Abstract
The ability to understand the molecular mechanisms by which secreted signaling proteins of the TGF-β superfamily assemble their cell surface receptors into complexes to initiate downstream signaling is dependent upon the ability to determine atomic-resolution structures of the signaling proteins, the ectodomains of the receptors, and the complexes that they form. The structures determined to date have revealed major differences in the overall architecture of the signaling complexes formed by the TGF-βs and BMPs, which has provided insights as to how they have evolved to fulfill their distinct functions. Such studies, have however, only been applied to a few members of the TGF-β superfamily, which is largely due to the difficulty of obtaining milligram-scale quantities of highly homogenous preparations of the disulfide-rich signaling proteins and receptor ectodomains of the superfamily. Here we describe methods used to produce signaling proteins and receptor ectodomains of the TGF-β superfamily using bacterial and mammalian expression systems and procedures to purify them to homogeneity.
Keywords: TGF-β, ligand and receptor, protein expression, protein purification, structural analysis
1. Introduction
The transforming growth factor-beta (TGF-β) superfamily is comprised of a diverse family of signaling proteins, with three known family members in C. elegans, seven in D. melanogastor, and more than thirty in humans and other vertebrates [1]. The proteins of the superfamily arose as developmental factors responsible for embryonic patterning and morphogenesis in invertebrates, but have further evolved to fulfill many extraembryonic roles as organisms have diversified.
The proteins of the superfamily are produced as pre-pro proteins and are secreted either as mature disulfide-linked dimers, or as mature disulfide-linked dimers non-covalently bound to their pro-domain [2,3]. The mature homodimers signal by binding and bringing together two transmembrane receptors, known as receptor types I and II, to form heterotetrameric complexes with two type I and two type II receptors [4,5]. The proteins of the superfamily can be divided into two phylogenetic clades based on the type I receptors they bind and Smad proteins they activate [6]. The more recently evolved members of the superfamily, including the TGF-βs, activins, nodal, and some of the GDFs and BMPs (GDF-9, -11, and -15 and BMP-15), bind and signal through type I receptors that couple to and activate R-Smads 2, 3, while the more distantly related GDFs (GDF-1, -3, -5, -7, and -10) and BMPs (BMP-2, -3, -4, -5, -6, -7, -8, -9, and -10) bind and signal through type I receptors that couple to and activate R-Smads 1, 5, and 8 [1]. The two subclasses of R-Smads, upon association with the co-mediator Smad, Smad4, assemble distinct transcriptional complexes and thus activate (or repress) distinct subsets of genes [7].
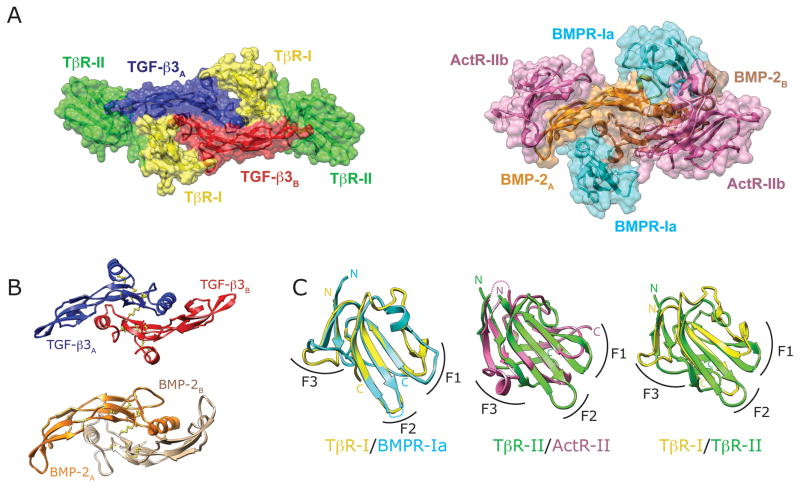
The recent structures of TGF-βs (TGF-β1 and -β3) and BMPs (BMP-2 and BMP-7) bound to the ectodomains of their receptors (TβRI and TβRII and BMPRIa/BMPRIb, ActRII/ActRIIb, respectively) show that although the TGF-βs and BMPs and their receptors share the same overall folds, they nevertheless bind and assemble their receptors in a distinct manner (Fig. 1) [8–13]. The differences are especially pronounced for the type II receptors: BMP type II receptors use the concave surface of their β-sheet to complement the convex surface of the knuckle epitope of the BMP, while the TGF-β type II receptor inserts one of its edge β-strands into the cleft between the fingertips of the TGF-β. Though the differences are less pronounced, the type I receptors also bind differently: BMP type I receptors bind to the wrist and have extensive contact with both BMP monomers, while the TGF-β type I receptor is shifted away from the wrist toward the fingertips where it contacts TβRII, the TGF-β monomer to which TβRII is bound, and to a limited extent, the adjacent monomer. These structural differences are significant as the distinct interfaces expand the range of specificity and segregate the actions of TGF-βs, which signal through receptors that activate Smads 2 and 3, away from the many BMPs and GDFs, which signal through receptors that activate Smads 1, 5, and 8 [14–17].
Figure 1.
Different modes of receptor complex assembly by TGF-βs and BMPs. (A) TGF-β (left) and BMP (right) type I receptor, type II receptor ternary complex structures. TGF-β type I and type II receptors are shaded yellow and green, respectively, and extensively contact one another. BMP type I and type II receptors are shaded cyan and magenta respectively, and do not contact one another. (B) Representative dimeric ligand structures, with the two monomers of TGF-β3 depicted in blue and red, and those of BMP-2 in orange and brown. (C) Receptor extracellular domains of the TGF-β superfamily adopt the same three finger toxin fold, as shown by an overlay of the BMP and TGF-β type I receptors on the left (cyan and yellow, respectively), the BMP and TGF-β type II receptors in the middle (magenta and green, respectively), and the TGF-β type I and type II receptors on the right (yellow and green, respectively). F1, F2, and F3 designate the three fingers of the receptor three-finger toxin fold.
The determination of the structures of the TGF-βs and BMPs bound to the ectodomains of their type I and type II receptors has been highly dependent on the ability to produce and isolate highly homogenous forms of each these proteins in quantities large enough for structural studies. The objective of this review is to provide an overview of the different strategies that have been used to obtain these proteins and to characterize their binding properties and structures in vitro. The high disulfide content of both the signaling proteins and receptor ectodomains of the superfamily necessitates that procedures specifically adapted for these types of proteins be used. The primary methods that have been used for these purposes include overexpression as secreted proteins in cultured eukaryotic cells, and overexpression in bacteria, followed by renaturation into native signaling proteins or receptors. The methods commonly used to obtain signaling proteins of the superfamily will be described first, followed by a summary of the methods used to obtain the receptor ectodomains of the superfamily.
2. Materials
2.1 Reagents and buffers for E. coli expression
E. coli host strain: BL21(DE3) (Stratagene)
Expression constructs: The coding sequences of mature human TGF-β2 and extracellular domain of TβRII were synthesized by Genscript (Piscataway, NJ) to optimize the codon usage for E.coli and minimize formation of secondary structure by the mRNA. The coding sequences were inserted between the NdeI and HindIII sites in plasmid pET32a (Novagen, Madison WI).
Standard LB medium: Dissolve 10 g tryptone, 5 g yeast extract, and 10 g NaCl in 1 L H2O and adjust to pH 7.4 by addition of 10 M NaOH, then sterilize by autoclaving. Add ampicillin (150 μg/mL) before use.
Ampicillin: Dissolve 1.5 g ampicillin in a final volume of 10 mL H2O to yield 150 mg/mL stock solution, sterilize by filtration through a 0.22 μm filter, and store in the dark at −20°C.
Isopropyl-β-D-thiogalactoside (IPTG): Dissolve 2.38 g IPTG in a final volume of 10 mL H2O to yield 1 M stock solution, sterilize by passage through a 0.22 μm filter, and store at −20°C.
2.2 Reagents and buffers for mammalian expression
Cell line for transfection: CHO-lec3.2.8.1 (see note 1 in section 4)
CMV-based expression construct: Construct is that initially reported by Zou, et. al [18] in which the human TGF-β1 open reading frame is subcloned into pcDNA3.1(+) modified to include a glutamine synthetase gene for gene amplification. The leader peptide of TGF-β1 was replaced with that of rat serum albumin, and an eight-histidine tag was inserted immediately after the leader sequence to facilitate protein purification. In addition, Cys 33 of TGF-β1, which forms a disulfide bond with latent TGF-β1-binding protein (LTBP) was replaced by a serine residue to increase secretion.
Cell culture medium: DMEM/F12 medium, 10% fetal bovine serum (FBS), antibiotics (penicillin-streptomycin), pre-warmed to 37°C.
Minimal Essential Medium (MEM): Opti-MEM medium (Invitrogen)
Lipid transfection reagent: Lipofectamine 2000 (Invitrogen)
Purified plasmid DNA: Maxi Prep (Qiagen)
Cell culture flasks and plates
Phosphate-buffered saline (PBS)
Glutamine-free Glasgow’s Minimal Essential Medium (GMEM) (SAFC biosciences)
Glutamine synthase (GS) supplement (50X) (SAFC Biosciences)
L-methionine sulfoximine (MSX) (Sigma)
Serum-free medium for protein expression: CHO-S-SFM II (Gibco) or SFM4CHO-A (HyClone)
Dimethyl sulfoxide (DMSO)
2.3 Reagents and buffers for protein purification
Tris(hydroxymethyl)aminomethane (Tris)
2-(N-morpholino)ethanesulfonic acid (MES)
Phenylmethanesulfonyl fluoride (PMSF)
Dithiothreitol (DTT)
3-[(3-Cholamidopropyl) dimethylammonio]-1-propanesulfonate (CHAPS)
Disruption buffer: 100 mM Tris, 10 mM EDTA, 1 mM PMSF, pH 8.0
TGF-β solubilizing buffer: 8 M urea, 20 mM Tris, 1% DTT (w/v), pH 8.0
SP denaturing buffer: 8 M urea, 20 mM sodium acetate, 0.1% DTT (w/v), pH 4.2
TGF-β folding buffer: 100 mM Tris, 30 mM CHAPS, 1 M NaCl, 5 mM reduced glutathione, pH 9.5
Source 15S buffer: 20 mM sodium acetate, 30 % isopropyl alcohol, pH 4.0
Ni buffer: 50 mM Tris, 150 mM NaCl and 10 mM imidazole at pH 8.0.
RII solubilizing buffer: 8M urea, 20 mM Tris, pH 7.0
RII folding buffer: 200 mM Tris, 2 mM reduced glutathione (GSH), 0.5 mM oxidized-glutathione (GSSG), pH 8.0
Source 15Q buffer: 20 mM MES, 1 mM PMSF, 1 mM EDTA, pH 6.0
2.4 Equipment and materials
Temperature-controlled orbital shaker
Water-jacketed CO2 incubator
Refrigerated high-speed centrifuge
Handheld glass/teflon homogenizer
UV spectrophotometer
Chromatography columns
Dialysis tubing
Centrifugal concentrators
Amicon stirred cell concentrator
Apparatus for SDS-PAGE
Äkta Fast Protein Liquid Chromatography (FPLC) system (GE Healthcare) or equivalent.
3. Methods
3.1 Overview of signaling protein production and isolation
TGF-βs are disulfide-linked dimers of identical 112-residue monomers. The monomers have nine cysteines, one of which participates in the formation of the inter-chain disulfide and eight of which form four internal disulfides [19–22]. Three of the four internal disulfide bonds form a conserved structure known as a cysteine knot [23]. BMPs, GDFs, activins, and most other signaling proteins of the TGF-β superfamily share a similar structure, though the number of internal disulfide bonds varies, with some, such as activins/inhibins possessing four disulfides, but others, such as BMPs and GDFs possessing only the three that form the cystine knot (Fig. 1B).
TGF-βs and other proteins of the superfamily are produced as pre-pro proteins [2]. The pro-domains promote the maturation, including the formation of the inter-chain disulfide bond that stabilizes their dimeric structure [24,25]. TGF-β’s pro-domain, also known as latency associated peptide, or LAP, is almost three times the length of the mature signaling protein (251 – 280 vs. 112 residues, respectively) and contains three cysteines, two of which (C223 and C225 in TGF-β1) pair cross-wise with the same cysteines in another molecule of the pro-domain to form the pro-domain homodimer [3]. The pro-domains for proteins of the superfamily vary widely in size and sequence: all are cleaved from the mature N-terminal signaling protein prior to secretion, and while the pro-domains of some superfamily proteins, such as TGF-β, associate tightly with the mature signaling dimer and are secreted in complex with them, some, such as those for activins, do not [2]. The approaches used to produce the proteins of the superfamily include a) overexpression of the full-length pre-pro proteins in cultured eukaryotic cells, and b) overexpression of the mature monomers in bacteria, followed by renaturation of the overexpressed polypeptide into native dimers. The procedures commonly used to obtain proteins of the superfamily by expression in eukaryotic cells will first be described, followed by a summary of procedures used to renature bacterially expressed protein into native dimers.
3.1.1 Expression of signaling proteins in eukaryotic cells
The majority of superfamily proteins produced in eukaryotic cells have been produced in mammalian cells, such as CHO or HEK-293 cells [18,26–41]. The preference for mammalian cells is largely for practical reasons, as most investigators are interested in producing mammalian signaling proteins and mammalian cells have the appropriate molecular machinery in the endoplasmic reticulum (ER) for folding and proteolytic processing. Though mammalian hosts are widely used, it has been observed that the proteolytic processing of the pro domain in the ER is often incomplete. This can sometimes complicate the purification as it then becomes necessary to not only separate the mature signaling dimer from its pro-domain, but also the pro-domain-mature precursor. There are several reports of the overexpression of mammalian signaling proteins in non-mammalian cells, including human BMP-2, bovine activin A, and bovine inhibin A in insect cells [42,43] and human activin A in yeast [44]. These expression hosts have some advantages over mammalian cells, including the absence of native binding partners that can interfere with maturation [18] and potentially higher yields [44,45]. The higher yields have only been obtained when the protease cleavage site between the pro-domain and mature signaling dimer have been altered to more closely match the endogenous proteases in the expression host [44,45]. Thus, successful heterologous expression has required some alterations to account for differences in molecular machinery relative to mammalian hosts, but so far these have been relegated to the enzymes responsible for proteolytic processing, not enzymes involved in catalyzing disulfide exchange or folding.
The signaling proteins are usually expressed along with their native pro-domains, although the signal peptides are often substituted to improve secretion [18,33]. The overexpressed proteins are purified from the conditioned medium using either conventional means (ion-exchange, gel filtration, etc.) or purification tags, such as hexahistdine tags. The N-terminus of the mature signaling dimer is accessible and flexible and thus provides a suitable site for tagging, while the C-terminus is structurally ordered and buried in the interior of the structure, and thus does not provide an appropriate site for tagging. If N-terminal tags are used, they should be removed after the signaling protein has been purified as the N-terminus lies near the binding site for the type I receptor and tags attached here have been found to block receptor binding and signaling [44]. The most straightforward means of removing the tag is to engineer a protease cleavage site between the purification tag and the N-terminus and then treat with the corresponding protease after the mature signaling protein has been purified. The alternative is to place the tag downstream of the predicted signal peptide cleavage site, but before the beginning of the pro-domain. Tagging at this position has the advantage that the tag is removed along with the pro-domain after the pro-domain has been separated away from the mature signaling dimer. The disadvantage of tagging at this position is that some pro-domains, such as those for activin A, associate rather weakly with their mature domain, which may lead to significant loss of the mature signaling protein during the wash steps of the purification.
The complexes between the pro-domains and the mature signaling dimer are generally soluble and can be isolated under non-denaturing conditions. The TGF-β1:TGF-β1 pro-domain complex, for example, is isolated in 50 mM Tris, 150 mM NaCl, pH 8.0 [18,33]. The mature signaling dimers, on the other hand, are generally poorly soluble in standard buffers at neutral pH after the pro-domain has been removed. The most common procedure is to acidify the pro-domain:signaling dimer complex after it has been purified from the medium. The acidification serves to release the mature signaling dimer from its pro-domain and to increase its charge, which generally improves solubility. Though acidification irreversibly unfolds many proteins, this is not generally observed with the disulfide-rich proteins of the TGF-β superfamily, and thus it is common for the final purification to be performed under acidic conditions. The procedures most commonly used include cation-exchange chromatography in acidic buffers (such as acetate at pH 4.0) or reverse phase chromatography with 0.05 –0.1% triflouracetic acid (and either acetonitrile or methanol for the gradient elution). The concentrated stocks of most superfamily proteins are also commonly stored under acidic conditions, such as 1 mM HCl or 100 mM acetic acid. Though it varies, most superfamily proteins are soluble when diluted 1:100 or 1:1000 from 1 mg/mL stocks in acidic conditions to phosphate buffered saline or other similar solutions near neutral pH. The procedure used to express and purify human TGF-β1 from CHO cells as initially described by Zou, et. al [18] is described below.
3.1.2 Protocol for expression and purification of human TGF-β1 using CHO cells
Establishment of a stable TGF-β1-expressing cell line
Seed CHO-lec3.2.8.1 cells at a density of 3 × 106 cells in 5 mL DMEM/F12 medium containing 10% FBS in T-25 flask and culture them at 37°C with 5% CO2.
After 24 h, replace the medium with 4 mL fresh DMEM/F12 medium containing 10% FBS.
Dilute 10 μg of CMV-based expression plasmid (see section 2.2 above) in 500 μL of Opti-MEM (mix A) and dilute 30 μL of lipofectamine 2000 in 500 μL Opti-MEM (mix B).
Incubate at room temperature for 20 min.
Add mix A to mix B with gentle pipetting up and down.
Incubate for 20 min at room temperature.
Add the mix A + B to cells.
Replace the medium (DMEM/F12 with 10% FBS) after 24 h. (see note 2).
After two days the cells were trypsinized, seeded into ten 96-well plates, and cultured with glutamine-free GMEM-S medium supplemented with GS supplement, 10% FBS, and 30 μM MSX. (see note 3)
After about three weeks, take the supernatant from growing wells (with visible colony) and assayed for TGF-β1 expression by ELISA. (see note 4)
Pick the ten highest expression clones and do the second round selection with 500 μM MSX. For each clone, seed one 96-well plate with 100–1000 cells per well. Wait for another three weeks and repeat the step above.
After two rounds of selection and amplification, the clone with the highest expression of TGF-β1 was chosen for large-scale recombinant protein production. Make stocks of the chosen clone and store them in liquid nitrogen.
Expression of TGF-β1 from CHO cells and purification
Expand the highest expression clone of TGF-β1 into T-225 flasks or T-500 triple layer flasks and culture the cells in glutamine-free GMEM-S medium supplemented with GS supplement, 10% FBS, and 500 μM MSX.
When the cultured cells reach confluence, wash the cells twice with PBS and change the medium to CHO-S-SFM II serum-free medium.
After 3 days, harvest the medium and replace with fresh CHO-S-SFM II serum-free medium, repeat 5–6 times or until desired volume. (see note 5)
Freeze harvested medium at −20 °C for further purification.
Thaw 500 mL harvested medium add imidazole to final concentration of 10 mM, filter with 0.22 μM membrane. (see note 6)
Equilibrate 20 mL Ni-NTA column with Ni buffer containing 50 mM Tris, 150 mM NaCl and 10 mM imidazole at pH 8.0.
Load 500 mL filtered medium onto Ni column and wash the column with Ni buffer for two column volumes.
Elute the protein by a linear concentration gradient of imidazole (10–500 mM) in Ni buffer.
Pool fractions that contain protein and adjust the pH to below 3.0 by adding 6 M HCl (see note 7); dialyze the solution against 100 mM acetic acid (minimum 20-fold dilution, three times).
Purify TGF-β1 on Source 15S column following the same protocol as TGF-β2 (steps 4–8 in 3.1.4 Isolation of native dimer) except using the linear gradient of 0–600 mM NaCl. (see note 8)
3.1.3 Expression of signaling protein monomers in bacteria and renaturation into native dimers
The other major avenue by which signaling proteins of the superfamily have been obtained is by expression of the mature monomers in bacteria, followed by renaturation of these into disulfide-linked dimers. The majority of family members are expressed using pET vectors, which are based on the T7 promoter [47]. To optimize expression, synthetic genes in which the codons have been optimized for bacteria may be used. Though N-terminal tags are sometimes added, these are not generally required, as the proteins invariably misfold and form highly refractile inclusion bodies. The formation of inclusion bodies aids in the purification since after disrupting the cells in non-denaturing buffer, most of the contaminating soluble proteins can be removed by repeatedly washing the inclusion bodies with non-denaturing buffers containing either salt (e.g. 0.5 – 1.0 M NaCl) or non-ionic detergents (e.g. 0.5 – 2.0% Triton X-100). The isolated inclusion bodies are almost always reconstituted in high concentrations of denaturant, such as 6 M GdmCl or 8 M urea. Typically, the denaturant is buffered at pH 8.0 – 9.0 and includes a reductant, such as dithiothreitol, to convert any disulfides into free cysteines. Tris is also often included, especially if urea is used as the denaturant, as its primary amine group will react with isocyanic acid which is formed from the breakdown of urea. Though typically already quite pure at this stage, the proteins are nevertheless often further purified in denaturant using ion-exchange, gel filtration, or metal affinity chromatography to remove residual contaminants that might interfere with the subsequent folding.
The native signaling dimers are most often formed by diluting the reduced denatured monomers into a much larger volume of folding buffer. The folding buffers used for most of the proteins of the superfamily are based on the buffers reported by Cerletti and co-workers for refolding the TGF-βs [48]. These include, among other components, low (mM) concentrations of redox agents, such as cysteine/cystine, reduced glutathione/oxidized glutathione, or dithiothreitol/trans-4,5-dihydroxy-1,2-dithiane (oxidized dithiothreitol) and relatively high concentrations (20 – 30 mM) of the non-denaturing detergent 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate, also known as CHAPS. The particular choice of redox agents is highly protein-dependent: sometimes only reductants are used, while in other cases a combination of reductants and oxidants are used. The majority of proteins folded have included CHAPS in the folding buffer, though notably, a recent study by Ejima and co-workers found that the CHAPS derivative sodium taurodeoxycholate (TDCA) was about 10 – 20 times more effective than CHAPS for folding human activin A [49]. The inclusion of CHAPS, or derivatives such as TDCA, is the most expensive reagent used in the folding since to be effective they must be used at concentrations comparable or higher than their critical micelle concentrations (which for CHAPS is near 10 mM). The folding mixture sometimes also include a co-solvent: TGF-βs, for example, have been folded in buffers containing 10 – 20 % DMSO, while activin A has been folded without any co-solvent. The requirement for co-solvents, such as DMSO, seems to be related to the solubility of the mature signaling dimer, as activins are reasonably soluble at milligram per milliliter concentrations at pH 8.0, while TGF-βs are not. The pH of the folding buffer is almost always 8.0 or higher, which is essential as cysteine thiols have a pKa of approximately 8.5 and are not capable of forming disulfides unless their sidechain thiols are deprotonated.
The concentration of protein in the folding buffer is important: concentrations higher than about 0.4 mg/mL are generally undesirable as this tends to lead to significant precipitate and lower yields of native dimers. Though less precipitate and greater yields of native dimers can generally be obtained with lower protein concentrations, there is a limit since the formation of disulfide-linked dimers is concentration-dependent and is favored at higher protein concentrations. Thus, the protein concentration must be adjusted to achieve a balance between minimizing loss due to increased formation of aggregates at higher concentration, and a slow rate of dimer formation at low protein concentrations. Though not yet in widespread use, it has been proposed that the folding be performed in two phases, one in which native monomers are formed, and another is which the native monomers are oxidized into disulfide-linked dimers [50]. To demonstrate feasibility, BMP-2 was initially folded under relatively dilute conditions (0.2 mg/mL) in the presence of redox buffers that promote disulfide exchange (0.1 mM reduced glutathione, 0.1 mM oxidized glutathione), and after a significant fraction of protein had folded into native monomers, the protein was concentrated five-fold (1 mg/mL) and transferred into buffer that only included oxidized glutathione (25 mM). The authors reasoned that this should lead mainly to the formation of native disulfide-linked dimers since all of the other cysteine residues had already formed their native disulfide-pairings (and were therefore not accessible for misfolding). This procedure was quite successful for BMP-2, with yields roughly 2-fold higher than that of single phase folding at 0.2 mg/mL for a period three times longer. The two-step folding method described above is predicated on the assumption that the monomers form the cystine knot and adopt their native folds independent of the other monomer. This may not be so for all family members, but it does appear to be for some, as native monomers of both GDF-5 and TGF-β3 have been isolated [10,51–53]. Thus, this procedure may lead to refolding of additional family members that have not been previously folded, but whether this procedure will be applicable to all family members has not yet been established.
The folding of most superfamily proteins can be monitored by running samples of the folding mixture on non-reducing SDS-gels; over a period of several days, one observes an increase in dimeric forms and a concomitant decrease in monomeric forms as the cysteines are oxidized and the proteins fold into native dimers. The primary species in the folding buffer after several days of folding include, in addition to native dimers, non-dimerized monomers and high molecular weight disulfide-linked aggregates. Ion-exchange chromatography is well-suited for isolation of the native dimers from folding mixtures owing to charge differences among the different species present. In practice, the folding mixtures are usually acidified, concentrated, and loaded onto cation-exchange columns under acidic conditions and eluted with a salt gradient. In some cases, the native dimers are directly isolated from the folding mixture using reverse-phase chromatography under acidic conditions as described above; more commonly, reverse phase chromatography is used as a polishing step following initial isolation of the native dimers using ion-exchange chromatography.
The yields of mature signaling dimer obtained using the procedures described here vary greatly. The procedure shown below typically yields 8 – 10 milligrams of TGF-β2 from each liter of bacteria, while similar procedures afford 3 – 4 milligrams of TGF-β3, and almost no TGF-β1. The differences in yield are due to differences in the efficiency of the folding – all three isoforms are produced at comparable levels and are present in equal amounts (ca. 50 – 60 milligrams per liter of culture medium) prior to folding. This emphasizes the point that the folding is highly dependent on the amino acid sequence and folding conditions. The most reasonable strategy that can pursued if the folding does not lead to native dimer would be to adhere to the procedures described above for isolating the purified denatured monomers, and then screen for formation of native dimers under different folding conditions using an ELISA-based approach. The types of screens that have been previously described for folding proteins of the superfamily can be tried [48,49] as well as other screens that have been reported for folding disulfide rich proteins more generally [54]. Though the development of a folding protocol that affords native dimers with high efficiency (defined as the amount of native dimer isolated relative to the amount of purified denatured monomer) can be time-consuming, this investment can pay off as folding efficiencies as high as 40% have been reported, affording large quantities (20 – 30 mg) of highly purified dimer from just a few liters of cultured bacteria. The procedure used to bacterially express, refold, and purify human TGF-β2 is described below.
3.1.4 Protocol for E. coli expression, refolding, and purification of human TGF-β2
Expression
Transform E. coli strain BL21(DE3) with the plasmid containing TGF-β2 sequence. Spread onto a LB-agar plate supplemented with ampicillin (150 μg/mL) and incubate overnight at 37°C.
Inoculate 1 L LB medium, supplemented with ampicillin (150 μg/mL), with colonies from the plate and incubate at 37°C with orbital shaking at 250 rpm in a 2.8 L flask. Add 0.8 mM IPTG at mid-log phase (0.6 OD600) to induce protein expression and incubate at 37°C for 3 h.
Harvest the cells by centrifuge at 6,000 × g for 15 min and freeze the pellet.
Recover insoluble, monomeric TGF-β2
Cell pellets from 1 L of culture were resuspended in 30 mL of disruption buffer (100 mM Tris, 10 mM EDTA, 1 mM PMSF, pH 8.0) and sonicated.
Centrifuge at 20,000 × g for 20 min and discard the supernatant (see note 1).
Resuspend pellet in 100 mL disruption buffer containing 1M NaCl.
Centrifuge at 20,000 × g for 20 min and discard the supernatant.
Resuspend pellet in 100 mL disruption buffer containing 1% Triton X-100 (v/v).
Centrifuge at 20,000 × g for 20 min and discard the supernatant.
Resuspend pellet in 100 mL TGF-β solubilizing buffer (8 M urea, 20 mM Tris, 1% DTT (w/v), pH 8.0), stir overnight at room temperature.
Add solid sodium acetate to a final concentration of 20 mM and adjust the pH to 4.0 using glacial acetic acid.
Centrifuge at 20,000 × g for 20 min and collect supernatant.
Initial purification
Equilibrate 20 mL SP-sepharose cation exchange column with SP denaturing buffer (8 M urea, 20 mM sodium acetate, 0.1% DTT (w/v), pH 4.2) for 2 column volumes.
Load the supernatant onto column and wash the column with SP denaturing buffer for 2 column volumes.
Elute with a linear 0–300 mM NaCl gradient in SP denaturing buffer.
Identify fractions containing purified TGF-β2 using SDS-PAGE; pool purified denatured TGF-β2 and dialyze against 100 mM acetic acid at 4°C (minimum 20-fold dilution, 3 times).
Folding
Isolation of native dimer
Adjust the pH of folding mix to 3.5 using acetic acid and concentrate the mixture to roughly 50 mL using an Amicon stirred cell concentrator with a YM-10 membrane (Millipore).
Dialyze the concentrated refolding mix against 100 mM acetic acid (minimum 20-fold dilution, three times) (see note 4).
Centrifuge at 20,000 × g for 20 min, collect supernatant and filter through 0.22 μM membrane (see note 5).
Equilibrate a Source 15S HR 10/10 column with 20 mM sodium acetate, 30 % isopropyl alcohol, pH 4.0 (Source 15S buffer) at a flow rate of 2.0 mL/min.
Load sample onto Source 15S column and wash column with two column volumes Source 15S buffer.
Elute with a linear gradient 0 – 400 mM NaCl over 10 column volumes in Source 15S buffer.
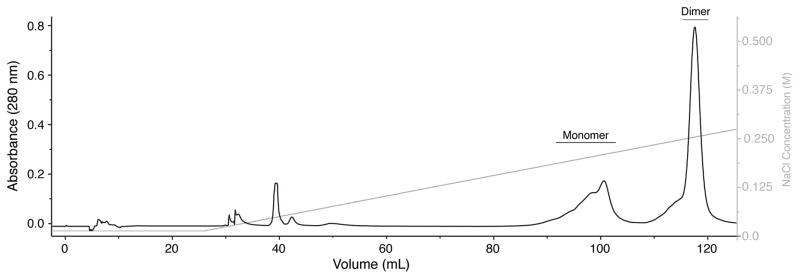
Collect peak fractions corresponding to the dimer peak (see Fig. 2) and then dialyze against 100 mM acetic acid (minimum 20-fold dilution, 3 times).
Measure the concentration of purified TGF-β2 by OD280 absorbance, aliquot, and lyophilize.
Figure 2.
Isolation of the human TGF-β2 dimer. Cation-exchange (Source 15S, GE Healthcare) elution profile of the TGF-β2 folding mixture. Peaks corresponding to monomeric and dimeric forms are labeled. Column was equilibrated in 20 mM sodium acetate, 30% isopropanol, pH 4.0 and eluted with a linear NaCl gradient in the same buffer (as shown by the grey line and accompanying axis on the right).
3.2 Overview of receptor ectodomain production and isolation
The structurally characterized receptor ectodomains of the TGF-β superfamily, which includes the type I receptors Alk1 [55,56], Alk3 [11,57], Alk5 [10,12,58], and Alk6 [59] and the type II receptors ActRII [8,60], ActRIIb [13,61], BMPRII [62], and TβRII [63–65], have each been shown to adopt a three finger toxin fold, consisting of three pairs of antiparallel β-strands stabilized by a characteristic pattern of four disulfides (Fig. 1C). The seven type I receptors, and all but one of the type II receptors of the superfamily, include one additional disulfide, while the TGF-β type II receptor, includes two. The procedures that have been used to produce the ectodomains of the superfamily are similar to those used to produce the signaling proteins and are described below.
3.2.1 Expression of receptor ectodomains in eukaryotic cells
The majority of receptor ectodomains that have been produced and studied are from mammalian species, such as humans and rodents, yet an almost equal number have been produced in mammalian and non-mammalian hosts. The mammalian hosts used include CHO, HEK-293, and mouse myeloma cells [56,66–70], while the non-mammalian hosts include insect cells and the methylotrophic yeast, Pichia pastoris [61,70–73]. The majority of receptors have been produced as secreted proteins, either alone as monomers, or as dimers by fusing them with the Fc region of an antibody. The Fc-dimerized receptors typically bind their cognate signaling proteins with affinities that are 1 – 2 orders of magnitude greater than their non Fc-dimerized counterparts [55,66,74], which is expected for a bivalent interaction between a covalently dimerized receptor and a covalently dimerized signaling protein. The Fc-dimerized receptor ectodomains are useful for increasing potency for use in binding studies [66] or as inhibitors in cells or animals [75–77].
The N- and C- termini are accessible and flexible in all of the type I and type II receptors of the superfamily, and thus affinity tags, such as hexahistidine tags, can be readily appended to the N- or C-termini without interfering with folding. To date, most of the ectodomains have been produced with a N-terminal tag followed by a protease cleavage site though a few have been produced with cleavable C-terminal tag. The receptors can be directly purified from the conditioned medium if they have an affinity tag. This generally yields protein free of most contaminants, though in many cases, some high molecular weight material may also be present. This high molecular weight material often disappears if the receptors are reduced prior to loading on the gel, indicating that while most of the overexpressed protein folds and adopts native disulfide pairings, some misfolds and forms disulfide-linked multimers. Thus, in many cases, receptors that have been purified with an affinity tag are further purified to eliminate multimers as well as other contaminating proteins. The multimers can be relatively easily eliminated using either size exclusion chromatography, which separates based on size, or ion-exchange chromatography, which separates based on charge (monomers have lower overall charge compared to multimers, and thus elute at lower salt concentrations than the multimers). The purification tag is often removed by treatment with appropriate protease prior to the second step of the purification, as such tags can potentially interfere crystallization. If an affinity tag was not included in the construct, the medium is usually desalted, and the dialyzed medium is then applied to anion or cation exchange column equilibrated at a pH where the protein is expected to bind (usually determined by calculating the net charge as a function of pH using a tool such as the Scripps Protein Calculator [78]). In many cases, this affords protein that is free of most other contaminating proteins, as well as disulfide-linked multimers, though if not, an additional purification step, such as size exclusion or hydrophobic interaction chromatography, can be used. Importantly, non-reducing SDS-PAGE must be used to analyze the purity of the final preparation since reducing agents, such as β-mercaptoethanol or dithiothreitol (DTT), will convert disulfide-linked oligomers or multimers into monomers. It is important to be aware of the presence of disulfide-linked oligomers or multimers and to remove them if they are present since these will interfere in subsequent binding and structural studies.
3.2.2 Protocol for expression of human TβRII-Fc using CHO cells
Establishment of a stable TβRII-Fc expressing cell line
The human TβRII ectodomain is inserted in the polylinker of plasmid pFUSE-hIgG4-Fc (Invivogen) to yield an expression construct in which TβRII is in frame with a C-terminal human IgG4 Fc domain. The TβRII-Fc expression cassette is then PCR amplified and inserted downstream of the rat serum albumin signal peptide in the modified form of pcDNA3.1(+) with a glutamine synthase gene described in Section 2.2.
The CMV-based TβRII-Fc expression plasmid is then transfected into CHO-lec3.2.8.1 cells and a stable expressing clone is selected using the same procedure described in Section 3.1.2 above.
Expression of TβRII-Fc from CHO cells and purification
Stably transfected cells are cultured in serum free medium in T-225 flasks and the conditioned medium is collected as described in Section 3.1.2 above.
Conditioned medium is diluted three-fold into 0.1 M Tris, pH 8.2 and passed over a HiTrap protein A column (GE Healthcare) equilibrated with 0.15 M NaCl, 0.01 M sodium phosphate, pH 7.2
Column is washed with ten column volumes of 0.15 M NaCl, 0.01 M sodium phosphate, pH 7.2 and the protein is then eluted by passing over five column volumes of 0.1 M citrate, pH 3.0.
Eluted protein is adjusted to pH 7.0 by addition of 0.1 M Tris base, followed by concentration to roughly 10 mg/mL using a Amicon ultrafiltration stirred cell (Millipore).
One milliliter aliquots of the TβRII-Fc fusion is passed over a 1.6 × 60 cm Superdex 200 gel filtration column (GE Healthcare) equilibrated in 0.1 M Tris, pH 7.0. Peak fractions are pooled, concentrated to 10 mg/mL using an Amicon ultrafiltration stirred cell. Protein purity is checked by non-reducing SDS-PAGE and pooled stocks are stored at −20 °C until ready for use.
3.2.3 Expression of receptor ectodomains in bacteria
The other major avenue by which the receptor ectodomains have been obtained is by expression in bacteria. The ectodomains, if expressed alone, form highly refractile inclusion bodies and must be renatured [10,52,55,58,64,73,79]. The overall procedure parallels that used for the signaling proteins, including initial isolation of the inclusion bodies, subsequent purification of the reduced protein in denaturant, and folding by dilution into non-denaturing buffers that include redox-active species that promote disulfide exchange. The procedure used to fold and purify the human TβRII ectodomain is described in Section 3.2.4 below.
The receptor ectodomains, like the signaling proteins, do not require a purification tag as the overexpressed protein forms inclusion bodies, and are therefore largely pure, even before the initial purification in denaturant. Tags are however sometimes included as they aid in the isolation of the protein from the folding buffer. The buffers used to fold the receptors are generally simpler than those used to the fold the signaling proteins: most include redox-active species, such as reduced and oxidized glutathione, but other additives such as non-denaturing detergents and co-solvents are not generally required. The ectodomains generally have favorable solubility properties and can be concentrated to high concentration (10 mg/mL, or even higher) in standard buffers (Tris, Hepes, phosphate, etc.) at neutral pH. Thus, the receptors are usually concentrated following folding, and then dialyzed into a buffer suitable for subsequent ion-exchange or gel filtration chromatography. These two methods are both effective for purifying the receptors following folding as both eliminate disulfide-linked oligomers, which are the major ‘contaminant’ present in the folding. These two purification methods do not, however, have sufficient resolution to separate native monomers from non-native monomers, and thus in cases where the monomer pool is heterogeneous, it is recommended that the receptor be further purified using C8 or C18 reverse phase chromatography. This method of purification, which separates based on hydrophobicity, has outstanding resolution and has been found to be effective for separating native monomers away from the non-native monomers. This type of purification denatures the receptors (since it is commonly performed in 0.1 % trifluoroacetic acid with either acetonitrile or methanol elution), but is not problematic since the disulfides remain intact and the receptors readily refold after the triflouroacetic acid and acetonitrile/methanol have been removed by either lyophilization or dialysis. The final purity of the receptor ectdomains, like the signaling proteins, should be assessed by non-reducing SDS-PAGE, since as before reductant will mask any misfolded disulfide-linked dimers and higher order oligomers.
Several receptors of the superfamily, including BMPRIa, BMPRIb, BMPRII, and TβRII have been previously produced as soluble proteins in E. coli by expressing them as fusion proteins with thioredoxin [62,80–84]. The advantage of producing the receptors in this manner is that the proteins remain soluble and can be extracted from the bacteria in their native form. These types of fusion proteins are usually constructed with an intervening hexahistidine tag and protease cleavage site between thioredoxin and the receptor. To increase the proportion of protein that remains soluble, the growth temperature can be lowered from 37 °C to 20 °C prior to the induction of protein expression. The protein is extracted from the cells by disrupting them in non-denaturing buffer with protease inhibitors. The proteins are usually purified by metal affinity chromatography, followed by a subsequent polishing step using gel filtration. The latter is important as the proteins produced in this manner often include some disulfide-linked dimer and higher order oligomers.
3.2.4 Protocol for expression of the human TβRII ectodomain using E. coli
Expression
Transform E. coli strain BL21(DE3) with the plasmid coding for the human TβRII ectodomain. Spread onto an LB-agar plate supplemented with ampicillin (150 μg/mL) and incubate overnight at 37°C.
Inoculate 1 L LB medium, supplemented with ampicillin (150 μg/mL), with colonies from the plate and incubate at 37°C with orbital shaking at 250 rpm in a 2.8 L flask. Add 0.8 mM IPTG at mid-log phase (0.6 OD600) to induce protein expression and incubate at 37°C for 3 h.
Harvest the cells by centrifugation at 6,000 × g for 15 min and freeze the pellet.
Recover non-soluble TβRII
Cell pellet from 1 L of culture is resuspended in 30 mL of disruption buffer (100 mM Tris, 10 mM EDTA, 1 mM PMSF, pH 8.0) and sonicated
Centrifuge at 20,000 × g for 20 min and discard the supernatant (see note 1)
Resuspend pellet in 100 mL disruption buffer containing 1 M NaCl
Centrifuge at 20,000 × g for 20 min and discard the supernatant
Resuspend pellet in 100 mL disruption buffer containing 1% Triton X-100 (v/v)
Centrifuge at 20,000 × g for 20 min and discard the supernatant
Resuspend pellet in 100 mL RII solubilizing buffer (8M urea, 20 mM Tris, pH 7.0), stir overnight at room temperature
Centrifuge at 20,000 × g for 20 min and collect supernatant
Initial purification
Equilibrate 20 mL DEAE-sepharose anion exchange column with RII solubilizing buffer (8M urea, 20 mM Tris, pH 7.0) for two column volumes.
Load the supernatant onto column and wash the column with RII solubilizing buffer for two column volumes.
Elute with a linear 0 – 300 mM NaCl gradient in RII solubilizing buffer.
Identify fractions containing purified TβRII using reducing SDS-PAGE; pool purified denatured TβRII; add solid DTT to final concentration of 25 mM, stir for 30 min and dialyze against 100 mM acetic acid at 4°C (minimum 20-fold dilution, 3 times).
Folding
Dialyzed protein is slowly diluted into RII folding buffer containing 200 mM Tris, 2 mM reduced-glutathione, 0.5 mM oxidized-glutathione, pH 8.0 to a final concentration of 0.5 mg/mL at 4°C. (see note 2). Stir gently for 18 – 24 h at 4 °C in an open vessel.
Isolation of native TβRII
Concentrate the folding solution to roughly 50 mL using an Amicon stirred cell concentrator with a YM-3 membrane (Millipore).
Dialyze the concentrated folding solution against 20 mM MES, pH 6.0 at 4°C (minimum 20-fold dilution, 3 times).
Centrifuge at 20,000 × g for 20 min, collect supernatant and filter through 0.22 μM membrane.
Equilibrate a Source 15Q HR 10/10 column with 20 mM MES, 1 mM PMSF, 1 mM EDTA, pH 6.0 (Source 15Q buffer) at a flow rate of 2.0 mL/min.
Load sample onto Source 15Q column and wash column with 2 column volumes Source 15Q buffer.
Elute with a linear gradient 0 – 300 mM NaCl in Source 15Q buffer over 10 column volumes.
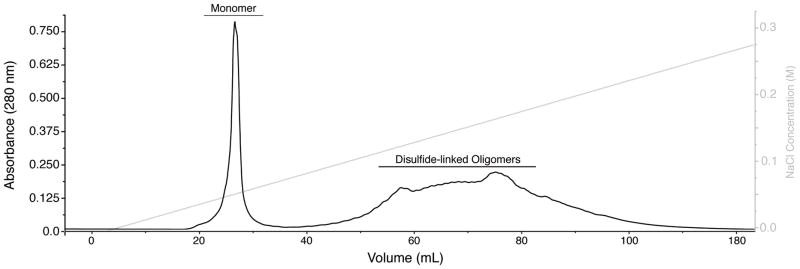
Pool the fractions corresponding to purified native receptor (see Fig. 3) and dialyze against Source 15Q buffer to remove salt (minimum 20-fold dilution, 3 times).
Measure the concentration of purified TβRII by OD280 absorbance, concentrate to 5 – 10 mg/mL, aliquot and store at −20°C.
Figure 3.
Isolation of the human TβRII ectodomain. Anion-exchange (Source 15Q, GE Healthcare) elution profile of the TβRII folding mixture. Peaks corresponding to native monomer and non-native disulfide-linked oligomeric forms are labeled. Column was equilibrated in 20 mM MES, pH 6.0 and eluted with a linear NaCl gradient in the same buffer (as shown by the grey line and accompanying axis on the right).
3.3 Methods to assess ligand-receptor binding
The binding properties of signaling proteins and receptors of the superfamily have been predominantly studied using surface plasmon resonance (SPR) [8–10,12,13,55,56,58,59,66,67,77,85–102]. The feature of this method that has led to its widespread use is that the signaling proteins can be readily coupled to SPR sensor surfaces at low pH where the proteins are soluble, and then the conditions can be changed to study receptor binding near neutral pH. The other advantage of performing SPR experiments with immobilized signaling proteins is that the amount of protein required is minimal (0.2 – 2 micrograms). These quantities can usually be purchased at a reasonable cost, though if they are, it is important to purchase ‘carrier-free’ protein so that the coupling of the signaling protein to the SPR sensor chip is not blocked by the carrier.
The signaling proteins are most commonly coupled to sensor chips by a carbodiimide coupling reaction in which amino groups of lysine residues on the signaling protein are coupled to carboxylates on the surface of the sensor chip. Though reliable and easy to perform, this has the disadvantage that it results in a random coupling of the signaling protein to the sensor chip. This can result in a heterogenous surface in which some of the protein is coupled such that receptor binding is unimpaired, but another portion in which receptor binding is partially of fully impaired. The heterogeneous surfaces are generally undesirable since this complicates the pattern of receptor binding. One way to minimize heterogeneity is to form the complex in solution between the signaling protein to be studied and its receptors and in turn biotinylate the complex using an amine-reactive biotin derivative. Once modified, the biotinylated signaling protein can be separated from the receptor(s) by purifying it under denaturing conditions (such as C8 or C18 reverse phase chromatography with either acetonitrile or methanol gradient elution). This separation step also removes free biotin from signaling protein. After it has been dialyzed to remove the acetonitrile or methanol, it can be captured onto a sensor chip coated with a high density of streptavidin or neutravidin.
The binding measurements are performed by using the microfluidic system of the SPR instrument to inject the receptor ectodomain for a given period of time, followed by a disassociation period in which the instrument is switched back to the running buffer. Two types of experiments are commonly performed, kinetic and equilibrium experiments. Kinetic experiments are performed by injecting at a high flow rate for a period long enough to determine the rate of binding, but not so long that the response reaches equilibrium, while equilibrium experiments are performed by injecting at a low flow rate for a period long enough so that equilibrium is reached. Kinetic experiments are analyzed by fitting the time-dependence of the association and disassociation to the simplest mathematical model describing the binding, while equilibrium experiments are analyzed by fitting the equilibrium response (Req) as a function of concentration of the injected receptor to a standard binding equation. The kinetic experiments therefore provide the association and disassociation rate constants (ka and kd, respectively, as well as the Kd from kd/ka) as well as the maximal response (Rmax), while the equilibrium experiments provide only the Kd and Rmax. Kinetic experiments therefore provide more information and are therefore generally desirable, though they have the disadvantage that this comes at the cost of using larger amounts of receptor (owing to the higher flow rates). The amount of receptor used in a typical kinetic SPR experiment in which the receptor concentration is varied from 0 – 4 μM is approximately 0.2 mg.
SPR experiments, in addition to providing rates and affinities for binding of a single receptor, also affords the opportunity to study binding of more than one receptor. Such measurements can provide insight into whether the receptors compete or cooperate with one another and have been instrumental in delineating the very different modes of receptor complex assembly for the TGF-βs and BMPs [9,10,12]. Such experiments can be performed in one of two ways: the first and simplest is to perform a co-injection experiment in which the first half of the injection loop is filled with a near-saturating concentration of the first receptor (e.g. type II receptor), while the second half is filled with a variable concentration of the second receptor (e.g. type I receptor) on top of the same concentration of the first receptor in the first half. Such experiments can therefore be used to determine whether the first receptor (e.g. type II receptor) alters the binding of the second receptor (e.g. type I receptor), by comparison to a control experiment in which the binding of the second receptor (e.g. type I receptor) is studied in the absence of the first receptor (e.g. type II receptor).
Several alternatives to SPR have been proposed in recent years, including bio-layer inferometry [103] and microscale thermophoresis [104]. Studies using these methods for studying interactions between signaling proteins and receptors of the TGF-β superfamily have not been reported in the literature, but it is expected that these will be increasingly used in the future, owing to their lower cost and ease of use. Binding studies using titration calorimetry have not been reported, presumably because most superfamily signaling proteins are poorly soluble and must therefore be stored under conditions (low pH) where they bind their cognate receptors poorly, or not at all. Such solubility properties require that the signaling protein and receptor be contained in different buffers, which has the disadvantage that the heat evolved from mixing solvents is large compared to the heat evolved by binding.
3.4 Structural analyses of ligand-receptor complexes
The relatively small size of the signaling proteins (ca. 25 kDa) and receptor ectodomains (11 – 16 kDa) has enabled studies of both using NMR [20,55,57,58,64,79,105–107] and X-ray crystallography [19,21,22,96,98,102,108–110]. The strategies used to obtain the NMR solution structures and X-ray crystal structures of the signaling proteins and receptor ectodomains more or less follow the standard methodologies. The larger sizes of the complexes, on the other hand, have relegated these to being studied mostly by X-ray crystallography [8–13,61,99,101]. The strategies used to obtain the structures of the signaling protein:receptor ectodomain complexes have mostly been performed by first adding an excess of receptors relative to signaling protein, followed by isolation of the complex by gel filtration. The rationale for this strategy is that it allows for the isolation of stoichiometric complexes, which due to their homogeneity, are more likely to form diffracting crystals. This strategy clearly has limits in light of weaker affinities and thus is likely to be successful for studying many of the signaling protein:receptor ectodomain complexes of the superfamily, but not all. The alternative strategy, in light of weaker affinities, is to combine the proteins in the appropriate stoichiometric ratio (two equivalents of type I or type II receptor ectodomain per equivalent of signaling homodimer), concentrate, and setup the crystallization trials at the highest protein concentration feasible (this has to be empirically determined based on the inherent solubility of the complex). The strategy is based on the presumption that the high protein concentration (typically on the order of 5 – 10 mg/mL) will drive the equilibrium toward complex formation given that the concentration of the complex (0.05 – 0.1 mM, depending on the size and concentration of the complex) is typically several orders of magnitude greater than the disassociation constant (typically 10 μM or lower). The alternative approach, in light of moderate to lower affinities, is to isotopically label either the signaling protein or receptor ectodomain with NMR-active stable isotopes (15N or 13C) and monitor the signals of the labeled component as increasing concentrations of the unlabeled partner are added [53]. The NMR signals of residues that lie directly in the binding site will be most strongly perturbed, thus providing a “map” of the contact surface. This procedure can be repeated with the labeling pattern reversed to obtain the complementary contact surface of the partner. These maps, together with the known structures of the two proteins, can in turn be used to construct a model of the complex using a data-driven docking algorithm, such as HADDOCK [111,112]. This strategy has not yet been used to determine the structures of any signaling protein:receptor complexes of the TGF-β superfamily, but has been widely used to study other systems [113], and may therefore be used to study signaling protein:receptor complexes of the superfamily, especially receptors, such as some of the BMP type II receptors, that bind their cognate signaling proteins weakly.
4. Notes
CHO-lec3.2.8.1 cells have multiple glycosylation defects for production of glycoproteins with minimal carbohydrate heterogeneity [46].
It is normal from some of the cells to detach and die after transfection.
In each well there should be 100–1000 cells and the transfection rate is approximately 0.1%, thus 0 −1 colonies are expected in each well.
Colonies that do form will grow at different rates and thus the medium should be removed only when the color of the medium turns yellow. Protein expression levels detected by ELISA do not generally correlate with growth rates.
Replace the medium when the color changes from red to yellow; can be repeated until the cells are no longer viable (as many as 10 times). Protein secretion levels generally decrease over time.
Add 10 mM imidazole in the sample to prevent non-specific binding to the Ni-NTA column
Acidification of the solution will dissociate the mature TGF-β1 from LAP; however, this step will not separate the mature TGF-β1 from incompletely processed pro-domain-mature precursor (usually 5 – 10%).
Only dimeric TGF-β will be observed (but not monomeric TGF-β as in the purification of refolded bacterial TGF-β)
The protein expressed in inclusion bodies will remain insoluble until dissolved in 8 M urea.
Small aliquots of 1 M NaOH are added during the addition to maintain the pH at 9.5.
The folding mixture will appear slightly cloudy and the folding can be monitored by running small aliquots of the folding mixture on a non-reducing SDS-PAGE gel.
Leave extra room in the dialysis tubing, because the volume will increase about 50% after dialysis.
The precipitate is mostly comprised of misfolded disulfide-linked multimers; the protein that remains soluble is mostly natively folded dimers and monomers.
The protein expressed in inclusion bodies will remain insoluble until dissolved in 8 M urea.
Small aliquots of 1 M NaOH are added to maintain the pH 8.0 during the addition.
5. Summary and future prospects
Structural studies of signaling proteins and signaling receptors of the TGF-β superfamily have shown that the TGF-βs, evolutionary latecomers to the superfamily, diverged from the BMPs and GDFs, the ancestral signaling proteins of the superfamily, to bind and assemble their type I and type II receptors in a distinct manner [14,16,17]. The receptors appear to have evolved these new modes of receptor binding in a stepwise manner, first by evolution of a new mode of type I receptor binding and later by evolution of a new mode of type II receptor binding. This idea is based on the observation that evolutionary intermediates of the superfamily, such as activins and nodal, bind and signal through type I receptors that are shared with the TGF-βs, evolutionary latecomers to the superfamily, and type II receptors that are shared with many of the distantly related BMPs and GDFs. There is at present only one structure of one of these intermediates bound to a receptor (activin A bound to its type II receptor, ActRIIb, or its close homolog, ActRII) [61,99], thus there is an urgent need to determine the structures of more of the evolutionary intermediates, such as activin, nodal, and myostatin, to their cognate type I and type II receptors. There are also several receptors yet to be structurally characterized, including the type I receptors Alk2, Alk4, and Alk7, and the type II receptor MISRII. The hope is that the principles and procedures described here for producing and studying the binding properties and structures of the signaling proteins and receptor ectodomains of the superfamily will lead to a better understanding of the different possible signaling complexes that the proteins of the superfamily form, and in turn, this will inform the underlying biology.
Acknowledgments
The author would also like to acknowledge the funding agencies that have supported the TGF-β research underway in his laboratory, including the NIH (GM58670 and CA172886), the Robert A. Welch Foundation (AQ-1842), and the Cancer Prevention and Research Institute in Texas (RP120867).
References
- 1.Kingsley DM. The TGF-beta superfamily: new members, new receptors, and new genetic tests of function in different organisms. Genes & development. 1994;8:133–46. doi: 10.1101/gad.8.2.133. [DOI] [PubMed] [Google Scholar]
- 2.Sengle G, Ono RN, Sasaki T, Sakai LY. Prodomains of transforming growth factor beta (TGFbeta) superfamily members specify different functions: extracellular matrix interactions and growth factor bioavailability. The Journal of biological chemistry. 2011;286:5087–99. doi: 10.1074/jbc.M110.188615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi M, Zhu J, Wang R, Chen X, Mi L, Walz T, Springer TA. Latent TGF-beta structure and activation. Nature. 2011;474:343–9. doi: 10.1038/nature10152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wrana JL, Attisano L, Carcamo J, Zentella A, Doody J, Laiho M, Wang XF, Massague J. TGF beta signals through a heteromeric protein kinase receptor complex. Cell. 1992;71:1003–14. doi: 10.1016/0092-8674(92)90395-s. [DOI] [PubMed] [Google Scholar]
- 5.Yamashita H, ten Dijke P, Franzen P, Miyazono K, Heldin CH. Formation of hetero-oligomeric complexes of type I and type II receptors for transforming growth factor-beta. J Biol Chem. 1994;269:20172–8. [PubMed] [Google Scholar]
- 6.Massague J. TGF-beta signal transduction. Annu Rev Biochem. 1998;67:753–91. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- 7.Massague J, Wotton D. Transcriptional control by the TGF-beta/Smad signaling system. EMBO J. 2000;19:1745–54. doi: 10.1093/emboj/19.8.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allendorph GP, Vale WW, Choe S. Structure of the ternary signaling complex of a TGF-beta superfamily member. Proc Natl Acad Sci U S A. 2006;103:7643–8. doi: 10.1073/pnas.0602558103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenwald J, Groppe J, Gray P, Wiater E, Kwiatkowski W, Vale W, Choe S. The BMP7/ActRII extracellular domain complex provides new insights into the cooperative nature of receptor assembly. Mol Cell. 2003;11:605–17. doi: 10.1016/s1097-2765(03)00094-7. [DOI] [PubMed] [Google Scholar]
- 10.Groppe J, et al. Cooperative assembly of TGF-beta superfamily signaling complexes is mediated by two disparate mechanisms and distinct modes of receptor binding. Mol Cell. 2008;29:157–68. doi: 10.1016/j.molcel.2007.11.039. [DOI] [PubMed] [Google Scholar]
- 11.Kirsch T, Sebald W, Dreyer MK. Crystal structure of the BMP-2-BRIA ectodomain complex. Nat Struct Biol. 2000;7:492–6. doi: 10.1038/75903. [DOI] [PubMed] [Google Scholar]
- 12.Radaev S, Zou Z, Huang T, Lafer EM, Hinck AP, Sun PD. Ternary complex of transforming growth factor-beta1 reveals isoform-specific ligand recognition and receptor recruitment in the superfamily. The Journal of biological chemistry. 2010;285:14806–14. doi: 10.1074/jbc.M109.079921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weber D, Kotzsch A, Nickel J, Harth S, Seher A, Mueller U, Sebald W, Mueller TD. A silent H-bond can be mutationally activated for high-affinity interaction of BMP-2 and activin type IIB receptor. BMC Struct Biol. 2007;7:6. doi: 10.1186/1472-6807-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Massagué J. A very private TGF-beta receptor embrace. Mol Cell. 2008;29:149–50. doi: 10.1016/j.molcel.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Nickel J, Sebald W, Groppe JC, Mueller TD. Intricacies of BMP receptor assembly. Cytokine & growth factor reviews. 2009;20:367–77. doi: 10.1016/j.cytogfr.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 16.Hinck AP, O’Connor-McCourt MD. Structures of TGF-beta Receptor Complexes: Implications for Function and Therapeutic Intervention Using Ligand Traps. Current pharmaceutical biotechnology. 2011 doi: 10.2174/138920111798808383. [DOI] [PubMed] [Google Scholar]
- 17.Hinck AP. Structural studies of the TGF-betas and their receptors - insights into evolution of the TGF-beta superfamily. FEBS letters. 2012;586:1860–70. doi: 10.1016/j.febslet.2012.05.028. [DOI] [PubMed] [Google Scholar]
- 18.Zou Z, Sun PD. Overexpression of human transforming growth factor-beta1 using a recombinant CHO cell expression system. Protein expression and purification. 2004;37:265–72. doi: 10.1016/j.pep.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Daopin S, Piez KA, Ogawa Y, Davies DR. Crystal structure of transforming growth factor-beta 2: an unusual fold for the superfamily. Science. 1992;257:369–73. doi: 10.1126/science.1631557. [DOI] [PubMed] [Google Scholar]
- 20.Hinck AP, et al. Transforming growth factor beta 1: three-dimensional structure in solution and comparison with the X-ray structure of transforming growth factor beta 2. Biochemistry. 1996;35:8517–34. doi: 10.1021/bi9604946. [DOI] [PubMed] [Google Scholar]
- 21.Mittl PR, Priestle JP, Cox DA, McMaster G, Cerletti N, Grutter MG. The crystal structure of TGF-beta 3 and comparison to TGF-beta 2: implications for receptor binding. Protein Science. 1996;5:1261–71. doi: 10.1002/pro.5560050705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schlunegger MP, Grutter MG. An unusual feature revealed by the crystal structure at 2.2 A resolution of human transforming growth factor-beta 2. Nature. 1992;358:430–4. doi: 10.1038/358430a0. [DOI] [PubMed] [Google Scholar]
- 23.Sun PD, Davies DR. The cystine-knot growth-factor superfamily. Annual review of biophysics and biomolecular structure. 1995;24:269–91. doi: 10.1146/annurev.bb.24.060195.001413. [DOI] [PubMed] [Google Scholar]
- 24.Gentry LE, Nash BW. The pro domain of pre-pro-transforming growth factor beta 1 when independently expressed is a functional binding protein for the mature growth factor. Biochemistry. 1990;29:6851–7. doi: 10.1021/bi00481a014. [DOI] [PubMed] [Google Scholar]
- 25.Gray AM, Mason AJ. Requirement for activin A and transforming growth factor--beta 1 pro-regions in homodimer assembly. Science. 1990;247:1328–30. doi: 10.1126/science.2315700. [DOI] [PubMed] [Google Scholar]
- 26.Bourdrel L, Lin CH, Lauren SL, Elmore RH, Sugarman BJ, Hu S, Westcott KR. Recombinant human transforming growth factor-beta 1: expression by Chinese hamster ovary cells, isolation, and characterization. Protein expression and purification. 1993;4:130–40. doi: 10.1006/prep.1993.1019. [DOI] [PubMed] [Google Scholar]
- 27.Bustos-Valenzuela JC, Halcsik E, Bassi EJ, Demasi MA, Granjeiro JM, Sogayar MC. Expression, purification, bioactivity, and partial characterization of a recombinant human bone morphogenetic protein-7 produced in human 293T cells. Molecular biotechnology. 2010;46:118–26. doi: 10.1007/s12033-010-9287-0. [DOI] [PubMed] [Google Scholar]
- 28.Chitty DW, Tremblay RG, Ribecco-Lutkiewicz M, Haukenfrers J, Zurakowski B, Massie B, Sikorska M, Bani-Yaghoub M. Development of BMP7-producing human cells, using a third generation lentiviral gene delivery system. Journal of neuroscience methods. 2012;205:17–27. doi: 10.1016/j.jneumeth.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 29.Israel DI, Nove J, Kerns KM, Kaufman RJ, Rosen V, Cox KA, Wozney JM. Heterodimeric bone morphogenetic proteins show enhanced activity in vitro and in vivo. Growth factors. 1996;13:291–300. doi: 10.3109/08977199609003229. [DOI] [PubMed] [Google Scholar]
- 30.Jones WK, et al. Osteogenic protein-1 (OP-1) expression and processing in Chinese hamster ovary cells: isolation of a soluble complex containing the mature and pro-domains of OP-1. Growth factors. 1994;11:215–25. doi: 10.3109/08977199409046919. [DOI] [PubMed] [Google Scholar]
- 31.Sampath TK, et al. Recombinant human osteogenic protein-1 (hOP-1) induces new bone formation in vivo with a specific activity comparable with natural bovine osteogenic protein and stimulates osteoblast proliferation and differentiation in vitro. The Journal of biological chemistry. 1992;267:20352–62. [PubMed] [Google Scholar]
- 32.Sathyamurthy M, Lee JS, Park JH, Kim YJ, Jeong JY, Jang JW, Lee GM. Overexpression of PACEsol improves BMP-7 processing in recombinant CHO cells. Journal of biotechnology. 2012;164:336–9. doi: 10.1016/j.jbiotec.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 33.Zou Z, Sun PD. An improved recombinant mammalian cell expression system for human transforming growth factor-beta2 and -beta3 preparations. Protein expression and purification. 2006;50:9–17. doi: 10.1016/j.pep.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 34.Lee SJ, McPherron AC. Regulation of myostatin activity and muscle growth. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:9306–11. doi: 10.1073/pnas.151270098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Q, et al. The conditioned medium from a stable human GDF3-expressing CHO cell line, induces the differentiation of PC12 cells. Molecular and cellular biochemistry. 2012;359:115–23. doi: 10.1007/s11010-011-1005-0. [DOI] [PubMed] [Google Scholar]
- 36.Peng J, et al. Growth differentiation factor 9:bone morphogenetic protein 15 heterodimers are potent regulators of ovarian functions. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E776–85. doi: 10.1073/pnas.1218020110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takaoka K, et al. Gene cloning and expression of a bone morphogenetic protein derived from a murine osteosarcoma. Clinical orthopaedics and related research. 1993:344–52. [PubMed] [Google Scholar]
- 38.Ulloa L, Creemers JW, Roy S, Liu S, Mason J, Tabibzadeh S. Lefty proteins exhibit unique processing and activate the MAPK pathway. The Journal of biological chemistry. 2001;276:21387–96. doi: 10.1074/jbc.M006933200. [DOI] [PubMed] [Google Scholar]
- 39.Ushiro Y, Hashimoto O, Seki M, Hachiya A, Shoji H, Hasegawa Y. Analysis of the function of activin betaC subunit using recombinant protein. The Journal of reproduction and development. 2006;52:487–95. doi: 10.1262/jrd.17110. [DOI] [PubMed] [Google Scholar]
- 40.Walton KL, Makanji Y, Wilce MC, Chan KL, Robertson DM, Harrison CA. A common biosynthetic pathway governs the dimerization and secretion of inhibin and related transforming growth factor beta (TGFbeta) ligands. The Journal of biological chemistry. 2009;284:9311–20. doi: 10.1074/jbc.M808763200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Papakostas TD, et al. Development of an efficiently cleaved, bioactive, highly pure FLAG-tagged recombinant human Mullerian Inhibiting Substance. Protein expression and purification. 2010;70:32–8. doi: 10.1016/j.pep.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cronin CN, Thompson DA, Martin F. Expression of bovine activin-A and inhibin-A in recombinant baculovirus-infected Spodoptera frugiperda Sf 21 insect cells. The international journal of biochemistry & cell biology. 1998;30:1129–45. doi: 10.1016/s1357-2725(98)00077-6. [DOI] [PubMed] [Google Scholar]
- 43.Maruoka Y, Oida S, Iimura T, Takeda K, Asahina I, Enomoto S, Sasaki S. Production of functional human bone morphogenetic protein-2 using a baculovirus/Sf-9 insect cell system. Biochemistry and molecular biology international. 1995;35:957–63. [PubMed] [Google Scholar]
- 44.Papakonstantinou T, Harris SJ, Fredericks D, Harrison C, Wallace EM, Hearn MT. Synthesis, purification and bioactivity of recombinant human activin A expressed in the yeast Pichia pastoris. Protein expression and purification. 2009;64:131–8. doi: 10.1016/j.pep.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 45.Fredericks D, Clay R, Warner T, O’Connor A, de Kretser DM, Hearn MT. Optimization of the expression of recombinant human activin A in the yeast Pichia pastoris. Biotechnology progress. 2010;26:372–83. doi: 10.1002/btpr.304. [DOI] [PubMed] [Google Scholar]
- 46.Rosenwald AG, Stanley P, Krag SS. Control of carbohydrate processing: increased beta-1,6 branching in N-linked carbohydrates of Lec9 CHO mutants appears to arise from a defect in oligosaccharide-dolichol biosynthesis. Molecular and cellular biology. 1989;9:914–24. doi: 10.1128/mcb.9.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Studier FW, Moffatt BA. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. Journal of molecular biology. 1986;189:113–30. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- 48.Cerletti N. Process for the production of biologically active dimeric protein. 6057430 US Patent. 2000
- 49.Ejima D, Ono K, Tsumoto K, Arakawa T, Eto Y. A novel “reverse screening” to identify refolding additives for activin-A. Protein expression and purification. 2006;47:45–51. doi: 10.1016/j.pep.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 50.von Einem S, Schwarz E, Rudolph R. A novel TWO-STEP renaturation procedure for efficient production of recombinant BMP-2. Protein expression and purification. 2010;73:65–9. doi: 10.1016/j.pep.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 51.Sieber C, et al. Monomeric and dimeric GDF-5 show equal type I receptor binding and oligomerization capability and have the same biological activity. Biological chemistry. 2006;387:451–60. doi: 10.1515/BC.2006.060. [DOI] [PubMed] [Google Scholar]
- 52.Zuniga JE, et al. Assembly of TbetaRI:TbetaRII:TGFbeta ternary complex in vitro with receptor extracellular domains is cooperative and isoform-dependent. J Mol Biol. 2005;354:1052–68. doi: 10.1016/j.jmb.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 53.Ilangovan U, Deep S, Hinck CS, Hinck AP. Sequential resonance assignments of the extracellular domain of the human TGFbeta type II receptor in complex with monomeric TGFbeta3. Journal of Biomolecular Nmr. 2004;29:103–4. doi: 10.1023/B:JNMR.0000019468.50957.42. [DOI] [PubMed] [Google Scholar]
- 54.Dechavanne V, Barrillat N, Borlat F, Hermant A, Magnenat L, Paquet M, Antonsson B, Chevalet L. A high-throughput protein refolding screen in 96-well format combined with design of experiments to optimize the refolding conditions. Protein expression and purification. 2011;75:192–203. doi: 10.1016/j.pep.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 55.Mahlawat P, Ilangovan U, Biswas T, Sun LZ, Hinck AP. Structure of the Alk1 extracellular domain and characterization of its bone morphogenetic protein (BMP) binding properties. Biochemistry. 2012;51:6328–41. doi: 10.1021/bi300942x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Townson SA, et al. Specificity and structure of a high affinity activin receptor-like kinase 1 (ALK1) signaling complex. The Journal of biological chemistry. 2012;287:27313–25. doi: 10.1074/jbc.M112.377960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Klages J, Kotzsch A, Coles M, Sebald W, Nickel J, Muller T, Kessler H. The solution structure of BMPR-IA reveals a local disorder-to-order transition upon BMP-2 binding. Biochemistry. 2008;47:11930–9. doi: 10.1021/bi801059j. [DOI] [PubMed] [Google Scholar]
- 58.Zuniga JE, Ilangovan U, Mahlawat P, Hinck CS, Huang T, Groppe JC, McEwen DG, Hinck AP. The TbetaR-I Pre-Helix Extension Is Structurally Ordered in the Unbound Form and Its Flanking Prolines Are Essential for Binding. Journal of molecular biology. 2011 doi: 10.1016/j.jmb.2011.07.046. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nickel J, Kotzsch A, Sebald W, Mueller TD. A single residue of GDF-5 defines binding specificity to BMP receptor IB. Journal of molecular biology. 2005;349:933–47. doi: 10.1016/j.jmb.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 60.Greenwald J, Fischer WH, Vale WW, Choe S. Three-finger toxin fold for the extracellular ligand-binding domain of the type II activin receptor serine kinase. Nature structural biology. 1999;6:18–22. doi: 10.1038/4887. [DOI] [PubMed] [Google Scholar]
- 61.Thompson TB, Woodruff TK, Jardetzky TS. Structures of an ActRIIB:activin A complex reveal a novel binding mode for TGF-beta ligand:receptor interactions. The EMBO journal. 2003;22:1555–66. doi: 10.1093/emboj/cdg156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mace PD, Cutfield JF, Cutfield SM. High resolution structures of the bone morphogenetic protein type II receptor in two crystal forms: implications for ligand binding. Biochemical and biophysical research communications. 2006;351:831–8. doi: 10.1016/j.bbrc.2006.10.109. [DOI] [PubMed] [Google Scholar]
- 63.Boesen CC, Radaev S, Motyka SA, Patamawenu A, Sun PD. The 1.1 A crystal structure of human TGF-beta type II receptor ligand binding domain. Structure. 2002;10:913–9. doi: 10.1016/s0969-2126(02)00780-3. [DOI] [PubMed] [Google Scholar]
- 64.Deep S, Walker KP, 3rd, Shu Z, Hinck AP. Solution structure and backbone dynamics of the TGFbeta type II receptor extracellular domain. Biochemistry. 2003;42:10126–39. doi: 10.1021/bi034366a. [DOI] [PubMed] [Google Scholar]
- 65.Hart PJ, Deep S, Taylor AB, Shu Z, Hinck CS, Hinck AP. Crystal structure of the human TbetaR2 ectodomain--TGF-beta3 complex. Nat Struct Biol. 2002;9:203–8. doi: 10.1038/nsb766. [DOI] [PubMed] [Google Scholar]
- 66.Alt A, et al. Structural and functional insights into endoglin ligand recognition and binding. PloS one. 2012;7:e29948. doi: 10.1371/journal.pone.0029948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Castonguay R, et al. Soluble endoglin specifically binds bone morphogenetic proteins 9 and 10 via its orphan domain, inhibits blood vessel formation, and suppresses tumor growth. The Journal of biological chemistry. 2011;286:30034–46. doi: 10.1074/jbc.M111.260133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.del Re E, Sidis Y, Fabrizio DA, Lin HY, Schneyer A. Reconstitution and analysis of soluble inhibin and activin receptor complexes in a cell-free system. The Journal of biological chemistry. 2004;279:53126–35. doi: 10.1074/jbc.M408090200. [DOI] [PubMed] [Google Scholar]
- 69.Lin HY, Moustakas A, Knaus P, Wells RG, Henis YI, Lodish HF. The soluble exoplasmic domain of the type II transforming growth factor (TGF)-beta receptor. A heterogeneously glycosylated protein with high affinity and selectivity for TGF-beta ligands. The Journal of biological chemistry. 1995;270:2747–54. doi: 10.1074/jbc.270.6.2747. [DOI] [PubMed] [Google Scholar]
- 70.Tsang ML, et al. Characterization of recombinant soluble human transforming growth factor-beta receptor type II (rhTGF-beta sRII) Cytokine. 1995;7:389–97. doi: 10.1006/cyto.1995.0054. [DOI] [PubMed] [Google Scholar]
- 71.Goetschy JF, Letourneur O, Cerletti N, Horisberger MA. The unglycosylated extracellular domain of type-II receptor for transforming growth factor-beta. A novel assay for characterizing ligand affinity and specificity. European journal of biochemistry / FEBS. 1996;241:355–62. doi: 10.1111/j.1432-1033.1996.00355.x. [DOI] [PubMed] [Google Scholar]
- 72.Greenwald J, Le V, Corrigan A, Fischer W, Komives E, Vale W, Choe S. Characterization of the extracellular ligand-binding domain of the type II activin receptor. Biochemistry. 1998;37:16711–8. doi: 10.1021/bi981939o. [DOI] [PubMed] [Google Scholar]
- 73.Boesen CC, Motyka SA, Patamawenu A, Sun PD. Development of a recombinant bacterial expression system for the active form of a human transforming growth factor beta type II receptor ligand binding domain. Protein expression and purification. 2000;20:98–104. doi: 10.1006/prep.2000.1306. [DOI] [PubMed] [Google Scholar]
- 74.Komesli S, Vivien D, Dutartre P. Chimeric extracellular domain type II transforming growth factor (TGF)-beta receptor fused to the Fc region of human immunoglobulin as a TGF-beta antagonist. European journal of biochemistry / FEBS. 1998;254:505–13. doi: 10.1046/j.1432-1327.1998.2540505.x. [DOI] [PubMed] [Google Scholar]
- 75.Muraoka RS, et al. Blockade of TGF-beta inhibits mammary tumor cell viability, migration, and metastases. J Clin Invest. 2002;109:1551–9. doi: 10.1172/JCI15234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang YA, et al. Lifetime exposure to a soluble TGF-beta antagonist protects mice against metastasis without adverse side effects. J Clin Invest. 2002;109:1607–15. doi: 10.1172/JCI15333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mitchell D, et al. ALK1-Fc inhibits multiple mediators of angiogenesis and suppresses tumor growth. Molecular cancer therapeutics. 2010;9:379–88. doi: 10.1158/1535-7163.MCT-09-0650. [DOI] [PubMed] [Google Scholar]
- 78.Putnam C. Scripps Protein Calculator v. 3.3. 2013. ed.^eds. [Google Scholar]
- 79.Hinck AP, Walker KP, 3rd, Martin NR, Deep S, Hinck CS, Freedberg DI. Sequential resonance assignments of the extracellular ligand binding domain of the human TGF-beta type II receptor. J Biomol NMR. 2000;18:369–70. doi: 10.1023/a:1026775321886. [DOI] [PubMed] [Google Scholar]
- 80.Gasparian ME, Elistratov PA, Yakimov SA, Dolgikh DA, Kirpichnikov MP. An efficient method for expression in Escherichia coli and purification of the extracellular ligand binding domain of the human TGFbeta type II receptor. Journal of biotechnology. 2010;148:113–8. doi: 10.1016/j.jbiotec.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 81.Hatta T, Konishi H, Katoh E, Natsume T, Ueno N, Kobayashi Y, Yamazaki T. Identification of the ligand-binding site of the BMP type IA receptor for BMP-4. Biopolymers. 2000;55:399–406. doi: 10.1002/1097-0282(2000)55:5<399::AID-BIP1014>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 82.Kirsch T, Nickel J, Sebald W. Isolation of recombinant BMP receptor IA ectodomain and its 2:1 complex with BMP-2. FEBS letters. 2000;468:215–9. doi: 10.1016/s0014-5793(00)01214-x. [DOI] [PubMed] [Google Scholar]
- 83.Marlow MS, Chim N, Brown CB, Barnett JV, Krezel AM. 1H, 13C, and 15N backbone assignments of the ligand binding domain of TGFbeta type II receptor. Journal of biomolecular NMR. 2000;17:349–50. doi: 10.1023/a:1008310912499. [DOI] [PubMed] [Google Scholar]
- 84.Yin H, Yeh LC, Hinck AP, Lee JC. Characterization of ligand-binding properties of the human BMP type II receptor extracellular domain. J Mol Biol. 2008;378:191–203. doi: 10.1016/j.jmb.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 85.Baardsnes J, Hinck CS, Hinck AP, O’Connor-McCourt MD. TbetaR-II discriminates the high- and low-affinity TGF-beta isoforms via two hydrogen-bonded ion pairs. Biochemistry. 2009;48:2146–55. doi: 10.1021/bi8019004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.De Crescenzo G, Grothe S, Lortie R, Debanne MT, O’Connor-McCourt M. Real-time kinetic studies on the interaction of transforming growth factor alpha with the epidermal growth factor receptor extracellular domain reveal a conformational change model. Biochemistry. 2000;39:9466–76. doi: 10.1021/bi992987r. [DOI] [PubMed] [Google Scholar]
- 87.De Crescenzo G, Grothe S, Zwaagstra J, Tsang M, O’Connor-McCourt MD. Real-time monitoring of the interactions of transforming growth factor-beta (TGF-beta ) isoforms with latency-associated protein and the ectodomains of the TGF-beta type II and III receptors reveals different kinetic models and stoichiometries of binding. The Journal of biological chemistry. 2001;276:29632–43. doi: 10.1074/jbc.M009765200. [DOI] [PubMed] [Google Scholar]
- 88.De Crescenzo G, et al. Three key residues underlie the differential affinity of the TGFbeta isoforms for the TGFbeta type II receptor. J Mol Biol. 2006;355:47–62. doi: 10.1016/j.jmb.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 89.De Crescenzo G, Pham PL, Durocher Y, Chao H, O’Connor-McCourt MD. Enhancement of the antagonistic potency of transforming growth factor-beta receptor extracellular domains by coiled coil-induced homo- and heterodimerization. The Journal of biological chemistry. 2004;279:26013–8. doi: 10.1074/jbc.M400655200. [DOI] [PubMed] [Google Scholar]
- 90.De Crescenzo G, Pham PL, Durocher Y, O’Connor-McCourt MD. Transforming growth factor-beta (TGF-beta) binding to the extracellular domain of the type II TGF-beta receptor: receptor capture on a biosensor surface using a new coiled-coil capture system demonstrates that avidity contributes significantly to high affinity binding. Journal of molecular biology. 2003;328:1173–83. doi: 10.1016/s0022-2836(03)00360-7. [DOI] [PubMed] [Google Scholar]
- 91.Huang T, et al. TGF-beta signalling is mediated by two autonomously functioning TbetaRI:TbetaRII pairs. The EMBO journal. 2011;30:1263–76. doi: 10.1038/emboj.2011.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li L, Orner BP, Huang T, Hinck AP, Kiessling LL. Peptide ligands that use a novel binding site to target both TGF-beta receptors. Molecular bioSystems. 2010;6:2392–402. doi: 10.1039/c0mb00115e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mendoza V, Vilchis-Landeros MM, Mendoza-Hernandez G, Huang T, Villarreal MM, Hinck AP, Lopez-Casillas F, Montiel JL. Betaglycan has two independent domains required for high affinity TGF-beta binding: proteolytic cleavage separates the domains and inactivates the neutralizing activity of the soluble receptor. Biochemistry. 2009;48:11755–65. doi: 10.1021/bi901528w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.O’Connor-McCourt MD, Segarubu O, Grothe S, Tsang M, Weatherbee JA. Analysis of the interaction between two TGF-beta-binding proteins and three TGF-beta isoforms using surface plasmon resonance. Annals of the New York Academy of Sciences. 1995;766:300–2. doi: 10.1111/j.1749-6632.1995.tb26682.x. [DOI] [PubMed] [Google Scholar]
- 95.Zwaagstra JC, et al. Engineering and therapeutic application of single-chain bivalent TGF-beta family traps. Molecular cancer therapeutics. 2012;11:1477–87. doi: 10.1158/1535-7163.MCT-12-0060. [DOI] [PubMed] [Google Scholar]
- 96.Allendorph GP, Isaacs MJ, Kawakami Y, Izpisua Belmonte JC, Choe S. BMP-3 and BMP-6 structures illuminate the nature of binding specificity with receptors. Biochemistry. 2007;46:12238–47. doi: 10.1021/bi700907k. [DOI] [PubMed] [Google Scholar]
- 97.Allendorph GP, Read JD, Kawakami Y, Kelber JA, Isaacs MJ, Choe S. Designer TGFbeta superfamily ligands with diversified functionality. PloS one. 2011;6:e26402. doi: 10.1371/journal.pone.0026402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brown MA, et al. Crystal structure of BMP-9 and functional interactions with pro-region and receptors. The Journal of biological chemistry. 2005;280:25111–8. doi: 10.1074/jbc.M503328200. [DOI] [PubMed] [Google Scholar]
- 99.Greenwald J, Vega ME, Allendorph GP, Fischer WH, Vale W, Choe S. A flexible activin explains the membrane-dependent cooperative assembly of TGF-beta family receptors. Molecular cell. 2004;15:485–9. doi: 10.1016/j.molcel.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 100.Keller S, Nickel J, Zhang JL, Sebald W, Mueller TD. Molecular recognition of BMP-2 and BMP receptor IA. Nature structural & molecular biology. 2004;11:481–8. doi: 10.1038/nsmb756. [DOI] [PubMed] [Google Scholar]
- 101.Kotzsch A, Nickel J, Seher A, Heinecke K, van Geersdaele L, Herrmann T, Sebald W, Mueller TD. Structure analysis of bone morphogenetic protein-2 type I receptor complexes reveals a mechanism of receptor inactivation in juvenile polyposis syndrome. The Journal of biological chemistry. 2008;283:5876–87. doi: 10.1074/jbc.M706029200. [DOI] [PubMed] [Google Scholar]
- 102.Saremba S, Nickel J, Seher A, Kotzsch A, Sebald W, Mueller TD. Type I receptor binding of bone morphogenetic protein 6 is dependent on N-glycosylation of the ligand. The FEBS journal. 2008;275:172–83. doi: 10.1111/j.1742-4658.2007.06187.x. [DOI] [PubMed] [Google Scholar]
- 103.Abdiche Y, Malashock D, Pinkerton A, Pons J. Determining kinetics and affinities of protein interactions using a parallel real-time label-free biosensor, the Octet. Analytical biochemistry. 2008;377:209–17. doi: 10.1016/j.ab.2008.03.035. [DOI] [PubMed] [Google Scholar]
- 104.Wienken CJ, Baaske P, Rothbauer U, Braun D, Duhr S. Protein-binding assays in biological liquids using microscale thermophoresis. Nature communications. 2010;1:100. doi: 10.1038/ncomms1093. [DOI] [PubMed] [Google Scholar]
- 105.Bocharov EV, Blommers MJ, Kuhla J, Arvinte T, Bürgi R, Arseniev AS. Sequence-specific 1H and 15N assignment and secondary structure of transforming growth factor beta3. J Biomol NMR. 2000;16:179–180. doi: 10.1023/a:1008315600134. [DOI] [PubMed] [Google Scholar]
- 106.Bocharov EV, Korzhnev DM, Blommers MJ, Arvinte T, Orekhov VY, Billeter M, Arseniev AS. Dynamics-modulated biological activity of transforming growth factor beta3. J Biol Chem. 2002;277:46273–9. doi: 10.1074/jbc.M206274200. [DOI] [PubMed] [Google Scholar]
- 107.Marlow MS, Brown CB, Barnett JV, Krezel AM. Solution structure of the chick TGFbeta type II receptor ligand-binding domain. Journal of molecular biology. 2003;326:989–97. doi: 10.1016/s0022-2836(03)00023-8. [DOI] [PubMed] [Google Scholar]
- 108.Griffith DL, Keck PC, Sampath TK, Rueger DC, Carlson WD. Three-dimensional structure of recombinant human osteogenic protein 1: structural paradigm for the transforming growth factor beta superfamily. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:878–83. doi: 10.1073/pnas.93.2.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Scheufler C, Sebald W, Hulsmeyer M. Crystal structure of human bone morphogenetic protein-2 at 2.7 A resolution. Journal of molecular biology. 1999;287:103–15. doi: 10.1006/jmbi.1999.2590. [DOI] [PubMed] [Google Scholar]
- 110.Schreuder H, Liesum A, Pohl J, Kruse M, Koyama M. Crystal structure of recombinant human growth and differentiation factor 5: evidence for interaction of the type I and type II receptor-binding sites. Biochemical and biophysical research communications. 2005;329:1076–86. doi: 10.1016/j.bbrc.2005.02.078. [DOI] [PubMed] [Google Scholar]
- 111.de Vries SJ, van Dijk ADJ, Krzeminski M, van Dijk M, Thureau A, Hsu V, Wassenaar T, Bonvin AMJJ. HADDOCK versus HADDOCK: New features and performance of HADDOCK2.0 on the CAPRI targets. Proteins: Struc Funct & Bioinformatics. 2007;69:726–733. doi: 10.1002/prot.21723. [DOI] [PubMed] [Google Scholar]
- 112.Dominguez C, Boelens R, Bonvin AMJJ. HADDOCK: A protein-protein docking approach based on biochemical or biophysical information. Journal of the American Chemical Society. 2003;125:1731–1737. doi: 10.1021/ja026939x. [DOI] [PubMed] [Google Scholar]
- 113.Ritchie DW. Recent progress and future directions in protein-protein docking. Current protein & peptide science. 2008;9:1–15. doi: 10.2174/138920308783565741. [DOI] [PubMed] [Google Scholar]
- 114.Papadopoulos JS, Agarwala R. COBALT: constraint-based alignment tool for multiple protein sequences. Bioinformatics. 2007;23:1073–9. doi: 10.1093/bioinformatics/btm076. [DOI] [PubMed] [Google Scholar]