Abstract
Background
Midodrine is prescribed to prevent symptomatic hypotension and decrease complications associated with hypotension during dialysis. We hypothesized that midodrine use before kidney transplantation may be a novel marker for posttransplant risk.
Methods
We analyzed integrated national U.S. transplant registry, pharmacy records and Medicare claims data for 16,308 kidney transplant recipients transplanted 2006–2008, of whom 308 (1.9%) had filled midodrine prescriptions in the year prior to transplantation. Delayed graft function (DGF), graft failure and patient death were ascertained from the registry. Posttransplant cardiovascular complications were identified using diagnosis codes on Medicare billing claims. Adjusted associations (adjusted hazards ratio) of pretransplant midodrine use with complications at 3 and 12 months posttransplant were quantified by multivariate Cox regression, including propensity for midodrine exposure.
Results
At 3 months, patients who used midodrine pretransplant had higher rates of DGF, 32% vs. 19%; hypotension, 14% vs. 4%; acute myocardial infarction, 4% vs. 2%; cardiac arrest, 2% vs. 0.9%, graft failure, 5% vs. 2%; and death, 4% vs. 1% than non-users (P<0.05). After multivariate adjustment including recipient, and donor factors, as well as for the propensity of midodrine exposure, pretransplant midodrine use was independently associated with risks of DGF (adjusted odd ratio 1.95; CI 1.49–2.56), death-censored graft failure (adjusted hazard ratio (aHR) 1.94; CI 1.14–3.27), and death (aHR 3.55; CI 1.99–6.33). Patterns were similar at 12 months.
Conclusions
Although associations may in part reflect underlying conditions, the need for midodrine before kidney transplantation is a risk marker for complications including DGF, graft failure, and death.
INTRODUCTION
Kidney transplant candidates undergo pretransplant assessment of overall and cardiovascular fitness, such that selected candidates are deemed to have acceptable risks of perioperative and longer-term complications to benefit from transplantation1. Nonetheless, with the aging and increasing comorbidity burden of the end stage renal disease (ESRD) population, many patients with stable but prognostically important comorbidities are listed for and receive transplants2. Despite the critical importance of baseline comorbidities for posttransplant patient and graft survival, only limited measures of comorbid conditions and no measures of medical or pharmaceutical care among candidates are captured in the national U.S. transplant registry. Thus, novel approaches to supplementing the registry, such as with integrated measures of medical care3–5, are needed to advance understanding of relationships of pretransplant comorbidities with posttransplant outcomes.
Symptomatic hypotension is a common complication in patients with ESRD, especially among those with prolonged dialysis dependence6,7. Symptomatic hypotension has been estimated to complicate approximately 25% of hemodialysis treatments, and may result in dialysis session interruptions and prevent delivery of adequate clearance and fluid removal8. Several therapeutic interventions are commonly used to prevent intradialytic hypotension including low temperature dialysate9, reduced ultrafiltration rate through longer and/or more frequent dialysis10, high dialysate calcium concentration11, and dialysate sodium modeling12. When these interventions fail to prevent intradialytic hypotension, pharmacological treatment with midodrine may be used13. Midodrine is an alpha-1 adrenergic receptor agonist that induces both arterial and venous vasoconstriction, leading to an increase in peripheral vascular resistance and a decrease in venous blood pooling. Midodrine is approved by the US Food and Drug Administration (FDA) for the treatment of orthostatic hypotension14–16 and may be used to manage resistant hypotension among ESRD patients in whom hypotension would otherwise compromise their dialysis17.
Despite available treatment options, hypotension in a dialysis patient is not a benign finding18,19. For example, one study of 1244 maintenance hemodialysis patients in Japan found that each 20 mmHg decrement in systolic blood pressure during was associated with a 21% reduction in the odds of 2-year survival (odds ratio 0.79, 95% CI 0.64–0.98)18. Low blood pressure may aggravate cardiovascular and other end-organ hypo-perfusion, leading to myocardial infarction, ventricular arrhythmias, stroke and death18. We hypothesized that requirement for midodrine use among kidney transplant candidates might also have prognostic implications for posttransplant patient and graft outcomes. To address this hypothesis, we examined a novel database that integrates national transplant registry data with pharmacy fill records and Medicare billing claims. Our goals were to identify pretransplant midodrine use as an indicator of symptomatic hypotension, examine correlates of midodrine use, and determine whether midodrine exposure before transplant predicts posttransplant cardiovascular complications, allograft dysfunction, and patient mortality.
METHODS
Data Sources
This study used data from the Organ Procurement and Transplantation Network (OPTN). The OPTN data system includes data on all donor, wait-listed candidates, and transplant recipients in the U.S., submitted by the members of the OPTN, and has been described elsewhere20. The Health Resources and Services Administration (HRSA), U.S. Department of Health and Human Services provides oversight to the activities of the OPTN contractor.
Pharmacy fill data were assembled by linking OPTN records for kidney transplant recipients with billing claims from a large U.S. pharmaceutical claims data (PCD) clearinghouse that captures prescription drug fill records including those reimbursed by private payers, public payers, and self-paid fills. The PCD comprises National Council for Prescription Drug Program (NCPDP) 5.1-format prescription claims aggregated from multiple sources including data clearinghouses, retail pharmacies, and prescription benefit managers for approximately 60% of U.S. retail pharmacy transactions. Individual claim records include the date of a given pharmacy fill with the National Drug Code (NDC) identifying agent and dosage. After Institutional Review Board and HRSA approvals, PCD records were linked with OPTN records for kidney transplant recipients. We applied a de-identification strategy wherein patient identifiers (last name, first name, date of birth, gender and ZIP code of residence) were transformed before delivery to the Saint Louis University researchers with encryption technology from Management Science Associates, Inc. The Patient De-Identification Software employs multiple encryption algorithms in succession to guarantee that the resulting “token” containing encrypted patient identifiers can never be decrypted. However, the algorithm yields the same results for a given set of data elements, such that linkages by unique anonymous tokens are possible.
Medicare billing claims data include diagnostic and procedure codes for patients with Medicare fee-for-service primary or secondary insurance (service information is submitted to and tracked by Medicare even if Medicare is not the primary payer). After regulatory approvals, beneficiary identifier numbers from Medicare’s electronic databases were linked using Social Security Number, sex, and birthdates to unique OPTN identification numbers. Finally, patients with PCD data before transplant and Medicare claims data after transplant were selected for inclusion using anonymous OPTN identification numbers.
Because of the large sample size, the anonymity of the patients studied, and the non-intrusive nature of the research, a waiver of informed consent was granted per the Department of Health and Human Services Code of Federal Regulations (Title 45, Part 46, Paragraph 46.116). Analyses were performed using Health Information Portability and Accountability Act (HIPAA)-compliant, limited datasets from which all direct identifiers were removed. This study was approved by the Institutional Review Board of Saint Louis University.
Sampling and Exposure Definitions
We selected kidney-only transplant recipients with at least one year of captured pharmaceutical fill records before transplant and Medicare eligibility at the time of transplant. As PCD data were available beginning in 2005, patients with linked pretransplant PCD data were transplanted beginning in 2006. The final analytic sample included patients transplanted in 2006 to 2008, based on overlap with the available Medicare claims data that ended in December 2008. Transplant recipient clinical and demographic factors, as well characteristics of the donated organ and other transplant factors, were defined by the OPTN Transplant Candidate (TCR) and Recipient Registration (TRR) forms (Table 1).
Table 1.
Distributions of clinical traits in the study sample of transplant recipients according to pretransplant midodrine use.
| Clinical Factors | Midodrine N= 308 |
No Midodrine n=16000 |
P value |
|---|---|---|---|
| % | % | % | |
| Recipient characteristics | |||
| Age, years | <.0001 | ||
| <18 | 0.3 | 2.9 | |
| 18 to 30 | 2.3 | 7.8 | |
| 31 to 44 | 15.6 | 19.5 | |
| 45 to 59 | 49.4 | 38.5 | |
| ≥60 | 32.5 | 31.3 | |
| Male | 48.7 | 60.7 | <.0001 |
| Race | <.0001 | ||
| White | 70.8 | 57.3 | |
| Black | 19.2 | 24.5 | |
| Other race | 10.1 | 18.2 | |
| Body mass index, kg/m2 | 0.001 | ||
| <18.5 | 3.6 | 3.8 | |
| 18.5 to 25 | 23.1 | 32.2 | |
| 25 to 30 | 32.5 | 32.9 | |
| >30 | 40.26 | 30.4 | |
| Employment status | <.0001 | ||
| Working | 14.3 | 24.7 | |
| Not working | 73.1 | 59.2 | |
| Unknown | 12.7 | 16.0 | |
| Insurance type | 0.0003 | ||
| Public | 75.3 | 67.2 | |
| Private | 24.0 | 32.6 | |
| Other/unknown | 0.7 | 0.1 | |
| Dialysis type | 0.0003 | ||
| Preemptive | 4.6 | 11.6 | |
| Peritoneal | 7.1 | 8.2 | |
| Hemodialysis | 88.3 | 80.2 | |
| Pretransplant ESRD duration, months | <.0001 | ||
| None (preemptive) | 4.6 | 11.6 | |
| >0 to 24 | 29.2 | 31.1 | |
| 25 to 60 | 34.4 | 34.8 | |
| >60 | 30.84 | 21.1 | |
| Cause of ESRD | <.0001 | ||
| Diabetes | 32.14 | 24.5 | |
| Glomerulonephritis | 12.01 | 20.1 | |
| Hypertension | 13.96 | 23.3 | |
| Polycystic kidney disease | 9.74 | 7.8 | |
| Other | 32.14 | 24.3 | |
| Comorbidities | |||
| Diabetes | 46.43 | 34.5 | <.0001 |
| Coronary disease/angina | 6.17 | 4.3 | 0.11 |
| COPD | 0.97 | 1.3 | 0.63 |
| Hypertension | 54.22 | 57.1 | 0.31 |
| Cerebral vascular disease | 3.57 | 2.3 | 0.14 |
| Peripheral vascular disease | 7.79 | 4.5 | 0.007 |
| Previous transplant | 21.75 | 14.6 | 0.0004 |
| Peak PRA level, % | <.0001 | ||
| <10 | 59.74 | 69.1 | |
| 10 to 79 | 19.48 | 18.5 | |
| ≥80 | 15.91 | 8.4 | |
| Missing | 4.87 | 4.0 | |
| Donor and Transplant Characteristics | |||
| Age, years, mean (SD) | 40.5 (15.9) | 39.7 (15.4) | 0.35 |
| Female donor | 47.73 | 45.9 | 0.52 |
| Donor race | 0.11 | ||
| White | 77.27 | 71.8 | |
| Black | 10.06 | 12.4 | |
| Other race | 12.66 | 15.8 | |
| CMV sero-pairing | 0.51 | ||
| Recipient −, Donor − | 14.61 | 15.2 | |
| Recipient +, Donor − | 23.7 | 23.1 | |
| Recipient −, Donor + | 16.23 | 17.1 | |
| Recipient +, Donor+ | 37.01 | 38.5 | |
| HLA mismatches | 0.32 | ||
| Zero A, B, and DR | 12.01 | 10.8 | |
| Zero DR | 40.91 | 45.1 | |
| Other | 47.08 | 44.1 | |
| Donor type | 0.0005 | ||
| Living | 21.1 | 31.9 | |
| Standard criteria deceased | 56.17 | 48.6 | |
| Donation after cardiac death | 8.12 | 8.0 | |
| Pumped expanded criteria deceased | 9.74 | 6.3 | |
| Non-pumped expanded criteria deceased | 4.87 | 5.2 | |
Abbreviations: CMV, cytomegalovirus; COPD, chronic obstructive pulmonary disease; ESRD, end-stage renal disease; HLA, human leukocyte antigen; PRA, panel reactive antibody.
Pretransplant midodrine use was defined as a midodrine-prescription fill within a year before transplant and was ascertained by submission of pharmacy fill records with a corresponding National Drug Code (NDC).
Posttransplant Outcomes
Delayed graft function (DGF) was defined as the need for dialysis within the first week of transplantation. Graft failure was defined as the earliest reported data of return to maintenance dialysis, re-transplantation, or patient death. Death-censored graft failure was defined as the earliest reported date of return to maintenance dialysis or re-transplantation. Posttransplant diagnoses of hypotension, acute myocardial infarction, stroke, ventricular arrhythmias, and cardiac arrest were ascertained by International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis codes on billing claims (SDC, Table 1), as previously defined in studies of these conditions after transplantation5,21–23.
Statistical Analyses
Data management and analyses were performed with SAS for Windows software, version 9.3 (SAS Institute Inc., Cary, NC). Distributions of clinical and demographics traits among patients with pretransplant midodrine exposure, compared to those without midodrine use, were compared by the Chi-square test.
Patient death, death-censored graft failure, and clinical complications were estimated at 3 and 12 months posttransplantation by the Kaplan-Meier method, with use of the Log-Rank test to assess the statistical significance of differences in unadjusted survival. At-risk time for all models was censored at end of assessment period, loss to follow-up, end of Medicare enrollment, end of study (December 31, 2008), or death for patients without concurrent study complication on date of death. Propensity scores for the likelihood of pretransplant midodrine use were estimated by logistic regression (SDC, Table 2). Adjusted associations of midodrine use with DGF, death-censored graft failure, posttransplant death, and clinical complications were quantified by multivariate Cox regression including adjustment for recipient, donor and transplant clinical factors listed in Table 1, and for quintile of propensity for midodrine exposure.
The combined impacts of pretransplant midodrine use and allograft type on one-year all-cause graft survival were estimated by the survival functions from multivariate Cox regression, stratified by donor type and including adjustment for characteristics of recipients of living donor, standard criteria organs donated (SCD) after brain death, expanded criteria organs donated (ECD) after brain death, and organs donated after cardiac death (DCD), respectively. ECD allografts were defined by the United Network for Organ Sharing (UNOS) definition active prior to the December 2014 revision of kidney allocation policy24. For a sub-analysis of deceased donor allograft survival, kidney donor profile index (KDPI) was computed by the UNOS definition25 and categorized as > 85%, 85–20%, and KDPI < 20%.
RESULTS
Sample Characteristics and Correlates for Pretransplant Midodrine Use
There were 31,197 U.S kidney-only transplant recipients from 2006 to 2010 with linked transplant registry and pharmacy claims in the year prior to transplantation. Of these, we identified 16,308 recipients with linked Medicare claims data from 2006 to 2008 for the current analysis. Of this study sample, 1.9 % (N=308) filled midodrine prescriptions in the year before transplant. Raw distributions of clinical traits according to midodrine use are shown in Table 1. Compared with transplant recipients who did not use midodrine before transplant, midodrine users were more commonly older (age category 45 to 59), women, white race, obese, and publicly insured. Patients who used midodrine before transplant also more commonly had diabetes, peripheral vascular disease, kidney failure due to diabetes, high levels of sensitization, longer dialysis dependence, and previous kidney transplantation compared to those who did not use midodrine. Allografts received by midodrine users more commonly included organs from deceased donors and pumped ECD kidneys, but other donor factors were similar in recipients with and without pretransplant midodrine exposure (Table 1).
Incidence of Posttransplant Clinical Complications
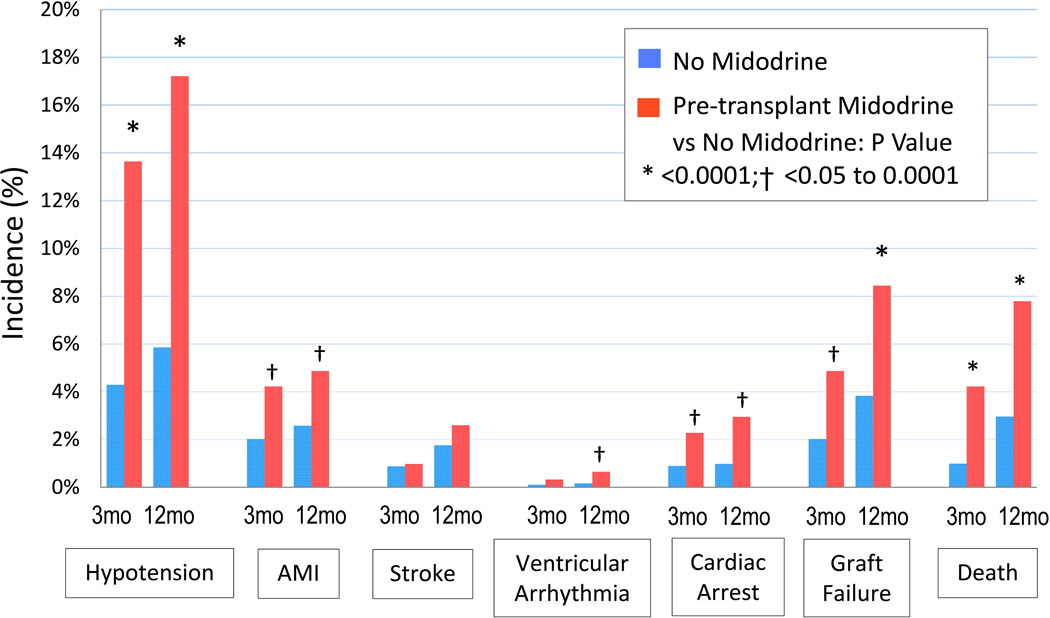
At three months posttransplant, patients who received midodrine before transplant had significantly higher rates of DGF, 32% vs. 19%; hypotension, 14% vs. 4%; acute myocardial infarction (AMI), 4% vs. 2%; cardiac arrest, 2% vs. 0.9%, graft failure, 5% vs. 2%; and death, 4% vs. 1% than midodrine non-users (Figure 1). Patterns were similar at 12 months posttransplantation. Ventricular arrhythmia was not higher in midodrine users compared to non-users at 3 months but was significantly higher at 12 months. There was no difference in stroke rates between midodrine users and non-users.
Figure 1.
Incidence of complications at 3-months and 12-months posttransplant according to pretransplant midodrine use.
Adjusted Associations of Pretransplant Midodrine with Clinical Complications
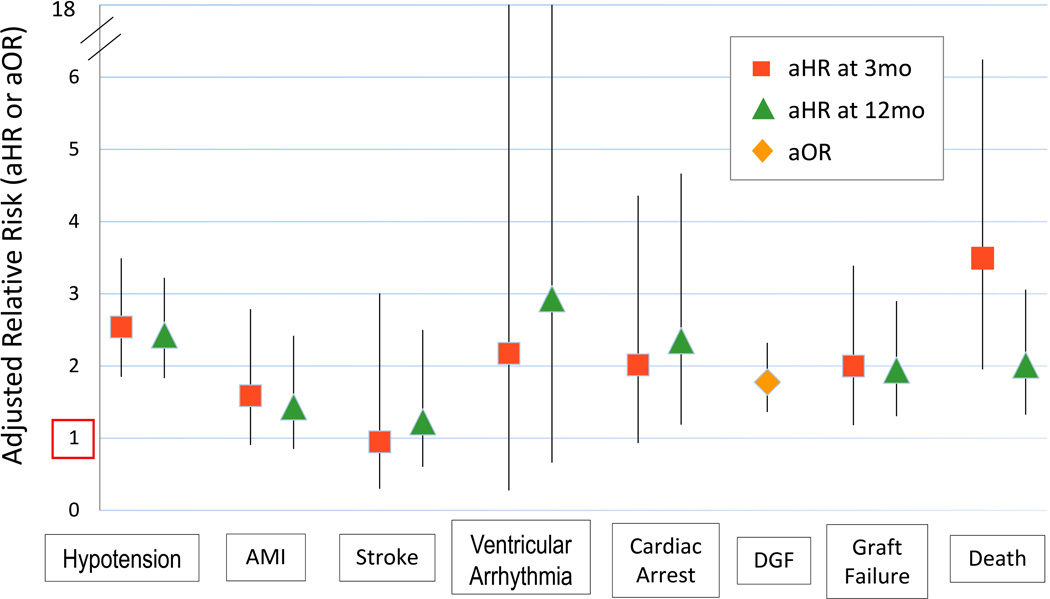
After multivariate adjustment for recipient, donor and transplant factors, as well as propensity adjustment for the likelihood of pretransplant midodrine use, midodrine exposure before transplant was independently associated with increased risk of DGF (aOR 1.78; 95% CI 1.36–2.32), death-censored graft failure (aHR 2; 95% CI 1.18–3.39), and death (aHR 3.49; 95% CI 1.95–6.24) at 3 months post transplantation (Figure 2). At 12 months, pretransplant midodrine was also associated with increased risk of death-censored graft failure (aHR 1.94; 95% CI 1.3–2.9), and patient death (aHR 2.01; 95% CI 1.33–3.06). Other factors associated with DGF, death-censored graft failure and death at 12 months are shown in SDC, Table 3.
Figure 2.
Adjusted associations of pretransplant midodrine use with posttransplant complications.
In the models adjusted for baseline clinical factors and propensity of midodrine exposure, midodrine use before transplant was also associated with increased risk of hypotension at 3 months (aHR 2.54; 95% CI 1.85–3.49) and 12 months (aHR 2.43; 95% CI 1.83–3.22) posttransplant (Figure 2). There were trends towards associations of pretransplant midodrine use with AMI (aHR 1.59; 95% CI 0.91–2.79; P=0.11) and cardiac arrest (aHR 2.02; 95% CI 0.93–4.36; P=0.08) at 3 months, and with ventricular arrhythmia at 12 months posttransplant (aHR 2.93; 95% CI 0.66–13; P=0.16). The association with cardiac arrest was statistically significant at 12 months posttransplantation (aHR 2.35; 95% CI 1.19–4.67). Pretransplant midodrine exposure was not significantly associated with stroke at 3 months (aHR 0.95; 95% CI 0.3–3.01) or 12 months (aHR 1.23; 95% CI 0.6–2.5) after transplantation.
All-Cause Graft Survival According to Pretransplant Midodrine Use and Donor Type
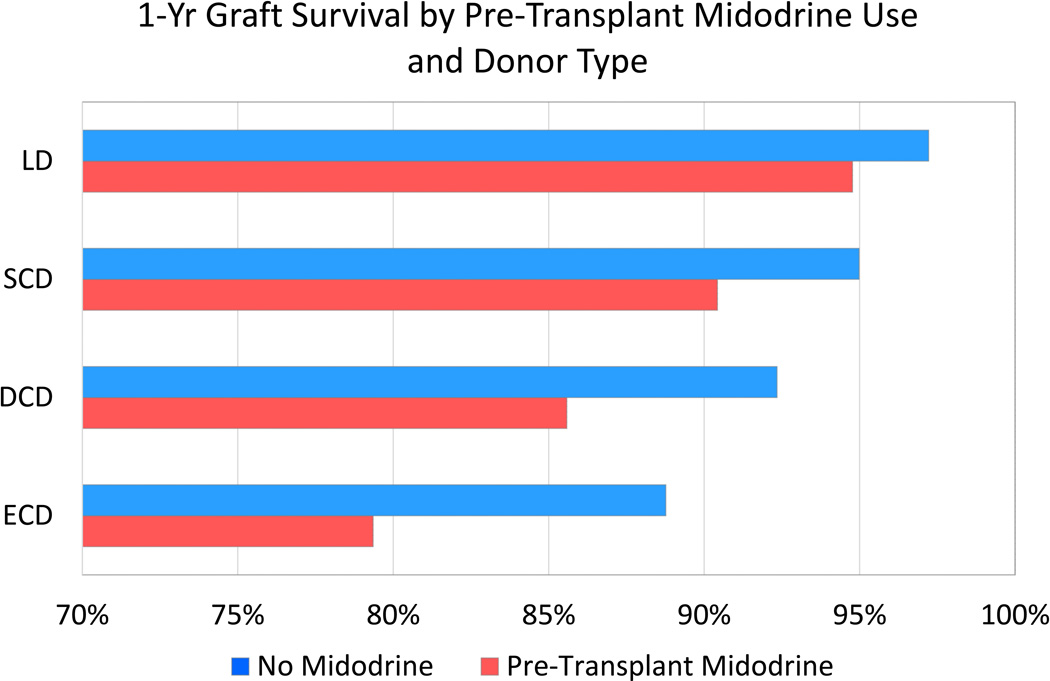
The absolute decrement in one-year adjusted graft survival associated with pretransplant midodrine use was greater in a graded manner among recipients of living donor, SCD, DCD and ECD allografts, respectively. Specifically, one-year graft survival among midodrine users and non-users according to type of allograft was: living donor transplant recipients, 94.8% (95% CI 93.1–96.5) vs. 97.2% (95% CI 96.8–97.2); SCD recipients, 90.4% (95% CI 87.7–93.3) vs. 95% (95% CI 94.5–95.5); DCD recipients, 85.6% (95% CI 81.1–90.3) vs. 92.3% (95% CI 91.0–93.7); ECD recipients, 79.4% (95% CI 73.7–85.5) vs. 88.8% (95% CI 87.4–90.1) (Figure 3). Among recipients of deceased donor allografts, the absolute decrement in one-year adjusted graft survival among midodrine users compared with non-users also rose in a graded manner according to KDPI level; KDPI <20%, 94.1% vs 96.4%; KDPI 20–85%, 91.4% vs 94.7%; KDPI >85%, 82.4% vs 88.9% (SCD, Figure 1).
Figure 3.
One-year graft survival according to pretransplant midodrine use and allograft type.
LD, living donor; SCD, standard criteria donor; DCD, donation after cardiac death; ECD, expanded criteria donor.
DISCUSSION
Novel approaches are needed to advance understanding of relationships of pretransplant comorbidities with posttransplant outcomes in large, representative samples. We examined integrated national transplant registry data, outpatient pharmacy fill records from a pharmaceutical claims clearinghouse, and Medicare billing claims to identify midodrine use in the year before kidney transplantation as a marker of patients affected by symptomatic hypotension and determine whether pretransplant midodrine use predicts posttransplant patient and graft outcomes. Among the findings, we observed that 2% percent of the sample filled midodrine prescriptions in the year before transplant, and that midodrine users were more likely to be on hemodialysis, to have a longer duration of dialysis dependence, and higher levels of sensitization compared to non-users. After adjustment for baseline recipient, donor, and transplant factors captured in the transplant registry as well as for propensity of midodrine use, midodrine users had twice the risks of developing DGF and graft failure at 3-months, and four times the risk of death at 3 months posttransplant than non-users. Similar patterns of graft failure and death occurred at 12 months posttransplantation. Pretransplant midodrine exposure was also associated with other medical complications after transplant including hypotension, AMI, ventricular arrhythmia, and cardiac arrest. While these relationships may be mediated by associations of midodrine requirements with underlying vascular calcification, arterial stiffness and autonomic dysfunction, our findings suggest that pretransplant midodrine use is a clinically relevant marker for increased risks of posttransplant complications.
A limited body of evidence supports the safety of midodrine as therapy for hypotension in dialysis patients. A systematic review of 10 studies including 117 ESRD patients with intradialytic hypotension reported that midodrine treatment resulted in improvements in systolic and diastolic blood pressure of 12 mmHg and 7 mmHg, respectively, and reduced symptoms of hypotension without appreciable adverse events13. The authors acknowledged the small sample sizes in each study and the variable timing of blood pressure measurements across the studies. A more recent study including 21 diabetic patients with chronic hypotension defined as systolic blood pressure less than 100 mmHg before dialysis found that midodrine therapy was associated with 10 mmHg and 5 mmHg increases in systolic and diastolic blood pressure, respectively. Vertigo and syncope significantly improved with midodrine therapy26. Again, no substantial side effects were noted.
Despite these observations of the safety of midodrine in the dialysis population13,26, intradialytic hypotension remains a significant risk factor for mortality among ESRD patients18,19. The present study advances knowledge of the outcome implications of symptomatic hypotension by identifying pretransplant midodrine use, a measure of clinically significant hypotension, as a risk marker for complications after kidney transplantation. Mechanisms of these associations may include ischemic complications of the vasoconstrictive actions of midodrine in those who continue the medication after transplant. However, while our study was designed to capture pharmacy claims data prior to transplantation, in our experience, midodrine is frequently discontinued after transplantation. It is likely that the observed prognostic impacts of midodrine are mediated, at least in part, by underlying complications of prolonged ESRD that predispose to symptomatic hypotension, including accelerated vascular aging with calcification and remodeling of large vessels, arterial stiffening and decreased vascular compliance, myocardial fibrosis and diastolic dysfunction, and autonomic dysfunction27.
Our study demonstrates that patients who require midodrine before transplant are prone to hypotension after transplant, which may lead to cardiovascular and other end-organ hypo-perfusion. Complementary to our findings, a recent observational analysis of associations of baseline blood pressure among kidney transplant recipients enrolled in the Folic Acid for Vascular Outcomes Reduction in Transplantation (FAVORIT) trial found that each 10 mmHg decrease in baseline diastolic BP below 70 mmHg was associated with 31% higher relative risks of both cardiovascular disease (HR, 1.31; 95% CI 1.06–1.62) and mortality (HR, 1.31; 95% CI, 1.03–1.66) over four years of follow-up28, supporting the adverse prognostic implications of hypotension in the kidney transplant recipient29.
We found that midodrine use before transplant impacts graft survival regardless of the donor type. However, this impact is augmented in recipients of non-standard organs, rising from a 4.5% decrement in one-year graft survival associated with midodrine use among recipients of SCD transplants to a 10% decrement among ECD transplant recipients. Similarly, among recipients of the deceased donor allografts, association of pretransplant midodrine use with decrements in one-year allograft failure larger with use of lower quality organs (7% difference with KDPI >85%) compared to higher quality organs (2% difference with KDPI <20%). This might be related to the observation that lower quality allografts are more likely to have arterial sclerosis and arteriolar hyalinosis30,31. We hypothesize that patients with symptomatic hypotension have impairment in the perfusion pressure required in marginal organs to achieve an adequate allograft function and that the impact of this impairment would be worse in marginal organs. Impaired perfusion leads to decreased hemoglobin delivery, resulting in hypoxemia and accumulation of reactive oxygen species, and finally, endothelial and tubular epithelial cell injury32. Importantly, older donor age has been identified as an independent correlate of progression of aortic stiffness after transplantation33. Therefore, inferior outcomes should be expected in utilizing non-standard deceased donor organs in recipients with symptomatic hypotension reflected by midodrine requirements. In the same line, the impact of midodrine use on graft function was more obvious in the recipients of high KDPI (>85%) with 7% decrement compared to 2% decrement in the recipients of low KDPI (KDPI <20%).
It is common and essential to determine whether a transplant candidate has comorbidities such as atherosclerotic vascular disease during evaluation for kidney transplantation. However, most guidelines on kidney transplant candidate evaluation do not include intradialytic hypotension in the assessment for transplant candidacy34–36. Herein, we provide evidence that symptomatic hypotension, as reflected by midodrine use, is relevant to risks of complications after transplantation. Regardless of the mechanisms of association, identification of novel markers of posttransplant outcomes is a timely concern for helping transplant programs better recognize and manage risk at the individual patient and programmatic level. Transplantation in the U.S. is an increasingly regulated field with a high level of public reporting. Centers are graded for recipient and graft survival using risk-adjusted equations developed by the Scientific Registry of Transplant Recipients (SRTR) for the prediction of expected one-year posttransplant patient and graft survival37. Importantly, SRTR equations do not adjust for cardiovascular comorbidity or hypotension as risk factors for posttransplant death or graft loss. Thus, centers transplanting patients requiring midodrine before transplantation should be aware of un-captured risk which will not be recognized by the SRTR, and in addition to attempting to optimize clinical status before transplant, should consider extra monitoring and focused posttransplant care of these recipients. To put this in perspective of the observed to expected outcome ratio (O:E), the impact of midodrine use would be worse for graft loss than many other identifiable risk factors in the multivariate model such as recipient age, obesity, cold ischemia time, and histocompatibility.
Our study has limitations. First, its retrospective design can identify associations but not prove causation. Second, the available data do not include relevant clinical information such as blood pressure readings or cardiac ejection fraction. Midodrine exposure was classified as a binary indicator, and we lacked a sufficient number of cases to discriminate risk by the midodrine dose or duration of use. Additionally, physical examination measurements, laboratory values, and diagnostic test results were not available to adjudicate the clinical diagnoses in our study. Another limitation is that our PCD data captures only 60% of U.S. retail pharmacy transactions and our sample was limited to Medicare beneficiaries for outcome ascertainment; thus, our results may not generalize to the full population of US transplant recipients.
Our study also has several important strengths. To our knowledge, this is the first study to examine the prognostic importance of midodrine use for outcomes after kidney transplantation. Second, use of a dataset linking national transplant registry data with records from a large pharmacy claims clearinghouse allowed us to capture more than 2.5-times the number of patients in a previous meta-analysis of midodrine use in ESRD patients that included 10 studies. Despite the relatively small number of exposed cases, we were able to detect clinically significant associations of midodrine use with important posttransplant outcomes that persisted after multivariate adjustment. Further research studies should seek to replicate these findings in larger samples, including patients transplanted after implementation of the 2014 revised Kidney Allocation System (KAS). While we observed that pretransplant midodrine use was fairly uncommon among recipients in the study period, use is expected to increase with prolonged dialysis times for some groups, which will allow confirmation of these results,
In summary, although associations likely in part reflect underlying conditions, the need for midodrine before kidney transplantation is a marker for increased risks of posttransplant complications including hypotension, DGF, graft failure and death. Importantly, because the new KAS prioritizes highly sensitized candidates and those with extended pretransplant dialysis durations – factors also associated with midodrine requirements – monitoring recipient comorbidity burden through novel methods including pharmacy claims and the associated impacts on transplant outcomes are important priorities. Our study informs transplant physicians and surgeons that many patients receiving midodrine will continue to be hypotensive after transplant, with relevance for the type of donor to accept, the immunosuppression to use, posttransplant clinical complications to expect, and impact on program performance grading. Transplant of a DCD or ECD allograft in a candidate receiving midodrine should be considered with caution, and future studies should seek to define the clinical circumstances under which non-standard organs may be safely transplanted in these higher-risk recipients.
Supplementary Material
Acknowledgments
DISCLOSURES: This work was supported by a grant from the National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) R01DK102981. DCB received support from the Eileen M. Brooks Transplant Nephrology Fund, the Donald F. Roach Transplant Nephrology Foundation, and the Alan A. and Edith L. Wolff Endowment Fund. The data reported here have been supplied by the United Network for Organ Sharing (UNOS) as the contractor for the Organ Procurement and Transplantation Network (OPTN). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the OPTN or the U.S. Government.
ABBREVIATIONS
- AMI
Acute myocardial infarction
- CAD
Coronary artery disease
- CVD
Cerebral vascular disease
- DGF
Delayed graft function
- DCD
Donation after cardiac death
- ECD
Expanded criteria donor
- ESRD
End stage renal disease
- PVD
Peripheral vascular disease
- KAS
Kidney allocation system
- KDPI
Kidney donor profile index
- SCD
Standard criteria donor
Footnotes
The authors declare no conflicts of interest
Tarek Alhamad, talhamad@dom.wustl.edu, Study design, interpretation, and writing of the manuscript
Daniel C. Brennan, dbrennan@dom.wustl.edu, Study design, interpretation, and writing of the manuscript
Zaid Brifkani, drzaidbrifkani@gmail.com, Study design, interpretation, and writing of the manuscript
Huiling Xiao, xiaoh@slu.edu, Data analysis and writing of the manuscript
Mark Schnitzler, schnitm@slu.edu, Study design, data acquisition, interpretation, and writing of the manuscript
Vikas R. Dharnidharka, Dharnidharka_V@kids.wustl.edu, Study design, interpretation, and writing of the manuscript
David Axelrod, David.A.Axelrod@Hitchcock.ORG, Study design, interpretation, and writing of the manuscript
Dorry L. Segev, dorry@jhmi.edu, Study design, interpretation, and writing of the manuscript
Krista L. Lentine, lentinek@slu.edu, Study design, data acquisition, data analysis, interpretation, and writing of the manuscript
Supplemental Digital Content (SDC):
SDC, Table 1. International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis codes used to define medical diagnoses from billing claims data.
SDC, Table 2. Propensity model for association of baseline recipient factors with pretransplant midodrine use.
SDC, Table 3. Adjusted associations of pretransplant midodrine use with risks of DGF, death-censored graft failure and death after transplant.
SDC, Figure 1. One-year graft survival according to the pretransplant midodrine use and KDPI. KDPI, kidney donor profile index.
REFERENCES
- 1.Lentine KL, Costa SP, Weir MR, et al. Cardiac disease evaluation and management among kidney and liver transplantation candidates: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. Journal of the American College of Cardiology. 2012 Jul 31;60(5):434–480. doi: 10.1016/j.jacc.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Matas AJ, Smith JM, Skeans MA, et al. OPTN/SRTR 2013 Annual Data Report: kidney. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2015 Jan;15(Suppl 2):1–34. doi: 10.1111/ajt.13195. [DOI] [PubMed] [Google Scholar]
- 3.Williams JM, Tuttle-Newhall JE, Schnitzler M, et al. Clopidogrel use as a risk factor for poor outcomes after kidney transplantation. American journal of surgery. 2014 Oct;208(4):556–562. doi: 10.1016/j.amjsurg.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Lentine KL, Yuan H, Tuttle-Newhall JE, et al. Quantifying prognostic impact of prescription opioid use before kidney transplantation through linked registry and pharmaceutical claims data. Transplantation. 2015 Jan;99(1):187–196. doi: 10.1097/TP.0000000000000248. [DOI] [PubMed] [Google Scholar]
- 5.Lentine KL, Lam NN, Xiao H, et al. Associations of pretransplant prescription narcotic use with clinical complications after kidney transplantation. American journal of nephrology. 2015;41(2):165–176. doi: 10.1159/000377685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sands JJ, Usvyat LA, Sullivan T, et al. Intradialytic hypotension: Frequency, sources of variation and correlation with clinical outcome. Hemodialysis International. 2014;18(2):415–422. doi: 10.1111/hdi.12138. [DOI] [PubMed] [Google Scholar]
- 7.Degoulet P, Reach I, Di Giulio S, et al. Epidemiology of dialysis induced hypotension. Proceedings of the European Dialysis and Transplant Association. European Dialysis and Transplant Association. 1980;18:133–138. [PubMed] [Google Scholar]
- 8.Passauer J, Büssemaker E, Gross P. Dialysis hypotension: do we see light at the end of the tunnel? Nephrology Dialysis Transplantation. 1998;13(12):3024–3029. doi: 10.1093/ndt/13.12.3024. [DOI] [PubMed] [Google Scholar]
- 9.Chesterton LJ, Selby NM, Burton JO, McINTYRE CW. Cool dialysate reduces asymptomatic intradialytic hypotension and increases baroreflex variability. Hemodialysis International. 2009;13(2):189–196. doi: 10.1111/j.1542-4758.2009.00355.x. [DOI] [PubMed] [Google Scholar]
- 10.Flythe JE, Kimmel SE, Brunelli SM. Rapid fluid removal during dialysis is associated with cardiovascular morbidity and mortality. Kidney international. 2011;79(2):250–257. doi: 10.1038/ki.2010.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alappan R, Cruz D, Abu-Alfa AK, Mahnensmith R, Perazella MA. Treatment of severe intradialytic hypotension with the addition of high dialysate calcium concentration to midodrine and/or cool dialysate. American Journal of Kidney Diseases. 2001;37(2):294–299. doi: 10.1053/ajkd.2001.21292. [DOI] [PubMed] [Google Scholar]
- 12.van der Sande FM, Kooman JP, Leunissen KM. Intradialytic hypotension—new concepts on an old problem. Nephrology Dialysis Transplantation. 2000;15(11):1746–1748. doi: 10.1093/ndt/15.11.1746. [DOI] [PubMed] [Google Scholar]
- 13.Prakash S, Garg AX, Heidenheim AP, House AA. Midodrine appears to be safe and effective for dialysis-induced hypotension: a systematic review. Nephrology Dialysis Transplantation. 2004;19(10):2553–2558. doi: 10.1093/ndt/gfh420. [DOI] [PubMed] [Google Scholar]
- 14.Low PA, Gilden JL, Freeman R, Sheng KN, McElligott MA. Efficacy of midodrine vs placebo in neurogenic orthostatic hypotension. A randomized, double-blind multicenter study. Midodrine Study Group. Jama. 1997 Apr 2;277(13):1046–1051. [PubMed] [Google Scholar]
- 15.Schirger A, Sheps SG, Thomas JE, Fealey RD. Midodrine. A new agent in the management of idiopathic orthostatic hypotension and Shy-Drager syndrome. Mayo Clinic proceedings. 1981 Jul;56(7):429–433. [PubMed] [Google Scholar]
- 16.Low PA, Gilden JL, Freeman R, Sheng K, McElligott M. Efficacy of midodrine vs placebo in neurogenic orthostatic hypotension: A randomized, double-blind multicenter study. Jama. 1997;277(13):1046–1051. [PubMed] [Google Scholar]
- 17.Lin YF, Wang JY, Denq JC, Lin SH. Midodrine improves chronic hypotension in hemodialysis patients. The American journal of the medical sciences. 2003 May;325(5):256–261. doi: 10.1097/00000441-200305000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Shoji T, Tsubakihara Y, Fujii M, Imai E. Hemodialysis-associated hypotension as an independent risk factor for two-year mortality in hemodialysis patients. Kidney international. 2004;66(3):1212–1220. doi: 10.1111/j.1523-1755.2004.00812.x. [DOI] [PubMed] [Google Scholar]
- 19.Tislér A, Akócsi K, Borbás B, et al. The effect of frequent or occasional dialysis-associated hypotension on survival of patients on maintenance haemodialysis. Nephrology Dialysis Transplantation. 2003;18(12):2601–2605. doi: 10.1093/ndt/gfg450. [DOI] [PubMed] [Google Scholar]
- 20.Department of Health and Human Services, Health Resources and Services Administration. Annual Report of the U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: Transplant Data 1994–2003. 2004 [Google Scholar]
- 21.Lentine KL, Schnitzler MA, Abbott KC, Bramesfeld K, Buchanan PM, Brennan DC. Sensitivity of billing claims for cardiovascular disease events among kidney transplant recipients. Clinical journal of the American Society of Nephrology : CJASN. 2009 Jul;4(7):1213–1221. doi: 10.2215/CJN.00670109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lentine KL, Rocca Rey LA, Kolli S, et al. Variations in the risk for cerebrovascular events after kidney transplant compared with experience on the waiting list and after graft failure. Clinical journal of the American Society of Nephrology : CJASN. 2008 Jul;3(4):1090–1101. doi: 10.2215/CJN.03080707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lentine KL, Brennan DC, Schnitzler MA. Incidence and predictors of myocardial infarction after kidney transplantation. Journal of the American Society of Nephrology : JASN. 2005 Feb;16(2):496–506. doi: 10.1681/ASN.2004070580. [DOI] [PubMed] [Google Scholar]
- 24.Metzger RA, Delmonico FL, Feng S, Port FK, Wynn JJ, Merion RM. Expanded criteria donors for kidney transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2003;3(Suppl 4):114–125. doi: 10.1034/j.1600-6143.3.s4.11.x. [DOI] [PubMed] [Google Scholar]
- 25.United Network Organ Sharing (UNOS), Kidney Donor Profile Index (KDPI) Calculator. [Accessed: Novemeber, 8, 2015]; https://www.unos.org/transplantation/allocation-calculators. [Google Scholar]
- 26.Sarikaya AM, Funda S, Güneş AJ. The Effect of Midodrine Hydrochloride on Chronic Hypotension in Patients with Diabetes Mellitus Undergoing Hemodialysis Therapy. Turkiye Klinikleri Journal of Medical Sciences. 2011;31(6):1527–1531. [Google Scholar]
- 27.Kersh ES, Kronfield SJ, Unger A, Popper RW, Cantor S, Cohn K. Autonomic Insufficiency in Uremia as a Cause of Hemodialysis-Induced Hypotension. New England Journal of Medicine. 1974;290(12):650–653. doi: 10.1056/NEJM197403212901203. 1974/03/21. [DOI] [PubMed] [Google Scholar]
- 28.Carpenter MA, John A, Weir MR, et al. BP, Cardiovascular Disease, and Death in the Folic Acid for Vascular Outcome Reduction in Transplantation Trial. Journal of the American Society of Nephrology : JASN. 2014 Mar 13; doi: 10.1681/ASN.2013040435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.2007 Annual Report of the OPTN and SRTR: CHAPTER VIII Calculating Life Years From Transplant (LYFT): Methods for Kidney and Kidney-Pancreas Candidates. doi: 10.1111/j.1600-6143.2008.02177.x. < http://www.optn.org/AR2007/chapter_vii_AR_cd.htm?cp=8>. [DOI] [PubMed] [Google Scholar]
- 30.Karpinski J, Lajoie G, Cattran D, et al. Outcome of kidney transplantation from high-risk donors is determined by both structure and function. Transplantation. 1999 Apr 27;67(8):1162–1167. doi: 10.1097/00007890-199904270-00013. [DOI] [PubMed] [Google Scholar]
- 31.Snoeijs MG, Boonstra LA, Buurman WA, et al. Histological assessment of pretransplant kidney biopsies is reproducible and representative. Histopathology. 2010 Jan;56(2):198–202. doi: 10.1111/j.1365-2559.2009.03469.x. [DOI] [PubMed] [Google Scholar]
- 32.Boros P, Bromberg JS. New cellular and molecular immune pathways in ischemia/reperfusion injury. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2006 Apr;6(4):652–658. doi: 10.1111/j.1600-6143.2005.01228.x. [DOI] [PubMed] [Google Scholar]
- 33.Delahousse M, Chaignon M, Mesnard L, et al. Aortic stiffness of kidney transplant recipients correlates with donor age. Journal of the American Society of Nephrology : JASN. 2008 Apr;19(4):798–805. doi: 10.1681/ASN.2007060634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bunnapradist S, Danovitch GM. Evaluation of adult kidney transplant candidates. American Journal of Kidney Diseases. 2007;50(5):890–898. doi: 10.1053/j.ajkd.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 35.Scandling JD. Kidney transplant candidate evaluation; Paper presented at: Seminars in dialysis; 2005. [DOI] [PubMed] [Google Scholar]
- 36.Pham PT, Pham PA, Pham PC, Parikh S, Danovitch G. Evaluation of adult kidney transplant candidates; Paper presented at: Seminars in dialysis; 2010. [DOI] [PubMed] [Google Scholar]
- 37.Scientific Registry of Transplant Recipients (SRTR) Risk Adjustment Models. [Accessed: July 24, 2015]; Available at: http://www.srtr.org/csr/current/modtabs.aspx. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.