In situ coma measurements of 67P suggest that volatiles in the nucleus are stored as clathrates and pure condensates.
Keywords: Space science, comets
Abstract
Cometary nuclei are considered to most closely reflect the composition of the building blocks of our solar system. As such, comets carry important information about the prevalent conditions in the solar nebula before and after planet formation. Recent measurements of the time variation of major and minor volatile species in the coma of the Jupiter family comet 67P/Churyumov-Gerasimenko (67P) by the ROSINA (Rosetta Orbiter Spectrometer for Ion and Neutral Analysis) instrument onboard Rosetta provide insight into the possible origin of this comet. The observed outgassing pattern indicates that the nucleus of 67P contains crystalline ice, clathrates, and other ices. The observed outgassing is not consistent with gas release from an amorphous ice phase with trapped volatile gases. If the building blocks of 67P were formed from crystalline ices and clathrates, then 67P would have agglomerated from ices that were condensed and altered in the protosolar nebula closer to the Sun instead of more pristine ices originating from the interstellar medium or the outskirts of the disc, where amorphous ice may dominate.
INTRODUCTION
Although there is no doubt that cometary nuclei are, to a large extent, composed of H2O ice, it is the structure and phase of this H2O ice that provide insight into the place of origin, formation temperature, and evolution of icy agglomerates in the protosolar nebula (PSN). Whether cometary H2O ice originated directly from the interstellar medium (ISM) or was derived from the PSN has been a topic of active debate over the past three decades. An origin from the ISM implies formation at larger heliocentric distances, where pristine amorphous H2O ice could be maintained in the extremely low temperature, nonturbulent protoplanetary disc (1–5). An origin from the PSN implies formation at smaller heliocentric distances where crystalline ice could form at a temperature of ~150 K in the cooling PSN (6, 7). The phase in which other volatile species are stored in the nucleus strongly depends on the phase of the H2O ice.
Amorphous H2O ice very efficiently traps large amounts of volatiles in its highly porous structure [for example, (8, 9)]. The trapped volatiles are then released simultaneously as a result of changes in the ice structure. The major release of trapped gases occurs during the exothermic transition from amorphous to crystalline ice (8).
On the other hand, free crystalline H2O ice could enable various volatile species to be encaged as guest species within clathrate hydrates. In a clathrate, volatile gases are locked inside cage-like structures of crystalline H2O ice. In the cooling PSN, H2O will be present in its crystalline structure, with no or very little amorphous H2O. The fraction of amorphous ice in the PSN would be negligible because condensation of the formerly vaporized H2O occurs at significantly higher temperatures in the crystalline phase than those needed for the formation of amorphous H2O ice. By the time the temperature of the PSN was low enough for amorphous H2O ice formation, most volatile gases themselves either had condensed or were trapped in clathrates. These processes leave no or very little H2O and other volatile gases available when conditions were right for amorphous ice to form (2, 6, 10). Lacking in situ measurements of the internal structure and ice phase of cometary nuclei, the composition of the coma and the outgassing pattern of volatile species of major and minor abundance provide the best clues about the ice structure and, as a result, the origin of cometary nuclei.
Recent measurements by the ROSINA/DFMS (Rosetta Orbiter Spectrometer for Ion and Neutral Analysis/Double-Focusing Mass Spectrometer) (11) instrument onboard the Rosetta spacecraft showed a strongly heterogeneous coma in the major (12) and minor volatile species (13) of the Jupiter family comet (JFC) 67P/Churyumov-Gerasimenko (hereinafter 67P). In addition, a strong north-south asymmetry was present in the measured abundances during October 2014 (14).
Here, we use these recent coma measurements by ROSINA/DFMS over the September to October 2014 time period (12–14) to infer the structure of the icy agglomerates from which 67P was assembled. In particular, we restrict our analysis to data obtained when the poorly illuminated, winter southern hemisphere of the comet was in the view of Rosetta. The mid-to-high southern latitude scans revealed an interesting feature in the coma signal over two narrow sub-spacecraft longitude regions (12, 13). Over these narrow southern hemisphere longitude regions, the signals of CO2, CO, and C2H6 clearly deviated from the overall H2O signal, showing maxima at times of deep H2O minima (figs. S1 to S3). This telling feature was not present over the well-illuminated northern hemisphere, which was experiencing summer at the time. The higher temperatures experienced by the northern hemisphere make it difficult to reliably infer whether minor species are being released from different ice phases. The observed outgassing over the northern hemisphere with substantial H2O ice sublimation due to the higher temperatures would be consistent with gas release from either amorphous ice or clathrates, or both. Once the temperature is high enough for H2O sublimation, as was the case in the northern hemisphere during the period of observation, differences in outgassing due to gas release from amorphous ice, clathrate structures, and nucleus heterogeneity cannot be clearly distinguished. In contrast, temporal variations in the outgassing of volatiles from the southern (winter) hemisphere are well resolved and distinguishable from each other. At these lower temperatures, volatile outgassing is expected to be different based on the phase of H2O ice in the nucleus. Hence, the clearly distinguishable outgassing features in the southern hemisphere coma provide insight into the structure and history of 67P’s nucleus.
RESULTS
Of the five species studied, CH4 was the only volatile species whose signal showed no apparent correlation with either CO2 or H2O (figs. S1 to S3) over the southern hemisphere scans (13). This outgassing behavior provides important clues about the nucleus of 67P. Coma heterogeneity was attributed to heterogeneity in the nucleus (13), including possible variations in surface properties (14, 15). These previous results clearly imply the presence of some kind of heterogeneity related to the properties of the nucleus, though the observed time variation of all volatile species would be difficult to explain solely with such variation. A heterogeneous nucleus and/or surface properties would certainly affect the composition of the coma, though it is unclear what surface properties would be able to affect CH4 differently from the other species. The distinct time variation displayed by CH4 also strongly suggests that it does not sublimate from a segregated nonpolar ice phase, as proposed for CO, CO2, and C2H6 (13).
We are also able to exclude gas release from amorphous H2O ice based on the available measurements. As has been shown in multiple laboratory experiments, large amounts of volatile gases are released in the phase transition from amorphous H2O ice to the cubic phase of crystalline H2O between 135 and 155 K [for example, (8)]. This transition is followed by the transition of cubic crystalline to hexagonal crystalline ice between 160 and 175 K [for example, (16)]. In these stages, the trapped volatile gases are released simultaneously and independently of their own volatility, with the exception of H2O. The simultaneous release that would occur with amorphous ice does not agree with the ROSINA/DFMS observations, in which not all minor volatile species were released together from the southern hemisphere nucleus (13). For instance, no HCN and CH3OH outgassing was observed at times of CO2, CO, and C2H6 outgassing maxima over the southern hemisphere during these measurements. The outgassing pattern of CH4 that is distinct from other major and minor volatile species is also inconsistent with gas release from amorphous ice as currently understood from laboratory studies.
The presence of clathrates would explain the observed time variation of CH4 over the southern hemisphere (figs. S1 to S3). Stability curves of CH4 and C2H6 single-guest clathrates, as well as CH4, C2H6, and CO2 condensation curves, are shown in Fig. 1 as a function of the total PSN gas pressure. In the cooling nebula, CH4 clathrate forms at temperatures 20 to 30 K higher than the CH4 condensate (Fig. 1). Figure 1 shows equilibrium curves with gas-phase mole fractions relative to H2 in the PSN, derived specifically from the measured cometary gas/H2O ratios of 67P’s southern hemisphere (17). Note that the abundances of volatiles measured by ROSINA/DFMS in the coma of 67P do not necessarily represent bulk abundances in the nucleus. Thus, several ratios were tested based on other available measurements for known comets. Indeed, CH4 clathrates will preferentially form instead of condensed CH4 ice in every case for gas-phase CH4 mole fractions varied within the range of known comets. Thus, if CH4 were present as clathrate in the nucleus of 67P, then CH4 outgassing from the nucleus would occur when the ambient pressure drops below the equilibrium pressure of the CH4 clathrate at a given temperature. In a cometary environment, the largest contribution to the total pressure within the nucleus comes from H2O, unlike in the PSN, where the total pressure is mainly given by H2. To represent clathrate decomposition in the nucleus of 67P, we calculated the equilibrium curves of volatile species as a function of total pressure (Fig. 2). For this calculation, the gas-phase mole fractions relative to H2O were taken directly from ROSINA/DFMS measurements derived for the southern hemisphere (14). We see from these curves that the decomposition of CH4 clathrate begins at temperatures significantly lower than those needed for the sublimation of the host H2O cages (Fig. 2). Thus, if CH4 is present as a clathrate structure in the nucleus of 67P, it is not required for the outgassing pattern of CH4 to follow that of H2O or CO2.
Fig. 1. Equilibrium curves of clathrates and condensation in the PSN.
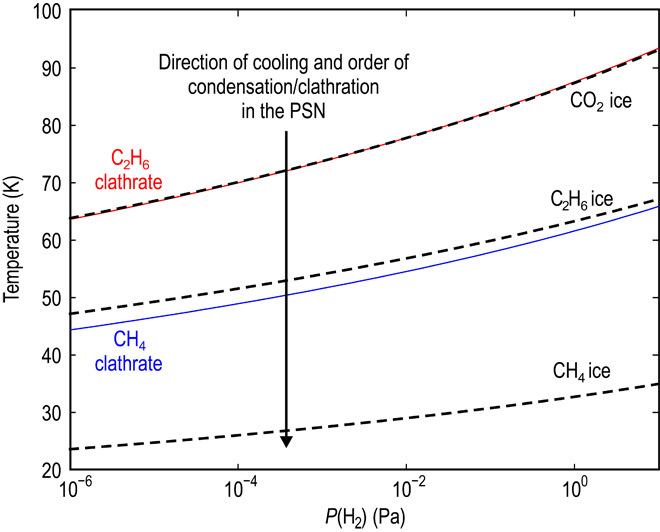
Equilibrium curves of C2H6 (red) and CH4 (blue) clathrates are shown with respect to the equilibrium curves of C2H6, CH4, and CO2 ices (black dashed lines) as a function of total nebular pressure. The arrow indicates the direction of cooling in the PSN. Above the clathrate stability/condensation curve, a volatile species exists in the gas phase. Below the clathrate stability/condensation curve, a volatile species may form clathrates or pure condensates. The gas-phase mole fractions relative to H2 were derived from the cometary X/H2O ratios (14) and solar system elemental abundances of O and H (40, 41).
Fig. 2. Equilibrium curves of clathrates and pure condensates in the cometary environment.
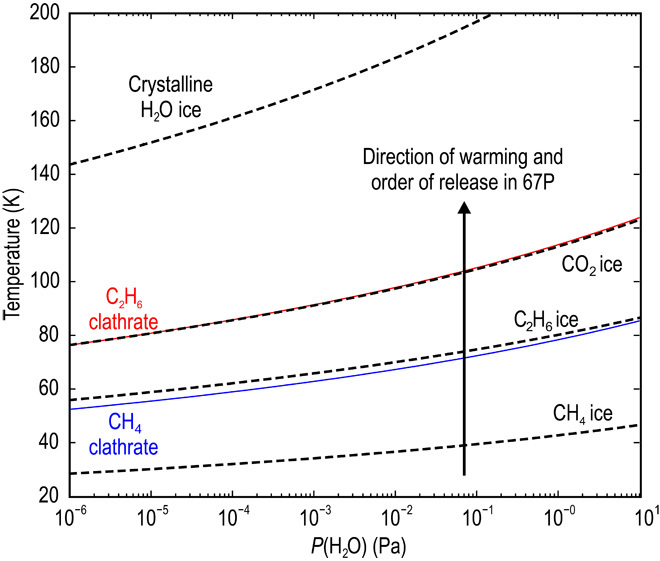
The equilibrium curves are shown here as a function of total H2O pressure. Decomposition of the clathrate structures and sublimation of pure ices occur at temperatures above the equilibrium curves, whereas clathrates/pure condensates remain stable below the curves. Mole fractions of each species shown are directly taken from ROSINA/DFMS measurements of the coma of 67P (14).
The curves in Fig. 2 are in agreement with the ROSINA/DFMS measurements that show poor correlations of CH4 with CO2 and H2O (R2 of 0.18 and 0.57, respectively) [figs. S1 to S3 (13)]. However, the same ROSINA/DFMS observations show that C2H6 follows closely and correlates very well with CO2 in the southern hemisphere (R2 of 0.77), unlike CH4 (13). At the same time, Fig. 1 suggests that C2H6 also preferentially forms clathrates instead of a pure condensate in the cooling PSN. We conclude that both CH4 and C2H6 are present as clathrates in the nucleus of 67P. In contrast with CH4, the strong correlation between C2H6 and CO2 is caused by the fact that the dissociation temperature of the C2H6 clathrate is coincidentally close to the sublimation temperature of CO2 ice. There is strong correlation between C2H6 and CO2, as shown by their overlapping equilibrium curves in Figs. 1 and 2. Outgassing as a result of decomposition of CH4 and C2H6 clathrates is in full agreement with the time variation of the volatile species in the southern hemisphere coma of 67P measured by ROSINA/DFMS (13). At the same time, the implications of our results do not depend on the measured abundance ratios in the coma of 67P, considering that abundances in the coma do not necessarily represent bulk abundances in the nucleus. Varying the abundances of CH4, C2H6, and CO2 within the range of other known comets results in the same ordering of species as shown in Figs. 1 and 2. C2H6 and CH4 clathrates always form before their pure condensates, that is, at temperatures about 20 K higher. In addition, C2H6 clathrates form at about the same time (within <1 K) as CO2 ice (fig. S4). In addition, the equilibrium pressure curves of the JFCs (67P and Hartley 2) have very similar temperature dependences, as do those of comets Halley and Hale-Bopp, which belong to the Oort cloud (fig. S4). On the basis of our results, clathrate and pure condensate formation temperatures change as a function of the abundance ratio used. Thus, although the specific formation temperature of the nucleus of 67P cannot be constrained reliably, our results provide a strong argument for the presence of CH4 and C2H6 clathrates in the nucleus of 67P.
DISCUSSION
Circumstantial evidence for the existence of amorphous ice in comets was previously given based on the onset of cometary activity at large heliocentric distances [for example, (18)], as well as distant and near-perihelion outbursts (19). These phenomena could be explained by the crystallization of amorphous ice, as was proposed for the distant outbursts of 1P/Halley (20) and Hale-Bopp (21–23), the perihelion activity of 2060 Chiron (24), and the erratic activity of comet Schwassmann-Wachmann (25). In the case of comet 17P/Holmes, however, the crystallization of H2O ice was likely not responsible for its 2007 post-perihelion megaburst (26). Recently, the exothermic phase transition of amorphous to crystalline H2O ice has been proposed as a potential cause for the observed active pits in the nucleus of 67P (27). At the same time, crystallization of amorphous H2O ice is not the only process capable of producing outbursts and pits. Pressure pulses resulting in outbursts could also be caused by clathrate decomposition at depths comparable to the depths of observed pits on time scales shorter than the lifetime of 67P, as has been shown recently (28).
The kinetics of clathrate formation at low temperatures are not well known, but the nature of the differences in prevailing thermodynamic conditions (P, T) in the PSN versus the ISM makes it more likely that clathrate will form in the former as opposed to the latter (5). Clathrates could also form later in the nucleus in the presence of free crystalline H2O ice, even if the original ice phase of 67P was amorphous H2O. If clathrates formed in the nucleus of 67P at a later period after the comet’s formation, then clathration would have had to occur on significantly shorter time scales than the formation of crystalline H2O and clathrate ice grains in the PSN. Unfortunately, the currently loosely constrained kinetics of clathrate formation (29) do not allow us to distinguish between a nebular and a postnebular formation of clathrate in 67P.
Our results do not exclude the existence of amorphous ice in the solar nebula, which may have been quite abundant in the low-temperature, outer regions of the disc [for example, (4, 30)]. In addition, the presence of clathrates in the nucleus of 67P does not prove that comets, including 67P, formed out of clathrates. Yet, our results, along with other recent efforts supporting the presence of N2, Ar, and CO clathrates in the nucleus of 67P (31, 32), suggest a picture of the origin of 67P that is different from what was envisaged before, where crystalline H2O ice, pure condensates, and clathrates, rather than amorphous ice, may play a leading role. If clathrates did form in the PSN, then 67P would have agglomerated from ices condensed and altered in the PSN instead of pristine ices from the ISM or the outskirts of the disc, where amorphous ice may dominate (4, 5). This idea is consistent with scenarios arguing that the building blocks of giant planets and satellites were formed in a similar manner in the nebula (33, 34) and that Titan accreted from clathrate-rich planetesimals originating from Saturn’s feeding zone (3). Dynamical model results suggest that both JFCs and Oort cloud comets may have formed in the same environment extending over heliocentric distances of tens of astronomical units (35). If the nucleus of 67P agglomerated from crystalline ices and clathrates, then it likely formed closer to the Sun than previously considered for JFCs (35). This also implies that other comets with measured D/H ratios lower than that of 67P (36) should have been formed from crystalline ices and clathrates, because they probably formed closer to the Sun than 67P (37). In any case, the presence of clathrates in 67P would indicate that the snow line, whose position is still loosely constrained (38), was located beyond the present asteroid belt in the PSN. This would suggest that comets formed from at least two distinct reservoirs: a crystalline H2O reservoir located inside the disc and an amorphous H2O ice reservoir located outside the disc (5). Future direct sampling of ices from other comets will be crucial in locating the boundary between these reservoirs and will better constrain the phase of ice in various comets at the time of their formation.
MATERIALS AND METHODS
Calculation of pure condensate and clathrate equilibrium curves
Condensation curves of CH4, C2H6, and CO2 were calculated based on Fray and Schmitt (39). The fitting expression used is . Fitting parameters Ai for CH4, C2H6, and CO2 are summarized in table S2. The condensation curve of H2O vapor was calculated via the following equations (39)
Pt and Tt are the triple point pressure and temperature of H2O, respectively, and parameters ei are given in table S3. Pcond and Pt are expressed in bar; T and Tt are expressed in kelvin.
The gas-phase X/H2 ratios in the PSN were determined using the X/H2O ratios measured for 67P, multiplied by the estimated H2O/H2 ratio in the PSN. The H2O/H2 ratio was derived from the elemental O and H abundances in the solar system (40), assuming that 57% of H2O was present in the form of H2O ice (41), leaving 43% of the total solar (O/H2)⊙ to exist in the gas phase. Because (O/H2)⊙ = 0.0011 (40), and assuming that O is in the form of H2O, we derive H2O/H2 = 0.5 × (O/H2)⊙ = 2.15 × 10−4. Then, the X/H2 ratio is calculated as the cometary X/H2O times the calculated H2O/H2.
To test the sensitivity of the clathrate and condensate equilibrium curves, the gas-phase X/H2O was varied within the range of known comets (14) and taken relative to H2 as described above. Varying the gas-phase X/H2O (hence, the X/H2) does not change the ordering of the curves as a function of temperature, and the CO2 condensate and C2H6 clathrate curves match each other well for the variety of X/H2O ranges of known comets. To demonstrate the latter point, equilibrium curves of four selected comets are shown in fig. S4.
Supplementary Material
Acknowledgments
We gratefully acknowledge the efforts of the ROSINA team, and all scientists, engineers, and technicians involved in the Rosetta mission, without whom this work would not have been possible. We also thank two anonymous reviewers for their valuable comments, which helped improve this paper. Funding: A.L.-K., M.H., and S.A.F. acknowledge support from the U.S. National Aeronautics and Space Administration Jet Propulsion Laboratory (subcontract no. 1496541). The work by O.M. was carried out thanks to the support of the A*MIDEX project (no. ANR-11-IDEX-0001-02) funded by the “Investissements d’Avenir” French government program, managed by the French National Research Agency. O.M. also acknowledges support from CNES (Centre National d’Études Spatiales). J.I.L. was supported by the JWST (James Webb Space Telescope) project. The work by K.E.M. was supported, in part, by the U.S. National Aeronautics and Space Administration through contract no. 1345493 with the Jet Propulsion Laboratory, California Institute of Technology. B.M. was supported by CNES and by the European Research Council (grant no. 267255). P.W. and M.R. were funded by the State of Bern, the Swiss National Science Foundation, and the European Space Agency PRODEX Program. Author contributions: A.L.-K. analyzed and interpreted the data, and wrote the manuscript; O.M. interpreted the data and wrote the manuscript; M.H. analyzed and interpreted the data; S.A.F. interpreted the data and supervised the work; J.I.L., B.M., K.E.M., P.W., and M.R. interpreted the data. All authors discussed the results and commented on the paper. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data used to obtain the conclusions in this paper are presented in the paper and/or the Supplementary Materials. Other data may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/2/4/e1501781/DC1
Fig. S1. Time variation of major and minor volatile species from ROSINA/DFMS on 18 September 2014.
Fig. S2. Time variation of major and minor volatile species from ROSINA/DFMS on 29 September 2014.
Fig. S3. Time variation of major and minor volatile species from ROSINA/DFMS on 11 October 2014.
Fig. S4. Equilibrium curves in the PSN for various known comets.
Table S1. Fitting parameters used in calculating clathrate equilibrium curves of CH4 and C2H6.
Table S2. Fitting coefficients used to calculate condensation curves.
Table S3. Fitting coefficients for the condensation pressure of crystalline H2O ice.
REFERENCES AND NOTES
- 1.Klinger J., Influence of a phase transition of ice on the heat and mass balance of comets. Science 209, 271–272 (1980). [DOI] [PubMed] [Google Scholar]
- 2.Mousis O., Gautier D., Bockelée-Morvan D., Robert F., Dubrulle B., Drouart A., Constraints on the formation of comets from D/H ratios measured in H2O and HCN. Icarus 148, 513–525 (2000). [Google Scholar]
- 3.Mousis O., Lunine J. I., Thomas C., Pasek M., Marboeuf U., Alibert Y., Ballenegger V., Cordier D., Ellinger Y., Pauzat F., Picaud S., Clathration of volatiles in the solar nebula and implications for the origin of Titan’s atmosphere. Astrophys. J. 691, 1780–1786 (2009). [Google Scholar]
- 4.Ciesla F. J., The phases of water ice in the solar nebula. Astrophys. J. 784, L1 (2014). [Google Scholar]
- 5.Willacy K., Alexander C., Ali-Dib M., Ceccarelli C., Charnley S. B., Doronin M., Ellinger Y., Gast P., Gibb E., Milam S. N., Mousis O., Pauzat E., Tornow C., Wirstrom E. S., Zicler E., The composition of the protosolar disk and the formation conditions for comets. Space Sci. Rev. 197, 151–190 (2015). [Google Scholar]
- 6.Kouchi A., Yamamoto T., Kozasa T., Kuroda T., Greenberg J. M., Conditions for condensation and preservation of amorphous ice and crystallinity of astrophysical ices. Astron. Astrophys. 290, 1009–1018 (1994). [Google Scholar]
- 7.Chick K. M., Cassen P., Thermal processing of interstellar dust grains in the primitive solar environment. Astrophys. J. 477, 398–409 (1997). [Google Scholar]
- 8.Bar-Nun A., Laufer D., First experimental studies of large samples of gas-laden amorphous “cometary” ices. Icarus 161, 157–163 (2003). [Google Scholar]
- 9.Notesco G., Bar-Nun A., Owen T., Gas trapping in water ice at very low deposition rates and implications for comets. Icarus 162, 183–189 (2003). [Google Scholar]
- 10.Iro N., Gautier D., Hersant F., Bockelée-Morvan D., Lunine J. I., An interpretation of the nitrogen deficiency in comets. Icarus 161, 511–532 (2003). [Google Scholar]
- 11.Balsiger H., Altwegg K., Bochsler P., Eberhardt P., Fischer J., Graf S., Jäckel A., Kopp E., Langer U., Mildner M., Müller J., Riesen T., Rubin M., Scherer S., Wurz P., Wüthrich S., Arijs E., Delanoye S., De Keyser J., Neefs E., Nevejans D., Rème H., Aoustin C., Mazelle C., Médale J.-L., Sauvaud J. A., Berthelier J.-J., Bertaux J.-L., Duvet L., Illiano J.-M., Fuselier S. A., Ghielmetti A. G., Magoncelli T., Shelley E. G., Korth A., Heerlein K., Lauche H., Livi S., Loose A., Mall U., Wilken B., Gliem F., Fiethe B., Gombosi T. I., Block B., Carignan G. R., Fisk L. A., Waite J. H., Young D. T., Wollnik H., ROSINA—Rosetta Orbiter Spectrometer for Ion and Neutral Analysis. Space Sci. Rev. 128, 745–801 (2007). [Google Scholar]
- 12.Hässig M., Altwegg K., Balsiger H., Bar-Nun A., Berthelier J. J., Bieler A., Bochsler P., Briois C., Calmonte U., Combi M., De Keyser J., Eberhardt P., Fiethe B., Fuselier S. A., Galand M., Gasc S., Gombosi T. I., Hansen K. C., Jäckel A., Keller H. U., Kopp E., Korth A., Kührt E., Le Roy L., Mall U., Marty B., Mousis O., Neefs E., Owen T., Rème H., Rubin M., Sémon T., Tornow C., Tzou C.-Y., Waite J. H., Wurz P., Time variability and heterogeneity in the coma of 67P/Churyumov-Gerasimenko. Science 347, aaa0276 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Luspay-Kuti A., Hässig M., Fuselier S. A., Mandt K. E., Altwegg K., Balsiger H., Gasc S., Jäckel A., Le Roy L., Rubin M., Tzou C.-Y., Wurz P., Mousis O., Dhooghe F., Berthelier J. J., Fiethe B., Gombosi T. I., Mall U., Composition-dependent outgassing of comet 67P/Churyumov-Gerasimenko from ROSINA/DFMS. Astron. Astrophys. 583, A4 (2015). [Google Scholar]
- 14.Le Roy L., Altwegg K., Balsiger H., Berthelier J.-J., Bieler A., Briois C., Calmonte U., Combi M. R., De Keyser J., Dhooghe F., Fiethe B., Fuselier S. A., Gasc S., Gombosi T. I., Hässig M., Jäckel A., Rubin M., Tzou C.-Y., Inventory of the volatiles on comet 67P/Churyumov-Gerasimenko from Rosetta/ROSINA. Astron. Astrophys. 583, A1 (2015). [Google Scholar]
- 15.Keller H. U., Mottola S., Davidsson B., Schröder S. E., Skorov Y., Kührt E., Groussin O., Pajola M., Hviid S. F., Preusker F., Scholten F., A’Hearn M. F., Sierks H., Barbieri C., Lamy P., Rodrigo R., Koschny D., Rickman H., Barucci M. A., Bertaux J.-L., Bertini I., Cremonese G., Da Deppo V., Debei S., De Cecco M., Fornasier S., Fulle M., Gutiérrez P. J., Ip W.-H., Jorda L., Knollenberg J., Kramm J. R., Küppers M., Lara L. M., Lazzarin M., Lopez Moreno J. J., Marzari F., Michalik H., Naletto G., Sabau L., Thomas N., Vincent J.-B., Wenzel K.-P., Agarwal J., Güttler C., Oklay N., Tubiana C., Insolation, erosion, and morphology of comet 67P/Churyumov-Gerasimenko. Astron. Astrophys. 583, A34 (2015). [Google Scholar]
- 16.Bar-Nun A., Kleinfeld I., Kochavi E., Trapping of gas mixtures by amorphous water ice. Phys. Rev. B Condens. Matter 38, 7749–7754 (1988). [DOI] [PubMed] [Google Scholar]
- 17.Materials and methods are available as supplementary materials on Science Online.
- 18.Meech K. J., Pittichov J., Bar-Nun A., Notesco G., Laufer D., Hainaut O. R., Lowry S. C., Yeomans D. K., Pitts M., Activity of comets at large heliocentric distances pre-perihelion. Icarus 201, 719–739 (2009). [Google Scholar]
- 19.D. Prialnik, J. Benkhoff, M. Podolak, Modeling the structure and activity of comet nuclei, in Comets II, M. C. Festou, H. U. Keller, H. A. Weaver, Eds. (University of Arizona Press, Tucson, AZ, 2004), pp. 359–387. [Google Scholar]
- 20.Prialnik D., Bar-Nun A., Crystallization of amorphous ice as the cause of comet P/Halley’s outburst at 14 AU. Astron. Astrophys. 258, L9–L12 (1992). [PubMed] [Google Scholar]
- 21.Prialnik D., Modelling gas and dust release from Comet Hale–Bopp. Earth Moon Planets 77, 223–230 (1999). [Google Scholar]
- 22.Prialnik D., Modeling the comet nucleus interior; application to Comet C/1995 O1 Hale-Bopp. Earth Moon Planets 89, 27–52 (2000). [Google Scholar]
- 23.Capria M. T., Coradini A., De Sanctis M. C., C/1995 O1 Hale–Bopp: Short and long distance activity from a theoretical model. Earth Moon Planets 90, 217–225 (2002). [Google Scholar]
- 24.Prialnik D., Brosch N., Ianovici D., Modelling the activity of 2060 Chiron. Mon. Not. R. Astron. Soc. 276, 1148–1154 (1995). [Google Scholar]
- 25.Klinger J., Levasseur-Regourd A.-C., Bouziani N., Enzian A., Towards a model of cometary nuclei for engineering studies for future space missions to comets. Planet. Space Sci. 44, 637–653 (1996). [Google Scholar]
- 26.Kossacki K. J., Szutowicz S., Crystallization of ice in comet 17P/Holmes: Probably not responsible for the explosive 2007 megaburst. Icarus 207, 320–340 (2010). [Google Scholar]
- 27.Vincent J.-B., Bodewits D., Besse S., Sierks H., Barbieri C., Lamy P., Rodrigo R., Koschny D., Rickman H., Keller H. U., Agarwal J., A’Hearn M. F., Auger A.-T., Barucci M. A., Bertaux J.-L., Bertini I., Capanna C., Cremonese G., Da Deppo V., Davidsson B., Debei S., De Cecco M., El-Maarry M. R., Ferri F., Fornasier S., Fulle M., Gaskell R., Giacomini L., Groussin O., Guilbert-Lepoutre A., Gutierrez-Marques P., Gutiérrez P. J., Güttler C., Hoekzema N., Höfner S., Hviid S. F., Ip W.-H., Jorda L., Knollenberg J., Kovacs G., Kramm R., Kührt E., Küppers M., La Forgia F., Lara L. M., Lazzarin M., Lee V., Leyrat C., Lin Z.-Y., Moreno J. J. L., Lowry S., Magrin S., Maquet L., Marchi S., Marzari F., Massironi M., Michalik H., Moissl R., Mottola S., Naletto G., Oklay N., Pajola M., Preusker F., Scholten F., Thomas N., Toth I., Tubiana C., Large heterogeneities in comet 67P as revealed by active pits from sinkhole collapse. Nature 523, 63–66 (2015). [DOI] [PubMed] [Google Scholar]
- 28.Mousis O., Guilbert-Lepoutre A., Brugger B., Jorda L., Kargel J. S., Bouquet A., Auger A.-T., Lamy P., Vernazza P., Thomas N., Sierks H., Pits formation from volatile outgassing on 67P/Churyumov-Gerasimenko. Astrophys. J. 814, L5 (2015). [Google Scholar]
- 29.Lunine J. I., Stevenson D. J., Thermodynamics of clathrate hydrate at low and high pressures with application to the outer solar system. Astrophys. J. Suppl. Ser. 58, 493–531 (1985). [Google Scholar]
- 30.Monga N., Desch S., External photoevaporation of the solar nebula: Jupiter’s noble gas enrichments. Astrophys. J. 798, 12 (2015). [Google Scholar]
- 31.Lectez S., Simon J. M., Mousis O., Picaud S., Altwegg K., Rubin M., Salazar J. M., ~32-70 K formation temperature range for the ice grains agglomerated by comet 67P/Churyumov-Gerasimenko. Astrophys. J. 805, L1 (2015). [Google Scholar]
- 32.Mousis O., Lunine J. I., Luspay-Kuti A., Guillot T., Marty B., Ali-Dib M., Wurz P., Altwegg K., Bieler A., Hässig M., Rubin M., Vernazza P., Waite J. H., A protosolar nebula origin for the ices agglomerated by comet 67P/Churyumov-Gerasimenko. Astrophys. J. 819, L33 (2016). [Google Scholar]
- 33.Mousis O., Lunine J. I., Picaud S., Cordier D., Volatile inventories in clathrate hydrates formed in the primordial nebula. Faraday Discuss. 147, 509–525 (2010). [DOI] [PubMed] [Google Scholar]
- 34.Mousis O., Lunine J. I., Fletcher L. N., Mandt K. E., Ali-Dib M., Gautier D., Atreya S., New insights on Saturn’s formation from its nitrogen isotopic composition. Astrophys. J. 796, L28 (2014). [Google Scholar]
- 35.Brasser R., Morbidelli A., Oort cloud and scattered disc formation during a late dynamical instability in the solar system. Icarus 225, 40–49 (2013). [Google Scholar]
- 36.Altwegg K., Balsiger H., Bar-Nun A., Berthelier J. J., Bieler A., Bochsler P., Briois C., Calmonte U., Combi M., De Keyser J., Eberhardt P., Fiethe B., Fuselier S., Gasc S., Gombosi T. I., Hansen K. C., Hässig M., Jäckel A., Kopp E., Korth A., LeRoy L., Mall U., Marty B., Mousis O., Neefs E., Owen T., Rème H., Rubin M., Sémon T., Tzou C.-Y., Waite H., Wurz P., 67P/Churyumov-Gerasimenko, a Jupiter family comet with a high D/H ratio. Science 347, 1261952 (2015). [DOI] [PubMed] [Google Scholar]
- 37.Kavelaars J. J., Mousis O., Petit J.-M., Weaver H. A., On the formation location of Uranus and Neptune as constrained by dynamical and chemical models of comets. Astrophys. J. 734, L30 (2011). [Google Scholar]
- 38.K. M. Pontoppidan, C. Salyk, E. A. Bergin, S. Brittain, B. Marty, O. Mousis, K. I. Ӧberg, Volatiles in protoplanetary disks, in Protostars and Planets VI, H. Beuther, R. Klessen, C. Dullemond, Th. Henning, Eds. (University of Arizona Press, Tucson, AZ, 2014), p. 363. [Google Scholar]
- 39.Fray N., Schmitt B., Sublimation of ices of astrophysical interest: A bibliographic review. Planet. Space Sci. 57, 2053–2080 (2009). [Google Scholar]
- 40.K. Lodders, H. Palme, H.-P. Gail, Abundances of elements in the solar system, in Landolt-Börnstein, New Series, J. E. Trümper, Ed. (Springer-Verlag, Berlin, 2009), vol. VI/4B, chap. 4.4. [Google Scholar]
- 41.Lodders K., Solar system abundances and condensation temperatures of the elements. Astrophys. J. 591, 1220–1247 (2003). [Google Scholar]
- 42.Thomas C., Mousis O., Ballenegger V., Picaud S., Clathrate hydrates as a sink of noble gases in Titan’s atmosphere. Astron. Astrophys. 474, L17–L20 (2007). [Google Scholar]
- 43.Marboeuf U., Fray N., Brissaud O., Schmitt B., Bockelée-Morvan D., Gautier D., Equilibrium pressure of ethane, acetylene, and krypton clathrate hydrates below the freezing point of water. J. Chem. Eng. Data 57, 3408–3415 (2012). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/2/4/e1501781/DC1
Fig. S1. Time variation of major and minor volatile species from ROSINA/DFMS on 18 September 2014.
Fig. S2. Time variation of major and minor volatile species from ROSINA/DFMS on 29 September 2014.
Fig. S3. Time variation of major and minor volatile species from ROSINA/DFMS on 11 October 2014.
Fig. S4. Equilibrium curves in the PSN for various known comets.
Table S1. Fitting parameters used in calculating clathrate equilibrium curves of CH4 and C2H6.
Table S2. Fitting coefficients used to calculate condensation curves.
Table S3. Fitting coefficients for the condensation pressure of crystalline H2O ice.


