A novel Amazonian reef biome was discovered, encompassing large rhodolith and sponge beds under low light, low oxygen, and high POC.
Keywords: coral reefs, mineralization, phase shifts, Marine biogeography, stepping stones
Abstract
Large rivers create major gaps in reef distribution along tropical shelves. The Amazon River represents 20% of the global riverine discharge to the ocean, generating up to a 1.3 × 106–km2 plume, and extensive muddy bottoms in the equatorial margin of South America. As a result, a wide area of the tropical North Atlantic is heavily affected in terms of salinity, pH, light penetration, and sedimentation. Such unfavorable conditions were thought to imprint a major gap in Western Atlantic reefs. We present an extensive carbonate system off the Amazon mouth, underneath the river plume. Significant carbonate sedimentation occurred during lowstand sea level, and still occurs in the outer shelf, resulting in complex hard-bottom topography. A permanent near-bottom wedge of ocean water, together with the seasonal nature of the plume’s eastward retroflection, conditions the existence of this extensive (~9500 km2) hard-bottom mosaic. The Amazon reefs transition from accretive to erosional structures and encompass extensive rhodolith beds. Carbonate structures function as a connectivity corridor for wide depth–ranging reef-associated species, being heavily colonized by large sponges and other structure-forming filter feeders that dwell under low light and high levels of particulates. The oxycline between the plume and subplume is associated with chemoautotrophic and anaerobic microbial metabolisms. The system described here provides several insights about the responses of tropical reefs to suboptimal and marginal reef-building conditions, which are accelerating worldwide due to global changes.
INTRODUCTION
Biogenic reefs are topographically significant structures built by benthic animals, plants, and microbes that mineralize carbonate or siliceous skeletons and/or induce carbonate precipitation (1). The most conspicuous biogenic reefs are the highly biodiverse coral reefs that occur in shallow, warm, and oligotrophic waters with a higher saturation state of calcium carbonate (Ω CaCO3). Under such optimal mineralization conditions, carbonate accumulation reaches up to 10 kg m−2 year−1, and structures may extend for thousands of kilometers (2). However, biogenic reefs develop under a much wider array of conditions that constrain mineralization and other core ecosystem processes typical of tropical coral reefs (for example, grazing by metazoans) (3, 4). The main controls over reef ecosystems interact and vary in a wide range of spatial and temporal scales. As a result, many types of reefs have been subjected to fruitless nomenclatural controversies since the 19th century (3).
Because of their impact on salinity, pH, light penetration, sedimentation, and nutrients, large tropical rivers typically exclude carbonate reef builders from continental shelves. The Amazon-Orinoco and the Ganges-Brahmaputra mouths are textbook examples of such major reef gaps (2). The wide (~300 km) Amazon continental shelf evolved from a carbonate to a siliciclastic system during the early Late Miocene (9.5 to 8.3 million years ago) (5, 6). By this time, under lowstand sea level, an incised canyon system directed sediment influx toward the slope and basin floor (7). Shelf edge reef buildups occurred peripherally to this deep Amazon Fan and were gradually overlain by siliciclasts during Neogene and Quaternary highstands (7, 8). At present, the high sediment load from the river settles relatively quickly in the inner and mid shelves, conditioning an unstable muddy benthic habitat with high bacterial biomass and low diversity and abundance of epifauna and meiofauna (9, 10). The region is also subjected to a highly energetic physical regime because of the fast-flowing North Brazil Current (NBC), strong wind stress, and high semidiurnal tidal ranges. Such conditions create a stressful habitat for benthic megafauna, especially in the areas with soft, fluid sediments. The massive sedimentation and sediment reworking in the inner and mid shelves have been comprehensively surveyed in the last decades, including the core river-ocean biogeochemical processes (9). On the other hand, the “relict magnesian calcite ooids” (11) and other carbonate sediments recorded along the outer shelf (5, 8) have received much less attention. For instance, it is unknown whether this surficial carbonate layer comprises living biomineralizers and other reef-associated organisms and how this benthic system may be coupled to the pelagic compartments. The only noteworthy exceptions to such knowledge gap about the outer shelf is a brief description of reef fishes associated with sponge bottoms (12) and a checklist of corals produced from specimens deposited in museums (13), both of which fail to report the presence of carbonate structures and rhodolith beds.
The Amazon River represents ~20% of the global riverine discharge to the ocean [~120 × 103 m3 s−1 in December to ~300 × 103 m3 s−1 in May; (14)], generating an up to 1.3 × 106–km2 offshore plume enriched with chromophoric dissolved organic matter (15, 16). This relatively shallow (5 to 25 m deep) and hyposaline layer is driven by seasonal winds and currents, flowing northward into the Caribbean and retroflecting eastward during September and October. Phytoplankton productivity is limited by low light penetration in the inner shelf, increasing only once sediments have cleared (16, 17). The resulting downward particle flow occurs away from the continental shelf (18). On the shelf break, sedimentation under the plume is limited by a permanent frontal process that draws near-bottom seawater landwards, coupled with Ekman veering (9). Oxygen levels are lowered in the subplume and near the bottom because of the high rates of organic matter mineralization in the inner and mid-shelf (10, 19). Although the plume has been the focus of recent studies (16, 17, 20), the subplume and the coupling between the plume, subplume, and outer-shelf benthic systems have been largely ignored.
The Amazon River mouth represents the distribution boundary for several sponges, scleractinian corals, and shallow water fishes, among other groups of coastal and reef-associated organisms, as a consequence of the massive oceanographic discontinuities that it imprints in the West Atlantic continental margin (21). On the other hand, many reef-associated species occur at both sides of the river mouth, with possible connectivity mechanisms related to long-range larval dispersal, rafting, or demersal migration through stepping stones (22). The operation of the Amazon mouth biogeographic filter is not completely known because information about the nature and extension of reef habitats off the Amazon mouth is still limited (11–13, 23, 24).
Here, we present the results of a multidisciplinary assessment of the outer Amazon shelf, where we found a unique carbonate reef system of ~9500 km2, between the French Guiana–Brazil border and the Maranhão State in Brazil (~1000 km). Our survey was carried out near the shelf edge and in the upper slope (25 to 120 m), and included geophysical and physical-chemical surveys, radiocarbon dating and petrographic characterization of reef samples, biogeochemical tracers, and microbial metagenomics. We provide a description of macrobenthic and demersal assemblages, including extensive rhodolith beds built by coralline algae and sponge-dominated hard bottom, and also adding primary and gray literature data about the large reef fisheries that operate off the Amazon mouth [for example, CREOCEAN (25) and IBAMA (26)]. The novel system presented here adds to the repertoire of “marginal” reef types shaped by conditions deviating from those of the archetypal tropical coral reefs. The ubiquity of large sponges and other filter feeders, as well as the increase of chemoautotrophic and anaerobic microbial metabolisms recorded in the subplume, provides insights about ecosystem-level responses to the globally accelerating conditions that select against photosymbiotic biocalcifiers (for example, scleractinian corals).
RESULTS
Structure, composition, and age of reef structures
An extensive carbonate reef system of ~9500 km2, spanning from 5°N to 1°S and 44° to 51°W, was recorded between the Brazil–French Guiana border and Maranhão State, Brazil (Fig. 1). Rhodolith beds and higher-relief structures were recorded across a relatively long (~1000 km) and narrow (~50 km) stretch in the outer shelf and upper slope, in depths ranging from 30 m to the shelf break at 90 to 120 m. This extensive submerged carbonate system extends from French Guiana southward to the Manuel Luis reef, the northernmost emerging reef within the Brazilian Biogeographic Province.
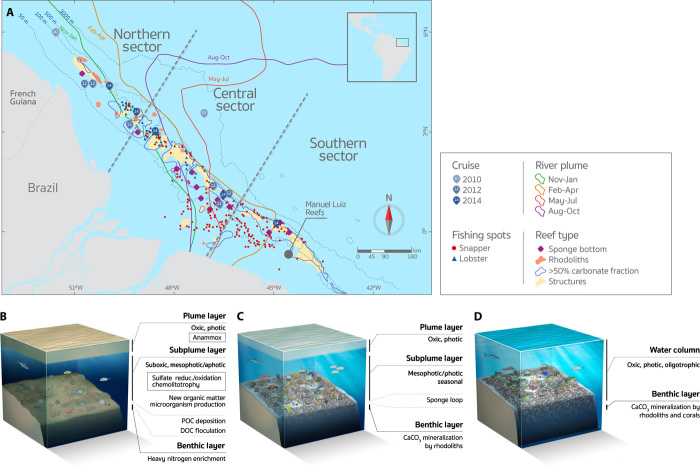
Fig. 1. Map of the Amazon shelf showing the benthic megahabitats and seasonal influence of the river plume.
(A) Distribution of reef fisheries and oceanographic stations. Manuel Luis reefs are the northernmost emerging reefs in Brazil. (B to D) Main structural and functional traits of the reefs in the Northern (120 m), Central (55 m), and Southern Sectors (25 m), respectively. Plume POC δ13C = −22.9 ± 0.7, δ15N = 4.0 ± 1.2; Plume DOC δ13C = −27.7 ± 1.0, δ15N = 1.3 ± 0.3. Subplume POC δ13C = −24.2 ± 1.3, δ15N = 5.1 ± 1.7; Subplume DOC δ13C = −26.6 ± 1.7, δ15N = 0.1 ± 1.8. Benthic (sediment) δ13C = −26.2 ± 0.6, δ15N = 2.2 ± 0.5. Some graphic elements are courtesy of the Integration and Application Network, University of Maryland Center for Environmental Science (http://ian.umces.edu/symbols/). The plume lines represent the outer edge of the plume during that season, according to satellite climatology (80).
In the Northern Sector of the study region, structures were recorded near the shelf edge, comprising widely spaced (hundreds to thousands of meters) patches with lengths of up to 300 m and heights of up to 30 m. These irregularly shaped reefs tended to be elongated with a parallel shelf edge orientation, resembling erosive structures (Fig. 1B). Dredged materials consist of carbonate fragments with incipient living cover of crustose coralline algae (<5%) and low-vitality rhodoliths recovered in the vicinity of the larger reef patches, and also include lateritic crusts. The dated sample (surficial carbonate fragment) presented a 2σ radiocarbon calibrated age of 13,382 to 13,749 years before present (BP), with microfacies typical of grainstone composed of skeleton fragments of tube worms, foraminifera, barnacles, bryozoans, and molluscs (Fig. 2, A and B). Dredge casts that did not hit structures recovered large sponges among soft sediments (fig. S1). In the Central Sector, the bottom was dominated by rhodoliths with high vitality (>50% of live coralline algae cover), as well as by complex sandwaves and gravel ripples between 20- and 100-m depths (Fig. 1C). Patches of carbonate blocks were small (<10 m2) and sparsely distributed. The core and surface of a ~70 × 40–cm block collected in this sector presented 2σ radiocarbon calibrated ages of 4487 to 4846 and 4157 to 4562 years BP, respectively. Microfacies is typical of boundstone and is mainly composed of crustose coralline algae and bryozoans (Fig. 2, C and D). The surface of this block presented small and sparse patches of living coralline algae. In the Southern Sector, structures were widespread and occurred between 30- and 90-m depths. Reef morphology consists of ridge-like features <5 m in height and irregular and low-relief patch reefs (<5 m in height) (Fig. 1D). Structures are surrounded by a high backscatter and flat hardground (fig. S2) dominated by high-vitality rhodoliths and carbonate sand. The dated sample (surficial carbonate fragment) presented a modern radiocarbon age (<150 years), with microfacies typical of boundstone composed of hydrocorals, crustose coralline algae, and corals (Fig. 2, E and F). The southern part of this sector encompasses one relatively shallow (<10-m depth) submerged reef (Banco do Álvaro reef) and the emerging Manoel Luís reef (~450 km2), both consisting of isolated and coalesced coralline pinnacles. None of the benthic casts in the Central and Southern Sectors recovered mud.
Fig. 2. Surficial reef fragments (left) and corresponding petrographic images (right) from the Northern (A and B, 120-m depth), Central (C and D, 60 m), and Southern Sectors (E and F, 23 m).
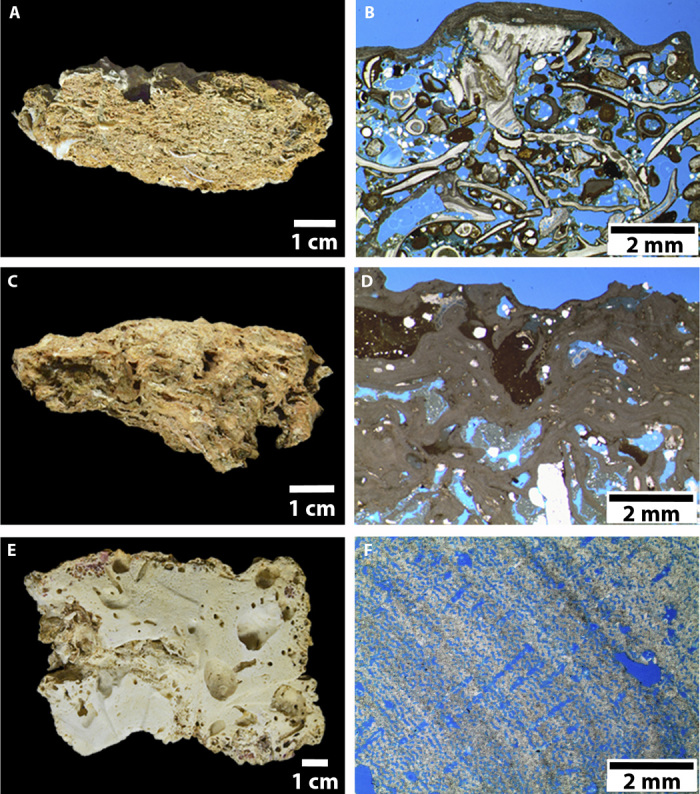
Microfacies transition from an older grainstone (12,100 ± 30 thousand years BP) composed of filter feeders (polychaetes, foraminifera, barnacles, bryozoans, and molluscs) under a thin veener of coralline algae in the Northern Sector (A and B) to a more recently turned-off (5220 ± 110 thousand years BP) boundstone composed of photosynthesizers (crustose coralline algae) and filter feeders (bryozoans) in the Central Sector (C and D) and, finally, to a recent boundstone typical of turbid zone reefs (hydrocorals, crustose coralline algae, and corals) in the Southern Sector (E and F).
Macrobenthos, demersal fish, and reef fisheries
Red algae (Rhodophyta, 25 species) were the predominant benthic plant group, followed by green (Chlorophyta, 6 species) and brown algae (Ochrophyta, 4 species) (table S1). Calcareous algae were ubiquitous (fig. S3), with a clear impoverishment gradient northward. Five encrusting calcareous algae taxa were identified in the surface of rhodoliths and carbonate blocks, with living Lithothamnion crispatum and Sporolithon ptychoides distributed across the entire outer shelf, including the subplume environment of the Northern Sector. A low-diversity assemblage (34 species) of typically tropical-subtropical and wide depth–ranging seaweeds was recorded in association with the rhodoliths in the Central and Southern Sector. These assemblages included greater functional diversity than those of the Northern Sector (table S1). Seaweeds recovered from the Northern Sector (for example, Gelidium and Anadyomene) consisted of detached and low-vitality fragments. With the exception of Anadyomene, green and brown algae were restricted to the South Sector.
The sponge assemblage comprised 61 species and was dominated by massive forms that were wide depth–ranging within the photic and mesophotic zones, but also included a few deep-water species (table S2 and fig. S4). Three Northern Sector stations were remarkable as they recovered sponges among soft sediments, including large-sized Xestospongia muta with unusual pale coloration and narrow atria and Tribrachium schmidtii with a buried bulbous base and an upward long papilla (fig. S4). The highest sponge diversity and biomass was recorded on the flatter rhodolith beds of the Central Sector. For instance, a single 20 minutes trawl (station 2014-6; 55-m depth) recovered about 30 species (150 specimens, ~900 kg), most of which exhibited large, erect, cup-like, and massive forms, growing attached to rhodoliths (table S2, fig. S4, and movie S1). The most common sponge species in the Central Sector were Agelas spp., Aplysina spp., Callyspongia vaginalis, Clathria nicoleae Geodia spp., Monanchora arbuscula, and Oceanapia bartschi. Encrusting species (for example, Clathria cf. calla) were overall rare and restricted to grow on other sponges. Lissodendoryx sp. and O. bartschi were heavily colonized by epibionts (ascidians, hydroids, and other sponges). Two excavating species of genus Cliona were found associated with scleractinian corals in the Southern Sector (table S2 and figs. S4 and S5), whereas no boring sponges were recorded in the Northern Sector.
Cnidarians were present at all stations, with hydroids (benthic colonial life stage of hydrozoans) being particularly abundant across the region. Two black coral species (Antipatharia), Antipathes furcata and Tanacetipathes tanacetum, typical of mesophotic zone reefs, were recorded at the Northern Sector (table S3). Octocorallia was the most speciose group (26 species), but most records are from sparse museum specimens without precise locality records (13). Scleractinians with symbiotic dinoflagellates (Symbiodinium spp.) were largely restricted to the Central and Southern Sectors. Where present (Central and Southern Sectors), scleractinians comprised impoverished (12 species, table S3) and low-density/cover assemblages (fig. S5) encompassing encrusting colonies of small-sized species (Meandrina braziliensis, Agaricia spp., Scolymia wellsii, and Favia gravida), small colonies of massive species (Montastraea cavernosa and Madracis decactis), and branching colonies of Millepora cf. alcicornis. With the exception of F. gravida and Millepora cf. alcicornis, all corals recorded off the Amazon mouth were wide depth–ranging species, occurring in photic and mesophotic habitats. An alien brittle star from the Pacific Ocean, Ophiothela mirabilis, was recorded in association with Leptogorgia miniata.
We recorded 73 reef fish species in the study region, most of them with wide depth and geographic ranges (table S4 and fig. S6). Most fish species were carnivores (86%), including piscivores and invertivores, whereas a few were planktivores or herbivore/detritivores (two species, 3% each). Four species (5.5%) of sponge-eating fishes of family Pomacanthidae (angelfishes) were recorded across the region. Significant fisheries for the Southern red snapper, Lutjanus purpureus (2900 metric tons year−1), and spiny lobsters, Palinurus spp. (1360 metric tons year−1), were recorded across the region, the latter being concentrated in the Northern and Central Sectors (Fig. 1). Reef fisheries are carried out by small- to medium-sized boats (8 to 20 m lengths) operating with traps (for lobsters) and hand lines or long lines (for reef fishes) in the outer shelf. Smaller dinghies with one to two crew (fig. S7) operating hand lines are also regularly spotted, and are used to increase fishing area and the chance of finding reef structures where fishes aggregate. At least 131 boats are currently registered to fish lobsters with traps (~3 boats per 10 km of the linear extension of the reef system), but a larger number of unregistered boats target reef fishes. Targeted species include a diverse assemblage of groupers (Serranidae, 321 metric tons year−1) and snappers (Lutjanidae, 4220 metric tons year−1), which are landed mainly in Pará and Amapá (26). Such intense reef fisheries (fig. S8) represent additional evidence for the wide distribution and importance of the reefs close to the Amazon mouth. In the inner shelf, fisheries are carried out over soft sediments, mostly with gillnets, trawls, and long lines.
Biogeographic patterns
All macroalgae recorded off the Amazon mouth are wide-ranging species that are distributed across large expanses of the Atlantic and Pacific basins. The sponge fauna was a typical tropical West Atlantic reef assemblage, with only three Brazilian endemics and two species that also occur in West Africa. Three new records were added to the Brazilian sponge fauna: Theonella atlantica, a typical deep-water species previously recorded in the Southern Caribbean; Clathria echinata, previously known from the Caribbean; and Didiscus verdensis, previously known from shallow waters in the Cape Verde Archipelago (27). The octocoral fauna (26 species) included typically mesophotic species, with 18 species that are wide-ranging in the West Atlantic, 7 Brazilian endemics, and 1 circum-globally distributed gorgonian. Anthipatarians included only three species that are widely distributed in the West Atlantic, including the black coral Anthipathes furcate, which is a new record to Brazilian tropical waters (previously known from the Caribbean and Southeastern Brazil). Of the 6 recorded scleractinians, 2 are Brazilian endemics and the remaining 4 are wide-ranging in the Atlantic Ocean. Brazilian-endemic scleractinians were restricted to the Central and Southern Sectors. The reef fish assemblage was also dominated by wide-ranging species (63% are widely distributed in the West Atlantic, 22% occur in the West and East Atlantic, and 11% occur in the Atlantic and Pacific), with the exception of Stegastes pictus, Halichoeres dimidiatus, and Sparisoma frondosum, which are Brazilian endemics with occasional records northward into the Caribbean and West Africa (S. frondosum). Pelagic spawners with high dispersal capabilities (80%) dominated the reef fish assemblage. Most recorded species (algae, sponges, cnidarians, and fishes) are wide depth–ranging, with a few exceptions restricted to the Southern Sector (tables S1 to S4).
Plume and nonplume water column
Water column profiles under nonplume conditions encompassed outer shelf, slope, and open-ocean/deep-sea stations (Fig. 3 and fig. S9). These profiles were well mixed to about 100 m, with near constant salinity at ~35.5 to 36. Temperatures near the surface were consistently ~28°C, cooling rapidly below 50 m, with 1% light level [photosynthetically active radiation (PAR)] reaching ~100 m. Dissolved inorganic nitrogen (DIN), a limiting nutrient, was near the detection limit in the upper 70 m, and particulate organic carbon (POC) and dissolved organic carbon (DOC) concentrations were generally low throughout the upper water column (Fig. 3). Oxygen was uniform at around 4 ml liter−1 throughout the upper 100 m (Fig. 3). Conversely, in profiles associated with the plume (Fig. 3 and fig. S9), the water column was strongly stratified with an evident lower salinity and higher temperature signal in the upper 10 to 15 m. Light attenuation was much stronger in plume profiles, with 1% levels no deeper than 50 m (Fig. 3), and nutrient concentrations (such as DIN) were consistently >0, with values depending on proximity to river mouth. Concentrations of POC and DOC were higher in the plume (Fig. 3), reflecting both riverine and marine organic inputs. Oxyclines were detectable at depths of ~5 to 10 m across the plume interface and 35 to 50 m within the subplume. Dissolved oxygen (DO) levels dropped to ≤ 3.5 ml liter−1 near the bottom at some stations on the outer shelf (fig. S9).
Fig. 3. Water column profiles under plume (A) and nonplume (B) conditions.
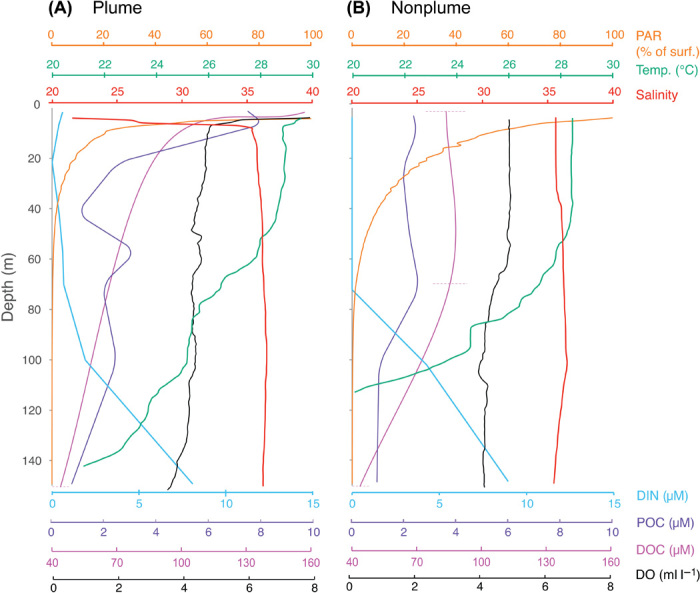
(A) Station 2010-04 (5.495°N, 51.488°W), under intense plume influence, Northern Sector. (B) Station 2010-08 (4.349°N, 46.852°W) under nonplume condition, Central Sector.
Isotopic analysis
The isotopic composition of POC was heavier in the plume (−22.9 ± 0.7‰) than in the subplume (−24.2 ± 1.3‰) and benthic (sediment) layers (−26.2 ± 0.6‰), whereas DOC showed a slight but not significant difference between the plume (−27.7 ± 1.0‰) and subplume (−26.6 ± 1.7‰) layers. The same trend was observed for nitrogen isotopic composition of particulate organic nitrogen (PON) in the plume (4.0 ± 1.2‰) and subplume (5.1 ± 0.7‰) layers, respectively, but significantly lower values were found in the benthic layer (2.2 ± 0.5‰) (Fig. 1). Dissolved organic nitrogen showed an opposite trend when compared with PON, with higher values in the plume (1.3 ± 0.3‰) than in the subplume (−0.1 ± 1.8‰) layer.
Transcriptome analysis
Compared to nonplume (oceanic) metatranscriptomes, more gene transcripts related to anaerobic metabolism were detected in the plume and subplume layers (fig. S10), corroborating the water column physical-chemical features. An opposite trend was observed for photosynthesis gene transcripts, except for the particle-associated fraction of the plume, reinforcing the increased contribution of chemosynthesis in the subplume. The adenylyl sulfate reductase subunits α and β (aprAB; responsible for dissimilatory sulfate reduction) and sulfur (thiosulfate and sulfide) oxidation (soxB) transcripts from free-living microbes were more abundant in the subplume layer. Anammox gene transcripts and respiratory nitrate/nitrite reductase (narB and nirB; responsible for nitrate respiration) transcripts were more abundant in the plume layer, from both free-living and particle-attached microbes in the plume.
DISCUSSION
Despite the iconic depiction of reefs as megadiverse systems thriving in warm, shallow, and oligotrophic waters, biogenic reefs develop under a much wider range of conditions. The benthic production by efficient mixotrophic holobionts that build carbonate structures (for example, scleractinian corals), together with the grazing by fish and macroinvertebrates, have been important drivers in the evolution of coral reef ecosystems (28, 29). However, these processes are constrained in the so-called marginal reef systems (4), which may share parts of their taxonomic structure and some functional properties with tropical coral reefs (29). Marginal reefs are subjected to environmental forcing that depart from the optimal mineralization conditions for corals, such as the rhodolith beds that occur at great depths and latitudes (30, 31), aphotic zone coralline and sponge reefs (32), and stromatolites that develop under extreme physicochemical conditions (3). These marginal reef systems share some common trends such as a lowered importance of photosymbioses, reduced diversity of macroorganisms (macroalgae and metazoans), reduced grazing, and increased microbial diversity. With a greater areal extent, depth range, and latitudinal extent than that of coral reefs, marginal reefs have been relatively neglected by science, especially because of the logistical constrains for direct observation and mapping with remote sensing in turbid waters (33). Here, we presented a major carbonate system that occurs off the Amazon River mouth, adding to the wide repertoire of marginal reefs that includes large megahabitats (thousands of square kilometers) that were only recently mapped (31, 34), despite occurring in continental shelves.
The extensive reef system off the Amazon River mouth presents erosive structures that ceased to grow during the late stages of the last post-glacial maximum transgression, as revealed by the carbonate rocks dated in the Northern (13,382 to 12,749 calibrated years BP) and Central Sectors (4487 to 4846 and 4157 to 4562 calibrated years BP). Dead rhodolith beds and relict magnesium calcite ooids (11) are recorded in the Northern Sector, extending into southern French Guiana (25), and their ages are compatible to the surface of the dated structure from this sector. The age of this structure also corresponds to the transitional period of the last turn off of the Amazon Fan because of widespread shelf flooding (sea level reaching 40 to 50 m below present-day sea level) (7). Besides the last post-glacial transgression and shifts in the sediment budget because of fluvial, oceanographic, and meteorological processes (35), the reef building turnoff (36) in the Northern Sector also seems related to shelf subsidence, which reached more than 100 m between 16 and 21 thousand years BP (35). Despite encompassing assemblages adapted to low light penetration, turbid zone reefs develop under narrow depth ranges and can be especially vulnerable to relative sea level changes (4).
Turbidity is elevated across the entire Equatorial Margin, but deposition is low in the outer shelf, especially in the Northern Sector, where the NBC reaches maximum speed (9) and prevents the burial of reefs by terrigenous sediments. Such high turbidity–low net sediment accumulation is also associated with the permanent frontal processes and Ekman pumping into the platform (9). From the Central Sector southward, turbidity decreases and the plume influence becomes more seasonal. The carbonate balance becomes positive from the Central Sector southward, mainly due to the high density of living rhodoliths covered by red algae (Corallinales), which are able to mineralize under very low light levels.
Although reef framework building has been “turned off” (35) in a significant portion of the Amazon reef range, in all sectors, there is a living assemblage of reef-associated organisms typical of West Atlantic mesophotic and deep reefs (37–39). The benthic assemblage of the Northern Sector is dominated by filter feeders adapted to strong currents, high suspended sediment, and lowered light and oxygen, such as octocorals and black corals, and especially by massive sponges with long papilla (O. bartschi), ball-shaped sponges (Cinachyrella kuekenthali), barrels with narrow atrium and high pumping rates (X. muta), and bulbs (T. schmidtii) (40). Besides bearing narrower atria typical of high current settings, the large barrel sponges, X. muta, were remarkable for being pale, possibly due to the lack of photosymbionts (fig. S1). An even more diverse assemblage of large sponges develops in association to the high-vitality rhodolith beds of the Central Sector, including growth forms adapted to steady currents, to light capture by photosynthetic symbionts, and to sediment resistance (for example, tubes—A. lacunosa; curled fan—C. vaginalis; branched—C. nicoleae; massive with long inhaling papillae and narrow elevated central oscule—O. bartschi) (table S2). There are few larger coalesced structures in the Central Sector, and the topography of the rhodolith beds is limited by the size of the nodules (centimeters to tens of centimeters). However, the great sponge abundance significantly increases habitat complexity and enhances nutrient supply to other organisms, reducing DOC concentration and providing significant benthic production (41). The high abundance of sponges in the outer shelf was recorded in an early survey targeting the discovery of shrimp-trawling beds (12), but it is now clear that sponge diversity and abundance peaks in the intermediary portion of the plume influence gradient. Turbidity and extreme limitations in light penetration may control the diversity and abundance of sponges in the Northern Sector, whereas competition with other benthic organisms (coralline algae, macroalgae, and corals) and predation by reef fishes may be the most important controls southward.
Large sponge reefs are well documented in aphotic areas in different oceans, but they are generally dominated by Hexactinellida (glass sponges), with a few exceptions in which Demospongiae dominate. Reefs dominated by few species of hexactinellids are well documented in the Northeast Canadian shelf, between 30- and 240-m depths (32). Deep-water aggregates of large Demospongiae are known as “sponge grounds” or “sponge gardens” and are widely distributed in the North Atlantic (42). These habitats may encompass up to 50 sponge species, including a strong contribution of Geodia spp. (42, 43), which is a ubiquitous genus in the Amazon reefs (table S2). A sponge garden hotspot in West Australia (tropical Carnarvon Shelf) also has high richness and biomass concentrated between 40- and 100-m depths (40). The Central Sector of the Amazon reefs system is similar to such sponge gardens, presenting (i) high sponge diversity and biomass in the mesophotic zone; (ii) large, erect, cup-like, and massive forms adapted to sedimentation; and (iii) species with low inorganic content (with few or no spicules) concentrated where the shelf is wider and currents are weaker.
The shallower Southern Sector is an area with higher wave energy and episodic plume influence (23, 44), resembling the typical turbid zone reefs [for example, Perry and Larcombe (4)] with few species and sparse corals and hydrocorals (Fig. 1C). When compared to other reefs within the Brazilian Province and the Caribbean [for example, Wilkinson (45)], coral and coralline algal diversity is still relatively low, but carbonate accumulation is positive, as indicated by the dating of the structures. Indeed, high coral diversity and framework accumulation are often uncoupled, and the former may not be a universal surrogate of reef health (4).
The Amazon reefs are also noteworthy for supporting considerable fisheries yields that span all sectors, especially lobsters (Crustacea: Palinuroidea) and snappers (Perciformes: Lutjanidae). Although extensive shrimp trawling and other fisheries (for example, gill nets and long lines) are well documented in the soft sediments of the inner and mid-shelf [for example, Pinheiro and Frédou (46)], hundreds of artisanal and commercial boats operate in the outer shelf with hand lines and traps. For instance, lobster yields in the Amazon reefs (mostly Panulirus argus, but also including five other species) (47) are equivalent to 5% of the total lobster capture in the 23 Caribbean countries that explore this resource (48, 49). Because of Brazil incipient fisheries management, the exact number of boats that operate in the Amazon reefs remains undisclosed, but tracking data show that fishing effort with hand lines and traps is concentrated in the outer shelf (fig. S8). Although some typical reef fisheries resources are lacking from the Amazon reefs (for example, parrot fishes), lobsters and other species (for example, red snapper and large groupers) may benefit from plume-related resources and conditions, showing that low-diversity reefs with incipient coral cover may still provide relevant and valuable ecosystem services.
The Amazon River mouth is the distribution boundary for several reef-associated organisms. Southward, the reef biota of the Brazilian Biogeographic Province is less diverse than that of the Caribbean and presents high endemism levels (24, 50). Although such lowered species richness seems to result from the relatively smaller area and suboptimal conditions for reef development (for example, high turbidity and river runoff), endemism seems to be largely driven by the partial isolation of the Southwestern Atlantic. The selective and intermittent nature of the Amazon mouth biogeographic filter may drive parapatric divergence (instead of allopatric speciation) because this model allows for restricted gene flow between diverging populations (51). Indeed, the novel information about the characteristics and extension of the Amazon mouth reef system provides additional support to the phylogeographic evidence for the operation of parapatric speciation, whereas our updated checklist of reef-associated organisms (tables S1 to S4) clarifies the selective nature of the biogeographic corridor.
The relatively low-diversity assemblage of algae, sponges, corals, and reef fishes is dominated by wide depth–ranging species that are broadly distributed in the Atlantic (or in the West Atlantic) (tables S1 to S4). Shallow-water dwellers, or species that depend on specific coralline microhabitats or resources, are not able to use the Amazon reef system as a stepping stone because reef structures and rhodolith beds are largely located in relatively deep areas (>40 m) with limited availability of habitat and food resources. At ecological time frames, such shallow-water dwellers must rely on larval dispersal or rafting (22) across the hyposaline plume within the unidirectional NBC, a fact that helps explain the higher Brazilian-endemism level within fish groups such as blennies (shallow-water dwellers) and parrot fishes (specialized herbivores) (52–54). Brazilian-endemic corals such as Mussimilia spp., which have expressive cover southward (55), are also shallow-water dwellers. These species only occur in deeper habitats in oceanic islands and offshore banks, where light penetration reaches greater depths. At larger time scales (thousands to tens of thousands years), lowered relative sea level (7) and other environmental fluctuations may “turn on” the Amazon Fan and widespread reef development in the Amazon reef system, providing a more permeable connectivity matrix between the Caribbean and the South Atlantic.
At least 29 sponge taxa are still identified only at supraspecific levels, indicating a source for new species. An alien brittle star from the Pacific Ocean, O. mirabilis, which was known from Brazil and French Guiana (56), was recorded in the Amazon reefs, showing that invasive species introduced in the Caribbean (for example, lionfishes) may reach the South Atlantic through this countercurrent dispersal route (57). Modeling of potential bioinvasions through this route may take depth range into account because of depth selectivity of the Amazon mouth biogeographic filter.
The inner Amazon shelf is known for high rates of benthic respiration, which is associated with the river-sourced terrestrial material (19). In the Amazon reefs, microbial metabolisms deviate from those commonly found in coralline reefs (39, 58) because they include chemosynthesis and heterotrophy, particularly in the Northern and Central Sectors. This particular functional structure is better understood from the layered structure comprising the plume, the subplume, and the benthic mosaic (Fig. 1 and fig. S2). Light reduction may condition heterotrophic and chemosynthetic microbial metabolisms (Fig. 3 and figs. S9 and S10). Whereas photosynthesis is the major carbon fixation process in nonplume waters, the subplume presents significant amounts of gene transcripts related to anaerobic respiration, resembling an oxygen minimum zone (OMZ), and corroborates the observed oxycline. Oxygen depletion in the subplume is not as drastic as in other OMZs (54), but oxygen levels near the bottom can be as low as 3 ml liter−1 and can potentially limit some benthic organisms.
At the Northern and Central Sectors, calcareous algae may photosynthesize at low light levels, and sponges may tolerate anoxic and suboxic conditions for several days (59). The sponge assemblage includes both high microbial abundance (HMA) and low microbial abundance (LMA) species (60); the former rely heavily on microbial symbionts, whereas the latter use water column microbes for nourishment. Symbiotic microbes associated with HMA sponges include chemosynthetic and fermenting taxa (for example, Proteobacteria, Firmicutes, Actinobacteria, Planctomycetes, and Thaumarchaeota) and Cyanobacteria (60) that help sponge metabolism. On the other hand, the high POC and DOC concentration in the Amazon mouth reefs may promote an intense development of LMA sponge loop (61, 62).
Although the low-salinity plume stays well above the seafloor, the plume may interact dynamically with benthic organisms through particle flux, shear, and enhanced eddy stirring and mixing (15). The clear marked difference in isotopic composition can be related to increased anoxia, with heavier N increased in the subplume (higher subplume N2 concentration). A significant fractionation in isotopic composition of N between suspended particles (plume and subplume layers) and surface sediment corroborates the presence of processes such as nitrogen fixation and denitrification/anammox (63). In addition, the isotopic analysis of plume and subplume DOC and POC indicates a strong contribution from terrestrial and mangrove-derived material, suggesting that the reefs in the Northern and Southern Sector are subjected to very specific biogeochemical conditions. Previous studies have suggested a rapid turnover of organic matter from terrestrial and mangrove origins, with a longer persistence of mangrove-derived DOC, with contribution to oceanic areas accounting for >10% of DOC (64, 65). Our results are in agreement with these patterns, and the isotopic signatures for Amazon rivers [−26.8 to −30.4‰ (DOC) and −27.4 ± 0.8‰ (POC)], mangrove waters [−31.4‰ (DOC) and −28.1 ± 1.5‰ (POC)], surface Atlantic waters [−20.8 ± 1.1‰ (DOC)], and deep Atlantic waters [−23.7‰ (DOC)] reinforce the contribution from terrestrial and mangrove-derived material to the reefs’ DOC and POC pools.
The rapid decline of coral reefs is drawing considerable attention because of the alarming forecasts of biodiversity losses from local (for example, pollution and overfishing) and global stressors (temperature anomalies and ocean acidification) (66, 67). Understanding the distribution of the several reef subtypes and how their biodiversity and functional properties are associated with different environmental forcing is a major and basic step toward forecasting generalized trajectories for reef systems (41, 68). In this regard, studies of marginal reef ecosystems have a major role to play in reef ecology because scleractinian-dominated communities may not be a universal baseline. The Amazon reef system comprises a gradient from marginal mineralization conditions (South Sector) to structures that are beyond CaCO3 mineralization thresholds for thousands of years (North Sector) but still supports significant reef-associated biodiversity and relevant ecosystem services. For low trophic level fisheries resources, such as lobsters, the system seems to support higher yields than coral-dominated reefs (49). The CaCO3 production by rhodolith beds (1.3 to 2.7 kg m−2 year−1), the dominant megahabitat in vast expanses of tropical and temperate shelves (30), as well as in the Amazon mouth, is close to the mean global coral reef rate (1.5 kg m−2 year−1) (31). Although the impoverished coral reefs in the Brazilian Province represent only 5% of the Atlantic reef area (33), the region’s extensive rhodolith beds produce >0.025 gigatons year−1, rivaling with the total CaCO3 production by coral reefs in the Caribbean (0.04 to 0.08 gigatons year−1). Although corals appear biologically fragile, they are geologically robust (“the most ingenious paradox”) (69), and there is mounting evidence that peripheral areas with reef-associated organisms may be a key to the evolution and survival of coral reef biota through geological time (70, 71).
The sponge dominance in the Central Sector provides support to the idea that coral domination may phase-shift to sponge domination as climate changes and some local stressors escalate (for example, nutrients) (40, 70). Sponges, corals, and coralline algae respond differently to ocean chemistry and environmental conditions, with sponges benefitting from increased DOC and POC while having broader tolerance to acidification and temperature anomalies. Indeed, sponges are the oldest reef-associated organisms; they dominated reef building during various stages of the Paleozoic and Mesozoic when conditions to biomineralizers deteriorated (28).
In conclusion, the novel reef system off the Amazon River is extensive, is impoverished in terms of biodiversity, and presents unique functional attributes due to the plume influence. The system provides relevant ecosystem services and functions as a selective biogeographic corridor between the Caribbean and the South Atlantic Ocean, and may give important insights in terms of future scenarios for forecasting coralline reefs trajectories under acute climate changes. Remarkably, 125 exploratory blocks for oil drilling in the Amazon shelf were offered in an international auction in 2013, 35 of which were acquired by domestic and transnational companies. In the past decade, a total of 80 exploratory blocks have been acquired for oil drilling in the study region, 20 of which are already producing. These blocks will soon be producing oil in close proximity to the reefs, but the environmental baseline compiled by the companies and the Brazilian government is still incipient and largely based on sparse museum specimens (13). Such large-scale industrial activities present a major environmental challenge, and companies should catalyze a more complete social-ecological assessment of the system before impacts become extensive and conflicts among the stakeholders escalate. The feasibility of oil and gas operations may be assessed by considering environmental and social sensibilities, but even the extent of the overlap of exploratory blocks with sensitive areas remains unclear. The context of great proximity to international waters and to the French border adds complexity. It is relevant to consider further studies on regional marine spatial planning, the functioning of the new reef biome in face of global changes, and sensitivities related to the hydrologic cycle of the Amazon—where extreme droughts and floods are on the increase and will influence the functioning of this novel carbonate reef system.
MATERIALS AND METHODS
Experimental design
Sampling was carried out onboard R/V Knorr (May 2010), R/V Atlantis (July 2012), and NHo Cruzeiro do Sul (September 2014). A complete station list of the three cruises is provided in table S5. To assess the effects of the dynamic river-ocean interface, sampling was stratified in (i) Northern Sector, representing the area under the strongest and permanent plume influence; (ii) Central Sector, under seasonal plume influence; and (iii) Southern Sector, under intermittent riverine influence (Fig. 1). Water column profiles were acquired with a conductivity-temperature-depth with a recorder (CTD), which was also equipped with sensors of PAR and DO at eight stations in 2010 and at nine stations in 2014 (table S5). Water was collected from near the surface, bottom, and in the chlorophyll maximum using Niskin bottles or surface pumps, and was analyzed for inorganic nutrients (16), DOC and POC (16, 72), and microorganisms (20, 73).
Bottom topography
We obtained 800 km of acoustic data with a Kongsberg EM122 Multibeam Echosounder in 2012. In 2014, we surveyed 500 km with two EdgeTech side scan sonars (model 4200, 100 to 400 kHz at stations 1 to 56; model 4100, 100 to 500 kHz at stations 67 to 100). Both surveys were carried out with ~300-m swath widths. Sonograms were processed with Sonar WizMap 5.03, converted into 1-m pixel images, and further vectored and submitted to supervised qualitative classification in a GIS environment. Classification was based on backscatter intensity and indirect topography (74).
Macrobenthic and demersal assemblages
We sampled 14 stations (5 in 2012 and 9 in 2014; Fig. 1) with heavy (150 kg) metal dredges with mouths of 100 to 150 by 40 to 80 cm and mesh nets of 1 cm2 that were trawled at 1 to 1.5 knots for 5 to 20 min. Two box corer launches were done in six stations of the 2014 cruise, and a flat shrimp net (15-m mouth, 1.5-cm mesh in the cod end, and two 150-kg trawl doors) was trawled in three stations. Dredging, trawling, or box-coring covered stations in all three sectors. Specimens were washed in seawater, sorted, and photographed on board, and were further preserved in 80% ethanol or 5% formalin. Frozen or dried subsamples were kept for microbiological, genetic, and chemical analyses. Vouchers were deposited at the Museu Nacional, Universidade Federal do Rio de Janeiro, and at the Jardim Botânico do Rio de Janeiro. Crustose coralline algae, sponges, and fishes were identified with standard methods (75, 76). Fisheries yields were obtained from unpublished governmental reports that refer to the last year during which Brazil monitored fisheries (2007). Only landings in Pará and Amapá were accounted for (Maranhão was excluded because its fleet extends southward to the Amazon River mouth).
Petrographic and isotopic analyses
Petrographic thin sections (30 μm) of carbonate rocks recovered in each sector were used to assess reef builders’ identities and relative importance. Radiocarbon (14C) ages were determined from the same samples, which included the surface and core of a larger carbonate block (~45 cm) from the Central Sector (80-m depth), and two superficial smaller (~20 cm) framework fragments from the North and Central Sector, obtained at depths of 120 and 23 m, respectively. Radiocarbon ages were derived from carbon reduction to graphite (100% C) after acid etch pretreatment, with subsequent detection in Accelerator Mass Spectrometry (Center for Applied Isotope Studies, University of Georgia). Dates were reported as 2σ calibrated (95% confidence) radiocarbon ages BP. Calibration was carried out using Calib 7.1 (available at http://calib.qub.ac.uk/calib/), Marine13 calibration curve, and assuming a global marine reservoir effect of 400 years (radiocarbon years before present, “present” = AD 1950). Organic matter samples were analyzed for C and N isotopes using an isotope ratio mass spectrometer (model DELTA V Advantage, Thermo Fisher Scientific) as described previously (77).
Secondary data sets
Literature data indicative of reefs and reef-associated biota were compiled and incorporated in the GIS (Fig. 2), including observations of high CaCO3 sediments, magnesium calcite ooids [for example, Barreto et al. (8)], sponges and reef fish (12), and reef fisheries (26).
Metatranscriptomes from the plume and subplume
Microbial genes and transcripts were obtained from water samples obtained at six stations of the 2010 cruise (73) and two stations of the 2012 cruise, inside and outside the plume, and in the subplume. Data sets were generated by Illumina sequencing (150 × 150 base pairs overlapping paired-end reads) and were deposited in GenBank under accession number SRP037995 (73). Ribosomal sequences in RNA-seq data (complementary DNA sequencing) were identified and removed from metatranscriptome data sets using riboPicker tool (73). Identification of chemosynthesis-related genes (that is, sulfur oxidation, sulfate reduction, and anammox transcripts in the plume and subplume interface) was performed based on profile hidden Markov model (pHMM) approach. Full-length sulfur oxidation (SoxA, SoxB, SoxX, SoxY, and SoxZ), sulfate reduction (DsrA, DsrB, DsrJ, DsrK, DsrL, DsrM, DrsO, DrsP, AprA, and AprB), and anammox (NarB, NarG, NarH, NarI, NirA, NirB, NirK, and NirS) amino acid sequences obtained from the UniProtKB database (www.uniprot.org) were used as seed alignments. pHMM profiles of protein subunits families related to photosynthesis were also used for contrasting water layers, using both photosystem complexes: I (PsaF, PsaM, PsaN, and PsaAB) and II (PsbN, PsbI, PsbH, and PSII). Profiles were obtained directly from the Pfam database (PsaN-PF05479, PsaM-PF07465, PsaAB-PF00223, PsaF-PF02507, PsbN- PF02468, PsbI- PF02532, PSII- PF00421, and PsbH-PF00737). Multiple alignments were conducted using MAFFT (version 6.717b) (78, 79) with the auto mode option, and pHMMs were built using hmmbuild functionality from HMMER package (version 3.0). Contigs assembled from metatranscriptomes were translated into six frames using the Transeq program from the EMBOSS package (v6.1.0) and used as the database for searching genes related to sulfur oxidation, sulfate reduction, and anammox metabolisms. Search was conducted using hmmersearch functionality from HMMER package of the pHMMs built against the plume database. Results were parsed and counted using Python, and shell scripts and relative abundance were calculated.
Supplementary Material
Acknowledgments
We thank J. C. Braga for the help with petrographic interpretations and J. Montoya, P. Medeiros, and V. Coles for providing oceanographic data. Funding: Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenadoria de Aperfeiçoamento de Pessoal de Nível Superior (CAPES),Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), and Brasoil provided essential funding. MCTI and the Brazilian Navy provided support with the NHo Cruzeiro do Sul in 2014. Expeditions in 2010 (R/V Knorr) and 2012 (R/V Atlantis) were funded by U.S. NSF (OCE-0934095 to P.L.Y.). Additional support was provided by the Gordon and Betty Moore Foundation (GBMF #2293 and #2928 to P.L.Y.). Author contributions: C.E.R. and F.L.T. coordinated the project. R..L.M., P.L.Y., C.E.R., and F.L.T. designed and coordinated the fieldwork. R.L.M., C.E.R., G.M.A.-F., A.C.B., F.C.M., P.L.Y., and F.L.T. wrote the paper. P.S.B., P.S.S., M.M.M., M.G.A., J.M.S., B.F.A., F.P.B., T.P.R., B.C.V.O., R.G.B., R.P.P., R.J.S.D., E.S., A.G.F., C.V.L., E.H., N.E.A., G.B.G., S.N.-L., P.L.Y., R.B.F.-F., A.F., M.C., B.S.S., A.P.B.M., L.O., A.C.S., R.C.P., L.A., N.L.O., J.B.T., C.C.T., and R.A.B.V. contributed with data and data analyses. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data used to obtain the conclusions in this paper are available online in the Brazilian Marine Biodiversity database (http://marinebiodiversity.lncc.br/files/index.php/s/TaLwiKC6QCiOvnY) and in the Biological and Chemical Oceanography Data Management Office (www.bco-dmo.org/project/2097).
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/2/4/e1501252/DC1
fig. S1. Trawl and dredge casts on ships’ deck.
fig. S2. Sonographic images of the main reef megahabitats off the Amazon River mouth.
fig. S3. Carbonate fragments (A and B) and rhodoliths (C and D) sampled off the Amazon River mouth.
fig. S4. Representative species of sponges collected off the Amazon River mouth.
fig. S5. Representative species of corals and hydrocoral collected off the Amazon River mouth.
fig. S6. Representative reef fish species collected off the Amazon River mouth.
fig. S7. Fishing boat operating dinghies with hand lines and long lines near the shelf edge in the Northern Sector during the 2014 cruise.
fig. S8. Density of fishing operations targeting red snapper (L. purpureus) in 2010 off the Amazon mouth.
fig. S9. Depth profiles of salinity and DO measured during the R/V Cruzeiro do Sul cruise (September 2014).
fig. S10. Relative contribution of functions related to chemosynthesis and photosynthesis recorded outside, within, and underneath the Amazon River plume.
table S1. Algae recorded off the Amazon River mouth.
table S2. Sponges recorded off the Amazon River mouth.
table S3. Corals, hydrocorals, and gorgonians recorded off the Amazon River mouth.
table S4. Reef fish species recorded off the Amazon mouth [does not include species recorded at the Manuel Luis reefs; see de Moura et al. (23) and Rocha and Rosa (44)].
table S5. Oceanographic stations (primary data sources).
movie S1. Sampling the plume, subplume, and reefs off the Amazon river mouth during the NHo Cruzeiro do Sul cruise (2014).
Supplementary file. Shape files.
REFERENCES AND NOTES
- 1.C. Birkeland, Ed. Life and Death of Coral Reefs (Chapman & Hall, New York, 1997). [Google Scholar]
- 2.W. N. Goldberg, The Biology of Reefs and Reef Organisms (The University of Chicago Press, Chicago, IL, 2013). [Google Scholar]
- 3.Riding R., Structure and composition of organic reefs and carbonate mud mounds: Concepts and categories. Earth Sci. Rev. 58, 163–231 (2002). [Google Scholar]
- 4.Perry C. T., Larcombe P., Marginal and non-reef-building coral environments. Coral Reefs 22, 427–432 (2003). [Google Scholar]
- 5.Milliman J. D., Summerhayes C. P., Barreto H. T., Quaternary sedimentation on the Amazon continental margin: A model. Geol. Soc. Amer. Bull. 86, 610–614 (1975). [Google Scholar]
- 6.Gorini C., Haq B. U., dos Reis A. T., Silva C. G., Cruz A., Soares E., Grangeon D., Late Neogene sequence stratigraphic evolution of the Foz do Amazonas Basin, Brazil. Terra Nova 26, 179–185 (2014). [Google Scholar]
- 7.Maslin M. A., Durham E., Burns S. J., Platzman E., Grootes P., Greig S. E. J., Nadeau M.-J., Schleicher M., Pflaumann U., Lomax B., Rimington N., Paleoreconstruction of the Amazon River freshwater and sediment discharge using sediments recovered at site 942 on the Amazon Fan. J. Quaternary Sci. 15, 419–434 (2000). [Google Scholar]
- 8.Barreto L. A., Milliman J. D., Amaral C. A. B., Francisconi O., Upper continental margin sedimentation off Brazil, northern Brazil. Contr. Sedimentol. 4, 11–43 (1975). [Google Scholar]
- 9.Nittrouer C. A., DeMaster D. J., The Amazon shelf setting: Tropical, energetic, and influenced by a large river. Cont. Shelf Res. 16, 553–573 (1996). [Google Scholar]
- 10.Aller J. Y., Stupakoff I., The distribution and seasonal characteristics of benthic communities on the Amazon shelf as indicators of physical processes. Cont. Shelf Res. 16, 717–751 (1996). [Google Scholar]
- 11.Milliman J. D., Barretto H. T., Relict magnesian calcite oolite on the Amazon shelf. Sedimentology 22, 137–145 (1975). [Google Scholar]
- 12.Collette B. B., Rützler K., Reef fishes over sponge bottoms off the mouth of the Amazon river. Proceedings of the 3rd International Coral Reef Symposium, Miami, FL, 1977 May. [Google Scholar]
- 13.Cordeiro R. T. S., Neves B. M., Rosa-Filho J. S., Pérez C. D., Mesophotic coral ecosystems occur offshore and north of the Amazon River. Bull. Mar. Sci. 91, 491–510 (2015). [Google Scholar]
- 14.Ward N. D., Krusche A. V., Sawakuchi H. O., Brito D. C., Cunha A. C., Moura J. M. S., da Silva R., Yager P. L., Keil R. G., Richey J. E., The compositional evolution of dissolved and particulate organic matter along the lower Amazon River—Óbidos to the ocean. Mar. Chem. 177, 244–256 (2015). [Google Scholar]
- 15.Coles V. J., Brooks M. T., Hopkins J., Stukel M. R., Yager P. L., Hood R. R., The pathways and properties of the Amazon River plume in the tropical North Atlantic Ocean. J. Geophys. Res. 118, 6894–6913 (2013). [Google Scholar]
- 16.Goes J. I., do Rosario Gomes H., Chekalyuk A. M., Carpenter E. J., Montoya J. P. , Coles V. J., Yager P. L., Berelson W. M., Capone D. G., Foster R. A., Steinberg D. K., Subramaniam A., Hafez M. A., Influence of the Amazon River discharge on the biogeography of phytoplankton communities in the western tropical north Atlantic. Prog. Oceanogr. 120, 29–40 (2014). [Google Scholar]
- 17.Subramaniam A., Yager P. L., Carpenter E. J., Mahaffey C., Björkman K., Cooley S., Kustka A. B., Montoya J. P., Sañudo-Wilhelmy S. A., Shipe R., Capone D. G., Amazon River enhances diazotrophy and carbon sequestration in the tropical North Atlantic Ocean. Proc. Natl. Acad. Sci. U.S.A. 105, 10460–10465 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chong L. S., Berelson W. M., McManus J., Hammond D. E., Rollins N. E., Yager P. L., Carbon and biogenic silica export influenced by the Amazon River Plume: Patterns of remineralization in deep-sea sediments. Deep Sea Res. Pt. I 85, 124–137 (2014). [Google Scholar]
- 19.Blair N. E., Aller R. C., Anaerobic methane oxidation on the Amazon shelf. Geochim. Cosmochim. Acta 59, 3707–3715(1995). [Google Scholar]
- 20.Satinsky B. M., Crump B. C., Smith C. B., Sharma S., Zielinski B. L., Doherty M., Meng J., Sun S., Medeiros P. M., Paul J. H., Coles V. J., Yager P. L., Moran M. A., Microspatial gene expression patterns in the Amazon River Plume. Proc. Natl. Acad. Sci. U.S.A. 111, 11085–11090 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miloslavich P., Klein E., Díaz J. M., Hernández C. E., Bigatti G., Campos L., Artigas F., Castillo J., Penchaszadeh P. E., Neill P. E., Carranza A., Retana M. V., Díaz de Astarloa J. M., Lewis M., Yorio P., Piriz M. L., Rodríguez D., Yoneshigue-Valentin Y., Gamboa L., Martín A., Marine biodiversity in the Atlantic and Pacific coasts of South America: Knowledge and gaps. PLOS One 6, e14631 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luiz O. J., Madin J. S., Robertson D. R., Rocha L. A., Wirtz P., Floeter S. R., Ecological traits influencing range expansion across large oceanic dispersal barriers: Insights from tropical Atlantic reef fishes. Proc. R. Soc. B 279, 1033–1040 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Moura R. L., Martins Rodrigues M. C., Francini-Filho R. B., Sazima I., Unexpected richness of reef corals near the southern Amazon River mouth. Coral Reefs 18, 170 (1999). [Google Scholar]
- 24.Rocha L. A., Patterns of distribution and processes of speciation in Brazilian reef fishes. J. Biogeogr. 30, 1161–1171 (2003). [Google Scholar]
- 25.CREOCEAN, Évaluation de l’évolution des peuplementshalieutiques des zones adjacentesetéloignées au site. Première campagne–avant acquisition sismique (Shell E&P France, Le Lamentin, Martinique, 2012). [Google Scholar]
- 26.IBAMA, Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis. Estatística da Pesca no Brasil. Grandesregiões e unidades da federação (Ministério do Meio Ambiente, Brasília, Brasil, 2007); http://www.ibama.gov.br/documentos-recursos-pesqueiros/estatistica-pesqueira.
- 27.Hiemstra F., van Soest R. W. M., Didiscus verdensis spec. nov. (Porifera: Halichondrida) from the Cape Verde Islands, with a revision and phylogenetic classification of the genus Didiscus. Zoölogische Mededelingen 65, 39–52 (1991). [Google Scholar]
- 28.Kiessling W., Geologic and biologic controls on the evolution of reefs. Annu. Rev. Ecol. Evol. Syst. 40, 173–192 (2009). [Google Scholar]
- 29.Bellwood D. R., Goatley C. H. R., Brandl S. J., Bellwood O., Fifty million years of herbivory on coral reefs: Fossils, fish and functional innovations. Proc. R. Soc. B. 281, 20133046 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foster M. S., Rhodoliths: Between rocks and soft places. J. Geophys. Res. 37, 659–667(2001). [Google Scholar]
- 31.Amado-Filho G. M., Moura R. L. , Bastos A. C., Salgado L. T., Sumida P. Y., Guth A. Z., Francini-Filho R. B., Pereira-Filho G. H., Abrantes D. P., Brasileiro P. S., Bahia R. G., Leal R. N., Kaufman L., Kleypas J. A., Farina M., Thompson F. L., Rhodolithbedsare beds are major CaCO3bio-factories in the Tropical South West Atlantic. PLOS One 7, e35171 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eluik L. S., Siliceous sponge communities, biological zonation, and recent sea-level change on the Arctic margin: Ice island results: Discussion. Can. J. Earth Sci. 28, 459–462 (1991). [Google Scholar]
- 33.M. D. Spalding, C. Ravilious, E. P. Green, World Atlas of Coral Reefs (University of California Press, Berkeley, CA, 2001). [Google Scholar]
- 34.Moura R. L., Secchin N. A., Amado-Filho G. M., Francini-Filho R. B., Freitas M. O., Minte-Vera C. V., Teixeira J. B., Thompson F. L., Dutra G. F., Sumida P. Y. G., Guth A. Z., Lopes R. M., Bastos A. C., Spatial patterns of benthic megahabitats and conservation planning in the Abrolhos Bank. Cont. Shelf Res. 70, 109–117 (2013). [Google Scholar]
- 35.Sommerfield C. K., Nittrouer C. A., Figueiredo A. G., Stratigraphic evidence of changes in Amazon shelf sedimentation during the late Holocene. Mar. Geol. 125, 351–371 (1995). [Google Scholar]
- 36.Perry C. T., Smithers S. G., Evidence for the episodic “turn-on” and “turn-off” of turbid-zone coral reefs during the late Holocene sea-level highstand. Geology 38, 119–122 (2010). [Google Scholar]
- 37.Olavo G., Costa P. C. S., Martins A. S., Ferreira B. P., Shelf-edge reefs as priority areas for conservation of reef fish diversity in the tropical Atlantic. Aquat. Conserv. 21, 199–209 (2011). [Google Scholar]
- 38.Pinheiro H. T., Mazzei E., Moura R. L., Amado-Filho G. M., Carvalho-Filho A., Braga A. C.; Costa P. A. S., Ferreira B. P., Ferreira C. E. L., Floeter S. R., Francini-Filho R. B., Gasparini J. L., Macieira R. M., Martins A. S., Olavo G., Pimentel C.R., Rocha L. A., Sazima I., Simon T., Teixeira J. B., Xavier L. B., Joyeaux J.-C., Fish biodiversity of the vitória-trindade seamount chain, Southwestern Atlantic: An updated database. PLOS One 10, e0118180 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meirelles P. M., Amado-Filho G. M., Pereira-Filho G. H., Pinheiro H. T., de Moura R. L., Joyeux J.-C., Mazzei E. F., Bastos A. C., Edwards R. A., Dinsdale E., Paranhos R., Santos E. O., Iida T., Gotoh K., Nakamura S., Sawabe T., Rezende C. E., Gadelha L. M. R. Jr, Francini-Filho R. B., Thompson C., Thompson F. L., Baseline assessment of mesophotic reefs of the Vitória-Trindade seamountchain based on water quality, microbial diversity, benthic cover and fish biomass data. PLOS One 10, e0130084 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schönberg C. H. L., Fromont J., Sponge gardens of Ningaloo Reef (Carnarvon Shelf, Western Australia) are biodiversity hotspots. Hydrobiologia 687, 143–161 (2011). [Google Scholar]
- 41.Bell J. J., Davy S. K., Jones T., Taylor M. W., Webster N. S., Could some coral reefs become sponge reefs as our climate changes? Glob. Change Biol. 19, 2613–2624 (2013). [DOI] [PubMed] [Google Scholar]
- 42.Klitgaard A. B., Tendal O. S., Distribution and species composition of mass occurrences of large-sized sponges in the northeast Atlantic. Prog. Oceanogr. 61, 57–98 (2004). [Google Scholar]
- 43.Beazley L. I., Kenchington E. L., Murillo F. J., del Mar Sacau M., Deep-sea sponge grounds enhance diversity and abundance of epibenthic megafauna in the Northwest Atlantic. ICES J. Mar. Sci. 70, 1471–1490 (2013). [Google Scholar]
- 44.Rocha L. A., Rosa I. L., Baseline assessment of reef fish assemblages of Parcel Manuel Luiz Marine State Park, Maranhão, north-east Brazil. J. Fish Biol. 58, 985–998 (2001). [Google Scholar]
- 45.C. R. Wilkinson, Ed., Status of Coral Reefs of the World: 2008 (Global Coral Reef Monitoring Network and Reef and Rainforest Research Centre, Townsville, Australia, 2008). [Google Scholar]
- 46.Pinheiro L. A., Frédou F. L., Caracterizaçãogeral de pesca industrial desembarcada no estado do Pará. Rev. Cient. Universidade Federal doPará 4, 1–16 (2004). [Google Scholar]
- 47.Araujo-Silva K. C., Cintra I. H. A., Ramos-Porto M., Viana G. F. S., Lagostas capturadas na plataforma continental do estado do Amapá (Crustacea, Nephropoidea, Palinuroidea). Bol. Téc. Cient. CEPNOR 7, 173–184 (2007). [Google Scholar]
- 48.A. A. Fonteles-Filho, Síntese sobre distribuição, abundância, potencial pesqueiro e biologia lagosta-vermelha Panulirus argus (Latreille) e a lagosta-verde Panulirus laevicauda (Latreille) do nordeste do Brasil (Ministério do Meio Ambiente/Recursos Vivos na Zona Econômica Exclusiva, Brasília, Brasil, 2008).
- 49.Chávez E. A., Potential production of the Caribbean spiny lobster (Decapoda, Palinura) fisheries. Crustaceana 82, 1393–1412 (2009). [Google Scholar]
- 50.G. Muricy, D. A. Lopes, E. Hajdu, M. S. Carvalho, F. C. Moraes, M. Klautau, C. Menegola, U. Pinheiro, Catalogue of Brazilian Porifera (Museu Nacional, Rio de Janeiro, 2011). [Google Scholar]
- 51.Rocha L. A., Bowen B. W., Speciation in coral reef fishes. J. Fish Biol. 72, 1101–1121 (2008). [Google Scholar]
- 52.R. L. Moura, I. Sazima, Species richness and endemism levels of the Southwestern Atlantic reef fish fauna, Ninth International Coral Reef Symposium, Bali, Indonesia, 23–27 October 2000.
- 53.Floeter S. R., , Rocha L. A., Robertson D. R., Joyeux J. C., Smith-Vaniz W. F., Wirtz P., Edwards A. J., Barreiros J. P., Ferreira C. E. L., Gasparini J. L., Brito A., Falcón J. M., Bowen B. W., Bernardi G., Atlantic reef fish biogeography and evolution. J. Biogeogr. 35, 22–47 (2008). [Google Scholar]
- 54.Canfield D. E., Stewart F. J., Thamdrup B., De Brabandere L., Dalsgaard T., Delong E. F., Revsbech N. P., Ulloa O., A cryptic sulfur cycle in oxygen-minimum–zone waters off the Chilean coast. Science 330, 1375–1378 (2010). [DOI] [PubMed] [Google Scholar]
- 55.Francini-Filho R. B., Coni E. O. C., Meirelles P. M., Amado-Filho G. M., Thompson F. L., Pereira-Filho G. H., Bastos A. C., Abrantes D. P., Ferreira C. M., Gibran F. Z., Güth A. Z., Sumida P. Y. G., Oliveira N. L., Kaufman L., Minte-Vera C. V., Moura R. L., Dynamics of coral reef benthic assemblages of the Abrolhos Bank, Eastern Brazil: Inferences on natural and anthropogenic drivers. PLOS One 8, 54260 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hendler G., Brugneaux S. J., New records of brittle stars from French Guiana: Ophiactissavignyi and the alien species Ophiothela mirabilis (Echinodermata: phiuroidea). Mar. Biodiv. Rec. 6, 113 (2013). [Google Scholar]
- 57.Luiz O. J., Floeter S. R., Rocha L. A., Ferreira C. E. L., Perspectives for the lionfish invasion in the South Atlantic: Are Brazilian reefs protected by the currents? Mar. Ecol. Prog. Ser. 485, 1–7 (2013). [Google Scholar]
- 58.Bruce T., Meirelles P. M., Garcia G., Paranhos R., Resende C. E., de Moura R. L., Filho R.-F., Coni E.O. C., Vasconcelos A. T., Filho G. A., Hatay M., Schmieder R., Edwards R., Dinsdale E., Thompson F. L., Abrolhos bank reef health evaluated by means of water quality, microbial diversity, benthic cover, and fish biomassa data. PLOS One 7, e36687 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mills D. B., Warda L. M., Jones C., Sweetena B., Forth M., Treusch A.H., Canfield D. E., Oxygen requirements of the earliest animals. Proc. Natl. Acad. Sci. U.S.A. 111, 4168–4172 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gloeckner V., Wehrl M., Moitinho-Silva L., Gernert C., Schupp P., Pawlik J. R., Lindquist N. L., Erpenbeck D., Wörheide G., Hentschel U., The HMA-LMA dichotomy revisited: An electron microscopical survey of 56 sponge species. Biol. Bull. 227, 78–88 (2014). [DOI] [PubMed] [Google Scholar]
- 61.de Goeij J. M., van Ovelen D., Vermeij M. J. A., Osinga R., Middelburg J. J., Goeij A. F. P. M., Admiraal W., Surviving in a marine desert: The sponge loop retains resources within coral reefs. Science 342, 108–110 (2013). [DOI] [PubMed] [Google Scholar]
- 62.Silveira C. B., Silva-Lima A. W., Francini-Filho R. B., Marques J. S.M., Almeida M. G., Thompson C. C., Rezende C. E., Paranhos R., Moura R. L., Salomon P. S., Thompson F. L., Microbial and sponge loops modify fish production in phase-shifting coral reefs. Environ. Microbiol. 17, 3832–3846 (2015). [DOI] [PubMed] [Google Scholar]
- 63.Robinson R. S., Kienast M., Albuquerque A. L., Altabet M., Contreras S., De Pol Holz R., Dubois N., Francois R., Galbraith E., Hsu T.-C., Ivanochko T., Jaccard S., Kao S.-J., Kiefer T., Kienast S., Lehmann M., Martinez P., Carthy M. M., Möbius J., Pedersen T., Quan T. M., Ryabenko E., Schmittner A., Schneider R., Schneider-Mor A., Shigemitsu M., Sinclair D., Somes C., Studer A., Thunell R., Yang J.-Y., A review of nitrogen isotopic alteration in marine sediments. Paleoceanography 27, PA4203 (2012). [Google Scholar]
- 64.Dittmar T., Lara R. J., Kattner G., River or mangrove? Tracing major organic matter sources in tropical Brazilian coastal waters. Mar. Chem. 73, 253–271 (2001). [Google Scholar]
- 65.Dittmar T., Hertkorn N., Kattner G., Lara R. J., Mangroves, a major source of dissolved organic carbon to the oceans. Global Biogeoch. Cycles 20, GB1012 (2006). [Google Scholar]
- 66.Hoegh-Guldberg O., Mumby P. J., Hooten A. J., Steneck R. S., Greenfield P., Gomez E., Harvell C. D., Sale P. F., Edwards A. J., Caldeira K., Knowlton N., Eakin C. M., Iglesias-Prieto R., Muthiga N., Bradbury R. H., Dubi A., Hatziolos M. E., Coral reefs under rapid climate change and ocean acidification. Science 318, 1737–1742 (2007). [DOI] [PubMed] [Google Scholar]
- 67.Descombes P., Wisz M. S., Leprieur F., Parravicini V., Heine C., Olsen S. M., Swingedouw D., Kulbicki M., Mouillot D., Pellissier L., Forecasted coral reef decline in marine biodiversity hotspots under climate change. Glob. Change Biol. 21, 2479–2487 (2015). [DOI] [PubMed] [Google Scholar]
- 68.Pandolfi J. M., Connolly S. R., Marshall D. J., Cohen A. L., Projecting coral reef futures under global warming and ocean acidification. Science 333, 418–422 (2011). [DOI] [PubMed] [Google Scholar]
- 69.Andersson A. J., Mackenzie F. T., Lerman A., Coastal ocean and carbonate systems in the high CO2 world of the anthropocene. Am. J. Sci. 305, 875–918(2005). [Google Scholar]
- 70.Bowen B. W., Rocha L. A., Toonen R. J., Karl S. A.; ToBo Laboratory, The origins of tropical marine biodiversity. Trends Ecol. Evol. 28, 359–366(2013). [DOI] [PubMed] [Google Scholar]
- 71.Norström A. V., Nyström M., Lokrantz J., Folke C., Alternative states on coral reefs: Beyond coral–macroalgal phase shifts. Mar. Ecol. Prog. Ser. 376, 295–306 (2009). [Google Scholar]
- 72.Medeiros P. M., Seidel M., Ward N. D., Carpenter E. J., Gomes H. R., Niggemann J., Krusche A. V., Richey J. E., Yager P. L., Dittmar T., Fate of the Amazon River dissolved organic matter in the tropical Atlantic Ocean. Global Biogeochem. Cy. 29, 677–690 (2015). [Google Scholar]
- 73.Satinsky B. M., Zielinski B. L., Doherty M., Smith C. B., Sharma S., Paul J. H., Crump B.C., Moran M. A., The Amazon continuum dataset: Quantitative metagenomic and metatranscriptomic inventories of the Amazon River plume, June 2010. Microbiome 2, 17 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cavalcanti G. S., Gregoracci G. B., dos Santos E. O., Silveira C. B., Meirelles P. M., Longo L., Gotoh K., Nakamura S., Iida T., Sawabe T., Rezende C. E., Francini-Filho R. B., Moura R. L., Amado-Filho G. M., Thompson F. L., Physiologic and metagenomic attributes of the rhodoliths forming the largest CaCO3 bed in the South Atlantic Ocean. ISME J. 8, 52–62 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.H. G. Greene, J. J. Bizzarro, V. M. O’Connell, C. K. Brylinsky, Mapp. Seafloor Habitat Charact. 141–155 (2007).
- 76.J. N. A. Hooper, R. W. M. van Soest, Eds., Systema Porifera: A Guide to the Classification of Sponges (Kluwer Academic/Plenum Press, New York, 2002). [Google Scholar]
- 77.Farquhar G. D., Ehleringer J. R., Hubick K. T., Carbon isotope discrimination and photosynthesis. Annu. Rev. Plant Phys. 40, 503–537 (1989). [Google Scholar]
- 78.Schmieder R., Lim Y. W., Edwards R., Identification and removal of ribosomal RNA sequences from metatranscriptomes. Bioinformatics 28, 433–435 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Katoh K., Misawa K., Kuma K.-i., Miyata T., MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 30, 3059–3066 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Salisbury J., Vandemark D., Campbell J., Hunt C., Wisser D., Reul N., Chapron B., Spatial and temporal coherence between Amazon River discharge, salinity, and light absorption by colored organic carbon in western tropical Atlantic surface waters. J. Geophys. Res. 116, C00H02 (2011). [Google Scholar]
- 81.Buddemeier R. W., Smith S. V., Coral adaptation and acclimatization: A most ingenious paradox. AmerZool 39, 1–9 (1999) [Google Scholar]
- 82.P. S. Brasileiro, thesis, EscolaNacional de Botânica Tropical, Rio de Janeiro (2013).
- 83.R. G. Bahia, thesis, EscolaNacional de Botânica Tropical, Rio de Janeiro (2014).
- 84.Pang R. K., The systematics of some Jamaican excavating sponges (Porifera). Postilla 161, 1–75 (1973). [Google Scholar]
- 85.Barros L. V., Santos G. G., Pinheiro U., Clathria (Clathria) Schmidt, 1862 from Brazil with description of a new species and a review of records (Poecilosclerida: Demospongiae: Porifera). Zootaxa 3640, 284–295 (2013). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/2/4/e1501252/DC1
fig. S1. Trawl and dredge casts on ships’ deck.
fig. S2. Sonographic images of the main reef megahabitats off the Amazon River mouth.
fig. S3. Carbonate fragments (A and B) and rhodoliths (C and D) sampled off the Amazon River mouth.
fig. S4. Representative species of sponges collected off the Amazon River mouth.
fig. S5. Representative species of corals and hydrocoral collected off the Amazon River mouth.
fig. S6. Representative reef fish species collected off the Amazon River mouth.
fig. S7. Fishing boat operating dinghies with hand lines and long lines near the shelf edge in the Northern Sector during the 2014 cruise.
fig. S8. Density of fishing operations targeting red snapper (L. purpureus) in 2010 off the Amazon mouth.
fig. S9. Depth profiles of salinity and DO measured during the R/V Cruzeiro do Sul cruise (September 2014).
fig. S10. Relative contribution of functions related to chemosynthesis and photosynthesis recorded outside, within, and underneath the Amazon River plume.
table S1. Algae recorded off the Amazon River mouth.
table S2. Sponges recorded off the Amazon River mouth.
table S3. Corals, hydrocorals, and gorgonians recorded off the Amazon River mouth.
table S4. Reef fish species recorded off the Amazon mouth [does not include species recorded at the Manuel Luis reefs; see de Moura et al. (23) and Rocha and Rosa (44)].
table S5. Oceanographic stations (primary data sources).
movie S1. Sampling the plume, subplume, and reefs off the Amazon river mouth during the NHo Cruzeiro do Sul cruise (2014).
Supplementary file. Shape files.