Abstract
Central neural circuits orchestrate the homeostatic repertoire that maintains body temperature during environmental temperature challenges and alters body temperature during the inflammatory response. This review summarizes the experimental underpinnings of our current model of the CNS pathways controlling the principal thermoeffectors for body temperature regulation: cutaneous vasoconstriction controlling heat loss, and shivering and brown adipose tissue for thermogenesis. The activation of these effectors is regulated by parallel but distinct, effector-specific, core efferent pathways within the CNS that share a common peripheral thermal sensory input. Via the lateral parabrachial nucleus, skin thermal afferent input reaches the hypothalamic preoptic area to inhibit warm-sensitive, inhibitory output neurons which control heat production by inhibiting thermogenesis-promoting neurons in the dorsomedial hypothalamus that project to thermogenesis-controlling premotor neurons in the rostral ventromedial medulla, including the raphe pallidus, that descend to provide the excitation of spinal circuits necessary to drive thermogenic thermal effectors. A distinct population of warm-sensitive preoptic neurons controls heat loss through an inhibitory input to raphe pallidus sympathetic premotor neurons controlling cutaneous vasoconstriction. The model proposed for central thermoregulatory control provides a useful platform for further understanding of the functional organization of central thermoregulation and elucidating the hypothalamic circuitry and neurotransmitters involved in body temperature regulation.
Keywords: Brown adipose tissue, shiver, cutaneous vasoconstriction, thermogenesis, fever, sympathetic nerve activity, preoptic hypothalamus, rostral raphe pallidus, dorsomedial hypothalamus
Introduction
Central neural circuits orchestrate a homeostatic repertoire to maintain body temperature during thermal challenges, both from the ambient and the internal (e.g., exercise) environment, and to alter body temperature during specific behavioral states (e.g., sickness, sleep, stress, etc.). Body temperature regulation is effected primarily through dedicated pathways in the brain which function to produce an optimal thermal environment for neurons and for the many tissues on which the brain depends for survival. The effector mechanisms for cold defense, recruited in order of increasing energy costs, include thermoregulatory behavior to reduce heat loss; cutaneous vasoconstriction (CVC) to conserve heat in the body core and limit heat loss to the environment; piloerection to supplement CVC in reducing heat loss; heat production (thermogenesis), a by-product of the inefficiency of mitochondrial ATP production and of ATP utilization, in skeletal muscle (shivering) and brown adipose tissue (BAT), the principal sources of metabolic heat production beyond those contributing to basal metabolic rate. Effector mechanisms for heat defense include thermoregulatory behavior to increase heat loss; cutaneous vasodilation, including an active vasodilation that appears to be specific to humans(Smith et al., 2016), that facilitates heat loss by conducting heat from the body core to the body surface; and evaporative cooling through sweating. The activation of these effectors is regulated by parallel but distinct, effector-specific, efferent pathways within the central nervous system that share common peripheral thermal sensory inputs. A wide variety of non-thermal physiological parameters, disease processes, neurochemicals and drugs can influence the central regulation of body temperature and their effects are hypothesized to result from an alteration of the activity within these core neural circuits for thermoregulation. For instance, since the high metabolic rate of BAT and shivering skeletal muscles during thermogenesis cannot be sustained without a dependable supply of metabolic fuels, particularly oxygen, lipolytic by-products and glucose, the CNS network driving cold-defensive and behavioral BAT activation or shivering is exquisitely sensitive to signals reflecting the short- and long-term availability of the fuel molecules essential for BAT and skeletal muscle metabolism.
The core central thermoregulatory network (Fig. 1), involving thermal afferent pathways, hypothalamic sensorimotor integration and descending efferent pathways to spinal motor neurons, comprises the fundamental pathways through which cutaneous cold and warm sensation and/or reductions or elevations in brain temperature elicit changes in thermoregulatory effectors to counter or protect against deviations from a homeostatic temperature of the brain and other critical organs. Although the experimental basis for this model depends largely on studies in rodents (reviewed in (Morrison, 2011; Morrison et al., 2011; Nakamura, 2011; Morrison et al., 2014a; Morrison et al., 2014b)) and often under anesthetized conditions, the fundamental neural circuits elucidated through this work are expected to be relevant to human thermoregulation since rodents have, with the exceptions of sweating and thermoregulatory behaviors, a repertoire of thermal effectors and thermal reflex responses that is similar to those in humans. This review will summarize the results of the principal studies leading to our current model (Fig. 1) of the core thermoregulatory neural circuits controlling CVC, and shivering and BAT thermogenesis.
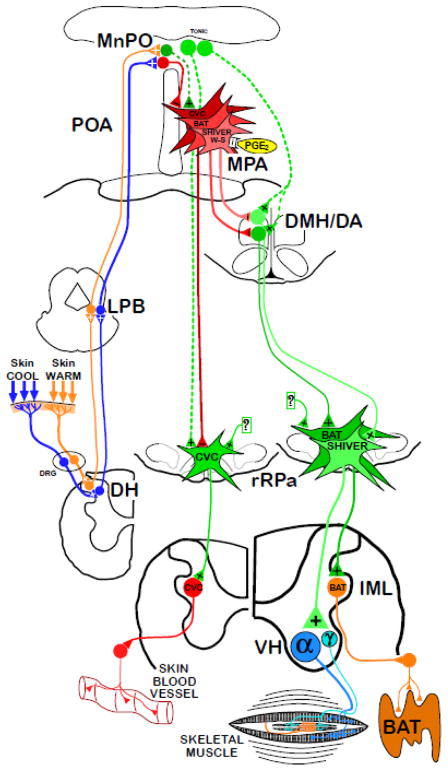
Figure 1.
Functional neuroanatomical model for the fundamental pathways providing the thermoregulatory control and pyrogenic activation of cutaneous vasoconstriction (CVC), brown adipose tissue (BAT) and shivering thermogenesis. Cool and warm cutaneous thermoreceptors transmit signals to respective primary sensory neurons in the dorsal root ganglia (DRG) which relay this information to second-order thermal sensory neurons in the dorsal horn (DH). Cool sensory DH neurons glutamatergically activate third-order sensory neurons in the external lateral subnucleus of the lateral parabrachial nucleus (LPB), while warm sensory DH neurons project to third-order sensory neurons in the dorsal subnucleus of the LPB. Thermosensory signals driving thermoregulatory responses are transmitted from the LPB to the preoptic area (POA) where GABAergic interneurons (red) in the median preoptic (MnPO) subnucleus are activated by glutamatergic inputs from cool-activated neurons in LPB and inhibit each of the distinct populations of warm-sensitive (W-S) neurons in the medial preoptic area (MPA) that control CVC, BAT and shivering. In contrast, glutamatergic interneurons (dark green) in the MnPO are postulated to be excited by glutamatergic inputs from warm-activated neurons in LPB and, in turn, excite W-S neurons in MPA. Prostaglandin (PG) E2 binds to EP3 receptors on each of the classes of W-S neurons in the POA to inhibit their activity. The MnPO also contains neurons (light green) that provide an excitatory input to CVC premotor neurons in the rostral raphe pallidus (rRPa), and neurons (light green) that excite BAT- and shivering- promoting neurons in the dorsomedial hypothalamus and dorsal hypothalamic area (DMH/DA). Preoptic W-S neurons provide inhibitory control of CVC by inhibiting CVC sympathetic premotor neurons in the rostral ventromedial medulla, including the rRPa, that project to CVC sympathetic preganglionic neurons in the intermediolateral nucleus (IML). Preoptic W-S neurons providing inhibitory thermoregulatory control of BAT and shivering thermogenesis inhibit BAT sympathoexcitatory neurons and shivering promoting neurons, respectively, in the DMH/DA, which, when disinhibited during skin and core cooling, provide respective excitatory drives to BAT sympathetic premotor neurons and to skeletal muscle shivering premotor neurons in the rRPa. These, in turn, project, respectively, to BAT sympathetic preganglionic neurons in the IML, and to alpha (α) and gamma (γ) motoneurons in the ventral horn (VH) of the spinal cord.
Afferents influencing thermoeffector activity
Cutaneous thermoreceptor afferent pathway
The central thermoregulatory system receives signals related to changes in the external environmental temperature through cutaneous thermoreceptors (primary sensory nerve endings distributed in the skin) and signals related to changes in the temperature of various tissues in the body core through local thermoreceptors, including intrinsically thermally-sensitive neurons in the brain (Boulant et al., 1986; Tabarean et al., 2004; Boulant, 2006; Lundius et al., 2010). Relatively little is known about visceral thermoreceptors; but, species and body location differences notwithstanding, many of the cutaneous thermoreceptors are cool thermoreceptors (Darian-Smith et al., 1973) that drive cold-defensive CVC and thermogenesis. The membranes of thermal afferent neurons contain transient receptor potential (TRP) cation channels whose temperature-dependent conductances transduce skin temperature into primary thermoreceptor afferent neuronal activity. The TRPM8 channel, activated by menthol and cooling, is the primary candidate for the cutaneous cold receptor TRP channel. Some non-thermal, unmyelinated afferents expressing TRP channels (Andresen et al., 2012) also have access to central thermoregulatory circuits: endogenous ligand-stimulated TRPV1 channels inhibit BAT thermogenesis (Steiner et al., 2007) and TRPV1 agonist infusion reduces thermogenesis and lowers body temperature (Feketa et al., 2013).
In addition to cutaneous thermoreception (reviewed in (Romanovsky, 2014)), thermoreceptive mechanisms exist in body core structures including the brain, spinal cord and abdomen. The afferent fibers from cold and warm receptors in the abdominal viscera are included among the splanchnic and vagus nerve afferent fibers and their responses to temperature changes are similar to those of cutaneous thermoreceptors (Riedel, 1976; Gupta et al., 1979). Temperature changes in the spinal cord can affect the activity of thermoregulatory neurons in more rostral areas of the brain (Guieu et al., 1970). TRP channels that are located in the central endings of primary somatosensory fibers in the spinal dorsal horn (Tominaga et al., 1998; Bautista et al., 2007) may sense spinal temperature and could underlie an integration of spinal thermal signals with cutaneous thermal signals at the spinal cord level. These could function to enhance thermoregulatory responses in extreme thermal environments when the feedforward thermoregulatory responses driven by changes in skin temperature prove inadequate to prevent changes in brain, spinal cord or body core temperatures.
Primary thermoreceptor dorsal root ganglion neurons synapse on thermoreceptive-specific, lamina I spinal (or trigeminal) dorsal horn cells that respond linearly to graded, innocuous cooling or warming stimuli, but are not activated further in the noxious temperature range (Craig, 2002). The TRP channels in the thermoreceptor central endings may also provide a substrate for spinal cord or trigeminal nucleus temperature to influence the level of thermal effector activity. In turn, spinal and trigeminal lamina I neurons collateralize and innervate the thalamus, providing the neural substrate for cutaneous thermosensory perception and localization (Craig et al., 1994; Craig, 2002), and the pontine lateral parabrachial nucleus (LPB) (Hylden et al., 1989; Li et al., 2006) (Fig. 1), responsible for triggering involuntary (e.g., autonomic, shivering and respiratory) thermoregulatory responses.
Spinal lamina I cold thermal responsive neurons provide a glutamatergic excitation to neurons in the external lateral subdivision of the lateral parabrachial nucleus (LPBel), which, in turn, project principally to the median preoptic subnucleus (MnPO) of the preoptic area (POA) (Nakamura et al., 2008b). In parallel, glutamatergic excitation of POA-projecting neurons in the dorsal subnucleus of the LPB (LPBd) (Nakamura et al., 2010) is necessary for the skin warming-evoked inhibition of CVC and BAT thermogenesis (Nakamura et al., 2010). The discharge rate of single, MnPO-projecting LPBel neurons recorded in vivo increased markedly in response to skin cooling in a manner paralleling the skin cooling-evoked increases in BAT sympathetic nerve activity (SNA) (Nakamura et al., 2008b). In contrast, single, MnPO-projecting LPBd neurons were excited by skin warming in parallel with the simultaneous inhibition of BAT SNA (Nakamura et al., 2010). Activation of LPBd or LPBel neurons evokes decreases or increases, respectively, in CVC, and in BAT and shivering thermogenesis that mimic respective skin warming-evoked or skin cooling-evoked physiological responses. The critical role of LPB neurons in transmitting cutaneous, and possibly visceral, thermal sensory information to the hypothalamus to drive thermoregulatory responses is demonstrated by the elimination of BAT, shivering and CVC responses to alterations in skin temperature following experimental inactivation of local neurons or blockade of local glutamate receptors in the LPB (Kobayashi et al., 2003; Nakamura et al., 2008a; Nakamura et al., 2010). Thus, activations of LPBd and LPBel neurons by glutamatergic inputs from lamina I neurons, driven respectively by cutaneous warming and cooling signals, transmit the respective warm and cold cutaneous thermal afferent stimuli, via a spinoparabrachiopreoptic pathway, that initiate heat defense and cold defense responses in CVC SNA, cutaneous blood flow, BAT SNA, shivering EMGs, and BAT and shivering thermogenesis to defend body temperature during thermal challenges from the environment.
Non-thermal afferents influence thermal effectors
Viscerosensory afferents, with axons in the vagus nerve, synapsing on second-order neurons in the nucleus of the solitary tract (NTS), and likely sensing metabolic rather than thermal parameters, can also influence BAT activity (Szekely, 2000) and shivering responses. For instance, the inhibition of BAT activity induced by upregulation of hepatic glucokinase (Tsukita et al., 2012) and the BAT activation following either intragastric delivery of the TRP agonist, capsiate (Ono et al., 2011) or the presence of lipids in the duodenum (Blouet et al., 2012) are mediated by vagal afferents. Arterial chemoreceptor afferents synapsing in the commissural NTS (commNTS) and signaling systemic hypoxia, elicit a marked inhibition of BAT SNA and BAT thermogenesis (Madden et al., 2005) to restrict oxygen consumption in the face of reduced oxygen availability. The NTS also receives inputs from brainstem and forebrain sites involved in metabolic regulation and these provide the additional potential for NTS neurons to integrate a variety of metabolic signals influencing BAT thermogenesis.
The NTS contains neurons whose activation reduces CVC and BAT SNAs and shivering EMGs (Johnson et al., 1998; Cao et al., 2010; Tupone et al., 2013). The bicuculline-evoked blockade of GABAA receptors in the intermediate NTS (iNTS) potently inhibits cooling-evoked and febrile increases in BAT SNA (Cao et al., 2010) and the inhibition of BAT SNA following injection of an adenosine A1 receptor agonist into iNTS is dependent on the activity of iNTS neurons (Tupone et al., 2013). As byproducts of ATP metabolism, adenosine and adenosine 5′-monophosphate, whose central administration also produces hypothermia (Muzzi et al., 2013), can diffuse from cells and may function to reduce energy consumption, such as BAT and shivering thermogenesis, in situations (e.g., hypoxia or caloric restriction) of reduced energy substrate availability. CVC SNA has a modest degree of baroreceptor sensitivity (Macefield et al., 1999; Owens et al., 2002), indicating that the circuits controlling CVC SNA also receive an inhibitory outflow from the NTS.
Central neural pathways to thermoregulatory effectors
Thermoregulatory behavior
Thermoregulatory behaviors, the stereotypical somatic motor acts directed primarily toward minimizing or optimizing heat transfer from the body to the environment or toward generating heat in the cold are triggered primarily by cutaneous thermal receptors. However, although skin thermal receptors can initiate human thermoregulatory behavior, adult humans have established such a rich repertoire of experience, learned responses and options for altering their environment that a variety of non-thermal cues (hearing a weather report) play a significant role in initiating thermoregulatory behaviors. Thermoregulatory behaviors are included in the category of “motivated” behaviors, which have important relationships to the limbic and hypothalamic emotional and dopaminergic reward systems in the brain. The emotional distress of being too hot or too cold is commonly a significant factor in motivating human behavior to seek or produce an ambient temperature that is more “comfortable” and thereby obtain the ‘reward’ of being in a pleasant thermal environment. Due to the complexity of the neural circuitry required for initiating, organizing, performing and controlling even simple thermoregulatory motor acts, we have only a minimal understanding of the organization of the neural pathways underlying thermoregulatory behavior (Satinoff et al., 1970; Nagashima et al., 2000; Almeida et al., 2006; Romanovsky, 2007). Lesion studies suggest, for instance, that a wide range of thermoregulatory behaviors can occur in the absence of the neurons in the classic thermoregulatory sensorimotor integration area of the hypothalamic preoptic area (POA) (Satinoff et al., 1970; Almeida et al., 2006).
Cutaneous vasoconstriction regulates heat loss
In the cold, increasing the sympathetic nerve activity to cutaneous blood vessels reduces skin blood flow and the loss of metabolic heat to the environment, thus helping to maintain a normal core body temperature. Conversely, in a hot environment, inhibition of the sympathetic nerve activity to cutaneous blood vessels (i.e., cutaneous vasodilation) increases skin blood flow and brings metabolic heat to the body surface where it is available for transfer to the environment. Simultaneously, visceral vaso- and venodilation in the cold and constriction in the heat contribute to the respective increases and decreases in core blood flow. In both humans and rodents, heating-evoked cutaneous vasodilation is accompanied by a marked increase in visceral (i.e., splanchnic and renal) vasoconstriction (Escourrou et al., 1982; Kregel et al., 1988; Minson et al., 1999). Sustained cutaneous vasoconstriction is an important factor in the defense of an elevated body temperature during fever. In humans, increased cutaneous vasoconstrictor sympathetic outflow mediates the reduction in cutaneous blood flow in a cold environment and during fever, while a sympathetic vasodilator outflow is principally responsible for the increase in cutaneous blood flow in hyperthermic environments (Wallin et al., 2007). Direct recordings of cutaneous vasoconstrictor postganglionic sympathetic nerve activity in several species, including humans, indicate a moderate level of cutaneous vasoconstrictor sympathetic activity at normothermic body temperatures.
CVC sympathetic preganglionic neurons (Fig. 1) are located in the intermediolateral cell column (IML) of the thoracolumbar spinal cord. These project primarily to paravertebral, cutaneous vasoconstrictor sympathetic ganglion cells that innervate the cutaneous blood vessels and anastomoses. Retrograde tracing studies indicate that premotor inputs to cutaneous vasoconstrictor spinal sympathetic networks including the preganglionic neurons, arise from neurons in the ventromedial medulla, including the rostral raphe pallidus (rRPa) and the parapyramidal (PaPy) region, some of which contain serotonin (5-HT) and others that express the vesicular glutamate transporter 3 (VGLUT3) (Smith et al., 1998; Nakamura et al., 2004; Toth et al., 2006). Additionally, premotor contributions arise from the rostral ventrolateral medulla (RVLM), some of which are C1 neurons, as well as from the A5 noradrenergic cell group, lateral hypothalamic area and paraventricular hypothalamic area (Smith et al., 1998).
Functionally, activation of neurons in the rostral medullary raphe (or the RVLM) elicits CVC (Blessing et al., 2001; Tanaka et al., 2002) and inhibition of neurons in either the rRPa or the RVLM prevents the cooling-evoked increases cutaneous vasoconstriction (Ootsuka et al., 2005b). An interaction of serotonin (via the 5-HT2A receptor) and glutamate neurotransmission in the IML is significant in determining the cutaneous vasoconstrictor outflow. Spinal application of a 5-HT2A receptor antagonist markedly reduces the CVC sympathetic response to rRPa stimulation and subsequent blockade of spinal glutamate receptors eliminates the residual cutaneous vasoconstrictor activation following rRPa stimulation (Ootsuka et al., 2005a). In summary, thermoregulatory alterations in cutaneous blood flow mediated by the CVC sympathetic outflow are determined by the activity of populations of CVC sympathetic premotor neurons located primarily in the rRPa (Fig. 1), PaPy and RVLM and involve release of glutamate and 5-HT within the CVC spinal sympathetic network.
Shivering thermogenesis
During the rapid, repeated skeletal muscle contractions of shivering, thermogenesis arises primarily from the inefficiency of energy utilization in cross-bridge cycling and calcium ion sequestration, and, to a lesser degree from mitochondrial membrane proton leak in the course of ATP production from fuel substrate oxidation. Heat generation through shivering has long been recognized as an essential mechanism in cold defense and in the elevated body temperature in fever in both experimental animals and in humans (Palmes et al., 1965; Saper et al., 1994). The muscle contractions of shivering result from rhythmic bursts of activity in the alpha-motoneurons innervating skeletal muscle fibers. The increased IA-afferent input in response to the simultaneous activation of gamma-motoneurons could play a role in determining alpha-motoneuron responsiveness and, in turn, in setting the shivering threshold and intensity (Schafer et al., 1973b; Schafer et al., 1973a) and frequency. The central neural network generating the cold-evoked bursts of alpha-motoneuron activity requires a supraspinal pathway that includes transmission of cutaneous cold afferent signals through the LPB (Nakamura et al., 2008a), hypothalamic integration and activation of descending outputs (Nakamura et al., 2011) to control the activity of somatic premotor neurons in the rostral ventromedial medulla (Tanaka et al., 2006; Brown et al., 2007; Nakamura et al., 2011). Anatomical tracing has established synaptic connections between rostral ventromedial medullary neurons, including those in rRPa, and skeletal muscle (Kerman et al., 2003). Activation of rostral ventromedial medullary neurons increases muscle EMG activity and elicits shivering (Nason et al., 2004; Nakamura et al., 2011).
BAT Thermogenesis
In contrast to the ancillary nature of thermogenic shivering in skeletal muscles that are normally used to produce movement and posture, non-shivering or adaptive thermogenesis in BAT is the specific metabolic function of this tissue and is accomplished by the heat generating capacity of a significant proton leak across the extensive mitochondrial membranes of the brown adipocytes facilitated by the high expression of uncoupling protein-1 (UCP1) in BAT mitochondrial membranes (Cannon et al., 2004). The level of BAT SNA and norepinephrine release and β3-adrenergic receptor binding to brown adipocytes determine the level of thermogenesis in BAT by regulating both the activity of lipases providing the immediate fuel molecules for BAT mitochondria and the level of expression of BAT mitochondrial UCP1(Cannon et al., 2004). BAT is an important thermoregulatory effector in rodents and other small mammals (Golozoubova et al., 2006), but also contributes to cold defense in both infant and adult humans (Nedergaard et al., 2007; Cypess et al., 2009; van Marken Lichtenbelt et al., 2009; Virtanen et al., 2009).
Similar to the medullospinal organization of the control of the CVC sympathetic outflow described above, the sympathetic outflow to BAT, which determines BAT thermogenesis, is driven by the activity of BAT sympathetic premotor neurons that provide an excitatory drive to BAT sympathetic preganglionic neurons in the thoracolumbar spinal cord, which, in turn, excite sympathetic ganglion cells innervating the BAT pads. A prominent location of BAT sympathetic premotor neurons is the rostral ventromedial medulla, centered in the rRPa and extending into nearby raphe magnus nucleus and over the pyramids to the PaPy area, initially suggested by transsynaptic retrograde tracing (Bamshad et al., 1999; Oldfield et al., 2002; Cano et al., 2003; Yoshida et al., 2003) and subsequently more directly demonstrated (Nakamura et al., 2004; Stornetta et al., 2005).
Glutamate and 5-HT play critical roles in the descending excitation of BAT sympathetic preganglionic neurons by their antecedent premotor neurons in the rRPa. Spinally-projecting neurons in the rRPa region can contain phenotypic markers for (a) the vesicular glutamate transporter 3 (VGLUT3), potentially indicative of glutamatergic neurons (Nakamura et al., 2004; Stornetta et al., 2005); (b) serotonin (5-HT) or tryptophan hydroxylase, a synthetic enzyme for 5-HT (Cano et al., 2003; Nakamura et al., 2004; Stornetta et al., 2005) and (c) glutamic acid decarboxylase-67 (GAD-67), a marker for GABAergic neurons (Stornetta et al., 2005). The majority of VGLUT3-containing neurons in the rRPa express Fos in response to cold exposure or to the febrile stimulus of prostaglandin (PG)E2 (Nakamura et al., 2004) or to psychological stress (Lkhagvasuren et al., 2011), nanoinjection of glutamate or NMDA into the upper thoracic IML activates BAT SNA and BAT thermogenesis (Nakamura et al., 2004; Madden et al., 2006), and blockade of glutamate receptors in the upper thoracic IML suppresses the increase in BAT thermogenesis evoked by bicuculline injection into rRPa (Nakamura et al., 2004). Putative serotonergic neurons in the rRPa increase their firing rate in response to cold (Martin-Cora et al., 2000; Nason et al., 2006) or PGE2 administration (Nason et al., 2006), serotonin injected into the IML can activate BAT SNA and BAT thermogenesis and potentiates the BAT SNA response to NMDA injections into the IML (Madden et al., 2006), and blockade of spinal serotonin receptors reverses the cold-evoked activation of BAT SNA (Madden et al., 2010).
BAT premotor neurons in the rRPa receive a potent glutamatergic excitation, as well as GABAergic inhibitory inputs, with the latter predominating under warm conditions to reduce BAT thermogenesis. Relief of this tonically-active, GABAergic inhibition as well as an increase in glutamate-mediated excitation, including that from the dorsomedial hypothalamus and dorsal hypothalamic area (DMH/DA) (Cao et al., 2006; Kataoka et al., 2014), contributes to the cold-evoked and febrile increases in BAT premotor neuronal discharge that drives BAT SNA and BAT heat production. Injections of glutamate agonists into the rRPa or blockade of local GABAA receptors in rRPa evoke intense activations of BAT SNA (Morrison et al., 1999; Cao et al., 2003; Madden et al., 2003). Conversely, inhibition of neuronal activity or blockade of glutamate receptors in the rRPa reverses the increases in BAT SNA and BAT thermogenesis and heart rate elicited by a variety of thermogenic stimuli, including skin cooling and fever (Nakamura et al., 2002; Madden et al., 2003; Morrison, 2003; Nakamura et al., 2007; Ootsuka et al., 2008), and produces dramatic falls in body temperature in awake rats (Zaretsky et al., 2003). Thus, the rRPa region of the ventromedial medulla contains the principal population of BAT sympathetic premotor neurons providing the final common medullospinal pathway for the sympathoexcitatory drive to the spinal network controlling BAT SNA and BAT thermogenesis (Fig. 1).
Evaporative cooling
Evaporative cooling, including sweating in humans, panting in dogs or thermoregulatory salivation in rodents, increases heat loss from wet body surfaces, and is a critical thermoregulatory strategy in humans, particularly when ambient temperature exceeds core temperature. Little is known about the pathway mediating the increased sympathetic cholinergic outflow to sweat glands in a warm environment, but it presumably includes the cutaneous warm thermoreceptor afferent pathway involving the dorsal horn and the LPBd warm-responsive neurons that project to the preoptic hypothalamus, as well as the warm-sensitive preoptic neurons responsive to elevated core temperature. Hypothalamic circuitry would then elicit an increase in the discharge of the supraspinal sympathetic premotor neurons (Shafton et al., 2013) that control sweat gland-specific, spinal sympathetic preganglionic neurons (Shafton et al., 1992). Cortical influences may also play a role (Farrell et al., 2015), although there is also a significant drive for thermally-motivated behavior in heat defense.
Evaporation occurs both from hairless skin and from the upper respiratory tract. The significant loss of body water during sweating, which usually occurs in parallel with a large increase in cutaneous blood flow, has a significant effect on blood volume and plasma osmolarity and emphasizes the strong interaction between thermoregulatory and cardiovascular and osmotic control circuits during prolonged heat exposure, dehydration and exercise (Whyte et al., 2005), much of which occurs through integration of these signals in the hypothalamus (McKinley et al., 2015).
The hypothalamus in body temperature regulation
As the central integrator of many dimensions of the homeostatic space, the hypothalamus is composed of several interconnected populations of neurons which receive a variety of signals relating to behavioral and emotional state, as well as the condition of the body and the interstitial fluid, and has outputs influencing emotional, behavioral, somatic and autonomic responses. For body temperature regulation, the hypothalamus occupies a pivotal integrative position between the sensation of skin and core temperatures and the sympathetic and somatic premotor pathways controlling thermoeffector activation. Additionally, through their responses to immune signaling molecules, hypothalamic neurons are also the primary site for the organization and maintenance of the febrile response to inflammation and infection, which includes the stimulation of CVC, and shivering (“chills”) and BAT thermogenesis mediated by the action of PGE2 on its EP3 receptors. Thermoregulatory control is but one of the myriad of interrelated homeostatic functions embedded in the hypothalamic matrix, thus, the latter also provides a rich substrate for non-thermal influences, such as those related to osmolarity and body water status and to metabolic energy stores, on thermoeffector activity. Hypothalamic neurons recognized to play significant roles in thermoregulation are located in the POA, including the MnPO and the medial (MPA) subnuclei, in the DMH/DA, in the paraventricular nucleus (PVN), and in the perifornical area of the lateral hypothalamus (PeF/LH).
Temperature sensation within the POA
The conceptual foundation of our current understanding of the role of the hypothalamus in normal body temperature regulation and in the elevated body temperature during fever is that the POA, including the MnPO and the MPA, contains a population of GABAergic neurons (Lundius et al., 2010) with intrinsic warm sensitivity (Boulant et al., 1974; Boulant et al., 1986) whose discharge frequency increases with local hypothalamic temperature. The altered discharge of such warm-sensitive neurons in response to their local environmental (i.e., blood) temperature could provide the fundamental mechanism through which the central thermoregulatory network is apprised of deep body temperature. Nakayama and colleagues made the first single-cell recording from thermosensitive neurons in the POA in vivo and found neurons with spontaneous discharge at thermoneutral temperatures that increased their discharge during local hypothalamic warming (Nakayama et al., 1961; Nakayama et al., 1963). The POA contains warm-sensitive neurons whose tonic discharge is reduced by skin cooling and whose thermosensitivity to preoptic temperature is increased when the skin is cooled (Boulant et al., 1974). Neurons sensitive to changes in bath temperature were found in subsequent recordings in the POA in hypothalamic slices and the majority of thermosensitive neurons were warm-sensitive neurons (Boulant et al., 1986).
Either skin cooling or direct cooling of the local environment of POA neurons evokes sympathetic thermogenesis in BAT as well as shivering thermogenesis (Hammel et al., 1960; Imai-Matsumura et al., 1984). Inhibition of neurons in the MPA, but not those in the MnPO, increases body core temperature, EMG activity (shivering), metabolism and heart rate (Osaka, 2004; Zaretsky et al., 2006; Yoshida et al., 2009). Transections immediately caudal to the POA elicit large increases in BAT temperature (Chen et al., 1998). Lesions in the MPA produce hyperthermia by increasing metabolism and by stimulating shivering thermogenesis and heat conservation through increased CVC (Szymusiak et al., 1982). In combination with the properties of warm-sensitive POA neurons, these findings support the hypothesis that warm-sensitive POA neurons integrate cutaneous and core thermal sensation, and provide a GABAergic inhibitory input that is tonically active at thermoneutral temperatures, to suppress the activity of BAT sympathoexcitatory and of shivering-promoting neurons in the DMH/DHA (Fig. 1), and/or that of CVC and BAT sympathetic premotor and of somatic shivering premotor neurons in the rostral raphe pallidus (rRPa) (Nakamura et al., 2002; Nakamura et al., 2009; Yoshida et al., 2009; Dimitrov et al., 2011). This mechanism would be consistent with the hypothesis that the firing rates of warm-sensitive, GABAergic MPA projection neurons are the principal neurophysiological substrate underlying the thermoregulatory “balance point” (Romanovsky, 2004), determined both by cutaneous thermosensory signals and the effect of local brain temperature, as well as a variety of non-thermal inputs. The different body temperature thresholds for activation of CVC, shivering and BAT are consistent with different populations of POA warm-sensitive neurons (Fig. 1) providing an inhibitory regulation of the CVC premotor neurons in rRPa and the different BAT- and shivering-promoting neurons in the DMH/DA (Fig. 1).
Thermoregulatory sensorimotor integration in the POA
As described above, the POA, predominantly the MnPO, contains neurons that receive skin cooling-driven, glutamatergic inputs from cool-responsive neurons in LPBel. Glutamatergic stimulation of the MnPO with NMDA evokes physiological responses mimicking cold-defensive responses, and inhibition of MnPO neurons completely blocks the activation of BAT and shivering thermogenesis evoked by skin cooling (Nakamura et al., 2008a; Nakamura et al., 2011). BAT and shivering thermogenesis evoked by skin cooling or by stimulation of MnPO neurons is blocked by antagonizing GABAA receptors in the MPA (Osaka, 2004; Nakamura et al., 2007; Nakamura et al., 2008a; Nakamura et al., 2011). Thus, skin cooling-evoked responses are postulated to require a local circuit in the POA in which cutaneous cool signals are received by GABAergic interneurons in MnPO that mediate an inhibition of the warm-sensitive, inhibitory projection neurons in the MPA, thereby reducing their tonic inhibition of neurons in caudal brain regions (e.g., DMH/DA, rRPa) whose increased activity leads to stimulation of the thermogenic effectors for cold defense (Fig. 1).
However, the MnPO also contains glutamatergic neurons that project to the DMH/DHA, that are synaptically connected to BAT, and that receive glutamatergic terminals containing tuberoinfundibular peptide of 39 residues (TIP39), which produces an increase in core temperature when injected into the MnPO (Dimitrov et al., 2011). MnPO neurons expressing the leptin receptor also project to DMH/DA (Zhang et al., 2011). Thus, glutamatergic inputs to DMH/DHA (Madden et al., 2004) from the MnPO could provide the excitation (Fig. 1) required to drive the BAT sympathoexcitatory neurons and the shivering-promoting neurons in DMH/DHA when their POA inhibitory input is reduced during skin cooling or fever. The strong activation of BAT and shivering thermogenesis following blockade of GABAA receptors in the MnPO (Nakamura et al., 2008a; Nakamura et al., 2011) could arise from a disinhibition of either (or both) the skin cooling-activated inhibitory interneurons in MnPO or a MnPO glutamatergic input to DMH/DHA, both of which would increase the activity of the thermogenesis-promoting neurons in the DMH/DA.
DMH/DA contains thermogenesis-promoting neurons
The observation that transection of the neuraxis immediately caudal to the POA increases BAT SNA and BAT thermogenesis (Chen et al., 1998) suggests that the POA projection neurons are inhibitory to BAT thermogenesis. In contrast, transections made in the midbrain, just caudal to the hypothalamus, do not increase basal levels of BAT thermogenesis in normothermic animals (Rothwell et al., 1983) and, in fact, reverse PGE2-evoked increases in BAT SNA and thermogenesis (Morrison et al., 2004; Rathner et al., 2006). These findings suggest that, although a long inhibitory pathway from the POA neurons to BAT sympathetic premotor neurons in rRPa (Nakamura et al., 2002; Tanaka et al., 2011) may contribute to the regulation of BAT thermogenesis and CVC, a source of excitatory drive to BAT thermogenesis must exist between the POA and the rostral midbrain.
The DMH contains neurons whose activity is necessary for both the cold-evoked and febrile activations of BAT SNA and BAT thermogenesis. Administration of endotoxin or cold exposure increases Fos expression in neurons in the DMH (Elmquist et al., 1996; Yoshida et al., 2002; Cano et al., 2003; Sarkar et al., 2007). Blockade of GABAA receptors in the DMH increases BAT SNA (Cao et al., 2004), suggesting a tonic GABAergic inhibitory input to BAT thermogenic neurons in the DMH (Fig. 1). This tonic GABAergic input to neurons within the DMH may originate in the POA since POA-derived GABAergic axon swellings make close appositions with DMH neurons, including those that project to the rRPa (Nakamura et al., 2005). In addition, inhibition of neurons in the DMH blocks febrile (Zaretskaia et al., 2003; Madden et al., 2004; Morrison et al., 2004; Nakamura et al., 2005; Nakamura et al., 2011) and cold-evoked (Nakamura et al., 2007; Nakamura et al., 2011) shivering and BAT thermogenesis. Blockade of ionotropic glutamate receptors within the DMH also blocks the increase in BAT SNA evoked by PGE2 within the POA (Madden et al., 2004), indicating that a glutamatergic input, potentially from the MnPO, to neurons in the DMH is essential for these febrile responses.
Since neurons in the DMH do not project directly to the spinal cord, DMH neurons likely contribute to BAT and shivering thermogenesis by directly influencing the activity of the BAT sympathetic and shivering somatic premotor neurons in the rRPa (Kataoka et al., 2014). The DMH contains neurons that project directly to the rRPa (Hermann et al., 1997; Samuels et al., 2002; Nakamura et al., 2005; Yoshida et al., 2009; Kataoka et al., 2014), some of which express Fos in response to thermogenic stimuli such as cold(Yoshida et al., 2009), endotoxin administration or stress (Sarkar et al., 2007; Kataoka et al., 2014) and some of which receive GABAergic putative synapses from neurons in the MPA (Nakamura et al., 2005). Indeed, glutamate receptor activation within the rRPa is necessary for the increase in BAT SNA evoked by disinhibition of neurons within the DMH (Cao et al., 2006).
Hypothalamic regulation of shivering thermogenesis
Shivering thermogenesis is the last cold-defense mechanism to be activated as its thermal threshold is at a lower core (i.e., POA) temperature than that for either CVC or BAT thermogenesis. This likely reflects the existence of a distinct population of preoptic warm-sensitive neurons that regulate shivering, but it is also in keeping with the high metabolic energy cost of shivering and the relative vulnerability of an animal during shivering as escape behavior would be more slowly mobilized. Although shivering is mediated by activation of somatic motoneurons rather than by the sympathetic nervous system that controls other thermoregulatory effectors, thermoregulatory and febrile shivering requires parallel changes in the activity of neurons in the same localized regions as does BAT thermogenesis (Nakamura et al., 2011). Briefly, cutaneous, and possibly visceral, cold afferent signals excite second-order cold sensory neurons in the spinal and trigeminal dorsal horns which project to thermally-responsive neurons in the LPBel (Nakamura et al., 2008b), as with the activation of other cold-defense effectors. Putative GABAergic interneurons in the MnPO are activated (Nakamura et al., 2008a; Nakamura et al., 2011) to reduce the activity of warm-sensitive, shivering-inhibiting MPA neurons (Zhang et al., 1995; Nakamura et al., 2011), thereby disinhibiting DMH neurons (Tanaka et al., 2001; Nakamura et al., 2011) that project to shivering-promoting premotor neurons in the rRPa providing an excitatory drive (Tanaka et al., 2006; Nakamura et al., 2011) to somatic alpha-motoneurons, and possibly also gamma-motoneurons, in the spinal ventral horn.
Hypothalamic regulation of CVC
Neurons within several brain regions influence cutaneous heat loss through their effects on the activity of CVC sympathetic premotor neurons in rRPa. The hypothalamic thermoregulatory control of CVC, similar to that of other thermal effectors, is mediated significantly by GABAergic, MPA projection neurons, perhaps with warm-sensitivity (Tanaka et al., 2011, Tanaka, 2009 #307). Consistent with this model, L- glutamate injections into the POA, electrical stimulation of the POA and preoptic warming each elicit vasodilation in the rat paw and the rat tail (Zhang et al., 1995). Injection of PGE2 or GABA into the POA increases CVC SNA (Tanaka et al., 2005; Rathner et al., 2008; Tanaka et al., 2009; Tanaka et al., 2013). Transection of the neuraxis immediately caudal to the POA increases CVC outflow (Rathner et al., 2008). The MnPO contains cold-activated neurons that project to the rRPa and inhibition of neuronal discharge in the MnPO inhibits cold-and PGE2-activated increases in CVC (Tanaka et al., 2011; Tanaka et al., 2013). Following the increase in CVC SNA after transection of the neuraxis immediately caudal to the POA, subsequent brain transections caudal to the DMH, but rostral to the rRPa, do not significantly reduce CVC outflow and injection of muscimol into DMH does not affect either spontaneous, thermally-sensitive, CVC neuronal discharge, or that evoked by injection of PGE2 into the POA (Rathner et al., 2008).
The absence of an effect of inhibition of DMH neurons on thermoregulatory control of CVC strongly supports the roles of direct pathways from the POA to the rRPa (Tanaka et al., 2011) in mediating CVC thermoregulatory responses. Indeed, the data of Tanaka and colleagues support a circuit for control of CVC with connections between both MnPO and MPA, and rRPa that parallels that proposed above with potential connections between both MnPO and MPA, and DMH/DA for the control of thermogenesis. PGE2-mediated inhibition of CVC inhibitory POA projection neurons, potentially those in the MnPO, contributes to the activation of CVC sympathetic premotor neurons during fever (Tanaka et al., 2013). The demonstration that POA contains GABAergic neurons that express EP3 receptors for PGE2 and project to the rRPa (Nakamura et al., 2002), and that these are distinct from those EP3-expressing POA neurons that project to the DMH (Nakamura et al., 2009) provides a potential anatomical substrate for this marked difference between the hypothalamic regulation of CVC and that of thermogenic effectors. The finding that inhibition of neurons in MnPO reduces the PGE2-stimulated CVC sympathetic discharge is consistent with a contribution of an MnPO-rRPa CVC excitatory pathway in the febrile increase in heat retention. Overall, these results support a model in which the discharge of CVC sympathetic premotor neurons in rRPa is determined by a balance of the inhibitory influence of warm-sensitive, inhibitory MPA-rRPa neurons and the excitatory input from cold-activated MnPO-rRPa neurons (Fig. 1). Importantly, another significant source of the excitatory drive to CVC sympathetic premotor neurons in the rRPa would appear to arise from neurons in the brainstem (Rathner et al., 2008).
The data derived from transection experiments could also be consistent with a PGE2-evoked and cooling-driven increase in CVC neuronal discharge arising from inhibition of POA neurons that excite neurons, located between the POA and the rRPa, which are inhibitory to CVC sympathetic premotor neurons in the rRPa. Several observations suggest that a population of such neurons may exist in the rostral periaqueductal gray (PAG). Neurons in the rostral PAG receive input from neurons in the POA that are activated (increased Fos expression) by environmental warming, but not cooling (Yoshida et al., 2005), environmental warming also increases Fos expression in the rostral PAG (Yoshida et al., 2002), and chemical excitation of neurons within the rostral PAG increases both tail temperature and blood flow (Zhang et al., 1997), presumably due to inhibition of CVC sympathetic outflow. The roles of direct and/or indirect pathways between CVC-regulating POA neurons and CVC sympathetic premotor neurons in the ventromedial and ventrolateral medulla remain to be elucidated.
Hypothalamic regulation of sweating
The thermal sensitivity of neurons in the POA provide the substrate for initiating and maintaining the sympathetic outflow to sweat glands, although little is known of the pathways connecting the POA neurons regulating sweating with the sympathetic preganglionic neurons for sweat glands (Shibasaki et al., 2010). The increased osmolarity during sustained sweating in a hot environment has central effects on thermoregulatory responses to heating, eliciting an increase the internal temperature threshold for cutaneous vasodilation (Shibasaki et al., 2009; Shibasaki et al., 2010)and evaporative heat loss (Baker et al., 1982) and a reduction in sweating (Takamata et al., 1995) and respiratory stimulation (Turlejska et al., 1986).
Perifornical lateral hypothalamus (PeF/LH) influences thermoregulatory effector output
The recent demonstration that neurons in the LH region can modulate the activity of thermogenic effectors, including BAT (Cerri et al., 2005; Zhang et al., 2010; Tupone et al., 2011) has sparked an interest in the role of LH and, in particular perifornical (PeF) area neurons in thermoregulation (Zhang et al., 2010; Takahashi et al., 2013). Orexinergic (hypocretin) neurons may provide the neurochemical and anatomical substrate responsible for the PeF/LH influence on BAT thermogenesis. Orexin-containing neurons within the LH are retrogradely infected following inoculation of BAT with the transsynaptic, retrograde tracer, pseudorabies virus (Tupone et al., 2011). Orexinergic neurons of the LH project directly to the rRPa and activation of orexin receptors within the rRPA potentiates cold-evoked BAT thermogenesis (Tupone et al., 2011). Interestingly, a co-transmitter in orexinergic neurons may be more important than orexin, at least in mice, for mediating the effects of orexin neurons on thermoregulatory responses (Zhang et al., 2010; Takahashi et al., 2013). In addition, orexinergic inputs to other brain regions involved in thermoregulation, including the median and medial preoptic areas and the DMH (Peyron et al., 1998) or non-orexinergic neurons within this area could contribute to the thermogenic tone elicited from this region. These pathways could play important roles in setting the tone of BAT thermogenesis and energy expenditure, and thereby body temperature, across sleep-wake cycles, during arousal or stress or in response to dietary influences.
The paraventricular hypothalamus (PVH) in thermoregulation
The PVH is a hypothalamic region that is not considered critical for thermoregulation since destruction of neurons in the PVH does not affect basal body temperature, the circadian rhythm of core body temperature (Lu et al., 2001), or cold-defense responses (Horn et al., 1994). However, lesions of the PVH attenuate febrile responses evoked by bacterial endotoxins such as lipopolysacharride (LPS) (Horn et al., 1994), which led to the speculation that neurons in the PVH may contribute to the febrile response by stimulating thermogenesis in BAT (Lu et al., 2001). Supporting this speculation were the findings that neurons in the PVH are labeled following injection of a transynaptic retrograde tracer into BAT (Bamshad et al., 1999; Oldfield et al., 2002; Cano et al., 2003); PVH neurons are activated (express Fos) in response to bacterial endotoxins (Elmquist et al., 1996); and neurons in the dorsal PVH with direct projections to the sympathetic preganglionic cell column are activated during fever (Zhang et al., 2000). However, a direct assessment of the role of neurons in the PVH on sympathetically-mediated BAT thermogenesis demonstrated that BAT SNA and the resulting thermogenesis are potently inhibited by activation of neurons in the PVH (Madden et al., 2009). Thus, while some PVH neurons likely contribute to fever responses, possibly by increasing CVC or by stimulating adrenal cortical or medullary hormone secretion, other PVH neurons exert a marked inhibitory influence on thermogenic effectors, preventing their activation and thus their consumption of metabolic energy. Whether this influence of PVH neurons plays a role in thermoregulation, perhaps by limiting thermogenesis during conditions of reduced energy availability, remains to be determined.
An important, albeit indirect role of the PVH in heat defense responses is their contribution to the visceral vasoconstriction (Leite et al., 2012), likely with an angiotensinergic component (Kregel et al., 1994), that supports the marked increase in cutaneous blood flow resulting from the warm-evoked inhibition of CVC. As mentioned above, a significant osmotic challenge can arise from an uncompensated water loss during evaporative cooling in heat defense. The PVH could play a role in the interaction of osmotic and temperature regulation in which thermoregulatory responses are overridden (Kregel et al., 1988) to maintain vascular volume and cardiac output.
Conclusion
The principal thermoregulatory effectors are the cutaneous blood vessels for control of heat loss, the BAT and skeletal muscle for thermogenesis and sweating for evaporative heat loss. The activation of these effectors is regulated by parallel but distinct, effector-specific, core efferent pathways within the central nervous system (Fig. 1) that are strongly influenced by shared cutaneous thermal afferent signals. Cutaneous (and likely visceral) thermal sensory information is integrated, in an as yet unknown manner, at synapses in the spinal and trigeminal dorsal horns and subsequently within the LPB, where cool and warm afferent signals are processed within anatomically distinct regions with projections to the POA. Within the POA, different, effector-specific populations of temperature-sensitive neurons, principally warm-sensitive neurons, provide the substrate for thermal sensory inputs, arriving via the LPB projection neurons, to be integrated with local (i.e., core) temperature to influence the activation of thermoregulatory effectors. Neurons in the MnPO provide an excitatory input to thermogenesis-promoting neurons in the DMH and to CVC premotor neurons in the rRPa. Different thermal sensitivities among populations of temperature-sensitive POA neurons may allow differential responsiveness of different effectors to changes in cutaneous vs. brain temperatures. It remains unknown whether and how these differing thermal sensitivities may be maintained under a variety of perturbing alterations in the external or internal thermal and neurochemical environments. The rostral ventromedial medulla, including the rRPa, contains the premotor neurons, including those containing serotonin, providing the essential excitation of thermoeffector spinal motor neurons.
Highlights.
Central neural circuits maintain a homeostatic body temperature during environmental temperature challenges and alter body temperature during the inflammatory response.
Activation of thermoeffectors is regulated by parallel but distinct, effector-specific, core efferent pathways within the CNS that share a common peripheral thermal sensory input.
Acknowledgments
This work was supported by NIH grants R01NS040987, R01NS091066.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Almeida MC, Steiner AA, Branco LG, Romanovsky AA. Neural substrate of cold-seeking behavior in endotoxin shock. PLoS ONE. 2006;1:e1. doi: 10.1371/journal.pone.0000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andresen MC, Hofmann ME, Fawley JA. The unsilent majority-TRPV1 drives “spontaneous” transmission of unmyelinated primary afferents within cardiorespiratory NTS. Am J Physiol Regul Integr Comp Physiol. 2012;303:R1207–1216. doi: 10.1152/ajpregu.00398.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker MA, Doris PA. Control of evaporative heat loss during changes in plasma osmolality in the cat. J Physiol. 1982;328:535–545. doi: 10.1113/jphysiol.1982.sp014282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamshad M, Song CK, Bartness TJ. CNS origins of the sympathetic nervous system outflow to brown adipose tissue. Am J Physiol. 1999;276:R1569–1578. doi: 10.1152/ajpregu.1999.276.6.R1569. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448:204–208. doi: 10.1038/nature05910. [DOI] [PubMed] [Google Scholar]
- Blessing WW, Nalivaiko E. Raphe magnus/pallidus neurons regulate tail but not mesenteric arterial blood flow in rats. Neuroscience. 2001;105:923–929. doi: 10.1016/s0306-4522(01)00251-2. [DOI] [PubMed] [Google Scholar]
- Blouet C, Schwartz GJ. Duodenal lipid sensing activates vagal afferents to regulate non-shivering brown fat thermogenesis in rats. PLoS One. 2012;7:e51898. doi: 10.1371/journal.pone.0051898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulant JA. Counterpoint: Heat-induced membrane depolarization of hypothalamic neurons: an unlikely mechanism of central thermosensitivity. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1481–1484. doi: 10.1152/ajpregu.00655.2005. discussion R1484. [DOI] [PubMed] [Google Scholar]
- Boulant JA, Dean JB. Temperature receptors in the central nervous system. Annu Rev Physiol. 1986;48:639–654. doi: 10.1146/annurev.ph.48.030186.003231. [DOI] [PubMed] [Google Scholar]
- Boulant JA, Hardy JD. The effect of spinal and skin temperatures on the firing rate and thermosensitivity of preoptic neurones. J Physiol. 1974;240:639–660. doi: 10.1113/jphysiol.1974.sp010627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JW, Sirlin EA, Benoit AM, Hoffman JM, Darnall RA. Activation of 5-HT1A receptors in medullary raphe disrupts sleep and decreases shivering during cooling in the conscious piglet. Am J Physiol Regul Integr Comp Physiol. 2007 doi: 10.1152/ajpregu.00655.2007. [DOI] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- Cano G, Passerin AM, Schiltz JC, Card JP, Morrison SF, Sved AF. Anatomical substrates for the central control of sympathetic outflow to interscapular adipose tissue during cold exposure. J Comp Neurol. 2003;460:303–326. doi: 10.1002/cne.10643. [DOI] [PubMed] [Google Scholar]
- Cao WH, Fan W, Morrison SF. Medullary pathways mediating specific sympathetic responses to activation of dorsomedial hypothalamus. Neuroscience. 2004;126:229–240. doi: 10.1016/j.neuroscience.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Cao WH, Madden CJ, Morrison SF. Inhibition of brown adipose tissue thermogenesis by neurons in the ventrolateral medulla and in the nucleus tractus solitarius. Am J Physiol Regul Integr Comp Physiol. 2010;299:R277–290. doi: 10.1152/ajpregu.00039.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao WH, Morrison SF. Disinhibition of rostral raphe pallidus neurons increases cardiac sympathetic nerve activity and heart rate. Brain Res. 2003;980:1–10. doi: 10.1016/s0006-8993(03)02981-0. [DOI] [PubMed] [Google Scholar]
- Cao WH, Morrison SF. Glutamate receptors in the raphe pallidus mediate brown adipose tissue thermogenesis evoked by activation of dorsomedial hypothalamic neurons. Neuropharmacology. 2006;51:426–437. doi: 10.1016/j.neuropharm.2006.03.031. [DOI] [PubMed] [Google Scholar]
- Cerri M, Morrison SF. Activation of lateral hypothalamic neurons stimulates brown adipose tissue thermogenesis. Neuroscience. 2005;135:627–638. doi: 10.1016/j.neuroscience.2005.06.039. [DOI] [PubMed] [Google Scholar]
- Chen XM, Hosono T, Yoda T, Fukuda Y, Kanosue K. Efferent projection from the preoptic area for the control of non-shivering thermogenesis in rats. J Physiol. 1998;512( Pt 3):883–892. doi: 10.1111/j.1469-7793.1998.883bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD, Bushnell MC, Zhang ET, Blomqvist A. A thalamic nucleus specific for pain and temperature sensation. Nature. 1994;372:770–773. doi: 10.1038/372770a0. [DOI] [PubMed] [Google Scholar]
- Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darian-Smith I, Johnson KO, Dykes R. “Cold” fiber population innervating palmar and digital skin of the monkey: responses to cooling pulses. J Neurophysiol. 1973;36:325–346. doi: 10.1152/jn.1973.36.2.325. [DOI] [PubMed] [Google Scholar]
- Dimitrov EL, Kim YY, Usdin TB. Regulation of hypothalamic signaling by tuberoinfundibular peptide of 39 residues is critical for the response to cold: a novel peptidergic mechanism of thermoregulation. J Neurosci. 2011;31:18166–18179. doi: 10.1523/JNEUROSCI.2619-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmquist JK, Scammell TE, Jacobson CD, Saper CB. Distribution of Fos-like immunoreactivity in the rat brain following intravenous lipopolysaccharide administration. Journal of Comparative Neurology. 1996;371:85–103. doi: 10.1002/(SICI)1096-9861(19960715)371:1<85::AID-CNE5>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Escourrou P, Freund PR, Rowell LB, Johnson DG. Splanchnic vasoconstriction in heat-stressed men: role of renin-angiotensin system. J Appl Physiol. 1982;52:1438–1443. doi: 10.1152/jappl.1982.52.6.1438. [DOI] [PubMed] [Google Scholar]
- Farrell MJ, Trevaks D, Taylor NA, McAllen RM. Regional brain responses associated with thermogenic and psychogenic sweating events in humans. J Neurophysiol. 2015;114:2578–2587. doi: 10.1152/jn.00601.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feketa VV, Balasubramanian A, Flores CM, Player MR, Marrelli SP. Shivering and tachycardic responses to external cooling in mice are substantially suppressed by TRPV1 activation but not by TRPM8 inhibition. Am J Physiol Regul Integr Comp Physiol. 2013;305:R1040–1050. doi: 10.1152/ajpregu.00296.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golozoubova V, Cannon B, Nedergaard J. UCP1 is essential for adaptive adrenergic nonshivering thermogenesis. Am J Physiol Endocrinol Metab. 2006;291:E350–357. doi: 10.1152/ajpendo.00387.2005. [DOI] [PubMed] [Google Scholar]
- Guieu JD, Hardy JD. Effects of heating and cooling of the spinal cord on preoptic unit activity. J Appl Physiol. 1970;29:675–683. doi: 10.1152/jappl.1970.29.5.675. [DOI] [PubMed] [Google Scholar]
- Gupta BN, Nier K, Hensel H. Cold-sensitive afferents from the abdomen. Pflugers Arch. 1979;380:203–204. doi: 10.1007/BF00582158. [DOI] [PubMed] [Google Scholar]
- Hammel HT, Hardy JD, Fusco MM. Thermoregulatory responses to hypothalamic cooling in unanesthetized dogs. Am J Physiol. 1960;198:481–486. doi: 10.1152/ajplegacy.1960.198.3.481. [DOI] [PubMed] [Google Scholar]
- Hermann DM, Luppi PH, Peyron C, Hinckel P, Jouvet M. Afferent projections to the rat nuclei raphe magnus, raphe pallidus and reticularis gigantocellularis pars alpha demonstrated by iontophoretic application of choleratoxin (subunit b) Journal of Chemical Neuroanatomy. 1997;13:1–21. doi: 10.1016/s0891-0618(97)00019-7. [DOI] [PubMed] [Google Scholar]
- Horn T, Wilkinson MF, Landgraf R, Pittman QJ. Reduced febrile responses to pyrogens after lesions of the hypothalamic paraventricular nucleus. American Journal of Physiology. 1994;267:R323–328. doi: 10.1152/ajpregu.1994.267.1.R323. [DOI] [PubMed] [Google Scholar]
- Hylden JL, Anton F, Nahin RL. Spinal lamina I projection neurons in the rat: collateral innervation of parabrachial area and thalamus. Neuroscience. 1989;28:27–37. doi: 10.1016/0306-4522(89)90229-7. [DOI] [PubMed] [Google Scholar]
- Imai-Matsumura K, Matsumura K, Nakayama T. Involvement of ventromedial hypothalamus in brown adipose tissue thermogenesis induced by preoptic cooling in rats. Jpn J Physiol. 1984;34:939–943. doi: 10.2170/jjphysiol.34.939. [DOI] [PubMed] [Google Scholar]
- Johnson CD, Gilbey MP. Effects of aortic nerve stimulation on discharges of sympathetic neurons innervating rat tail artery and vein. Am J Physiol. 1998;275:R942–949. doi: 10.1152/ajpregu.1998.275.4.R942. [DOI] [PubMed] [Google Scholar]
- Kataoka N, Hioki H, Kaneko T, Nakamura K. Psychological stress activates a dorsomedial hypothalamus-medullary raphe circuit driving brown adipose tissue thermogenesis and hyperthermia. Cell Metab. 2014;20:346–358. doi: 10.1016/j.cmet.2014.05.018. [DOI] [PubMed] [Google Scholar]
- Kerman IA, Enquist LW, Watson SJ, Yates BJ. Brainstem substrates of sympatho-motor circuitry identified using trans-synaptic tracing with pseudorabies virus recombinants. J Neurosci. 2003;23:4657–4666. doi: 10.1523/JNEUROSCI.23-11-04657.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi A, Osaka T. Involvement of the parabrachial nucleus in thermogenesis induced by environmental cooling in the rat. Pflugers Arch. 2003;446:760–765. doi: 10.1007/s00424-003-1119-7. [DOI] [PubMed] [Google Scholar]
- Kregel KC, Stauss H, Unger T. Modulation of autonomic nervous system adjustments to heat stress by central ANG II receptor antagonism. Am J Physiol. 1994;266:R1985–1991. doi: 10.1152/ajpregu.1994.266.6.R1985. [DOI] [PubMed] [Google Scholar]
- Kregel KC, Wall PT, Gisolfi CV. Peripheral vascular responses to hyperthermia in the rat. J Appl Physiol. 1988;64:2582–2588. doi: 10.1152/jappl.1988.64.6.2582. [DOI] [PubMed] [Google Scholar]
- Leite LH, Zheng H, Coimbra CC, Patel KP. Contribution of the paraventricular nucleus in autonomic adjustments to heat stress. Exp Biol Med (Maywood) 2012;237:570–577. doi: 10.1258/ebm.2011.011286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Xiong K, Pang Y, Dong Y, Kaneko T, Mizuno N. Medullary dorsal horn neurons providing axons to both the parabrachial nucleus and thalamus. J Comp Neurol. 2006;498:539–551. doi: 10.1002/cne.21068. [DOI] [PubMed] [Google Scholar]
- Lkhagvasuren B, Nakamura Y, Oka T, Sudo N, Nakamura K. Social defeat stress induces hyperthermia through activation of thermoregulatory sympathetic premotor neurons in the medullary raphe region. Eur J Neurosci. 2011;34:1442–1452. doi: 10.1111/j.1460-9568.2011.07863.x. [DOI] [PubMed] [Google Scholar]
- Lu J, Zhang YH, Chou TC, Gaus SE, Elmquist JK, Shiromani P, Saper CB. Contrasting effects of ibotenate lesions of the paraventricular nucleus and subparaventricular zone on sleep-wake cycle and temperature regulation. J Neurosci. 2001;21:4864–4874. doi: 10.1523/JNEUROSCI.21-13-04864.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundius EG, Sanchez-Alavez M, Ghochani Y, Klaus J, Tabarean IV. Histamine influences body temperature by acting at H1 and H3 receptors on distinct populations of preoptic neurons. J Neurosci. 2010;30:4369–4381. doi: 10.1523/JNEUROSCI.0378-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macefield VG, Wallin BG. Respiratory and cardiac modulation of single sympathetic vasoconstrictor and sudomotor neurones to human skin. J Physiol. 1999;516( Pt 1):303–314. doi: 10.1111/j.1469-7793.1999.303aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden CJ, Morrison SF. Excitatory amino acid receptor activation in the raphe pallidus area mediates prostaglandin-evoked thermogenesis. Neuroscience. 2003;122:5–15. doi: 10.1016/s0306-4522(03)00527-x. [DOI] [PubMed] [Google Scholar]
- Madden CJ, Morrison SF. Excitatory amino acid receptors in the dorsomedial hypothalamus mediate prostaglandin-evoked thermogenesis in brown adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2004;286:R320–325. doi: 10.1152/ajpregu.00515.2003. [DOI] [PubMed] [Google Scholar]
- Madden CJ, Morrison SF. Hypoxic activation of arterial chemoreceptors inhibits sympathetic outflow to brown adipose tissue (BAT) in rats. J Physiol. 2005 doi: 10.1113/jphysiol.2005.086322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden CJ, Morrison SF. Serotonin potentiates sympathetic responses evoked by spinal NMDA. J Physiol. 2006;577:525–537. doi: 10.1113/jphysiol.2006.116574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden CJ, Morrison SF. Neurons in the paraventricular nucleus of the hypothalamus inhibit sympathetic outflow to brown adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2009;296:R831–843. doi: 10.1152/ajpregu.91007.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden CJ, Morrison SF. Endogenous activation of spinal 5-hydroxytryptamine (5-HT) receptors contributes to the thermoregulatory activation of brown adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2010;298:R776–783. doi: 10.1152/ajpregu.00614.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Cora FJ, Fornal CA, Metzler CW, Jacobs BL. Single-unit responses of serotonergic medullary and pontine raphe neurons to environmental cooling in freely moving cats. Neuroscience. 2000;98:301–309. doi: 10.1016/s0306-4522(00)00133-0. [DOI] [PubMed] [Google Scholar]
- McKinley MJ, Yao ST, Uschakov A, McAllen RM, Rundgren M, Martelli D. The median preoptic nucleus: front and centre for the regulation of body fluid, sodium, temperature, sleep and cardiovascular homeostasis. Acta Physiol (Oxf) 2015;214:8–32. doi: 10.1111/apha.12487. [DOI] [PubMed] [Google Scholar]
- Minson CT, Wladkowski SL, Pawelczyk JA, Kenney WL. Age, splanchnic vasoconstriction, and heat stress during tilting. Am J Physiol. 1999;276:R203–212. doi: 10.1152/ajpregu.1999.276.1.r203. [DOI] [PubMed] [Google Scholar]
- Morrison SF. Raphe pallidus neurons mediate prostaglandin E2-evoked increases in brown adipose tissue thermogenesis. Neuroscience. 2003;121:17–24. doi: 10.1016/s0306-4522(03)00363-4. [DOI] [PubMed] [Google Scholar]
- Morrison SF. 2010 Carl Ludwig Distinguished Lectureship of the APS Neural Control and Autonomic Regulation Section: Central neural pathways for thermoregulatory cold defense. J Appl Physiol. 2011;110:1137–1149. doi: 10.1152/japplphysiol.01227.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SF, Cao WH, Madden CJ. Dorsomedial hypothalamic and brainstem pathways controlling thermogenesis in brown adipose tissue. Journal of Thermal Biology. 2004;29:333–337. [Google Scholar]
- Morrison SF, Madden CJ. Central nervous system regulation of brown adipose tissue. Compr Physiol. 2014a;4:1677–1713. doi: 10.1002/cphy.c140013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SF, Madden CJ, Tupone D. Central neural regulation of brown adipose tissue thermogenesis and energy expenditure. Cell Metab. 2014b;19:741–756. doi: 10.1016/j.cmet.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SF, Nakamura K. Central neural pathways for thermoregulation. Front Biosci (Landmark Ed) 2011;16:74–104. doi: 10.2741/3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SF, Sved AF, Passerin AM. GABA-mediated inhibition of raphe pallidus neurons regulates sympathetic outflow to brown adipose tissue. Am J Physiol. 1999;276:R290–297. doi: 10.1152/ajpregu.1999.276.2.R290. [DOI] [PubMed] [Google Scholar]
- Muzzi M, Blasi F, Masi A, Coppi E, Traini C, Felici R, Pittelli M, Cavone L, Pugliese AM, Moroni F, Chiarugi A. Neurological basis of AMP-dependent thermoregulation and its relevance to central and peripheral hyperthermia. J Cereb Blood Flow Metab. 2013;33:183–190. doi: 10.1038/jcbfm.2012.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima K, Nakai S, Tanaka M, Kanosue K. Neuronal circuitries involved in thermoregulation. Auton Neurosci. 2000;85:18–25. doi: 10.1016/S1566-0702(00)00216-2. [DOI] [PubMed] [Google Scholar]
- Nakamura K. Central circuitries for body temperature regulation and fever. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1207–1228. doi: 10.1152/ajpregu.00109.2011. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Matsumura K, Hubschle T, Nakamura Y, Hioki H, Fujiyama F, Boldogkoi Z, Konig M, Thiel HJ, Gerstberger R, Kobayashi S, Kaneko T. Identification of sympathetic premotor neurons in medullary raphe regions mediating fever and other thermoregulatory functions. J Neurosci. 2004;24:5370–5380. doi: 10.1523/JNEUROSCI.1219-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Matsumura K, Kaneko T, Kobayashi S, Katoh H, Negishi M. The rostral raphe pallidus nucleus mediates pyrogenic transmission from the preoptic area. J Neurosci. 2002;22:4600–4610. doi: 10.1523/JNEUROSCI.22-11-04600.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Morrison SF. Central efferent pathways mediating skin cooling-evoked sympathetic thermogenesis in brown adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2007;292:R127–136. doi: 10.1152/ajpregu.00427.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Morrison SF. Preoptic mechanism for cold-defensive responses to skin cooling. J Physiol. 2008a;586:2611–2620. doi: 10.1113/jphysiol.2008.152686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Morrison SF. A thermosensory pathway that controls body temperature. Nat Neurosci. 2008b;11:62–71. doi: 10.1038/nn2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Morrison SF. A thermosensory pathway mediating heat-defense responses. Proc Natl Acad Sci U S A. 2010;107:8848–8853. doi: 10.1073/pnas.0913358107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Morrison SF. Central efferent pathways for cold-defensive and febrile shivering. J Physiol. 2011;589:3641–3658. doi: 10.1113/jphysiol.2011.210047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Nakamura K, Matsumura K, Kobayashi S, Kaneko T, Morrison SF. Direct pyrogenic input from prostaglandin EP3 receptor-expressing preoptic neurons to the dorsomedial hypothalamus. Eur J Neurosci. 2005;22:3137–3146. doi: 10.1111/j.1460-9568.2005.04515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Nakamura K, Morrison SF. Different populations of prostaglandin EP3 receptor-expressing preoptic neurons project to two fever-mediating sympathoexcitatory brain regions. Neuroscience. 2009;161:614–620. doi: 10.1016/j.neuroscience.2009.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama T, Eisenman JS, Hardy JD. Single unit activity of anterior hypothalamus during local heating. Science. 1961;134:560–561. doi: 10.1126/science.134.3478.560. [DOI] [PubMed] [Google Scholar]
- Nakayama T, Hammel H, Hardy J, Eisenman J. Thermal stimulation of electrical activity of single units of the preoptic region. Am J Physiol. 1963;204:1122–1126. [Google Scholar]
- Nason MW, Jr, Mason P. Modulation of sympathetic and somatomotor function by the ventromedial medulla. J Neurophysiol. 2004;92:510–522. doi: 10.1152/jn.00089.2004. [DOI] [PubMed] [Google Scholar]
- Nason MW, Jr, Mason P. Medullary raphe neurons facilitate brown adipose tissue activation. Journal of Neuroscience. 2006;26:1190–1198. doi: 10.1523/JNEUROSCI.4707-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2007;293:E444–452. doi: 10.1152/ajpendo.00691.2006. [DOI] [PubMed] [Google Scholar]
- Oldfield BJ, Giles ME, Watson A, Anderson C, Colvill LM, McKinley MJ. The neurochemical characterisation of hypothalamic pathways projecting polysynaptically to brown adipose tissue in the rat. Neuroscience. 2002;110:515–526. doi: 10.1016/s0306-4522(01)00555-3. [DOI] [PubMed] [Google Scholar]
- Ono K, Tsukamoto-Yasui M, Hara-Kimura Y, Inoue N, Nogusa Y, Okabe Y, Nagashima K, Kato F. Intragastric administration of capsiate, a transient receptor potential channel agonist, triggers thermogenic sympathetic responses. J Appl Physiol (1985) 2011;110:789–798. doi: 10.1152/japplphysiol.00128.2010. [DOI] [PubMed] [Google Scholar]
- Ootsuka Y, Blessing WW. Inhibition of medullary raphe/parapyramidal neurons prevents cutaneous vasoconstriction elicited by alerting stimuli and by cold exposure in conscious rabbits. Brain Res. 2005a;1051:189–193. doi: 10.1016/j.brainres.2005.05.062. [DOI] [PubMed] [Google Scholar]
- Ootsuka Y, Blessing WW, Steiner AA, Romanovsky AA. Fever response to intravenous prostaglandin E2 is mediated by the brain but does not require afferent vagal signaling. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1294–1303. doi: 10.1152/ajpregu.00709.2007. [DOI] [PubMed] [Google Scholar]
- Ootsuka Y, McAllen RM. Interactive drives from two brain stem premotor nuclei are essential to support rat tail sympathetic activity. Am J Physiol Regul Integr Comp Physiol. 2005b;289:R1107–1115. doi: 10.1152/ajpregu.00005.2005. [DOI] [PubMed] [Google Scholar]
- Osaka T. Cold-induced thermogenesis mediated by GABA in the preoptic area of anesthetized rats. Am J Physiol Regul Integr Comp Physiol. 2004;287:R306–313. doi: 10.1152/ajpregu.00003.2004. [DOI] [PubMed] [Google Scholar]
- Owens NC, Ootsuka Y, Kanosue K, McAllen RM. Thermoregulatory control of sympathetic fibres supplying the rat’s tail. J Physiol. 2002;543:849–858. doi: 10.1113/jphysiol.2002.023770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmes ED, Park CR. The regulation of body temperature during fever. Arch Environ Health. 1965;11:749–759. doi: 10.1080/00039896.1965.10664295. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathner JA, Madden CJ, Morrison SF. Central pathway for spontaneous and prostaglandin E2-evoked cutaneous vasoconstriction. Am J Physiol Regul Integr Comp Physiol. 2008;295:R343–354. doi: 10.1152/ajpregu.00115.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathner JA, Morrison SF. Rostral ventromedial periaqueductal gray: a source of inhibition of the sympathetic outflow to brown adipose tissue. Brain Res. 2006;1077:99–107. doi: 10.1016/j.brainres.2006.01.035. [DOI] [PubMed] [Google Scholar]
- Riedel W. Warm receptors in the dorsal abdominal wall of the rabbit. Pflugers Arch. 1976;361:205–206. doi: 10.1007/BF00583468. [DOI] [PubMed] [Google Scholar]
- Romanovsky A. Skin temperature: its role in thermoregulation. Acta Physiol (Oxf) 2014;210:498–507. doi: 10.1111/apha.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanovsky AA. Do fever and anapyrexia exist? Analysis of set point-based definitions. Am J Physiol Regul Integr Comp Physiol. 2004;287:R992–995. doi: 10.1152/ajpregu.00068.2004. [DOI] [PubMed] [Google Scholar]
- Romanovsky AA. Thermoregulation: some concepts have changed. Functional architecture of the thermoregulatory system. American Journal of Physiology - Regulatory Integrative & Comparative Physiology. 2007;292:R37–46. doi: 10.1152/ajpregu.00668.2006. [DOI] [PubMed] [Google Scholar]
- Rothwell NJ, Stock MJ, Thexton AJ. Decerebration activates thermogenesis in the rat. J Physiol (Lond) 1983;342:15–22. doi: 10.1113/jphysiol.1983.sp014836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels BC, Zaretsky DV, DiMicco JA. Tachycardia evoked by disinhibition of the dorsomedial hypothalamus in rats is mediated through medullary raphe. J Physiol. 2002;538:941–946. doi: 10.1113/jphysiol.2001.013302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB, Breder CD. The neurologic basis of fever. N Engl J Med. 1994;330:1880–1886. doi: 10.1056/NEJM199406303302609. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Zaretskaia MV, Zaretsky DV, Moreno M, DiMicco JA. Stress- and lipopolysaccharide-induced c-fos expression and nNOS in hypothalamic neurons projecting to medullary raphe in rats: a triple immunofluorescent labeling study. European Journal of Neuroscience. 2007;26:2228–2238. doi: 10.1111/j.1460-9568.2007.05843.x. [DOI] [PubMed] [Google Scholar]
- Satinoff E, Rutstein J. Behavioral thermoregulation in rats with anterior hypothalamic lesions. J Comp Physiol Psychol. 1970;71:77–82. doi: 10.1037/h0028959. [DOI] [PubMed] [Google Scholar]
- Schafer SS, Schafer S. The behavior of the proprioceptors of the muscle and the innervation of the fusimotor system during cold shivering. Exp Brain Res. 1973a;17:364–380. doi: 10.1007/BF00234100. [DOI] [PubMed] [Google Scholar]
- Schafer SS, Schafer S. The role of the primary afference in the generation of a cold shivering tremor. Exp Brain Res. 1973b;17:381–393. doi: 10.1007/BF00234101. [DOI] [PubMed] [Google Scholar]
- Shafton AD, McAllen RM. Location of cat brain stem neurons that drive sweating. Am J Physiol Regul Integr Comp Physiol. 2013;304:R804–809. doi: 10.1152/ajpregu.00040.2013. [DOI] [PubMed] [Google Scholar]
- Shafton AD, Oldfield BJ, McAllen RM. CRF-like immunoreactivity selectively labels preganglionic sudomotor neurons in cat. Brain Res. 1992;599:253–260. doi: 10.1016/0006-8993(92)90399-t. [DOI] [PubMed] [Google Scholar]
- Shibasaki M, Aoki K, Morimoto K, Johnson JM, Takamata A. Plasma hyperosmolality elevates the internal temperature threshold for active thermoregulatory vasodilation during heat stress in humans. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1706–1712. doi: 10.1152/ajpregu.00242.2009. [DOI] [PubMed] [Google Scholar]
- Shibasaki M, Crandall CG. Mechanisms and controllers of eccrine sweating in humans. Front Biosci (Schol Ed) 2010;2:685–696. doi: 10.2741/s94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CJ, Johnson JM. Responses to hyperthermia. Optimizing heat dissipation by convection and evaporation: Neural control of skin blood flow and sweating in humans. Auton Neurosci. 2016 doi: 10.1016/j.autneu.2016.1001.1002. [DOI] [PubMed] [Google Scholar]
- Smith JE, Jansen AS, Gilbey MP, Loewy AD. CNS cell groups projecting to sympathetic outflow of tail artery: neural circuits involved in heat loss in the rat. Brain Res. 1998;786:153–164. doi: 10.1016/s0006-8993(97)01437-6. [DOI] [PubMed] [Google Scholar]
- Steiner AA, Turek VF, Almeida MC, Burmeister JJ, Oliveira DL, Roberts JL, Bannon AW, Norman MH, Louis JC, Treanor JJ, Gavva NR, Romanovsky AA. Nonthermal activation of transient receptor potential vanilloid-1 channels in abdominal viscera tonically inhibits autonomic cold-defense effectors. J Neurosci. 2007;27:7459–7468. doi: 10.1523/JNEUROSCI.1483-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stornetta RL, Rosin DL, Simmons JR, McQuiston TJ, Vujovic N, Weston MC, Guyenet PG. Coexpression of vesicular glutamate transporter-3 and gamma-aminobutyric acidergic markers in rat rostral medullary raphe and intermediolateral cell column. J Comp Neurol. 2005;492:477–494. doi: 10.1002/cne.20742. [DOI] [PubMed] [Google Scholar]
- Szekely M. The vagus nerve in thermoregulation and energy metabolism. Auton Neurosci. 2000;85:26–38. doi: 10.1016/S1566-0702(00)00217-4. [DOI] [PubMed] [Google Scholar]
- Szymusiak R, Satinoff E. Acute thermoregulatory effects of unilateral electrolytic lesions of the medial and lateral preoptic area in rats. Physiol Behav. 1982;28:161–170. doi: 10.1016/0031-9384(82)90118-4. [DOI] [PubMed] [Google Scholar]
- Tabarean IV, Behrens MM, Bartfai T, Korn H. Prostaglandin E2-increased thermosensitivity of anterior hypothalamic neurons is associated with depressed inhibition. Proc Natl Acad Sci U S A. 2004;101:2590–2595. doi: 10.1073/pnas.0308718101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Zhang W, Sameshima K, Kuroki C, Matsumoto A, Sunanaga J, Kono Y, Sakurai T, Kanmura Y, Kuwaki T. Orexin neurons are indispensable for prostaglandin E2-induced fever and defence against environmental cooling in mice. J Physiol. 2013 doi: 10.1113/jphysiol.2013.261271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamata A, Mack GW, Gillen CM, Jozsi AC, Nadel ER. Osmoregulatory modulation of thermal sweating in humans: reflex effects of drinking. Am J Physiol. 1995;268:R414–422. doi: 10.1152/ajpregu.1995.268.2.R414. [DOI] [PubMed] [Google Scholar]
- Tanaka M, McAllen RM. A subsidiary fever center in the medullary raphe? Am J Physiol Regul Integr Comp Physiol. 2005;289:R1592–1598. doi: 10.1152/ajpregu.00141.2005. [DOI] [PubMed] [Google Scholar]
- Tanaka M, McKinley MJ, McAllen RM. Roles of two preoptic cell groups in tonic and febrile control of rat tail sympathetic fibers. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1248–1257. doi: 10.1152/ajpregu.91010.2008. [DOI] [PubMed] [Google Scholar]
- Tanaka M, McKinley MJ, McAllen RM. Preoptic-raphe connections for thermoregulatory vasomotor control. J Neurosci. 2011;31:5078–5088. doi: 10.1523/JNEUROSCI.6433-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, McKinley MJ, McAllen RM. Role of an excitatory preoptic-raphe pathway in febrile vasoconstriction of the rat’s tail. Am J Physiol Regul Integr Comp Physiol. 2013;305:R1479–1489. doi: 10.1152/ajpregu.00401.2013. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Nagashima K, McAllen RM, Kanosue K. Role of the medullary raphe in thermoregulatory vasomotor control in rats. J Physiol. 2002;540:657–664. doi: 10.1113/jphysiol.2001.012989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Owens NC, Nagashima K, Kanosue K, McAllen RM. Reflex activation of rat fusimotor neurons by body surface cooling, and its dependence on the medullary raphe. J Physiol. 2006;572:569–583. doi: 10.1113/jphysiol.2005.102400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Tonouchi M, Hosono T, Nagashima K, Yanase-Fujiwara M, Kanosue K. Hypothalamic region facilitating shivering in rats. Jpn J Physiol. 2001;51:625–629. doi: 10.2170/jjphysiol.51.625. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Toth IE, Toth DE, Boldogkoi Z, Hornyak A, Palkovits M, Blessing WW. Serotonin-synthesizing neurons in the rostral medullary raphe/parapyramidal region transneuronally labelled after injection of pseudorabies virus into the rat tail. Neurochem Res. 2006;31:277–286. doi: 10.1007/s11064-005-9018-2. [DOI] [PubMed] [Google Scholar]
- Tsukita S, Yamada T, Uno K, Takahashi K, Kaneko K, Ishigaki Y, Imai J, Hasegawa Y, Sawada S, Ishihara H, Oka Y, Katagiri H. Hepatic glucokinase modulates obesity predisposition by regulating BAT thermogenesis via neural signals. Cell Metab. 2012;16:825–832. doi: 10.1016/j.cmet.2012.11.006. [DOI] [PubMed] [Google Scholar]
- Tupone D, Madden CJ, Cano G, Morrison SF. An orexinergic projection from perifornical hypothalamus to raphe pallidus increases rat brown adipose tissue thermogenesis. J Neurosci. 2011;31:15944–15955. doi: 10.1523/JNEUROSCI.3909-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupone D, Madden CJ, Morrison SF. Central activation of the A1 adenosine receptor (A1AR) induces a hypothermic, torpor-like state in the rat. J Neurosci. 2013;33:14512–14525. doi: 10.1523/JNEUROSCI.1980-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turlejska E, Baker MA. Elevated CSF osmolality inhibits thermoregulatory heat loss responses. Am J Physiol. 1986;251:R749–754. doi: 10.1152/ajpregu.1986.251.4.R749. [DOI] [PubMed] [Google Scholar]
- van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerback S, Nuutila P. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- Wallin BG, Charkoudian N. Sympathetic neural control of integrated cardiovascular function: insights from measurement of human sympathetic nerve activity. Muscle Nerve. 2007;36:595–614. doi: 10.1002/mus.20831. [DOI] [PubMed] [Google Scholar]
- Whyte DG, Johnson AK. Lesions of the anteroventral third ventricle region (AV3V) disrupt cardiovascular responses to an elevation in core temperature. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1783–1790. doi: 10.1152/ajpregu.00524.2004. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Konishi M, Nagashima K, Saper CB, Kanosue K. Fos activation in hypothalamic neurons during cold or warm exposure: projections to periaqueductal gray matter. Neuroscience. 2005;133:1039–1046. doi: 10.1016/j.neuroscience.2005.03.044. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Li X, Cano G, Lazarus M, Saper CB. Parallel preoptic pathways for thermoregulation. J Neurosci. 2009;29:11954–11964. doi: 10.1523/JNEUROSCI.2643-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Maruyama M, Hosono T, Nagashima K, Fukuda Y, Gerstberger R, Kanosue K. Fos expression induced by warming the preoptic area in rats. Brain Res. 2002;933:109–117. doi: 10.1016/s0006-8993(02)02287-4. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Nakamura K, Matsumura K, Kanosue K, Konig M, Thiel HJ, Boldogkoi Z, Toth I, Roth J, Gerstberger R, Hubschle T. Neurons of the rat preoptic area and the raphe pallidus nucleus innervating the brown adipose tissue express the prostaglandin E receptor subtype EP3. Eur J Neurosci. 2003;18:1848–1860. doi: 10.1046/j.1460-9568.2003.02919.x. [DOI] [PubMed] [Google Scholar]
- Zaretskaia MV, Zaretsky DV, DiMicco JA. Role of the dorsomedial hypothalamus in thermogenesis and tachycardia caused by microinjection of prostaglandin E2 into the preoptic area in anesthetized rats. Neuroscience Letters. 2003;340:1–4. doi: 10.1016/s0304-3940(03)00047-8. [DOI] [PubMed] [Google Scholar]
- Zaretsky DV, Hunt JL, Zaretskaia MV, DiMicco JA. Microinjection of prostaglandin E2 and muscimol into the preoptic area in conscious rats: comparison of effects on plasma adrenocorticotrophic hormone (ACTH), body temperature, locomotor activity, and cardiovascular function. Neurosci Lett. 2006;397:291–296. doi: 10.1016/j.neulet.2005.12.032. [DOI] [PubMed] [Google Scholar]
- Zaretsky DV, Zaretskaia MV, DiMicco JA. Stimulation and blockade of GABAA receptors in the raphe pallidus: effects on body temperature, heart rate, and blood pressure in conscious rats. Am J Physiol Regul Integr Comp Physiol. 2003;285:R110–116. doi: 10.1152/ajpregu.00016.2003. [DOI] [PubMed] [Google Scholar]
- Zhang W, Sunanaga J, Takahashi Y, Mori T, Sakurai T, Kanmura Y, Kuwaki T. Orexin neurons are indispensable for stress-induced thermogenesis in mice. J Physiol. 2010;588:4117–4129. doi: 10.1113/jphysiol.2010.195099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Kerman IA, Laque A, Nguyen P, Faouzi M, Louis GW, Jones JC, Rhodes C, Munzberg H. Leptin-receptor-expressing neurons in the dorsomedial hypothalamus and median preoptic area regulate sympathetic brown adipose tissue circuits. J Neurosci. 2011;31:1873–1884. doi: 10.1523/JNEUROSCI.3223-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YH, Hosono T, Yanase-Fujiwara M, Chen XM, Kanosue K. Effect of midbrain stimulations on thermoregulatory vasomotor responses in rats. J Physiol (Lond) 1997;503:177–186. doi: 10.1111/j.1469-7793.1997.177bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YH, Lu J, Elmquist JK, Saper CB. Lipopolysaccharide activates specific populations of hypothalamic and brainstem neurons that project to the spinal cord. J Neurosci. 2000;20:6578–6586. doi: 10.1523/JNEUROSCI.20-17-06578.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YH, Yanase-Fujiwara M, Hosono T, Kanosue K. Warm and cold signals from the preoptic area: which contribute more to the control of shivering in rats? J Physiol. 1995;485(Pt 1):195–202. doi: 10.1113/jphysiol.1995.sp020723. [DOI] [PMC free article] [PubMed] [Google Scholar]