Abstract
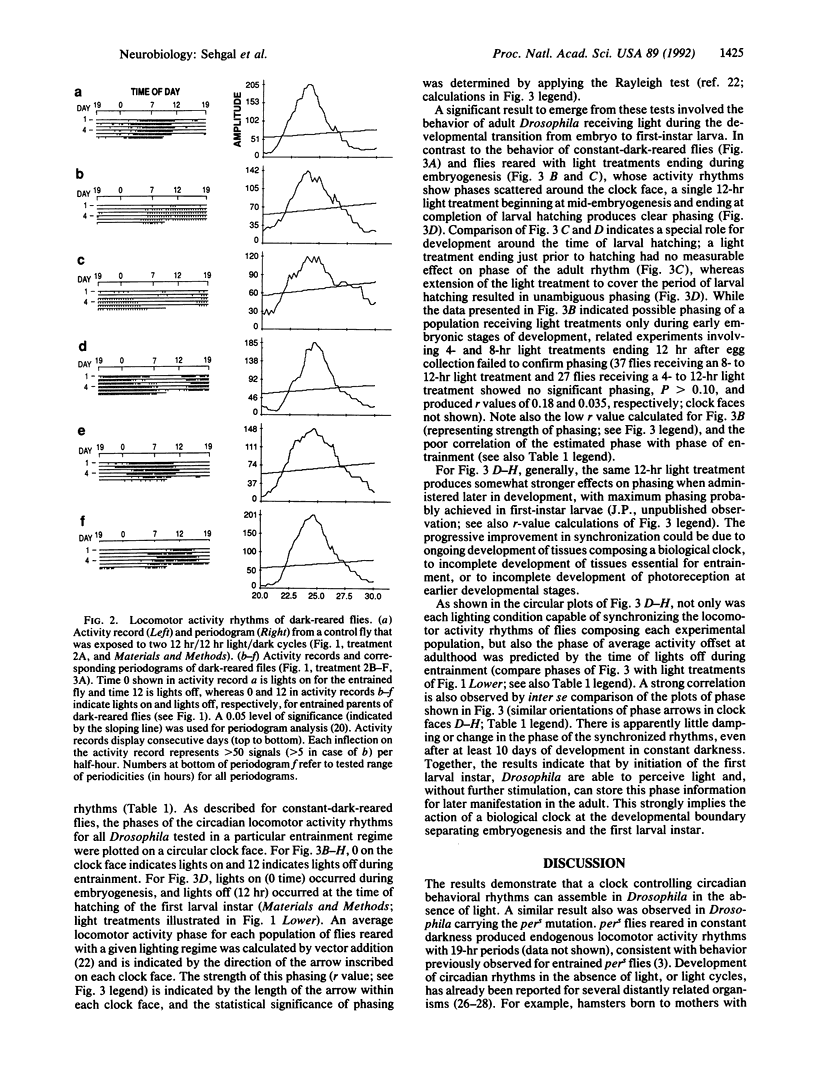
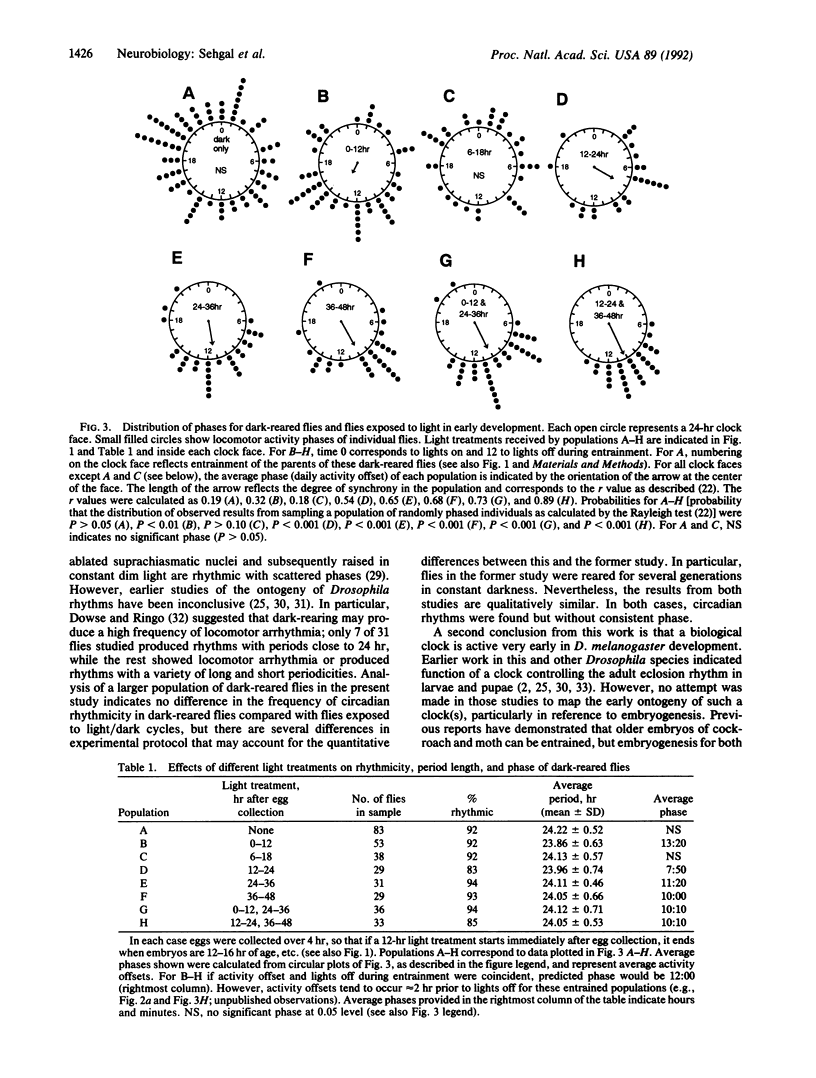
Drosophila melanogaster born and reared in constant darkness exhibit circadian locomotor activity rhythms as adults. However, the rhythms of the individual flies composing these populations are not synchronized with one another. This lack of synchrony is evident in populations of flies commencing development at the same time, indicating that a biological clock controlling circadian rhythmicity in Drosophila begins to function without a requirement for light and without a developmentally imparted phase. It is possible to synchronize the phases of rhythms produced by dark-reared flies with light treatments ending as early as the developmental transition from embryo to first-instar larva: Light treatments occurring at developmental times preceding hatching of the first-instar larva fail to synchronize adult locomotor activity rhythms, while treatments ending at completion of larval hatching entrain these rhythms. The synchronized rhythmic behavior of adult flies receiving such light treatments suggests that a clock controlling circadian rhythms may function continuously from the time of larval hatching to adulthood.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASCHOFF J. Exogenous and endogenous components in circadian rhythms. Cold Spring Harb Symp Quant Biol. 1960;25:11–28. doi: 10.1101/sqb.1960.025.01.004. [DOI] [PubMed] [Google Scholar]
- BROWMAN L. G. Artificial sixteen-hour day activity rhythms in the white rat. Am J Physiol. 1952 Mar;168(3):694–697. doi: 10.1152/ajplegacy.1952.168.3.694. [DOI] [PubMed] [Google Scholar]
- Bargiello T. A., Saez L., Baylies M. K., Gasic G., Young M. W., Spray D. C. The Drosophila clock gene per affects intercellular junctional communication. Nature. 1987 Aug 20;328(6132):686–691. doi: 10.1038/328686a0. [DOI] [PubMed] [Google Scholar]
- Bargiello T. A., Young M. W. Molecular genetics of a biological clock in Drosophila. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2142–2146. doi: 10.1073/pnas.81.7.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylies M. K., Bargiello T. A., Jackson F. R., Young M. W. Changes in abundance or structure of the per gene product can alter periodicity of the Drosophila clock. 1987 Mar 26-Apr 1Nature. 326(6111):390–392. doi: 10.1038/326390a0. [DOI] [PubMed] [Google Scholar]
- Citri Y., Colot H. V., Jacquier A. C., Yu Q., Hall J. C., Baltimore D., Rosbash M. A family of unusually spliced biologically active transcripts encoded by a Drosophila clock gene. Nature. 1987 Mar 5;326(6108):42–47. doi: 10.1038/326042a0. [DOI] [PubMed] [Google Scholar]
- Davis F. C., Gorski R. A. Development of hamster circadian rhythms: role of the maternal suprachiasmatic nucleus. J Comp Physiol A. 1988 Apr;162(5):601–610. doi: 10.1007/BF01342635. [DOI] [PubMed] [Google Scholar]
- Dushay M. S., Konopka R. J., Orr D., Greenacre M. L., Kyriacou C. P., Rosbash M., Hall J. C. Phenotypic and genetic analysis of Clock, a new circadian rhythm mutant in Drosophila melanogaster. Genetics. 1990 Jul;125(3):557–578. doi: 10.1093/genetics/125.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dushay M. S., Rosbash M., Hall J. C. The disconnected visual system mutations in Drosophila melanogaster drastically disrupt circadian rhythms. J Biol Rhythms. 1989 Spring;4(1):1–27. doi: 10.1177/074873048900400101. [DOI] [PubMed] [Google Scholar]
- Hamblen M., Zehring W. A., Kyriacou C. P., Reddy P., Yu Q., Wheeler D. A., Zwiebel L. J., Konopka R. J., Rosbash M., Hall J. C. Germ-line transformation involving DNA from the period locus in Drosophila melanogaster: overlapping genomic fragments that restore circadian and ultradian rhythmicity to per0 and per- mutants. J Neurogenet. 1986 Sep;3(5):249–291. doi: 10.3109/01677068609106855. [DOI] [PubMed] [Google Scholar]
- Helfrich C., Cymborowski B., Engelmann W. Circadian activity rhythm of the house fly continues after optic tract severance and lobectomy. Chronobiol Int. 1985;2(1):19–32. doi: 10.3109/07420528509055538. [DOI] [PubMed] [Google Scholar]
- Helfrich C. Role of the optic lobes in the regulation of the locomotor activity rhythm of Drosophila melanogaster: behavioral analysis of neural mutants. J Neurogenet. 1986 Nov;3(6):321–343. doi: 10.3109/01677068609106857. [DOI] [PubMed] [Google Scholar]
- Jackson F. R., Bargiello T. A., Yun S. H., Young M. W. Product of per locus of Drosophila shares homology with proteoglycans. Nature. 1986 Mar 13;320(6058):185–188. doi: 10.1038/320185a0. [DOI] [PubMed] [Google Scholar]
- Jackson F. R. The isolation of biological rhythm mutations on the autosomes of Drosophila melanogaster. J Neurogenet. 1983 Sep;1(1):3–15. doi: 10.3109/01677068309107068. [DOI] [PubMed] [Google Scholar]
- James A. A., Ewer J., Reddy P., Hall J. C., Rosbash M. Embryonic expression of the period clock gene in the central nervous system of Drosophila melanogaster. EMBO J. 1986 Sep;5(9):2313–2320. doi: 10.1002/j.1460-2075.1986.tb04499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka R. J., Benzer S. Clock mutants of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2112–2116. doi: 10.1073/pnas.68.9.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Lorenz L., Yu Q. N., Hall J. C., Rosbash M. Spatial and temporal expression of the period gene in Drosophila melanogaster. Genes Dev. 1988 Feb;2(2):228–238. doi: 10.1101/gad.2.2.228. [DOI] [PubMed] [Google Scholar]
- Minis D. H., Pittendrigh C. S. Circadian oscillation controlling hatching: its ontogeny during embryogenesis of a moth. Science. 1968 Feb 2;159(3814):534–536. doi: 10.1126/science.159.3814.534. [DOI] [PubMed] [Google Scholar]
- Newby L. M., White L., DiBartolomeis S. M., Walker B. J., Dowse H. B., Ringo J. M., Khuda N., Jackson F. R. Mutational analysis of the Drosophila miniature-dusky (m-dy) locus: effects on cell size and circadian rhythms. Genetics. 1991 Jul;128(3):571–582. doi: 10.1093/genetics/128.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page T. L. Circadian rhythms of locomotor activity in cockroach nymphs: free running and entrainment. J Biol Rhythms. 1990 Winter;5(4):273–289. doi: 10.1177/074873049000500401. [DOI] [PubMed] [Google Scholar]
- Pollock J. A., Benzer S. Transcript localization of four opsin genes in the three visual organs of Drosophila; RH2 is ocellus specific. Nature. 1988 Jun 23;333(6175):779–782. doi: 10.1038/333779a0. [DOI] [PubMed] [Google Scholar]
- Saez L., Young M. W. In situ localization of the per clock protein during development of Drosophila melanogaster. Mol Cell Biol. 1988 Dec;8(12):5378–5385. doi: 10.1128/mcb.8.12.5378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siwicki K. K., Eastman C., Petersen G., Rosbash M., Hall J. C. Antibodies to the period gene product of Drosophila reveal diverse tissue distribution and rhythmic changes in the visual system. Neuron. 1988 Apr;1(2):141–150. doi: 10.1016/0896-6273(88)90198-5. [DOI] [PubMed] [Google Scholar]
- Steller H., Fischbach K. F., Rubin G. M. Disconnected: a locus required for neuronal pathway formation in the visual system of Drosophila. Cell. 1987 Sep 25;50(7):1139–1153. doi: 10.1016/0092-8674(87)90180-2. [DOI] [PubMed] [Google Scholar]
- Sulzman F. M. Microcomputer monitoring of circadian rhythms. Comput Biol Med. 1982;12(4):253–261. doi: 10.1016/0010-4825(82)90030-0. [DOI] [PubMed] [Google Scholar]
- Young M. W., Jackson F. R., Shin H. S., Bargiello T. A. A biological clock in Drosophila. Cold Spring Harb Symp Quant Biol. 1985;50:865–875. doi: 10.1101/sqb.1985.050.01.104. [DOI] [PubMed] [Google Scholar]
- Yu Q., Jacquier A. C., Citri Y., Hamblen M., Hall J. C., Rosbash M. Molecular mapping of point mutations in the period gene that stop or speed up biological clocks in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1987 Feb;84(3):784–788. doi: 10.1073/pnas.84.3.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman W. F., Goldsmith T. H. Photosensitivity of the circadian rhythm and of visual receptors in carotenoid-depleted Drosophila. Science. 1971 Mar 19;171(3976):1167–1169. doi: 10.1126/science.171.3976.1167. [DOI] [PubMed] [Google Scholar]
- Zimmerman W. F. On the absence of circadian rhythmicity in Drosophila Pseudoobscura pupae. Biol Bull. 1969 Jun;136(3):494–500. doi: 10.2307/1539691. [DOI] [PubMed] [Google Scholar]



