Abstract
Purpose
Although success rates are reported to be high, radiographic followup after pyeloplasty to correct ureteropelvic junction obstruction varies in intensity and modality. We characterized postoperative care after pyeloplasty to identify imaging trends.
Materials and Methods
Using the MarketScan® database we identified patients 17 to 65 years old treated with pyeloplasty from 2007 to 2010. Followup imaging was classified as functional (diuretic renogram or excretory urogram) and nonfunctional (ultrasound, computerized tomography or magnetic resonance imaging). The postoperative period was divided into intervals of less than 6, 6 to 12, 12 to 24, 24 to 36 and greater than 36 months. We excluded from study patients with less than 24 months of postoperative enrollment in MarketScan. Multivariate logistic regression was used to determine associations between demographic variables and imaging utilization patterns.
Results
We identified 742 patients with a mean ± SD followup of 36.8 ± 3.7 months, of whom 65% underwent minimally invasive pyeloplasty. Of the patients 12% underwent no postoperative imaging. Within the first 6 months 554 patients (75%) underwent at least 1 imaging study and within the first 12 months 82% underwent at least 1 imaging study, which was most commonly functional. After 12 months 54% of patients underwent any imaging, which was most commonly nonfunctional. At least annual imaging was significantly associated with older age, female gender and longer hospital stay. Secondary procedures were required in 62 patients (8%).
Conclusions
After pyeloplasty in adulthood most patients undergo a functional imaging study within 6 months. However, after 1 year only half of patients undergo followup imaging. Variability and insufficient radiological followup may bias the belief of pyeloplasty success.
Keywords: kidney, ureteral obstruction, hydronephrosis, diagnostic imaging, follow-up studies
Anderson-Hynes dismembered pyeloplasty is considered the gold standard to correct UPJO with reported success rates greater than 90%.1–4 With the development of minimally invasive techniques there has been a dramatic increase in the last decade in the use of laparoscopic and robotic pyeloplasty.5 The 2 techniques appear to have equivalent success rates and risks of complications compared with open pyeloplasty in nonrandomized comparative studies.6 Contemporary series show success rates between 87% and 98% for laparoscopic,4,7–10 and 93% and 100% for robotic7,10–14 pyeloplasty.
Success in most published series has been defined by clinical and radiographic criteria but the duration and type of radiographic followup that should be performed are unclear. Late failures beyond 2 years have been reported.9,10,15,16 We hypothesize that there is substantial variation in radiographic followup after pyeloplasty in terms of imaging type and timing. We characterized imaging followup using a large administrative database to identify trends in use and duration.
MATERIALS AND METHODS
Data Source
The MarketScan database contains information from American employer based commercial health plans, including records captured longitudinally from inpatient admissions and outpatient visits.17 Individual level health services records include patient demographics, service dates, length of stay, ICD-9-CM diagnostic codes and CPT codes. The data set contains approximately 60 million inpatient records, comprising approximately 50% of annual discharges from American hospitals.18 Race/ethnicity and socioeconomic data are unavailable. Because patients are de-identified in the database, institutional review board approval was not obtained for this study.
Study Population
We identified patients treated with pyeloplasty from 2007 to 2010 using CPT codes 50400, 50405 and 50544. A total of 1,535 patients were excluded from analysis due to age less than 17 years at surgery or greater than 65 years at the time of the last enrollment data, or there were less than 24 months of enrollment data after the index surgery. Patients older than 65 years were also excluded because they may have had concurrent Medicare insurance coverage.
Patient and Hospital Characteristics
Patient characteristics were evaluated, including age, gender, CCI, operative approach, surgery year, hospital region, patient insurance status and length of stay. CCI was calculated from inpatient and outpatient claims in the 6 months before the date of surgery.19 Operative approach was categorized as open or minimally invasive. Insurance status was stratified as HMO or nonHMO.
Radiographic Followup
Imaging use after discharge from the index hospital admission was identified for abdominal and renal ultrasound, abdominal CT, abdominal MRI, renogram with and without diuretic administration, and IVP using CPT and the ICD-9-CM codes (see Appendix, http://jurology.com/). Imaging type was categorized as functional (renogram or IVP) or nonfunctional (ultrasound, CT or MRI). CPT and ICD-9-CM codes for CT with intravenous contrast medium do not allow for the specification of CT IVP/urogram. CPT codes 77160, 77170, 74177 and 74178 (CT with contrast) represented 376 of all 502 CTs (53.6%) in eligible patients. The use of magnetic resonance urography as a functional study could not be determined from the codes.
We examined certain postoperative intervals, including 0 to 6, 6 to 12, 12 to 24, 24 to 36 and greater than 36 months from the date of surgery. Observed imaging patterns were classified into 1 of 4 categories, including 1–no imaging, 2–imaging within 12 months only, 3–imaging after 12 months only, and 4–imaging before and after 12 months.
Secondary Interventions
We identified secondary interventions using CPT and ICD-9-CM codes. The Appendix (http://jurology.com/) lists abstracted diagnosis and procedure codes. For analysis stent/drain procedures and procedures corresponding to salvage endoscopic correction were grouped together. When patients had multiple codes corresponding to secondary interventions, only the most invasive procedure was counted. For instance, endopyelotomy with a stent was counted as endoscopic management and not a stent/drain. A pattern of repeat stent exchanges was counted as a single stent/drain management strategy.
Statistical Analysis
Unadjusted and adjusted multivariate logistic regression was used to determine associations between demographic factors with at least annual radiographic followup. Exploratory univariate analysis was done to determine demographic factors associated with no imaging followup. Statistical analysis was performed using Stata® 12.1 with 2-sided p <0.05 considered statistically significant.
RESULTS
A total of 742 patients met study inclusion criteria. Mean ± SD followup was 36.8 ± 3.7 months. Table 1 lists patient demographics. The proportion of minimally invasive pyeloplasties increased from 61% in 2007 to 79% in 2010. Complicated pyeloplasty (CPT 50405) was performed in 146 patients (20%) in the cohort.
Table 1.
Demographics of 742 patients
| No. Pts (%) | |
|---|---|
| Age: | |
| 17–39 | 321 (43.3) |
| 40–49 | 191 (25.7) |
| 50–63 | 230 (31.0) |
| Male | 310 (41.8) |
| Female | 432 (58.2) |
| CCI: | |
| 0 | 478 (64.4) |
| 1 | 49 (6.6) |
| 2 | 22 (3.0) |
| 3 or Greater | 8 (1.1) |
| Unknown | 185 (24.9) |
| Operative approach: | |
| Open | 258 (34.8) |
| Minimally invasive | 484 (65.2) |
| Surgery yr: | |
| 2007 | 207 (27.9) |
| 2008 | 259 (34.9) |
| 2009 | 257 (34.6) |
| 2010 | 19 (2.6) |
| Region: | |
| Northeast | 130 (17.5) |
| North Central | 202 (27.2) |
| South | 269 (36.3) |
| West | 120 (16.2) |
| Unknown | 21 (2.8) |
| HMO: | |
| Yes | 94 (12.7) |
| No | 630 (84.9) |
| Unknown | 18 (2.4) |
| Length of stay (days): | |
| 2 or Less | 437 (58.9) |
| 3–5 | 263 (35.4) |
| 6 or Greater | 42 (5.7) |
| Need for secondary procedure(s): | |
| Yes | 62 (8.4) |
| No | 680 (91.6) |
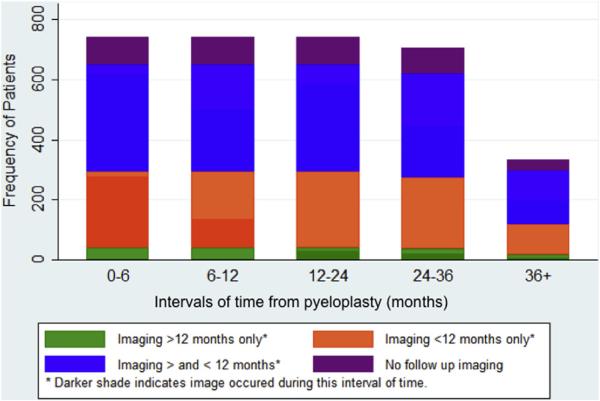
Figure 1 shows imaging utilization patterns after pyeloplasty. Followup imaging was done in 88% of patients, including 34% in the first 12 months only, 48.5% before and after 12 months postoperatively, and 5.5% only after 12 months postoperatively. Within the first 6 months after pyeloplasty 75% of patients underwent at least 1 imaging study and after 12 months only 54% of patients received followup imaging. In the 471 patients with multiple imaging studies during 2 years postoperatively the average number of imaging studies was 3.7 ± 2.3. Of the 554 patients with imaging in the first 6 months postoperatively at least 1 study was done within the first 3 months in 413 (75%). Of all 925 imaging studies done in the first 6 months 563 (61%) were done within the first 3 months.
Figure 1.
Imaging by time after pyeloplasty in all patients with enrollment data during each interval, categorized into 1 of 4 imaging patterns. Intervals indicate number censored with time.
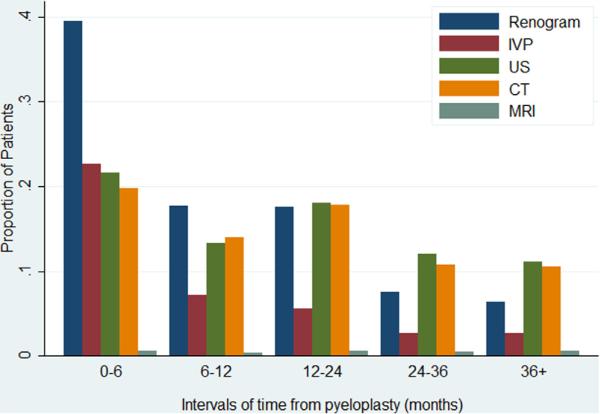
Figure 2 shows specific imaging modalities by time, counted by the proportion of patients who underwent at least 1 study per category. Renal scans were the most common study in patients in whom imaging was done in the first 12 months. In the 12 to 24-month period renal scans remained common but most studies were ultrasound and CT. MRI represented less than 1% of imaging during each period.
Figure 2.
Imaging type by time after pyeloplasty. Patients may have undergone multiple imaging types in same period. US, ultrasound.
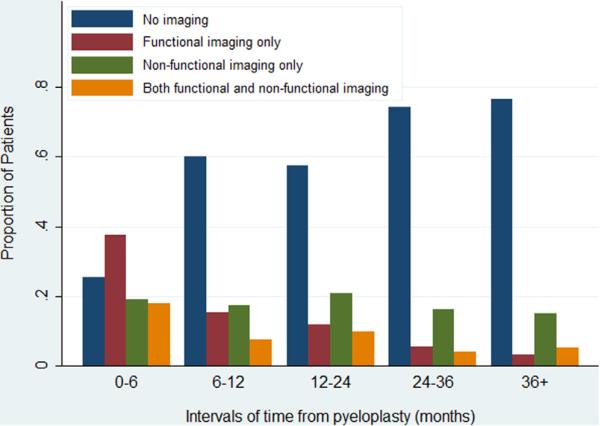
Figure 3 shows the proportion of patients who underwent functional and/or nonfunctional imaging. The proportion with no imaging during each interval increased from 25% in the 0 to 6-month period to 74% after 3 years. The proportion with only functional imaging decreased from 38% in the 0 to 6-month period to 6% beyond 3 years. The use of nonfunctional imaging alone remained stable at between 17% to 21% throughout the study period. Of patients with functional and nonfunctional imaging 18% underwent each type of imaging in the first 6 months and 3% underwent each type after 3 years.
Figure 3.
Functional (renogram or IVP) or nonfunctional (ultrasound, CT or MRI) imaging type by time after pyeloplasty.
On univariate and multivariate analysis older age (50 to 63 years), female gender and length of stay 6 days or greater were associated with at least annual radiographic followup (table 2). Other demographic factors, including CCI, operative approach, surgery year, geographic region, HMO insurance status and need for secondary procedures, showed no statistically significant associations.
Table 2.
Univariate and multivariate analyses of association of demographic factors with at least annual radiographic followup in 742 patients
| No. Annual Screening (%) |
OR (95% CI) |
|||
|---|---|---|---|---|
| Yes | No | Unadjusted* | Multivariate† | |
| Age: | ||||
| 17–39 | 139 (43.3) | 182 (56.7) | Referent | Referent |
| 40–49 | 97 (50.8) | 94 (49.2) | 1.35 (0.94–1.93) | 1.30 (0.91–1.88) |
| 50–63 | 122 (53) | 108 (47) | 1.47 (1.05–2.08)‡ | 1.41 (1.00–2.00)‡ |
| Male | 131 (42.3) | 179 (57.7) | Referent | Referent |
| Female | 227 (52.5) | 205 (47.5) | 1.51 (1.13–2.03)‡ | 1.48 (1.10–2.00)‡ |
| Length of stay (days): | ||||
| 2 or Less | 208 (47.6) | 229 (52.4) | Referent | Referent |
| 3–5 | 123 (46.8) | 140 (53.2) | 0.97 (0.71–1.31) | 0.92 (0.67–1.26) |
| 6 or Greater | 27 (64.3) | 15 (35.7) | 1.98 (1.03–3.83)‡ | 1.92 (0.99–3.73) |
CCI, operative approach, surgery year, geographic region, HMO insurance status and need for secondary procedure showed no statistically significant association with annual screening status.
Adjusted for CCI, operative approach, surgery year, geographic region, HMO insurance status and need for secondary procedure.
Significant (p <0.05).
The 12% of patients who underwent no imaging were more likely to be male than female (17% vs 9%, p = 0.004). There was a higher proportion of no imaging in those with 2 or more comorbidities, those with a length of stay of less than 2 days and those younger than 30 or older than 50 years. However, none of these differences attained significance on univariate modeling.
Secondary procedures were performed in 62 patients (8.4%) at a mean of 10.9 ± 9.6 months after initial pyeloplasty (table 3). Drain placement was needed in 27 patients (44%), 19 (31%) required redo pyeloplasty and another 19 (31%) required nephrectomy.
Table 3.
Secondary procedures after initial pyeloplasty
| Events* | No Pts (%) | Mean ± SD Mos to Secondary Procedure (range) |
|---|---|---|
| Drain† | 27 (44) | 11.5 ± 10.9 (0.1, 41.7) |
| Endoscopic‡ | 5 (8) | 9.7 ± 8.4 (1.4, 21.0) |
| Pyeloplasty§ | 19 (31) | 11.6 ± 7.3 (1.4, 26.3) |
| Nephrectomy | 19 (31) | 7.5 ± 5.0 (0.2, 18.6) |
| Transplantation | 1 (2) | 44.3 |
| Totals | 62 | 10.9 ± 9.6 (0.0, 44.3) |
Tallied and categorized by management strategy, ie subsequent stent or drain exchanges after initial procedure were not counted and endopyelotomy with stent placement was counted as endopyelotomy only.
Including ureteral stent and nephrostomy.
Including endopyelotomy, endoscopic dilation or stricture incision.
Including ureteropyelostomy.
DISCUSSION
There are several important findings from this study. 1) Most patients underwent at least 1 imaging study in the first 12 months after pyeloplasty in adulthood. Most of these studies were functional imaging. 2) The use of imaging beyond 1 year after pyeloplasty decreased dramatically. Approximately half of the patients did not undergo radiographic followup after followup year 1. This suggests that the current belief of long-term pyeloplasty success for UPJO may be confounded by insufficient followup. Of patients with continued radiographic followup after 1 year the imaging type was most commonly nonfunctional. 3) Lack of annual followup imaging through 24 months was associated with younger age, male gender and shorter hospital stay. Patients with a more prolonged and presumably complicated hospital course appear to have been followed more closely with imaging. 4) Secondary procedures were performed in 8% of patients with pyeloplasty, usually within postoperative year 1.
Although reported success rates in the literature are high, true success rates may be overestimated by short followup and under diagnosis of asymptomatic failure based on imaging. Published series vary in followup and criteria for success. Jarrett et al reported on 100 laparoscopic pyeloplasties with a mean followup of just greater than 2 years, describing 96% radiographic success based on hydronephrosis improvement on IVP or improved drainage on renal scan.8 IVP or renogram was obtained 2 to 3 months postoperatively and then annually. Zhang et al found equivalent radiographic success rates (98%) for 56 retroperitoneal laparoscopic and 40 open pyeloplasties at a mean followup of 30.2 and 23.4 months, respectively.4 IVP and renal ultrasound were performed at 3 and 6 months with followup imaging annually. Rassweiler et al reported success rates of 73% and 94% in 113 endopyelotomies and 143 retroperitoneal laparoscopic pyeloplasties, respectively, at a mean followup of 63 months.9 IVP was done after stent removal, followed by renal scan at 3 months and annually. Success was defined as a combination of symptomatic resolution, stable or improved renal function and/or stable or improved washout on functional imaging with a resistance index of less than 0.7. They noted 3 failures, which occurred after 30 months. Yanke reported on 116 laparoscopic pyeloplasties with Kaplan-Meier 1, 3 and 7-year failure-free estimates of 93%, 86% and 76%, respectively, at a mean followup of 20 months.10 Renal scan was obtained at 3, 6 and 12 months. If the initial renogram was normal, ultrasound was occasionally done if the patient remained asymptomatic.
Robotic pyeloplasty appears to have comparable success rates. Schwentner et al reported a 97% symptom and radiographic success rate for 92 robotic pyeloplasties with a mean followup of 39.1 months.12 IVP and renal scan were done at 3 months and renal ultrasound was obtained annually. Mufarrij et al reported on 134 robotic pyeloplasties with a mean followup of 29 months.13 The success rate was 96% based on renal scan or IVP at 1 month. Subsequent imaging included renal scan or IVP every 3 to 6 months for at least 2 years. Gupta et al reported 85 robotic pyeloplasties with a 97% success rate at a mean followup of 13.6 months.14 Surveillance was performed with clinical assessment and IVP or renal scan at 3, 6 and 12 months, and annually thereafter. Followup was completed in 71, 59, 41 and 21 patients at 6, 12, 18 and 24 months, respectively. Etafy et al reported 61 robotic pyeloplasties with a mean followup of 19 months.20 The success rate was 81% using a strict definition of failure with a renogram half-life of less than 10 minutes and symptomatic relief using a validated pain analog score. The success rate was 93% based on radiographic criteria only. Imaging included renal scan at 3, 6, 12 and 24 months, which was repeated after this period in symptomatic patients.
The timing and duration of functional studies has been better studied in the pediatric population, in which there are concerns regarding radiation risk. Pohl et al recommended that if a 3-month renal scan shows a half-time of less than 20 minutes, a 12-month scan is unnecessary.21 van den Hoek et al evaluated 138 children and found that renal scans 3.5 and 5.5 years after surgery showed stable split renal function.22 They recommended that scans are unnecessary beyond 5 to 7 years. Almodhen et al obtained postoperative renal ultrasound only at 3 months and if hydronephrosis was downgraded, a subsequent renal scan was unnecessary because it would not show obstruction.23 Based on 77 pediatric pyeloplasty patients with greater than 5-year followup Psooy et al recommended that a combination of functional and nonfunctional imaging extending to 2 years is sufficient.24
However, extrapolating these data to adults should be performed with caution since the patient, disease and aim of pyeloplasty differ. Adults who present with UPJO are typically symptomatic with pain, infection and/or calculi. Pediatric patients are commonly asymptomatic and the goal of treatment is primarily renal preservation.
In this study only 54% of patients underwent at least 1 followup imaging study after 12 months. While the appropriate followup is not well defined, failure has been reported beyond 2 years.9,10,15,16 Dimarco et al compared antegrade endopyelotomy to pyeloplasty with a mean 3.9-year followup (range 0.3 to 20) in the pyeloplasty cohort of 174 patients.15 Three, 5 and 10-year estimated recurrence-free survival rates in the pyeloplasty group were 85%, 80% and 75%, respectively. Of the failures 42% occurred after 1 year. The group noted an asymptomatic failure rate in 2.3% of patients with radiographic failure despite the resolution of flank pain. Madi et al reported 60 laparoscopic pyeloplasties, including 35 with at least 1-year radiographic followup.16 In that cohort 30% of failures developed after 2 years but all patients were symptomatic at presentation. Therefore, the group proposed that radiographic followup after 1 year may not be needed, although 35% of their cohort had less than 1 year of radiographic followup or none at all.
Ultimately, the goal of imaging surveillance is to diagnose obstruction early and allow for intervention that may lead to renal preservation. It is unclear whether more imaging or a specific imaging pattern is associated with early detection of recurrent UPJO. A prospective study with longer term followup is needed to determine the role of imaging in defining and maintaining pyeloplasty success.
Our findings must be interpreted in the context of the limitations of our study design. In this data set of administrative claims certain demographic information (income and ethnicity), specific disease characteristics (body habitus), operative details (aberrant vessels and case complexity) and patient reported symptoms were not available. In our study cohort 20% of cases were coded as complex. It was also assumed that the need for secondary procedures was in the ipsilateral kidney since the data set has no information on procedure laterality. Patients in this employer based database do not reflect the entire American population, which limits the generalizability of our findings. Furthermore, since only half of patients underwent any imaging after 1 year, asymptomatic failures were missed. Asymptomatic failures may have been diagnosed by imaging but it was decided not to intervene. It is expected that more imaging was performed around the time of the secondary procedures, which may confound a descriptive analysis of routine radio-graphic followup. In addition, CT and MRI can be functional studies but the billing codes did not allow us to determine delayed contrast medium use. Therefore, they were categorized as nonfunctional imaging.
CONCLUSIONS
There is substantial variation in the timing and modality of radiographic followup after pyeloplasty. Although most patients underwent imaging within year 1, only half continued to receive radiographic followup. The current belief of success may be overestimated since asymptomatic failures go undiagnosed. On average younger patients, males and those with shorter hospital stays are less likely to undergo at least annual imaging. Further study of the sources of these variations is needed and efforts should be made to standardize followup protocols.
Supplementary Material
Abbreviations and Acronyms
- CCI
Charlson comorbidity index
- CT
computerized tomography
- HMO
health maintenance organization
- IVP
excretory pyelogram
- MRI
magnetic resonance imaging
- UPJO
ureteropelvic junction obstruction
REFERENCES
- 1.O'Reilly PH, Brooman PJ, Mak S, et al. The long-term results of Anderson-Hynes pyeloplasty. BJU Int. 2001;87:287. doi: 10.1046/j.1464-410x.2001.00108.x. [DOI] [PubMed] [Google Scholar]
- 2.Persky L, Krause JR, Boltuch RL. Initial complications and late results in dismembered pyeloplasty. J Urol. 1977;118:162. doi: 10.1016/s0022-5347(17)57936-7. [DOI] [PubMed] [Google Scholar]
- 3.Gogus C, Karamursel T, Tokatli Z, et al. Long-term results of Anderson-Hynes pyeloplasty in 180 adults in the era of endourologic procedures. Urol Int. 2004;73:11. doi: 10.1159/000078796. [DOI] [PubMed] [Google Scholar]
- 4.Zhang X, Li HZ, Ma X, et al. Retrospective comparison of retroperitoneal laparoscopic versus open dismembered pyeloplasty for ureteropelvic junction obstruction. J Urol. 2006;176:1077. doi: 10.1016/j.juro.2006.04.073. [DOI] [PubMed] [Google Scholar]
- 5.Sukumar S, Sun M, Karakiewicz PI, et al. National trends and disparities in the use of minimally invasive adult pyeloplasty. J Urol. 2012;188:913. doi: 10.1016/j.juro.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 6.Braga LH, Pace K, DeMaria J, et al. Systematic review and meta-analysis of robotic-assisted versus conventional laparoscopic pyeloplasty for patients with ureteropelvic junction obstruction: effect on operative time, length of hospital stay, postoperative complications, and success rate. Eur Urol. 2009;56:848. doi: 10.1016/j.eururo.2009.03.063. [DOI] [PubMed] [Google Scholar]
- 7.Uberoi J, Disick GI, Munver R. Minimally invasive surgical management of pelvic-ureteric junction obstruction: update on the current status of robotic-assisted pyeloplasty. BJU Int. 2009;104:1722. doi: 10.1111/j.1464-410X.2009.08682.x. [DOI] [PubMed] [Google Scholar]
- 8.Jarrett TW, Chan DY, Charambura TC, et al. Laparoscopic pyeloplasty: the first 100 cases. J Urol. 2002;167:1253. doi: 10.1016/s0022-5347(05)65276-7. [DOI] [PubMed] [Google Scholar]
- 9.Rassweiler JJ, Subotic S, Feist-Schwenk M, et al. Minimally invasive treatment of ureteropelvic junction obstruction: long-term experience with an algorithm for laser endopyelotomy and laparoscopic retroperitoneal pyeloplasty. J Urol. 2007;177:1000. doi: 10.1016/j.juro.2006.10.049. [DOI] [PubMed] [Google Scholar]
- 10.Yanke BV, Lallas CD, Pagnani C, et al. The minimally invasive treatment of ureteropelvic junction obstruction: a review of our experience during the last decade. J Urol. 2008;180:1397. doi: 10.1016/j.juro.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 11.Lucas SM, Sundaram CP, Wolf JS, Jr, et al. Factors that impact the outcome of minimally invasive pyeloplasty: results of the Multi-institutional Laparoscopic and Robotic Pyeloplasty Collaborative Group. J Urol. 2012;187:522. doi: 10.1016/j.juro.2011.09.158. [DOI] [PubMed] [Google Scholar]
- 12.Schwentner C, Pelzer A, Neururer R, et al. Robotic Anderson-Hynes pyeloplasty: 5-year experience of one centre. BJU Int. 2007;100:880. doi: 10.1111/j.1464-410X.2007.07032.x. [DOI] [PubMed] [Google Scholar]
- 13.Mufarrij PW, Woods M, Shah OD, et al. Robotic dismembered pyeloplasty: a 6-year, multi-institutional experience. J Urol. 2008;180:1391. doi: 10.1016/j.juro.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 14.Gupta NP, Nayyar R, Hemal AK, et al. Outcome analysis of robotic pyeloplasty: a large single-centre experience. BJU Int. 2010;105:980. doi: 10.1111/j.1464-410X.2009.08983.x. [DOI] [PubMed] [Google Scholar]
- 15.Dimarco DS, Gettman MT, McGee SM, et al. Long-term success of antegrade endopyelotomy compared with pyeloplasty at a single institution. J Endourol. 2006;20:707. doi: 10.1089/end.2006.20.707. [DOI] [PubMed] [Google Scholar]
- 16.Madi R, Roberts WW, Wolf JS., Jr Late failures after laparoscopic pyeloplasty. Urology. 2008;71:677. doi: 10.1016/j.urology.2007.10.070. [DOI] [PubMed] [Google Scholar]
- 17. [May 31, 2013];Truven Health Analytics: Databases and Online Tools. Available at http://www.truvenhealth.com/your_healthcare_focus/pharmaceutical_and_medical_device/data_databases_and_online_tools.aspx.
- 18.Hansen LG, Chang S. [May 31, 2013];White Paper. Health Research Data for the Real World: The Market-Scan Databases. 2012 Jul; Available at http://www.truvenhealth.com/assets/2012_Truven_MarketScan_white_paper.pdf.
- 19.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 20.Etafy M, Pick D, Said S, et al. Robotic pyeloplasty: the University of California-Irvine experience. J Urol. 2011;185:2196. doi: 10.1016/j.juro.2011.02.054. [DOI] [PubMed] [Google Scholar]
- 21.Pohl HG, Rushton HG, Park JS, et al. Early diuresis renogram findings predict success following pyeloplasty. J Urol. 2001;165:2311. doi: 10.1016/S0022-5347(05)66192-7. [DOI] [PubMed] [Google Scholar]
- 22.van den Hoek J, de Jong A, Scheepe J, et al. Prolonged follow-up after paediatric pyeloplasty: are repeat scans necessary? BJU Int. 2007;100:1150. doi: 10.1111/j.1464-410X.2007.07033.x. [DOI] [PubMed] [Google Scholar]
- 23.Almodhen F, Jednak R, Capolicchio JP, et al. Is routine renography required after pyeloplasty? J Urol. 2010;184:1128. doi: 10.1016/j.juro.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 24.Psooy K, Pike JG, Leonard MP. Long-term followup of pediatric dismembered pyeloplasty: how long is long enough? J Urol. 2003;169:1809. doi: 10.1097/01.ju.0000055040.19568.ea. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.