Abstract
Purpose
The aim of this study is to examine the reproducibility of anti-1-amino-3-[18F]fluorocyclobutane-1-carboxylic acid (anti-3-[18F]FACBC) quantitative measurements in key background structures and untreated malignant lesions.
Procedures
Retrospective review of 14 patients who underwent follow-up anti-3-[18F]FACBC positron emission tomography-X-ray computed tomography (PET-CT) for prostate carcinoma recurrence. Standard uptake values (SUV) were measured in both original and follow-up scans in key background structures and untreated malignant lesions. Absolute and percent mean difference in SUV between scans and interclass correlation coefficients (ICC) were also computed.
Results
Mean (±SD, range) scan interval was 17.4 months (±7.1, 4–29). %Mean difference in SUVmean was <20 % in background structures with low absolute differences. ICCs were >0.6 except for early-phase blood pool (ICC=0.4). SUVmax in malignant lesions without interim therapy increased or remained stable over time.
Conclusions
Despite variable time interval between scans, FACBC PET-CT demonstrates acceptable reproducibility in key background structures. Untreated malignant lesions showed stable or increased uptake over time. A formal test-retest study is planned.
Keywords: FACBC, Prostate cancer, Reproducibility, PET-CT, Radiotracer, Reliability
Introduction
Radiolabeled amino acid imaging targets the upregulation of amino acid transport and utilization in neoplastic cells [1]. Anti-1-amino-3-[18F]fluorocyclobutane-1-carboxylic acid (anti-3-[18F]FACBC) is an investigational synthetic amino acid analog which has been studied in the diagnosis of prostate and other cancers [2–11]. Anti-3-[18F]FACBC has been studied most extensively in the restaging of recurrent prostate cancer where it has demonstrated enhanced lesion detection compared with other imaging modalities such as indium-111 capromab-pendetide single-photon emission computerized tomography/computerized tomography (SPECT-CT) (ProstaScint; Jazz Pharmaceuticals, Dublin, Ireland) and 11C-choline positron emission tomography-X-ray/computed tomography (PET-CT) [4, 6, 10–12].
The PET radiopharmaceutical 2-deoxy-2-[18F]fluoro-D-glucose ([18F]FDG), a glucose analog, is used extensively in oncologic imaging as well as functional imaging in the brain and heart [13, 14]. Several studies have explored the repeatability and reproducibility of [18F]FDG uptake in background structures and various tumors [15–20]; however, the reproducibility of anti-3-[18F]FACBC quantitative parameters has not been studied. In our ongoing and completed clinical trials with anti-3-[18F]FACBC in prostate cancer, 14 patients underwent repeat scanning. Thus, we were presented with an opportunity to perform an exploratory analysis of the reproducibility and reliability of scan to scan measurements of uptake in key background structures and untreated malignant lesions. This exploratory data may then be utilized to design a formal prospective test-retest study.
Materials and Methods
Patient Population
After institutional review board (IRB) approval, we performed a retrospective review of 115 patients who had anti-3-[18F]FACBC PET-CT at Emory University Hospital as part of two prostate carcinoma clinical trials from November 28, 2007 to June 18, 2013. Fourteen of these 115 patients underwent follow-up anti-3-[18F]FACBC PET-CT for suspected prostate carcinoma recurrence and were thus included in our analysis. Patients were enrolled based on inclusion criteria which have been previously reported [4, 11]. Informed consent was obtained from all individual participants included in these studies. All procedures performed in studies involving human participants were in accordance with the ethical standards of the Emory University IRB.
Imaging Protocols
Preparation of anti-3-[18F]FACBC and acquisition protocols have been described elsewhere [4, 21]. The radiotracer was produced under investigational new drug (IND) application 72,437. Scanning was conducted on a Discovery DLS in 13 patients and 690 PET-CT scanner in one patient (GE Healthcare; Milwaukee, WI). All repeat scans were performed on the same scanner. Images were interpreted on a MimVista workstation (MimSoftware, Cleveland, OH). Patients fasted for 4–6 h before the anti-3-[18F]FACBC scan. Anti-3-[18F]FACBC (dose ranging from 292.3 to 418.1 MBq) was injected IV over 2 min. After a 3-min delay for blood pool clearance, abdominopelvic PET-CT imaging was completed with 5–16-min (early) and 17–28-min (delayed) acquisitions [11]. Visual inspection of the PET-CT images was performed by a board certified nuclear radiologist. Semi-quantitative measurements were computed using standardized uptake values (SUV). SUV is defined as tissue concentration (MBq/ml) divided by activity injected per body weight (MBq/g) [22].
Mean SUV (SUVmean) for background structures including blood pool (abdominal aorta), liver, bone marrow (L3), pancreas (measured at pancreatic head), muscle (gluteus), and spleen were recorded. Maximum SUV (SUVmax) of untreated target lesions within the prostate bed or lymph nodes were also obtained for each acquisition time point. We believe that SUVmean reflects overall uptake in background structures while SUVmax is best employed for target lesions. Regions of interest (ROI) conforming as best as possible to the organ to be measured were utilized in order to decrease variation introduced from noise and then propagated through all the scans in that patient. For malignant lesions, the MimVista PET-EDGE tool was used which draws a conforming region of interest based on the differences in intensity between a lesion and background structures and SUVmax was recorded. Simultaneous quantitative measurements of the same structures were undertaken at baseline (test) and second scan (retest) using a customized workflow designed by MimVista (MimSoftware, Cleveland, OH).
Statistical Analysis
Analysis of reproducibility of anti-3-[18F]FACBC PET quantitative parameters was done on a per lesion/background structure basis. The difference between both tumor and background structure uptake in both scans (initial and follow-up) was compared on a per lesion basis using the two-tailed pairwise t test and Wilcoxon signed rank test (when the number of patients was too small for comparison with a t test). Absolute SUVmean differences in background structures and SUVmax for untreated malignant lesions were computed. Intrasubject variability (relative differences) of all background structure parameters was assessed by calculating percent change in mean which is the difference between test and retest values divided by the mean of both values as described by Minn et al. [23]. Analysis of the reliability of PET pharmacokinetic parameters was done on an organ basis by computing Pearson’s correlation coefficients and interclass correlation coefficients (ICCs). The ICC is a scale free reliability ratio which ranges between 0 and 1 where 0 represents an independence of measurements (measurements are dissimilar) and 1 indicates perfect reliability of the measurements under consideration [24]. For the purpose of this paper, we considered ICC values <0.20 as low, 0.40 to 0.59 as moderate, 0.60–0.79 as high, and ICC values between 0.80 and 1.00 as being very high. Statistical significance was determined using a type I error rate of α=0.05, all p values are two-tailed and are reported with the results. Statistical analyses were done using statistical analyses software (SAS version 9.3 SAS Institute Inc., Cary, NC, USA).
Results
The 14 patients ranged in age from 57 to 74 years (mean± SD, 67.2±5.3). The average injected dose of anti-3-[18F]FACBC was 366.3±29.6 MBq for the initial scan and 362.6±33.3 MBq for the follow-up scan (no significant difference; p=0.9). The time interval between both scans ranged from 4 to 29 months (mean±SD, 17.4±7.1).
Background Structures
Table 1 contains the average SUVmean for background structures on both initial and follow-up scans at early and delayed time points. There was no statistically significant difference in the average SUVmean on both initial and follow-up scans at early and delayed imaging except in the marrow at 4 min (p=0.04). The average absolute mean differences (±SD) in background SUVmean uptake on both scans ranged from 0.1 (±0.1) at 4 min and 0.1 (±0.2) at 17 min in blood pool to 1.2 (±0.8) at 4 min and 0.9 (±0.7) at 17 min in the liver.
Table 1.
Reproducibility of anti-3-[18F]FACBC uptake in background structures and malignant lesions on follow-up PET-CT in prostate carcinoma: an exploratory analysis
| SUVmean of background structures at early (4 min) imaging when comparing original to follow-up scans | ||||||
|---|---|---|---|---|---|---|
| Organ | Scan 1, mean (SD) | Scan 2, mean (SD) | p value (t test or Wilcoxon) | Average absolute difference at 4 min (SD) | % Mean difference at 4 min (SD) | ICC (95 % CI) |
| Aorta (blood pool) | 1.41 (0.15) | 1.44 (0.19) | 0.47 | 0.1 (0.1) | 14.2 (11.1) | 0.4 (−0.1–0.7) |
| Liver | 7.54 (1.16) | 7.85 (1.91) | 0.41 | 1.2 (0.8) | 14.9 (9.2) | 0.6 (0.2–0.9) |
| Marrow | 3.06 (0.58) | 3.26 (0.71) | 0.04 | 0.3 (0.2) | 10.7 (6.5) | 0.8 (0.6–0.9) |
| Pancreas | 13.16 (3.92) | 12.77 (3.33) | 0.38 | 1.0 (1.2) | 7.2 (8.1) | 0.9 (0.8–1.0) |
| Muscle | 1.13 (0.38) | 1.25 (0.44) | 0.12 | 0.2 (0.2) | 18.2 (17.8) | 0.8 (0.4–0.9) |
| Spleen | 2.96 (0.44) | 3.11 (0.35) | 0.12 | 0.3 (0.2) | 10.6 (7.3) | 0.6 (0.1–0.8) |
| SUVmean of background structures at delayed (17 min) imaging when comparing original to follow-up scans | ||||||
| Organ | Scan 1, mean (SD) | Scan 2, mean (SD) | p value (t test or Wilcoxon) | Average absolute difference at 17 min (SD) | % Mean difference at 17 min (SD) | ICC (95 % CI) |
| Aorta (blood pool) | 1.12 (0.40) | 1.12 (0.36) | 0.91 | 0.1 (0.2) | 13.3 (10.1) | 0.7 (0.2–0.9) |
| Liver | 7.18 (1.08) | 7.29 (1.88) | 0.75 | 0.9 (0.7) | 12.5 (7.1) | 0.7 (0.3–0.9) |
| Marrow | 2.66 (0.65) | 2.66 (0.56) | 1.00 | 0.2 (0.1) | 6.9 (6.6) | 0.9 (0.8–1.0) |
| Pancreas | 8.72 (3.75) | 8.28 (2.60) | 0.33 | 1.1 (1.1) | 13.8 (13.4) | 0.9 (0.7–1.0) |
| Muscle | 1.44 (0.47) | 1.53 (0.61) | 0.32 | 0.2 (0.3) | 13.8 (13.3) | 0.8 (0.5–0.9) |
| Spleen | 2.05 (0.51) | 2.18 (0.33) | 0.20 | 0.3 (0.2) | 14.9 (11.0) | 0.7 (0.3–0.9) |
The percent mean differences (±SD) in SUVmean at 4 min was 14.2 (±11.1) in blood pool, 14.9 (±9.2) in the liver, 10.7 (±6.5) in the marrow, 7.2 (±8.1) in the pancreas, 18.2 (±17.8) in the muscle, and 10.6 (±7.3) in the spleen, while at 17 min, the percent mean differences (±SD) in SUVmean was 13.3 (±10.1), 12.5 (±7.1), 6.9 (±6.6), 13.8 (±13.4), 13.8 (±13.3), and 14.9 (±11.0) in those same background structures, respectively (Table 1).
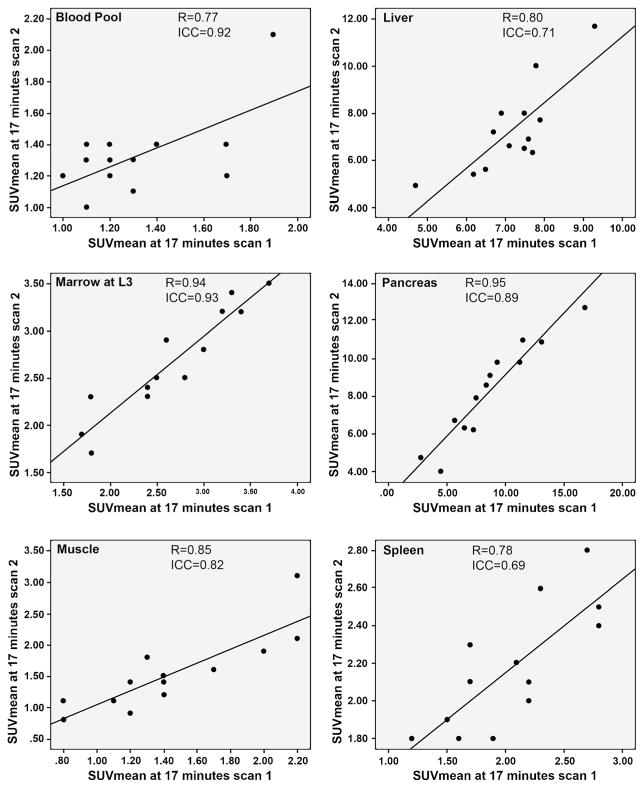
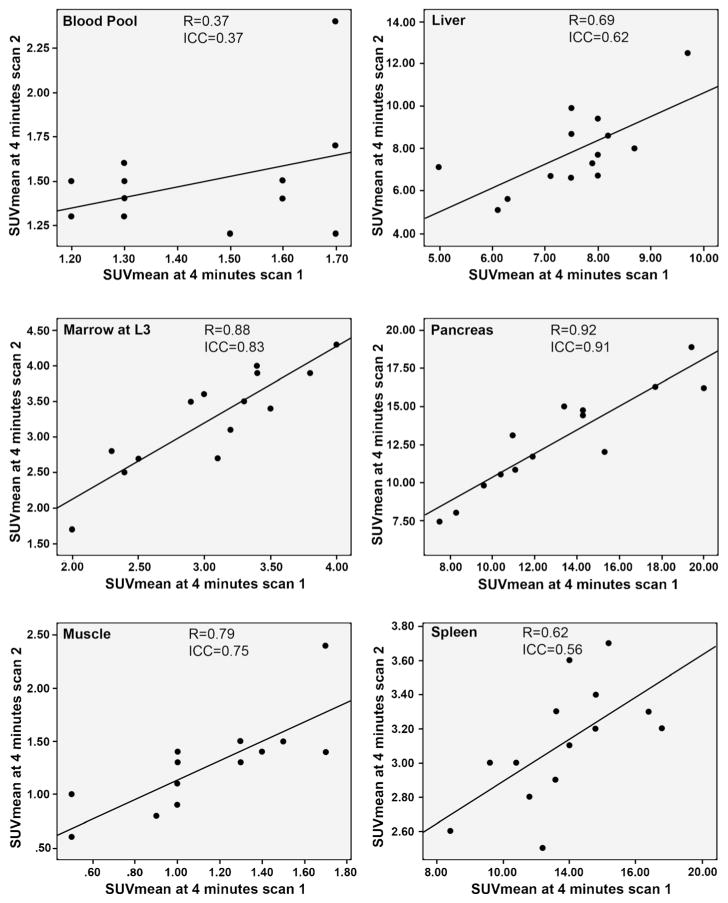
At 4 min, all ICCs were >0.6 with the exception of blood pool, which was 0.4 (moderate to very high reliability). At 17 min, there was high to very high reliability of anti-3-[18F]FACBC quantitative measurement with ICCs in background structures ranging from 0.7 to 0.9 (Table 1). Scatterplots of the correlation of background SUV across initial and follow-up scans at early and delayed imaging are shown in Figs. 1 and 2, respectively.
Fig. 1.
Correlation of early (4 min) SUVmean on both initial and follow-up scans in background structures.
Fig. 2.
Correlation of delayed (17 min) SUVmean of both initial and follow-up scans in background structures.
Untreated Malignant Lesions
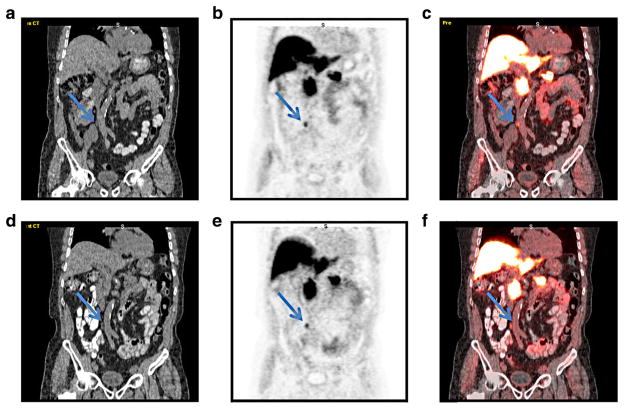
SUVmax and SUVmean in five malignant lesions (two prostate; three lymph nodes) in four patients without interim therapy increased or was essentially stable in the time interval between initial and follow-up scans on early and delayed imaging (Table 2). Table 2 also contains reasons for lack of interim treatment in those malignant lesions. Figure 3 shows images from both initial and follow-up scans in the same patient, illustrating the similarities in background uptake and the appearance of a malignant lymph node without interim treatment between scans.
Table 2.
SUV of untreated malignant lesions at early and delayed time points on both scans
| Lesion | First scan at 4 min | Second scan at 4 min | First scan at 17 min | Second scan at 17 min | Reason for lack of treatment | ||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||
| SUVmax | SUVmean | SUVmax | SUVmean | SUVmax | SUVmean | SUVmax | SUVmean | ||
| Patient 1: prostate lesion | 2.7 | 1.8 | 4.2 | 2.4 | 2.5 | 1.6 | 3.6 | 2.1 | Biopsy after the first scan was negative, and PSA continued to rise. Subsequent positive biopsy after repeat scan |
| Patient 2: prostate lesion | 5.6 | 3.9 | 8.4 | 5.4 | 6.7 | 4.7 | 9.0 | 4.3 | Biopsy after the first scan was negative, and PSA continued to rise. Subsequent positive biopsy after repeat scan |
| Patient 3: right internal iliac node | 9.4 | 4.0 | 10.4 | 4.8 | 5.1 | 2.6 | 5.9 | 3.2 | Node (3.1×1.8 cm) positive on FACBC at first scan, however, laparoscopic biopsy was negative. Biopsy subsequently was positive after repeat second FACBC scan at same location. Original biopsy did not sample lesion in retrospect. |
| Patient 3: left obturator node | 11.1 | 5.6 | 11.3 | 6.0 | 9.3 | 4.4 | 7.5 | 4.4 | Same patient as above. Node (2.3×2.1 cm) positive on FACBC on both scans with similar uptake to right internal iliac node. Though this node was not biopsied, clinically believed to be metastatic. |
| Patient 4: aortocaval node | 4.8 | 2.9 | 5.7 | 3.7 | 4.7 | 3 | 5.1 | 3.4 | Node (0.6×0.5 cm) positive on FACBC at first scan, however, area not accessible by CT-guided biopsy and patient chose to have follow-up. After PSA rose and second FACBC scan was positive at same location, patient agreed to laparoscopic biopsy. |
Fig. 3.
a CT, b FACBC PET, and c co-registered image from initial study in a patient with recurrent prostate carcinoma (patient 4 in Table 2) demonstrates abnormal uptake in a 0.6×0.7 cm aortocaval node (arrow). Follow-up study obtained 14 months later with d CT, e FACBC PET, and f co-registered image demonstrates similar uptake in the node (arrow), as well as visualized background structures. The node was biopsy proven to be metastatic prostate carcinoma.
Discussion
We set out to examine the test-retest reproducibility and reliability of quantitative measurements in key background structures and untreated malignant lesions among patients with prostate cancer recurrence. We found acceptable reproducibility of anti-3-[18F]FACBC measurements on initial and follow-up imaging. The percent mean difference in SUVmean was less than 20 % in all background structures with relatively low absolute SUVmean differences. There was also no statistically significant difference between SUV on both original and follow-up scans except in the marrow at 4 min (p=0.04); however, this had a low absolute mean difference (0.3). There was generally high reliability of anti-3-[18F]FACBC measurements with all ICCs being >0.6 with the exception of aortic blood pool on early imaging which had an ICC of 0.4. We also found that radiotracer uptake in malignant lesions without interim therapy increased or remained stable over time at both early and delayed imaging.
Our findings are important as they will facilitate the development of objective quantitative criteria for interpretation of anti-3-[18F]FACBC PET-CT scans especially as its use migrates toward influencing clinical decisions in recurrent prostate cancer diagnosis and possibly for therapy response monitoring. The precise quantification of radio-tracer uptake measurement reliability, reproducibility, and variability is a vital step in the clinical interpretation and utilization of any imaging test, including anti-3-[18F]FACBC PET-CT. Although this retrospective analysis was not meant to replace a formal prospective test-retest, this data will be helpful in designing such a study.
While the ICC of most key background structures was high or very high, we noted an ICC of 0.4 in blood pool (aorta) on early imaging which is a moderate value. We believe this is likely due to the fact that at early imaging, blood pool is still clearing and is thus more sensitive to small changes in acquisition timing [9]. Even so, absolute difference of SUV was quite low (0.1) and blood pool ICC subsequently improved at delayed imaging to 0.7. Although we observed a significant difference in average marrow SUVmean at 4 min (p=0.04), the average absolute mean difference was minimal (0.3), mean percent difference was 10.7 %, and there was a very high ICC (0.8).
Test-retest reliability and variability of quantitative parameters have been studied mostly for [18F]FDG and in a more limited manner for most radiotracers, including amino acid based radiotracers [15, 17–20, 25–29]. In examining within patient variability of [18F]FDG standardized uptake values in normal tissues, Paquet found stable uptake in the liver (0.6 ICC) and mediastinum over time [30]; in our population, we found similar reliability of anti-3-[18F]FACBC liver uptake measurements (ICC 0.6 at early and 0.7 at delayed imaging for anti-3-[18F]FACBC) [30].
We found that SUVmax of untreated malignant lesions on repeat studies either increased or was essentially stable, as expected. Several studies have examined the reliability of [18F]FDG parameters in malignant lesions [15, 17–20]. Nahmias and Weber in separate studies with [18F]FDG found reproducible SUVs in malignant lesions [18, 20]. In both studies, patients were scanned ≤10 days apart and had various cancers [18, 20]. Since there was a greater time difference between scans and only patients with prostate cancer were studied, our findings are not directly comparable.
The limitations of our study include its retrospective nature and that the study was not a formal test-retest protocol. The time between the first and second scans was variably lengthy. Though firm conclusions cannot be made due to the prolonged time differences between studies, reliability of quantitative measurements in key background structures and reproducibility of uptake in lesions was acceptable. In addition, this study had a limited sample size and was carried out in patients only with prostate cancer. Ideally, a test-retest study for background structures is best performed on normal volunteers within a short temporal sequence. Another limitation of our analysis is that we did not account for partial volume effects in small lesions which can affect SUV, especially in lesions that grew with time. In spite of these limitations, our study provides preliminary data on the reliability of anti-3-[18F]FACBC quantitative measurements as it moves into multicenter clinical trials and also supplies information that would be useful in the design of a formal test-retest study.
Conclusion
Despite variable time interval between scans, anti-3-[18F]FACBC PET-CT demonstrates acceptably reproducible uptake in key background structures and malignant lesions. A formal test-retest study is being planned.
Acknowledgments
We would like to acknowledge Eric Jablonowski for his help with the figures included in the manuscript. This study was funded by the National Institutes of Health (5R01CA129356) and (P50 CA 128301), and Georgia Cancer Coalition.
Footnotes
Conflict of Interest. Emory University and Dr. Mark Goodman are eligible to receive royalties from anti-3-[18F]FACBC.
References
- 1.Jager PL, Vaalburg W, Pruim J, et al. Radiolabeled amino acids: basic aspects and clinical applications in oncology. J Nucl Med. 2001;42:432–445. [PubMed] [Google Scholar]
- 2.Shoup TM, Olson J, Hoffman JM, et al. Synthesis and evaluation of [18F] 1-amino-3-fluorocyclobutane-1-carboxylic acid to image brain tumors. J Nucl Med. 1999;40:331–338. [PubMed] [Google Scholar]
- 3.Schuster DM, Nye JA, Nieh PT, et al. Initial experience with the radiotracer anti-1-amino-3-[18F]fluorocyclobutane-1-carboxylic acid (anti[18F]FACBC) with PET in renal carcinoma. Mol Imaging Biol. 2009;11:434–438. doi: 10.1007/s11307-009-0220-5. [DOI] [PubMed] [Google Scholar]
- 4.Schuster DM, Savir-Baruch B, Nieh PT, et al. Detection of recurrent prostate carcinoma with anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid PET/CT and 111In-capromab pendetide SPECT/CT. Radiology. 2011;259:852–861. doi: 10.1148/radiol.11102023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schuster DM, Taleghani PA, Nieh PT, et al. Characterization of primary prostate carcinoma by anti-1-amino-2-[(18)F]-fluorocyclobutane-1-carboxylic acid (anti-3-[(18) F]FACBC) uptake. Am J Nucl Med Mol Imaging. 2013;3:85–96. [PMC free article] [PubMed] [Google Scholar]
- 6.Schuster DM, Votaw JR, Nieh PT, et al. Initial experience with the radiotracer anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid with PET/CT in prostate carcinoma. J Nucl Med. 2007;48:56–63. [PubMed] [Google Scholar]
- 7.Amzat R, Taleghani P, Miller DL, et al. Pilot study of the utility of the synthetic PET amino-acid radiotracer anti-1-amino-3-[(18) F]fluorocyclobutane-1-carboxylic acid for the noninvasive imaging of pulmonary lesions. Mol Imaging Biol. 2013;15:633–643. doi: 10.1007/s11307-012-0606-7. [DOI] [PubMed] [Google Scholar]
- 8.Sorensen J, Owenius R, Lax M, Johansson S. Regional distribution and kinetics of [18F]fluciclovine (anti-[18F]FACBC), a tracer of amino acid transport, in subjects with primary prostate cancer. Eur J Nucl Med Mol Imaging. 2013;40:394–402. doi: 10.1007/s00259-012-2291-9. [DOI] [PubMed] [Google Scholar]
- 9.Asano Y, Inoue Y, Ikeda Y, et al. Phase I clinical study of NMK36: a new PET tracer with the synthetic amino acid analogue anti[18F]FACBC. Ann Nucl Med. 2011;25:414–418. doi: 10.1007/s12149-011-0477-z. [DOI] [PubMed] [Google Scholar]
- 10.Nanni C, Schiavina R, Boschi S, et al. Comparison of 18F-FACBC and 11C-choline PET/CT in patients with radically treated prostate cancer and biochemical relapse: preliminary results. Eur J Nucl Med Mol Imaging. 2013;40(Suppl 1):S11–17. doi: 10.1007/s00259-013-2373-3. [DOI] [PubMed] [Google Scholar]
- 11.Schuster DM, Nieh PT, Jani AB, et al. Anti-3-[(18) F]FACBC positron emission tomography-computerized tomography and (111)In-capromab pendetide single photon emission computerized tomography-computerized tomography for recurrent prostate carcinoma: results of a prospective clinical trial. J Urol. 2014;191:1446–1453. doi: 10.1016/j.juro.2013.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nanni C, Schiavina R, Brunocilla E, et al. 18F-FACBC compared with 11C-choline PET/CT in patients with biochemical relapse after radical prostatectomy: a prospective study in 28 patients. Clin Genitourin Cancer. 2013;12(2):106–10. doi: 10.1016/j.clgc.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Fletcher JW, Djulbegovic B, Soares HP, et al. Recommendations on the use of 18F-FDG PET in oncology. J Nucl Med. 2008;49:480–508. doi: 10.2967/jnumed.107.047787. [DOI] [PubMed] [Google Scholar]
- 14.Rohren EM, Turkington TG, Coleman RE. Clinical applications of PET in oncology. Radiology. 2004;231:305–332. doi: 10.1148/radiol.2312021185. [DOI] [PubMed] [Google Scholar]
- 15.Minn H, Zasadny KR, Quint LE, Wahl RL. Lung cancer: reproducibility of quantitative measurements for evaluating 2-[F-18]-fluoro-2-deoxy-D-glucose uptake at PET. Radiology. 1995;196:167–173. doi: 10.1148/radiology.196.1.7784562. [DOI] [PubMed] [Google Scholar]
- 16.Velasquez LM, Boellaard R, Kollia G, et al. Repeatability of 18F-FDG PET in a multicenter phase I study of patients with advanced gastrointestinal malignancies. J Nucl Med. 2009;50:1646–1654. doi: 10.2967/jnumed.109.063347. [DOI] [PubMed] [Google Scholar]
- 17.de Langen AJ, Vincent A, Velasquez LM, et al. Repeatability of 18F-FDG uptake measurements in tumors: a metaanalysis. J Nucl Med. 2012;53:701–708. doi: 10.2967/jnumed.111.095299. [DOI] [PubMed] [Google Scholar]
- 18.Weber WA, Ziegler SI, Thödtmann R, et al. Reproducibility of metabolic measurements in malignant tumors using FDG PET. J Nucl Med. 1999;40:1771–1777. [PubMed] [Google Scholar]
- 19.Kamibayashi T, Tsuchida T, Demura Y, et al. Reproducibility of semi-quantitative parameters in FDG-PET using two different PET scanners: influence of attenuation correction method and examination interval. Mol Imaging Biol. 2008;10:162–166. doi: 10.1007/s11307-008-0132-9. [DOI] [PubMed] [Google Scholar]
- 20.Nahmias C, Wahl LM. Reproducibility of standardized uptake value measurements determined by 18F-FDG PET in malignant tumors. J Nucl Med. 2008;49:1804–1808. doi: 10.2967/jnumed.108.054239. [DOI] [PubMed] [Google Scholar]
- 21.McConathy J, Voll RJ, Yu W, et al. Improved synthesis of anti[18F]FACBC: improved preparation of labeling precursor and automated radiosynthesis. Appl Radiat Isot. 2003;58:657–666. doi: 10.1016/s0969-8043(03)00029-0. [DOI] [PubMed] [Google Scholar]
- 22.Zasadny KR, Wahl RL. Standardized uptake values of normal tissues at PET with 2-[fluorine-18]-fluoro-2-deoxy-D-glucose: variations with body weight and a method for correction. Radiology. 1993;189:847–850. doi: 10.1148/radiology.189.3.8234714. [DOI] [PubMed] [Google Scholar]
- 23.Minn H, Zasadny KR, Quint LE, Wahl RL. Lung cancer: reproducibility of quantitative measurements for evaluating 2-[F-18]-fluoro-2-deoxy-D-glucose uptake at PET. Radiology. 1995;196:167–173. doi: 10.1148/radiology.196.1.7784562. [DOI] [PubMed] [Google Scholar]
- 24.Shou H, Eloyan A, Lee S, et al. Quantifying the reliability of image replication studies: the image intraclass correlation coefficient (I2C2) Cogn Affect Behav Neurosci. 2013;13:714–724. doi: 10.3758/s13415-013-0196-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheebsumon P, van Velden FH, Yaqub M, et al. Effects of image characteristics on performance of tumor delineation methods: a test-retest assessment. J Nucl Med. 2011;52:1550–1558. doi: 10.2967/jnumed.111.088914. [DOI] [PubMed] [Google Scholar]
- 26.Okamoto S, Shiga T, Yasuda K, et al. High reproducibility of tumor hypoxia evaluated by 18F-fluoromisonidazole PET for head and neck cancer. J Nucl Med. 2013;54:201–207. doi: 10.2967/jnumed.112.109330. [DOI] [PubMed] [Google Scholar]
- 27.Costes N, Zimmer L, Reilhac A, et al. Test-retest reproducibility of 18F-MPPF PET in healthy humans: a reliability study. J Nucl Med. 2007;48:1279–1288. doi: 10.2967/jnumed.107.041905. [DOI] [PubMed] [Google Scholar]
- 28.Kenny LM, Contractor KB, Hinz R, et al. Reproducibility of [11C] choline-positron emission tomography and effect of trastuzumab. Clin Cancer Res. 2010;16:4236–4245. doi: 10.1158/1078-0432.CCR-10-0468. [DOI] [PubMed] [Google Scholar]
- 29.Koeppe RA, Shulkin BL, Rosenspire KC, et al. Effect of aspartame-derived phenylalanine on neutral amino acid uptake in human brain: a positron emission tomography study. J Neurochem. 1991;56:1526–1535. doi: 10.1111/j.1471-4159.1991.tb02047.x. [DOI] [PubMed] [Google Scholar]
- 30.Paquet N, Albert A, Foidart J, Hustinx R. Within-patient variability of (18) F-FDG: standardized uptake values in normal tissues. J Nucl Med. 2004;45:784–788. [PubMed] [Google Scholar]