Abstract
Room-temperature carbon monoxide oxidation, important for maintaining clean air among other applications, is challenging even after a century of research into carbon monoxide oxidation. Here we report using time-resolved diffuse reflectance infrared spectroscopy, X-ray absorption fine structure spectroscopy and mass spectrometry a platinum carbonate-mediated mechanism for the room-temperature oxidation of carbon monoxide. By applying a periodic reduction–oxidation mode of operation we further show that this behaviour is reversible and can be formed into a catalytic cycle that requires molecular communication between metallic platinum nanoparticles and highly dispersed oxidic platinum centres. A new possibility for the attainment of low-temperature oxidation of carbon monoxide is therefore demonstrated.
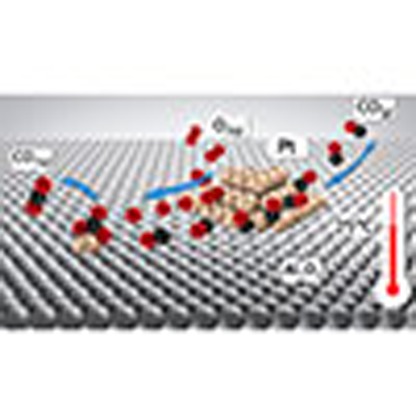 There is economic impetus to achieve low-temperature carbon monoxide oxidation. Here, the authors use time-resolved diffuse reflectance infrared spectroscopy and mass spectrometry to show that platinum carbonates formed reversibly from platinum/aluminium oxide can oxidize carbon monoxide at room temperature.
There is economic impetus to achieve low-temperature carbon monoxide oxidation. Here, the authors use time-resolved diffuse reflectance infrared spectroscopy and mass spectrometry to show that platinum carbonates formed reversibly from platinum/aluminium oxide can oxidize carbon monoxide at room temperature.
The catalytic oxidation of CO by O2 by platinum is one of the longest established and industrially important heterogeneous catalytic conversions. The generally accepted Langmuir–Hinshelwood (LH) mechanism requires the adsorption and reaction of molecular CO with atomic oxygen over metallic platinum surfaces1,2,3,4,5. At temperatures where adsorbed molecular CO becomes stable, the catalytic cycle cannot be completed as the Pt surfaces become poisoned by adsorbed CO, which prevents dissociation of O2. Catalysis therefore becomes efficient only when the steady-state coverage of CO is diminished to the degree where dissociation of O2 can efficiently occur. Practically speaking, this restricts effective CO oxidation using Pt-Al2O3 catalysts to temperatures >400 K (Supplementary Fig. 1 shows this for a conventional test of catalytic light off using the 5 wt% Pt/Al2O3 used in this study). However, Pt remains the metal of choice in many applications for effective CO conversion to CO2 where sufficient temperatures are intrinsic or can be easily applied.
There are strong drivers, be they economic or regulatory in nature, to achieve CO oxidation at lower, ideally ambient, temperatures. This is especially the case in the purification of fuel (H2) feeds, emission control and the maintenance of clean air. Given the fundamental limitations of the LH mechanism for simple Pt catalysts, research to achieve low-temperature CO oxidation has proceeded in other directions: Pt may be favourably modified through contact with more exotic oxides, for instance CeO2 (refs 6, 7) and, most recently, iron–nickel hydroxides8, or other metals, most notably Au, may be employed to achieve low-temperature CO conversion9,10,11.
Beyond the classic LH mechanism, some attention has recently been drawn to reactive Pt surface oxides and carbonates in CO conversion12,13,14,15,16,17. Ackermann et al.12 determined the presence of two reactive, single-layer surface oxides formed on Pt(110). The incommensurate oxide (essentially a sheet of hexagonal PtO2) has been latterly observed during CO oxidation over Al2O3-supported Pt nanoparticles13. The second reactive and commensurate (1 × 2) surface oxide observed by Ackermann et al.12 has yet to be directly observed on high surface area nanosized catalysts. Intriguingly, however, calculations12 suggested that this oxide requires the stabilization of an additional carbonate species.
Most recently, Moses-DeBusk et al.17 have theoretically constructed an entire catalytic cycle that converts CO to CO2 solely through isolated Pt centres, adsorbed on Al2O3 (010) surfaces, which requires the involvement of reactive carbonates. The existence of such Pt carbonates on low loaded (0.18 and 1 wt%) Pt/Al2O3 catalysts was verified using diffuse reflectance infrared Fourier transform (DRIFTS), although their intrinsic reactivity was not addressed.
Herein, we reveal a route to CO2 that is mediated through such Pt carbonates and active at ambient temperature. This is achieved using a commercially available catalyst system comprising 5 wt% Pt (average particle diameter 3 nm, see Supplementary Fig. 5) supported on a majority θ-Al2O3 co-existing with some γ-Al2O3 phase (see Supplementary Fig. 4). We show, using periodic redox operation, that this route to CO2 can be made catalytic, and that achieving such reversibility requires a synergy between metallic Pt nanoparticles and non-metallic Pt centres that are the precursors to the carbonates. A new mechanism for low-temperature CO conversion using Pt/Al2O3-based catalysts is therefore demonstrated.
Results
Periodic CO oxidation of the 5 wt% Pt/Al2O3 catalyst at 298 K
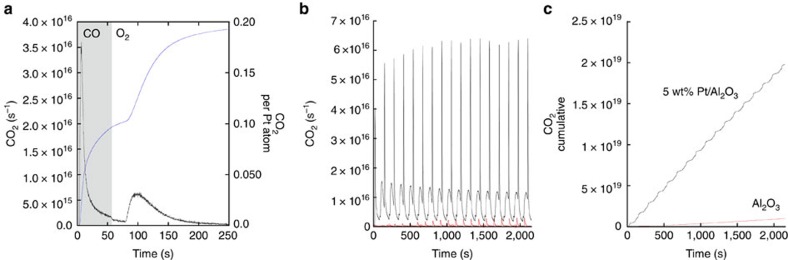
Figure 1a shows variations in the CO2 production measured by mass spectrometry (MS) during room-temperature exposure of a 5-wt% Pt/Al2O3 (Type-94, Johnson Matthey) to 5 vol% CO/Ar (50 ml min−1). Supplementary Fig. 3 shows equivalent data for a second commercial catalyst (2 wt% Pt/γ-Al2O3, Umicore), whereas Supplementary Fig. 4 compares both catalysts from the perspective of X-ray diffraction, see also Supplementary Discussion. The feed is then switched to 21 vol% O2/Ar at the same flow rate. This sample had been previously reduced in situ (5 vol% H2/Ar to 573 K) before being returned to an Ar flow before the subsequent CO/O2 exposure cycle at 298 K. Figure 1b shows the CO2 production resulting from extending the experimental protocol of Fig. 1a to a periodic operation over repeated CO/O2 switches. Alongside the CO2 production from the 5 wt% Pt/Al2O3 the red curve shows that obtained from an unloaded Al2O3 sample (Condea Puralox (γ)). Finally, Fig. 1c shows the cumulative CO2 production achieved over the duration of the periodic operation of Fig. 1b.
Figure 1. Periodic redox operation of the 5 wt% Pt/Al2O3 catalyst at 298 K.
(a) Transient evolution of CO2 observed during exposure of a pre-reduced Type-94 (Johnson Matthey) 5 wt% Pt/Al2O3 catalyst to 5 vol% CO/Ar (shaded area), followed by a switch to 21 vol% O2/He at 298 K. The left-hand axis reports the evolution of CO2 in terms of molecules per second (black), the right-hand axis shows the cumulative CO2 production as a fraction of the total number of Pt atoms in the catalyst bed (blue). (b) Repeated cycles of a similar (shorter oxidizing cycle) experiment shown in a: black=5 wt% Pt/Al2O3; red=Al2O3. (c) Cumulative CO2 (molecules) production during the experiment shown in b.
CO2 is produced instantaneously on the admission of CO before rapidly returning towards baseline levels within the cycle. At the same time (not shown), an exotherm (ca. 3 K) is also transiently observed. On O2/Ar admission, CO2 is again observed to be formed but with some delay from the switching out of CO in favour of O2. The integrals show that in each branch of the redox switch practically the same number of CO2 molecules are produced, indicating a quantitative reversibility. This reversibility is confirmed (Fig. 1b,c) by repeated gas switching and is compared with a similar experiment carried out over a Pt-free Al2O3 (red lines). The total number of CO2 molecules produced over the first cycle shown in Fig. 1a is equivalent to almost 0.2 CO2 per Pt atom present in the bed. This implies that in each half of the first cycle the active phase comprises only ca. 10% of the Pt. Figure 1b shows that the CO2 production in the CO cycle actually improves significantly after the first cycle (Fig. 1a) and is maintained at a higher level thereafter; as such, the 10% estimate of the active fraction of the Pt represents a lower limit, as subsequent cycles might indicate that a range of 10–20% may be appropriate. Over the extended period of the experiment (Fig. 1c), the total amount of CO2 produced is some five times the number of Pt atoms present in the catalyst bed.
DRIFTS during periodic CO oxidation operation at 298 K
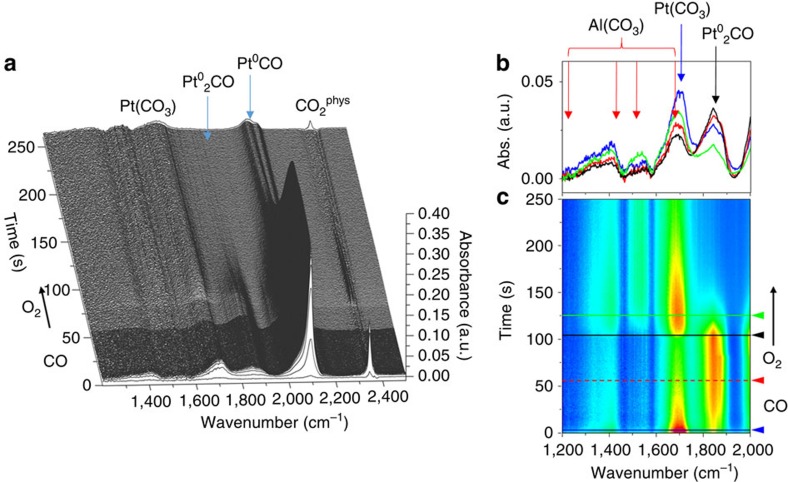
Figure 2a shows the evolution of infrared bands during the single CO/O2 cycle of Fig. 1a. Figure 2c shows a colour map of the 1,200–2,000 cm−1 region of the DRIFTS spectra to emphasize the temporal behaviour of infrared-active species in this region.
Figure 2. DRIFTS during periodic redox operation at 298 K.
(a) DRIFTS spectra derived from a first CO/O2 cycle shown in Fig. 1. (b) The 1,200–2,000 cm−1 region of the DRIFTS shown to emphasize the reactive behaviour of species below ca. 1,750 cm−1 as compared with the bridging 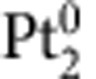 CO (ca. 1,850 cm−1). (b) Individual absorbance spectra corresponding to the different arrow/lines shown in c. The band positions expected for different aluminum carbonates (red), Pt(CO3) (blue) and
CO (ca. 1,850 cm−1). (b) Individual absorbance spectra corresponding to the different arrow/lines shown in c. The band positions expected for different aluminum carbonates (red), Pt(CO3) (blue) and 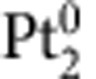 CO (black) are also given21,22. (c) Colour map representation of the same set of DRIFTS spectra of a. The red arrow shows the changeover point in time between the CO/Ar flow and the O2/Ar flow; the solid black line highlights the delay between the switch to O2, the removal of bridging
CO (black) are also given21,22. (c) Colour map representation of the same set of DRIFTS spectra of a. The red arrow shows the changeover point in time between the CO/Ar flow and the O2/Ar flow; the solid black line highlights the delay between the switch to O2, the removal of bridging 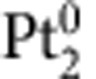 CO species and the transient re-appearance of the bridging
CO species and the transient re-appearance of the bridging 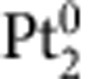 CO band at ca. 1,700 cm−1.
CO band at ca. 1,700 cm−1.
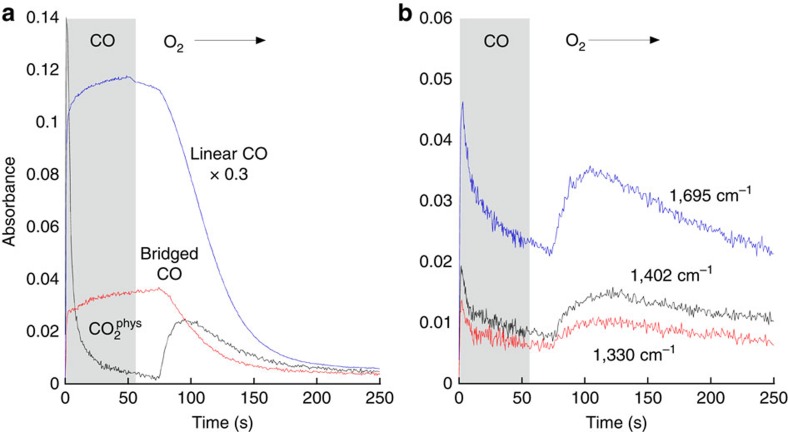
Figure 3a,b shows the temporal variations in a number of different infrared-visible bands observed in Fig. 2 and how they relate to the observed production of CO2. As might be expected, under CO the DRIFTS spectra are dominated by bands due to linear (2,094 cm−1) and bridged CO (1,845 cm−1) adsorbed on metallic Pt18. These appear instantaneously on introduction of CO and persist beyond the introduction of O2 before attenuating to any significant degree. Alongside these bands a number of other transient species are also seen, to rapidly evolve and then attenuate. The largest of these under CO is a sharp feature at 2,345 cm−1 due to physisorbed CO2 (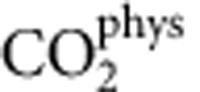 ). Concomitantly, a strong band at 1,695 cm−1 transiently appears along with less intense bands at 1,547, 1,402 and 1,330 cm−1.
). Concomitantly, a strong band at 1,695 cm−1 transiently appears along with less intense bands at 1,547, 1,402 and 1,330 cm−1.
Figure 3. Temporal evolution of adsorbed species at 298 K.
(a) Linear CO (blue: 2,094 cm−1), bridged CO (red: 1,845 cm−1) and 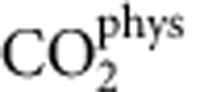 (black: 2,345 cm−1). (b) Other bands at 1,695, 1,402 and 1,330 cm−1 as indicated. The shaded area indicates the period of exposure to 5% CO.
(black: 2,345 cm−1). (b) Other bands at 1,695, 1,402 and 1,330 cm−1 as indicated. The shaded area indicates the period of exposure to 5% CO.
As can be seen in Fig. 2b the bands due to CO adsorbed on metallic Pt have little relation to the evolution of gas phase CO2 (CO2(g)): their temporal character indicates little more than adsorption/desorption processes related to the presence in this sample of metallic Pt nanoparticles. However, it may be noted that the lower wavenumber bands, and that due to 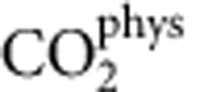 , only appear in the oxygen cycle when the removal of linear and bridged, adsorbed CO species starts to become appreciable. This shows that the reserve of ‘inactive' CO adsorbed on metallic Pt nanoparticles is the source through which the low-temperature active phase of Pt in this system is replenished and can convert CO to CO2(g) in the absence of gas phase CO. This can occur as a result of CO desorption being mediated by physisorbed precursor states19,20. At ambient temperature, these have an appreciable lifetime and can therefore search out the active Pt centres required for reaction. The low-temperature catalytic production of CO2 observed therefore requires a communication between reduced nanoparticles and other highly dispersed, oxidic Pt centres.
, only appear in the oxygen cycle when the removal of linear and bridged, adsorbed CO species starts to become appreciable. This shows that the reserve of ‘inactive' CO adsorbed on metallic Pt nanoparticles is the source through which the low-temperature active phase of Pt in this system is replenished and can convert CO to CO2(g) in the absence of gas phase CO. This can occur as a result of CO desorption being mediated by physisorbed precursor states19,20. At ambient temperature, these have an appreciable lifetime and can therefore search out the active Pt centres required for reaction. The low-temperature catalytic production of CO2 observed therefore requires a communication between reduced nanoparticles and other highly dispersed, oxidic Pt centres.
The bands at lower wavenumber all show similar, although not identical, profiles that correlate well with CO2 production. The strongest correlation in both halves of the cycle (both in MS and DRIFTS via the 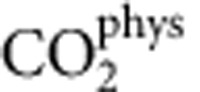 band at 2,345 cm−1) is found for the band at ca. 1,695 cm−1. This principal band is very similar to that calculated by Moses-Debusk et al.17 for bidentate carbonates formed at oxidized and atomic Pt centres, lying midway between that calculated for a Pt(CO3) species (1,730 cm−1) and that observed by experiment (1,659 cm−1–0.18 wt% Pt and 1,637 cm−1–1 wt% Pt)17.
band at 2,345 cm−1) is found for the band at ca. 1,695 cm−1. This principal band is very similar to that calculated by Moses-Debusk et al.17 for bidentate carbonates formed at oxidized and atomic Pt centres, lying midway between that calculated for a Pt(CO3) species (1,730 cm−1) and that observed by experiment (1,659 cm−1–0.18 wt% Pt and 1,637 cm−1–1 wt% Pt)17.
Although the summation of the evidence derived from MS and DRIFTS strongly suggests that a single Pt carbonate species is responsible for the majority of the room-temperature production of CO2, it would be remiss of us not to note that the number of bands, however weak, observed to correlate with the CO2 production would indicate the presence of more than a single carbonate species. However, and in addition to the negligible activity of an unloaded alumina support (Fig. 1c, red line), we may exclude the possibility that carbonates formed at the Al2O3 surface are participating in the chemistry shown in Fig. 2. Although such species show infrared absorption in the region of interest21,22 (Fig. 2b), none of them, by way of band position, combinations of bands and their expected relative intensities22, explains our observations. For instance, Al bicarbonates (1,655 cm−1)21,22 and bidentate carbonates (1,660–1,730 cm−1)21 always show bands in the 1,200–1,300 cm−1 region that should be detectable if they were contributing significantly to the chemistry21,22. Similarly, Al monodentate carbonates show spectral features only between 1,400 and 1,650 cm−1 (refs 21, 22). In this region, however, only the weak band at 1,402 cm−1 shows evidence for a diminution after its formation (although, by and large, it persists) and one that is very much slower than the majority CO2 production. As such, it cannot be deemed responsible for the majority CO turnover we observe that is far more highly correlated to the much stronger band at 1,695 cm−1.
Therefore, the above demonstrates that Pt carbonates are intrinsically capable of forming rapidly under CO and converting that CO to CO2 at 298 K. It also shows that re-oxidation of the Pt sites responsible for this chemistry is much slower and acts to limit the overall efficiency of this process.
XAFS during periodic CO oxidation on 5 wt% Pt/Al2O3 at 298 K
DRIFTS and MS analyses tell us little about the Pt itself during these events. Importantly, these measurements cannot discriminate between a direct re-oxidation of isolated Pt centres that is subject to a relatively high activation energy—as considered by Moses-DeBusk et al.17—or whether, and as might be implied by the DRIFTS, desorption of molecular CO from metallic Pt nanoparticles has to occur first. To resolve this issue we therefore conducted static and time-resolved X-ray absorption fine structure (XAFS) spectroscopy at the Pt L3 edge under identical experimental conditions. Some results of this are shown in Fig. 4.
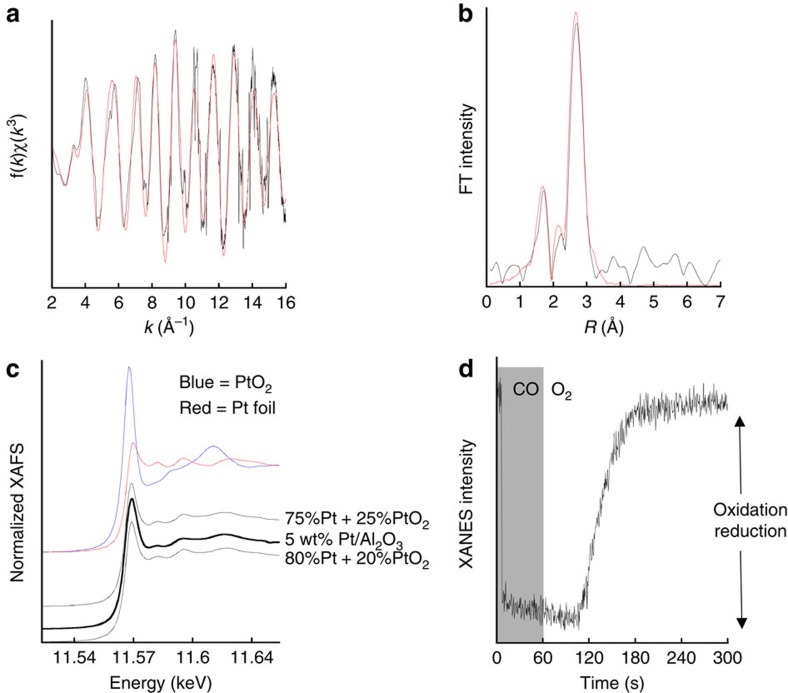
Figure 4. XAFS during periodic redox operation at 298 K.
(a) k3-weighted EXAFS derived in situ from the 5 wt% Pt/Al2O3 catalyst along with theoretical fit (red); (b) the corresponding Fourier transform (again with the theoretical fit in red). (c) Comparison of Pt L3 edge X-ray absorption near edge structure from this sample with that from Pt foil (red) and PtO2 (blue). The sample spectrum is also compared with linear combinations (80% Pt0 and 75% Pt0 as indicated) of these two reference spectra. (d) The temporal variation observed in the Pt L3 white line intensity during exposure of the catalyst to 5 vol% CO/Ar and then 21 vol% O2/Ar.
Figure 4a shows the k-weighted extended XAFS (EXAFS) obtained from the 5 wt% Pt/Al2O3 catalyst, whereas Fig. 4b shows the corresponding Fourier transform. The results of best fitting the EXAFS spectrum are given in Table 1. These measurements show that, as might be deduced from the dominance of linear and bridging CO bands in DRIFTS, the Pt in this sample principally comprises metallic nanoparticles. This is verified for a fresh sample by transmission electron microscopy (TEM, Supplementary Fig. 5). However, alongside this, and to a first approximation (Fig. 4c), a significant proportion (20–25%) of the Pt is present in a Pt (IV) oxidation state that would correspond to the relatively large, low Z (oxygen) coordination detected by the EXAFS (Fig. 4a,b and and Table 1). All or a portion of this oxidized Pt may correspond to the smaller Pt entities unambiguously detected by high-angle annular dark-field scanning TEM analysis (Supplementary Fig. 6). Moreover, the spatial proximity of these species to the reduced Pt nanoparticles makes the required molecular communication between these two types of Pt plausible.
Table 1. Best-fit parameters obtained for the 5 wt% Pt/Al2O3 sample measured in situ at room temperature and after reduction at 573 K.
EXAFS, extended X-ray absorption fine structure.
EF=−10.31=the edge position relative to the vacuum zero (Fermi energy).
R%=33.37=(∫[χT−χE]k3dk/[χE]k3dk) × 100%, where χT and χE are the theoretical and experimental EXAFS and k is the photoelectron wave vector.
The Debye–Waller factor=2σ2, where σ is the root mean square internuclear separation.
Other parameters: Attentuation factor (AFAC), related to the proportion of electrons performing an EXAFS-type scatter on absorption, is 0.875. Structural data were obtained by fitting the EXAFS in k space in the range: k=2.5–16.5 Å−1.
*Coordination number (±ca. 10% stated value).
†Distance of scatterer atom from central atom (±ca. 1.5% stated value).
‡Debye–Waller factor.
Figure 4d shows the behaviour of the Pt during room-temperature exposure to CO and then 21 vol% O2/Ar, from the perspective of the height of the Pt L3 edge white line. This reveals that the reduction of the Pt—corresponding with the onset of CO2 production in the CO cycle—is extremely rapid and essentially complete in <10 s. On returning the sample to the oxidizing flow we clearly see that re-oxidation of the active Pt is much slower and subject to an induction time that matches well with the observations made using DRIFTS. From this we may conclude that re-oxidation of the carbonate precursor requires dissociation of O2 at the metallic sites, and that this can only occur as and when the linear and bridging CO species start to desorb.
Discussion
The combined (DRIFTS, MS and time-resolved XAFS) evidence obtained allows us to derive a room-temperature cyclic mechanism, involving both Pt carbonates and Pt nanoparticles for the CO oxidation under the periodic conditions of operation employed.
Under CO:
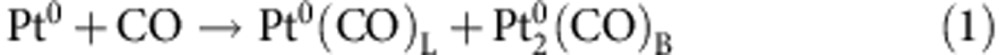 |
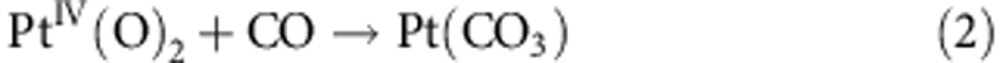 |
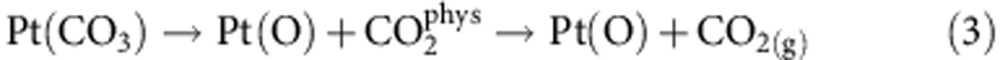 |
Under O2:
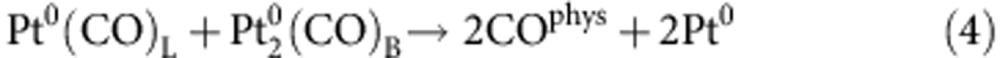 |
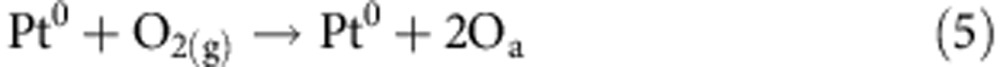 |
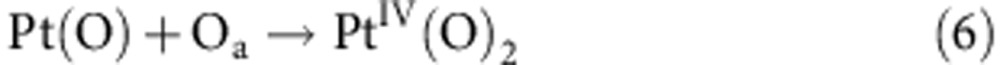 |
 |
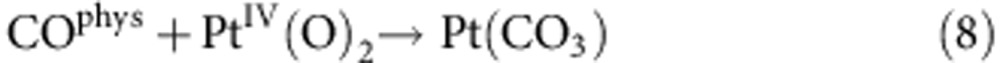 |
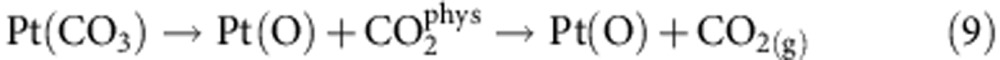 |
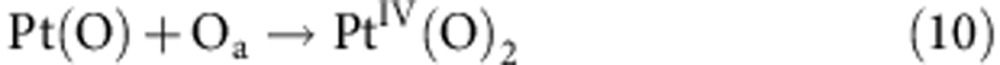 |
where Pt0 is the metallic platinum, Pt0(CO)L is the CO adsorbed in linear geometry on metallic Pt, 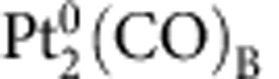 is the CO adsorbed in twofold bridge geometry on metallic Pt, Pt(CO3) is the platinum carbonate intermediate, PtIV(O)2 is the isolated oxidic platinum species, Oa is the adsorbed oxygen, Pt(O) is the product of carbonate decomposition,
is the CO adsorbed in twofold bridge geometry on metallic Pt, Pt(CO3) is the platinum carbonate intermediate, PtIV(O)2 is the isolated oxidic platinum species, Oa is the adsorbed oxygen, Pt(O) is the product of carbonate decomposition, 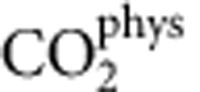 and COphys are physisorbed CO2 and CO, respectively, and O2(g), CO2(g) and CO(g) are gas phase species.
and COphys are physisorbed CO2 and CO, respectively, and O2(g), CO2(g) and CO(g) are gas phase species.
Within this mechanism, the oxidative regeneration of the precursor to carbonate formation, equation (6), limits the reformation of the carbonates in oxidizing conditions. However, the combined evidence suggests that this is mediated via the Pt0 nanoparticles and therefore can only happen once free sites are created on the Pt nanoparticles rather than through a direct dissociation of O2 at the precursor sites to the carbonates. As such, we suggest that it is CO desorption (equation (4)) that effectively limits the efficacy of this reactive pathway. In this respect, our mechanism differs from that of Moses-DeBusk et al.17 who considered only a direct dissociation of O2 by atomically dispersed Pt precursors to the carbonate species.
As a result, and in respect of steady-state operation, our room-temperature mechanism still suffers from the poisoning effect of CO that hobbles the classic LH mechanism. Indeed, steady-state operation (Supplementary Fig. 2) under a 4O2:CO flow leads to a very small, but non-zero, production of CO2 at room temperature; at present, it is only through adopting a periodic operation that this reactive impasse may be, to some degree, circumvented.
A priori we cannot definitively say where these carbonates actually reside. It is clear from our experimental methodology that the oxidized Pt entities that lead to their formation can survive reduction in H2/Ar to at least 573 K. This characteristic mitigates against their existence within a commensurate and reactive surface oxide in the manner proposed by Ackermann et al.12 Instead, in this respect we would tend to concur with the propositions of Moses-DeBusk et al.17 who only considered atomically dispersed oxidic Pt centres adsorbed on alumina surfaces. Further, high-angle annular dark-field scanning TEM measurements (Supplementary Fig. 6) also support this view and are consistent with recent works that have shown that similar atomic or quasi atomically adsorbed Pt and Pd species can be highly reactive for oxidation reactions23,24. Although we cannot rule out a role for interfacial Pt-O-Al sites, these would not be sufficient (based on the particle size distribution from TEM, see Supplementary Fig. 5) to yield the ca. 10–20% of active Pt that our results indicate are mediating carbonate formation and CO turnover in the current case.
To summarize, we have demonstrated that room-temperature CO oxidation over Pt/Al2O3 is feasible and mediated by oxidized, isolated and reduction-resistant Pt centres that may form reactive carbonates from CO and be re-oxidized under O2. Using periodic operation we have shown that this process is quantitatively reversible and can form a catalytic cycle. At present, the reactive Pt centres necessary for this low-temperature conversion exist as a minority species within the conventional catalyst used for these studies: most of the Pt (estimated to be ca. ≥80%) is not directly active but does have a role to play in completing the catalytic cycle. We have shown that at room temperature metallic nanoparticulate Pt may act as a reservoir for CO, which may be transferred to the minority quasi atomic oxidic Pt phase, and converted to CO2. The rate-limiting re-oxidation of the carbonate precursor sites (Pt(O)) also requires the presence of Pt nanoparticles that can facilitate O2 dissociation and oxygen transfer to these sites.
As such, this new pathway for achieving low-temperature CO oxidation immediately poses a number of further challenges to material synthesis and fundamental understanding. Foremost among these is the fundamental question of whether a direct oxidation of the precursor to the Pt carbonates can be attained and how we may promote CO desorption from the Pt nanoparticles to permit more efficient O2 dissociation. Second would be to understand, from a material synthesis perspective, how to optimize the levels of such carbonate forming Pt species on Al2O3 from ca. 10–20% minority to a working majority.
Methods
Infrared spectroscopy
Combined DRIFTS/MS experiments were carried at the Swiss Light Source using a Bruker Vertex 80V infrared spectrometer fitted with a narrow-band high-sensitivity mercury cadmium telluride (MCT) detector and with a Pfeiffer mass spectrometer. DRIFTS spectra were collected with a time resolution of 0.433 s and 2 cm−1 resolution.
The in-situ cell used for these experiments was that recently described by Chiarello et al.25 for transient experimentation and was windowed with CaF2. This cell was connected to a gas handling system equipped with fast solenoid switching valves (Parker) controlled using the infrared spectrometer. The reactor exit was connected to a Pfeiffer mass spectrometer that recorded both the sample temperature and the gas switching events alongside a range of masses compatible with the experiments being made.
Gas flows (50 ml min−1) were controlled by Bronkhorst mass flow controllers with a 2-bar pressure of gases behind the controllers themselves. This set-up allowed the purging of the system with Ar, then reduction using 5 vol% H2/Ar and, finally, gas switching experiments between 5 vol%CO/Ar and 21 vol%O2/Ar.
In the experiments described, 25–30 mg of the 5 wt% Pt/Al2O3 catalyst (Type-94, Johnson Matthey) was loaded into the DRIFTS cell. After purging in Ar, this sample was then reduced under 5 vol% H2/Ar to 573 K under a linear heating ramp (10 K min−1) and then held at 573 K for 30 min. The sample was then cooled under 5 vol% H2/Ar to 323 K whereon the reducing flow was substituted for flowing Ar once more. At 298 K, the sample was then exposed to 5 vol% CO/Ar for 52 s before the flow is switched to 21 vol% O2/He for 208 s, whereas DRIFTS and MS data were collected. This experiment was then repeated for a total of 16 CO/O2 cycles.
Subsequently, the sample was then exposed to a 4O2:1CO reaction mixture (again 50 ml min−1 total flow, 4.2 × 1017 molecules CO per second) and the temperature incremented until light-off of conventional CO oxidation catalysis was initiated (T∼423 K). At each temperature, the DRIFTS and MS data were collected, with the latter being used to calibrate the CO2 response in terms of the overall CO conversions.
Having conducted this calibration experiment, the sample was re-cooled under the reaction mixture, purged again before a second CO/O2 switching experiment was conducted and again DRIFTS and MS data simultaneously acquired. It is the results of this second switching experiment that are reported in Figs 1, 2, 3. We note that both first and second switching experiments, as well as the 16 cycle experiment, return the same global results save for some minor differences in overall CO2 production. In addition, although not shown here, essentially the same results can be achieved without any pre-reduction of the catalyst and the species responsible for the chemistry we have reported are present in the as-received catalyst.
X-ray absorption spectroscopy
Pt L3 edge XAFS was collected in transmission mode at the SuperXAS beamline at the Swiss Light source (Villigen, Switzerland) using a newly installed fast, Si (111) channel cut monochromator system coupled to gridded N2-filled ionization chambers for detection. This bidirectional scanning system was operated at 2 Hz, yielding four spectra per second. The static and reference spectra are obtained as averages over 3 min of acquisition.
The in-situ time-resolved X-ray absorption near edge structure data were extracted from individual (250 ms time resolution) spectra during CO switching from 5 vol% CO/Ar and 21 vol% O2/Ar, and using the same sample environment as for the DRIFT/MS. Online MS was recorded as for the DRIFTS-based measurements. Data reduction was made using PAXAS26 and analysis of the EXAFS using EXCURV27.
Additional information
How to cite this article: Newton, M. A. et al. Room-temperature carbon monoxide oxidation by oxygen over Pt/Al2O3 mediated by reactive platinum carbonates. Nat. Commun. 6:8675 doi: 10.1038/ncomms9675 (2015).
Supplementary Material
Supplementary Figures 1-6, Supplementary Discussion and Supplementary References
Acknowledgments
M.A.N. thanks the Swiss Light Source/Paul Scherrer Institut and the Department of Physics at the University of Warwick, for the visiting scientist positions that facilitated this work. Johnson Matthey (Dr D. Thompsett) and Umicore are also thanked for provision of the samples used in these studies. We thank Frank Krumeich and Maksym Kovalenko (ETH Zürich) for enabling high-angle annular dark-field scanning TEM. Electron microscopy was carried out at the Scientific Center for Optical and Electron Microscopy (ETH Zurich). We are thankful to Lorenz Bäni for technical support. Financial support from the Swiss National Science Foundation (SNF, project number 200021–138068) is greatly acknowledged.
Footnotes
Author contributions Experiments were conceived and carried out by M.A. Newton with experimental assistance from V. Marchionni and D. Ferri (DRIFTS/MS) and M. Nachtegaal and G. Smolentsev (XAFS). M.A. Newton wrote the manuscript with D. Ferri and M. Nachtegaal. All authors have proofread the manuscript.
References
- Langmuir I. The mechanism of the catalytic action of platinum in the reactions of 2CO+O2=CO2 and 2H2+O2=H2O. Trans. Faraday Soc. 17, 0621–0654 (1921). [Google Scholar]
- Campbell C. T., Ertl G., Kuipers H. & Segner J. A molecular beam study of the catalytic-oxidation of CO on a Pt(111) surface. J. Chem. Phys. 73, 5862–5873 (1980). [Google Scholar]
- Wintterlin J., Volkening J., Janssens T. V. W., Zambelli T. & Ertl G. Atomic and macroscopic reaction rates of a surface-catalyzed reaction. Science 278, 1931–1934 (1997). [DOI] [PubMed] [Google Scholar]
- Alavi A., Hu P., Deutsch T., Silvestri P. & Hutter P. L. CO oxidation on Pt(111): an ab initio density functional theory study. Phys. Rev. Lett. 80, 3650–3653 (1998). [Google Scholar]
- Allian A. D. et al. Chemisorption of CO and mechanism of CO oxidation on supported platinum nanoclusters. J. Am. Chem. Soc. 133, 4498–4517 (2011). [DOI] [PubMed] [Google Scholar]
- Trovarelli A. Catalysis by Ceria and Related Materials Imperial College Press (2002). [Google Scholar]
- Vayssilov G. N. et al. Support nanostructure boosts oxygen transfer to catalytically active platinum nanoparticles. Nat. Mater. 10, 310–315 (2011). [DOI] [PubMed] [Google Scholar]
- Chen G. et al. Interfacial effects in iron-nickel hydroxide–platinum nanoparticles enhance catalytic oxidation. Science 344, 495–499 (2014). [DOI] [PubMed] [Google Scholar]
- Haruta M. Size- and support-dependency in the catalysis of gold. Catal. Today 36, 153–166 (1997). [Google Scholar]
- Bond G. C. & Thompson D. T. Catalysis by gold. Catal. Rev. Sci. Eng. 41, 319–388 (1999). [Google Scholar]
- Hashmi A. S. K. & Hutchings G. J. Gold catalysis. Angew. Chem. Int. Ed. 45, 7896–7936 (2006). [DOI] [PubMed] [Google Scholar]
- Ackermann M. D. et al. Structure and reactivity of surface oxides on Pt(110) during catalytic CO oxidation. Phys. Rev. Lett. 95, 255505 (2005). [DOI] [PubMed] [Google Scholar]
- Newton M. A., Chapman K. W., Thompsett D. & Chupas P. J. Chasing changing nanoparticles: a time resolved total X-ray scattering study of the behavior of Supported Pt nanoparticles during CO oxidation catalysis. J. Am. Chem. Soc. 134, 5036–5039 (2012). [DOI] [PubMed] [Google Scholar]
- Butcher D. R. et al. In situ oxidation study of Pt(110) and its interaction with CO. J. Am. Chem. Soc. 133, 20319–20325 (2011). [DOI] [PubMed] [Google Scholar]
- Miller D. et al. Different reactivity of the various platinum oxides and chemisorbed oxygen in CO oxidation on Pt(111). J. Am. Chem. Soc. 136, 6340–6347 (2014). [DOI] [PubMed] [Google Scholar]
- Szlachetko J. et al. Sub-second and in situ chemical speciation of Pt/Al2O3 during oxidation reduction cycles monitored by high-energy resolution off-resonant X-ray spectroscopy. J. Am. Chem. Soc. 135, 19071–19074 (2013). [DOI] [PubMed] [Google Scholar]
- Moses-DeBusk M. et al. CO oxidation on supported single Pt atoms: experimental and ab initio density functional studies of CO interaction with Pt atom on theta-Al2O3 (010) surface. J. Am. Chem. Soc. 135, 12634–12645 (2013). [DOI] [PubMed] [Google Scholar]
- Hollins P. The influence of surface-defects on the infra-red spectra of adsorbed species. Surf. Sci. Rep. 16, 51–94 (1992). [Google Scholar]
- Kisliuk P. The sticking probabilities of gases chemisorbed on the surfaces of solids. J. Phys. Chem. Solids 3, 95–101 (1957). [Google Scholar]
- Bowker M., Bowker L. J., Bennett R. A., Stone P. & Ramirez-Cuesta A. In consideration of precursor states, spillover and Boudart's ‘collection zone' and of their role in catalytic processes. J. Mol. Cat. A 163, 221–232 (2000). [Google Scholar]
- Busca G. & Lorenzelli V. Infrared spectroscopic identification of species arising from reactive adsorption of carbon oxides on metal oxide surfaces. Mater. Chem. 7, 89–126 (1982). [Google Scholar]
- Liu X., Korotkikh O. & Farrauto R. Selective catalytic oxidation of CO in H2: structural study of Fe oxide-promoted Pt/alumina catalyst. Appl. Catal. A 226, 293–303 (2002). [Google Scholar]
- Narula C. K., Allard L. F., Stocks G. M. & Moses-DeBusk M. Remarkable NO oxidation on single supported platinum atoms. Sci. Rep. 4, 7238 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson E. J. et al. Low temperature carbon monoxide oxidation catalyzed by regenerable atomically dispersed Palladium on Alumina. Nat. Commun. 5, 4885 (2014). [DOI] [PubMed] [Google Scholar]
- Chiarello G. L. et al. Adding diffuse reflectance infrared Fourier transform spectroscopy capability to extended X-ray absorption fine structure in a new cell to study solid catalysts in combination with a modulation approach. Rev. Sci. Instr. 85, 074102 (2014). [DOI] [PubMed] [Google Scholar]
- Binsted N. PAXAS: Programme for the Analysis of X-ray Adsorption Spectra University of Southampton (1988). [Google Scholar]
- Binsted N. EXCURV98 CCLRC Daresbury Laboratory Computer Programme (1998). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1-6, Supplementary Discussion and Supplementary References