Abstract
Rationale
N-(4-hydroxyphenyl)-arachidonamide (AM404) is an anandamide transport inhibitor shown to reduce rewarding and relapse-inducing effects of nicotine in several animal models of tobacco dependence. However, the reinforcing/rewarding effects of AM404 are not clear.
Objectives
We investigated whether AM404 maintains self-administration behavior or reinstates extinguished drug seeking in squirrel monkeys.
Methods and Results
In monkeys with a history of anandamide or cocaine self-administration, we substituted injections of AM404 (1–100 µg/kg/injection). Using a 10-response, fixed-ratio schedule, self-administration behavior was maintained by AM404. Dose-response curves had inverted-U shapes, with peak response rates occurring at a dose of 10 µg/kg/injection. In anandamide-experienced monkeys, we also demonstrated self-administration of another anandamide transport inhibitor VDM11. In addition to supporting self-administration, priming injections of AM404 (0.03–0.3 mg/kg) reinstated drug-seeking behavior previously reinforced by cannabinoids (THC or anandamide) or cocaine. Both, AM404 self-administration behavior and reinstatement of drug seeking by AM404 were reduced by treatment with the cannabinoid CB1 receptor antagonist/inverse agonist rimonabant (0.3 mg/kg). Moreover, the reinforcing effects of AM404 were potentiated by the treatment with the FAAH inhibitor URB597 (0.3 mg/kg) suggesting the major role of anandamide in these effects. Finally, AM404 (0.3 mg/kg) potentiated the reinforcing effects of anandamide, but not those of cocaine.
Conclusions
In non-human primates, AM404 effectively reinforced self-administration behavior and induced reinstatement of drug-seeking behavior in abstinent monkeys. These effects appeared to be mediated by cannabinoid CB1 receptors. Therefore, compounds that promote actions of endocannabinoids throughout the brain by inhibiting their membrane transport may have a potential for abuse.
Keywords: Ananadamide, AM404, self-administration, reinstatement, rimonabant, squirrel monkeys
Introduction
With few exceptions, drugs that are abused by humans are self-administered by animals. Prior to 2000, one of those exceptions was Δ9-tetrahydrocannabinol (THC), the active ingredient for marijuana. However, in 2000, Tanda and colleagues were able to show that THC would be reliably self-administered by squirrel monkeys that had been previously trained to self-administer cocaine (Tanda et al. 2000). Justinova et al. (Justinova et al. 2003) later showed that naïve monkeys could also be trained to self-administer THC. These studies were followed by reports of self-administration of major endogenous cannabinoids anandamide and 2-arachidonoylglycerol (2-AG) by the same species (Justinova et al. 2011). The effects of THC, anandamide, and 2-AG were blocked by the cannabinoid CB1 antagonist/inverse agonist rimonabant, suggesting that self-administration was mediated by cannabinoid CB1 receptors. Rimonabant is highly selective at CB1 over CB2 receptors, but was also shown to be an antagonist at mu opioid receptors and possibly other receptors and ion channels (Raffa and Ward 2012; Seely et al. 2012). If endocannabinoids are self-administered when administered systemically, then compounds that enhance the action of endocannabinoids might also be self-administered. Anandamide is primarily metabolized by the enzyme fatty acid amide hydrolase (FAAH) and a number of compounds that inhibit FAAH has been developed, e.g. URB597, URB694, AM3506, and PF-04457845 to name a few (Ahn et al. 2011; Alapafuja et al. 2012; Clapper et al. 2009b; Piomelli et al. 2006). FAAH inhibitors were proposed as potentially safer alternatives to direct CB1 agonists for treatment of pain and many neuropsychiatric disorders, including addiction, anxiety, cognitive and movement disorders (Clapper et al. 2010; Janero et al. 2009; Pertwee 2014; Petrosino and Di Marzo 2010; Piomelli 2005). The best characterized FAAH inhibitor, URB597, increases the levels of anandamide in the squirrel monkey brain (Justinova et al. 2008a). Interestingly, the increase in anandamide levels was accompanied by a decrease in 2-AG levels in that study, but this effect was not shown in a later study in squirrel monkeys (Justinova et al. 2015). Despite the increase in anandamide levels, URB597 was not self-administered by squirrel monkeys and it did not reinstate extinguished THC or anandamide self-administration (Justinova et al. 2008a). However, other studies from our laboratory indicate that the reinforcing effects of FAAH inhibitors in non-human primates are not uniform and outcomes range from no or moderate self-administration (URB597, URB694) to significant self-administration (PF-04457845, AM3506) (Bergman et al. 2011; Justinova et al. 2008a; Justinova et al. 2014; Justinova et al. 2015).
In addition to metabolism, evidence suggests the action of anandamide is also terminated though reuptake into neurons (Beltramo et al. 1997; Di Marzo et al. 1994; Hillard et al. 1997). The processes involved in anandamide uptake have long been a matter of a considerable debate and both passive and active mechanisms have been proposed (Di Marzo et al. 1994; Fowler et al. 2004; Fowler 2012; Fu et al. 2012; Glaser et al. 2003; Hillard and Jarrahian 2003; Kaczocha et al. 2009; Piomelli et al. 1999). The most studied anandamide transport inhibitor, AM404 (N-(4-hydroxyphenyl)-arachidonamide), has been shown to increase the endogenous brain levels of anandamide in both mice and rats (Bortolato et al. 2006; Fegley et al. 2004), as well as the levels of anandamide in rat plasma (Beltramo et al. 1997; Giuffrida et al. 2000) for at least one to two hours. VDM-11, UCM-707 and OMDM-2 have been shown to increase whole brain levels of anandamide in rats (de Lago et al. 2005).
As with self-administration, conditioned place preference (CPP) is typically seen with drugs that are abused in humans. In rat studies, AM404 has been shown to induce CPP, but the results are mixed and point to a need for special conditions (like enriched environment) in order to capture the rewarding effects of AM404 in rodents (Bortolato et al. 2006; Gamaleddin et al. 2013; Scherma et al. 2012). Also, the role of dopamine in the effects of AM404 is unclear (Scherma et al. 2012; Solinas et al. 2007), as well as the effects of AM404 on endocannabinoid levels in the brain reward (Wiskerke et al. 2012). There is a need for careful work to explore direct and indirect effects of AM404 using proven and reliable methods.
To date, there are no published rodent (or monkey) self-administration studies with AM404, thus there is no clear picture of the reinforcing/rewarding effects of AM404. Therefore, the purpose of the current study was to study the effects of AM404 in a non-human primate self-administration model where previous research has shown that a variety of cannabinoids will be self-administered. As our results showed that AM404 was self-administered, we further determined whether there was involvement of cannabinoid CB1 receptors in the reinforcing effects of AM404. Although, it is known that AM404 itself binds to CB1 receptors with very low affinity (Ki = 1.8 µM) and does not activate these receptors either in vitro or in vivo (Beltramo et al. 2000). Moreover, since AM404 also activates vanilloid TRPV1 receptors (Zygmunt et al. 2000), we compared its reinforcing effects with those of VDM11, the anandamide transport inhibitor that is not active at TRPV1 receptors and has only a very weak action at CB1 receptors (Ki > 5–10 µM) (De Petrocellis et al. 2000). We also studied how AM404 would interact with other self-administered drugs (cocaine, anandamide) and determined the effects of FAAH inhibition by URB597 on self-administration of AM404. Finally, the effects of AM404 were also compared with other cannabinoids in a model of relapse.
Method
Subjects
Seventeen adult male squirrel monkeys (Saimiri sciureus) housed in individual cages in rooms in which temperature and humidity were controlled were used as subjects. Room lights were on a 12:12-h cycle with lights on at 0700 hours. Fresh water was continuously available. Monkeys were fed a daily food ration consisting of high-protein monkey diet (Lab Diet 5045, PMI Nutrition International, Richmond, IN) and banana flavored food treats (Banana Softies, Bio-Serv, Frenchtown, NJ) that maintained their body weight throughout the course of the experiment (800–1,200 g). The number of biscuits (8–14) was determined for each monkey individually to maintain their body weights at a constant level throughout the study. Monkeys self-administering food pellets had the number of biscuits adjusted to maintain the motivation to perform a food-reinforced task. Fresh fruits, vegetables and environmental enrichment were provided daily. Each animal had a unique numeric or alphanumeric identificator. These monkeys were implanted with venous catheters for the delivery of drug. The general surgical procedure has been described in detail elsewhere (Goldberg 1973). Monkeys wore nylon jackets (Lomir Biomedical, Canada) at all times to protect the catheters. Catheters were flushed with saline daily and sealed with stainless steel obturators when not in use. Following a 2-week recovery period, experiments were begun.
All animals used in this study were maintained in facilities fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care, and experiments were conducted in accordance with guidelines of the Institutional Animal Care and Use Committee of the Intramural Research Program, National Institute of Drug Abuse, National Institutes of Health, and the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Research (Council 2003).
Apparatus
During experimental sessions, the monkeys sat in Plexiglas chairs and were loosely restrained in the seated position by a waist lock (see Justinova et al. 2005). The chair contained a response lever (no. 121-05, BRS/LVE Corp., Laurel, MD, USA) mounted on a transparent front wall. Pairs of green and amber stimulus lights, mounted behind the transparent wall of the chair, could be illuminated and used as visual stimuli. A food trough was located in the bottom middle of the same panel as the levers. The monkey’s catheter was connected to polyethylene tubing that passed out of the isolation chamber where it was attached to a motor-driven syringe pump. A computer located in an adjacent room using the MED Associates MED-PC software package (East Fairfield, VT, USA) controlled the operation of the experimental events and data collection.
Procedure
Self-Administration
One-hour sessions were typically conducted Monday-Friday at around the same time each day. Monkeys were transported from the holding room to the experimental room where they were seated in the Plexiglas chairs. Catheters were flushed with saline and connected to the syringe pump. An injection of drug calculated to fill the dead space of the catheter was then administered. Sessions began with the illumination of the green stimulus lights. When the green stimulus lights were on, 10 responses on the response lever (FR 10) lead to the green light being turned off, the amber lights being turned on and the operation of the infusion pump (0.2 ml in 0.2 sec). After 2 sec, the amber lights were turned off and a timeout began where no stimulus lights were illuminated. Reponses during the timeout were counted, but had no scheduled effect. After a 1-min timeout, the green stimulus lights were turned on signaling that responses on the lever could again be reinforced on the FR 10 schedule.
Monkeys were initially trained with anandamide (40 µg/kg/injection), cocaine (30 µg/kg/injection) or THC (4 µg/kg/injection). Self-administration of AM404 was tested over a range of doses (1 to 100 µg/kg/injection) in anandamide- and cocaine-trained monkeys. Self-administration of VDM11 was tested over a similar range of doses (0.3 to 56 µg/kg/injection) in anandamide-trained monkeys. Each AM404 or VDM11 dose was made available for 4–5 consecutive sessions each to obtain dose-effect functions. Testing of each dose was always preceded and followed with 4–5 sessions of extinction. The doses of AM404 for this study were selected by conservative extrapolation from rodent pretreatment studies. Doses of VDM11 were based on our results with AM404.
To assess the effects of CB1-receptor blockade on reinforcing effects of AM404, rimonabant (0.1 and 0.3 mg/kg, i.m.) was given before AM404 (10 µg/kg/injection) self-administration sessions. Each rimonabant dose was tested for four consecutive sessions that were preceded and followed by 3–5 sessions with vehicle pretreatment. Then, monkeys were returned to self-administration of anandamide and three doses of AM404 (0.03, 0.1, and 0.3 mg/kg, i.v.) were compared for their effects on self-administration of the 40-µg/kg/injection dose of anandamide. Each dose was tested for five consecutive sessions that were preceded and followed by 3–5 sessions with vehicle pretreatment. The dose (0.3 mg/kg) that most effectively decreased anandamide self-administration was selected for further determination of the effects of AM404 on anandamide or cocaine self-administration dose-effect curves. Here, AM404 (0.3 mg/kg) was administered i.v. before each of five consecutive anandamide or cocaine self-administration sessions, preceded and followed by 3–5 consecutive baseline self-administration sessions with vehicle pretreatment. These sets of baseline and test sessions were conducted at anandamide doses of 2.5, 10, 40, and 80 µg/kg/injection and cocaine doses 3, 30, and 100 µg/kg/injection to detect changes in the anandamide and cocaine dose-effect curves. AM404 at a dose 0.3 mg/kg was also tested with food under self-administration conditions that paralleled the drug self-administration procedure.
Reinstatement procedure
Prior to the experiments described here, the monkeys were repeatedly exposed to extinction conditions where vehicle replaced the self-administered drug (responses still produced the 2-sec amber stimulus light and time-out on the FR schedule) and thus their responding typically decreased rapidly, usually within 1 day. For the reinstatement procedure, anandamide-, THC-, and cocaine-trained monkeys were first exposed to extinction conditions until responding decreased to a low level. For the reinstatement test, priming injection of test drug (0.04 mg/kg of THC, 0.3 mg/kg of anandamide, or 0.3 mg/kg of AM404) was administered i.v. prior to the session, during which completion of the FR produced the vehicle injections, the brief stimulus and time-out. In anandamide-trained monkeys, we previously determined the effects of three doses of AM404 (0.03, 1, and 0.3 mg/kg) and the most effective dose (0.3 mg/kg) was then used alone and in combination with rimonabant (0.3 mg/kg, i.m.) in all three groups of monkeys. Each reinstatement test was preceded by 1–2 extinction sessions with vehicle pretreatment.
Drugs
AM404 (N-(4-hydroxyphenyl)-arachidonamide) and anandamide (N-Arachidonoylethanolamine) were synthesized in the laboratory of Dr. Makriyannis at the Center for Drug Discovery, Northeastern University (Boston, MA, USA). AM404 was dissolved in a vehicle containing 2% Tween 80, ethanol 2%, and saline to obtain stock solution (1.5 mg/ml) that was further diluted with saline as needed. When used as a pretreatment, AM404 was injected i.v. (1 ml/kg) 5 min before the session. Anandamide was dissolved in a vehicle containing 2% Tween 80, 2% ethanol, and saline to obtain a stock solution (10 mg/ml) and further diluted with saline as needed. (−)-Cocaine HCl (Sigma Chemical Co., St. Louis, MO, USA) was dissolved in saline (doses expressed as salt). Δ9-tetrahydrocannabinol (THC, NIDA Drug Supply Program, Bethesda, MD, USA) was dissolved in a vehicle containing 1% Tween 80, 1% ethanol, and saline to obtain stock solution (0.4 mg/ml) and further diluted with saline. VDM11 [(5Z,8Z,11Z,14Z)-N-(4-Hydroxy-2-methylphenyl)-5,8,11,14-eicosatetraenamide] (Tocris Bioscience, Ellisville, MO, USA) was dissolved in a vehicle containing 2% Tween 80, 6% ethanol, and saline to obtain stock solution (0.3 mg/ml) and further diluted with saline as needed. Rimonabant (SR141716; N-piperidino-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methylpyrazole-3-carboxamide), provided by NIDA Drug Supply Program (Bethesda, MD, USA), was dissolved in a vehicle of 2% Tween 80, 2% ethanol, and sterile water and injected i.m. (in a volume of 0.3–0.5 ml/kg) 60 min before the session. URB597 (cyclohexyl carbamic acid 3’-carbamoyl-3-yl ester) was kindly provided by Dr. Daniele Piomelli (University of California, Irvine, CA, USA). URB597 was dissolved in vehicle containing 5% Tween 80, and saline and injected i.v. (1 ml/kg) 30 min before the session. In the reinstatement tests, THC, anandamide, and AM404 were injected i.v. (1 ml/kg) immediately before the session.
Data analysis
Cumulative response records were obtained during all sessions to assess within-session patterns of responding. The number of reinforcements (injections or pellets) per session represents total number of injections delivered during each 1-hour session. The rates of responding are expressed as responses per second averaged over the 1 h session, with time and responding during time-outs not included in calculations. Data for dose–effect curves are expressed as mean response rates and numbers of reinforcements per session ± SEM over the last three sessions. In addition, total intake for each self-administered dose was calculated. For statistical evaluation of effects over consecutive sessions, the average of the last three sessions prior to the experimental manipulation (extinction or pretreatment) was used as a control value to allow comparisons with subsequent sessions. Statistical analysis was performed using one-way or two-way repeated measures ANOVA (data met the assumptions of the test). Post hoc analysis was performed either by Tukey pairwise multiple comparisons or Bonferroni t-test (multiple comparisons versus control group). Statistical significance was accepted at the p < 0.05 level. SigmaStat software (Systat Software Inc.) was used for all statistical analyses.
Results
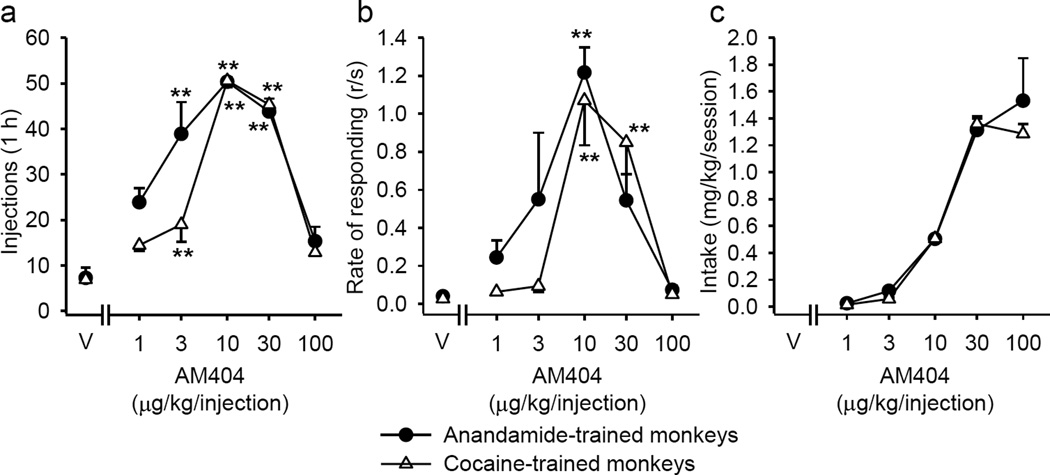
Monkeys initially trained to self-administer either anandamide or cocaine also self-administered AM404 (Figure 1). For both groups of monkeys, a typical inverted U-shaped dose-effect function was observed, with peak responding occurring at a dose of 10 µg/kg/injection AM404. Both the number of injections per session (Figure 1a; anandamide group: F(5,10) = 17.91, p < 0.001; cocaine group: F(5,10) = 115.23, p < 0.001) and the rate of responding (Figure 1b; anandamide group: F(5,10) = 6.85, p = 0.005; cocaine group: F(5,10) = 11.72, p < 0.001) were significantly above vehicle levels at a number of doses. The total AM404 intake per session reached 1.53 ± 0.32 mg/kg in anandamide group and 1.29 ± 0.07 mg/kg in cocaine group (Figure 1c). The number of 10-µg/kg injections of AM404 received per session (anandamide group: 50.33 ± 1.02 ; cocaine group: 50.58 ± 1.40) was near available maximum and the rates of responding (anandamide group: 1.22 ± 0.13 ; cocaine group: 1.07 ± 0.24) were typical of other drugs of abuse in squirrel monkeys, including THC (Justinova et al. 2003) and anandamide (Justinova et al. 2005).
Figure 1.
Dose-effect functions for self-administration of various doses of AM404 under a fixed-ratio 10 (FR10) schedule. Total number of AM404 injections self-administered per 1-h session (a), overall response rates in the presence of the green light signaling drug availability (b), and total AM404 intake per 1-h session (c) are shown as a function of AM404 dose (abscissae log scale) in anandamide- (n=3) and cocaine-trained (n=3) monkeys. **p < 0.01, post-hoc comparisons with vehicle (V) self-administration, Bonferroni test.
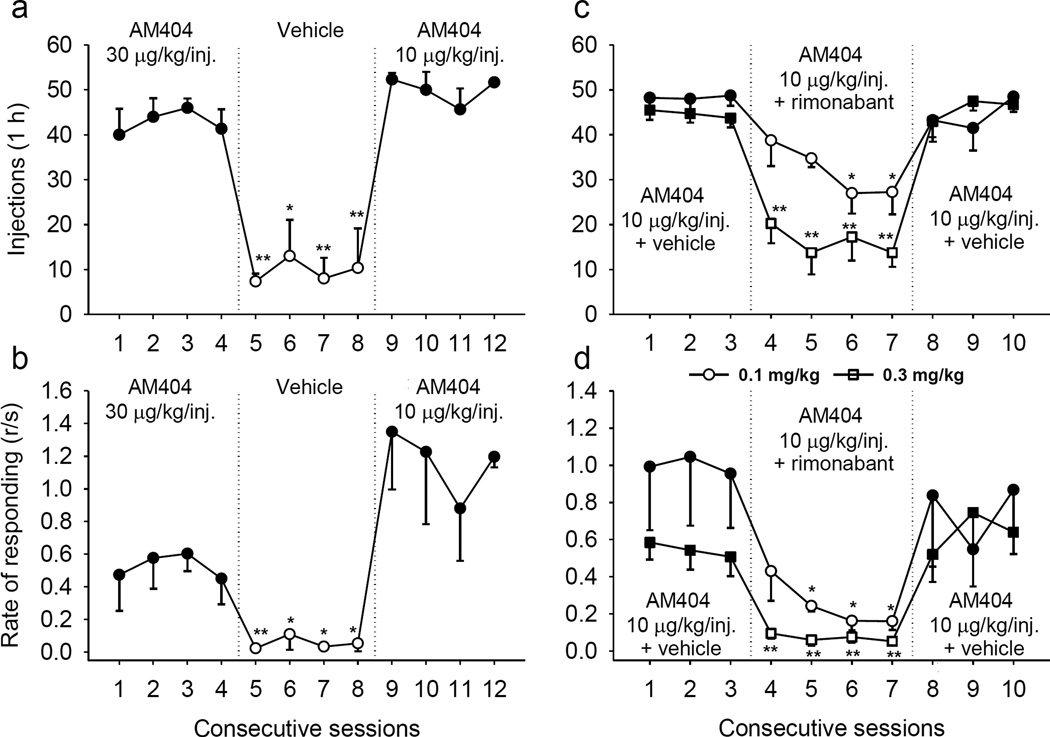
When vehicle was substituted for AM404 (30 µg/kg/injection), but the brief stimulus and time-out still followed each FR completion, both the number of injections (Figure 2a; F(4,8) = 9.28, p = 0.004) and the rate of responding (Figure 2b; F(4,8) = 6.99, p = 0.01) dropped immediately. When AM404 (10 µg/kg/injection) was again made available for self-administration, injections and rate of responding recovered rapidly. Self-administration of AM404 (10 µg/kg/injection) was attenuated by pretreatment with the CB1 antagonist/inverse agonist rimonabant, indicating a role for CB1 receptors in AM404 self-administration (Figure 2c,d). A dose of 0.3 mg/kg rimonabant reduced the number of injections per session by over half (Figure 2c; F(4,12) = 8.5, p = 0.002) and reduced response rates by more than 80% (Figure 2d; F(4,12) = 19.18, p < 0.001), effects not much different from that observed with vehicle substitution (Figure 2a,b). A lower dose of rimonabant (0.1 mg/kg) produced smaller, but still significant reductions in responding (Figure 2c, injections: F(4,12) = 3.90, p = 0.03; Figure 2d, response rate: F(4,12) = 4.61, p = 0.017). Cumulative records from the sessions with the rimonabant treatment showed that all the responding occurred in the first half of the session. The pattern of responding was indicative of attenuation of the effects of AM404. The reduction in responding and cumulative records were comparable to those seen with the same rimonabant pretreatment doses in monkeys self-administering THC (Tanda et al. 2000) or anandamide (Justinova et al. 2005).
Figure 2.
Extinction and reacquisition of AM404 self-administration. Number of injections per session (a) and overall rates of responding (b) during AM404 (30 µg/kg/injection) self-administration (sessions 1–4), vehicle extinction (sessions 5–8), and AM404 (10 µg/kg/injection) self-administration (sessions 9–12) are shown (n=3). Attenuation of AM404 self-administration by cannabinoid CB1 antagonist/inverse agonist rimonabant. Number of injections per session (c) and overall rates of responding (d) during AM404 (10 µg/kg/injection) self-administration are shown (n=4) after i.m. pretreatment with vehicle (sessions 1–3 and 8–11) or rimonabant (0.1 and 0.3 mg/kg; sessions 4–7). Points represent means ± SEM. *p < 0.05, **p < 0.01, post-hoc vs. the mean of the last three sessions of AM404 self-administration (a,b; sessions 2–4) or the mean of the last three sessions of vehicle pretreatment (c,d; sessions 1–3), Bonferroni test.
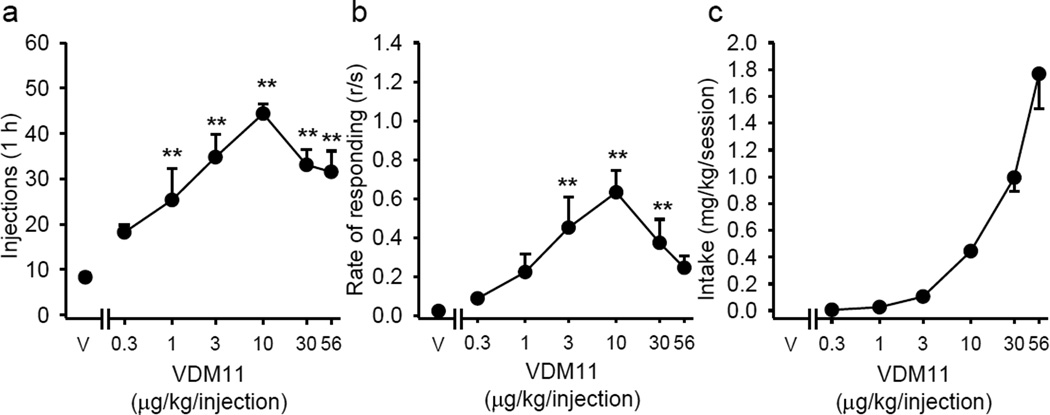
Like AM404, the anandamide uptake inhibitor VDM11 that is not active at TRPV1 receptors also supported self-administration behavior in anandamide-experienced squirrel monkeys (Figure 3). Inverted U-shaped dose-effect functions were observed for both total number of injections and response rates. A number of VDM11 doses maintained infusions (Figure 3a; F(6,22) = 14.38, p < 0.001) and response rate (Figure 3b; F(6,22) = 8.82, p < 0.001) at levels above those maintained by vehicle and total intake reached 1.77 ± 0.26 mg/kg at the highest dose tested (Figure 3c).
Figure 3.
Dose-effect functions for self-administration of various doses of VDM11 under a fixed-ratio 10 (FR10) schedule. Total number of VDM11 injections self-administered per 1-h session (a), overall response rates in the presence of the green light signaling drug availability (b), and total VDM11 intake per 1-h session (c) are shown as a function of VDM11 dose (abscissae log scale) in anandamide-trained monkeys (n=5; except for the dose 0.3 µg/kg/injection where n=3). *p < 0.05, **p < 0.01, post-hoc comparisons with vehicle (V) self-administration, Bonferroni test.
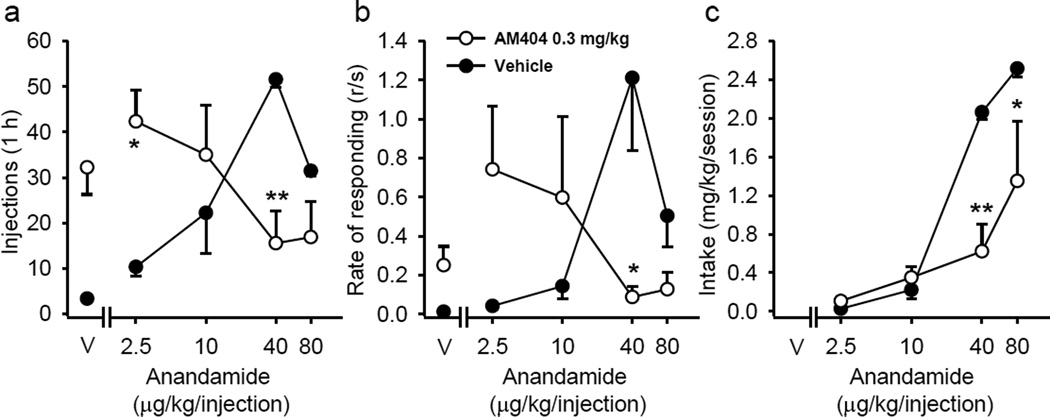
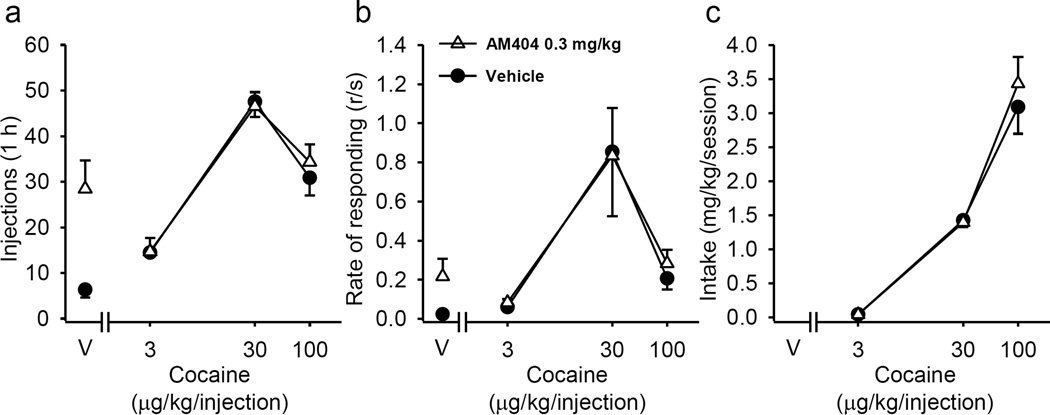
In monkeys self-administering anandamide (40 µg/kg/injection), pretreatment with 0.03 and 0.1 mg/kg of AM404 did not significantly decrease number of self-administered injections. However, a dose of 0.3 mg/kg AM404 significantly (injections: F(5,10) = 5.42; p = 0.011; response rate: F(5,10) = 4.04; p = 0.029) reduced anandamide self-administration (not shown) over 5 consecutive sessions. To further investigate the interaction between AM404 and anandamide, the effect pretreatment with 0.3 mg/kg AM404 on the anandamide self-administration dose-effect function was determined. Figure 4 shows that AM404 pretreatment shifts the anandamide dose-effect function for both injections (Figure 4a; anandamide dose×AM404 interaction: F(3,6) = 21.59; p = 0.001) and rate of responding (Figure 4b; anandamide dose×AM404 interaction: F(3,6) = 11.70; p = 0.006) to the left. Pretreatment with AM404 decreased the amount of anandamide self-administered at the two highest doses (Figure 4c; anandamide dose×AM404 interaction: F(3,6) = 7.95; p = 0.016). Unlike with anandamide, pretreatment with AM404 did not affect cocaine self-administration (Figure 5). Like with cocaine, when monkeys responding for food pellets on the same FR 10 schedule were pretreated with 0.3 mg/kg AM404 responding was not affected (not shown; pre AM404 treatment number of pellets: 49.67 ± 1.76, response rate: 0.96 ± 0.2; post AM404 treatment number of pellets: 48.89 ± 0.35, response rate: 0.79 ± 0.02). Thus, the effects of AM404 pretreatment appear to be specific to cannabinoid responding.
Figure 4.
Effect of AM404 on anandamide self-administration in squirrel monkeys under a fixed-ratio 10 (FR10) schedule. Number of anandamide injections per 1-h session (a), overall response rates in the presence of the green light signaling anandamide availability (b) and total anandamide intake per session (c) are shown as a function of the anandamide dose (abscissae log scale) after vehicle or AM404 (0.3 mg/kg) pretreatment. Each data point represents the mean ± SEM of the last three sessions under each anandamide and vehicle (V) conditions (n=3). *p < 0.05, **p < 0.01, post-hoc comparisons of the effects of pretreatment with AM404 vs. vehicle treatment within each anandamide dose, Tukey test.
Figure 5.
Effect of AM404 on cocaine self-administration in squirrel monkeys under a fixed-ratio 10 (FR10) schedule. Number of cocaine injections per 1-h session (a), overall response rates in the presence of the green light signaling cocaine availability (b) and total cocaine intake per session (c) are shown as a function of the cocaine dose (abscissae log scale) after vehicle or AM404 (0.3 mg/kg) pretreatment. Each data point represents the mean ± SEM of the last three sessions under each cocaine and vehicle (V) conditions (n=3).
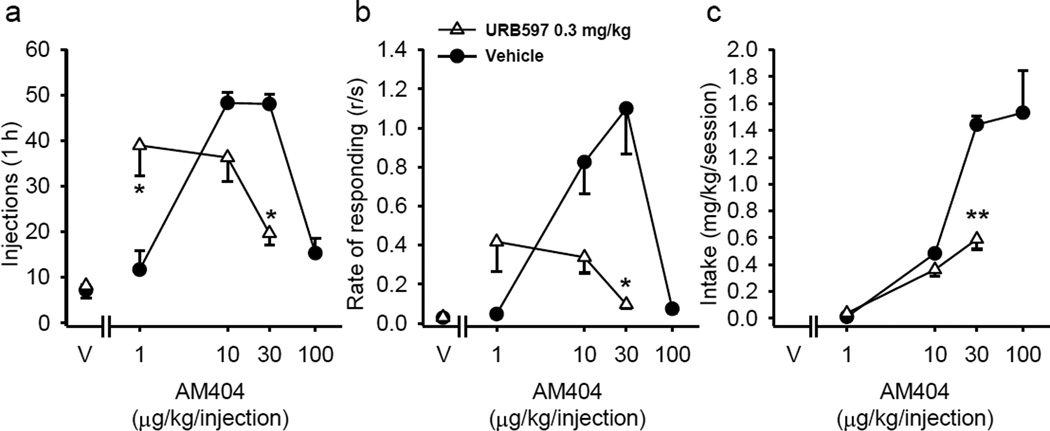
Any compound that further enhances the effects of released anandamide might be expected to affect the self-administration of AM404. One such compound is URB597 that reduces anandamide metabolism by inhibiting the enzyme that metabolizes anandamide (FAAH). Figure 6 shows that pretreatment with 0.3 mg/kg URB597 shifts the AM404 dose-effect functions to the left, which is indicative of enhanced reinforcing effects of AM404 (AM404 dose×URB597 interactions; Figure 6a, injections: F(2,4) = 49.03; p = 0.002; Figure 6b, response rates: F(2,4) = 15.47; p = 0.013; Figure 6c, intake: F(2,4) = 37.66; p = 0.003).
Figure 6.
Effect of URB597 on AM404 self-administration in squirrel monkeys under a fixed-ratio 10 (FR10) schedule. Number of AM404 injections per 1-h session (a), overall response rates in the presence of the green light signaling AM404 availability (b) and total AM404 intake per session (c) are shown as a function of the AM404 dose (abscissae log scale) after vehicle or URB597 (0.3 mg/kg) pretreatment. Each data point represents the mean ± SEM of the last three sessions under each AM404 and vehicle (V) conditions (n=4). *p < 0.05, **p < 0.01, post-hoc comparisons of the effects of pretreatment with URB597 vs. vehicle treatment within each AM404 dose, Tukey test.
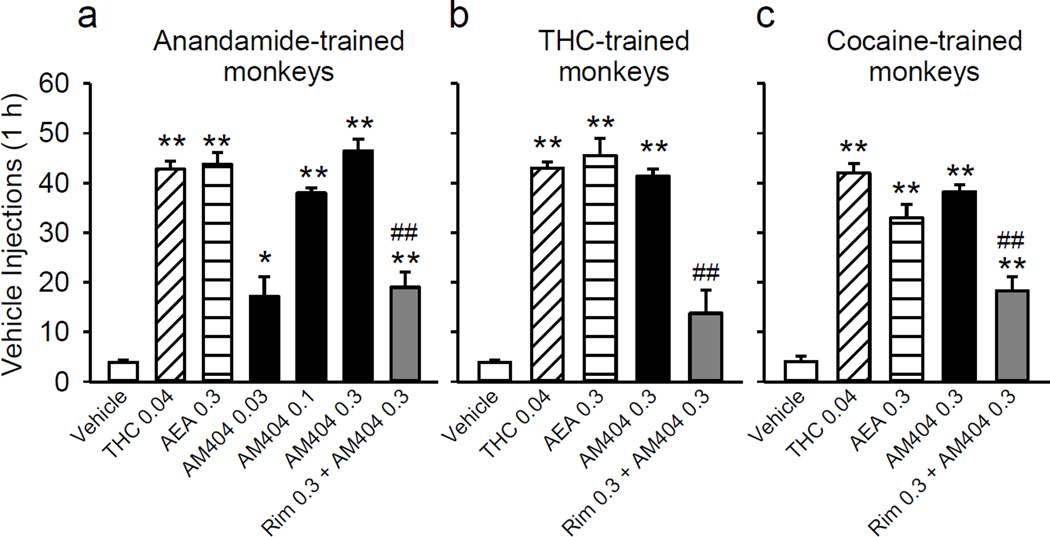
Finally, we tested the effects of priming injections of AM404 in comparison with cannabinoid agonists (anandamide, THC) on extinguished drug seeking behavior in monkeys trained to self-administer anandamide, THC, or cocaine. When vehicle was substituted for the training drug in the self-administration procedure (extinction), monkeys’ lever-pressing decreased to very low rates in all three groups (Figure 7a,b,c). In anandamide-trained monkeys, we tested the priming effects of non-contingent i.v. injections of AM404 at three doses (0.03, 0.1, and 0.3 mg/kg) and observed a dose-dependent reinstatement of extinguished drug seeking behavior with the dose 0.3 mg/kg producing an effect comparable to the effects of priming injections of THC (0.04 mg/kg) or anandamide (0.3 mg/kg) (Figure 7a; F(6,15) = 57.79; p < 0.001). In THC- and cocaine-trained monkeys, the effects of priming with AM404 (0.3 mg/kg) were also virtually the same as the effects of THC or anandamide (Figure 7b, F(4,15) = 55.23; p < 0.001; Figure 7c, F(4,11) = 59.80; p < 0.001, respectively). The lever pressing was reinstated to approximately the same levels that had been obtained during self-administration of the training dose of each drug. Pretreatment with rimonabant (0.3 mg/kg) prevented the priming effects of AM404 in all three groups of monkeys (Figures 7a,b,c; all p´s < 0.001).
Figure 7.
Anandamide (AEA), THC, and AM404 priming-induced reinstatement of extinguished drug seeking behavior in anandamide-, THC- and cocaine-experienced monkeys. Priming i.v. injection with THC (0.04 mg/kg), anandamide (0.3 mg/kg), or AM404 (0.03–0.3 mg/kg) reinstated extinguished self-administration behavior in anandamide- (a; n=4), THC- (b; n=5), and cocaine-trained (c; n=4) monkeys. Pretreatment with rimonabant (0.3 mg/kg) blocked the reinstatement induced by priming injection of AM404 (0.3 mg/kg) in all three groups of monkeys. Number of vehicle injections self-administered during 1-h sessions are shown. Bars represent means ± SEM. “Vehicle” bars represent the average of 4–6 extinction sessions prior to the test sessions. *p < 0.05, **p < 0.01, post-hoc comparisons with “Vehicle” condition; ##p < 0.01, post-hoc comparisons with “AM404 0.3” condition; Tukey test.
Discussion
Using the same intravenous self-administration procedure that had been used previously to show that cannabinoids are self-administered, we show that the anandamide transport inhibitor AM404 is also robustly self-administered by squirrel monkeys. The rates of self-administration were similar to those previously seen for other cannabinoids, including THC (Justinova et al. 2003; Tanda et al. 2000), anandamide (Justinova et al. 2005) and 2-AG (Justinova et al. 2011). Substituting vehicle for AM404 led to immediate reductions in self-administration responding and rates of self-administration immediately recovered when AM404 was again available. Self-administration of AM404 showed the typical inverted U-shaped dose-effect function that is seen for most drugs of abuse, with peak self-administration rates occurring at 10-µg/kg injection dose. AM404 was reliably self-administered by monkeys previously trained to self-administer anandamide, but also by monkeys trained on cocaine, indicating that a prior history of cannabinoid self-administration was not a necessary prerequisite to AM404 self-administration.
The mechanisms underlying the reinforcing effects of AM404 almost certainly involve cannabinoid CB1 receptors, as the CB1 antagonist/inverse agonist rimonabant attenuated AM404 self-administration. In addition to anandamide (Beltramo et al. 1997; Di Marzo et al. 1994; Hillard et al. 1997), AM404 and other anandamide uptake inhibitors have also been shown to block the uptake of 2-AG (Bisogno et al. 2001; de Lago et al. 2005). As noted above, squirrel monkeys will self-administer both anandamide (Justinova et al. 2005) and 2-AG (Justinova et al. 2011), however the involvement of 2-AG in the effects of AM404 in this study is not clear. AM404 increases 2-AG levels in the nucleus accumbens of rats, an area where changes in the levels of endocannabinoids are associated with motivation properties of some drugs (Caille et al. 2007), but does not increase the levels of anandamide there (Wiskerke et al. 2012). Here we observed that URB597, which is not self-administered by monkeys, was able to potentiate the reinforcing effects of AM404 by shifting the self-administration dose-effect functions to the left, which is indicative of interference with anandamide levels (Justinova et al. 2008a). Further, the effect of URB597 on AM404 self-administration was identical to the effect of URB597 on anandamide self-administration shown previously in monkeys (Justinova et al. 2008a). These results suggest that blockade of anandamide transport may be crucial in mediating AM404 self-administration. It is unlikely that the self-administration observed with AM404 is related to its action at vanilloid TRPV1 receptors, as VDM11, an uptake inhibitor that does not affect TRPV1 receptors (De Petrocellis et al. 2000), was also self-administered by squirrel monkeys.
In addition to supporting self-administration, AM404 administration also reinstates extinguished drug seeking behavior that was previously reinforced by cannabinoids (THC or anandamide) or cocaine. The reinstating effect of AM404 was identical to the effects of other CB1 agonists, anandamide and THC, in cannabinoid- and cocaine-experienced monkeys. It has previously been shown that AM404 reinstates self-administration responding that was previously maintained on a second-order schedule of THC reinforcement (Justinova et al. 2008b), but this is the first demonstration of reinstatement of cocaine-seeking behavior by AM404. This result suggests that the reinforcing effects of AM404 share properties with other self-administered drugs, including drugs from different chemical classes. It could also mean that AM404 at the tested dose triggered general increases in operant responding, regardless of the previous reinforcer. However, the fact that AM404 did not increase responding at low cocaine self-administration doses would argue against a general increase in operant responding. Nevertheless, there was involvement of CB1 receptors in the reinstatement effects of AM404, as rimonabant attenuated the reinstatement of drug seeking induced by AM404 in both the cannabinoid- and cocaine-experienced monkeys.
The success of methadone treatment for heroin abuse and the use of nicotine replacement as smoking cessation treatments have led a number of investigators to propose the use of agonist replacement therapy for other drugs of abuse (Grabowski et al. 2004; Rothman et al. 2008), including cannabinoid abuse (Clapper et al. 2009a). The findings with AM404 pretreatment in monkeys self-administering anandamide support this approach. AM404 reduced the self-administration of anandamide at doses near the peak of the anandamide dose-effect function and shifted the entire anandamide dose-effect function to the left. This effect was specific for cannabinoid self-administration, as AM404 did not alter self-administration of cocaine. The fact that AM404 is itself self-administered would appear to detract from its use as a replacement therapy, although this fact alone should not be disqualifying if the treatment reduces the overall negative effects of cannabinoid use. Further developments of uptake inhibitors can be directed to producing compounds that have slower onsets and longer durations of action that may reduce the abuse potential of the candidate medications (Gorelick 1998; Schindler et al. 2011).
In conclusion, the anandamide transport inhibitors AM404 and VDM11 were reliably self-administered by squirrel monkeys at rates that matched those seen with other cannabinoids. AM404 also reinstated extinguished drug-seeking responding for the cannabinoids THC and anandamide, as well as the psychostimulant cocaine. Both, the reinforcing effects of AM404 and its priming effects in the model of relapse were blocked by the cannabinoid CB1 antagonist/inverse agonist rimonabant suggesting involvement of CB1 receptors.
Acknowledgments
This research was supported by the Intramural Research Program of NIDA, NIH and by NIDA grants R01DA003801 and R01DA007215 (to Dr. Makriyannis). We thank Dr. Daniele Piomelli for providing URB597 and NIDA Drug Supply Program for providing THC and rimonabant.
Footnotes
The authors have no conflicts of interest to report.
References
- Ahn K, Smith SE, Liimatta MB, Beidler D, Sadagopan N, Dudley DT, Young T, Wren P, Zhang Y, Swaney S, Van Becelaere K, Blankman JL, Nomura DK, Bhattachar SN, Stiff C, Nomanbhoy TK, Weerapana E, Johnson DS, Cravatt BF. Mechanistic and pharmacological characterization of PF-04457845: a highly potent and selective fatty acid amide hydrolase inhibitor that reduces inflammatory and noninflammatory pain. J Pharmacol Exp Ther. 2011;338:114–124. doi: 10.1124/jpet.111.180257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alapafuja SO, Nikas SP, Bharathan IT, Shukla VG, Nasr ML, Bowman AL, Zvonok N, Li J, Shi X, Engen JR, Makriyannis A. Sulfonyl fluoride inhibitors of fatty acid amide hydrolase. J Med Chem. 2012;55:10074–10089. doi: 10.1021/jm301205j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltramo M, Stella N, Calignano A, Lin SY, Makriyannis A, Piomelli D. Functional role of high-affinity anandamide transport, as revealed by selective inhibition. Science. 1997;277:1094–1097. doi: 10.1126/science.277.5329.1094. [DOI] [PubMed] [Google Scholar]
- Beltramo M, de Fonseca FR, Navarro M, Calignano A, Gorriti MA, Grammatikopoulos G, Sadile AG, Giuffrida A, Piomelli D. Reversal of dopamine D(2) receptor responses by an anandamide transport inhibitor. J Neurosci. 2000;20:3401–3407. doi: 10.1523/JNEUROSCI.20-09-03401.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman J, Shakiru O, Makriyannis A, Justinova Z, Goldberg SR. Discriminative-stimulus and reinforcing effects of FAAH inhibitors in CB-1 trained subjects. FASEB J. 2011;25:796–794. [Google Scholar]
- Bisogno T, MacCarrone M, De Petrocellis L, Jarrahian A, Finazzi-Agro A, Hillard C, Di Marzo V. The uptake by cells of 2-arachidonoylglycerol, an endogenous agonist of cannabinoid receptors. Eur J Biochem. 2001;268:1982–1989. doi: 10.1046/j.1432-1327.2001.02072.x. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Campolongo P, Mangieri RA, Scattoni ML, Frau R, Trezza V, La Rana G, Russo R, Calignano A, Gessa GL, Cuomo V, Piomelli D. Anxiolytic-like properties of the anandamide transport inhibitor AM404. Neuropsychopharmacology. 2006;31:2652–2659. doi: 10.1038/sj.npp.1301061. [DOI] [PubMed] [Google Scholar]
- Caille S, Alvarez-Jaimes L, Polis I, Stouffer DG, Parsons LH. Specific alterations of extracellular endocannabinoid levels in the nucleus accumbens by ethanol, heroin, and cocaine self-administration. J Neurosci. 2007;27:3695–3702. doi: 10.1523/JNEUROSCI.4403-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapper JR, Mangieri RA, Piomelli D. The endocannabinoid system as a target for the treatment of cannabis dependence. Neuropharmacology. 2009a;561(Suppl 1):235–243. doi: 10.1016/j.neuropharm.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapper JR, Vacondio F, King AR, Duranti A, Tontini A, Silva C, Sanchini S, Tarzia G, Mor M, Piomelli D. A second generation of carbamate-based fatty acid amide hydrolase inhibitors with improved activity in vivo. ChemMedChem. 2009b;4:1505–1513. doi: 10.1002/cmdc.200900210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapper JR, Moreno-Sanz G, Russo R, Guijarro A, Vacondio F, Duranti A, Tontini A, Sanchini S, Sciolino NR, Spradley JM, Hohmann AG, Calignano A, Mor M, Tarzia G, Piomelli D. Anandamide suppresses pain initiation through a peripheral endocannabinoid mechanism. Nat Neurosci. 2010;13:1265–1270. doi: 10.1038/nn.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Council NR. Guidelines for the care and use of mammals in neuroscience and behavioral research. Washington DC: The National Academies Press; 2003. [PubMed] [Google Scholar]
- de Lago E, Petrosino S, Valenti M, Morera E, Ortega-Gutierrez S, Fernandez-Ruiz J, Di Marzo V. Effect of repeated systemic administration of selective inhibitors of endocannabinoid inactivation on rat brain endocannabinoid levels. Biochem Pharmacol. 2005;70:446–452. doi: 10.1016/j.bcp.2005.05.011. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Bisogno T, Davis JB, Pertwee RG, Di Marzo V. Overlap between the ligand recognition properties of the anandamide transporter and the VR1 vanilloid receptor: inhibitors of anandamide uptake with negligible capsaicin-like activity. FEBS Lett. 2000;483:52–56. doi: 10.1016/s0014-5793(00)02082-2. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz JC, Piomelli D. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 1994;372:686–691. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- Fegley D, Kathuria S, Mercier R, Li C, Goutopoulos A, Makriyannis A, Piomelli D. Anandamide transport is independent of fatty-acid amide hydrolase activity and is blocked by the hydrolysis-resistant inhibitor AM1172. Proc Natl Acad Sci U S A. 2004;101:8756–8761. doi: 10.1073/pnas.0400997101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CJ, Tiger G, Ligresti A, Lopez-Rodriguez ML, Di Marzo V. Selective inhibition of anandamide cellular uptake versus enzymatic hydrolysis--a difficult issue to handle. Eur J Pharmacol. 2004;492:1–11. doi: 10.1016/j.ejphar.2004.03.048. [DOI] [PubMed] [Google Scholar]
- Fowler CJ. Anandamide uptake explained? Trends Pharmacol Sci. 2012;33:181–185. doi: 10.1016/j.tips.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Fu J, Bottegoni G, Sasso O, Bertorelli R, Rocchia W, Masetti M, Guijarro A, Lodola A, Armirotti A, Garau G, Bandiera T, Reggiani A, Mor M, Cavalli A, Piomelli D. A catalytically silent FAAH-1 variant drives anandamide transport in neurons. Nat Neurosci. 2012;15:64–69. doi: 10.1038/nn.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamaleddin I, Guranda M, Scherma M, Fratta W, Makriyannis A, Vadivel SK, Goldberg SR, Le Foll B. AM404 attenuates reinstatement of nicotine seeking induced by nicotine-associated cues and nicotine priming but does not affect nicotine- and food-taking. J Psychopharmacol. 2013;27:564–571. doi: 10.1177/0269881113477710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuffrida A, Rodriguez de Fonseca F, Nava F, Loubet-Lescoulie P, Piomelli D. Elevated circulating levels of anandamide after administration of the transport inhibitor, AM404. Eur J Pharmacol. 2000;408:161–168. doi: 10.1016/s0014-2999(00)00786-x. [DOI] [PubMed] [Google Scholar]
- Glaser ST, Abumrad NA, Fatade F, Kaczocha M, Studholme KM, Deutsch DG. Evidence against the presence of an anandamide transporter. Proc Natl Acad Sci U S A. 2003;100:4269–4274. doi: 10.1073/pnas.0730816100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg SR. Comparable behavior maintained under fixed-ratio and second-order schedules of food presentation, cocaine injection or d-amphetamine injection in the squirrel monkey. J Pharmacol Exp Ther. 1973;186:18–30. [PubMed] [Google Scholar]
- Gorelick DA. The rate hypothesis and agonist substitution approaches to cocaine abuse treatment. Adv Pharmacol. 1998;42:995–997. doi: 10.1016/s1054-3589(08)60914-x. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Shearer J, Merrill J, Negus SS. Agonist-like, replacement pharmacotherapy for stimulant abuse and dependence. Addict Behav. 2004;29:1439–1464. doi: 10.1016/j.addbeh.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Hillard CJ, Edgemond WS, Jarrahian A, Campbell WB. Accumulation of N-arachidonoylethanolamine (anandamide) into cerebellar granule cells occurs via facilitated diffusion. J Neurochem. 1997;69:631–638. doi: 10.1046/j.1471-4159.1997.69020631.x. [DOI] [PubMed] [Google Scholar]
- Hillard CJ, Jarrahian A. Cellular accumulation of anandamide: consensus and controversy. Br J Pharmacol. 2003;140:802–808. doi: 10.1038/sj.bjp.0705468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janero DR, Vadivel SK, Makriyannis A. Pharmacotherapeutic modulation of the endocannabinoid signalling system in psychiatric disorders: drug-discovery strategies. Int Rev Psychiatry. 2009;21:122–133. doi: 10.1080/09540260902782778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Tanda G, Redhi GH, Goldberg SR. Self-administration of delta9-tetrahydrocannabinol (THC) by drug naive squirrel monkeys. Psychopharmacology (Berl) 2003;169:135–140. doi: 10.1007/s00213-003-1484-0. [DOI] [PubMed] [Google Scholar]
- Justinova Z, Solinas M, Tanda G, Redhi GH, Goldberg SR. The endogenous cannabinoid anandamide and its synthetic analog R(+)-methanandamide are intravenously self-administered by squirrel monkeys. J Neurosci. 2005;25:5645–5650. doi: 10.1523/JNEUROSCI.0951-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Mangieri RA, Bortolato M, Chefer SI, Mukhin AG, Clapper JR, King AR, Redhi GH, Yasar S, Piomelli D, Goldberg SR. Fatty acid amide hydrolase inhibition heightens anandamide signaling without producing reinforcing effects in primates. Biol Psychiatry. 2008a;64:930–937. doi: 10.1016/j.biopsych.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Munzar P, Panlilio LV, Yasar S, Redhi GH, Tanda G, Goldberg SR. Blockade of THC-seeking behavior and relapse in monkeys by the cannabinoid CB(1)-receptor antagonist rimonabant. Neuropsychopharmacology. 2008b;33:2870–2877. doi: 10.1038/npp.2008.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Yasar S, Redhi GH, Goldberg SR. The endogenous cannabinoid 2-arachidonoylglycerol is intravenously self-administered by squirrel monkeys. J Neurosci. 2011;31:7043–7048. doi: 10.1523/JNEUROSCI.6058-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Mascia P, Secci ME, Redhi GH, Piomelli D, Goldberg SR. The FAAH inhibitor PF-04457845 has THC-like rewarding and reinstatement effects in squirrel monkeys and increases dopamine levels in the nucleus accumbens shell in rats. FASEB J. 2014;28:838–836. [Google Scholar]
- Justinova Z, Panlilio LV, Moreno-Sanz G, Redhi GH, Auber A, Secci ME, Mascia P, Bandiera T, Armirotti A, Bertorelli R, Chefer SI, Barnes C, Yasar S, Piomelli D, Goldberg SR. Effects of Fatty Acid Amide Hydrolase (FAAH) Inhibitors in Non-Human Primate Models of Nicotine Reward and Relapse. Neuropsychopharmacology. 2015;40:2185–2197. doi: 10.1038/npp.2015.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczocha M, Glaser ST, Deutsch DG. Identification of intracellular carriers for the endocannabinoid anandamide. Proc Natl Acad Sci U S A. 2009;106:6375–6380. doi: 10.1073/pnas.0901515106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. Elevating endocannabinoid levels: pharmacological strategies and potential therapeutic applications. Proc Nutr Soc. 2014;73:96–105. doi: 10.1017/S0029665113003649. [DOI] [PubMed] [Google Scholar]
- Petrosino S, Di Marzo V. FAAH and MAGL inhibitors: therapeutic opportunities from regulating endocannabinoid levels. Curr Opin Investig Drugs. 2010;11:51–62. [PubMed] [Google Scholar]
- Piomelli D, Beltramo M, Glasnapp S, Lin SY, Goutopoulos A, Xie XQ, Makriyannis A. Structural determinants for recognition and translocation by the anandamide transporter. Proc Natl Acad Sci U S A. 1999;96:5802–5807. doi: 10.1073/pnas.96.10.5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piomelli D. The endocannabinoid system: a drug discovery perspective. Curr Opin Investig Drugs. 2005;6:672–679. [PubMed] [Google Scholar]
- Piomelli D, Tarzia G, Duranti A, Tontini A, Mor M, Compton TR, Dasse O, Monaghan EP, Parrott JA, Putman D. Pharmacological profile of the selective FAAH inhibitor KDS-4103 (URB597) CNS Drug Rev. 2006;12:21–38. doi: 10.1111/j.1527-3458.2006.00021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffa RB, Ward SJ. CB(1)-independent mechanisms of Delta(9)-THCV, AM251 and SR141716 (rimonabant) J Clin Pharm Ther. 2012;37:260–265. doi: 10.1111/j.1365-2710.2011.01284.x. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Blough BE, Baumann MH. Dopamine/serotonin releasers as medications for stimulant addictions. Prog Brain Res. 2008;172:385–406. doi: 10.1016/S0079-6123(08)00919-9. [DOI] [PubMed] [Google Scholar]
- Scherma M, Justinova Z, Zanettini C, Panlilio LV, Mascia P, Fadda P, Fratta W, Makriyannis A, Vadivel SK, Gamaleddin I, Le Foll B, Goldberg SR. The anandamide transport inhibitor AM404 reduces the rewarding effects of nicotine and nicotine-induced dopamine elevations in the nucleus accumbens shell in rats. Br J Pharmacol. 2012;165:2539–2548. doi: 10.1111/j.1476-5381.2011.01467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler CW, Cogan ES, Thorndike EB, Panlilio LV. Rapid delivery of cocaine facilitates acquisition of self-administration in rats: an effect masked by paired stimuli. Pharmacol Biochem Behav. 2011;99:301–306. doi: 10.1016/j.pbb.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seely KA, Brents LK, Franks LN, Rajasekaran M, Zimmerman SM, Fantegrossi WE, Prather PL. AM-251 and rimonabant act as direct antagonists at mu-opioid receptors: implications for opioid/cannabinoid interaction studies. Neuropharmacology. 2012;63:905–915. doi: 10.1016/j.neuropharm.2012.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Tanda G, Justinova Z, Wertheim CE, Yasar S, Piomelli D, Vadivel SK, Makriyannis A, Goldberg SR. The endogenous cannabinoid anandamide produces delta-9-tetrahydrocannabinol-like discriminative and neurochemical effects that are enhanced by inhibition of fatty acid amide hydrolase but not by inhibition of anandamide transport. J Pharmacol Exp Ther. 2007;321:370–380. doi: 10.1124/jpet.106.114124. [DOI] [PubMed] [Google Scholar]
- Tanda G, Munzar P, Goldberg SR. Self-administration behavior is maintained by the psychoactive ingredient of marijuana in squirrel monkeys. Nat Neurosci. 2000;3:1073–1074. doi: 10.1038/80577. [DOI] [PubMed] [Google Scholar]
- Wiskerke J, Irimia C, Cravatt BF, De Vries TJ, Schoffelmeer AN, Pattij T, Parsons LH. Characterization of the effects of reuptake and hydrolysis inhibition on interstitial endocannabinoid levels in the brain: an in vivo microdialysis study. ACS Chem Neurosci. 2012;3:407–417. doi: 10.1021/cn300036b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygmunt PM, Chuang H, Movahed P, Julius D, Hogestatt ED. The anandamide transport inhibitor AM404 activates vanilloid receptors. Eur J Pharmacol. 2000;396:39–42. doi: 10.1016/s0014-2999(00)00207-7. [DOI] [PubMed] [Google Scholar]