Abstract
Background & Aims
Cirrhosis is associated with significant pain and disability, the etiologies of which are poorly understood. We investigated whether the pain and disability in patients with cirrhosis are associated with systemic inflammation and psychiatric symptoms.
Methods
In a prospective study, we recruited 193 patients with cirrhosis caused by hepatitis C virus (HCV) infection, non-alcoholic steatohepatitis (NASH), or alcohol from the hepatology clinic at the University of Pittsburgh. Patients were assessed using the McGill Pain Questionnaire, Hospital Anxiety and Depression Scale (HADS), Pittsburgh Sleep Quality Index (PSQI), and the Pain Disability Index. Serum samples were collected and markers of inflammation were measured using standardized Luminex assays. We evaluated factors associated with pain, pain-related disability, and chronic opioid use using multivariable regression models.
Results
Pain was reported by 79% of patients, pain-related disability by 75%, and depression and/or anxiety by 47%; the average Model for End-Stage Liver Disease (MELD) score was=12±5. Serum samples from 58% percent of patients had increased levels of c-reactive protein (CRP). Opioids were prescribed for 30% of patients with pain. In multivariate analysis, factors significantly associated with pain included younger age (odds ratio [OR]/year=0.93; 95% confidence interval [CI], 0.90–0.99), serum level of interleukin 6 (OR=1.63, 95% CI=1.09–2.58), HADS score (OR/point=1.14; 95% CI, 1.07–1.24), and etiology (HCV infection vs alcohol, OR=3.70; 95% CI, 1.27–11.11). Disability scores were significantly related to psychiatric symptoms (incidence rate ratio [IRR]/point=1.04; 95% CI, 1.02–1.05), prescription opioid use (IRR=1.49; 95% CI, 1.14–1.94), MELD score (IRR/point=1.02; 95% CI, 1.0001–1.05), level of CRP (IRR=1.13; 95% CI, 1.02–1.24), and pain severity (IRR/point=1.19; 95% CI, 1.08–1.32).
Conclusion
Pain and disability are common among patients with cirrhosis, and are associated with inflammation, psychiatric symptoms, and opioid use, which are potentially modifiable. Although opioids are commonly used to treat pain, psychiatric symptoms and inflammation might also be treatment targets in this population.
Keywords: IL6, CRP, opioid use, pain management, cirrhosis, hepatitis C
Introduction
Pain is common among patients with cirrhosis1, 2 and is associated with poor quality of life3 and increased healthcare utilization.4 Pain adds to the functional impairment of cirrhosis with its high rate of disability.5 The treatment of pain and poses a significant treatment dilemma. Current guidelines are based on limited data,6 and evidence-based approaches to pain management in this high risk population have yet to be established. Subsequently, many patients receive prescription opioids,2 though experts caution about their regular use.6
Understanding pain mechanisms may guide development of alternative treatment strategies. Compared with healthy controls, hepatitis C (HCV)-infected patients have more pain and functional impairment, with associated increases in pro-inflammatory markers.7 Progression of cirrhosis has been linked to increasing C-reactive protein (CRP) and pro-inflammatory cytokines.8 These markers also correlate with pain, sleep disorders, and depression, indicating a possible connection between these often comorbid entities via inflammation.9, 10 Thus, pain may indeed be a consequence of the systemic inflammation related to liver disease, which together with other physical and emotional symptoms, may contribute to worsening function.
To date there have been no prospective assessments of modifiable factors associated with pain and pain-related disability in cirrhosis. Thus we aimed to 1) understand the relationship between pain, inflammation, and psychiatric symptoms, 2) evaluate the factors associated with abdominal pain, 3) understand the current management of pain and predictors of opioid use, and 4) assess the predictors of pain-related disability. We hypothesized that pain and disability in patients with cirrhosis would be associated with inflammation and psychiatric symptoms.
Methods
Subjects
We screened the medical records of patients scheduled for outpatient hepatology appointments. Patients ≥18 years old with cirrhosis due to alcohol, HCV, or non-alcoholic steatohepatitis (NASH), as defined by a hepatologist, were eligible. Exclusion criteria included any known causes of abdominal pain and/or systemic inflammation (eg.cancer) (Figure 1). Clinicians were asked to refer eligible patients who were able to consent. Once consented, participants completed study instruments and had blood drawn. The study was approved by the University of Pittsburgh Internal Review Board (PRO12060413).
Figure 1.
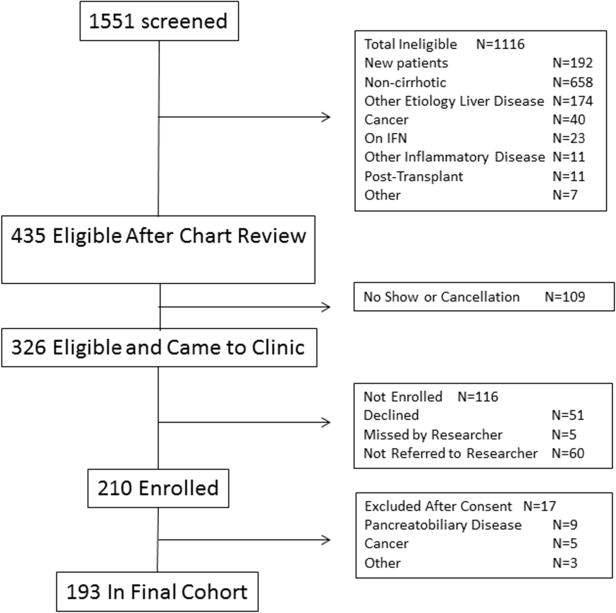
Subject Enrollment
Surveys and Measurements
Participants self-reported demographic and substance abuse information. Pain medications were self-reported and confirmed in the medical record. Prescription opioids were converted into IV morphine equivalents using standard conversion tables. Subjects were asked to describe the potential presence, treatment, and presumed cause of their pain using free texting. When they wrote “liver” or “cirrhosis” complications as the cause of their pain this was coded as “liver-related”. Participants completed the McGill Pain Questionnaire (MPQ)11, Hospital Anxiety and Depression Scale (HADS)12, Pittsburgh Sleep Quality Inventory (PSQI)13, and the Pain Disability Index (PDI).14, 15 The ROME III criteria16 functional abdominal pain module was used to evaluate functional abdominal pain and irritable bowel syndrome (IBS)17. The MPQ provides a pain intensity scale from 0–5, pain descriptors (including location), and a Pain Rating Index assigned to each descriptor. These descriptors can be categorized on subscales as sensory, affective, or evaluative. The HADS quantifies symptoms of depression and anxiety and can be used as a continuous scale of 0–42, or with scores of ≥8 on either sub-scale as thresholds for disorders.18 PSQI scores range from 0–21, with a threshold of 5 having a 90% sensitivity and 87% specificity for identifying sleep disordered patients.19 The questions in the PDI assess the degree to which pain disrupts aspects of daily life and range from 0–10. The burden of comorbid disease was quantified using the Charlson Comorbidity Index20 using medical record review.
CT scans obtained for routine clinical care were assessed, with MRIs or ultrasounds substituted when necessary. The radiologists’ description of spleen size determined whether patients had splenomegaly. Laboratory values in closest proximity to the time of the visit were used to calculate Model for End-Stage Liver Disease (MELD) scores. Clinician notes were reviewed in order to determine whether patients had a history of ascites, active ascites, or encephalopathy. The most recent endoscopy report was evaluated to assess for esophageal varices.
Serum Analysis
Participant blood (10ml) was drawn after their clinic visits. Vials were left upright at room temperature for 60 minutes prior to centrifuging at 3000 RPM for 11 minutes. Serum was stored at −80° C. Commercially available Luminex assays were used to evaluate CRP, TNFα, IL-1β, and IL-6 levels in the University of Pittsburgh Luminex Core Laboratory.21 Values were standardized based on established protocols of the Core Laboratory. Normative data were derived from pooled and individual volunteer samples.
Statistical Analysis
Analyses were completed using R Version 2.15.222. Differences in the baseline characteristics were assessed for participants with and without pain using Chi-square, Student’s T, and Wilcoxon Rank-Sum tests for categorical, continuous, and non-normal variables, respectively. Characteristics that differed with a p<0.2 were included in the pool of factors in the logistic regression analysis, which was completed using AIC optimization with the MASS package23 in order to reach a parsimonious final model of factors associated with pain and opioid use. Inflammatory markers were normalized using a natural log transformation for statistical evaluation. Abdominal pain was defined by the MPQ and assessed using logistic regression. Disability scores from PDI questions were assessed using negative binomial regression models. In order to further validate our findings, models were made assessing predictors of pain severity using negative binomial regression. All models were checked for multicollinearity using a pre-specified variance inflation factor (VIF) of 5.
Results
Among the final cohort of 193 participants, cirrhosis was due to HCV in 78, NASH in 66, and ETOH in 49. The cohort was 40% female with an average age of 58±9 and average MELD of 12±5 (Table 1). Depression and/or anxiety were found in 47%. The mean PSQI score was 10.4±4.1 (>5 represents disordered sleep).19 Pain was found in 79% of the cohort and clustered with inflammation and depression (Figure 2). Aside from IL-1β, all inflammatory markers were significantly(p<0.05) correlated with MELD scores. Among the 49 subjects with alcohol as their primary etiology, 2 were actively drinking at the time of assessment. Among the subjects with HCV, 15 had responded to prior HCV therapy, 21 were treatment naïve, and 31 had failed prior therapy. Among the subjects, 11 patients had prior TIPS, one of whom had recurrent ascites. Sixty-eight had diuretic-controlled ascites at the time of their visit, and 25 had clinically evident ascites on exam.
Table 1.
Baseline Characteristics*
| Characteristic | No Pain (N=41) | Pain (N=152) | P | No Abdominal pain (N=113) | Abdominal Pain (N=80) | P | |
|---|---|---|---|---|---|---|---|
| Age (mean,yrs) | 60.7±8.8 | 57.1±8.9 | 0.03 | 59.1±8.9 | 56.2±8.8 | 0.02* | |
| Female n(%) | 14 (34) | 63 (41) | 0.50 | 39 (35) | 38 (48) | 0.10 | |
| Non-white n(%) | 3 (7) | 14 (9) | 1.00 | 12 (11) | 5 (6) | 0.44 | |
| Latino n(%) | 0 | 4 (3) | 0.58 | 2 (2) | 2 (3) | 1.00 | |
| Income (median,$10k/person) | 2.5(1.9,3.5) | 1.5(1.0,3.3) | 0.08 | 2.3 (1.0,3.8) | 1.5 (1.0,2.5) | 0.01* | |
| BMI (mean, kg/m2) | 32.1±4.6 | 29.1±5.7 | 0.21 | 30.8±4.8 | 27.4±8.6 | 0.57 | |
| Tobacco n(%) | 7 (17) | 35 (23) | 0.54 | 23 (20) | 19 (24) | 0.70 | |
| Heroin/narcotics n(%) | 4 (10) | 26 (17) | 0.37 | 13 (12) | 17 (21) | 0.10 | |
| Illicit drug use n(%) | 13 (32) | 56 (37) | 0.67 | 42 (37) | 27 (34) | 0.74 | |
| ETOH overuse n(%) | 24 (59) | 76 (50) | 0.34 | 68 (60) | 32 (40) | <0.01* | |
| CCI (mean) | 3.8±1.0 | 4.0±1.2 | 0.33 | 3.9±1.1 | 4.0±1.2 | 0.59 | |
| Disability (median PDI) | 0 (0,0) | 4 (2,7) | <0.01 | 2 (0,5) | 5 (2,7) | <0.01* | |
| Liver-Related Variables | |||||||
| Etiology of Liver Diseas | 0.04* | 0.01* | |||||
| HCV n(%) | 13 (32) | 65 (43) | 45 (40) | 33 (41) | |||
| ETOH n(%) | 17 (42) | 32 (21) | 37 (33) | 12 (15) | |||
| NASH n(%) | 11 (27) | 55 (36) | 31 (27) | 35 (44) | |||
| MELD (Mean±sd) | 12.3±5.2 | 12.0±4.7 | 0.79 | 12.2±5.2 | 12.0±4.2 | 0.80 | |
| Child Score (Mean±sd) | 7.4±2 | 7.7±2 | 0.43 | 7.4±2 | 7.9±2 | 0.09 | |
| Ascites n(%) | 20 (49) | 73 (48) | 1.00 | 49 (43) | 44 (55) | 0.15 | |
| Varices n(%) | 10 (24) | 49 (32) | 0.55 | 37 (33) | 22 (28) | 0.55 | |
| Splenomegaly n(%) | 23 (56) | 99 (65) | 0.56 | 62 (55) | 60 (75) | 0.01* | |
| Psychiatric Variables | |||||||
| Anxiety (median) | 2 (1,6) | 7 (4,11) | <0.01* | 4 (2,7) | 9 (5,11) | <0.01* | |
| Depression (median) | 4 (1,5) | 7 (3, 9) | <0.01* | 4 (2,7) | 8 (5,10) | <0.01* | |
| Total HADS (median) | 6 (1,10) | 13 (8,19) | <0.01* | 9 (4,14) | 17 (10,21) | <0.01* | |
| Sleep (median) | 8.4±3.8 | 10.9±4.0 | <0.01* | 9.7±4.1 | 11.5±4.0 | <0.01* | |
| Inflammatory Markers | Normative Values | ||||||
| IL-1β (mean,pg/L) | 3.0±0.2 | 3.3±0.9 | 3.4±0.9 | 0.40 | 3.4±0.9 | 3.4±1.0 | 0.85 |
| IL-6 (mean,pg/L) | 4.0±0.5 | 5.2±1.1 | 5.6±1.1 | 0.04* | 5.4±1.2 | 5.6±1.1 | 0.33 |
| TNFα (mean,pg/L) | 5.6±0.5 | 5.8±0.6 | 5.9±0.6 | 0.23 | 5.9±0.6 | 5.9±1.2 | 0.91 |
| CRP (median,mg/dL) | 1.0 (0.3,1.1) | 1.0(0.5,3.0) | 1.4(0.6,2.9) | 0.48 | 0.9 (0.5,2.5) | 1.8 (0.8,3.1) | <0.01* |
| Medication Use | |||||||
| Opioids N(%) | 0 | 45 (30) | <0.01* | 18 (16) | 27 (34) | <0.01* | |
| SSRI/SNRI N(%) | 13 (32) | 56 (37) | 0.67 | 40 (35) | 29 (36) | 1.00 | |
| Tramadol N(%) | 1 (2) | 12 (8) | 0.22 | 5 (4) | 8 (10) | 0.22 | |
| NSAIDS N(%) | 3 (7) | 19 (13) | 0.58 | 13 (12) | 9 (11) | 1.00 | |
| Acetaminophen N(%) | 0 | 10 (7) | 0.12 | 5 (4) | 5 (6) | 0.82 | |
| Zolpidem N(%) | 2 (5) | 18 (12) | 0.26 | 12 (11) | 8 (10) | 1.00 | |
| Benzodiazepines N(%) | 3 (7) | 19 (13) | 0.58 | 9 (8) | 13 (16) | 0.12 | |
| Sleep Medication N(%) | 9 (22) | 54 (36) | 0.30 | 33 (29) | 30 (38) | 0.15 | |
indicates p<0.05, CCI=Charlson Comorbidity Index, cytokines have been In transformed to normalize, mean shown with ±sd, median with (IQR), and N(column %)
Figure 2.
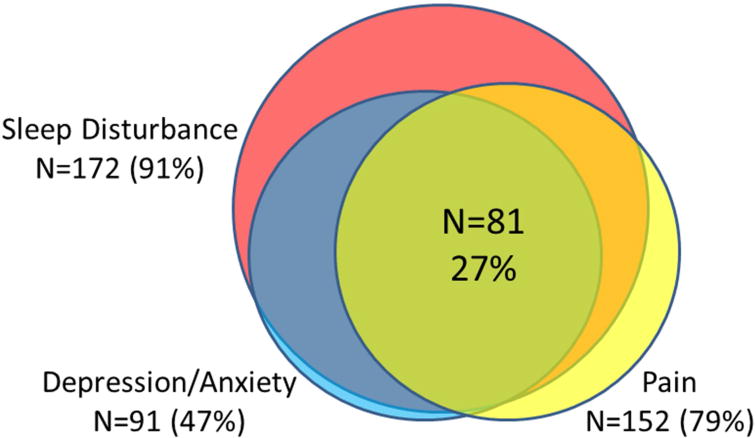
Symptom Clustering
Pain Characteristics
Among the 79% of participants with pain, the median pain score was 2/5 (IQR=1,3) with 66% having pain scores ≥2. The median Pain Rating Index from the MPQ was 8 (2,23) for the entire cohort and 15 (5,27) among those with pain. The most common locations of pain were the abdomen (N=80), lower back (N=63), and large joints (N=60) (Figure 3). Of those reporting pain, the median number of words chosen to described the pain was 6 (IQR=2,12; with mostly sensory (median=11, IQR=4,19), as opposed to affective terms (median=0, IQR=0,2). Most patients with pain described a component of their pain as constant, steady, or continuous in nature (54%), with 35% choosing words such as rhythmic, periodic, or intermittent, and 11% reporting pain that was brief, momentary, or transient. Most (56%) said their pain occurred at least daily.
Figure 3.
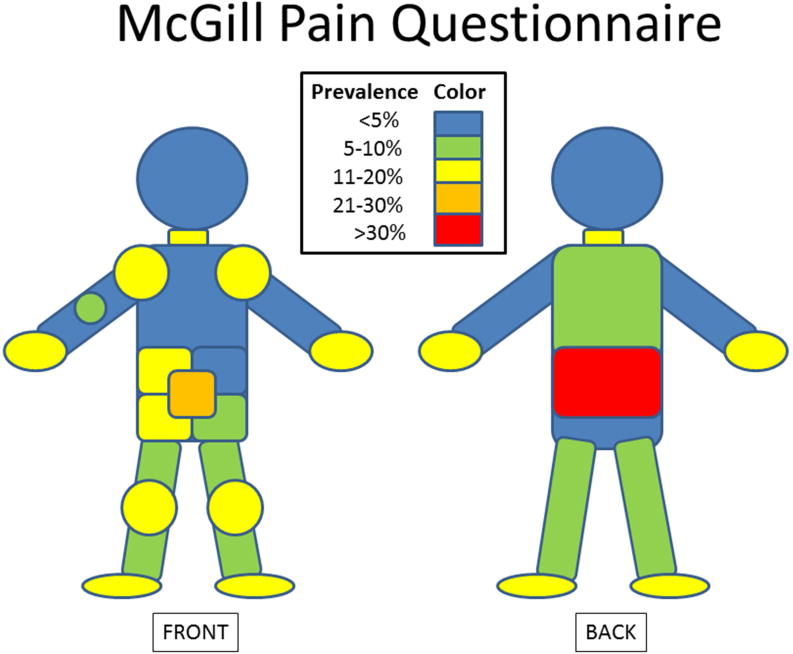
Distribution of Pain: the percentage of participants selecting various regions of pain on the MPQ is denoted with color coding
Factors Associated with Pain
In univariate analysis, pain was significantly associated with cirrhosis etiology (83% NASH or HCV=83% vs. alcohol=65%,p<0.01), younger age, psychiatric symptoms, opioid use, PDI, and IL-6 (Table 1). In multivariate analysis, age, IL-6, total HADS score, and etiology remained significantly related to pain prevalence (Table 2). The predictors of pain intensity in multivariable negative binomial regression modeling were HADS score (IRR=1.02 per point, 95% CI=1.01,1.04, p<0.01) and income per person in household (IRR per $10,000=0.89, 95% CI=0.82,0.97, p<0.01).
Table 2.
Multivariable Regression Models of Pain, Abdominal Pain, and Pain-Related Disability
| Pain | Abdominal Pain | Pain-Related Disability | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Covariate | OR | 95% CI | P | OR | 95% CI | P | Covariate | IRR | 95% CI | p |
| Age | 0.93 | 0.90,0.99 | <0.01 | 0.96 | 0.92,1.01 | 0.11 | ||||
| Income | 0.69 | 0.43,1.08 | 0.10 | |||||||
| Alcohol Abuse | 0.27 | 0.12,0.59 | <0.01* | |||||||
| MELD | 1.02 | 1.0001,1.05 | 0.05 | |||||||
| Etiology (vs. HCV) | 0.02* | Etiology (vs. HCV) | 0.10 | |||||||
| NASH | 1.23 | 0.39,3.92 | 0.72 | NASH | 0.95 | 0.72,1.25 | 0.70 | |||
| ETOH | 0.27 | 0.09,0.79 | 0.04* | ETOH | 0.73 | 0.54,0.98 | 0.08 | |||
| HADS | 1.14 | 1.07,1.24 | <0.01* | 1.10 | 1.04,1.16 | <0.01* | HADS | 1.04 | 1.02,1.05 | <0.01* |
| IL-6 | 1.63 | 1.09,2.58 | 0.03* | |||||||
| CRP | 1.44 | 1.08,1.93 | 0.03* | CRP | 1.13 | 1.02,1.24 | 0.01* | |||
| Splenomegaly | 1.96 | 0.83,4.75 | 0.13 | |||||||
| Pain Severity | 1.19 | 1.08,1.32 | <0.01* | |||||||
| Prescription | 1.49 | 1.14,1.94 | <0.01* | |||||||
| Opioids | ||||||||||
p<0.05, P for etiology is the overall p for the variable and the individual levels were compared to HCV and were adjusted using Holm-Bonferroni correction for multiple comparisons.
Pain-Related Disability
Among the cohort, 145 individuals (75%) reported some pain-related disability. The median score of items was 3.0 (0.17,5.83). Pain-related disability was most influential in the area of occupation, where the median score was 4 out of 10 for all patients in the cohort. Notably, only 11% of the cohort was working even though only 19% were of retirement age. In univariate testing, disability score was correlated with age, depression and anxiety symptoms, MELD score, pain severity, number of painful locations, and levels of CRP, TNFα, and IL-6. Disability was also significantly related to opioid use with opioid users vs. non-users having median scores of 6.5 (4.5,7.8) vs. 1.7 (0.0,4.7), p<0.01. Multivariable modeling is shown in Table 2, with HADS, opioid use, MELD, CRP, and severity of pain remaining statistically significant in this model.
Abdominal pain
Despite excluding patients with known causes of abdominal pain, abdominal pain was still found in 40% of the patients. Out of this cohort, 40% cited their liver as the presumed etiology of their abdominal pain. On univariate testing (Table 1) the patients with abdominal pain were significantly younger, had decreased income, increased likelihood of splenomegaly, higher CRP levels and increased psychiatric and sleep symptoms. With multivariate logistic regression modeling, abdominal pain was significantly related to symptoms of mood disorders, higher CRP, and was less common in persons with past heavy alcohol use (Table 2). The rates of pain and abdominal pain were similar between those with and without clinically evident ascites. Prior reports demonstrated that inflammatory markers vary according to age, sex and race24, but forcing these factors into the models did not substantially change the results, nor did substituting Child-Turcotte-Pugh for MELD.
Among patients with abdominal pain, the severity of pain was significantly related to household income (IRR=0.99, 95%CI=0.99,0.99, p<0.01) and anxiety score (IRR=1.05, 95%CI=1.01,1.10, p=0.01) with a trend towards higher CRP values (IRR=1.13, 95%CI=0.97,1.32, p=0.11).
The words most commonly used to describe the abdominal pain included sharp (n=42), cramping (n=37), stabbing (n=32), shooting (29), throbbing (n=28), and hurting (n=27). Most of the patients described abdominal pain as a daily occurrence (63%). Notably, 5 patients with abdominal pain met criteria for functional abdominal pain and 19 met criteria for IBS based on the ROME criteria.
Analgesic Use
The most commonly prescribed analgesics were opioids, used by 23% of the cohort (Table 1). The majority were taking oxycodone or an equivalent with a median IV morphine equivalent of 25mg/day (IQR=4,30) per day. While 23 participants admitted to taking pain medications not prescribed to them at some point, only 3 of these participants were being prescribed opioids at the time of the study. In univariate analysis, opioid prescription was significantly related to younger age (55±8 vs. 59±9, p<0.01), lower income (median/household member/yr=$10,000 vs. 23,000, p<0.01) lower comorbidity scores (3.6±0.8 vs. 4.0±1.2, p=0.02), HCV etiology (37% HCV on opioids vs. 14% of others, p<0.01), psychiatric symptoms (median HADS 18 vs. 10, p<0.01), past illicit drug use (53% vs. 53%, p<0.01) and increased pain scores (median pain score 2; IQR=2,3 vs. 1; IQR=0,2 in non-users; p<0.01). There was a trend towards more non-white vs. white subjects taking opioids (43% vs. 21%, p=0.1). There were no significant differences in inflammatory markers by opioid use. In multivariate analysis prescription of opioids was significantly associated with lower comorbidity scores (OR=0.56, 95%CI=0.33,0.88, p=0.02), more sleep disturbance (OR=1.22, 95%CI=1.07,1.41, p<0.01), past illicit drug use (OR=2.65, 95%CI=1.14,6.32, p=0.03), with a trend towards increased psychiatric symptoms (OR=1.07, 95%CI=1.00,1.14, p=0.06)
Discussion
Using a large cohort of patients with cirrhosis, our data highlight the importance of pain in cirrhosis, which affected 79% of individuals and was associated with significant functional impairment. Importantly, more than half of our participants experienced chronic daily and even constant pain. The presence of pain not surprisingly correlated with opioid use (25% of participants). The use of prescription opioids correlated with higher pain intensity, lower ratings for functional status and more sleep and emotional problems.
The high prevalence of pain was strikingly similar to the rates among patients wait-listed for liver transplantation2 and Veterans with chronic HCV25 yet higher than rates of approximately 50% in chronic renal disease.26 Chronic pain carries a significant burden as shown by pain-related disability in 75% of our patients, even though we excluded individuals with known diseases commonly associated with pain and/or inflammation. Only 11% of our cohort was actively working, and 79% of non-working participants reported pain-related occupational disability. The prevalence and severity of pain-related disability for this cohort exceeds reported findings for patients with coronary artery disease and chest pain post-bypass grafting27, and was only slightly lower than findings for patients with chronic back pain undergoing evaluations for chronic disability.28
Abdominal pain was common in this cohort. Although we previously reported an association between progressive liver disease, abdominal pain, and ascites1, the presence of ascites did not correlate with pain in this cohort likely because large, active ascites was rare. Despite the limited afferent innervation of the liver, patients often attribute abdominal pain to their liver disease. Based on our exclusion criteria and review of imaging studies, known structural abnormalities other than the liver disease were not present. Also typical functional disorders cannot fully explain pain in our cohort. The prevalence of IBS among those with pain in this study was 12.5%, which is nearly identical to global prevalence estimates29.
Similar to other findings in other studies30 etiology was significantly related to pain, with those with alcohol-related liver disease having less pain in multivariable analyses. There are several possible explanations for this. Alcohol itself can cause anti-nociceptive effects.31 Additionally, pain has been associated with adiposity32, and BMI was significantly higher among members of our cohort with HCV and NASH than alcohol. BMI fell out of the models because it was not related to pain in this group, but this factor should be further explored in larger studies.
We identified two distinct potential mechanistic pathways for pain: affect and inflammation. Our results fit into the framework of existing information that suggest a role of psychiatric comorbidity in pain severity, pain-related disability, and the effectiveness of pain management.33 The cross-sectional design did not allow us to consider whether the experience of chronic pain drives negative affect or latent affective spectrum disorders lead to physical symptoms, including onset of pain, as indicated in studies on mechanisms of central sensitization.34 Increases in systemic inflammatory may also contribute to the high prevalence of pain, with IL-6 and CRP levels correlating with pain prevalence, abdominal pain and pain-related disability. Cirrhosis and progression of fibrosis have been associated with increase in pro-inflammatory markers, including IL-635 and CRP.8 Pro-inflammatory cytokines have been found to be elevated among patients with cirrhosis in the past. We here show that the level of inflammation was associated with symptoms, independent of the severity of liver disease. Consistent with our results, pain and disability in HCV patients were linked to increased inflammatory markers in a recently published study.7 Pain, depression, and inflammation have been linked in other disease processes.36 Experimentally, IL-6 triggers depressive-like behavior in animals.37 Interestingly, stress can also increase systemic inflammatory response, pointing at a complex and potentially reciprocal relationship between affect and inflammatory signaling38. Independent of its effect on mood, inflammatory mediators also affect central pain pathways and processing39, thus providing yet another potential explanation for our observations as well as a possible justification for trying medications that impact on pain, inflammation, and mood symptoms, such as anti-inflammatories, serotonin norepinephrine reuptake inhibitors. These pathways, along with others such as bone demineralization and postural changes associated with liver disease, may explain how seemingly unrelated types of pain (eg. in the lower back) may actually be exacerbated by liver disease.
The most commonly used analgesic in this cohort was opioids, despite concerns about their risks in cirrhosis.6 These risks may be particularly relevant in patients with cirrhosis and substance abuse histories, for whom the risks are likely greater than the general population. Interestingly, pain and disability ratings were significantly higher in patients on opioids compared to the remainder of the cohort despite the use of often high dose opioids. These results are consistent with prior data showing persistently high pain intensity and functional impairment despite opioid therapy for non-cancer pain.40, 41 Importantly, opioid therapy independently predicted pain-related disability, corresponding to findings from prospective trials.42 Additionally, opioid use was common among participants with past opioid abuse and diversion, further demonstrating an urgent need to define safe and effective pain medications for patients with comorbid substance abuse and liver disease. Opioids use has been highlighted as problematic in other gastrointestinal disease such as inflammatory bowel disease, where 5% of patients take opioids, which have been associated with increased pain and depression, similar to our cohort, as well as mortality.43, 44
While our study provides a complex set of data, obtained prospectively in a large and diverse group of patients with advanced liver disease, its cross-sectional design only enables us to identify correlations and limits our ability to determine causality, which will require longitudinal evaluations. The study was conducted in a single center and the majority of patients were Caucasian, which may limit the generalizability of the results. Additionally, substance abuse was self-reported, which could lead to a reporting bias. We focused on three etiologies of cirrhosis, and further study will be required to evaluate pain in other patient populations. Finally, we did not have access to pharmacy data to assess whether prescriptions were indeed filled and did not perform toxicological drug screens to independently confirm opioid use.
In summary, pain and pain management are critical issues for patients with advanced liver disease. Pain and disability are common among patients with cirrhosis, even in a group with relatively low MELD scores. Opioids were the most commonly used medications for pain in this group despite potential morbidity and likely higher abuse risk, indicating a need for systematic investigation into the optimal treatments for pain in patients with cirrhosis. Two factors, a pro-inflammatory cytokine profile and mood symptoms, likely contribute to the pathogenesis of chronic pain in this group of patients and are possible targets for future interventions.
Acknowledgments
We would like to thank Amy Schmotzer, Becca, Chip, Pat, Anita, Sarah, and the attendings and staff of the CLD for their help and support with this project.
Grant Support:
This project used the UPCI Luminex Core Laboratory that is supported in part by award P30CA047904. This project was supported by The Starzl Transplant Young Investigator Award of the University of Pittsburgh. Dr. Zickmund’s time was supported in part by the Veteran Healthcare Adminstration.
Abbreviations
- CRP
C reactive protein
- HADS
Hospital Anxiety and Depression Score
- HCV
Hepatitis C Virus
- IL
Interleukin
- MELD
Model for End-Stage Liver Disease
- NASH
Non-alcoholic steatohepatitis
- PDI
Pain Disability Index
- PSQI
Pittsburgh Sleep Quality Index
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions: study concept and design:SR, KB, FL, ES, AD; acquisition of data:SR; data interpretation of data:all; drafting of the manuscript: SR; critical revision of the manuscript for important intellectual content: all authors; statistical analysis: SR and AD; obtained funding: SR and AD.
Disclosures: No authors have disclosures relevant to this manuscript.
Disclaimer: The views expressed in this article are those of the authors and do not necessarily reflect the position or the policy of the Department of Veterans Affairs or the United States Government.
Writing Assistance: None
References
- 1.Rogal SS, Winger D, Bielefeldt K, et al. Pain and opioid use in chronic liver disease. Dig Dis Sci. 2013;58:2976–85. doi: 10.1007/s10620-013-2638-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Madan A, Barth KS, Balliet WE, et al. Chronic pain among liver transplant candidates. Prog Transplant. 2012;22:379–84. doi: 10.7182/pit2012535. [DOI] [PubMed] [Google Scholar]
- 3.Gutteling JJ, de Man RA, van der Plas SM, et al. Determinants of quality of life in chronic liver patients. Aliment Pharmacol Ther. 2006;23:1629–35. doi: 10.1111/j.1365-2036.2006.02934.x. [DOI] [PubMed] [Google Scholar]
- 4.Rogal SS, Winger D, Bielefeldt K, et al. Healthcare utilization in chronic liver disease: the importance of pain and prescription opioid use. Liver Int. 2013;33:1497–503. doi: 10.1111/liv.12215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rakoski MO, McCammon RJ, Piette JD, et al. Burden of cirrhosis on older Americans and their families: analysis of the health and retirement study. Hepatology. 2012;55:184–91. doi: 10.1002/hep.24616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chandok N, Watt KD. Pain management in the cirrhotic patient: the clinical challenge. Mayo Clin Proc. 2010;85:451–8. doi: 10.4065/mcp.2009.0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huckans M, Fuller BE, Olavarria H, et al. Multi-analyte profile analysis of plasma immune proteins: altered expression of peripheral immune factors is associated with neuropsychiatric symptom severity in adults with and without chronic hepatitis C virus infection. Brain Behav. 2014;4:123–42. doi: 10.1002/brb3.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wiese S, Mortensen C, Gotze JP, et al. Cardiac and proinflammatory markers predict prognosis in cirrhosis. Liver Int. 2013 doi: 10.1111/liv.12428. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez-Pinto I, Agmon-Levin N, Howard A, et al. Fibromyalgia and cytokines. Immunol Lett. 2014 doi: 10.1016/j.imlet.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 10.Benson S, Kattoor J, Wegner A, et al. Acute experimental endotoxemia induces visceral hypersensitivity and altered pain evaluation in healthy humans. Pain. 2012;153:794–9. doi: 10.1016/j.pain.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain. 1975;1:277–99. doi: 10.1016/0304-3959(75)90044-5. [DOI] [PubMed] [Google Scholar]
- 12.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 13.Buysse DJ, Reynolds CF, 3rd, Monk TH, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 14.Tait RC, Pollard CA, Margolis RB, et al. The Pain Disability Index: psychometric and validity data. Arch Phys Med Rehabil. 1987;68:438–41. [PubMed] [Google Scholar]
- 15.Tait RC, Chibnall JT, Krause S. The Pain Disability Index: psychometric properties. Pain. 1990;40:171–82. doi: 10.1016/0304-3959(90)90068-O. [DOI] [PubMed] [Google Scholar]
- 16.Drossman DA, Dumitrascu DL. Rome III: New standard for functional gastrointestinal disorders. J Gastrointestin Liver Dis. 2006;15:237–41. [PubMed] [Google Scholar]
- 17.Engsbro AL, Begtrup LM, Kjeldsen J, et al. Patients suspected of irritable bowel syndrome–cross-sectional study exploring the sensitivity of Rome III criteria in primary care. Am J Gastroenterol. 2013;108:972–80. doi: 10.1038/ajg.2013.15. [DOI] [PubMed] [Google Scholar]
- 18.Bjelland I, Dahl AA, Haug TT, et al. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52:69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 19.Buysse DJ, Hall ML, Strollo PJ, et al. Relationships between the Pittsburgh Sleep Quality Index (PSQI), Epworth Sleepiness Scale (ESS), and clinical/polysomnographic measures in a community sample. J Clin Sleep Med. 2008;4:563–71. [PMC free article] [PubMed] [Google Scholar]
- 20.Staud C.M.E. Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 21.Vignali DA. Multiplexed particle-based flow cytometric assays. J Immunol Methods. 2000;243:243–55. doi: 10.1016/s0022-1759(00)00238-6. [DOI] [PubMed] [Google Scholar]
- 22.R_Core_Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2012. [Google Scholar]
- 23.Venables WN, Ripley BD. Modern Applied Statistics with S. Fourth. New York: Springer; 2002. [Google Scholar]
- 24.Woloshin S, Schwartz LM. Distribution of C-reactive protein values in the United States. N Engl J Med. 2005;352:1611–3. doi: 10.1056/NEJM200504143521525. [DOI] [PubMed] [Google Scholar]
- 25.Lovejoy TI, Dobscha SK, Cavanagh R, et al. Chronic pain treatment and health service utilization of veterans with hepatitis C virus infection. Pain Med. 2012;13:1407–16. doi: 10.1111/j.1526-4637.2012.01476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Solano JP, Gomes B, Higginson IJ. A comparison of symptom prevalence in far advanced cancer, AIDS, heart disease, chronic obstructive pulmonary disease and renal disease. J Pain Symptom Manage. 2006;31:58–69. doi: 10.1016/j.jpainsymman.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 27.Eisenberg E, Pultorak Y, Pud D, et al. Prevalence and characteristics of post coronary artery bypass graft surgery pain (PCP) Pain. 2001;92:11–7. doi: 10.1016/s0304-3959(00)00466-8. [DOI] [PubMed] [Google Scholar]
- 28.Crighton AH, Wygant DB, Applegate KC, et al. Can brief measures effectively screen for pain and somatic malingering? Examination of the Modified Somatic Perception Questionnaire and Pain Disability Index. Spine J. 2014;14:2042–50. doi: 10.1016/j.spinee.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 29.Canavan C, West J, Card T. The epidemiology of irritable bowel syndrome. Clin Epidemiol. 2014;6:71–80. doi: 10.2147/CLEP.S40245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barkhuizen A, Rosen HR, Wolf S, et al. Musculoskeletal pain and fatigue are associated with chronic hepatitis C: a report of 239 hepatology clinic patients. Am J Gastroenterol. 1999;94:1355–60. doi: 10.1111/j.1572-0241.1999.01087.x. [DOI] [PubMed] [Google Scholar]
- 31.Gatch MB, Lal H. Effects of ethanol and ethanol withdrawal on nociception in rats. Alcohol Clin Exp Res. 1999;23:328–33. [PubMed] [Google Scholar]
- 32.Yoo JJ, Lim SH, Cho NH, et al. Relationships between body mass index, fat mass, muscle mass, and musculoskeletal pain in community residents. Arthritis Rheumatol. 2014 doi: 10.1002/art.38861. [DOI] [PubMed] [Google Scholar]
- 33.Jamison RN, Edwards RR, Liu X, et al. Relationship of negative affect and outcome of an opioid therapy trial among low back pain patients. Pain Pract. 2013;13:173–81. doi: 10.1111/j.1533-2500.2012.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anand P, Aziz Q, Willert R, et al. Peripheral and central mechanisms of visceral sensitization in man. Neurogastroenterol Motil. 2007;19:29–46. doi: 10.1111/j.1365-2982.2006.00873.x. [DOI] [PubMed] [Google Scholar]
- 35.Kao JT, Lai HC, Tsai SM, et al. Rather than interleukin-27, interleukin-6 expresses positive correlation with liver severity in naive hepatitis B infection patients. Liver Int. 2012;32:928–36. doi: 10.1111/j.1478-3231.2011.02742.x. [DOI] [PubMed] [Google Scholar]
- 36.Kojima M, Kojima T, Suzuki S, et al. Depression, inflammation, and pain in patients with rheumatoid arthritis. Arthritis Rheum. 2009;61:1018–24. doi: 10.1002/art.24647. [DOI] [PubMed] [Google Scholar]
- 37.Sukoff Rizzo SJ, Neal SJ, Hughes ZA, et al. Evidence for sustained elevation of IL-6 in the CNS as a key contributor of depressive-like phenotypes. Transl Psychiatry. 2012;2:e199. doi: 10.1038/tp.2012.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rohleder N. Stimulation of systemic low-grade inflammation by psychosocial stress. Psychosom Med. 2014;76:181–9. doi: 10.1097/PSY.0000000000000049. [DOI] [PubMed] [Google Scholar]
- 39.Clark AK, Old EA, Malcangio M. Neuropathic pain and cytokines: current perspectives. J Pain Res. 2013;6:803–14. doi: 10.2147/JPR.S53660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wasserman RA, Brummett CM, Goesling J, et al. Characteristics of chronic pain patients who take opioids and persistently report high pain intensity. Reg Anesth Pain Med. 2014;39:13–7. doi: 10.1097/AAP.0000000000000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Valkanoff TA, Kline-Simon AH, Sterling S, et al. Functional disability among chronic pain patients receiving long-term opioid treatment. J Soc Work Disabil Rehabil. 2012;11:128–42. doi: 10.1080/1536710X.2012.677653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chu LF, D’Arcy N, Brady C, et al. Analgesic tolerance without demonstrable opioid-induced hyperalgesia: a double-blinded, randomized, placebo-controlled trial of sustained-release morphine for treatment of chronic nonradicular low-back pain. Pain. 2012;153:1583–92. doi: 10.1016/j.pain.2012.02.028. [DOI] [PubMed] [Google Scholar]
- 43.Targownik LE, Nugent Z, Singh H, et al. The prevalence and predictors of opioid use in inflammatory bowel disease: a population-based analysis. Am J Gastroenterol. 2014;109:1613–20. doi: 10.1038/ajg.2014.230. [DOI] [PubMed] [Google Scholar]
- 44.Hanson KA, Loftus EV, Jr, Harmsen WS, et al. Clinical features and outcome of patients with inflammatory bowel disease who use narcotics: a case-control study. Inflamm Bowel Dis. 2009;15:772–7. doi: 10.1002/ibd.20847. [DOI] [PubMed] [Google Scholar]


