Abstract
Perineural invasion (PNI) is the neoplastic invasion of nerves. PNI is widely recognized as an important adverse pathological feature of many malignancies, including pancreatic, prostate, and head and neck cancers and is associated with a poor prognosis. Despite widespread acknowledgment of the clinical significance of PNI, the mechanisms underlying its pathogenesis remain largely unknown. Recent theories of PNI pathogenesis have placed a significant emphasis on the active role of the nerve microenvironment, with PNI resulting from well-orchestrated reciprocal interactions between cancer and host. Elucidating the mechanisms involved in PNI may translate into targeted therapies for this ominous process.
Keywords: perineural invasion, neurotrophic factors, chemokine, head and neck cancer
Introduction
Perineural invasion (PNI) has been defined as the ability of cancer cells to invade in, around, and through nerves.1 PNI is an ominous clinical and pathological finding for a variety of cancer types that is associated with elevated recurrence rates and diminished survival.2 3 4 Despite widespread acknowledgment of the clinical significance of PNI, the mechanisms underlying its pathogenesis remain largely unknown and specific therapies targeting nerve invasion are entirely lacking.
The pathological description and clinical significance of PNI, particularly in head and neck cancers, was described and recognized long ago.5 Initial theories suggested that PNI was simply an extension of lymphatic metastasis, which was eventually disproven. Subsequent theories on PNI pathogenesis centered on neural sheaths serving as a low-resistance, conduit for tumor cell spread.6 However, careful study of nerves with electron microscopy and other techniques demonstrated that the nerve sheath is actually a higher resistance path with several layers of collagen and concentrically arranged endothelial cells.7 Modern studies have demonstrated that PNI is a directed process, which results from reciprocal molecular interactions between cancer and host, challenging the historical notion that this is a purely cancer-induced event.
The research paradigm has more recently shifted for PNI with current studies now focusing on neurotrophic factors, chemokines, cellular adhesion molecules, and the nerve microenvironment. The picture of PNI that is emerging is one of a conserved, symbiotic relationship between cancer and host in which both parties facilitate metastasis. A mechanistic understanding of this entity may potentially facilitate the development of targeted therapies for PNI.
Peripheral Nerve Sheath
An understanding of the peripheral nerve structure is necessary to study PNI and elucidate its pathogenesis. A nerve sheath is composed of three tissue layers (from outside in): the epineurium, perineurium, and endoneurium (Fig. 1). The epineurium is comprised of dense connective tissue consisting of both collagen and elastin fibers, which surrounds an entire nerve.8 This layer contains the vascular supply of the nerve. Underlying this is a layer of flat cells, the perineurium that forms a complete sleeve around a bundle of axons. Tightly fitting endothelial cell with basement membranes constitute this layer, which functions as a permeable but selective barrier.8 The endoneurium represents the innermost layer which invests single nerve fibers and the Schwann cells, which myelinate them.9 The endoneurium, which consists of a layer of delicate connective tissue, forms an uninterrupted sheath that extends from the surface of the brain or spinal cord to the level at which the axon synapses. Within the endoneurium, individual fibers are surrounded by endoneurial fluid, which is similar to cerebrospinal fluid and helps constitute a blood–nerve barrier. The endoneurial cells also function to support and protect the delicate axons.
Fig. 1.
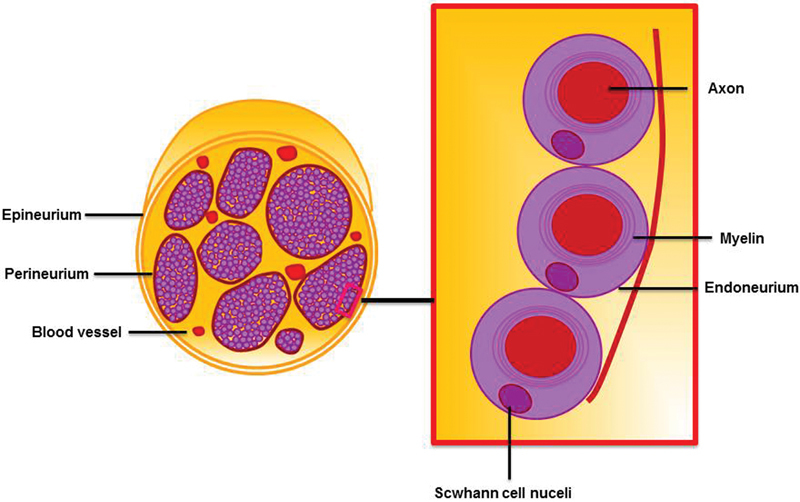
Peripheral nerve structure. A nerve sheath is composed of three tissue layers consisting of the epineurium, perineurium, and endoneurium.
Cancer within any of the three layers of the nerve sheath constitutes PNI. It is important to note that many pathologists also classify tumor in close proximity to the nerve and involving at least one-third of its circumference as PNI. However, a recent study suggests that it may be invasion within these three layers, termed intraneural invasion that is clinically relevant.10 The mechanism by which cancer is able to breach these sheaths remains to be elucidated.
Nerve Microenvironment
The nerve microenvironment is a rich network of cells whose role is to maintain and support the surrounding neuronal cells. There is growing evidence that these supportive cells interact with the cancer and promote the invasion and spread along nerves. Here, we review the major cell types within and adjacent to the peripheral nerve sheath.
Macrophages
There is strong evidence that indicates that macrophages promote tumor progression and metastasis.11 Endoneurial macrophages, represent a subpopulation that participates in nerve homeostasis and regeneration of peripheral nerves following injury.12 Endoneurial macrophages were demonstrated to produce glial cell line-derived neurotrophic factor (GDNF) and promote PNI in response to CSF-1 secretion by pancreatic cancer.13 There have been clinical correlates associating macrophages with PNI in human samples as well.14
Fibroblasts
Like macrophages, fibroblasts have also been shown to have a profound influence on the development and progression of cancers.15 Fibroblasts can synthesize growth factors and chemokines that may inadvertently fuel cancer invasion.16 Importantly, the perineurium originates from fibroblasts.17 Any alteration in the composition of the perineurium can result in increased permeability and impaired protection of nerve fascicles from invasion. Currently, there is no direct evidence linking fibroblasts with PNI; however, this is an area worthy of investigation.
Schwann cells
Schwan cells represent a major constituent of the nerve microenvironment and serve several integral roles. Schwann cells help nurture neurons during development as well as myelinate mature peripheral nerves.18 They also promote neuronal repair and regeneration following traumatic injury.19 There is recent evidence that Schwann cells have an affinity for certain gastrointestinal cancer types, and that their migration toward cancer may precede any invasion suggesting the nerve milieu may play a role in initiating PNI.20 Recent work from our laboratory has revealed that Schwann cells may promote PNI through the disruption and cancer clusters. Schwann cells make contact with cancer cells, intercalate between them, and disperse cells. These events ultimately promote cancer migration and invasion along nerves. Interestingly, such Schwann cell behavior appear to recapitulate its function in nerve development and repair.21
Model Systems
Our ability to elucidate the mechanisms involved in PNI has been restricted by the limited number of models for this intricate process. To appreciate modern theories of PNI pathogenesis, it is essential to review both in vitro and in vivo models that have been utilized.
In vitro Model
In vitro models of a complex disease process are inherently limited. However, in vitro models do allow for a highly controlled experimental setting, which has led to a better understanding of the contributions of several soluble factors in promoting PNI. Ayala et al originally described an in vitro system in which murine dorsal root ganglia (DRG) are cocultured with prostate cancer in Matrigel (Corning Lifesciences, Tewksbury, Massachusetts, United States).22 Once suspended in the Matrigel matrix, the DRG sprouts neurites (Fig. 2), which for experimental purposes are similar to individual nerve fibers. These neurites both grow toward a cancer colony and become invaded by cancer. Notably, in this model cancer migrates unidirectionally toward the central ganglion, which mimics the clinical observation that PNI typically spreads centripetally. Utilizing this model Ayala et al was the first to describe the symbiotic nature of PNI in which both the cancer and the nerve have a growth advantage when cocultured.23
Fig. 2.
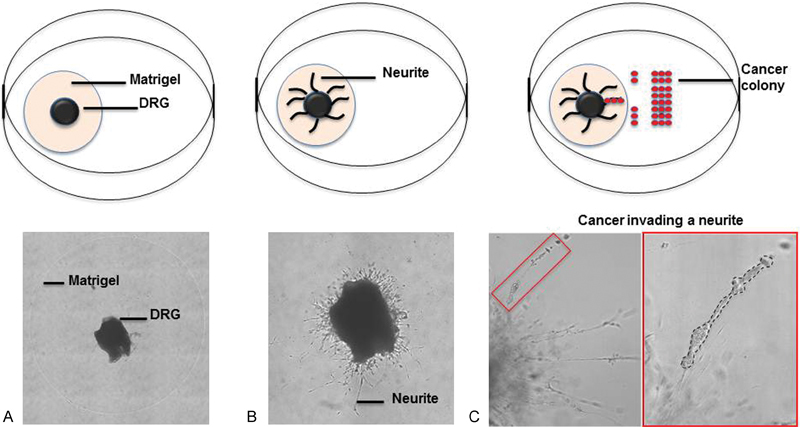
In vitro dorsal root ganglion (DRG) model of perineural invasion. (A) Murine DRG are suspended in a Matrigel matrix (Corning Lifesciences, Tewksbury, Massachusetts, United States) following explant. (B) Over the next few days, neurites sprout from the central ganglion. (C) Cancer is cocultured adjacent to the DRG and over time invades the neurites (dotted black line), which can be quantified.
A modified model has been utilized by our laboratory. In our version, we have cocultured either pancreatic or prostate cancer cell lines with the DRG, which allows the migration of cancer along individual neurites as well extent of invasion to be quantified.24 25 26 The cancer can be cocultured with DRG using different techniques. In one version, the cancer can either be grown in a colony directly adjacent to the DRG in an impermeable barrier. Once the barrier is removed, the cancer will migrate toward the DRG and eventually invade the adjacent neurites making it easy to quantify the degree of invasion (Fig. 2C). In another variation, the DRG may be grown in the Matrigel and then the cancer added to the media. In this approach, the cancer is widely dispersed and has the opportunity to interact with a large number of individual neurites.
Ex vivo models of PNI using explanted rat vagal nerve tissue and a chamber system have also been described.27 While the nerves utilized in this model may better mimic those of the large scale nerves that become infiltrated in humans, the same major limitation of being unable to replicate the nerve microenvironment applies. In vivo models of PNI which are described below have been more promising in this regard.
In vivo Model
Carcinogenesis-Induced Models
The carcinogenesis-induced models have the benefit of both mimicking their human counterparts histologically as well as studying PNI in the relevant tumor and nerve microenvironment. KPC mice, which contain a conditional mutant Kras allele among other mutations, develop pancreatic cancer.28 This model has been used to characterize the linear progression of neuroplastic changes in surrounding nerves as the cancer progresses.29 Similar genetic models have been used to study the role of the autonomic nervous system in prostate cancer with Hi-Myc transgenic mice that develop prostate cancer similar to human tumors.30 31 To date, these models have been most helpful in elucidating the contribution of the nervous system to the development and progression of cancer and less to understanding the basic biology of PNI. The clear benefit of these systems is that the nerve microenvironment remains intact and the cancer naturally invades the nerves as opposed to orthotopic or heterotopic models in which the cancer and PNI develop iatrogenically.
Orthotopic Models
Orthotopic models of PNI in which cancer is transplanted into its native organ have been present for several years.32 These exist for several different cancer types and organs including the pancreas,32 head and neck (squamous cell carcinoma),33 prostate,30 and salivary glands.34 Orthotopic models are ideal for studies which aim to investigate specific cancer properties that promote PNI. These models also allow for transplanted cells to be labeled and followed over time; however, functional and endpoints may be limited depending on the organ of interest. Further the iatrogenic introduction of cancer is not ideal for investigating early events involved in PNI development.
Heterotopic Models
Our laboratory utilizes a murine sciatic nerve model to study PNI in vivo (Fig. 3).26 The sciatic nerve can be easily identified and cancer microscopically injected into the nerve, distal to its bifurcation of the tibial and common peroneal nerves. Neurotropic cancers including prostate and pancreatic cell lines invade toward the spinal cord causing hind limb paralysis, which can be followed over time. Validated functional metrics such the sciatic neurological score and hind paw width can be used to assess invasion clinically over time.24 25 26 35 Further, the sciatic nerve is easily amenable to magnetic resonance imaging (Fig. 3C).25 This model allows for a wide range of cancer histologies to be utilized and for PNI to be followed closely over time either clinically and/or with imaging.
Fig. 3.

In vivo sciatic nerve model of perineural invasion (PNI). (A) Cancer is injected into the distal sciatic nerve. (B) With time, the sciatic nerve becomes infiltrated and thickened as cancer invades unidirectionally toward the spinal cord as seen grossly, (C) on magnetic resonance imaging (dotted white line), and (D) histologically. (E) PNI can be functionally followed by monitoring hind limb function.
Molecular Mechanisms
Recent studies have identified several potential molecular mechanisms that drive PNI. Many different classes of proteins and cellular processes have been invoked in promoting PNI (Fig. 4). The picture of PNI pathogenesis that is emerging is one of a deliberate, reciprocal process between the cancer and the surrounding nerve microenvironment.
Fig. 4.
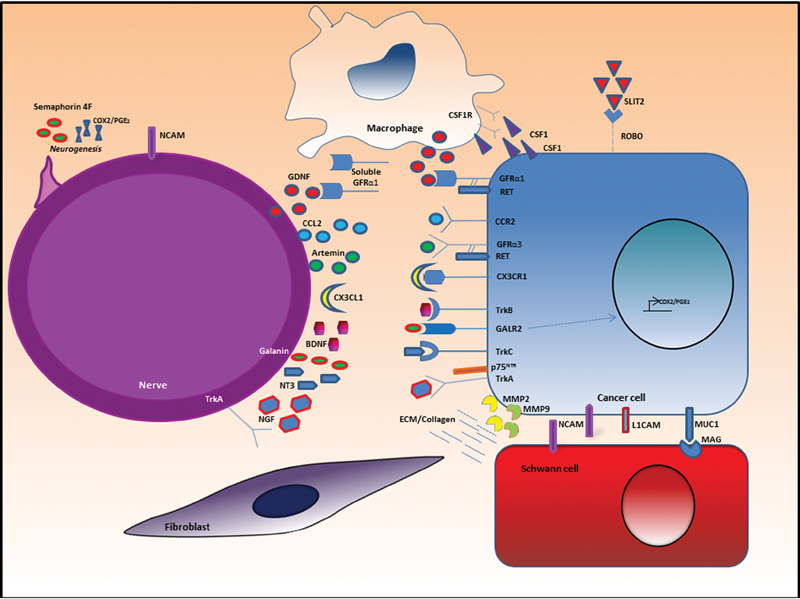
Molecular mechanisms of perineural invasion (PNI). PNI results from multidirectional signaling between the cancer and the nerve microenvironment involving various signaling and cellular adhesion molecules. BDNF, brain-derived neurotrophic factor; COX2, cyclooxygenase-2; CSF1, colony stimulating factor 1; CSF1R, colony stimulating factor 1 receptor; ECM, extracellular matrix; GAL; galanin; GDNF, glial cell line-derived neurotrophic factor; GFRα, GDNF receptor-α; L1CAM, L1 cell adhesion molecule; MAG, myelin-associated glycoprotein; MMP, matrix metalloproteinase; NCAM, neural cell adhesion molecule; NGF, nerve growth factor; NT3, neurotrophin-3; PGE2, prostaglandin E2.
Neurotrophic Factors
Neurotrophic factors are a family of proteins that regulate the growth of development of axons as well as the maintenance of mature neurons. While their primary role is in the development of the peripheral and central nervous system, recent evidence has suggested that both cancers and neuronal cells upregulate neurotrophic factors, which may facilitate the growth and directional spread of cancer along nerves.
Glial Cell Line-Derived Neurotrophic Factor
The GDNF family of growth factors has been demonstrated by many groups to play an important role in PNI. The GDNF family consists of four members: GDNF, neurturin, artemin, and persephin. Each protein has a specific GDNF receptor-α (GFRα), which signals through its cognate receptor RET.36
Pancreatic cancer has been shown to both overexpress GDNF and migrate toward it in a dose-dependent fashion.37 GDNF is known to be secreted by both nerves and its supporting cells, including macrophages.13 Work from our laboratory demonstrated that pancreatic cancer invasion and migration along nerves is driven by cancer cell chemotaxis toward GDNF.26 Further, the reduction of GDNF expression or the blockade of its receptor, RET, has been shown to reduce nerve invasion both in vitro and in vivo, respectively.26 Therapeutic radiation, which is often administered adjuvantely in cases of PNI along the course of invaded nerves, may act not only by inducing cancer cell death but by also suppressing GDNF levels.24 In addition to effects on cancer motility, GDNF has also been shown to induced higher expression of matrix metalloproteinases (MMPs) in human oral squamous cell carcinomas, which may promote a more invasive cancer phenotype.38
RET is overexpressed on several neurotrophic cancers.24 26 For GDNF to activate RET, it must first bind to GFRα1. It is this GDNF–GFRα1 complex that binds to RET on the cancer cell surface and initiates signal transduction.39 Recent work has demonstrated that soluble GFRα1 is released from nerves and interacts with secreted GDNF to activate RET on cancer cells and induce migration and invasion along nerves.35 Collectively, the GDNF–GFRα1–RET complex activates downstream pathways including the mitogen-activated protein kinase pathway, which control cell migration, directionality, and growth.40 41 The contribution of both GDNF and GFRα1 by the nerve microenvironment, underscores its participatory role in facilitating PNI.
Artemin
The evidence implicating other members of the GDNF family in PNI is not as robust. However, there are studies suggesting that artemin and its receptor GFRα3 may play a role in promoting PNI. Elevated levels of both artemin and GFRα3/RET were detected in specimens of pancreatic ductal adenocarcinoma.42 Interestingly, levels of artemin and GFRα3 were also upregulated in the setting of pancreatitis suggesting that it may function to repair ongoing neural damage incurred by inflammation.43 Similar to GDNF and GFRα1, both artemin and GFRα3 are supplied by the nerve microenvironment.43 Corollary in vitro studies have also demonstrated that overexpression of artemin in pancreatic cancer cell lines promoted neurotrophic invasion.44
Brain-Derived Neurotrophic Factor
Brain-derived neurotrophic factor (BDNF) is a member of the neurotrophin family of growth factors and plays critical role in aiding the survival of existing neurons and promoting the growth of new neurons and synapses. The contribution of BDNF toward PNI has been examined in pancreatic cancer, gastric cancer, and adenoid cystic carcinoma.45 46 47 48 Most of the studies investigating BDNF have correlated expression levels in human samples with PNI. However, there is mechanistic in vitro data which demonstrates that the overexpression of BDNF increases proliferation and invasiveness of both pancreatic and gastric cancers.48 49 50 TrkB, the high-affinity receptor for BDNF has also been shown to be overexpressed in metastatic human pancreatic ductal adenocarcinoma cells, which is associated with PNI.51 The evidence to date implicates BDNF/TrkB in promoting cancer invasiveness, proliferation, and progression.
Neurotrophin-3
Neurotrophin-3 (NT3) is a growth factor which supports the growth and differentiation of both existing and new neurons. Early studies demonstrated that the presence of NT3 was elevated in pancreatic ductal adenocarcinoma specimens in comparison to normal pancreas tissue.49 Notably, NT3 and its receptor TrkC were more highly expressed in intratumoral nerves within pancreatic adenocarcinoma carcinoma specimens.45 Blockade of NT3 in vivo inhibited the growth and progression of prostate and pancreatic adenocarcinoma in murine xenograft models.52
Nerve Growth Factor
Nerve growth factor (NGF) and its receptor TrKA have been associated with PNI in several cancer types. NGF expression has been well studied in pancreatic cancer. Increased levels of NGF and its high-affinity receptor, TrKA, and low-affinity receptor p75NTR have been reported in pancreatic cancer and the surrounding nerves, which correlated significantly with the presence of PNI.53 Interestingly, TrKA is expressed on both the surrounding nerves and on pancreatic cancer cells.54 Thus, NGF, which is produced by pancreatic cancer, may function to promote the growth and invasion of the tumor in an autocrine fashion and fuel PNI in a paracrine fashion.53 Exposure of pancreatic cancer to exogenous NGF resulted in a dose-dependent increase in MMP-2 expression potentially accounting for the increased invasive behavior associated with NGF.55 NGF expression has also been linked to PNI in squamous cell carcinoma of the head and neck. In oral tongue specimens, robust staining for NGF and TrKA was present in specimens with PNI in comparison to cases of the same stage without PNI.56
Chemokines
Chemokines are a family of signaling proteins with the ability to induce chemotaxis in nearby responsive cells. Chemokines have long been known to play a role in cancer progression57; however, only more recently have they been investigated in PNI. Given the directional nature of cancer cell migration in PNI, this class of proteins represents a logical area for investigation.
CCL2
CCL2 also referred to as monocyte chemotactic protein 1 predominately functions to recruit monocytes, which express its receptor CCR2, to sites of inflammation or infection.58 In the peripheral nervous system, CCL2 plays a critical role in promoting nerve repair and its expression is elevated in the nerve following injury.59 Given that the presence of cancer within a nerve is a traumatic event, CCL2 may be a potential mediator of PNI. Work from our group demonstrated that CCL2 supported prostate cancer migration and PNI through CCR2-mediated signaling.25 The CCL2–CCR2 signaling axis may also fuel cancer growth and invasion through indirect effects on the tumor microenvironment in addition to direct effects on the cancer.60
CX3CL1
CX3CL1 also known as fractalkine, is expressed by neurons and activated endothelial cells.61 CX3CR1 exclusively binds CX3CL1, and its expression has also been explored in several cancers. Notably, high expression levels of CX3CR1 were seen in pancreatic cancer with PNI.62 Pancreatic cancer cell lines migrate toward CX3CL1 in vitro and pancreatic tumors transfected with CX3CR1 infiltrated local nerves, suggesting that this signaling axis is important for the neurotrophic behavior.62 A recent study demonstrated that expression of both CX3CL1 and CX3CR1 was higher in pancreatic intraepithelial neoplasias (PanINs) than matched invasive cancers, suggesting that this chemokine axis may play a role in the early involvement of pancreatic cancer progression. Prostate cancer, which also displays a high incidence of PNI, expresses high levels of CX3CR1 as well.63 CX3CL1–CX3CR1 signaling plays an important role in the activation of adhesion molecules to neural cells making it an appealing axis to target in cases of PNI.62
Galanin
Galanin (GAL) is a neuropeptide that has been well studied in the context of nociception, cellular survival, and regeneration.64 65 GAL has been demonstrated to be expressed by peripheral nerves following injury.66 Only more recently has GAL been investigated in the context of cancer.67 GAL has three G protein receptors: GALR1, GALR2, and GALR3. GALR2 is known to have proliferative effects on squamous cell carcinoma.68 Recently, Scanlon et al elucidated its role in promoting PNI of squamous cell carcinoma of the head and neck.69 A meta-analysis-based screen of potential neuropeptides involved in head and neck squamous cell carcinoma was performed, which identified a high GAL expression correlation with poor clinical outcome. They then demonstrated that nerve-secreted GAL binds to GALR2 on cancer cells. This initiates nuclear factor of activated T cells, cytoplasmic, calcineurin-dependent-2 (NFATC2)-mediated transcription of cyclooxygenase-2 in the cancer cell, which enzymatically facilitates synthesis of prostaglandin E2. In turn this induces neurogenesis in the adjacent nerves thereby promoting PNI. In a feedback mechanism, GAL is also expressed by the cancer furthering this proinvasive cycle. This work underscores the importance of neurogenesis in PNI pathogenesis as well as the reciprocal nature of PNI, as originally observed by Ayala et al.
Extracellular Matrix
As previously discussed, the peripheral nerve sheath represents a multilayer selective barrier, which tightly protects and regulates the neurons within them. Migration within the layers of the peripheral nerve sheath likely requires a manipulation of the nerve extracellular matrix to make it more hospitable to cancer growth and invasion.
Matrix Metalloproteinases
MMPs are a family of endopeptidases, which are responsible for tissue remodeling and degradation of extracellular matrix. Several collagens are known to be expressed in peripheral nerves, including collagen IV, which is a major component of the Schwann cell basement membrane.70 MMP2 and MMP9, which are both type IV collagenases, have been shown to be expressed by NGF and GDNF, respectively, potentially implicating them in PNI.55 71 Further, both of these MMPs have also been found to be overexpressed in pancreatic cancers.72 73 MMP2 has also been found to be overexpressed by myofibroblasts in adenoid cystic carcinoma of the head and neck, a cancer with a very high prevalence of PNI. While MMPs represent logical therapeutic targets in PNI, it should be noted that the broad-spectrum MMP inhibitors have been failed to show any significant impact on tumor development in several clinical trials.74
Neurogenesis
PNI results from a symbiotic relationship between cancer and nerves, in which both parties are guilty in propelling this ominous behavior. In the original DRG model, described by Ayala et al, it was noted that neurite formation increased in the presence of cancer suggesting that axonal growth may be a key element of PNI.22 Subsequent studies went on to show that in patients with prostate cancer there were increased numbers of neurons in their prostatic ganglia compared with controls, corroborating this phenomenon of cancer-related neurogenesis.75 76 Increased nerve density in these specimens was correlated with increased expression of proteins involved in survival pathways on tissue microarray.76 In this study, PNI diameter but not nerve density was very strongly correlated with tumor volume suggesting that early neurogenesis is a critical factor in PNI initiation and progression. A similar observation was seen in colon cancer specimens, in which the degree of neurogenesis correlated with survival.77 Thus PNI may result in part from a growth advantage for both the cancer and the nerve.
Axonal Guidance Molecules
Given the potential role that axonogenesis plays, it is reasonable to investigate axonal guidance proteins in PNI. Semaphorins represent a large class of proteins that act as axonal growth cone guidance molecules that can serve as both a chemoattractant and repellent depending on the context and class of semaphorin.78 Overexpression of semaphorin 3A in pancreatic cancer specimens was associated with poor clinical outcome though not directly linked to PNI.79 In vitro, overexpression of semaphorin 4F by prostate cancer induced neurogenesis suggesting that it may play a role in the increased nerve density observed in prostate cancer specimens.75 Subsequent studies demonstrated that semaphorin 4F expression by human prostate cancer correlates with both nerve density and PNI diameter.80 Semaphorin 4F may also act on the cancer itself to promote PNI. Overexpression of semaphorin 4F in prostate cancer resulted in a growth and migratory advantage in vitro, and correlated an NF-κB expression.80
SLITs and their ROBO receptors represent another class of guidance factors that serve as chemorepellent cues controlling axonal growth and branching.81 SLIT2 expression was reduced in pancreatic specimens when compared with normal pancreatic tissue. Restoration of low-SLIT2 expression in pancreatic cancer cell lines resulted in impaired directional migration of pancreatic cancer along neurites in vitro.82 Importantly, restoration of SLIT2 signaling was sufficient to inhibit the directed movement of pancreatic cancer toward known chemoattractants but not their random movement suggesting that escape from this repellent may be essential to allow pancreatic cancer to disseminate along nerves. This work implies that PNI pathogenesis may be more complex than initially thought and may not simply result from just chemoattraction but also an alteration in chemorepulsion.
The Eph receptors and their Eph receptor-interacting (ephrin) ligands represent another class of proteins important in axonal guidance with strong links to cancer formation.83 The Eph receptors represent a large family of tyrosine kinase receptors. Of the members, EphA2 has the strongest link to cancer progression.84 Only more recently has it been studied in the context of PNI. Expression of EphA2/ephrinA1 has been correlated with PNI in adenoid cystic carcinoma; however, mechanistic studies are currently lacking.85
Cellular Adhesion Molecules
Cell adhesion molecules are a diverse group of transmembrane proteins, which mediate cell–cell and cell–extracellular matrix adhesion and also serve as the receptor for different ligands. Cell adhesion molecules have been implicated in the invasion and metastases of several cancers.86 Further, nerve invading cancers have been known to associate with Schwann cells via cell surface proteins suggesting that cell adhesion molecules may be implicated in PNI.87
Neural Cell Adhesion Molecule
Neural cell adhesion molecule (NCAM) is a hemophilic-binding protein that is known to be expressed on the surface of neurons and Schwann cells.88 NCAM plays a role in cell–cell adhesion as well as nerve growth.89 There have been several correlative studies demonstrating expression in tumors with PNI.90 91 Additionally, NCAM expression has also been detected in nerves with PNI.92 There are however, conflicting studies from cutaneous cancers of the head and neck which, paradoxically, suggest that NCAM may not play a strong role in PNI.93 94 A recent study has demonstrated that NCAM also plays a key role in a promoting Schwann cell-induced cancer cell dispersion and invasion, which facilitates PNI.21
MUC1/Myelin-Associated Glycoprotein
MUC1 is a transmembrane mucin known to affect the adhesive properties of cells. Myelin-associated glycoprotein (MAG) is a membrane glycoprotein that is expressed by both oligodendrocytes and Schwann cells and serves to bind myelin to neurons.95 Swanson et al demonstrated that MUC1 expressed on pancreatic cancer interacts with MAG on Schwann cells in cases of PNI.96 The authors speculate that the pancreatic cells which overexpress MUC1 may have an adhesive advantage, which results in an improved capacity to survive within the nerve.
L1 Cell Adhesion Molecule
L1 cell adhesion molecule (L1CAM) is a transmembrane protein whose role was initially characterized in the setting of neuronal cell migration.97 Its expression was later correlated with tumor progression and metastasis in several cancers.98 99 L1CAM expression has been investigated in pancreatic cancer in the context of PNI and demonstrated to be overexpressed along with GDNF in tumor specimens.100 However, mechanistic studies supporting its role in PNI are lacking.
Innervation and Cancer Progression
It is clear that PNI results from a symbiotic relationship between cancer and nerve elements. While the focus of this review has been on cancer invasion of nerves, there is a growing body of literature which suggests that innervation of tumors, specifically from the autonomic nervous system, is critical to cancer progression. Elegant studies have demonstrated that surgical or pharmacological sympathectomy prevented the early phases of prostate cancer development.30 By contrast, inhibition of cholinergic fibers reduced invasion and metastasis in murine models of prostate cancers.30 The authors demonstrate that adrenergic signaling may play a role in the initial phases of prostate cancer development whereas the cholinergic fibers play a role in invasion and metastases. A similar paradigm has been studied in gastric cancer. Zhao et al demonstrate that surgical or pharmacological denervation of the stomach markedly reduced gastric tumor incidence and progression.101 Currently, this phenomenon of autonomic regulation of cancer development and progression appears distinct from PNI but further study is needed to determine the degree of overlap if any between these two processes. Sensory innervation has also been shown to increase dramatically when only PanIN was present suggesting that the nervous system participates in the early development of pancreatic cancer as well.29 Collectively, this work further highlights the importance of understanding how the cancer–nerve relationship promotes cancer invasion and growth. These findings also raise important questions regarding the earliest events in both cancer and PNI development. Do the nerves grow to innervate the cancer first, or does the cancer invade the nerve first? This review provides evidence that both processes may be at play. If true, this concept suggests that an optimal therapeutic strategy may entail targeting both nerve and cancer growth.
Future Directions
The current body of research has highlighted the reciprocal nature of PNI between the cancer and its microenvironment. Future work must delve into mechanisms involved in the interactions between cancer, nerves, and the supporting cells with the notion that all such parties may be collaborating together to promote invasion. While our understanding of the mechanisms underlying PNI is still incomplete, the molecular and cellular processes that have been implicated so far have been studied in the context of neurodevelopment. Finally, the contribution of innervation toward cancer progression is just starting to be appreciated and may provide valuable insights into how neuronal signaling may fuel PNI.
Conclusions
PNI results from a multidirectional dialogue between cancer and its microenvironment. While traditionally viewed as a cancer-driven process, PNI is equally facilitated by the host. Multiple processes are likely corrupted at the cancer and nerve interface. There is reciprocity in these interactions, resulting in a migratory and growth advantages for both the cancer and the nerve. A mechanistic understanding of PNI is critical to the development of targeted therapeutics to inhibit this adverse behavior. Future therapeutic strategies will likely target not just the cancer cell, but also the nerve microenvironment.
References
- 1.Batsakis J G. Nerves and neurotropic carcinomas. Ann Otol Rhinol Laryngol. 1985;94(4 Pt 1):426–427. [PubMed] [Google Scholar]
- 2.Soo K C, Carter R L, O'Brien C J, Barr L, Bliss J M, Shaw H J. Prognostic implications of perineural spread in squamous carcinomas of the head and neck. Laryngoscope. 1986;96(10):1145–1148. doi: 10.1288/00005537-198610000-00015. [DOI] [PubMed] [Google Scholar]
- 3.Fagan J J, Collins B, Barnes L, D'Amico F, Myers E N, Johnson J T. Perineural invasion in squamous cell carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg. 1998;124(6):637–640. doi: 10.1001/archotol.124.6.637. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi T, Ishikura H, Motohara T, Okushiba S, Dohke M, Katoh H. Perineural invasion by ductal adenocarcinoma of the pancreas. J Surg Oncol. 1997;65(3):164–170. doi: 10.1002/(sici)1096-9098(199707)65:3<164::aid-jso4>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 5.Cruveilheir J. Paris, France: JB Bailliere; 1835. Maladies Des Nerfs Anatomie Pathlogique Du Corps Humain. [Google Scholar]
- 6.Larson D L, Rodin A E, Roberts D K, O'Steen W K, Rapperport A S, Lewis S R. Perineural lymphatics: myth or fact. Am J Surg. 1966;112(4):488–492. doi: 10.1016/0002-9610(66)90309-6. [DOI] [PubMed] [Google Scholar]
- 7.Akert K, Sandri C, Weibel E R, Peper K, Moor H. The fine structure of the perineural endothelium. Cell Tissue Res. 1976;165(3):281–295. doi: 10.1007/BF00222433. [DOI] [PubMed] [Google Scholar]
- 8.Stolinski C. Structure and composition of the outer connective tissue sheaths of peripheral nerve. J Anat. 1995;186(Pt 1):123–130. [PMC free article] [PubMed] [Google Scholar]
- 9.Olsson Y. Microenvironment of the peripheral nervous system under normal and pathological conditions. Crit Rev Neurobiol. 1990;5(3):265–311. [PubMed] [Google Scholar]
- 10.Amit M, Binenbaum Y, Trejo-Leider L. et al. International collaborative validation of intraneural invasion as a prognostic marker in adenoid cystic carcinoma of the head and neck. Head Neck. 2015;37(7):1038–1045. doi: 10.1002/hed.23710. [DOI] [PubMed] [Google Scholar]
- 11.Pollard J W. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4(1):71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 12.Kieseier B C, Hartung H P, Wiendl H. Immune circuitry in the peripheral nervous system. Curr Opin Neurol. 2006;19(5):437–445. doi: 10.1097/01.wco.0000245365.51823.72. [DOI] [PubMed] [Google Scholar]
- 13.Cavel O, Shomron O, Shabtay A. et al. Endoneurial macrophages induce perineural invasion of pancreatic cancer cells by secretion of GDNF and activation of RET tyrosine kinase receptor. Cancer Res. 2012;72(22):5733–5743. doi: 10.1158/0008-5472.CAN-12-0764. [DOI] [PubMed] [Google Scholar]
- 14.Zeng L, Guo Y, Liang J. et al. Perineural Invasion and TAMs in Pancreatic Ductal Adenocarcinomas: Review of the Original Pathology Reports Using Immunohistochemical Enhancement and Relationships with Clinicopathological Features. J Cancer. 2014;5(9):754–760. doi: 10.7150/jca.10238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhowmick N A, Neilson E G, Moses H L. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432(7015):332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6(5):392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 17.Bunge M B, Wood P M, Tynan L B, Bates M L, Sanes J R. Perineurium originates from fibroblasts: demonstration in vitro with a retroviral marker. Science. 1989;243(4888):229–231. doi: 10.1126/science.2492115. [DOI] [PubMed] [Google Scholar]
- 18.Jessen K R. Glial cells. Int J Biochem Cell Biol. 2004;36(10):1861–1867. doi: 10.1016/j.biocel.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 19.Scheib J, Höke A. Advances in peripheral nerve regeneration. Nat Rev Neurol. 2013;9(12):668–676. doi: 10.1038/nrneurol.2013.227. [DOI] [PubMed] [Google Scholar]
- 20.Demir I E, Boldis A, Pfitzinger P L. et al. Investigation of Schwann cells at neoplastic cell sites before the onset of cancer invasion. J Natl Cancer Inst. 2014;106(8):pii: dju184. doi: 10.1093/jnci/dju184. [DOI] [PubMed] [Google Scholar]
- 21.Deborde S Omelchenko T Lyubchik A et al. Schwann cells induce cancer dispersion and invasion J Clin Invest 2016. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ayala G E, Wheeler T M, Shine H D. et al. In vitro dorsal root ganglia and human prostate cell line interaction: redefining perineural invasion in prostate cancer. Prostate. 2001;49(3):213–223. doi: 10.1002/pros.1137. [DOI] [PubMed] [Google Scholar]
- 23.Dai H, Li R, Wheeler T. et al. Enhanced survival in perineural invasion of pancreatic cancer: an in vitro approach. Hum Pathol. 2007;38(2):299–307. doi: 10.1016/j.humpath.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Bakst R L, Lee N, He S. et al. Radiation impairs perineural invasion by modulating the nerve microenvironment. PLoS ONE. 2012;7(6):e39925. doi: 10.1371/journal.pone.0039925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He S, He S, Chen C H. et al. The chemokine (CCL2-CCR2) signaling axis mediates perineural invasion. Mol Cancer Res. 2015;13(2):380–390. doi: 10.1158/1541-7786.MCR-14-0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gil Z, Cavel O, Kelly K. et al. Paracrine regulation of pancreatic cancer cell invasion by peripheral nerves. J Natl Cancer Inst. 2010;102(2):107–118. doi: 10.1093/jnci/djp456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abiatari I, DeOliveira T, Kerkadze V. et al. Consensus transcriptome signature of perineural invasion in pancreatic carcinoma. Mol Cancer Ther. 2009;8(6):1494–1504. doi: 10.1158/1535-7163.MCT-08-0755. [DOI] [PubMed] [Google Scholar]
- 28.Braun B S, Tuveson D A, Kong N. et al. Somatic activation of oncogenic Kras in hematopoietic cells initiates a rapidly fatal myeloproliferative disorder. Proc Natl Acad Sci U S A. 2004;101(2):597–602. doi: 10.1073/pnas.0307203101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stopczynski R E, Normolle D P, Hartman D J. et al. Neuroplastic changes occur early in the development of pancreatic ductal adenocarcinoma. Cancer Res. 2014;74(6):1718–1727. doi: 10.1158/0008-5472.CAN-13-2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Magnon C, Hall S J, Lin J. et al. Autonomic nerve development contributes to prostate cancer progression. Science. 2013;341(6142):1.236361E6. doi: 10.1126/science.1236361. [DOI] [PubMed] [Google Scholar]
- 31.Meng E, Sun G H, Wu S T. et al. Value of prostate-specific antigen in the staging of Taiwanese patients with newly diagnosed prostate cancer. Arch Androl. 2003;49(6):471–474. doi: 10.1080/01485010390249971. [DOI] [PubMed] [Google Scholar]
- 32.Pour P M, Egami H, Takiyama Y. Patterns of growth and metastases of induced pancreatic cancer in relation to the prognosis and its clinical implications. Gastroenterology. 1991;100(2):529–536. doi: 10.1016/0016-5085(91)90226-b. [DOI] [PubMed] [Google Scholar]
- 33.Cabanillas R, Secades P, Rodrigo J P, Astudillo A, Suárez C, Chiara M D. Orthotopic murine model of head and neck squamous cell carcinoma [in Spanish] Acta Otorrinolaringol Esp. 2005;56(3):89–95. doi: 10.1016/s0001-6519(05)78579-4. [DOI] [PubMed] [Google Scholar]
- 34.Chernichenko N, Linkov G, Li P. et al. Oncolytic vaccinia virus therapy of salivary gland carcinoma. JAMA Otolaryngol Head Neck Surg. 2013;139(2):173–182. doi: 10.1001/jamaoto.2013.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He S, Chen C H, Chernichenko N. et al. GFRα1 released by nerves enhances cancer cell perineural invasion through GDNF-RET signaling. Proc Natl Acad Sci U S A. 2014;111(19):E2008–E2017. doi: 10.1073/pnas.1402944111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Durbec P, Marcos-Gutierrez C V, Kilkenny C. et al. GDNF signalling through the Ret receptor tyrosine kinase. Nature. 1996;381(6585):789–793. doi: 10.1038/381789a0. [DOI] [PubMed] [Google Scholar]
- 37.Okada Y, Takeyama H, Sato M. et al. Experimental implication of celiac ganglionotropic invasion of pancreatic-cancer cells bearing c-ret proto-oncogene with reference to glial-cell-line-derived neurotrophic factor (GDNF) Int J Cancer. 1999;81(1):67–73. doi: 10.1002/(sici)1097-0215(19990331)81:1<67::aid-ijc13>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 38.Chuang J Y, Tsai C F, Chang S W. et al. Glial cell line-derived neurotrophic factor induces cell migration in human oral squamous cell carcinoma. Oral Oncol. 2013;49(12):1103–1112. doi: 10.1016/j.oraloncology.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 39.Treanor J J, Goodman L, de Sauvage F. et al. Characterization of a multicomponent receptor for GDNF. Nature. 1996;382(6586):80–83. doi: 10.1038/382080a0. [DOI] [PubMed] [Google Scholar]
- 40.Airaksinen M S, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci. 2002;3(5):383–394. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- 41.Trupp M, Scott R, Whittemore S R, Ibáñez C F. Ret-dependent and -independent mechanisms of glial cell line-derived neurotrophic factor signaling in neuronal cells. J Biol Chem. 1999;274(30):20885–20894. doi: 10.1074/jbc.274.30.20885. [DOI] [PubMed] [Google Scholar]
- 42.Ceyhan G O, Giese N A, Erkan M. et al. The neurotrophic factor artemin promotes pancreatic cancer invasion. Ann Surg. 2006;244(2):274–281. doi: 10.1097/01.sla.0000217642.68697.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ceyhan G O, Bergmann F, Kadihasanoglu M. et al. The neurotrophic factor artemin influences the extent of neural damage and growth in chronic pancreatitis. Gut. 2007;56(4):534–544. doi: 10.1136/gut.2006.105528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gao L, Bo H, Wang Y, Zhang J, Zhu M. Neurotrophic Factor Artemin Promotes Invasiveness and Neurotrophic Function of Pancreatic Adenocarcinoma In Vivo and In Vitro. Pancreas. 2015;44(1):134–143. doi: 10.1097/MPA.0000000000000223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ketterer K, Rao S, Friess H, Weiss J, Büchler M W, Korc M. Reverse transcription-PCR analysis of laser-captured cells points to potential paracrine and autocrine actions of neurotrophins in pancreatic cancer. Clin Cancer Res. 2003;9(14):5127–5136. [PubMed] [Google Scholar]
- 46.Jia S, Wang W, Hu Z. et al. BDNF mediated TrkB activation contributes to the EMT progression and the poor prognosis in human salivary adenoid cystic carcinoma. Oral Oncol. 2015;51(1):64–70. doi: 10.1016/j.oraloncology.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 47.Kowalski P J, Paulino A F. Perineural invasion in adenoid cystic carcinoma: Its causation/promotion by brain-derived neurotrophic factor. Hum Pathol. 2002;33(9):933–936. doi: 10.1053/hupa.2002.128249. [DOI] [PubMed] [Google Scholar]
- 48.Okugawa Y, Tanaka K, Inoue Y. et al. Brain-derived neurotrophic factor/tropomyosin-related kinase B pathway in gastric cancer. Br J Cancer. 2013;108(1):121–130. doi: 10.1038/bjc.2012.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miknyoczki S J, Lang D, Huang L, Klein-Szanto A J, Dionne C A, Ruggeri B A. Neurotrophins and Trk receptors in human pancreatic ductal adenocarcinoma: expression patterns and effects on in vitro invasive behavior. Int J Cancer. 1999;81(3):417–427. doi: 10.1002/(sici)1097-0215(19990505)81:3<417::aid-ijc16>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 50.Amit M, Na'ara S, Sharma K. et al. Elective neck dissection in patients with head and neck adenoid cystic carcinoma: an international collaborative study. Ann Surg Oncol. 2015;22(4):1353–1359. doi: 10.1245/s10434-014-4106-7. [DOI] [PubMed] [Google Scholar]
- 51.Sclabas G M, Fujioka S, Schmidt C. et al. Overexpression of tropomysin-related kinase B in metastatic human pancreatic cancer cells. Clin Cancer Res. 2005;11(2 Pt 1):440–449. [PubMed] [Google Scholar]
- 52.Miknyoczki S J, Wan W, Chang H. et al. The neurotrophin-trk receptor axes are critical for the growth and progression of human prostatic carcinoma and pancreatic ductal adenocarcinoma xenografts in nude mice. Clin Cancer Res. 2002;8(6):1924–1931. [PubMed] [Google Scholar]
- 53.Zhu Z, Friess H, diMola F F. et al. Nerve growth factor expression correlates with perineural invasion and pain in human pancreatic cancer. J Clin Oncol. 1999;17(8):2419–2428. doi: 10.1200/JCO.1999.17.8.2419. [DOI] [PubMed] [Google Scholar]
- 54.Ma J, Jiang Y, Jiang Y, Sun Y, Zhao X. Expression of nerve growth factor and tyrosine kinase receptor A and correlation with perineural invasion in pancreatic cancer. J Gastroenterol Hepatol. 2008;23(12):1852–1859. doi: 10.1111/j.1440-1746.2008.05579.x. [DOI] [PubMed] [Google Scholar]
- 55.Okada Y, Eibl G, Guha S, Duffy J P, Reber H A, Hines O J. Nerve growth factor stimulates MMP-2 expression and activity and increases invasion by human pancreatic cancer cells. Clin Exp Metastasis. 2004;21(4):285–292. doi: 10.1023/b:clin.0000046131.24625.54. [DOI] [PubMed] [Google Scholar]
- 56.Kolokythas A, Cox D P, Dekker N, Schmidt B L. Nerve growth factor and tyrosine kinase A receptor in oral squamous cell carcinoma: is there an association with perineural invasion? J Oral Maxillofac Surg. 2010;68(6):1290–1295. doi: 10.1016/j.joms.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 57.Murphy P M. Chemokines and the molecular basis of cancer metastasis. N Engl J Med. 2001;345(11):833–835. doi: 10.1056/NEJM200109133451113. [DOI] [PubMed] [Google Scholar]
- 58.Mackay C R. Chemokines: immunology's high impact factors. Nat Immunol. 2001;2(2):95–101. doi: 10.1038/84298. [DOI] [PubMed] [Google Scholar]
- 59.Van Steenwinckel J, Reaux-Le Goazigo A, Pommier B. et al. CCL2 released from neuronal synaptic vesicles in the spinal cord is a major mediator of local inflammation and pain after peripheral nerve injury. J Neurosci. 2011;31(15):5865–5875. doi: 10.1523/JNEUROSCI.5986-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang J, Lu Y, Pienta K J. Multiple roles of chemokine (C-C motif) ligand 2 in promoting prostate cancer growth. J Natl Cancer Inst. 2010;102(8):522–528. doi: 10.1093/jnci/djq044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Verge G M, Milligan E D, Maier S F, Watkins L R, Naeve G S, Foster A C. Fractalkine (CX3CL1) and fractalkine receptor (CX3CR1) distribution in spinal cord and dorsal root ganglia under basal and neuropathic pain conditions. Eur J Neurosci. 2004;20(5):1150–1160. doi: 10.1111/j.1460-9568.2004.03593.x. [DOI] [PubMed] [Google Scholar]
- 62.Marchesi F, Piemonti L, Fedele G. et al. The chemokine receptor CX3CR1 is involved in the neural tropism and malignant behavior of pancreatic ductal adenocarcinoma. Cancer Res. 2008;68(21):9060–9069. doi: 10.1158/0008-5472.CAN-08-1810. [DOI] [PubMed] [Google Scholar]
- 63.Marchesi F, Locatelli M, Solinas G, Erreni M, Allavena P, Mantovani A. Role of CX3CR1/CX3CL1 axis in primary and secondary involvement of the nervous system by cancer. J Neuroimmunol. 2010;224(1–2):39–44. doi: 10.1016/j.jneuroim.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 64.Alier K A, Chen Y, Sollenberg U E, Langel U, Smith P A. Selective stimulation of GalR1 and GalR2 in rat substantia gelatinosa reveals a cellular basis for the anti- and pro-nociceptive actions of galanin. Pain. 2008;137(1):138–146. doi: 10.1016/j.pain.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 65.Hobson S A, Bacon A, Elliot-Hunt C R. et al. Galanin acts as a trophic factor to the central and peripheral nervous systems. EXS. 2010;102:25–38. doi: 10.1007/978-3-0346-0228-0_3. [DOI] [PubMed] [Google Scholar]
- 66.Hulse R P, Wynick D, Donaldson L F. Activation of the galanin receptor 2 in the periphery reverses nerve injury-induced allodynia. Mol Pain. 2011;7:26. doi: 10.1186/1744-8069-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rauch I, Kofler B. The galanin system in cancer. EXS. 2010;102:223–241. doi: 10.1007/978-3-0346-0228-0_16. [DOI] [PubMed] [Google Scholar]
- 68.Banerjee R, Henson B S, Russo N, Tsodikov A, D'Silva N J. Rap1 mediates galanin receptor 2-induced proliferation and survival in squamous cell carcinoma. Cell Signal. 2011;23(7):1110–1118. doi: 10.1016/j.cellsig.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Scanlon C S, Banerjee R, Inglehart R C. et al. Galanin modulates the neural niche to favour perineural invasion in head and neck cancer. Nat Commun. 2015;6:6885. doi: 10.1038/ncomms7885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen P, Cescon M, Bonaldo P. The Role of Collagens in Peripheral Nerve Myelination and Function. Mol Neurobiol. 2015;52(1):216–225. doi: 10.1007/s12035-014-8862-y. [DOI] [PubMed] [Google Scholar]
- 71.Okada Y, Eibl G, Duffy J P, Reber H A, Hines O J. Glial cell-derived neurotrophic factor upregulates the expression and activation of matrix metalloproteinase-9 in human pancreatic cancer. Surgery. 2003;134(2):293–299. doi: 10.1067/msy.2003.239. [DOI] [PubMed] [Google Scholar]
- 72.Ellenrieder V, Alber B, Lacher U. et al. Role of MT-MMPs and MMP-2 in pancreatic cancer progression. Int J Cancer. 2000;85(1):14–20. doi: 10.1002/(sici)1097-0215(20000101)85:1<14::aid-ijc3>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 73.Durlik M, Gardian K. Metalloproteinase 2 and 9 activity in the development of pancreatic cancer. Pol Przegl Chir. 2012;84(8):377–382. doi: 10.2478/v10035-012-0064-6. [DOI] [PubMed] [Google Scholar]
- 74.Overall C M, Kleifeld O. Tumour microenvironment - opinion: validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy. Nat Rev Cancer. 2006;6(3):227–239. doi: 10.1038/nrc1821. [DOI] [PubMed] [Google Scholar]
- 75.Ayala G E, Dai H, Powell M. et al. Cancer-related axonogenesis and neurogenesis in prostate cancer. Clin Cancer Res. 2008;14(23):7593–7603. doi: 10.1158/1078-0432.CCR-08-1164. [DOI] [PubMed] [Google Scholar]
- 76.Olar A, He D, Florentin D, Ding Y, Wheeler T, Ayala G. Biological correlates of prostate cancer perineural invasion diameter. Hum Pathol. 2014;45(7):1365–1369. doi: 10.1016/j.humpath.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Albo D, Akay C L, Marshall C L. et al. Neurogenesis in colorectal cancer is a marker of aggressive tumor behavior and poor outcomes. Cancer. 2011;117(21):4834–4845. doi: 10.1002/cncr.26117. [DOI] [PubMed] [Google Scholar]
- 78.de Wit J, Verhaagen J. Role of semaphorins in the adult nervous system. Prog Neurobiol. 2003;71(2–3):249–267. doi: 10.1016/j.pneurobio.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 79.Müller M W, Giese N A, Swiercz J M. et al. Association of axon guidance factor semaphorin 3A with poor outcome in pancreatic cancer. Int J Cancer. 2007;121(11):2421–2433. doi: 10.1002/ijc.22949. [DOI] [PubMed] [Google Scholar]
- 80.Ding Y, He D, Florentin D. et al. Semaphorin 4F as a critical regulator of neuroepithelial interactions and a biomarker of aggressive prostate cancer. Clin Cancer Res. 2013;19(22):6101–6111. doi: 10.1158/1078-0432.CCR-12-3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li H S, Chen J H, Wu W. et al. Vertebrate slit, a secreted ligand for the transmembrane protein roundabout, is a repellent for olfactory bulb axons. Cell. 1999;96(6):807–818. doi: 10.1016/s0092-8674(00)80591-7. [DOI] [PubMed] [Google Scholar]
- 82.Göhrig A, Detjen K M, Hilfenhaus G. et al. Axon guidance factor SLIT2 inhibits neural invasion and metastasis in pancreatic cancer. Cancer Res. 2014;74(5):1529–1540. doi: 10.1158/0008-5472.CAN-13-1012. [DOI] [PubMed] [Google Scholar]
- 83.Pasquale E B. Eph receptors and ephrins in cancer: bidirectional signalling and beyond. Nat Rev Cancer. 2010;10(3):165–180. doi: 10.1038/nrc2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wykosky J, Debinski W. The EphA2 receptor and ephrinA1 ligand in solid tumors: function and therapeutic targeting. Mol Cancer Res. 2008;6(12):1795–1806. doi: 10.1158/1541-7786.MCR-08-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shao Z, Zhu F, Song K, Zhang H, Liu K, Shang Z. EphA2/ephrinA1 mRNA expression and protein production in adenoid cystic carcinoma of salivary gland. J Oral Maxillofac Surg. 2013;71(5):869–878. doi: 10.1016/j.joms.2012.10.026. [DOI] [PubMed] [Google Scholar]
- 86.Cavallaro U, Christofori G. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat Rev Cancer. 2004;4(2):118–132. doi: 10.1038/nrc1276. [DOI] [PubMed] [Google Scholar]
- 87.Bockman D E, Büchler M, Beger H G. Interaction of pancreatic ductal carcinoma with nerves leads to nerve damage. Gastroenterology. 1994;107(1):219–230. doi: 10.1016/0016-5085(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 88.Neuberger T J, Cornbrooks C J. Transient modulation of Schwann cell antigens after peripheral nerve transection and subsequent regeneration. J Neurocytol. 1989;18(5):695–710. doi: 10.1007/BF01187088. [DOI] [PubMed] [Google Scholar]
- 89.Maness P F, Schachner M. Neural recognition molecules of the immunoglobulin superfamily: signaling transducers of axon guidance and neuronal migration. Nat Neurosci. 2007;10(1):19–26. doi: 10.1038/nn1827. [DOI] [PubMed] [Google Scholar]
- 90.Kameda K, Shimada H, Ishikawa T. et al. Expression of highly polysialylated neural cell adhesion molecule in pancreatic cancer neural invasive lesion. Cancer Lett. 1999;137(2):201–207. doi: 10.1016/s0304-3835(98)00359-0. [DOI] [PubMed] [Google Scholar]
- 91.Shang J, Sheng L, Wang K, Shui Y, Wei Q. Expression of neural cell adhesion molecule in salivary adenoid cystic carcinoma and its correlation with perineural invasion. Oncol Rep. 2007;18(6):1413–1416. [PubMed] [Google Scholar]
- 92.Li R, Wheeler T, Dai H, Ayala G. Neural cell adhesion molecule is upregulated in nerves with prostate cancer invasion. Hum Pathol. 2003;34(5):457–461. doi: 10.1016/s0046-8177(03)00084-4. [DOI] [PubMed] [Google Scholar]
- 93.Solares C A, Brown I, Boyle G M, Parsons P G, Panizza B. Neural cell adhesion molecule expression: no correlation with perineural invasion in cutaneous squamous cell carcinoma of the head and neck. Head Neck. 2009;31(6):802–806. doi: 10.1002/hed.21037. [DOI] [PubMed] [Google Scholar]
- 94.Chen-Tsai C P, Colome-Grimmer M, Wagner R F Jr. Correlations among neural cell adhesion molecule, nerve growth factor, and its receptors, TrkA, TrkB, TrkC, and p75, in perineural invasion by basal cell and cutaneous squamous cell carcinomas. Dermatol Surg. 2004;30(7):1009–1016. doi: 10.1111/j.1524-4725.2004.30306.x. [DOI] [PubMed] [Google Scholar]
- 95.Vyas A A, Patel H V, Fromholt S E. et al. Gangliosides are functional nerve cell ligands for myelin-associated glycoprotein (MAG), an inhibitor of nerve regeneration. Proc Natl Acad Sci U S A. 2002;99(12):8412–8417. doi: 10.1073/pnas.072211699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Swanson B J, McDermott K M, Singh P K, Eggers J P, Crocker P R, Hollingsworth M A. MUC1 is a counter-receptor for myelin-associated glycoprotein (Siglec-4a) and their interaction contributes to adhesion in pancreatic cancer perineural invasion. Cancer Res. 2007;67(21):10222–10229. doi: 10.1158/0008-5472.CAN-06-2483. [DOI] [PubMed] [Google Scholar]
- 97.Lindner J, Rathjen F G, Schachner M. L1 mono- and polyclonal antibodies modify cell migration in early postnatal mouse cerebellum. Nature. 1983;305(5933):427–430. doi: 10.1038/305427a0. [DOI] [PubMed] [Google Scholar]
- 98.Thies A, Schachner M, Moll I. et al. Overexpression of the cell adhesion molecule L1 is associated with metastasis in cutaneous malignant melanoma. Eur J Cancer. 2002;38(13):1708–1716. doi: 10.1016/s0959-8049(02)00105-3. [DOI] [PubMed] [Google Scholar]
- 99.Boo Y J, Park J M, Kim J. et al. L1 expression as a marker for poor prognosis, tumor progression, and short survival in patients with colorectal cancer. Ann Surg Oncol. 2007;14(5):1703–1711. doi: 10.1245/s10434-006-9281-8. [DOI] [PubMed] [Google Scholar]
- 100.Ben Q W, Wang J C, Liu J. et al. Positive expression of L1-CAM is associated with perineural invasion and poor outcome in pancreatic ductal adenocarcinoma. Ann Surg Oncol. 2010;17(8):2213–2221. doi: 10.1245/s10434-010-0955-x. [DOI] [PubMed] [Google Scholar]
- 101.Zhao C M, Hayakawa Y, Kodama Y. et al. Denervation suppresses gastric tumorigenesis. Sci Transl Med. 2014;6(250):250ra115. doi: 10.1126/scitranslmed.3009569. [DOI] [PMC free article] [PubMed] [Google Scholar]


