Abstract
This article provides an overview of perineural spread of head and neck malignancy. It defines the problem and explores some of the unique features, which occur with this pathology. The expectation is for a better understanding of this extraordinary disease, hopefully leading to earlier diagnosis and for a more consistent reporting of results. It summarizes the topics to be covered in this special edition, which should leave the reader with a fairly complete understanding of the contemporary issues of perineural spread.
Keywords: perineural invasion, perineural spread, head and neck, skin cancer, squamous cell carcinoma, adenoid cystic carcinoma, skull base surgery
Introduction
Perineural spread (PNS) is a unique and often misunderstood form of malignant spread. This special issue of the Journal of Neurological Surgery; Part B: Skull Base is dedicated to this topic with the aim of better defining the disease. By bringing together world experts in the field, a contemporary summary of the nature of the disease and current investigative and treatment strategies, including follow-up, is provided. The mechanism of how and why tumor spreads along nerves is currently poorly understood but only progress in this area will allow for novel methods of future treatment to improve outcomes.
Nature of the Disease and Confusion in the Literature
Tumors can involve nerves and cause dysfunction by differing mechanisms, which have an effect not only on how we should treat, but also on how we interpret our treatment outcomes and guidelines for management. Nerves can be involved by (1) access into and spread along a nerve, (2) infiltrating and destroying nerves, and (3) external compression of nerves. In each of these cases, the tumor cells are phenotypically and probably genotypically distinct (see Table 1) and, as such, should not be bundled together when we analyze them, either from a molecular, epidemiological, or treatment outcome perspective.
Table 1. Comparison of tumor types and nerve involvement.
| Tumors invading into nerves | Tumors accessing nerve endings and spreading | |
|---|---|---|
| Nerve access | Anywhere | At terminal end |
| Primary tumor or nodal deposit | Always present | Often not present |
| MR neurography findings | Negative | Positive |
| Nerve size | Small nerve/nerve fibers | Large (named nerves) |
| Clinical deficits | Variable | Present (V/VII) |
| Spread along nerve | No | Always by definition |
| Nodal metastatic risk | High | Low (<10%) |
| Failure | Local/regional/distant metastatic | Centrally (brainstem) |
| Perineurium | Easily transgressed | A barrier to spread |
| Molecular basis example | ↑ GALR23 | ↓ GALR2a |
From the author's data on whole-genome expression profiling of PNS specimens.
This issue is dedicated to the PNS of malignancy, a unique pathological event where a malignant cell gains access to a nerve and then spreads centripetally away from the primary tumor within the peri- and endoneural spaces, with eventual failure in the brainstem if left untreated. It is a disease process that is detected clinically and radiologically prior to treatment and thus dictates how the patient should be managed. In the cases of cutaneous malignancy with PNS, the primary tumor has most often been treated or resolved by the body's immune system,1 months to years earlier (see article “The Natural History and Treatment Outcomes of Perineural Spread of Malignancy within the Head and Neck” in this issue). As such, it can be a form of metastatic disease as there is most often no physical connection to the primary tumor.
To explore PNS further, it is worthwhile defining other pathological process that can either involve nerves or cause nerve dysfunction, which behave differently. There are tumors that have an ability to invade nerves. These are usually large tumors, and to see the remnant nerves that have been invaded, special stains such as S100 are required. These have been reported to have a more aggressive nature but do not travel along nerves away from the main tumor.2 They are identified pathologically only after surgery and so are prognostic and may affect postsurgical treatment (Table 1). In addition, tumors may cause external compression and dysfunction of a nerve without spread, and these tumors and those that spread along the fissures and foramina of the skull base will not be discussed.
In addition, the relationship between perineural infiltration (PNI) and PNS needs to be defined. PNI can be divided into the following: (1) PNI that is found incidentally by the pathologist and gives us prognostic information along with other standard pathological criteria such as size, depth of invasion, or degree of differentiation and (2) PNI that manifests as a clinical sign such as dysesthesia or paralysis and almost always can be identified on a dedicated magnetic resonance imaging (MRI) scan. The former occurs when the tumor obtains access to a peripheral nerve ending, within or adjacent to the tumor, sitting within the perineural space, and is termed incidental or microscopic. The latter occurs when the tumor has spread along a nerve away from the tumor, respecting the perineurium as a barrier, and is called clinical PNI, named nerve PNI, or PNS.
The terms small and large nerve PNI have led to confusion between different specialists, as pathologists reporting on incidental PNI will give a measurement on the size of nerves involved as another prognostic indicator, with 0.1 mm the arbitrary size between small and large.4 It should be noted that clinical PNI or PNS involving named nerves and causing a neurological deficit are usually more than 2 mm in size.
Types of Perineural Spread and How They Present
PNS can present from either cutaneous, or mucosal, or salivary gland malignancies. A lot of the literature and certainly our own experience is with cutaneous origin and the Sun Belt areas of Queensland, Florida, and Texas have contributed greatly to our understanding of PNS. As the face is the most heavily sun-exposed part of the body and has a rich neural network, it is no surprise that the trigeminal and facial nerves are most often involved, around 85 and 25%, respectively in our experience. The trigeminal nerve branch most frequently involved is V2. Because of the rich cross-innervations between nerves, in 35% of cases multiple nerves are involved, with the combination of VII and V3 occurring most frequently. Occasionally, the cervical plexus can be involved.
Cutaneous malignancies leading to PNS are most frequently squamous cell carcinoma (SCC; ∼90% in our series with known pathology), followed by melanoma and basal cell carcinoma (BCC). Interestingly, we have looked at a similar drainage population with incidental PNI, and absolute numbers are higher for BCC than SCC (although frequency is higher for SCCs), confirming SCC to have the much greater potential to spread along nerves. The type of pathology is an important prognostic indicator in the literature, with BCC doing twice as well as SCC, while melanoma usually carries the poorest prognosis.
Our own experience with mucosal SCC with PNS is limited, whereas adenoid cystic carcinomas may spread from a primary but also are frequently seen without primaries, suggesting an origin from salivary rests near or in nerves (Fig. 1).
Fig. 1.
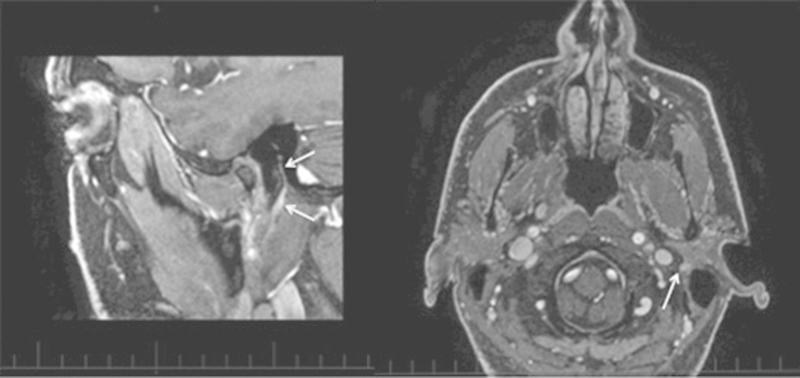
Coronal and axial T1-weighted Gadolinium MRI showing an adenoid cystic carcinoma in the facial nerve (arrows) without any apparent primary. Patient presented with a slowly progressive and complete facial nerve palsy initially, favoring a facial nerve schwannoma.
Presentation is with a slowly progressive dysesthesia conforming to the distribution of one of the branches of the trigeminal nerve or a slowly progressive facial nerve palsy, which distinguishes the disease process quite clearly from a Bell palsy, which is of rapid onset. This is often on the background of not having had any skin cancers removed for months or years prior or in over 20% having no history of ever having had a skin lesion removed or treated by nonsurgical means.5 In addition, when the likely index lesions histopathology is available, in our series one-third did not have incidental PNI and in 10% no comment on PNI is made.5 The point here is that if a patient presents with a good story of a progressive dysesthesia or has a progressive facial nerve palsy regardless of previous skin lesions, the patient should be considered to have PNS until proven otherwise. The disease process is missed by many types of specialists and the average delay, from symptoms to diagnosis, in our series is 6 months. As the progression along the nerve has been shown to affect prognosis, early recognition and diagnosis are crucial in this disease process.6 Finally, even though the Princess Alexandra is a high-volume transplant hospital, it is rare for these patients to be immunosuppressed (n = 5/200).
Radiology, Pathology, and Staging
Imaging of PNS has improved dramatically over the years, and the articles “The imaging of large nerve perineural spread” and “Postoperative imaging and surveillance in large nerve perineural spread” by Gandhi and Sommerville demonstrate the current state of the art with MR neurography in diagnosis, treatment planning, and follow-up. In the past, many reports on outcome involved so-called MR-negative PNS without histological evidence of disease. The dilemma has always been the patient who has had a skin cancer removed with incidental PNI and dysesthesia from the surgical excision, which is then treated as “MR-negative PNS.” As most incidental PNI does not proceed onto PNS, many of these, if not most, probably did not have PNS, thus favorably distorting the results presented. The sensitivity of 3T MR neurography has been shown to be 95%, and it is able to predict the zonal extent of the disease in almost 90% of cases.7 It is also important to look for the secondary effects of the nerve involved (Fig. 2).
Fig. 2.
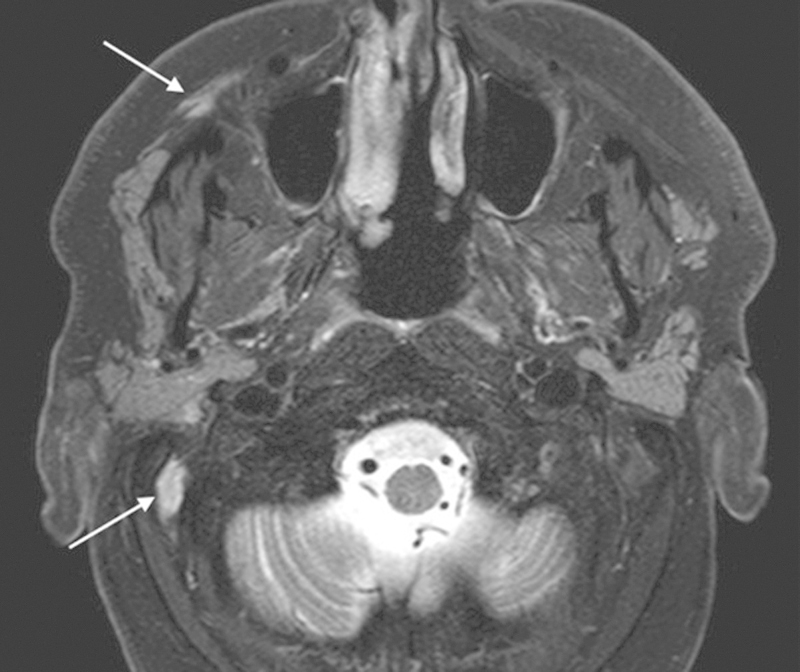
T2-weighted MRI showing the denervation changes in the facial muscles and posterior belly of digastric (arrows). The tumor can be seen in the facial nerve near the stylomastoid foramen.
Follow-up with MRI is preferred by almost all patients to be kept informed. The changed anatomy and stump neuromas, which occur postsurgery, need to be understood to give meaningful reports. In addition to providing reassurance to the patient, salvage surgery is possible with early detection of recurrence. In our hands, of those who recur, 65% have been offered salvage surgery, with a no further recurrence rate of 37.5% at the last review.
The histopathological analysis of en-bloc surgical specimens has been important in establishing the patterns of disease spread and is discussed in the article “Pathology of perineural spread” by Brown. This analysis has helped guide not only the design of surgical procedures but also the radiation fields used in treatment. We know that the concept of skip lesions, which has been propagated in the literature, if it does exists, occurs so infrequently that it should not affect treatment strategies. In addition, the barrier function of the nerve lining is such that surgical specimens and radiation fields are confined to that anatomical region that contains the nerve, its ganglion, and the branch points. This information and the accuracy of imaging in defining the existence and extent of disease provide an excellent guide to treatment planning.
Staging also remains confusing, and it is unusual that the American Joint Committee on Cancer (AJCC) uses a staging system that incorporates a method of metastasis into the primary classification.8 Cutaneous malignancy with “perineural invasion of the skull base” is staged as T4. The zonal system (see article “The Imaging of Large Nerve Perineural Spread” in this issue) gives information on the degree of spread along a nerve and has been shown to have prognostic significance (see the article “The Natural History and Treatment Outcomes of Perineural Spread of Malignancy within the Head and Neck” in this issue). The current AJCC staging gives no meaning/emphasis to zone 1 disease, but once disease has reached or extended through the skull base (zone 2/3), it is staged as T4.
I have never seen a primary skin cancer with tumor spreading up to the skull base. Almost all our primary tumors have been dealt with months to years prior, and in a recent report of 120 cases, the primary stages were roughly equally distributed between TX, T0, T1, and T2, with no T3 or T4 lesions.5 The staging of PNS should reflect the fact that this is recurrent disease and so the recognized rT classification should be used rather than applying T4 to all the disease as most authors do.
Being able to separate the disease into prognostic groups using something similar to the zonal system of spread would seem useful. However, further modification of the zonal system is due. Currently, we deem zone 1 and 2 disease as resectable and zone 3 as not, due to the risks of spreading disease and dismantling the dural barriers, which when in place allow a more focused radiation field. Disease along the trigeminal nerve when it approaches the gasserian ganglion has the ability to either stay in the ganglion or spread into the cavernous sinus. This status separates a tumor from being resected and contained or resected and probably spilled. A further division into zone 2a for tumor confined to the ganglion and zone 2b for tumor up to the ganglion with spread into the cavernous sinus should probably be used.
Treatment Strategies, Mechanism of Spread, and Current Research
The current treatment of PNS is covered in the articles on radiation and surgical management. It is not surprising that for such advanced disease, surgery combined with radiation gives the best results. It is also no surprise that clear margins give improved survival. The focus therefore is on how to interpret the preoperative imaging and to design operations to encompass and eradicate the disease while minimizing the morbidity to the patient. Reconstruction of these complex defects is integral to successful long-term outcomes, and Rowe and Emmett have covered this topic in their article “Reconstruction of base of skull defects—lessons learned over 25 combined years.” However, the success in preventing the progression of the tumor centrally, which has led to improved survival, has also changed the pattern of failure. The natural history of failing centrally with a hidden tumor has been replaced by a delayed failure peripherally, which can be morbid. We must therefore ask ourselves whether the improved cure rates and delays to recurrence, with the change in failure from central to peripheral, are justified.
Future advances will only come from an understanding of the mechanisms leading to PNS, and the summary by Bakst and Wong (see article “Mechanisms of Perineural Invasion”) discusses our current knowledge. In addition to this, we will soon be publishing our results on the different gene expression, using whole-genome expression profiling, between cutaneous SCC, SCC with incidental PNI, and SCC with PNS. We have also successfully developed a mouse tumor model where cutaneous SCCs have had candidate genes up- or downregulated and injected subcutaneously in the midface, which then gain access by themselves to nerves and spread centripetally. In addition, we have recently shown the importance of the perineural tumor microenvironment and the role immune cells and molecules, particularly galectin-1, have on antitumor immunity.9 Hopefully, this will one day soon lead to targeted therapy, thus limiting the morbidity of current treatment.
Conclusion
PNS of cancer, although a relatively rare disease, is more prevalent than thought. During my training and fellowship years, I never saw a case. Between 2000 and 2015, my unit has managed over 180 cases and we now see 25 new cases per year. The previous idiopathic cranial nerve palsies or the skin cancer patients with their progressive dysesthesias previously untreated will now be recognized for what they are. Hopefully, this special issue will help to make this unique pathology less confusing and our patients detected at an earlier stage, so that better outcomes can occur. I thank the Editors for their foresight of seeing the need for such an issue and to my colleague and co-editor for all his help in its assembly.
Acknowledgment
Alando J. Ballantyne from the MD Anderson Hospital in Houston, Texas, wrote a seminal paper on PNS in 1963 and in it he made two prescient comments that are worth repeating.10 The first, which was the opening sentence of the paper, stated, “As long as surgery and radiation therapy remain the principal methods of treatment of cancer, anatomic and pathologic studies designed to delineate the routes of spread will be necessary in order to try and improve survival rates.” We hope the works on radiology and pathology presented in this issue go some way in helping improve survival rates.
The second in the comments section of the paper stated “as long as the extension of the carcinoma is recognized and adequately treated prior to entry within the cranial cavity, or spinal canal, the possibility of cure exists.” Certainly earlier detection will effect better cures, but with the advances in skull base surgery and with radiotherapy over the past 50 years, we can now also aim for cure even in those with extension into the cranial cavity.
References
- 1.Swann J B, Smyth M J. Immune surveillance of tumors. J Clin Invest. 2007;117(5):1137–1146. doi: 10.1172/JCI31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cernea C R, Ferraz A R, de Castro I V. et al. Perineural invasion in aggressive skin carcinomas of the head and neck. Potentially dangerous but frequently overlooked. ORL J Otorhinolaryngol Relat Spec. 2009;71(1):21–26. doi: 10.1159/000165171. [DOI] [PubMed] [Google Scholar]
- 3.Scanlon C S, Banerjee R, Inglehart R C. et al. Galanin modulates the neural niche to favour perineural invasion in head and neck cancer. Nat Commun. 2015;6:6885. doi: 10.1038/ncomms7885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ross A S, Whalen F M, Elenitsas R, Xu X, Troxel A B, Schmults C D. Diameter of involved nerves predicts outcomes in cutaneous squamous cell carcinoma with perineural invasion: an investigator-blinded retrospective cohort study. Dermatol Surg. 2009;35(12):1859–1866. doi: 10.1111/j.1524-4725.2009.01354.x. [DOI] [PubMed] [Google Scholar]
- 5.Warren T A Whiteman D C Porceddu S V Panizza B J Insight into the epidemiology of cutaneous squamous cell carcinoma with perineural spread Head Neck 2016. (e-pub ahead of print). doi: 10.1002/hed.24453 [DOI] [PubMed] [Google Scholar]
- 6.Warren T A Panizza B Porceddu S V et al. Outcomes after surgery and postoperative radiotherapy for perineural spread of head and neck cutaneous squamous cell carcinoma Head Neck 2014. (e-pub ahead of print). doi:10.1002/hed.23982 [DOI] [PubMed] [Google Scholar]
- 7.Baulch J, Gandhi M, Sommerville J, Panizza B. 3T MRI evaluation of large nerve perineural spread of head and neck cancers. J Med Imaging Radiat Oncol. 2015;59(5):578–585. doi: 10.1111/1754-9485.12338. [DOI] [PubMed] [Google Scholar]
- 8.AJCC . New York, NY: Springer; 2010. Cutaneous squamous cell carcinoma and other cutaneous carcinomas; pp. 301–314. [Google Scholar]
- 9.Chawla S, Warren T A, Wockner L F. et al. Galectin-1 is associated with poor prognosis in patients with cutaneous head and neck cancer with perineural spread. Cancer Immunol Immunother. 2016;65(2):213–222. doi: 10.1007/s00262-015-1788-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ballantyne A J, McCarten A B, Ibanez M L. The extension of cancer of the head and neck through peripheral nerves. Am J Surg. 1963;106:651–667. doi: 10.1016/0002-9610(63)90074-6. [DOI] [PubMed] [Google Scholar]


