Abstract
The perineural space is a compartment located between the nerve axons, supporting cells and tissues, and the epineural fibrous sheath. Tumor cells invade this space in response to a complex interplay of trophic factors in the local microenviroment. This attraction of tumor cells to nerves is referred to as neurotropism. The perineural space provides a conduit for tumor spread beyond the primary site of tumor occurrence. Perineural tumor growth is of two types: perineural invasion, affecting small unnamed nerves; and perineural spread, affecting larger, named nerves and presenting with clinical symptoms related to the involved nerve. Both forms of perineural tumor growth represent an adverse prognostic feature and are an essential element of the histopathologic reporting of malignancies of the head and neck region. Perineural spread is associated with decreased overall survival. Endoneurial invasion frequently accompanies perineural spread. The epineurium is more resistant to invasion and represents an important barrier to tumor spread. Immunohistochemical stains such as broad-spectrum keratin can aid in defining the proximal extent of perineural tumor spread.
Keywords: perineural invasion, perineural spread, endoneurium, epineurium, neurotropism
Introduction
The perineural space is a potential space that exists between the neural axons and their surrounding perineural layer. Perineural tumor growth is one of the important mechanisms by which cancers extend beyond their primary site of development. Infrequently, it may be the only mechanism of metastatic spread. Two interrelated types of perineural tumor growth exist: perineural invasion (PNI) and perineural spread (PNS). PNI occurs when tumor cells access the perineural space aided by a complex interplay between tumor cell factors and local nerves. PNI is typically a process of small, microscopically identified, unnamed peripheral nerves in the immediate vicinity of the invasive neoplasm. By contrast PNS involves larger, typically named, central nerves. The boundary point at which PNI becomes PNS is not exactly defined; however, PNS is evident on magnetic resonance imaging (MRI) and clinical manifestations directly related to the nerve involved are usual and clinically more aggressive.1 2
Perineural tumor growth has been best described in malignancies of the pancreas, prostate, colorectum, and head and neck.3 4 5 6 At each site it represents an adverse prognostic feature associated with both increased locoregional recurrence following complete surgical resection and with decreased patient survival. Histopathologists should routinely examine for and report perineural space invasion in all tumor resection specimens. This feature is a required data element of the structured histopathologic reporting protocols for cutaneous and head and neck malignancies for the College of American Pathologists.7
The pathogenesis of PNS has undergone considerable reassessment. Initially it was believed that tumor cells gained access to the perineural space by direct invasion or via transepineural lymphatic permeation, and once inside the perineural space, the low-resistance environment would favor central growth.8 However, it is now realized that lymphatic channels do not penetrate into the inner epineural sheath and that the perineural space is not the low-resistance tissue plane once thought.8 Currently, most attention regarding the pathogenesis of PNS has focused on various factors produced by the neoplastic cells and from the local microenvironment, which induce an interaction between tumor cells, nerve cells, and the microenvironment. These factors appear to be important in facilitating tumor cells to gain access to the perineural space and proliferate once within the space.8 The heterogeneity in production of these trophic factors by tumor cells goes some way to explaining why certain malignancies have a greater predilection for PNI. Into the future, an enhanced understanding of these cellular factors might yield immunohistochemical and/or molecular markers for tumors most at risk for PNS and potentially provide for targeted molecular treatment.
Histological Anatomy and Definition of Terms
The cranial and peripheral nerves are characterized by bundles of axons originating from the brainstem or spinal cord respectively. These axons are supported by Schwann cells, which provide myelin, fibroblasts and the collagen matrix they produce, capillaries, and scattered mast cells. The axon bundle with associated cells and matrix is referred to as the endoneurium.8 9 10 The nerve cell bodies remain closely associated with either the spinal cord or brainstem in local collections known as ganglia (Fig. 1).
Fig. 1.
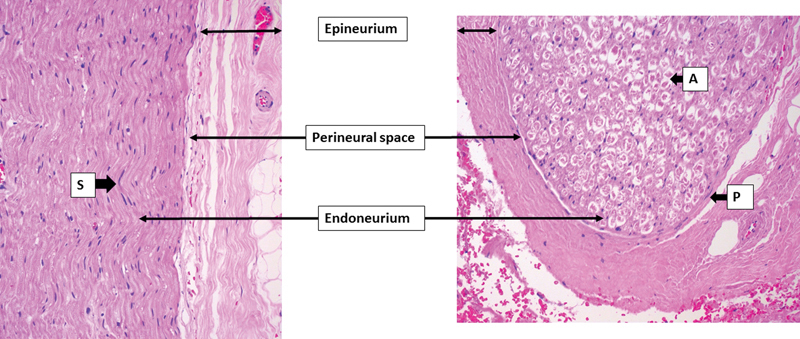
Normal histological anatomy of a cranial nerve demonstrating the epineurial layer, perineural space, and endoneurium. A, axon; P, perineural cell; S, Schwann cells supporting the axons. The perineural layer is thin and merges with the epineurium in this peripheral aspect of the nerve. The perineural space is only partly appreciable.
Circumferentially surrounding the endoneurium is the perineurium. This comprises one to many layers of flat perineural cells resting on a collagen-rich basement membrane. Near the brain stem, there may be as many as 10 to 15 concentric, “onion skin-like layers” comprising the perineurium. The number of layers progressively reduces as the nerve is followed peripherally, until a point where the nerve consists of a single nonmyelinated axon surrounded by a single layer of perineural cells.9 10 At the brainstem, the perineural cells continue into the central nervous system as the pia arachnoid layer.10 Peripherally, the perineurium merges with the sensory end organ, in the case of sensory nerves, and flares to form a funnel-like arrangement at the motor end plate in motor nerves.10 The perineurium is tightly opposed to the endoneurium. Hence the perineural space is normally only a potential zone, although it is continuous from end organ to brainstem. Perineural cells are adherent to one another via tight junctions (Zonulae occludentes) and rest on an intact basement membrane. This forms an effective barrier between the nerve and the bloodstream, known as the blood nerve barrier.8 9 10 In addition to this function, the perineurium provided mechanical support to the nerve and proliferates after injury effectively walling off the damaged nerve. Perineural cells also have surface receptors for complement and most likely play a role in local immune regulation.9 10 Fibroblasts and collagen contribute to the layered arrangement of the perineurium, which can be likened to the layers of an onion. The flattened nature of perineural cells makes them difficult to appreciate in standard histologic sections; however, immunohistochemical reaction for epithelial membrane antigen (EMA), GLUT-1, and claudin-1 are useful to demonstrate the cells (see the following text).9
Encasing the axon bundles and their associated perineurium is a connective tissue-rich structure referred to as the epineurium.8 9 10 The cells of this layer comprise fibroblasts and some mast cells. It is continuous with the dura mater at the brainstem, whereas peripherally it entirely disappears by the stage where the nerve consists of a single axon, the perineurium then being the most peripheral layer of the nerve. The epineurium merges imperceptibly into the surrounding connective tissue. Small vessels of the vaso nervosum and perineural lymphatic vessels reside in the outer layers of the epineurium.9 10
What Is Perineural Invasion?
The definition of what constitutes PNI has been subject to various proposals. The most widely recognized definition is the broader interpretation proposed by Batsakis of tumor cell invasion “in, around, and through the nerve.”11 Under this definition, tumor cells need not be strictly within in the perineural space but rather may involve any of the three layers of the nerve. Conceivably the tumor may not even be within the nerve itself, because focal abutment would achieve this proposed definition. Because of this, a requirement that at least one-third of the nerve circumference must be involved has been added as a refinement to exclude most of the latter situation.8 This definition is useful in small nerves where the anatomical layers may not be well defined. However, in the large nerves, such as those involved in PNS of head and neck malignancy, the layers are well defined and PNS occurs within the true perineural space. In this circumstance it is more exact to define the type of spread by the anatomical location it involves. Hence we define tumor spread in large nerves as showing (1) epineural invasion, (2) PNI, and/or (3) intraneural (endoneural) invasion. As implied, the pattern of spread need not be restricted to a single layer of the nerve.
Prognostic Implications of Perineural Growth in Tumors
Perineural growth in head and neck tumors is of two types: PNI and PNS. Both are adverse prognostic indicators, although they predict for different outcomes. PNI is an adverse prognostic feature in malignancies of the prostate, pancreas, colorectum, stomach, and head and neck.3 4 5 6 8 Poorer prognosis relates first to the provision of a mechanism enabling direct spread beyond the primary site of malignancy. This may be beyond the surgical margin or effective radiation field. Second, a finding of PNI marks a malignancy as innately aggressive. Underlining this are studies showing malignancies demonstrating PNI have a high likelihood to also demonstrate nodal or systemic metastases. Similarly, PNI is an adverse factor in colorectal carcinoma that does not demonstrate lymph node metastases. Because of this adverse risk prediction, PNI is now a recommended standard feature of histopathology reports for resected malignancies of most organs.
With respect to the head and neck squamous cell carcinoma (SCC), PNI is detected in up to 80% of cases and predicts for local recurrence and adverse prognosis (decreased disease free survival).2 Local recurrence rates are increased from 5–9% to 23–36% when PNI is detected. Branches of the trigeminal and facial nerves are most commonly involved, although any nerve, sensory, motor, or autonomic, of the head and neck region may potentially be involved.
PNS in head and neck tumors involves larger nerves, essentially always beyond the site of primary tumor. The prognostic implication is a reduced overall survival. In contrast to PNI, PNS does not predict local tumor recurrence or increased risk of lymph node metastasis.
Mechanisms of Perineural Spread
The physical mechanism by which cancer gains access to the perineural space remains unclear. Potential weakness exists near the nerve ending where the perineurial layer no longer exists, at the site where the vessels of the vaso nervosa penetrate the epineurium to supply the nerves, and at the site where reticular fibers penetrate the epineurium.10 Direct invasion through the epineurium and perineurium layers may be aided by production of various proteases produced by the tumor cells and the peritumoral inflammatory cells. In addition, matrix metalloproteinases act to enhance tumor cell proliferation.12 Neoplastic cells may also undergo epithelial mesenchymal transition leading to loss of cell to cell connection and the acquisition of pseudopodic motion (“invadopodia”) allowing individual tumor cell invasion.13 14 15 16
As mentioned previously, various chemical factors in the local environment modulate the risk for PNI by several mechanisms.2 8 17 First, they induce proliferation of neurites increasing the physical contact of tumor cells and nerves. Second, they promote, via reciprocal signaling, interactions between neoplastic cells and nerves, which promote homing of tumor to nerve (“neurotropism”). Third, they induce a change in the stromal microenvironment with the net effect of promoting tumor invasion through the stroma. Clearly, it would be beneficial to determine which tumors are producing these factors and to what extent these factors increase the risk for perineural space invasion. To date several tumor secreted neuropeptides have been studied in various tumor types. These include neurotrophic factors (brain-derived neurotrophic factor [BDNF], glial cell line–derived neurotrophic factor [GDCF]), nerve growth factor (NGF), neurotrophins 3 and 4 (NT-3, NT-4), the neural cell adhesion molecule (NCAM), substance P (SP), chemokines, and galanin.2 8 17 18 These factors bind to cell surface receptors, mainly tyrosine kinase (TrK) family (neurotrophins) or G protein coupled receptors (galanin). Once receptor bound, signaling pathways are activated leading to induction of nuclear transcription factors that induce neural cell proliferation and growth and promote PNI. A recent meta-analysis specific for head and neck squamous cell carcinoma (SCC) revealed galanin and BDNF as the only significantly upregulated neuropeptides over all studies when compared with noncancerous tissue.18
While most attention has focused on the positive association with PNI and tumor cell secreted neurotrophic factors, there is a similar effect observed with increased growth factor receptor production. A recent study has highlighted the increased risk of PNI when a high-affinity NGF receptor (TrkA) is overexpressed in tumor cells.19
Approach to Pathological Specimens from the Head and Neck Region
Macroscopic Approach
Pathologic specimens demonstrating PNS include regional dissections, such as a parotidectomy, resection of the pterygopalatine fossa, resection of the infratemporal fossa, or may simply include a dissection of the involved nerve for variable length along its course. In radical resection specimens the main aim is to remove the primary tumor, PNS being an accessory feature. Pathologists should approach the dissection of these specimens with regard to the characterization of the primary tumor and documentation of soft tissue and neurovascular margin status (Figs. 2 and 3) For parotidectomy specimens we aim, where practical, to take sections perpendicular to the long axis of the facial nerve because the tissue sections then present a complete transverse section of the facial nerve allowing the highest likelihood to demonstration PNS. In the case of neurectomy specimens, two approaches may be taken. The first is to embed the nerve such that the long axis of the nerve runs parallel to the subsequent sections (Fig. 2). This approach has the advantage of demonstrating continuous spread along the nerve and allows for the most accurate measurement of the length of nerve involved by PNS (Fig. 3A). The disadvantage is the potential to not identify PNS that involves only part of the circumference of the nerve. For example, if a nerve with partial involvement is embedded longitudinally such that the PNS involvement is on the aspect of the nerve away from the plane of section, PNS will not appear in the subsequent histologic sections. The other approach is to take serial transverse sections along the nerve. This overcomes the issue of missing noncircumferential nerve involvement but diminishes the accuracy of determination of the length of nerve involvement because multiple sections are usually placed in a single block. Additionally, if multiple transverse sections are placed in a single block, it is not possible to separate discontinuous spread (“skip lesions”) from continuous spread, which ceases at some point within the sections taken. Both situations produce sections of nerve with and without PNS in the same histologic slide. This could be overcome by placing only a single transverse section in a tissue block; however, this produces many additional slides with significant additional cost. Additionally, this approach will still not enable an accurate demonstration of the length of nerve involved.
Fig. 2.
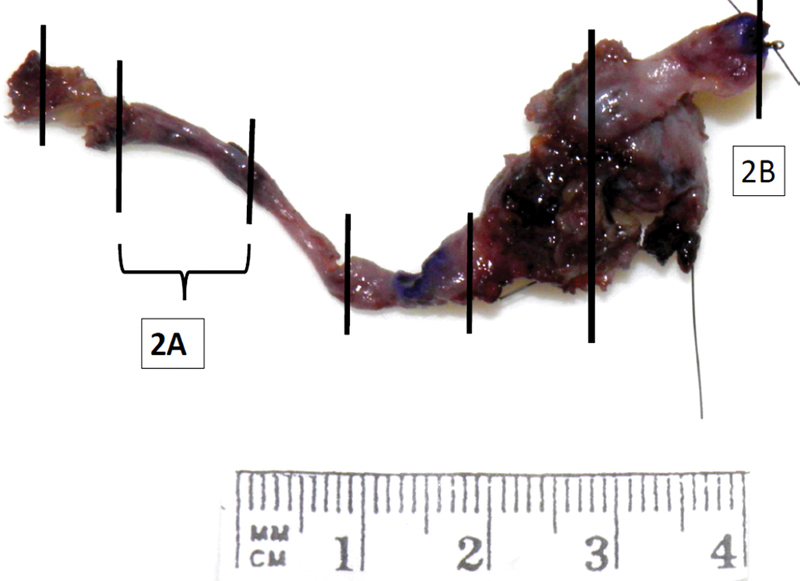
Neurectomy specimen of infraorbital nerve to V2 ganglion. Vertical lines demonstrate the position of tissue sections. Intervening segments of nerve are embedded in a longitudinal fashion. Full-face transverse sections are taken of the cutaneous and brainstem margins.
Fig. 3.

Corresponding histologic sections. (A) Longitudinal section with peri- and intraneural spread. (B) Transverse en face section of the brainstem margin. Note the presence of ganglion cells (G).
Practically, it matters little whether the nerve is sectioned transversely or longitudinally in most cases. If a specific question needs to be answered, one technique may be favored; for example, longitudinal sections are best to demonstrate potential skip lesions and to determine distance to an end margin or length of nerve involvement. Transverse sections are best for all other situations. For routine cases we favor longitudinal sections for the reasons presented above. Marking ink should be applied to the external surface of the nerve as this represents the surgical soft tissue margin. The end margins of the nerve also represent resection margins.
End margins of resection are best performed as en face transverse sections as they demonstrate the entire area of the margin (Figs. 2 and 3B). This is also the best approach to document margin clearance at the time of frozen section. The presence of ganglion cells, sometime numerous, at the proximal margin can cause difficulties in excluding malignancy during frozen section.
Microscopic Approach
PNS is in the main easy to appreciate histologically. At low power, the nerve appears expanded and hypercellular. A lymphoid infiltrate surrounding the involved section of the nerve may also be encountered, and is occasionally florid and may partly obscure the tumor. At higher power, malignant cells generally display an epithelioid morphology, although sometimes a spindled appearance is seen. Most commonly PNS of carcinoma is within the perineural layers creating an onion skin–like arrangement (Fig. 4A). Intraneural spread within the substance of the nerve is the second most common pattern of involvement but can be subtle and may require immunohistochemistry to demonstrate tumor cells. Occasionally tumor is observed invading beyond the perineurium into the epineurium. This is normally seen only when the perineural space is markedly expanded by tumor.
Fig. 4.
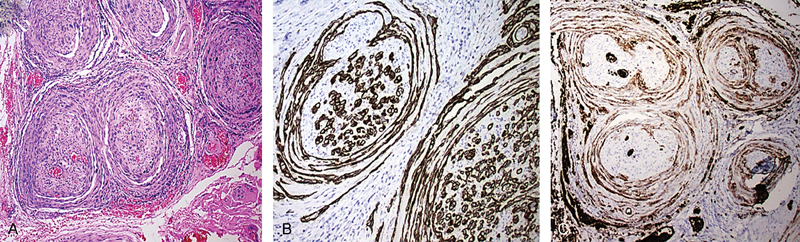
Perineural spread by carcinoma. (A) H&E stain displaying characteristic onion skin pattern. (B) Endoneurial pattern of spread is also highlighted by the AE1/AE3 keratin stain. (C) GLUT 1 immunohistochemical stain highlighting the perineural layers.
Benign histologic mimics of PNS should always be considered. The most important of these include (1) ganglion cells resembling the cells of well-differentiated SCC; (2) intraneural blood vessels with prominent endothelial cells; and (3) reactive perineural cell hypertrophy that may be observed near PNS or as reaction to inflammation around tumor cells. In most cases close attention to the histology will allow separation, since malignant cells display nuclear hyperchromasia, irregular enlarged nucleoli, variable nuclear size and shape, and importantly the presence of mitoses and/or apoptotic bodies. However, in problematic cases, special stains may be required (see the following text).
Use of Special Stains
Immunohistochemical stains may be helpful in (1) defining the extent of PNS; (2) demonstrating the presence of tumor in subtle cases; and (3) separating carcinoma from a benign mimic. The most useful stains are an S-100 to demonstrate the nerve itself and a broad-spectrum keratin stain (AE1/AE3) to demonstrate carcinoma cells (Fig. 4B). It is possible to “double stain” tissue sections simultaneously for both markers, although we have not found this to be of additional benefit in most cases. Broad-spectrum keratin is useful in demonstrating single carcinoma cells or small collections of cells that extend beyond the main tumor mass. It is also helpful in demonstrating intraneural spread. One particular important use for broad-spectrum keratin is in formally excluding the presence of tumor cells at the surgical margin. Hence we have a low threshold for applying this stain when end margins or soft tissue margins appear close to tumor.
Ganglion cells exhibit a positive reaction for S-100 immunohistochemical whereas they are nonreactive with an AE1/AE3 keratin stain. A more specific marker of ganglion cells such as chromogranin can also be useful in this differential diagnosis as it does not stain the background neural cells and is therefore easier to review. Demonstration of perineural cells can be useful to demonstrate that tumor is within the perineural space or to demonstrate that cells at the edge of the nerve are hypertrophic perineural cells rather than malignant cells. We have found claudin 1 and GLUT-1 (Fig. 4C) to be more useful perineural markers than EMA for these purposes.
Special Issues
1. Absence of cutaneous primary lesion
Up to one-third of clinical PNS of SCC occurs in the absence of a known primary cutaneous lesion.1 Potential reasons for this include, first the patient being unable to accurately recall excisions or topical treatments of a cutaneous lesion. This is particularly true in patients with multiple cutaneous cancers in which numerous lesions may have been treated over time. Because almost half of cutaneous SCCs subsequently presenting with PNS do not demonstrate PNI, any risk for PNS will likely not be communicated to the patient. Second, the primary lesion may have undergone complete regression. How often this occurs is unknown; however, it is described in cutaneous SCC and other cutaneous cancers, in particular Merkel cell carcinoma and melanoma.20 21
2. Involvement of more than one nerve
In general, PNS is restricted to the distribution of a named nerve within which it occurs. However, it is potentially possible for PNS to involve more than one nerve distribution. This arises most commonly between the facial and trigeminal nerves that have three points of interaction where tumor may cross. These include (1) at the sphenopalatine ganglion; (2) the junction between the lingual nerve and the chorda tympani; and (3) within the parotid gland involving the auricular temporal branch of the mandibular nerve. Involvement of more than one nerve might also occur in large cutaneous lesions in which tumor might invade both trigeminal and facial nerve branches. Theoretically, extensive lymphatic permeation could allow access to the epineural region of more than one nerve branch, although we believe this would be uncommon.
Often, we have observed the retrograde spread form one division of the trigeminal nerve into another division to occur centrally at the level of the ganglia.
3. Continuous growth versus skip lesion
In our published experience, once PNS becomes established in a nerve, it continues centrally in a continuous fashion. Our examination of more than 50 longitudinally sectioned specimens of resected nerves with PNS revealed no instances where there was a “skip” in the pattern of spread.22 We believe that the concept to skip lesions may be the result of a misinterpretation arising from Mohs procedure specimens that is now perpetuated in the literature. The extension of SCC into side branches from the main nerve (retrograde spread) or adjacent but separate nerves can in the certain planes of section give the appearance of a skip along the nerve. This is especially true in PNS involving the facial nerve within the parotid gland. However, if sufficient sections are cut and the 3D anatomy reconstructed, the apparent skip areas can be demonstrated to be in continuity.
4. Perineural versus endoneural spread
We have not found it helpful to subcategorize neural spread as to whether it involves either perineurium or endoneurium. This is because both patterns of spread are often concurrently found, especially when clinically evident PNS is manifest. We occasionally encounter cases where there is only PNS, generally a more peripheral nerve. We have never encountered a case of neural spread restricted solely to the endoneural layer.
5. Epineurium as a barrier and hence a surgical margin (Fig. 5A)
Fig. 5.

Barriers to extraneural spread. (A) Epineurium as a barrier. The dense collagen layer is highlighted. Tumor remains confined to the perineural space. (B) The central ganglion causing dispersion of the invasive carcinoma.
The epineurium is the collar of collagen-rich connective tissue surrounding the nerve bundles located external to the perineurium. The epineurium is particularly well developed in the central few centimeters of the nerve. To invade the epineurium, tumor must first be able to invade through the perineurium. Our experience is that the perineurium provides a significant barrier. This is because the initial reaction to tumor invasion is perineural cell hypertrophy with production of more extracellular matrix creating thicker collagen rich layers effectively reinforcing the walls of this space. If tumor eventually breaches the perineural space, it encounters a further dense connective tissue barrier, the epineurium (Fig. 4). We have noticed that tumor is more likely to grow along the perineural spaces than to invade directly through the epineurium. Hence, we believe that this layer represents a path of greater resistance. Our collective experience is that very few PNS cases we have examined demonstrate a positive soft tissue/epineural margin.
6. The ganglia as a relative barrier (Fig. 5B)
The ultimate destination for PNS is the brainstem and hence access to the cerebrospinal fluid with dissemination to the leptomeninges and spinal cord. Between the nerve and the brainstem lies a relative barrier comprising the neurone cell bodies and the junction between the perineurium/epineurium and meninges. At a histologic level, this transition zone is disordered, and the accompanying anatomical spaces that are well defined distally, become more convoluted. Of course they do not cease to exist; however, we have observed that at the level of the ganglia, perineural tumor spread becomes more dispersed and seemingly less organized as it ramifies through a more maze-like architecture (Fig. 5).
7. Difficulties encountered at frozen section assessment
Frozen section is typically performed to document the presence of PNS by tumor and clearance at the resection margin. It is a technically difficult exercise, best performed by a technician experienced in sectioning frozen tissue and a pathologist experienced in examining frozen sections. Technical issues for PNS specimens relate to the size of tissue required to be frozen and issues of orientation of the specimen piece in the freezing medium. The main issues for the pathologist interpretation include difficulty in separating malignancy, especially when poorly differentiated, from inflammatory cells, reactive perineural or endothelial cells or from ganglion cells. In some cases assessment will be necessarily deferred to paraffin sections.
Conclusion
The adverse prognostic implications of PNS of head and neck SCC are well recognized. Pathologists play an important role by confirming the clinical diagnosis, documenting the extent of tumor spread, determining the patterns of spread, excluding benign mimics of tumor, and, importantly, assessing for margin involvement. Conceivably in the future, pathologists may be required to perform additional stains or undertake molecular studies that could predict the risk for PNS in a tumor, or provide evidence of a target protein within the tumor amenable to biological therapy.
References
- 1.Panizza B, Warren T. Perineural invasion of head and neck skin cancer: diagnostic and therapeutic implications. Curr Oncol Rep. 2013;15(2):128–133. doi: 10.1007/s11912-012-0288-y. [DOI] [PubMed] [Google Scholar]
- 2.Roh J, Muelleman T, Tawfik O, Thomas S M. Perineural growth in head and neck squamous cell carcinoma: a review. Oral Oncol. 2015;51(1):16–23. doi: 10.1016/j.oraloncology.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu B, Lu K Y. Neural invasion in pancreatic carcinoma. Hepatobiliary Pancreat Dis Int. 2002;1(3):469–476. [PubMed] [Google Scholar]
- 4.Harnden P, Shelley M D, Clements H. et al. The prognostic significance of perineural invasion in prostatic cancer biopsies: a systematic review. Cancer. 2007;109(1):13–24. doi: 10.1002/cncr.22388. [DOI] [PubMed] [Google Scholar]
- 5.Liebig C, Ayala G, Wilks J. et al. Perineural invasion is an independent predictor of outcome in colorectal cancer. J Clin Oncol. 2009;27(31):5131–5137. doi: 10.1200/JCO.2009.22.4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnston M, Yu E, Kim J. Perineural invasion and spread in head and neck cancer. Expert Rev Anticancer Ther. 2012;12(3):359–371. doi: 10.1586/era.12.9. [DOI] [PubMed] [Google Scholar]
- 7.http://www.cap.org/web/home/resources/cancer-reporting-tools/cancer-protocol-templates? Accessed July 13, 2015
- 8.Liebig C, Ayala G, Wilks J A, Berger D H, Albo D. Perineural invasion in cancer: a review of the literature. Cancer. 2009;115(15):3379–3391. doi: 10.1002/cncr.24396. [DOI] [PubMed] [Google Scholar]
- 9.Piña-Oviedo S, Ortiz-Hidalgo C. The normal and neoplastic perineurium: a review. Adv Anat Pathol. 2008;15(3):147–164. doi: 10.1097/PAP.0b013e31816f8519. [DOI] [PubMed] [Google Scholar]
- 10.Ortiz-Hidalgo Weller R O In: Mills S E ed.Peripheral Nervous System in Histology for Pathologists. 4th ed Philadelphia, PA: Lippincott Williams & Wilkins; 2012261–294.: chap 10 [Google Scholar]
- 11.Batsakis J G. Nerves and neurotropic carcinomas. Ann Otol Rhinol Laryngol. 1985;94(4 Pt 1):426–427. [PubMed] [Google Scholar]
- 12.Stetler-Stevenson W G, Yu A E. Proteases in invasion: matrix metalloproteinases. Semin Cancer Biol. 2001;11(2):143–152. doi: 10.1006/scbi.2000.0365. [DOI] [PubMed] [Google Scholar]
- 13.Shinto E, Jass J R, Tsuda H. et al. Differential prognostic significance of morphologic invasive markers in colorectal cancer: tumor budding and cytoplasmic podia. Dis Colon Rectum. 2006;49(9):1422–1430. doi: 10.1007/s10350-006-0595-1. [DOI] [PubMed] [Google Scholar]
- 14.Weaver A M. Invadopodia: specialized cell structures for cancer invasion. Clin Exp Metastasis. 2006;23(2):97–105. doi: 10.1007/s10585-006-9014-1. [DOI] [PubMed] [Google Scholar]
- 15.Smith A, Teknos T N, Pan Q. Epithelial to mesenchymal transition in head and neck squamous cell carcinoma. Oral Oncol. 2013;49(4):287–292. doi: 10.1016/j.oraloncology.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jimenez L, Jayakar S K, Ow T J, Segall J E. Mechanisms of Invasion in Head and Neck Cancer. Arch Pathol Lab Med. 2015;139(11):1334–1348. doi: 10.5858/arpa.2014-0498-RA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marchesi F, Piemonti L, Mantovani A, Allavena P. Molecular mechanisms of perineural invasion, a forgotten pathway of dissemination and metastasis. Cytokine Growth Factor Rev. 2010;21(1):77–82. doi: 10.1016/j.cytogfr.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Scanlon C S, Banerjee R, Inglehart R C. et al. Galanin modulates the neural niche to favour perineural invasion in head and neck cancer. Nat Commun. 2015;6:6885. doi: 10.1038/ncomms7885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frydenlund N, Leone D A, Mitchell B, Abbas O, Dhingra J, Mahalingam M. Perineural invasion in cutaneous squamous cell carcinoma: role of immunohistochemistry, anatomical site, and the high-affinity nerve growth factor receptor TrkA. Hum Pathol. 2015;46(8):1209–1216. doi: 10.1016/j.humpath.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Roseman J M. Regression of locally recurrent squamous cell carcinoma of the skin following excision of a metastasis: with review of the literature. J Surg Oncol. 1988;39(3):213–214. doi: 10.1002/jso.2930390317. [DOI] [PubMed] [Google Scholar]
- 21.Al-Hadad I, Kotecha S, Webster K. Multiple self-healing squamous cell carcinomas of the face. Br J Oral Maxillofac Surg. 2009;47(8):635–637. doi: 10.1016/j.bjoms.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 22.Panizza B, Warren T A, Solares C A, Boyle G M, Lambie D, Brown I. Histopathological features of clinical perineural invasion of cutaneous squamous cell carcinoma of the head and neck and the potential implications for treatment. Head Neck. 2014;36(11):1611–1618. doi: 10.1002/hed.23509. [DOI] [PubMed] [Google Scholar]


