hz), 6.55 (1H, d, J=11.8 Hz), 6.46 (2H, s), 3.83 (3H, s), 3.71 (3H, s), 3.60 (6H, s). 13C NMR (125 MHz, CD3OD): δ 160.11, 152.83, 152.82, 133.86, 132.04, 131.52, 130.51, 123.86, 122.08, 113.51, 108.42, 106.21, 59.66, 54.89, 54.83. HRMS: m/z: obsd 316.1544 [M−Cl]+, calcd for C18H22NO4+, 316.1543. HPLC (Method B): 13.02 min.
(Z)-2-(2′,3′-Diamine hydrochloride-4′-methoxyphenyl)-1-(3,4,5-trimethoxyphenyl)ethene (10)
To a solution of CA1-diamine 8 (0.320 g, 0.970 mmol) in anhydrous CH2Cl2 (50 mL) was added a HCl solution (4 N in dioxane, 5 equiv.), and the reaction mixture was stirred for 5 h. The resulting crystals were filtered and re-crystallized from anhydrous methanol at −20 °C. The brown crystals thus obtained were washed with dry CH2Cl2 to obtain the salt 10 (0.130 g, 0.320 mmol, 30%, melting point range 184-186 °C). 1H NMR (600 MHz, CD3OD): δ 6.98 (1H, d, J=8.5 Hz), 6.60 (1H, d, J=11.9 Hz), 6.47 (1H, d, J=8.6 Hz), 6.42 (2H, s), 6.37 (1H, d, J=11.8 Hz), 3.83 (3H, s), 3.62 (3H, s), 3.52 (6H, s). 13C NMR (150 MHz, CD3OD): δ 152.7, 152.3, 137.1, 137.0, 132.6, 132.2, 129.2, 124.0, 119.0, 107.4, 106.0, 101.4, 59.7, 55.4, 54.9. HRMS: m/z: obsd 353.1476 [M −2HCl+ Na]+, calcd for C18H22N2O4Na+, 353.1472. HPLC (Method C): 13.06 min.
4.1.7. Preparation of amino acid prodrug conjugates of 2' combretastatin A-4 amines15,53
(S,Z)-2-((((9H-fluoren-9-yl)methoxy)carbonyl)amino)-3-((5-methoxy-2-(3,4,5-trimethoxystyryl)phenyl)amino)-3-oxopropyl acetate (11)15
To a solution of 2' CA4-amine 7 (0.220 g, 0.700 mmol) in anhydrous CH2Cl2 (30 mL) was added Et3N (0.110 mL, 0.760 mmol), peptide coupling reagent T3P (50% in EtOAc, 0.830 mL, 1.40 mmol) and Fmoc-L-ser(Ac)-OH (0.284 g, 0.760 mmol). The reaction mixture was stirred for 8 h at room temperature. H2O (20 mL) was added, and the reaction mixture was extracted with CH2Cl2. The organic extract was washed with brine, dried over Na2SO4 and concentrated under reduced pressure, and the residue was purified using flash column chromatography (50% EtOAc/hexanes) to afford the desired Fmoc-L-serinamide acetate 11 (0.388 g, 0.580 mmol, 83%) as a colorless solid. 1H NMR (500 MHz, CDCl3): δ 8.23 (1H, b), 7.93 (2H, d, J = 7.5), 7.60 (2H, d, J = 7.5), 7.60 (2H, d, J = 7.5), 7.40 (2H, dd, J = 7.5, 7.0), 7.33 (2H, dd, J = 7.5, 7.0), 6.98 (1H, d, J = 8.5), 6.65 (1H, d, J = 9.0), 6.56 (1H, s), 6.00 (1H, t, J = 4.5), 5.75 (1H, d, J = 6.5), 4.47 (4H, m), 4.23 (1H, d, J = 11.5), 3.89 (3H, s), 3.84 (6H, s), 3.79 (3H, s), 2.67 (2H, t, J = 7.5), 2.30 (2H, td, J = 7.5, 4.5), 2.11 (3H, s). 13C NMR (150 MHz, CDCl3): δ 171.2, 166.6, 159.8, 153.2, 143.9, 141.7, 138.1, 135.3, 133.2, 131.5, 130.2, 128.2, 127.5, 127.5, 125.2, 125.1, 124.2, 120.4, 120.4, 111.7, 105.8, 105.8, 67.2, 63.8, 61.1, 56.43, 56.0, 55.8, 47.4, 20.8.
(S,Z)-2-amino-3-hydroxy-N-(5-methoxy-2-(3,4,5-trimethoxystyryl)phenyl)propanamide (13)
Fmoc-L-serinamide acetate 11 (0.290 g, 0.435 mmol) was dissolved in a CH2Cl2/MeOH mixture (10 mL, 1:1 ratio), a NaOH solution (2 M, 0.650 mL, 1.30 mmol) was added, and the mixture was stirred for 30 min. After the evaporation of solvent, the resulting oil was purified by normal phase preparative TLC (95% CH2Cl2/MeOH) to obtain 2' CA4-L-serinamide 13 (0.125 g, 0.311 mmol, 71%) as a colorless solid. 1H NMR (500 MHz, CDCl3): δ 9.65 (1H, s), 8.04 (1H, d, J=2.6 Hz), 7.16 (1H, dd, J=8.5, 0.9 Hz), 6.69 (1H, dd, J=8.5, 2.6 Hz), 6.61 (1H, d, J=12.0 Hz), 6.50 (1H, d, J=12.0 Hz), 6.42 (2H, s), 3.82 (3H, s), 3.80 (3H, s), 3.76 (1H, dd, J=10.8, 5.2 Hz), 3.61 (7H, s), 3.37 (1H, t, J=5.4 Hz). 13C NMR (90 MHz, CDCl3): δ 171.7, 159.5, 152.8, 137.9, 135.8, 132.6, 131.8, 130.1, 124.3, 120.1, 110.8, 106.1, 105.6, 64.8, 60.8, 56.8, 55.9, 55.4. HRMS: m/z: obsd 403.1866 [M+H]+, calcd for C21H27N2O6+, 403.1864. HPLC (Method A): 12.97 min.
(S,E)-2-amino-3-hydroxy-N-(5-methoxy-2-(3,4,5-trimethoxystyryl)phenyl)propanamide hydrochloride (17)53
To a well stirred solution of 2' CA4-L-serinamide 13 (0.0950 g, 0.236 mmol) in CH2Cl2 (10 mL) was added a HCl solution (4 N in dioxane, 0.8 mmol) at room temperature. After completion of the reaction (monitored by TLC), diethyl ether (20 mL) was added to the reaction mixture, producing a colorless solid that was recovered by filtration through a membrane filter. After the solid was washed with diethyl ether (10 mL), the water soluble 2' CA4-L-serinamide 17 (0.0780 g, 0.178 mmol, 75%, melting point range 208-212 °C) was obtained as a colorless solid. 1H NMR (600 MHz, CD3OD): δ 7.72 (1H, d, J=8.8 Hz), 7.23 (1H, d, J=16.2 Hz), 7.09 (1H, d, J=2.6 Hz), 7.01 (1H, d, J=16.1 Hz), 6.92 (1H, dd, J=8.7, 2.6 Hz), 6.89 (2H, s), 4.26 (1H, t, J=5.2 Hz), 4.12 (2H, d, J=5.2 Hz), 3.91 (6H, s), 3.84 (3H, s), 3.79 (3H, s). 13C NMR (150 MHz, CD3OD): δ 166.2, 159.5, 153.2, 137.4, 134.5, 133.9, 128.6, 126.5, 124.9, 122.4, 112.8, 111.0, 103.5, 60.5, 59.8, 55.3, 55.1, 54.5. HRMS: m/z: obsd 403.1873 [M−Cl]+, calcd for C21H27N2O6+ 403.1864. HPLC (Method C): 13.23 min.
(S,Z)-2-amino-3-hydroxy-N-(5-methoxy-2-(3,4,5-trimethoxystyryl)phenyl)propanamide hydrochloride (15)
To 2' CA4-L-serinamide 13 (0.0500 g, 0.124 mmol) was added a HCl solution (4 N in dioxane, 0.36 mmol) at 0 °C. The resulting reaction mixture was stirred for 3 h, followed by evaporation of the solvent under reduced pressure at 30 °C. The resulting green oil was washed with anhydrous diethyl ether to furnish the water soluble HCl salt of 2' CA4-L-serinamide 15 (0.0360 g, 0.0820 mmol, 66%, melting point range 55-61 °C) as a dark green solid. 1H NMR (600 MHz, CD3OD): δ 7.37 (1H, d, J=2.4 Hz), 7.12 (1H, dd, J=8.5, 2.5 Hz), 6.75 (1H, d, J=7.8 Hz), 6.56 (1H, d, J=11.8 Hz), 6.47 (1H, d, J=11.9 Hz), 6.42 (2H, s), 3.98 (1H, t, J=5.9 Hz), 3.75 (3H, s), 3.72 (1H, d, J=4.5 Hz), 3.67 (3H, s), 3.61 (1H, m), 3.55 (6H, s). 13C NMR (150 MHz, CD3OD): δ 165.35, 159.38, 152.70, 137.08, 135.26, 132.31, 131.57, 130.38, 124.80, 123.57, 111.27, 109.52, 105.93, 60.09, 59.70, 55.08, 54.87, 54.52. HRMS: m/z: obsd 403.1885 [M+H]+, calcd for C21H27N2O6+, 403.1884. HPLC (Method C): 13.05 min.
Tert-butyl (Z)-(2-((5-methoxy-2-(3,4,5-trimethoxystyryl)phenyl)amino)-2-oxoethyl) carbamate (12)
To a solution of 2' CA4-amine 7 (0.363 g, 0.700 mmol) in anhydrous CH2Cl2 (75 mL) was added Et3N (0.300 mL, 1.72 mmol), peptide coupling reagent T3P (50% in EtOAc, 1.70 mL, 2.30 mmol) and Boc-glycine-OH (0.311 g, 1.44 mmol). The reaction mixture was stirred for 3 h at room temperature. Water (25 mL) was added, and the organic solvent was removed by evaporation under reduced pressure. The resulting crude mixture was extracted with EtOAc, and the organic extract was washed with brine, dried over Na2SO4, and concentrated under reduced pressure. The residue was purified using flash column chromatography (50% EtOAc/hexanes) to afford the desired Boc-protected glycinamide 12 (0.490 g, 1.04 mmol, 90%) as a colorless solid. 1H NMR (500 MHz, CDCl3): δ 7.95 (1H, s), 7.93 (1H, bs), 7.15 (1H, d, J = 8.5 Hz), 6.68 (1H, d, J = 7.7 Hz), 6.61 (1H, d, J = 12.0 Hz), 6.46 (1H, d, J = 12.0 Hz), 6.41 (2H, s), 4.80 (1H, s), 3.81 (3H, s), 3.80 (3H, s), 3.67 (1H, s), 3.61 (6H, d, J = 1.4 Hz), 1.46 (9H, s). 13C NMR (125 MHz, CDCl3): δ 167.26, 159.51, 155.67, 152.86, 137.83, 135.32, 132.51, 131.43, 130.06, 124.34, 119.50, 110.91, 109.99, 105.67, 105.64, 60.91, 55.80, 55.46, 45.10, 28.29.
(Z)-2-amino-N-(5-methoxy-2-(3,4,5-trimethoxystyryl)phenyl)acetamide (14)81
The Boc- protected glycinamide 12 (0.128 g, 0.270 mmol) in anhydrous CH2Cl2 (2 mL) was reacted with TFA (0.650 mL, 1.30 mmol) and stirred for 30 min.81 After evaporation of the solvent, the resulting oily residue was diluted with water (2 mL) and washed with ether (2 × 2 mL). The aqueous phase was treated with NaHCO3 (1 N) until pH 7-8 and extracted with CH2Cl2 (3 × 5 mL). The combined organic phase was dried over Na2SO4, concentrated under reduced pressure and purified using flash column chromatography (50% EtOAc/hexanes) to obtain 2' CA4 glycinamide prodrug 14 (0.0680 g, 0.183 mmol, 71%) as a brown oil. 1H NMR (500 MHz, CDCl3): δ 9.52 (1H, s), 8.08 (1H, d, J=2.6 Hz), 7.14 (1H, d, J=8.5 Hz), 6.65 (1H, dd, J=8.6, 2.5 Hz), 6.60 (1H, d, J=12.0 Hz), 6.50 (1H, d, J=12.1 Hz), 6.41 (2H, s), 3.82 (3H, s), 3.80 (3H, s), 3.60 (6H, s), 3.28 (2H, s). 13C NMR (125 MHz, CDCl3): δ 170.62, 159.52, 152.73, 137.63, 136.05, 132.44, 131.77, 130.01, 124.43, 119.59, 110.51, 105.85, 105.21, 60.89, 55.82, 55.45, 45.42. HRMS: m/z: obsd 373.1759 [M+H]+, calcd for C20H25N2O5+, 373.1758. HPLC (Method A): 8.14 min.
(E)-2-amino-N-(5-methoxy-2-(3,4,5-trimethoxystyryl)phenyl)acetamide hydrochloride (18)53
To a well stirred solution of Boc-protected 2' CA4 glycinamide 12 (0.146 g, 0.310 mmol) in CH2Cl2 (10 mL) was added a HCl solution (4 N in dioxane, 1.20 mmol) at room temperature. After completion of the reaction (monitored by TLC), diethyl ether (25 mL) was added to the reaction mixture, producing a colorless solid that was filtered through a membrane filter. After washing the product on the membrane with diethyl ether (10 mL), the HCl salt of the E-isomer of 2' CA4 glycinamide 18 (0.109 g, 0.226 mmol, 79%, melting point range 240-243 °C) was obtained as a colorless solid. 1H NMR (500 MHz, CD3OD): δ 7.66 (1H, d, J=8.8 Hz), 7.20 (1H, d, J=15 Hz), 7.19 (1H, d, J=2.7 Hz), 6.98 (1H, d, J=16.1 Hz), 6.88 (1H, d, J=2.5 Hz), 6.87 (2H, s), 3.98 (2H, s), 3.88 (6H, s), 3.81 (3H, s), 3.77 (3H, s). 13C NMR (125 MHz, CD3OD): δ 165.04, 159.47, 153.25, 137.58, 134.59, 133.83, 129.04, 126.75, 124.24, 122.22, 112.35, 110.48, 103.75, 59.76, 55.31, 54.46, 40.66. HRMS: m/z: obsd 373.1769 [M−Cl]+, calcd for C20H25N2O5+ 373.1758. HPLC (Method B): 6.661 min.
(Z)-2-amino-N-(5-methoxy-2-(3,4,5-trimethoxystyryl)phenyl)acetamide hydrochloride (16)73
To Boc-protected 2' CA4 glycinamide 12 (0.0898 g, 0.190 mmol) was added a HCl solution (4 N in dioxane, 0.270 mmol) at 0 °C. The resulting reaction mixture was stirred for 30 min., followed by evaporation of the solvent under reduced pressure at 30 °C. The resulting brownish oil, on washing with anhydrous diethyl ether, furnished the water soluble HCl salt of 2' CA4 glycinamide 16 (0.0691 g, 0.171 mmol, 90%, melting point range 59-63 °C) as a colorless solid. 1H NMR (600 MHz, CD3OD): δ 7.37 (1H, d, J=2.6 Hz), 7.12 (1H, d, J=8.4 Hz), 6.73 (1H, dd, J=8.3, 2.4 Hz), 6.57 (1H, d, J=11.8 Hz), 6.48 (1H, d, J=11.9 Hz), 6.43 (2H, s), 3.75 (3H, s), 3.67 (3H, d, J=1.6 Hz), 3.63 (2H, s), 3.56 (6H, s). 13C NMR (150 MHz, CD3OD): δ 164.38, 159.37, 152.65, 136.96, 135.29, 132.57, 131.37, 130.58, 124.93, 123.22, 111.06, 109.45, 105.84, 59.75, 54.89, 54.51, 40.59. HRMS: m/z: obsd 373.1759 [M−Cl]+, calcd for C20H25N2O5+, 373.1759. HPLC (Method C): 12.94 min.
4.1.8. Preparation of amino acid prodrug conjugates of combretastatin A-1 diamines15,16,53
(Z)-2-(2',3′-Diamino-4′-methoxyphenyl)-1-(3,4,5-trimethoxyphenyl)ethene-Fmoc-L-serinamide (19)
To a solution of CA1-diamine 8 (0.780 g, 2.37 mmol) in dry CH2Cl2 (150 mL) was added Fmoc-L-serine (Ac)-OH (2.40 g, 7.11 mmol), Et3N (1.00 mL, 7.11 mmol), and T3P (50% in EtOAc, 5.62 mL, 9.48 mmol). The resulting solution was stirred for 8 h at room temperature. At this point, the reaction mixture was washed with water (3 × 50 mL) and brine and dried over Na2SO4. The organic solvent was removed under reduced pressure, and the crude product was purified by flash column chromatography (50% EtOAc/hexanes) to afford the Fmoc-L-diserinamide acetate 19 (1.31 g, 1.30 mmol, 55%) as a colorless solid. 1H NMR (500 MHz, CDCl3): δ 8.35 (1H, s), 8.05 (1H, s), 7.71 (2H, d, J = 6.6 Hz), 7.66 (2H, s), 7.49 (4H, d, J = 6.0 Hz), 7.33 (4H, m), 7.23 – 7.15 (4H, m), 7.18 (1H, d, J = 9.0 Hz), 6.78 (1H, d, J = 8.0 Hz), 6.46 (1H, d, J = 12.0 Hz), 6.43 (2H, s), 6.39 (1H, d, J = 12.0 Hz), 6.09 (1H, s), 5.84 (1H, s), 4.68 – 4.02 (12H, m), 3.79 (6H, s), 3.62 (6H, s), 2.04 (6H, s). 13C NMR (150 MHz, CDCl3): δ 171.0, 171.0, 168.1, 167.5, 156.6, 156.4, 152.9, 152.5, 143.6, 143.6, 143.5, 143.5, 141.3, 141.2, 137.3, 132.1, 131.0, 130.4, 128.8, 127.8, 127.8, 127.1, 127.1, 125.9, 125.1, 125.1, 125.0, 120.0, 109.6, 105.9, 67.6, 67.5, 63.7, 63.6, 60.9, 56.2, 55.9, 54.8, 46.9, 34.7, 29.1, 25.3, 20.8.
(2S, 2'S)-N, N’-(3-methoxy-6-((Z)-3,4,5-trimethoxystyryl)-1,2-phenylene)bis(2-amino-3-hydroxypropanamide) (22)
Fmoc-L-diserinamide acetate 19 (0.501 g, 0.485 mmol) was dissolved in a CH2Cl2/MeOH mixture (20 mL, 1:1 ratio) to which a NaOH solution (2 M, 0.970 mL, 1.98 mmol) was added, followed by stirring (30 min). After the evaporation of solvent, the resulting oil was purified by normal phase preparative TLC (90% CH2Cl2/MeOH. 0.2% Et3N) to obtain CA1-L-diserinamide prodrug 22 (0.0650 g, 0.128 mmol, 27%, melting point range 75-80 °C) as a colorless solid. 1H NMR (600 MHz, CD3OD): δ 7.17 (1H, d, J=8.6 Hz), 6.98 (1H, d, J=8.7 Hz), 6.54 (1H, d, J=12 Hz ), 6.49 (2H, s), 6.48 (1H, d, J=13.3 Hz), 3.90 (2H, d, J=5.7 Hz), 3.89 – 3.87 (1H, m), 3.86 (3H, s), 3.85 – 3.80 (2H, m), 3.76 (1H, m), 3.71 (3H, s), 3.61 (6H, s), 1.95 (4H, s). 13C NMR (150 MHz, CD3OD): δ 180.70, 173.77, 155.83, 154.95, 139.05, 134.89, 133.67, 132.82, 130.62, 130.45, 128.38, 124.07, 111.92, 108.28, 65.55, 65.50, 61.94, 58.85, 58.54, 57.51, 57.20. HRMS: m/z: obsd 505.2289 [M+H]+, calcd for C24H33N4O8+, 505.2293. HPLC (Method C): 10.94 min.
(2S, 2'S)-((3-methoxy-6-((Z)-3,4,5-trimethoxystyryl)-1,2-phenylene)bis(azanediyl))bis(2-((tert-butoxycarbonyl)amino)-3-oxopropane-3,1-diyl) diacetate (20)
To a solution of CA1 diamine 8 (0.270 g, 0.817 mmol) in anhydrous CH2Cl2 (50 mL) was added Boc-serine (Ac)-OH·DCHA (0.875 g, 2.04 mmol), Et3N (0.290 mL, 2.04 mmol), and T3P (50% in EtOAc, 2.10 mL, 3.27 mmol). The resulting solution was stirred for 8 h at room temperature. At this point, the reaction mixture was washed with water (3 × 20 mL) and brine and dried over Na2SO4. The organic solvent was removed under reduced pressure, and the crude reaction mixture was purified by flash column chromatography (50% EtOAc/hexanes) to afford the Boc-protected-L-serinamide acetate 20 (0.251 g, 0.318 mmol, 39%) as a colorless solid. 1H NMR (500 MHz, CDCl3): δ 8.40 (1H, s), 8.21 (1H, s), 7.14 (1H, d, J=8.6 Hz), 6.75 (1H, d, J=8.6 Hz), 6.45 (1H, d, J=12.0 Hz), 6.41 (2H, s), 6.38 (1H, d, J=11.9 Hz), 5.66 (1H, s), 5.48 – 5.41 (1H, m), 4.62 (1H, s), 4.48 (2H, d, J=5.2 Hz), 4.40 (2H, d, J=7.5 Hz), 4.30 (1H, dd, J=7.0 Hz), 3.80 (3H, s, 1 Hz), 3.79 (3H, s), 3.62 (6H, s), 2.05 (3H, s), 2.02 (3H, s), 1.46 (9H, s), 1.43 (9H, s). 13C NMR (125 MHz, CDCl3): δ 170.97, 170.80, 168.40, 167.67, 155.56, 155.33, 152.82, 152.40, 137.27, 132.00, 130.91, 130.42, 128.50, 128.36, 125.95, 121.24, 109.53, 105.98, 64.00, 63.92, 60.80, 60.38, 56.13, 55.83, 54.05, 28.31, 28.28, 20.66, 20.65.
(2S, 2'S)-N, N’-(3-methoxy-6-((Z)-3,4,5-trimethoxystyryl)-1,2-phenylene)bis(2-amino-3-hydroxypropanamide) dihydrochloride (23)
Boc-L-serinamide acetate 20 (0.203 g, 0.258 mmol) was dissolved in a CH2Cl2/MeOH mixture (20 mL, 1:1 ratio) to which a NaOH solution (2 M, 0.650 mL, 1.30 mmol) was added. The reaction mixture was stirred for 30 min. After the evaporation of solvent, the resulting colorless solid (0.130 g, 0.184 mmol) in CH2Cl2 (15 mL) was reacted with a HCl solution (4 N in dioxane, 2.27 mmol) at room temperature for 2 h. Following evaporation of solvent under reduced pressure, the solid was purified by reversed phase column chromatography (RP-18 silica column, acetonitrile/water) to afford compound 20 (0.0590 g, 0.102 mmol, 40%) as an off-white solid. 1H NMR (500 MHz, CD3OD): δ 7.16 (1H, d, J = 8.7 Hz), 6.99 (1H, d, J = 8.7 Hz), 6.55 (1H, d, J = 12.0 Hz), 6.49 (2H, s), 6.48 (1H, d, J = 12.0 Hz), 4.32 (1H, dd, J = 5.5, 4.3 Hz), 4.27 (1H, dd, J = 6.1, 4.8 Hz), 4.12 (1H, dd, J = 11.5, 4.5 Hz), 4.03 (1H, dd, J = 11.5, 4.5 Hz), 4.00 (1H, dd, J = 12.0, 8.5 Hz), 3.89 (1H, dd, J = 12.0, 8.5 Hz), 3.85 (3H, s), 3.71 (3H, s), 3.61 (6H, s). 13C NMR (150 MHz, CD3OD): δ 166.1, 165.6, 153.9, 152.6, 136.8, 132.4, 131.4, 131.0, 129.0, 128.7, 125.9, 121.7, 110.3, 106.2, 60.4, 59.7, 55.4, 55.1, 55.1, 55.0, 55.0. HRMS: m/z: obsd 527.2102 [M −HCl+ Na]+, calcd for C24H32N4O8Na+, 527.2112. HPLC (Method C): 11.17 min.
Di-tert-butyl (((3-methoxy-6-(3,4,5-trimethoxystyryl)-1,2-phenylene)bis(azanediyl))bis(2-oxoethane-2,1-diyl))(Z)-dicarbamate (21)
To a solution of CA1-diamine 8 (0.300 g, 0.908 mmol) in anhydrous CH2Cl2 (50 mL) was added Boc-glycine-OH (0.398 g, 2.27 mmol), Et3N (0.270 mL, 1.91 mmol), and T3P (50% in EtOAc, 2.25 mL, 3.63 mmol). The resulting solution was stirred for 3 h at room temperature. At this point, the reaction mixture was washed with water (3 × 20 mL) and brine and dried over Na2SO4. The organic solvent was removed under reduced pressure, and the crude product was purified by flash column chromatography (50% EtOAc/hexanes) to afford the Boc-protected CA1-diglycinamide 21 (0.503 g, 0.780 mmol, 86%) as a colorless solid. 1H NMR (600 MHz, CDCl3): δ 8.21 (1H, bs), 7.96 (1H, bs), 7.14 (1H, d, J=8.6 Hz), 6.74 (1H, d, J=8.6 Hz), 6.49 (1H, d, J=12.0 Hz), 6.44 (2H, s), 6.43 (1H, d, J=11.6 Hz), 5.54 (1H, bs), 5.38 (1H, bs), 3.96 (2H, d, J=5.6 Hz), 3.83 (2H, d, J=5.8 Hz), 3.80 (6H, s), 3.63 (6H, s), 1.47 (9H, s), 1.45 (9H, s). 13C NMR (150 MHz, CDCl3): δ 169.2, 168.8, 156.6, 156.4, 153.1, 152.8, 137.4, 132.6, 131.2, 128.9, 128.7, 126.5, 121.7, 109.8, 106.2, 61.2, 56.4, 56.2, 45.2, 44.8, 28.7, 28.7.
(Z)-N,N'-(3-methoxy-6-(3,4,5-trimethoxystyryl)-1,2-phenylene)bis(2-aminoacetamide) (24)
To a solution of Boc-protected CA1-diglycinamide 21 (0.355 g, 0.550 mmol) in CH2Cl2 (50 mL) was added a HCl solution (4 N in dioxane, 2.75 mmol) at room temperature, and the mixture was stirred for 3 h. On adding diethyl ether (25 mL) to the reaction mixture, a colorless solid formed, and it was filtered through a membrane filter. After washing the solid with diethyl ether (20 mL), CA1-diglycinamide 24 (0.142 g, 0.275 mmol, 50%, melting point range 81-84 °C) was obtained as a colorless solid. 1H NMR (600 MHz, CD3OD): δ 7.18 (1H, d, J=8.3 Hz), 7.01 (1H, d, J=8.4 Hz), 6.59 (1H, d, J=11.8 Hz), 6.53 (1H, d, J=12 Hz), 6.51 (2H, s), 4.02 (4H, bs, NH2, CH2), 3.93 (2H, bs, NH2), 3.92 (2H, bs, CH2), 3.87 (3H, s), 3.73 (3H, s), 3.63 (6H, s). 13C NMR (150 MHz, CD3OD): δ 164.9, 164.7, 153.9, 152.6, 136.8, 132.5, 131.4, 131.0, 128.9, 128.7, 126.0, 121.7, 110.2, 106.1, 59.7, 55.3, 55.1, 40.7, 40.6. HRMS: m/z: obsd 467.1897 [M −HCl+ Na]+, calcd for C22H28N4O6Na+, 467.1901. HPLC (Method A): 6.06 min.
4.1.9. Synthesis of Amino Acid Prodrug Conjugates of Amino Dihydronaphthalene and Amino Benzosuberene42,43,46
6-Methoxy-5-nitro-1-tetralone (25)
To a well-stirred solution of 6-methoxy-1-tetralone (20.0 g, 114 mmol) in acetic anhydride (200 mL), a solution of HNO3 (10 mL) and AcOH (10 mL) was slowly added over the course of 1 h at 0 °C. The reaction was stirred for another 20 h and was allowed to reach room temperature. Water (20 mL) was added to the reaction mixture, and the crude product was extracted with CH2Cl2 (3 × 50 mL). The organic phase was dried with Na2SO4 and concentrated under reduced pressure. The residue was purified by flash column chromatography (20% EtOAc/hexanes) to give the desired 6-methoxy-5-nitro-tetralone 25 (8.28 g, 37.5 mmol, 33%) as a light yellow solid. 1H NMR (600 MHz, CDCl3): δ 8.18 (1H, d, J = 8.8 Hz), 7.02 (1H, d, J = 8.8 Hz), 3.97 (3H, s), 2.86 (2H, t, J = 6.5 Hz), 2.65 (2H, t, J = 6.5 Hz), 2.15 (2H, p, J = 6.5 Hz).13C NMR (150 MHz, CDCl3): δ 195.5, 154.4, 140.3, 137.3, 131.2, 126.1, 110.7, 56.8, 38.2, 24.7, 22.3.
2-Methoxy-1-nitro-5-(3,4,5-trimethoxyphenyl)-6,7,8,9-tetrahydro-5H-benzo[7]annulen-5-ol (30)
To a solution of 5-bromo-1,2,3-trimethoxybenzene (1.11 g, 4.49 mmol) in THF (20 mL) at −78 °C, n-BuLi (1.80 mL, 2.50 M) was added, and the reaction mixture was stirred for 1 h. Compound 25 (0.500 g, 2.30 mmol) in THF (3 mL) was added to the reaction mixture dropwise. The reaction mixture was stirred for 18 h and was allowed to warm to room temperature. The reaction mixture was quenched with H2O (20 mL) and extracted with CH2Cl2 (3 × 25 mL). The organic extract was dried over Na2SO4, filtered, and concentrated under reduced pressure, and the residue was purified by flash column chromatography (40% EtOAc/hexanes) to yield alcohol 30 (0.521 g, 1.34 mmol, 59% yield) as a light yellow solid. 1H NMR (500 MHz, CDCl3): δ 7.18 (1H, d, J=8.8 Hz), 6.85 (1H, d, J=8.8 Hz), 6.54 (2H, s), 3.87 (3H, s), 3.85 (3H, s), 3.81 (6H, s), 2.79 (2H, t, J=7.4 Hz), 2.12 (1H, s), 2.10 (2H, m), 2.04 (1H, m), 1.86 (1H, m). 13C NMR (125 MHz, CDCl3): δ 152.9, 150.0, 143.7, 137.0, 135.3, 132.1, 130.6, 110.8, 103.8, 74.9, 61.0, 56.5, 56.4, 40.6, 24.6, 18.6.
2-Methoxy-5-(3,4,5-trimethoxyphenyl)-7,8-dihydronaphthalen-1-amine (32)
Alcohol 30 (0.521 g, 1.34 mmol) was dissolved in AcOH (18 mL) in a round-bottom reaction flask, followed by the addition of Zn (1.75 g, 26.8 mmol). The reaction mixture was stirred for 6 h at room temperature and then filtered through Celite®, which was washed with CH2Cl2. The filtrate was concentrated under reduced pressure and the residue was purified by flash column chromatography (30% EtOAc/hexanes) to give the desired amino-dihydronaphthalene (32) (0.410 g, 1.27 mmol, 89% yield, melting point range 183-185 °C) as a dark brown solid. 1H NMR (500 MHz, CDCl3): δ 6.59 (1H, d, J = 8.5 Hz), δ 6.56 (2H, s), δ 6.51 (1H, d, J = 8.5 Hz), δ 5.92 (1H, t, J = 4.5 Hz), δ 3.89 (3H, s), δ 3.85 (3H, s), δ 3.83 (6H, s), δ 2.67 (2H, t, J = 7.5 Hz), δ 2.41 (2H, td, J = 7.5, 4.5 Hz). 13C NMR (125 MHz, DMSO-d6): δ 152.9, 146.7, 140.0, 137.1, 136.9, 134.0, 127.8, 124.2, 120.3, 115.1, 107.6, 106.2, 60.4, 56.2, 55.7, 23.0, 21.7. HRMS: m/z: obsd 342.1698 [M+H]+, calcd for C20H24NO4+, 342.1705. HPLC (Method B): 14.18 min.
5-(3'-Methoxy-2'-nitrophenyl)pent-4-enoic acid (27)
To a well-stirred solution of 3-(carboxypropyl)triphenylphosphonium bromide (21.5 g, 50.1 mmol) in THF (400 mL), potassium tert-butoxide (13.4 g, 119 mmol) was added, and the reaction mixture was stirred for 1 h at ambient temperature. The reaction mixture was cooled to 0 °C, and 3-methoxy-2-nitrobenzaldehyde (9.00 g, 47.7 mmol) in THF (100 mL) was added dropwise. After the reaction mixture was stirred for 16 h, warming from 0 °C to room temperature, the reaction was quenched with HCl (2 M, 200 mL). The organic solvent was removed by evaporation under reduced pressure, and the residue was extracted with EtOAc (4 × 300 mL). The combined organic phase was washed with brine, dried over Na2SO4, filtered, and evaporated under reduced pressure. The resulting organic crude product was purified by flash column chromatography using a pre-packed 100 g silica column [solvent A: EtOAc; solvent B: hexanes; gradient: 12%A / 88%B (1 V), 12%A / 88%B → 100%A / 0%B (15 V), 100%A / 0%B (4 CV); flow rate: 40 mL/min; monitored at 254 and 280 nm] affording a mixture of E and Z-isomers 27 (8.78 g, 34.9 mmol, 73%) as a red solid. 1H NMR (500 MHz, CDCl3): δ 7.38 (1H, t, J=8.1 Hz), 7.33 (1H, t, J=8.2 Hz), 7.12 (1H, d, J=7.8 Hz), 6.95 (1H, d, J=8.3 Hz), 6.89 (2H, d, J=7.7 Hz), 6.37 (1H, d, J=11.3 Hz), 6.31 (2H, m), 5.83 (1H, dt, J=11.5, 7.0 Hz), 3.89 (3H, s), 3.87 (3H, s), 2.53 (4H, m), 2.45 (4H, m). 13C NMR (125 MHz, CDCl3): δ 178.5, 178.4, 150.9, 150.9, 134.9, 134.5, 130.8, 130.7, 123.7, 123.6, 121.7, 118.2, 111.4, 111.0, 56.5, 56.5, 33.6, 33.3, 28.1, 23.9.
5-(3'-Methoxy-2'-nitrophenyl)pentanoic acid (28)
Anhydrous ethanol (18 mL) was added to a flask containing pentenoic acid 27 (0.750 g, 2.98 mmol) and Pd/C (10%, 0.830 g), and the solution was stirred for 10 min at ambient temperature. 1,4-Cyclohexadiene (8.90 mL, 93.8 mmol) was added to the solution, which was then stirred for another 3 h. The reaction mixture was filtered through Celite®, and the Celite® was washed with EtOAc (3 × 20 mL). The combined filtrate was removed by evaporation under reduced pressure, and the residue was purified by flash column chromatography using a pre-packed 100 g silica column [solvent A: Et Ac; solvent B: hexanes; gradient: 12%A / 88%B (1 V), 12%A / 88%B → 100%A / 0%B (12 CV), 100%A / 0%B (2 CV); flow rate: 40 mL/min; monitored at 254 and 280 nm] to afford pentanoic acid analogue 28 (0.490 g, 1.93 mmol, 65%) as a tan solid. 1H NMR (500 MHz, CDCl3): δ 7.33 (1H, t, J=8.1 z), 6.87 (2H, dd, J=8.1, 3.7 Hz), 3.87 (3H, s), 2.58 (2H, m), 2.36 (2H, m), 1.67 (4H, m). 13C NMR (125 MHz, CDCl3): δ 179.3, 150.8, 142.0, 134.8, 130.8, 121.7, 110.3, 56.5, 33.7, 30.8, 29.9, 24.4.
2-Methoxy-1-nitro-benzosuber-5-one (29)
Pentanoic acid analogue 28 (0.490 g, 1.93 mmol) was dissolved in Eaton’s reagent (14.5 mL, 7.7% P2O5 in CH3SO3H) under N2, and the reaction mixture was stirred for 16 h at ambient temperature. To the solution was added ice, which allowed to melt, and the reaction mixture was neutralized slowly with NaHCO3 (aq.) and extracted with EtOAc (3 × 20 mL). The combined organic phase was dried over Na2SO4, filtered, and removed by evaporation under reduced pressure, and the residue was purified by flash chromatography using a pre-packed 50 g silica column [solvent A: Et Ac; solvent B: hexanes; gradient: 7%A / 93%B (1 V), 7%A / 93%B → 60%A / 40%B (13 CV), 60%A / 40%B (1 CV); flow rate: 25 mL/min; monitored at 254 and 280 nm] to afford benzosuberone analogue 29 (0.340 g, 1.45 mmol, 75%) as a colorless solid. 1H NMR (500 MHz, CDCl3): δ 7.84 (1H, d, J=8.8 Hz), 6.98 (1H, d, J=8.8 Hz), 3.95 (3H, s), 2.78 (2H, m), 2.71 (2H, m), 1.91 (2H, m), 1.81 (2H, m).13C NMR (125 MHz, CDCl3): δ 203.2, 153.4, 141.5, 134.1, 132.3, 131.9, 110.4, 56.7, 40.4, 26.3, 24.5, 20.3.
2-Methoxy-1-nitro-5-(3',4',5'-trimethoxyphenyl)-benzosuber-5-ol (31)
To a well-stirred solution of 3,4,5-trimethoxyphenyl bromide (0.680 g, 2.75 mmol) in THF (25 mL) at −78 °C, n-BuLi (1.00 mL, 2.50 M in hexanes) was added, and the reaction mixture was stirred for 1 h. Benzosuberone analogue 29 (0.390 g, 1.66 mmol) in THF (5 mL) was added dropwise into the reaction mixture, which was then stirred for 18 h and allowed to warm from −78 °C to room temperature. The reaction was quenched with H2O (20 mL), and the solvent was removed under reduced pressure. The mixture was extracted with EtOAc (3 × 25 mL), and the combined organic phase was washed with brine, dried over Na2SO4, filtered, and concentrated under reduced pressure. The residue was purified by flash column chromatography using a pre-packed 50 g silica column [solvent A: EtOAc; solvent B: hexanes; gradient: 5%A / 95%B (1 CV), 5%A / 95%B → 100%A / 0%B (13 CV), 100%A / 0%B (2 CV); flow rate: 40 mL/min; monitored at 254 and 280 nm] to afford alcohol 31 (0.468 g, 1.16 mmol, 70%) as a colorless solid. 1H NMR (500 MHz, CDCl3): δ 7.68 (1H, d, J=8.7 Hz), 6.88 (1H, d, J=8.7 Hz), 6.46 (2H, s), 3.90 (3H, s), 3.84 (3H, s), 3.76 (6H, s), 2.60 (2H, m), 2.41 (1H, m), 2.37 (1H, s), 2.11 (1H, m), 1.95 (1H, m), 1.79 (2H, m), 1.53 (1H, m). 13C NMR (125 MHz, CDCl3): δ 153.3, 149.4, 142.4, 140.5, 138.6, 137.6, 133.6, 129.4, 109.3, 104.1, 79.7, 60.9, 56.3, 56.2, 40.9, 28.7, 26.4, 26.0.
1-Amino-2-methoxy-5-(3’,4’,5’-trimethoxyphenyl)-benzosuber-5-ene (33)
To a solution of alcohol 31 (0.498 g, 1.16 mmol) in AcOH (15 mL) was added Zn dust (1.52 g, 23.2 mmol), and the reaction was stirred for 6 h at ambient temperature. The reaction mixture was filtered through Celite®, which was washed with CH2Cl2, and the filtrate was concentrated under reduced pressure. The concentrated residue was neutralized with NaHCO3 and extracted with EtOAc (3 × 20 mL). The combined extracts were washed with brine, dried over Na2SO4, filtered, and removed by evaporation under reduced pressure. The crude product was purified by flash column chromatography using a pre-packed 25 g silica column [solvent A: EtOAc; solvent B: hexanes; gradient: 5%A / 95%B (1 V), 5%A / 95%B → 60%A / 40%B (13 CV), 60%A / 40%B (2 CV); flow rate: 40 mL/min; monitored at 254 and 280 nm] to afford benzosuberene 33 (KGP156, 0.350 g, 0.980 mmol, 85%, melting point range 140-145 °C) as a colorless solid. 1H NMR (500 MHz, CDCl3): δ 6.67 (1H, d, J=8.4 Hz), 6.52 (2H, s), 6.49 (1H, d, J=8.4 Hz), 6.30 (1H, t, J=7.3 Hz), 3.88 (3H, s), 3.86 (3H, s), 3.80 (6H, s), 2.59 (2H, t, J=6.9 Hz), 2.12 (2H, p, J=7.0 Hz), 1.95 (2H, q, J=7.2 Hz). 13C NMR (125 MHz, CDCl3): δ 152.9, 146.5, 143.7, 138.7, 137.4, 133.7, 132.6, 126.9, 126.4, 120.0, 107.7, 105.4, 61.1, 56.3, 55.7, 33.4, 25.8, 25.4. HRMS: m/z: obsd 356.1863 [M+H]+, calcd for C21H26NO4+, 356.1862. HPLC (Method B): 15.34 min.
(S)-2-((((9H-fluoren-9-yl)methoxy)carbonyl)amino)-3-((2-methoxy-5-(3,4,5-trimethoxyphenyl)-7,8-dihydronaphthalen-1-yl)amino)-3-oxopropyl acetate (34)
To a well-stirred solution of amino compound 32 (0.500 g, 1.45 mmol) in CH2Cl2 (30 mL), Fmoc-L-ser(Ac)-OH (0.648 g, 2.18 mmol), T3P (50% in EtOAc, 2.60 mL, 4.35 mmol), and Et3N (0.31 mL, 2.18 mmol) were added, and the reaction mixture was stirred for 17 h at room temperature. Water (30 mL) was added, and the reaction mixture was extracted with EtOAc (3 × 30 mL). The organic phase was rinsed with brine, dried over Na2SO4, and concentrated under reduced pressure. The residue was purified by flash column chromatography (35% EtOAc/hexanes) to afford the desired Fmoc-L-serinamide acetate 34 (0.695 g, 1.12 mmol, 77%) as a yellow solid. 1H NMR (500 MHz, CDCl3): δ 7.77 (2H, d, J = 7.5 Hz), 7.60 (2H, d, J = 7.5 Hz), 7.60 (1H, s), 7.40 (2H, dd, J = 7.5, 7.0 Hz), 7.33 (2H, dd, J = 7.5, 7.0 Hz), 6.98 (1H, d, J = 8.5 Hz), 6.65 (1H, d, J = 9.0 Hz), 6.56 (2H, s), 6.00 (1H, t, J = 4.5 Hz), 5.75 (1H, d, J = 6.5 Hz), 4.75 (1H, t, J = 11.5 Hz), 4.47 (4H, m), 4.23 (1H, t, J = 11.5 Hz), 3.89 (3H, s), 3.84 (6H, s), 3.79 (3H, s), 2.67 (2H, t, J = 7.5 Hz), 2.30 (2H, td, J = 7.5, 4.5 Hz), 2.11 (3H, s). 13C NMR (125 MHz, CDCl3): δ 170.77, 167.42, 152.96, 143.62, 143.55, 141.32, 139.47, 137.11, 136.55, 135.32, 128.81, 127.80, 127.11, 125.30, 125.16, 124.98, 121.79, 120.03, 120.01, 107.62, 105.78, 67.27, 64.47, 60.92, 56.15, 55.67, 54.36, 47.15, 23.99, 22.92, 20.78. HRMS: m/z: obsd 715.2625 [M+Na]+, calcd for C40H40N2O9Na+, 715.2630.
(S)-2-((((9H-fluoren-9-yl)methoxy)carbonyl)amino)-3-((3-methoxy-9-(3,4,5-trimethoxyphenyl)-6,7-dihydro-5H-benzo[7]annulen-4-yl)amino)-3-oxopropyl acetate (35)47
This compound was synthesized using the procedure as described for compound 34. Starting from amino compound 33 (0.355 g, 1.00 mmol), Fmoc-L-ser(Ac)-OH (0.553 g, 1.50 mmol), T3P (50% in EtOAc, 1.50 mL, 2.50 mmol), and Et3N (0.210 mL, 1.50 mmol) in CH2Cl2 (25 mL), the desired product 35 (0.393 g, 0.556 mmol, 56%) was obtained as a colorless solid. 1H NMR (500 MHz, CDCl3): δ 7.74 (2H, d, J = 7 Hz), 7.65 (1H, s), 7.57 (2H, d, J = 7.5 Hz), 7.38 (2H, dd, J = 7.5, 7.0 Hz), 7.27 (2H, dd, J = 7.5, 7.0 Hz), 6.97 (1H, d, J = 8.5 Hz), 6.73 (1H, d, J = 8.5 Hz), 6.49 (2H, s), 6.36 (1H, t, J = 7.5 Hz), 5.94 (1H, d, J = 6.5 Hz), 4.82 (1H, bs), 4.49 (4H, m), 4.23 (1H, t, J = 6.5 Hz), 3.86 (3H, s), 3.79 (6H, s), 3.74 (3H, s), 2.59 (2H, t, J = 7.5 Hz), 2.14 (2H, m,), 2.10 (3H, s). 1.95 (2H, m). 13C NMR (125 MHz, CDCl3): δ 170.90, 168.51, 156.24, 153.31, 152.94, 143.69, 143.60, 142.46, 141.32, 140.74, 138.38, 137.42, 133.53, 129.45, 127.84, 127.15, 125.04, 122.19, 120.04, 108.42, 105.36, 67.32, 64.61, 60.95, 56.20, 55.71, 54.26, 47.14, 33.91, 27.09, 25.63, 20.82. [Note: A portion of the experimental data for this compound was reproduced from reference 47 with permission from Elsevier.]
(S)-2-Amino-3-hydroxy-N-(2-methoxy-5-(3,4,5-trimethoxyphenyl)-7,8-dihydronaphthalen-1-yl)propanamide (36)
To a stirred solution of CH2Cl2/MeOH (6 mL, 1:1 ratio) were added Fmoc-L-serinamide acetate 34 (0.30 g, 0.43 mmol) and a NaOH solution (2 M, 0.48 mL, 0.97 mmol). After stirring for 18 h at room temperature, the solvent was evaporated under reduced pressure, and water (6 mL) was added. The solution was extracted with EtOAc (3 × 10 mL), and then the combined organic phase were rinsed with brine, dried with Na2SO4, and concentrated under reduced pressure. The residue was purified by flash column chromatography (5% MeOH/ CH2Cl2) to afford the desired serinamide 36 (0.12 g, 0.28 mmol, 65%, melting point range 176-178 °C) as a yellow solid. 1H NMR (500 MHz, CDCl3): δ 8.91 (1H, s), 6.98 (1H, d, J = 8.5 Hz), 6.65 (1H, d, J = 9.0 Hz), 6.56 (2H, s), 6.00 (1H, t, J = 4.5 Hz), 4.00 (1H, dd, J = 11, 4.5 Hz), 3.88 (3H, s), 3.84 (6H, s), 3.81 (3H, s), 3.81 (1H, dd, J = 11, 4.5 Hz), 3.69 (1H, t, J = 4.5 Hz), 2.67 (2H, t, J = 7.5 Hz), 2.30 (2H, td, J = 7.5, 4.5 Hz). 13C NMR (125 MHz, CDCl3): δ 172.6, 152.9, 152.8, 139.4, 137.0, 136.6, 135.2, 128.7, 125.1, 125.0, 122.3, 107.7, 105.7, 65.3, 60.8, 56.6, 56.1, 55.7, 23.9, 22.9. HRMS: m/z: obsd 429.2041 [M+H]+, calcd for C23H29N2O6+, 429.2026. HPLC (Method A): 7.80 min.
(S)-2-Amino-3-hydroxy-N-(3-methoxy-9-(3,4,5-trimethoxyphenyl)-6,7-dihydro-5H-benzo[7]annulen-4-yl)propanamide (37)47
This compound was synthesized using the procedure as described for compound 36. Starting from Fmoc-L-serinamide acetate 35 (0.393 g, 0.556 mmol) and a NaOH solution (2 N, 0.630 mL, 1.26 mmol) in CH2Cl2/MeOH (6 mL, 1:1 ratio), the desired product 37 (0.185 g, 0.418 mmol, 75%, melting point range 234-235 °C) was obtained as a colorless solid. 1H NMR (500 MHz, CDCl3): δ 8.73 (1H, s), 6.97 (1H, d, J = 8.5 Hz), 6.78 (1H, d, J = 8.5 Hz), 6.49 (2H, s), 6.36 (1H, t, J = 7.5 Hz), 4.04 (1H, dd, J = 11, 4.5 Hz), 3.86 (3H, s), 3.84 (6H, s), 3.80 (3H, s), 3.80 (1H, dd, J = 11, 4.5 Hz), 3.71 (1H, t, J = 4.5 Hz), 2.62 (2H, t, J = 6.5 Hz), 2.16 (2H, m), 1.98 (2H, q, J = 7.0 Hz). 13C NMR (125 MHz, CDCl3): δ 173.5, 153.1, 152.8, 142.5, 140.6, 138.3, 133.5, 129.1, 127.6, 122.5, 109.9, 108.4, 105.3, 65.9, 60.9, 56.3, 56.1, 55.8, 34.0, 27.0, 25.5. HRMS: m/z: obsd 443.2190 [M+H]+, calcd for C24H31N2O6+, 443.2177. HPLC (Method A): 8.23 min. [Note: A portion of the experimental data for this compound was reproduced from reference 47 with permission from Elsevier.]
(S)-2-Amino-3-hydroxy-N-(2-methoxy-5-(3,4,5-trimethoxyphenyl)-7,8-dihydronaphthalen-1-yl)propanamide hydrochloride (38)
Serinamide 36 (0.055 g, 0.13 mmol) was dissolved in CH2Cl2 (0.8 mL), and HCl (4 N in dioxane, 0.080 mL, 0.32 mmol) was added to the solution. After stirring for 1 h, the mixture was removed by evaporation to dryness, and the crude solid was re-crystallized using EtOAc/MeOH to afford desired serinamide salt 38 (0.015 g, 0.032 mmol, 25%, melting point range 241-242 °C) as a light yellow solid 1H NMR (500 MHz, CD3 D): δ 6.98 (1H, d, J = 8.5 Hz), 6.83 (1H, d, J = 8.5 Hz), 6.58 (2H, s), 6.01 (1H, t, J = 4.5 Hz), 4.20 (1H, dd, J = 7.0, 4.0 Hz), 4.15 (1H, dd, J = 11, 4.0 Hz), 3.97 (1H, dd, J = 11, 4.0 Hz), 3.82 (3H, s), 3.80 (6H, s), 3.79 (3H, s), 2.67 (2H, m), 2.30 (2H, m). 13C NMR (125 MHz, CD3 D): δ 166.1, 153.7, 152.9, 139.4, 136.9, 136.8, 135.6, 128.3, 125.3, 124.6, 121.6, 107.8, 105.7, 60.6, 59.7, 55.1, 55.1, 54.8, 23.2, 22.4. HRMS: m/z: obsd 429.2063 [M−Cl]+, calcd for C23H29N26O+, 429.2026. HPLC (Method A): 7.94 min.
(S)-2-Amino-3-hydroxy-N-(3-methoxy-9-(3,4,5-trimethoxyphenyl)-6,7-dihydro-5H-benzo[7]annulen-4-yl)propanamide hydrochloride (39)47
This compound was synthesized using the procedure as described for compound 38. Starting from serinamide 37 (0.10 g, 0.23 mmol) and HCl (4 N in dioxane, 0.28 mL, 1.1 mmol) in CH2Cl2/MeOH (6 mL, 1:1 ratio), the desired product 39 (0.074 g, 0.15 mmol, 67%, melting point range 260-263 °C) was obtained as a colorless solid. 1H NMR (500 MHz, CD3 D): δ 6.96 (1H, d, J = 8.5 Hz), 6.92 (1H, d, J = 8.5 Hz), 6.53 (2H, s), 6.39 (1H, t, J = 7.5 Hz), 4.26 (1H, dd, J = 7.5, 4.5 Hz), 4.19 (1H, dd, J = 11, 4.0 Hz), 3.99 (1H, dd, J = 11, 7.5 Hz), 3.82 (3H, s), 3.76 (6H, s), 3.75 (3H, s), 2.62 (2H, m), 2.16 (2H, m), 1.92 (2H, m). 13C NMR (125 MHz, CD3 D): δ 166.9, 153.9, 152.8, 142.5, 140.7, 138.4, 137.1, 133.3, 129.3, 126.9, 121.7, 108.5, 105.1, 60.7, 59.7, 55.1, 55.1, 54.8, 33.5, 26.2, 24.9. HRMS: m/z: obsd 443.2214 [M−Cl]+, calcd for C24H31N2O6+, 443.2177. HPLC (Method A): 8.32 min. [Note: A portion of the experimental data for this compound was reproduced from reference 47 with permission from Elsevier.]
(9H-fluoren-9-yl)methyl (2-((2-methoxy-5-(3,4,5-trimethoxyphenyl)-7,8-dihydronaphthalen-1-yl)amino)-2-oxoethyl)carbamate (40)
This compound was synthesized using the procedure as described for compound 34. Starting from amino compound 32 (0.500 g, 1.45 mmol), Fmoc-glycine-OH (0.648 g, 2.18 mmol), T3P (50% in EtOAc, 2.60 mL, 4.35 mmol), and Et3N (0.310 mL, 2.18 mmol) in CH2Cl2 (30 mL) with 12 h stirring, the desired product 40 (0.678 g, 1.10 mmol, 75%) was obtained as a yellow solid. 1H NMR (500 MHz, CDCl3): δ 7.75 (2H, d, J = 7.5 Hz), 7.60 (2H, d, J = 7.5 Hz), 7.46 (1H, s), 7.38 (2H, t, J = 7.5 Hz), 7.29 (2H, t, J = 7.5 Hz), 6.97 (1H, d, J = 8.5 Hz), 6.65 (1H, d, J = 9.0 Hz), 6.57 (2H, s), 5.99 (1H, t, J = 4.5 Hz), 5.61 (1H, s), 4.48 (2H, d, J = 7 Hz), 4.24 (1H, t, J = 7 Hz), 4.12 (2H, s), 3.88 (3H, s), 3.84 (6H, s), 3.76 (3H, s), 2.67 (2H, t, J = 7.5 Hz), 2.30 (2H, td, J = 7.5, 4.5 Hz). 13C NMR (125 MHz, CDCl3): δ 167.71, 156.57, 152.94, 152.63, 143.84, 143.66, 141.31, 139.47, 137.09, 136.61, 135.38, 128.80, 127.76, 127.07, 125.19, 125.00, 121.92, 120.00, 107.57, 105.80, 67.19, 60.92, 56.16, 55.65, 47.17, 44.86, 24.05, 22.94.
(9H-fluoren-9-yl)methyl (2-((3-methoxy-9-(3,4,5-trimethoxyphenyl)-6,7-dihydro-5H-benzo[7]annulen-4-yl)amino)-2-oxoethyl)carbamate (41)
This compound was synthesized using the procedure as described for compound 34. Starting from amino compound 33 (0.200 g, 0.563 mmol), Fmoc-glycine-OH (0.250 g, 0.841 mmol), T3P (50% in EtOAc, 1.07 mL, 1.68 mmol), and Et3N (0.118 mL, 0.841 mmol) in CH2Cl2 (10 mL) with 12 h stirring, the desired product 41 (0.350 g, 0.552 mmol, 98%) was obtained as a colorless solid. 1H NMR (500 MHz, CDCl3): δ 7.73 (2H, d, J = 7.04 Hz), 7.58 (2H, d, J = 7.04 Hz), 7.42 (1H, s), 7.35 (2H, t, J = 6.80 Hz), 7.27 (2H, t, J = 6.80), 6.96 (1H, d, J = 8.20 Hz), 6.73 (1H, d, J = 7.87 Hz), 6.49 (2H, s), 6.35 (1H, t, J = 6.45 Hz), 5.80 (1H, s), 4.45 (2H, m), 4.22 (1H, t, J = 6.93 Hz), 4.13 (2H, s), 3.86 (3H, s), 3.79 (6H, s), 3.76 (3H, s), 2.58 (2H, t, J = 6.93 Hz), 2.14 (2H, td, J = 6.93, 7.32 Hz), 1.95 (2H, q, J = 7.32 Hz). 13C NMR (125 MHz, CDCl3): δ 168.93, 156.84, 153.43, 152.97, 143.76, 142.50, 141.36, 140.86, 138.45, 137.46, 133.53, 129.40, 127.89, 127.82, 127.14, 125.10, 122.34, 120.05, 108.44, 105.41, 67.26, 60.99, 56.26, 55.75, 47.23, 44.93, 33.97, 27.18, 25.69.
2-Amino-N-(2-methoxy-5-(3,4,5-trimethoxyphenyl)-7,8-dihydronaphthalen-1-yl)acetamide (42)
This compound was synthesized using the procedure as described for compound 36. Starting from Fmoc-glycinamide 40 (0.477 g, 0.768 mmol) and a NaOH solution (2 M, 0.770 mL, 1.54 mmol) in CH2Cl2/MeOH (9 mL/ 1:1 ratio) with 13 h stirring, the desired product 42 (0.285 g, 0.715 mmol, 93%, melting point range 180-181 °C) was obtained as a tan solid. 1H NMR (500 MHz, CDCl3): δ 8.84 (1H, s), 6.93 (1H, d, J = 8.5 Hz), 6.63 (1H, d, J = 9.0 Hz), 6.55 (2H, s Hz), 5.95 (1H, t, J = 4.5 Hz), 3.85 (3H, s), 3.80 (6H, s), 3.77 (3H, s), 3.55 (2H, s), 2.67 (2H, t, J = 7.5 Hz), 2.26 (2H, td, J = 7.5, 4.5 Hz), 2.02 (2H, s). 13C NMR (125 MHz, CDCl3): δ 171.5, 152.8, 152.8, 139.5, 136.9, 136.7, 135.1, 128.6, 125.0, 124.7, 122.5, 107.6, 105.7, 60.8, 56.1, 55.6, 45.0, 24.1, 22.9. HRMS: m/z: obsd 399.1930 [M+H]+, calcd for C22H27N2O5+, 399.1914. HPLC (Method A): 8.10 min.
2-Amino-N-(3-methoxy-9-(3,4,5-trimethoxyphenyl)-6,7-dihydro-5H-benzo[7]annulen-4-yl)acetamide (43)
This compound was synthesized using the procedure as described for compound 36. Starting from Fmoc-glycinamide 41 (0.350 g, 0.552 mmol) and a NaOH solution (2 M, 0.560 mL, 1.12 mmol) in CH2Cl2/MeOH (6 mL, 1:1 ratio) with 13 h stirring, the desired product 43 (0.193 g, 0.468 mmol, 83%, melting point range 123-128 °C) was obtained as a colorless solid. 1H NMR (500 MHz, CD3OD): δ 6.92 (1H, d, J = 8.58 Hz), 6.88 (1H, d, J = 8.61 Hz), 6.53 (2H, s), 6.37 (1H, t, J = 7.31 Hz), 3.80 (3H, s), 3.76 (3H, s), 3.74 (6H, s), 3.53 (2H, s), 2.60 (2H, t, J = 6.81 Hz), 2.13 (2H, p, J = 6.88 Hz), 1.93 (2H, q, J = 7.11 Hz). 13C NMR (125 MHz, CD3OD): δ 175.20, 155.54, 154.20, 144.12, 142.12, 139.97, 138.54, 134.61, 130.37, 128.23, 123.91, 109.79, 106.61, 61.15, 56.57, 56.19, 45.06, 34.96, 27.69, 26.38. HRMS: m/z: obsd 413.2074 [M+H]+, calcd for C23H29N2O5+, 413.2071. HPLC (Method A): 8.45 min.
2-Amino-N-(2-methoxy-5-(3,4,5-trimethoxyphenyl)-7,8-dihydronaphthalen-1-yl)acetamide hydrochloride (44)
This compound was synthesized using the procedure as described for compound 38. Starting from glycinamide 42 (0.274 g, 0.688 mmol) and HCl (4 N in dioxane, 0.510 mL, 2.04 mmol) in MeOH (5 mL), the desired product 44 (0.171 g, 0.393 mmol, 57%, melting point range 238-239 °C) was obtained as a light yellow solid. 1H NMR (500 MHz, CD3 D): δ 6.97 (1H, d, J = 8.5 Hz), 6.82 (1H, d, J = 8.5 Hz), 6.58 (2H, s), 6.01 (1H, t, J = 4.5 Hz), 3.97 (2H, s), 3.81 (3H, s), 3.80 (6H, s), 3.79 (3H, s), 2.69 (2H, m), 2.31 (2H, m). 13C NMR (125 MHz, CD3 D): δ 165.1, 153.7, 152.9, 139.5, 136.9, 136.8, 135.6, 128.3, 125.3, 124.6, 121.6, 107.8, 105.7, 59.7, 55.1, 54.7, 40.2, 33.2, 22.47. HRMS: m/z: obsd 399.1919 [M−Cl]+, calcd for C22H27N2O5+, 399.1914. HPLC (Method A): 8.03 min.
2-Amino-N-(3-methoxy-9-(3,4,5-trimethoxyphenyl)-6,7-dihydro-5H-benzo[7]annulen-4-yl)acetamide hydrochloride (45)
This compound was synthesized using the procedure as described for compound 38. Starting from glycinamide 43 (0.150 g, 0.364 mmol) and HCl (4 N in dioxane, 0.450 mL, 1.80 mmol) in MeOH/CH2Cl2 (4 mL, 1:1 ratio), the desired product 45 (0.0847 g, 0.189 mmol, 53%, melting point range 274-276 °C) was obtained as a light yellow solid. 1H NMR (500 MHz, CD3OD): δ 6.98 (1H, d, J = 8.59 Hz), 6.94 (1H, d, J = 8.62 Hz), 6.54 (2H, s), 6.41 (1H, t, J = 7.31 Hz), 3.99 (2H, s), 3.84 (3H, s), 3.77 (3H, s), 3.77 (6H, s), 2.64 (2H, t, J = 6.81 Hz), 2.16 (2H, p, J = 6.72 Hz), 1.95 (2H, q, J = 7.13 Hz). 13C NMR (125 MHz, CD3OD): δ 167.39, 155.48, 154.25, 144.05, 142.07, 139.85, 138.63, 134.75, 130.80, 128.30, 123.13, 109.93, 106.63, 61.17, 56.59, 56.24, 41.63, 35.01, 27.70, 26.35. HRMS: m/z: obsd 413.2074 [M−Cl]+, calcd for C23H29N2O5+, 413.2071. HPLC (Method A): 8.47 min.
4.2. Biological Evaluations
4.2.1. Cell Culture and SRB Cytotoxicity Assay16,82–84
Three cancer cell lines ( DU-145, prostate; SK-OV-3, ovarian; and NCI-H460, lung cancer) were grown and passaged using DMEM media supplemented with 10% FBS (Gibco One Shot®) and 1% gentamycin sulfate (Teknova, Hollister, CA). Cells were maintained at 37 °C in a humidified atmosphere of 5% CO2, up to passage 15 for use in these experiments. The sulforhodamine B (SRB) assay was used to assess inhibition of human cell line growth as previously described.16,82–84 Briefly, cancer cells were plated at 7500 cells/well using DMEM supplemented with 5% FBS and 1% gentamycin sulfate in 96-well plates and incubated for 24 h. Subsequently, 10-fold serial dilutions of the compounds to be tested were then added to the wells. After 48 h, the cells were fixed with 10% trichloroacetic acid (final concentration), stained with sulforhodamine B (Acid Red 52) (TKI, Tokyo), read at 540 nm, and normalized at 630 nm with an automated Biotek Elx800 plate reader (Biotek, Winooski, VT). A growth inhibition of 50% (GI50 or the drug concentration causing a 50% reduction in the net protein staining relative to controls) was calculated from optical density data with Excel software.
4.2.2. Inhibition of Tubulin Polymerization85
Tubulin assembly experiments were performed using 0.25 mL reaction mixtures (final volume),85 which contained 1.0 mg/mL (10 μM) purified bovine brain tubulin, 0.8 M monosodium glutamate (pH 6.6 in a 2 M stock solution) 4% (v/v) dimethyl sulfoxide, 0.4 mM GTP, and varying compound concentrations. Initially, all components except GTP were preincuabted for 15 min at 30 °C in 0.24 mL. After chilling the mixtures on ice, 10 μL of 10 mM GTP was added. The reaction mixtures were placed in cuvettes held at 0 °C in Beckman DU-7400 and DU-7500 spectrophotometers equipped with electronic temperature controllers. The temperature was jumped to 30 °C over about 30 s, and polymerization was followed turbidimetrically at 350 nM for 20 min. Each reaction set included a reaction mixture without compound, and the IC50 was defined as the compound concentration that inhibited extent of assembly by 50% after 20 min at 30 °C.
4.2.3. Colchicine Binding Assay86
Inhibition of [3H]colchicine binding to tubulin was determined using 100 μL reaction mixtures, each containing 1.0 μM tubulin, 5.0 μM [3H]colchicine (from Perkin-Elmer), 5% (v/v) dimethyl sulfoxide, potential inhibitors at 1.0 or 5.0 μM and components demonstrated to stabilize the colchicine binding activity of tubulin86 (1.0 M monosodium glutamate [adjusted to pH 6.6 with HCl in a 2.0 M stock solution], 0.5 mg/mL bovine serum albumin, 0.1 M glucose-1-phosphate, 1.0 mM MgCl2, and 1.0 mM GTP). Incubation was for 10 min at 37 °C, a time point at which the binding reaction in the control is 40-60% complete. Reactions were stopped by adding 2.0 mL of ice-cold water and placing the samples on ice. Each sample was poured onto a stack of two DEAE-cellulose filters, followed immediately by 6 mL of ice-cold water, and the water was aspirated under reduced vacuum. The filters were washed three times with 2 mL of water and, following removal of excess water under a strong vacuum, placed into vials containing 5 mL of Biosafe II scintillation cocktail. Samples were counted the next day in a Beckman scintillation counter. Samples with potential inhibitors were compared to controls with no inhibitor to determine percent inhibition. All samples were corrected for the amount of colchicine that bound to the filters in the absence of tubulin.
4.2.4. In Vitro LAP Cleavage Assay
LAP (from porcine kidney microsomes) was purchased from Sigma-Aldrich or Calzyme Laboratories Inc, and its activity (enzyme units) was determined with 24 mM L-leucine p-nitroanilide as substrate in 50 mM sodium phosphate buffer (pH 7.2) at 37 °C.5 In preliminary experiments, the extent of cleavage of individual amino acid prodrug conjugates were determined. In a general procedure, LAP (appropriate amount of units) was pre-incubated in 50 mM sodium phosphate buffer (pH 7.2) at 37 °C. The enzymatic reaction was initiated by the addition of substrate. After varying periods of incubation, a small portion of the reaction mixture was quenched by the addition of acetonitrile on ice and centrifuged. The resulting supernatant was diluted by HPLC solvent (acetonitrile/water) and filtered through a 0.2 μm nylon syringe filter and analyzed by HPLC (Agilent Technologies 1200 series) with an Agilent ZORBAX Eclipse XDB-18 column (4.6 × 150 mm, 5 μm). HPL operating conditions were: mobile phase, acetonitrile/water containing 0.05% TFA, flow rate, 1.0 mL/min; column temperature, 22 °C; diode array UV detector. For rate studies, aliquots were withdrawn from incubation mixtures at different time points (1-15 min), and they were immediately quenched with acetonitrile and analyzed by HPLC (see supplemental data for more information). Standard calibration curves were used to determine the concentration of individual amino acid prodrug conjugates and their parent compounds.87
4.2.5. In Vitro HUVEC Tubule Disruption Assay
A layer of MatrigelTM (9.5 mg/mL) was plated manually into each well of a 24-well plate, which was then placed into an incubator at 37 ° C for 60 min prior to cell plating. HUVECs (124,000 cells suspended in 300 μL of M200 growth factors) were plated into 24 wells and allowed to incubate at 37 ° C for 16 h. The incubated HUVECs were treated with varying concentrations of inhibitors (0.01 μM, 0.1 μM, 1.0 μM), and a photographic record was obtained (9 fields/well, 40x objective).
4.2.5. In Vivo Tumor Model78
Human breast cancer cells, MDA-MB-231(ATCC), were transfected with a lentivirus containing a firefly luciferase reporter. Induction of tumors was carried out by injecting 106 cells mixed with 30% MatrigelTM (BD Biosciences, San Jose, CA) into the mammary fat pads of female SCID mice (UTSW breeding colony). Tumors were allowed to grow to about 5 mm in diameter, determined by calipers, before BLI and assessment of VDAs. All animal procedures were approved by the University of Texas Southwestern Medical Center Institutional Animal Care and Use Committee.
4.2.6. In Vivo Bioluminescence Imaging (BLI)79
BLI was performed as described previously.79 Briefly, anesthetized, tumor bearing mice (O2, 2% isoflurane, Henry Schein Inc., Melville, NY) were injected subcutaneously in the fore-back neck region with 80 μL of a solution of luciferase substrate, D-luciferin (sodium salt, 120 mg/kg, in saline, Gold Biotechnology, St. Louis, MO). Mice were maintained under anesthesia (2% isoflurane in oxygen, 1 dm3/min,), while baseline BLI was performed using a Xenogen IVIS® Spectrum (Perkin-Elmer, Alameda, CA). A series of BLI images was collected over 35 min using the following settings: auto exposure time, f-stop = 2, Field of view = D, binning = 4 (medium). Light intensity-time curves obtained from these images were analyzed using Living Image® software. Mice were injected intraperitoneally with either 120 μL of saline (vehicle), CA4P (provided by OXiGENE at 120 mg/kg in saline as used previously9) compound 10 (80 mg/kg), or compound 44 (5, 10, 15, or 20 mg/kg) in saline immediately after baseline BLI. Bioluminescence imaging was repeated, with new luciferin injections 2 and/or 4 and 24 h later. Dosing with 20 and 30 mg/kg was repeated in a separate cohort of mice. Analysis of variance was used to compare the maximum BLI light intensity observed on each occasion with respect to drug administration. Time-point and significance assessed using Fisher’s PLSD (protected least squares difference) using Statview software with p<0.05 considered significant.
Supplementary Material
Acknowledgements
The authors are grateful to the National Cancer Institute of the National Institutes of Health (Grant No. 5R01CA140674 to K.G.P, M.L.T, and R.P.M), the Cancer Prevention and Research Institute of Texas (CPRIT, Grant No. RP140399 to K.G.P, M.L.T, and R.P.M), and OXiGENE, Inc. (grant to K.G.P. and M.L.T.) for their financial support of this project. The content is solely the responsibility of the authors and does not necessarily reflect the official views of the National Institutes of Health. The authors also thank Dr. Michelle Nemec (Director) for the use of the shared Molecular Biosciences Center at Baylor University and Dr. Alejandro Ramirez (Mass Spectrometry Core Facility, Baylor University). The authors are grateful to Mr. Jarrod Tunnell (Baylor University) for his contributions to the synthesis of an advanced intermediate, and to Jeni Gerberich (UTSW) for valuable technical assistance. Imaging (at UTSW) was facilitated with the assistance of Resources of the Harold C. Simmons Cancer Center supported through an National Institutes of Health National Cancer Institute Cancer Center Support Grant [Grant 1P30 CA142543], specifically, the Southwestern Small Animal Imaging Resource, and Live Cell Imaging Resource. The IVIS Spectrum was purchased with support of 1S10RR024757.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Data
Supplementary data including the synthesis of AVE8062, characterization data (1H NMR, 13C NMR, 31P NMR, HPLC, HRMS) for final compounds and intermediates (1H NMR, 13C NMR), HPLC chromatograms, calibration curves and rate studies related to LAP enzymatic assays, and aqueous solubility data for representative compounds associated with this article are available online.
References
- 1.Tozer GM, Kanthou C, Baguley BC. Nat. Rev. Cancer. 2005;5:423–435. doi: 10.1038/nrc1628. [DOI] [PubMed] [Google Scholar]
- 2.Baluk P, Hashizume H, McDonald DM. Curr. Opin. Genet. Dev. 2005;15:102–111. doi: 10.1016/j.gde.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 3.Kanthou C, Tozer GM. Expert Opin. Ther. Targets. 2007;11:1443–1457. doi: 10.1517/14728222.11.11.1443. [DOI] [PubMed] [Google Scholar]
- 4.Denekamp J. Br. J. Cancer. 1982;45:136–139. doi: 10.1038/bjc.1982.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denekamp J. Br. J. Radiol. 1993;66:181–196. doi: 10.1259/0007-1285-66-783-181. [DOI] [PubMed] [Google Scholar]
- 6.Pinney KG, Jelinek C, Edvardsen K, Chaplin DJ, Pettit GR. In: Anticancer Agents from Natural Products. Kingston D, Newman D, Cragg G, editors. CRC Press, Taylor and Francis Group; Boca Raton, FL: 2005. pp. 23–46. [Google Scholar]
- 7.Siemann DW. Cancer Treat. Rev. 2011;37:63–74. doi: 10.1016/j.ctrv.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tozer GM, Kanthou C, Parkins CS, Hill SA. Int. J. Exp. Pathol. 2002;83:21–38. doi: 10.1046/j.1365-2613.2002.00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mason RP, Zhao D, Liu L, Trawick ML, Pinney KG. Integr. Biol. 2011;3:375–387. doi: 10.1039/c0ib00135j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pettit GR, Singh SB, Niven ML, Hamel E, Schmidt JM. J. Nat. Prod. 1987;50:119–131. doi: 10.1021/np50049a016. [DOI] [PubMed] [Google Scholar]
- 11.Pettit GR, Singh SB, Hamel E, Lin CM, Alberts DS, Garcia-Kendall G. Experientia. 1989;45:209–211. doi: 10.1007/BF01954881. [DOI] [PubMed] [Google Scholar]
- 12.Pettit GR, Temple C, Jr, Narayanan VL, Varma R, Simpson MJ, Boyd MR, Rener GA, Bansal N. Anticancer. Drug Des. 1995;10:299–309. [PubMed] [Google Scholar]
- 13.Pettit GR, Singh SB, Boyd MR, Hamel E, Pettit RK, Schmidt JM, Hogan F. J. Med. Chem. 1995;38:1666–1672. doi: 10.1021/jm00010a011. [DOI] [PubMed] [Google Scholar]
- 14.Pettit GR, Grealish MP, Herald DL, Boyd MR, Hamel E, Pettit RK. J. Med. Chem. 2000;43:2731–2737. doi: 10.1021/jm000045a. [DOI] [PubMed] [Google Scholar]
- 15.Monk KA, Siles R, Hadimani MB, Mugabe BE, Ackley JF, Studerus SW, Edvardsen K, Trawick ML, Garner CM, Rhodes MR, Pettit GR, Pinney KG. Bioorg. Med. Chem. 2006;14:3231–3244. doi: 10.1016/j.bmc.2005.12.033. [DOI] [PubMed] [Google Scholar]
- 16.Siles R, Ackley JF, Hadimani MB, Hall JJ, Mugabe BE, Guddneppanavar R, Monk KA, Chapuis J-C, Pettit GR, Chaplin DJ, Edvardsen K, Trawick ML, Garner CM, Pinney KG. J. Nat. Prod. 2008;71:313–320. doi: 10.1021/np070377j. [DOI] [PubMed] [Google Scholar]
- 17.Pinney KG, Pettit GR, Trawick ML, Jelinek C, Chaplin DJ. In: Anticancer Agents from Natural Products. 2nd Kingston D, Newman D, Cragg G, editors. CRC Press, Taylor and Francis Group; Boca Raton, FL: 2012. pp. 27–63. [Google Scholar]
- 18.Lippert JW. Bioorg. Med. Chem. 2007;15:605–615. doi: 10.1016/j.bmc.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 19.Patterson DM, Rustin GJS. Clin. Oncol. 2007;19:443–456. doi: 10.1016/j.clon.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 20.Dark GG, Hill SA, Prise VE, Tozer GM, Pettit GR, Chaplin DJ. Cancer Res. 1997;57:1829–1834. [PubMed] [Google Scholar]
- 21.Pettit GR, Rhodes MR. Anticancer. Drug Des. 1998;13:183–191. [PubMed] [Google Scholar]
- 22.Kirwan IG, Loadman PM, Swaine DJ, Anthoney DA, Pettit GR, Lippert JW, Shnyder SD, Cooper PA, Bibby MC. Clin. Cancer Res. 2004;10:1446–1453. doi: 10.1158/1078-0432.ccr-0518-03. [DOI] [PubMed] [Google Scholar]
- 23.Cooney MM, Ortiz J, Bukowski RM, Remick SC. Curr. Oncol. Rep. 2005;7:90–95. doi: 10.1007/s11912-005-0033-x. [DOI] [PubMed] [Google Scholar]
- 24.Chaplin DJ, Horsman MR, Siemann DW. Curr. Invest. Drugs. 2006;7:522–528. [PubMed] [Google Scholar]
- 25.Baguley BC, McKeage MJ. Clin. Investig. 2012;2:985–993. [Google Scholar]
- 26.Kretzschmann VK, Fürst R. Phytochem. Rev. 2014;13:191–206. [Google Scholar]
- 27.Liu P, Qin Y, Wu L, Yang S, Li N, Wang H, Xu H, Sun K, Zhang S, Han X, Sun Y, Shi Y. Anticancer. Drugs. 2014;25:462–471. doi: 10.1097/CAD.0000000000000070. [DOI] [PubMed] [Google Scholar]
- 28.Jordan MA, Wilson L. Nat. Rev. Cancer. 2004;4:253–265. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 29.Folkes LK, Christlieb M, Madej E, Stratford MRL, Wardman P. Chem. Res. Toxicol. 2007;20:1885–1894. doi: 10.1021/tx7002195. [DOI] [PubMed] [Google Scholar]
- 30.Rice L, Pampo C, Lepler S, Rojiani AM, Siemann DW. Microvasc. Res. 2011;81:44–51. doi: 10.1016/j.mvr.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patterson DM, Zweifel M, Middleton MR, Price PM, Folkes LK, Stratford MRL, Ross P, Halford S, Peters J, Balkissoon J, Chaplin DJ, Padhani AR, Rustin GJS. Clin. Cancer Res. 2012;18:1415–1425. doi: 10.1158/1078-0432.CCR-11-2414. [DOI] [PubMed] [Google Scholar]
- 32.Chaplin DJ, Garner CM, Kane RR, Pinney KG, Prezioso JA. From PCT Int. Appl. 2003 WO 2003035008 A2. [Google Scholar]
- 33.Hadimani MB, Hua J, Jonklaas MD, Kessler RJ, Sheng Y, Olivares A, Tanpure RP, Weiser A, Zhang J, Edvardsen K, Kane RR, Pinney KG. Bioorg. Med. Chem. Lett. 2003;13:1505–1508. doi: 10.1016/s0960-894x(03)00206-3. [DOI] [PubMed] [Google Scholar]
- 34.Chang J-Y, Yang M-F, Chang C-Y, Chen C-M, Kuo C-C, Liou J-P. J. Med. Chem. 2006;49:6412–6415. doi: 10.1021/jm060616k. [DOI] [PubMed] [Google Scholar]
- 35.Pettit GR, Anderson CR, Herald DL, Jung MK, Lee DJ, Hamel E, Pettit RK. J. Med. Chem. 2003;46:525–531. doi: 10.1021/jm020204l. [DOI] [PubMed] [Google Scholar]
- 36.Ohsumi K, Nakagawa R, Fukuda Y, Hatanaka T, Morinaga Y, Nihei Y, Ohishi K, Suga Y, Akiyama Y, Tsuji T. J. Med. Chem. 1998;41:3022–3032. doi: 10.1021/jm980101w. [DOI] [PubMed] [Google Scholar]
- 37.Pinney KG, Mejia MP, Villalobos VM, Rosenquist BE, Pettit GR, Verdier-Pinard P, Hamel E. Bioorg. Med. Chem. 2000;8:2417–2425. doi: 10.1016/s0968-0896(00)00176-0. [DOI] [PubMed] [Google Scholar]
- 38.Hadimani MB, MacDonough MT, Ghatak A, Strecker TE, Lopez R, Sriram M, Nguyen BL, Hall JJ, Kessler RJ, Shirali AR, Liu L, Garner CM, Pettit GR, Hamel E, Chaplin DJ, Mason RP, Trawick ML, Pinney KG. J. Nat. Prod. 2013;76:1668–1678. doi: 10.1021/np400374w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hsieh H, Liou J, Mahindroo N. Curr. Pharm. Des. 2005;11:1655–1677. doi: 10.2174/1381612053764751. [DOI] [PubMed] [Google Scholar]
- 40.Tron GC, Pirali T, Sorba G, Pagliai F, Busacca S, Genazzani AA. J. Med. Chem. 2006;49:3033–3044. doi: 10.1021/jm0512903. [DOI] [PubMed] [Google Scholar]
- 41.Alloatti D, Giannini G, Cabri W, Lustrati I, Marzi M, Ciacci A, Gallo G, Tinti MO, Marcellini M, Riccioni T, Guglielmi MB, Carminati P, Pisano C. J. Med. Chem. 2008;51:2708–2721. doi: 10.1021/jm701362m. [DOI] [PubMed] [Google Scholar]
- 42.Pinney KG, Mocharla VP, Chen Z, Garner CM, Ghatak A, Hadimani MB, Kessler J, Dorsey JM. From PCT Int. Appl. 2001 WO 2001068654 A2. [Google Scholar]
- 43.Pinney KG, Mocharla VP, Chen Z, Garner CM, Ghatak A, Hadimani MB, Kessler J, Dorsey JM, Edvardsen K, Chaplin DJ, Prezioso J, Ghatak UR. From U.S. Pat. Appl. Publ. 2004 US 20040043969 A1. [Google Scholar]
- 44.Hadimani MB. Studies Toward the Discovery of New Classes of Privileged Molecules as Colchicine-Site Binding Ligands for Tubulin: Structure-Based Design, Synthesis and Bioactivity of Small Ligands Targeted at Tumor Vasculature. Ph. D. dissertation, Baylor University; Waco, TX: 2004. [Google Scholar]
- 45.Sriram M, Hall JJ, Grohmann NC, Strecker TE, Wootton T, Franken A, Trawick ML, Pinney KG. Bioorg. Med. Chem. 2008;16:8161–8171. doi: 10.1016/j.bmc.2008.07.050. [DOI] [PubMed] [Google Scholar]
- 46.Tanpure RP, George CS, Sriram M, Strecker TE, Tidmore JK, Hamel E, Charlton-Sevcik AK, Chaplin DJ, Trawick ML, Pinney KG. MedChemComm. 2012;3:720–724. doi: 10.1039/C2MD00318J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tanpure RP, George CS, Strecker TE, Devkota L, Tidmore JK, Lin C-M, Herdman CA, MacDonough MT, Sriram M, Chaplin DJ, Trawick ML, Pinney KG. Bioorg. Med. Chem. 2013;21:8019–8032. doi: 10.1016/j.bmc.2013.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen Z, Maderna A, Sukuru SCK, Wagenaar M, O’Donnell CJ, Lam M-H, Musto S, Loganzo F. Bioorg. Med. Chem. Lett. 2013;23:6688–6694. doi: 10.1016/j.bmcl.2013.10.039. [DOI] [PubMed] [Google Scholar]
- 49.Rasolofonjatovo E, Provot O, Hamze A, Rodrigo J, Bignon J, Wdzieczak-Bakala J, Desravines D, Dubois J, Brion J-D, Alami M. Eur. J. Med. Chem. 2012;52:22–32. doi: 10.1016/j.ejmech.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 50.Pochopin NL, Charman WN, Stella VJ. Int. J. Pharm. 1995;121:157–167. [Google Scholar]
- 51.Vig BS, Huttunen KM, Laine K, Rautio J. Adv. Drug Deliv. Rev. 2013;65:1370–1385. doi: 10.1016/j.addr.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 52.Mittal S, Song X, Vig BS, Amidon GL. Pharm. Res. 2007;24:1290–1298. doi: 10.1007/s11095-007-9249-9. [DOI] [PubMed] [Google Scholar]
- 53.Ohsumi K, Hatanaka T, Nakagawa R, Fukuda Y, Morinaga Y, Suga Y, Nihei Y, Ohishi K, Akiyama Y, Tsuji T. Anticancer. Drug Des. 1999;14:539–548. [PubMed] [Google Scholar]
- 54.Delmonte A, Sessa C. Expert Opin. Investig. Drugs. 2009;18:1541–1548. doi: 10.1517/13543780903213697. [DOI] [PubMed] [Google Scholar]
- 55.Blay J-Y, Pápai Z, Tolcher AW, Italiano A, Cupissol D, López-Pousa A, Chawla SP, Bompas E, Babovic N, Penel N, Isambert N, Staddon AP, Saâda-Bouzid E, Santoro A, Franke FA, Cohen P, Le-Guennec S, Demetri GD. Lancet Oncol. 2015;16:531–540. doi: 10.1016/S1470-2045(15)70102-6. [DOI] [PubMed] [Google Scholar]
- 56.Hori K, Saito S. Br. J. Cancer. 2003;89:1334–1344. doi: 10.1038/sj.bjc.6601261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Subbiah IM, Lenihan DJ, Tsimberidou AM. The Oncologist. 2011;16:1120–1130. doi: 10.1634/theoncologist.2010-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Linderstrom-Lang K. Physiol. Chem. 1929;182:151–174. [Google Scholar]
- 59.Taylor A, Volz KW, Lipscomb WN, Takemoto LJ. J. Biol. Chem. 1984;259:14757–14761. [PubMed] [Google Scholar]
- 60.Sanderink GJ, Artur Y, Seist G. J. Clin. Chem. Clin. Biochem. 1988;26:795–807. doi: 10.1515/cclm.1988.26.12.795. [DOI] [PubMed] [Google Scholar]
- 61.Niinobe M, Hitomi Y, Fujii S. J. Biochem. 1980;87:779–783. doi: 10.1093/oxfordjournals.jbchem.a132807. [DOI] [PubMed] [Google Scholar]
- 62.Fleisher GA, Pankow M, Warmka C. Clin. Chim. Acta. 1964;9:259–268. doi: 10.1016/0009-8981(64)90105-6. [DOI] [PubMed] [Google Scholar]
- 63.Matsui M, Fowler JH, Walling LL. Biol. Chem. 2006;387:1535–1544. doi: 10.1515/BC.2006.191. [DOI] [PubMed] [Google Scholar]
- 64.Burley SK, David PR, Lipscomb WN. Proc. Natl. Acad. Sci. U.S.A. 1991;88:6916–6920. doi: 10.1073/pnas.88.16.6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kanno T, Maekawa M, Kohno H, Sudo K. Am. J. Clin. Pathol. 1984;82:700–705. doi: 10.1093/ajcp/82.6.700. [DOI] [PubMed] [Google Scholar]
- 66.Kohno H, Kanda S, Kanno T. J. Biol. Chem. 1986;261:10744–10748. [PubMed] [Google Scholar]
- 67.Mericas G, Anagnostou E, Hadziyannis St., Kakari S. J. Clin. Path. 1964;17:52–55. doi: 10.1136/jcp.17.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Miller AL, Worsley L. Br. Med. J. 1960;2:1419–1422. doi: 10.1136/bmj.2.5210.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Garg LN, Yadav SP, Lal H. J. Laryngol. Otol. 1994;108:660–662. doi: 10.1017/s0022215100127756. [DOI] [PubMed] [Google Scholar]
- 70.Pinney KG. Vascular-Targeted Therapies in Oncology. John Wiley & Sons, Ltd; 2006. pp. 95–121. [Google Scholar]
- 71.Chen Z. Synthesis of Tubulin Binding Ligands Incorporating Dihydronaphthalene, Indene, and Benzo[b]thiophene Molecular Frameworks and Preparation of Their Corresponding Phosphorus Containing Prodrugs as Tumor Specific Vascular Targeting Agents. MS dissertation, Baylor University; Waco, TX: 2000. [Google Scholar]
- 72.Ninomiya M, Tanaka K, Tsuchida Y, Muto Y, Koketsu M, Watanabe K. J. Med. Chem. 2011;54:1529–1536. doi: 10.1021/jm1015457. [DOI] [PubMed] [Google Scholar]
- 73.Han G, Tamaki M, Hruby VJ. J. Pept. Res. 2001;58:338–341. doi: 10.1034/j.1399-3011.2001.00935.x. [DOI] [PubMed] [Google Scholar]
- 74.Tanpure RP, Nguyen BL, Strecker TE, Aguirre S, Sharma S, Chaplin DJ, Siim BG, Hamel E, Lippert JW, Pettit GR, Trawick ML, Pinney KG. J. Nat. Prod. 2011;74:1568–1574. doi: 10.1021/np200104t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Smith EL, Spackman H, Polglase WJ. J. Biol. Chem. 1952;199:801–817. [PubMed] [Google Scholar]
- 76.Smith EL, Spackman H. J. Biol. Chem. 1955;212:271–299. [PubMed] [Google Scholar]
- 77.Devkota L, Strecker TE, Tunnell JP, Tidmore JK, Lopez R, Li L, Mason RP, Trawick ML, Pinney KG. Abstract of Papers, 246th ACS National Meeting & Exposition of the American Chemical Society, Indianapolis, IN. American Chemical Society; Washington, DC: 2013. MEDI-100. [Google Scholar]
- 78.Zhao D, Richer E, Antich PP, Mason RP. FASEB J. 2008;22:2445–2451. doi: 10.1096/fj.07-103713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu L, Beck H, Wang X, Hsieh H-P, Mason RP, Liu X. PLoS ONE. 2012;7:e43314. doi: 10.1371/journal.pone.0043314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tanpure RP, Strecker TE, Chaplin DJ, Siim BG, Trawick ML, Pinney KG. J. Nat. Prod. 2010;73:1093–1101. doi: 10.1021/np100108e. [DOI] [PubMed] [Google Scholar]
- 81.Cheguillaume A, Salaün A, Sinbandhit S, Potel M, Gall P, Baudy-Floc’h M, Le Grel P. J. Org. Chem. 2001;66:4923–4929. doi: 10.1021/jo001500d. [DOI] [PubMed] [Google Scholar]
- 82.Monks A, Scudiero D, Skehan P, Shoemaker R, Paull K, Vistica D, Hose C, Langley J, Cronise P, Vaigro-Wolff A, Gray-Goodrich M, Campbell H, Mayo J, Boyd M. J. Natl. Cancer Inst. 1991;83:757–766. doi: 10.1093/jnci/83.11.757. [DOI] [PubMed] [Google Scholar]
- 83.Vichai V, Kirtikara K. Nat. Protoc. 2006;1:1112–1116. doi: 10.1038/nprot.2006.179. [DOI] [PubMed] [Google Scholar]
- 84.Voigt W. Methods Mol. Med. 2005;110:39–48. doi: 10.1385/1-59259-869-2:039. [DOI] [PubMed] [Google Scholar]
- 85.Hamel E. Cell Biochem. Biophys. 2003;38:1–21. doi: 10.1385/CBB:38:1:1. [DOI] [PubMed] [Google Scholar]
- 86.Hamel E, Lin CM. Biochim. Biophys. Acta. 1981;675:226–231. doi: 10.1016/0304-4165(81)90231-2. [DOI] [PubMed] [Google Scholar]
- 87.Pfleiderer G. Methods in Enzymology. 1970;19:514–521. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.