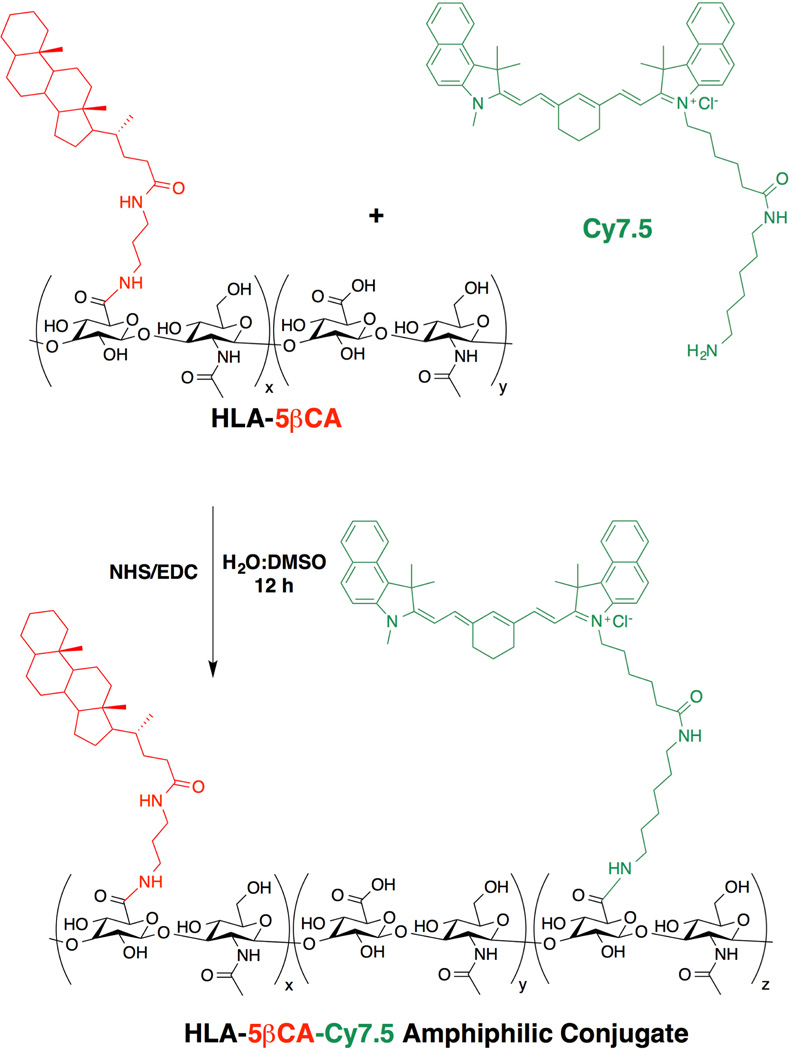
Abstract
Fluorescent imaging agents that can specifically highlight tumor cells could have a significant impact on image-guided tumor removal. Here, fluorescent nanoparticles (NPs) derived from hyaluronic acid (HA) are investigated. HA is a ligand for the receptor CD44, which is a common biomarker present on many primary tumor cells, cancer-initiating cells, and tumor-associated fibroblasts. In addition, a family of enzymes that degrade HA, called hyaluronidases (HYALs), are also overexpressed with increased activity in many tumors. We report the design and development of a panel of targeted imaging agents using the near-infrared (NIR) dye, Cy7.5, that was directly conjugated to hydrophobically-modified HA. Two different molecular weights of HA, 10 kDa and 100 kDa, and three different degrees of hydrophobic moiety conjugation (0, 10, and 30 mol%) were utilized to develop a panel of NPs with variable size that ranged from 50 to 400 nm hydrodynamic diameter (HD) depending HA molecular weight, extent of fluorescence quenching (25–50%), kinetics of cellular uptake, and targeting to CD44+ cells. The kinetics and energy-dependence of cellular uptake in breast and prostate cancer cell lines, MDA-MB 231 and PC-3 cells, respectively, showed increased uptake with longer incubation times (at 4 and 8 h compared to 1h), as well as uptake at 37°C but not 4°C, which indicated energy-dependent endocytosis. NP uptake studies in the presence of excess free HA showed that pre-treatment of cells with excess high molecular weight (MW) free HA decreased NP uptake by up to 50%, while no such trend was observed with low MW HA. These data lay the foundation for selection of optimized HA-derived NPs for image-guided surgery.
Keywords: hyaluronic acid, image-guided surgery, intraoperative imaging, near-infrared fluorescence, Cy7.5
Graphical Abstract
1. Introduction
Surgery is a primary method of treatment for more than 50% of patients with some form of cancer [1]. Current diagnostic imaging techniques are primarily based on computed tomography, positron emission tomography, magnetic resonance imaging, and ultrasound. These can be limited in their ability to differentiate benign and malignant lesions, especially for small tumors [2]. Detection of small malignant lesions and residual cancer cells remaining at the surgical margin impacts tumor recurrence, follow-up treatment, and ultimately, patient survival.
Use of NIR fluorescence imaging in cancer surgery could be paradigm shifting, resulting in decreased healthcare costs and better patient survival. NIR light can penetrate tissue on a millimeter scale, while visible light can only penetrate on the order of micrometers. NIR fluorophores offer several advantages over UV-Vis dyes, such as deeper tissue penetration due to lower absorbance and scatter from hemoglobin and water, decreased autofluorescence from endogenous fluorophores, and minimum photo damage to the native tissue [2–4]. Intraoperative NIR imaging enables visualization of superficial tumors (<8 mm below surface of tissue) and local metastatic lesions, is relatively safe for patients and surgeons, and has the potential for specific targeting [5]. NIR dyes have been used to explore various applications regarding in vitro and in vivo imaging, including, pH changes, biomolecules, reactive oxygen and reactive nitrogen species signaling, metal ions and enzymes [3]. Currently available FDA-approved NIR dyes, indocyanine green (ICG) and methylene blue, are non-specific [6,7]. Demand for new probes that have higher photo-stability, quantum yield, Stoke’s shift, and ease of modification for various applications is required for the next generation of specifically targeted NIR fluorescent bioconjugates. For example, IR800-CW (LI-COR Biosciences, Lincoln, NE) conjugated to bevacizumab (against VEGF) is being studied in patients in Phase I clinical trials [8].
NPs have gained recent attention in biomedical imaging and especially for image-guided surgery [9–11]. Fluorescent dyes and drugs embedded in the core of nanomaterials have been shown to possess higher chemical stability (against enzymes or ROS) and photo stability, improved water solubility, and targetability. Furthermore, macromolecules in general, including NPs, can preferentially accumulate in tumors due to enhanced vascular permeability and poor lymphatic drainage [12]. Specificity can be enhanced by conjugation of targeting ligands to improve intra-tumoral accumulation, drug efficacy, and reduced off-target toxicity [13,14]. To that end, HA, a non-sulfated glycosaminoglycan comprised of disaccharide repeat units of alternating (1–3)-β linked N-acetyl-D-glucosamine and (1–4)-β linked D-glucuronic acid, is a biopolymer uniquely suited for biomedical applications. HA is biocompatible and biodegradable by HYALs, which are overexpressed in many tumors [15–17]. Of note, HA is a ligand for CD44, which is receptor commonly present on many primary tumor cells, cancer-initiating cells, and tumor-associated fibroblasts [18,19].
Several groups have attempted to take advantage of CD44’s endogenous ligand, HA, to develop NPs that can target multiple types of cancer for drug and/or imaging agent delivery [20– 22]. One common method of NP formation using HA is modification with hydrophobic ligands and polymers. For example, ceramide, bile acids, or polymers such as poly [lactide-(co-glycolic acid)] (PLGA), poly ( -amino ester) have been conjugated or grafted to HA to drive self-assembly into nanoparticles for targeted delivery of therapeutic moieties. [20,21,23–28]. Likewise, we have recently described HA-derived NPs that entrap ICG, termed NanoICG, for image-guided surgery using human breast tumor xenografts in mice [28]. In this case, HA was modified with different hydrophobic ligands to form self-assembled micelle-like structures that could entrap ICG. Although NanoICG demonstrated significantly higher contrast to noise compared to ICG alone, the physical entrapment of ICG has inherent limitations in determining the role and distribution of amphiphilic HA, as ICG can also associate with serum proteins [7,29].
Furthermore, it has been shown that for a polymer, molecular weight plays a large role in fundamental NP properties such as size, toxicity and in vivo behavior [30,31]. This work presents an initial analysis into the effect of HA MW and hydrophobic ligand content on NP properties. Accordingly, we have designed and developed a panel of HA-derived nanoparticles using the NIR dye Cy7.5 directly conjugated to HA. We report here the effect of HA molecular weight, hydrophobic ligand content, and direct dye conjugation on the physical, chemical, optical, and biological properties of HA-derived nanoparticles in vitro. These studies lay the foundation for the next generation of HA-based NIR imaging agents for image-guided surgery.
2. Materials and Methods
2.1. Materials
HA (10 and 100 kg/mol) was purchased from LifeCore Biomedical (Chaska, MN). 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC), N-hydroxysuccinimide (NHS) and N,N-dimethylformamide (DMF) were purchased from Fisher Scientific. 5-beta cholanamide (5 CA) was synthesized from 5-beta cholanic acid (Fisher Scientific) using a previously published procedure [22,28]. All reagents were used without further purification unless specified otherwise. Cy7.5-amine was obtained from Lumiprobe Corporation (Hallandale Beach, FL). Desalting PD10 columns and dialysis membranes (3,500 MWCO and 6,000–8,000 MWCO) were purchased from GE healthcare and Fisher Scientific, respectively. NMR was performed on a 500 MHz Bruker or 600 MHz Varian system using a 5 mm probe at room temperature. Size exclusion chromatography (SEC) was performed in an aqueous mobile phase containing 0.1 M sodium phosphate (NaH2PO4) with 250 ppm sodium azide in water at a flow rate 0.45 mL/min. HA-conjugates dissolved in the mobile phase were separated using Ultrahydrogel™ 250 and 1000 columns (Waters Corporation). The SEC system was equipped with a Waters 2998 photodiode array, 2414 refractive index detector, and a Wyatt miniDAWN TREOS multiangle laser light scattering detector. Data was recorded and analyzed using the ASTRA (version 6.1) software.
DMEM, fetal bovine serum, and penicillin/streptomycin were obtained from Cell and Viral Vector lab at Wake Forest Health Sciences. PBS was obtained from Hyclone™ Laboratories Inc. (GE Healthcare). Human breast (MDA-MB 231) and prostate (PC-3) cancer cell lines were obtained from ATCC (Manassas, VA).
2.2. General procedure for conjugation of 5-beta cholanamide to hyaluronic acid
Sodium hyaluronate (100 mg of 10 or 100 kg/mol) was dissolved into 12.5 mL nanopure water. HA polymer conjugates or nanoparticles derived from 10 kDa HA are identified with subscript “10”, while those from 100 kDa HA are identified with subscript “100”. Next, EDC and NHS (78–154 mmol each) were dissolved into the HA/nanopure water solution to give a tenfold molar excess of coupling agent. HA solution was stirred at room temperature for 30 min to allow activation of carboxylic acid groups before further functionalization and then 12.5 mL of DMF was slowly added to this solution. Zero (no ligand, subscript Ø), 10 (low content, subscript L), or 25–30 (high content, subscript H) mol% 5 CA was dissolved into 12.5 ml of DMF under stirring and low heat. After cooling to room temperature, 12.5 ml of nanopure water was added to the 5 CA/DMF solution. Finally, 5 CA solution was added to the activated HA mixture at room temperature and stirred for 12–15 h. The reaction mixture was dialyzed against 1:1 ethanol: ultrapure water for one day and ultrapure water for next two days using 3500 Da (for 10 kg/mol HA derivatives) or 6000–8000 Da (for 100 kg/mol HA derivatives) MWCO dialysis tubing to remove excess of small molecular reactants and DMF. This product was then lyophilized to obtain white fluffy polymer (Scheme 1). NMR integration was used to determine conjugation ratio of 5 CA to HA as previously reported [28]. Yield: HA10-5βCAL (yield = 83.0%), HA10-5βCAH (yield = 66.5%), HA100-5βCAL (yield = 98.0%) and HA100-5βCAH (yield = 68.5%). Conjugation efficiency of 5βCA to HA is summarized in Table 1.
Scheme 1.
Synthetic Conjugation of Cy7.5 to HA-5 CA polymers.
Table 1.
Chemical composition of NPs functionalized with 5βCA and Cy7.5 as determined by NMR and UV-Vis spectroscopy.
| Namea | Polymer Conjugate |
Theoretical 5βCA content (mol %) |
Actual 5βCA content (mol %) |
Theoretical Cy7.5 loading (mol %) |
Actual Cy7.5 loading (mol %) |
Zeta Potential (mV) |
|---|---|---|---|---|---|---|
| NanoCy7.510-Ø | HA10-Cy7.5 | 0 | 0 | 2.0 | 0.23 | −34.4 ± 2.1 |
| NanoCy7.510-L | HA10-5βCAL-Cy7.5 | 10.4 | 9.0 | 3.2 | 0.42 | −34.2 ± 1.9 |
| NanoCy7.510-H | HA10-5βCAH-Cy7.5 | 25.7 | 22.5 | 2.5 | 1.21 | −33.5 ± 0.4 |
| NanoCy7.5100-Ø | HA100-Cy7.5 | 0 | 0 | 1.8 | 0.2 | −39.8 ± 1.0 |
| NanoCy7.5100-L | HA100-5βCAL-Cy7.5 | 10.4 | 8.6 | 3.9 | 0.4 | −32.1 ± 0.4 |
| NanoCy7.5100-H | HA100-5βCAH-Cy7.5 | 32.1 | 29.0 | 3.05 | 1.25 | −31.9 ± 0.3 |
NP nomenclature is as follows: NanoCy7.5x-y, where NanoCy7.5 refers to HA conjugated to Cy7.5, x = HA molecular weight used for conjugates, and y refers to either no (Ø), low (L) or high (H) 5βCA content.
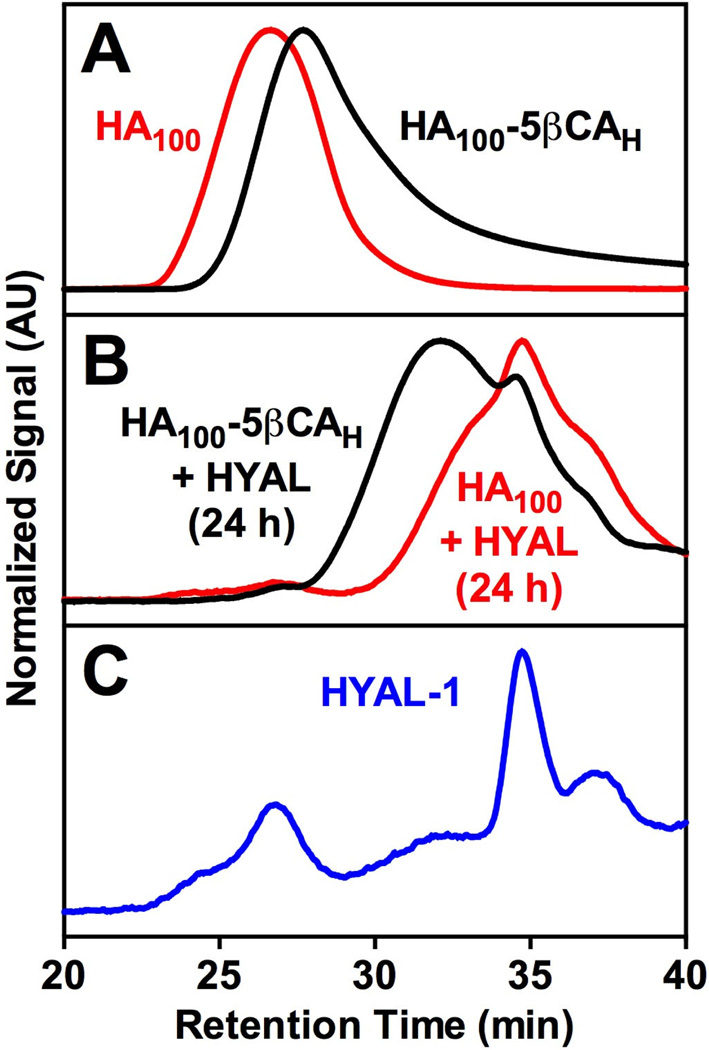
SEC was used to measure the degradability of HA by HYAL after modification with a hydrophobic moiety. Briefly, either HA (100 kDa) or HA100-5βCAH (2 mg/ml) was dissolved in PBS (pH 7) containing 0.5 mg/mL (200–500 units) of HYAL (from bovine testes, Sigma Aldrich) and incubated at 37 °C for 24 h. Each sample, including HYAL alone, was then subjected to SEC using the parameters above. Changes in retention time and chromatogram shape between HA (100 kDa) and HA100-5βCAH were then compared.
2.3. General procedure for conjugation of Cy7.5-amine to hyaluronic acid and HA-5 CA
HA-5 CA conjugate, 23.8 mg (54.0 µmol), was dissolved in 2.8 mL of ultrapure water. EDC (0.7mg, 4.0 µmol) and NHS (0.4 mg, 4.0 µmol) were dissolved in 100 µL of ultrapure water were added to the respective HA-5 CA solutions. The reaction mixture was stirred at room temperature for 30 min to allow activation of carboxylic acid groups on HA. Next, a solution of Cy7.5-amine (1.1 mg, 1.34 µmol) in dimethyl sulfoxide (DMSO) (3.0 mL) was prepared. This solution was added to the above HA mixture dropwise under constant stirring. The reaction mixture was allowed to stir for 12 h at room temperature. The reaction vial was covered with a foil to avoid light exposure. After the reaction mixture was stirred for at least 12 h, the product was purified from excess reactants, DMSO and other impurities using dialysis. The reaction mixture was placed in a dialysis bag with MWCO of 3500 (10kDa HA-5 CA derivatives) or 6000–8000 Da (100kDa HA-5 CA derivatives) and dialyzed against water for 24h with 4–6 water changes. Residual unconjugated Cy7.5 dye was removed from the HA-conjugates via a PD10 desalting columns with ultrapure water as a mobile phase. The product fraction was freeze-dried to obtain fluffy, light-green product (NanoCy7.510-Ø, NanoCy7.510-L, NanoCy7.510-H, NanoCy7.5100-Ø, NanoCy7.5100-L and NanoCy7.5100-H). Yields: NanoCy7.510-Ø (yield= 56%), NanoCy7.510-L (yield = 48%), NanoCy7.510-H (yield = 52.3%), NanoCy7.5100-Ø (yield = 64.2%), NanoCy7.5100-L (yield = 44%), NanoCy7.5100-H (yield = 53.3%).
2.4. Nanoparticle Characterization
Particle size and hydrodynamic diameter (HD) of all the NPs were obtained using dynamic light scattering (DLS) with a ZetaPlus system (Brookhaven Instruments Corporation; Holtsville, NY). Zeta potential of all NPs was obtained using a ZetaSizer Nano ZS90 (Malvern; Malvern, UK). NP samples were prepared by dissolving in ultrapure water (1mg/mL) and filtered through 0.45 µm syringe filter. Absorbance spectra of the NPs were obtained with UV-2600 spectrometer (Shimadzu Scientific Instruments; Columbia MD) in water and 1:1 mixture of water:DMSO, to study NP assembly. Fluorescence spectra were obtained on a FluoroMax-4, fluorescence spectrometer equipped with a NIR extended range PMT (Horiba Jobin Yvon; Edison, NJ, USA), and a Pearl Impulse Animal Imaging System (LI-COR; Lincoln, NE). Conjugation ratio of Cy7.5 dye to HA was determined using standard curve developed for Cy7.5 dye in 1:1 water:DMSO.
2.5. Nanoparticle Cytotoxicity
NP toxicity was studied using the CCK-8 assay (Dojindo, Japan). Human breast (MDA-MB-231) or prostate cancer (PC-3) cells were seeded in 96-well plate at 25,000 cells/well density using DMEM media with 10% FBS and 1% P/S a day before NP treatment. NPs were dissolved in serum-free media at several concentrations (0, 0.01, 0.05, 0.1 mg/mL) and 200 µL of NP solution was added to the each well. Cells were treated with NPs for 24 h and then washed twice with PBS. Next, 100 µL of 10% solution of CCK-8 reagent in media was added to each well and incubated at 37 °C for 1 h. After 1 h, 90 µL of CCK-8 solution was transferred to new 96-well plate to prepare for the absorbance assay. The absorbance of each solution was measured at 450 nm using Spectramax M5 spectrophotometer (Molecular Devices, Sunnyvale, CA). Viability of NP treated cells was compared to untreated control cells.
2.6. Characterization of NP Uptake with Flow Cytometry
NP uptake in human breast and prostate cancer cell lines at 37 °C and 4 °C was studied using flow cytometry (FACSAria II, BD Bioscience, San Jose, CA, USA). Cells were seeded in 12-well plates at 105 cells/well using DMEM culture media with 10% FBS and 1% P/S 24 h prior to NP treatment. Next, 1 mL of DMEM (serum-free) media containing 10 kDa and 100 kDa NPs with variable ligand content (NanoCy7.510-Ø NanoCy7.510-L, NanoCy7.510-H, NanoCy7.5100-Ø, NanoCy7.5100-L and NanoCy7.5100-H ) at equimolar Cy7.5 dye concentrations of 1.5 mM was added to each well and incubated for 4 h at 37°C and 4°C. Incubation times were based on kinetics study performed on 10 kDa and 100 kDa MW NPs separately and selected with more than 95% cells population positive for NP fluorescence. After NP incubation, cells were washed with PBS, trypsinized and re-suspended in 400 µL of FACS buffer (3% FBS in PBS). CD44 (PE Mouse Anti-Human CD44, BD Pharmingen) or isotype (PE mouse IgG1k isotype control, BD Pharmingen) antibodies were added (20 µL for 106 cells) to cell suspension and incubated in the dark at 4°C for 30min. Next, cells were fixed with 10% neutral buffered formalin (300 µL) for 15 min at room temperature. Cells were stored at 4°C in FACS buffer (200 µL) and filtered through 70 µm cell strainer before FACS analysis. All flow cytometry data was analyzed using FlowJo software.
2.7. Confocal Microscopy
MDA-MB-231 cells were seeded (105 cells/well) onto the coverslips fitted inside 12-well plates. 24 h after plating, cells were treated with NanoCy7.5 at a concentration of 3 mM in serum-free media and incubated at 37 °C or 4 °C. After incubation, cells were washed twice with PBS (5 min each), and then fixed with neutral buffered formalin (NBF) for 15 min, followed by three PBS washings. Next, cells were washed with 0.25% Triton in PBS to permeabilize the cell membrane, followed by blocking with 1% BSA in PBS. Next, primary antibody for early endosomal marker Rab 5 (Rabbit polyclonal IgG, Santa Cruz Biotechnology Inc., Dallas, TX) was added in 1% BSA solution and incubated with the cells overnight at 4°C. Secondary antibody diluted in 1% BSA was added to each well along with the untreated control well and incubated at room temperature for 30 min. After washing three times with PBS, cells were stained with NucBlue® fixed cell stain (Molecular Probes, Life Technologies, Carlsbad, CA) and cover slips were mounted onto a glass slide with Prolong® Gold anti-fading reagent (Molecular probes, Life Technologies, Carlsbad, CA). Slides were stored at 4 °C until imaging.
Confocal images were taken with Olympus Fluoview FV10i Microscope, 60X with 1 µm cell slices. DAPI, Alexa Flour 488 and Alexa Fluor 647 filters were used to detect blue, green and NIR signal from the cells. Images were analyzed by ImageJ software (National Institutes of Health; Bethesda, MD).
2.8. Nanoparticle Uptake in Presence of Excess HA
NanoCy7.5 targeting was evaluated in the presence of excess HA in MDA-MB-231 cells; in this case HA, the endogenous ligand of CD44 and can act as an inhibitor of NP uptake [27]. Flow cytometry (FACSAria II, BD Bioscience, San Jose, CA, USA) and confocal microscopy (Olympus Fluoview FV10i) were performed after incubation with NanoCy7.5 and HA as follows: cells were pre-incubated with unmodified HA for 1 h at 37 °C in serum-free DMEM media. After 1 h, media was removed and NPs were added to the pretreated cells along with free HA and incubated further for 1 h. After NP incubation, the cells were treated as described above. Cy7.5 fluorescence from cells pretreated with HA was compared with fluorescence from untreated cells for the same incubation time.
3. Results
3.1. Synthesis of Amphiphilic Hyaluronic Acid Conjugates and Self-Assembled Nanoparticles
HA was functionalized with the hydrophobic ligand, 5 CA, to form amphiphilic conjugates that could self-assemble in water. 5 CA was synthesized from commercially available 5-beta cholanic acid via two-step process as described previously [22,28]. The extent of 5 CA functionalization was characterized with NMR by integrating N-acetyl protons (at 2.03 ppm) on HA with methyl protons on 5 CA (0.65 ppm) as previously reported [22,28]. For both the high and low 5 CA feed ratios with HA, conjugation efficiency of 80–90% was obtained (Table 1). Cy7.5 conjugation, also via EDC/NHS, significantly increased in efficiency with an increase in 5βCA content on HA. Conjugation efficiency was ∼11 % when coupling Cy7.5 directly to HA, which increased to average of ∼35% efficiency for NPs with 30% ligand conjugation (Table 1). Thus, we speculate that the increase in hydrophobicity of HA may have created more favorable conditions for hydrophobic dye functionalization.
3.2. Degradation with HYAL
HA (100 kDa) and HA100-5 CAH were subjected to HYAL degradation for 24 h and analyzed using SEC for changes in MW (Figure 1). The chromatogram for HA100 prior to HYAL treatment (Figure 1A) was to the left of HA100-5 CAH even though MW of the amphiphilic conjugate is higher. This is likely due to more compact structure of HA100-5 CAH compared to random coil and expanded morphology of free HA during its elution through size exclusion column in a mobile phase. Both the chromatograms for HA100 and HA100-5 CAH shifted to the right upon treatment with HYAL indicating a decrease in MW. The peak from HYAL in each chromatogram is readily identifiable. HA100-5 CAH was chosen for this analysis because 100 kDa could be readily distinguished from HYAL and the high hydrophobic content represented the highest degree of HA modification. HA remains biodegradable after synthetic modification with a hydrophobic moiety as demonstrated HA100-5 CAH degradation when incubated with HYAL.
Figure 1.
Degradation of HA100 and HA100-5 CAH in presence of HYAL enzyme before (A) and after (B) chemical modification with hydrophobic ligand studied using size exclusion chromatography. Shift in light scattering chromatogram toward right with increase in time indicates degradation of pure HA and HA-derivatives due to HYAL enzymes. The SEC chromatogram of HYAL enzyme under same conditions (C) is added for the reference.
3.3. Particle Size and Zeta Potential Determination
NanoCy7.5 diameters were determined by DLS and TEM (Figure 2). NP size was highly dependent on MW of the HA backbone. NanoCy7.5 based on 10 kDa HA had effective diameters that ranged from 100 to 130 nm, while those based on 100 kDa HA were significantly larger, ranging between 300 and 600 nm (Figure 2A). Overall, we observed an increase in particle size upon conjugation of HA with a hydrophobic moiety since NanoCy7.510-L, NanoCy7.510-H, NanoCy7.5100-L, and NanoCy7.5100-H have higher particle sizes compared to their analogues without 5βCA, NanoCy7.510-Ø and NanoCy7.5100-Ø. For NanoCy7.510-Ø and NanoCy7.5100-Ø, i.e. when no hydrophobic moiety is present, Cy7.5 in a small quantity ∼0.2 mol% is not enough to drive stable self-assembly of HA polymer chains. This is evidenced by the optical properties (Figure 3A), where NP containing no 5βCA had only a modest effect on fluorescence-quenching, compared to NanoCy7.510-L, NanoCy7.510-H, NanoCy7.5100-L, and NanoCy7.5100-H. With the introduction of 5βCA, a more well-defined hydrophobic region is formed resulting in an increase in particle size and fluorescence quenching as described below in Section 3.4.
Figure 2.
DLS analysis of 10 kDa and 100 kDa HA NPs functionalized with 5 CA and Cy7.5 dye was performed in water to determine particle sizes (A). Number average size distribution profiles of HA NPs formed with variable hydrophobic ligand content: no ligand, 10%, and 30% (theoretical). B) TEM of NanoCy7.510-H and NanoCy7.5100-H showed significant reduction in particle size (40nm for NanoCy7.510-H and ∼200 nm for NanoCy7.5100-H) compared to DLS. Scale bars are 500 nm.
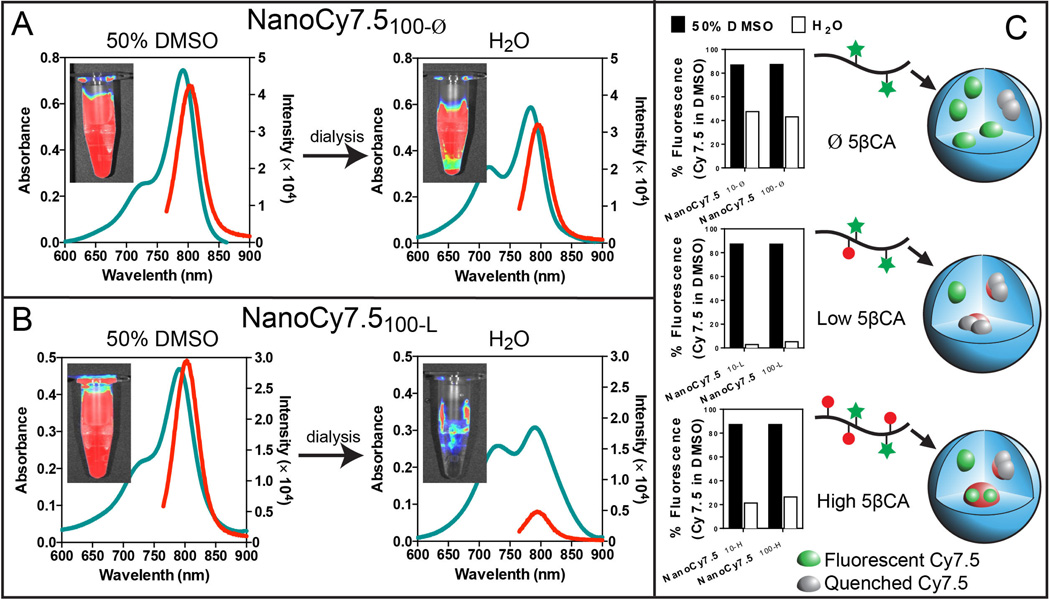
Figure 3.
Absorbance and fluorescence properties of NPs upon self-assembly (in H2O) and disassembly (1:1 H2O:DMSO). NanoCy7.5100-Ø (A) and NanoCy7.5100-L (B) are shown to illustrate optical properties of lowest (quenched) and highest (activated) fluorescence quenching NPs. NP fluorescence was also monitored in activated (H2O) and quenched state (1:1 H2O:DMSO) using LI-COR NIR imaging system, with 800 nm channel. Fluorescence activation and quenching of Cy7.5 in different NPs formulations in water and 1:1 H2O:DMSO was studied to deduce patterns in quenching-activation properties as a function of amount of hydrophobic ligand (C).
The particle size determined with TEM was smaller than DLS due to dehydration of the HA component of the NP during TEM sample preparation [32,33]. For example, in Figure 2B, NanoCy7.510-H diameter was 80% smaller (∼20 nm) compared to the HD determined by DLS; NanoCy7.5100-H, diameter was approximately 75% smaller as measured by TEM.
Zeta potential of each NP was negative, as expected due to the carboxylate groups on HA (Table 1). Zeta potential values ranged from −39.8 mV for NanoCy7.5100-Ø to −31.9 mV for NanoCy7.5100-H. For NP series based on either 100 kDa HA or 10 kDa HA zeta potential increased with increasing hydrophobic moiety content; however, this trend was not statistically significant.
3.4. Optical Properties
The NIR absorption and fluorescence of the NanoCy7.5 NPs were studied in 1:1 solution of H2O:DMSO to study the effect of self-assembly and disassembly on the optical properties. All the NPs were studied at concentration of 0.2 mg/mL, where no aggregation behavior was observed (determined by DLS, data not shown). NanoCy7.5 NPs exhibited a broad extinction spectrum in water (Figure 3A,B). In the H2O:DMSO solution, the absorbance spectra regained the shape characteristic of Cy7.5. Correspondingly, Cy7.5 fluorescence was quenched to varying degrees in H2O compared to the strong fluorescence in H2O:DMSO (Figure 3A,B). The combined observation of a broad extinction spectrum in water and quenched fluorescence is indicative of fluorophores that closely pack as part of the nanoparticle self-assembly process [28,34].
The effect of hydrophobic moiety content and HA MW on extinction and fluorescence properties was also compared (Figure 3C). Conjugation of Cy7.5 to HA (both 10 and 100 kDa) resulted in attenuated fluorescence signal, likely due to the relatively hydrophobic nature of the dye. Similar quenching was observed by Mok, et al., when ICG was directly conjugated to HA [34]. NanoCy7.510-L and NanoCy7.5100-L had nearly complete quenching. Unexpectedly, NanoCy7.5 that had higher 5βCA content had a lower degree of fluorescence quenching. We hypothesize that higher 5βCA content created “soluble” domains for Cy7.5 resulting in reduced self-aggregation and self-quenching. This is further corroborated with NIR imaging of NanoCy7.5100-Ø and NanoCy7.5100-L with a NIR imaging system in 800 nm channel (Figure 3A,B inset).
3.5. Nanoparticle Cytotoxicity
NanoCy7.5 NPs were tested for cytotoxicity using CCK-8 assay. NP groups were normalized to untreated cells. Overall, all MDA-MB-231 and PC-3 cells treated with NanoCy7.5 had viability > 80% (Figure 4). A distinct relationship between NPs dependent on molecular weights or extent of ligand conjugation was not observed.
Figure 4.
CCK-8 assay was performed on MDA-MB-231 (A) and PC-3 (B) cells incubated with the library 10 kDa and 100 kDa HA NPs for 24h.
3.6. Nanoparticle Uptake with Flow Cytometry
NanoCy7.5 NPs were studied for uptake into the breast and prostate cancer cell lines, MDA-MB 231 and PC-3, using flow cytometry. Both of these cell lines are positive for CD44 as confirmed by flow cytometry with anti-CD44-PE. We first examined the uptake kinetics of each NP by monitoring % cells positive for Cy7.5 fluorescence at 1, 4, 8, and 24 h. For NPs based on 10 kDa and 100 kDa HA, uptake increased through 8 hours. Next, NanoCy7.5 uptake was performed at 37 °C vs. 4 °C to determine if the NPs are internalized through an energy dependent process, such as endocytosis, which would be prevalent at 37°C (Figure 5B and E) and not 4 °C. We observed a 35–50% decrease in cells positive for Cy7.5 fluorescence at 4 °C compared to 37 °C, in MDA-MB 231 cells. For PC-3 cells, we observed a similar trend of significantly decreased NP uptake at 4°C. Overall, a decrease of 50–90% in cells positive for Cy7.5 was identified for each NanoCy7.5 tested (Figure 5E).
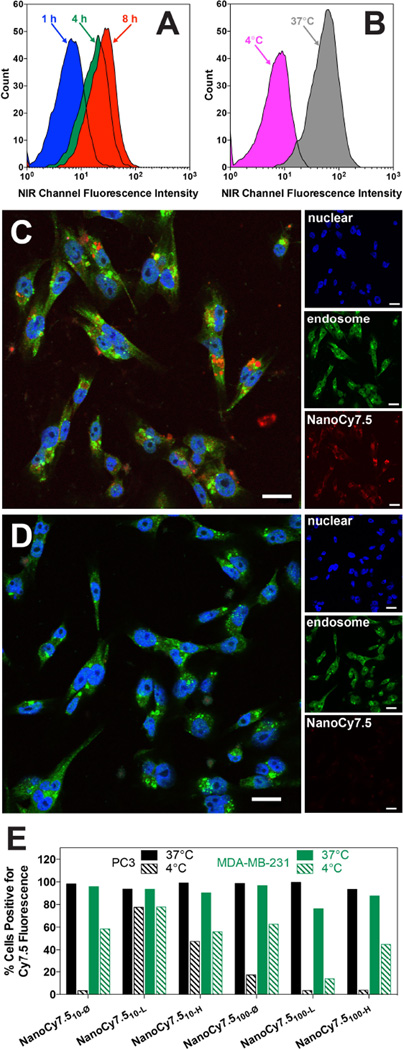
Figure 5.
Kinetics and thermodynamics of NanoCy7.5 uptake (A–E). FACS data for progression of NanoCy7.510-Ø NP uptake in MDA-MB 231 cells at 1, 4, and 8 h time points (A) as well as 37 °C vs. 4°C temperatures (B). Confocal microscopy was performed on MDA-MB 231 cells at 37 °C (C) and using 4 °C (D) with NucBlue® (blue, nucleus) and Rab5 (green, early endosomes) stains to visualize uptake of NPs (red, Cy7.5). There was minimal uptake (red fluorescence) at 4 °C compared to 37 °C. The scale bar represents 20 µm on all images. NP uptake at 37 °C vs. 4 °C was studied for NanoCy7.5 functionalized with variable ligand content (0–30 mol %) in MDA-MB 231 (black bars) and PC-3 cells (green bars) using FACS analysis (E). Results are represented in terms of % cells positive for Cy7.5 fluorescence.
Confocal microscopy was used visualize the localization of NanoCy7.5 within the cells. At 37 °C, co-localization of NanoCy7.5 fluorescence (red) with endosomal marker, Rab 5 (green) was confirmed and localized predominantly near the nucleus (blue), Figure 5C. No difference was observed between NPs prepared with 10 kDa or 100 kDa HA with respect to localization (data not shown). NPs fluorescence (Cy7.5, red) was significantly diminished at 4 °C compared to 37 °C, while the nuclear stain and Rab5 signal remained consistent at 4 °C, which suggests decreased NP uptake due to inhibition of energy-dependent endocytosis at 4 °C.
3.7. Nanoparticle Uptake in Presence of Excess Hyaluronic Acid
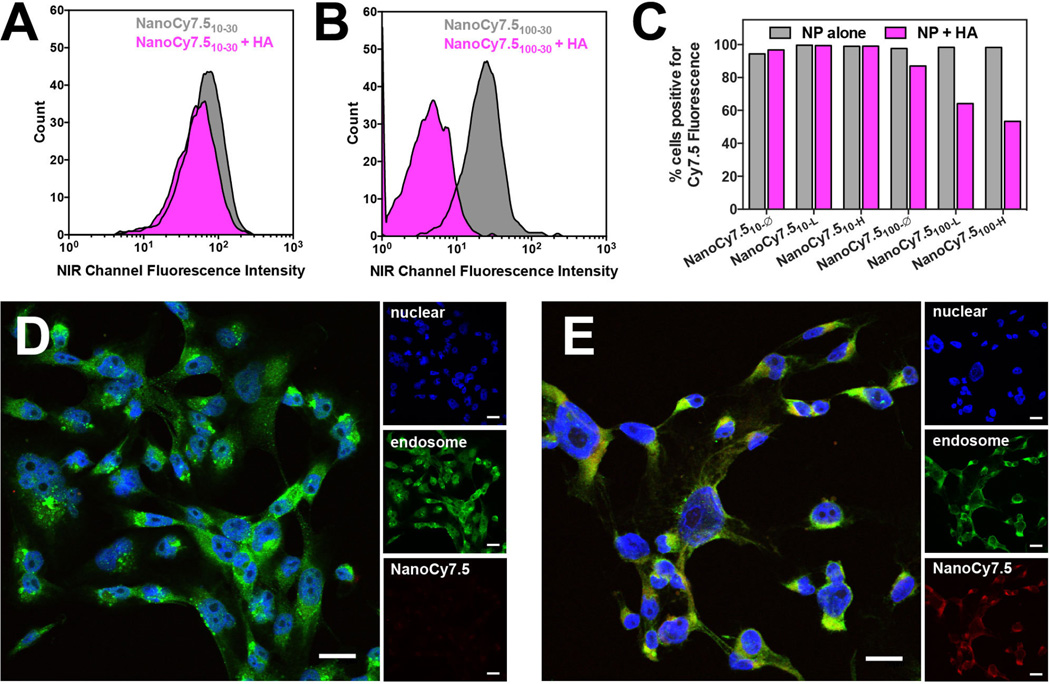
Uptake of NanoCy7.5 NPs in MDA-MB 231 cells was carried out in the presence of excess HA. HA is an endogenous ligand for CD44 and RHAMM receptors which are overexpressed on several cancer types. Thus, we postulated that NanoCy7.5 cellular uptake could be blocked by pretreatment with an excess of HA and serve as a measure of CD44 targeting by NanoCy7.5. NanoCy7.5 uptake was determined by flow cytometry after a 1 h incubation in cells with and without pretreatment of HA. Unexpectedly, when NanoCy7.510-Ø, NanoCy7.510-L and NanoCy7.510-H were pre-incubated with 10kDa HA (which corresponds with MW in those NPs), no significant decrease Cy7.5 signal was observed. In contrast, pre-incubation of cells with 100 kDa HA inhibited uptake (as measured by % cells positive for Cy7.5) of NanoCy7.5100-Ø, NanoCy7.5100-L and NanoCy7.5100-H (Figure 6) depended on 5 CA content. NanoCy7.5100-H had the highest uptake inhibition (up to 50%) compared to NanoCy7.5100-L and NanoCy7.5100-Ø. These results demonstrate that HA MW strongly contributes to NP targeting. It is interesting to note that longer incubation times, e.g. 8 h and 24 h, of NPs with cells that had been pre-treated with HA resulted in a high percentage of cells positive for Cy7.5, indicating that free HA was only capable of blocking NanoCy7.5 uptake over relatively short periods of time, which could potentially be attributed to NP and HA cycling through endo- and exocytosis and reached equilibrium with the receptor.
Figure 6.
Targeting ability of NanoCy7.5 NPs to CD44 positive cells was studied using FACS in MDA-MB 231cells (A–C) and confocal microscopy (D and E). NanoCy7.5 uptake was studied by pretreating MDA-MB 231 cells with 10 kDa or 100 kDa HA for 1 h prior to NP treatment. NanoCy7.510-Ø, NanoCy7.510-L, NanoCy7.510-H were pretreated with 10 kDa HA and NanoCy7.5100-Ø, NanoCy7.5100-L, NanoCy7.5100-H pretreated with 100 kDa HA. There was no significant change in NP uptake (A) upon treatment of NanoCy7.510-H with 10kDa MW HA. However, a significant decrease in NP uptake was observed upon pretreatment of NanoCy7.5100-H with 100 kDa HA (B).
The comprehensive FACS results for all the NPs types are presented in (C). Using confocal microscopy analysis, pretreatment with 100 kDa HA resulted in a significant decrease NP fluorescence (D) compared to control (E). The scale bar represents 20 µm for all microscopy images.
4. Discussion
We sequentially modified native HA with hydrophobic ligand 5 CA and the NIR dye Cy7.5 with routine EDC/NHS chemistry in mild aqueous reaction conditions. Functionalization efficiency of the hydrophobic ligand was close to 90% for both MW HA as determined from NMR integration. The extent of Cy7.5 conjugation was primarily quantified via UV-Vis absorption of NanoCy7.5 in water: DMSO mixture and was up to 1.25 mol%. The particle size of the NP as determined by both DLS and TEM was dependent on the MW of HA, producing significantly larger particles (∼300–400 nm) with 100 kDa HA compared to 10 kDa (∼50–100 nm). The critical micelle concentration of similar NPs has previously been reported between 25–170 µg/ml, depending on the degree of substitution [25].
The fluorescence quenching observed with NanoCy7.5 NPs due to aggregation of hydrophobic fluorescence molecules in aqueous solvent conditions has been reported in the literature using other HA-derived NP formulations. For example, Wang et al. modified HA with a newly developed NIR fluorescent marker in varying molar ratios 1–17% [35]. Their studies showed an increase in the extent of fluorescence quenching with NIR fluorophore content. This is because of increased aggregation and energy transfer between dye molecules. In another study, Mok, et al. developed ICG-encapsulated HA based nanogels for NIR imaging of tumors and lymph nodes [34]. Due to self-assembly and energy transfer between dye molecules in the close proximity of each other, ICG fluorescence signal was quenched under aqueous conditions in absence of HYAL. However, a significant increase in fluorescence signal was observed with increase in addition of hyaluronidase enzymes that can degrade the ICG nanogels and disrupt the self-assembly. The quantum yield of these conjugates is likely to be similar to that of non-conjugated Cy7.5, which is estimated to have a quantum yield similar to that of indocyanine green and IR800 of between 5–10% [36,37].
Cytotoxicity studies performed on CD44+ breast cancer and prostate cancer cell lines. NanoCy7.5 NPs were found to be non-toxic at the concentration tested under physiological concentrations using CCK-8 viability assay, consistent with previously developed HA NP formulations [22,28]. Cellular NP uptake was significantly reduced at 4 °C compared to 37 °C for both MDA-MB 231 and PC-3 cell lines. NP fluorescence was observed throughout the cell and prominently around peri-nuclear region. This is similar to results obtained by Choi et al. using HA-based NPs conjugated with Cy5.5 dye [25], who observed Cy5.5 fluorescence throughout carcinoma cells due to NP uptake via CD44 receptor-mediated endocytosis. In another study, Li et al. developed HA-deoxycholic acid based micelles for redox-sensitive release of paclitaxel in the tumor cytosol [38]. They studied localization of these drug carriers via confocal microscopy in MDA-MB 231 cells by loading Nile red dye into the NP micelles. The results showed NP micelles throughout the cytosol and especially around the nucleus; however none were taken up by the nucleus. We also observed co-localization of NPs with Rab5 indicating these NPs are present in early endosome. This mechanistic information is important to further design and develop these NPs toward tuning fluorophore or therapeutic release based on endo-lysosomal environment.
The targeting ability of HA derived NPs was investigated by pretreatment of MDA-MB-231 cells with free HA. The targeting ability of the NPs was found to be dependent on its MW, which is consistent with the literature [39–41]. The effect of molecular weight on the targeting of HA toward CD44 has been studied by Wolny et al [40]. who identified that interaction of 1–20 kDa HA with the CD44 receptors are more reversible than the higher molecular weight biopolymers, as higher MW HA (100 kDa or 500 kDa) forms multivalent and longer lasting interactions. For example, Choi et al. pretreated SCC7 cancer cells with 250 kDa MW HA prior to incubation with HA-derived NPs, and observed a significant decrease in NP uptake in vitro, with similar results seen in vivo [25]. The study reported here suggests that both the HA MW in the NP and the MW of HA acting as the inhibitor can significantly affect cellular targeting and uptake. We postulate that pre-incubation of cells with 100 kDa HA leads to multivalent, tighter binding with the receptors, which prevents further uptake of NPs through receptor-mediated endocytosis. It is interesting to point out, that uptake inhibition was correlated with amount of hydrophobic ligand present in 100 kDa HA NPs, NanoCy7.5100-H had the highest inhibition (50% of cells positive for Cy7.5) compared to NanoCy7.5100-L (60% Cy7.5 positive) and NanoCy7.5100-∅︀ (90% Cy7.5 positive). In the case of 10 kDa HA NPs, no change in NP uptake was observed with increase in hydrophobic content. Thus the possibility exists that NanoCy7.5 based on 10 kDa HA NPs can be internalized by mechanisms in addition to CD44 or RHAMM-receptor mediated endocytosis. However, for NanoCy7.5 based on 100 kDa HA, the primary pathway of cellular uptake is dependent HA-receptor interaction, e.g. CD44 or RHAMM, since uptake was inhibited by free HA. Uptake was not inhibited entirely by free HA, however, indicating that modification of 100 kDa HA by 5βCA and Cy7.5 influences the cellular interaction. Rather unexpectedly, 100 kDA with the highest degree of modification, NanoCy7.5100-H was inhibited to the greatest extent by free HA. An additional explanation is the relationship between NP size and uptake. Each of the NPs based on 10 kDa HA showed strong uptake (Figure 6C) and each is below 120 nm (hydrodynamic diameter). NanoCy7.5100-H and NanoCy7.5100-L are significantly larger (> 400 nm) and are inhibited to 50% or 60% of cells positive for Cy7.5. On the other hand, NanoCy7.5100-Ø had strong uptake relative to the other 100 kDa HA derived NPs, but its HD was around 100 nm, which was consistent with the 10 kDa-based NPs. Smaller nanoparticles, e.g. <50 nm have the highest rate of endocytosis, whereas those larger than 100 nm get progressively slower as the NP gets bigger[42]. It is likely that NanoCy7.5 size, specific and nonspecific receptor interaction, and extent of hydrophobic conjugation all have an effect on HA-based NP cell interaction.
A panel of NanoCy7.5 NPs developed in this report demonstrate interesting properties with respect to their size, fluorescence activation-quenching as well as CD44 targeting ability. The multifunctionality of these nanoparticle conjugates can open up numerous formulation possibilities for imaging and therapeutic delivery. One can select a combination of different NP to achieve high vs. low targeting ability, NP size and optical properties.
5. Conclusion
We have developed new biodegradable, NIR fluorescent NP imaging agents based on low (10kDa) and high (100kDa) molecular weight HA. This is an initial study of variable MW HA NP imaging agents for studying cancer cell targeting ability and optical properties. We observed variable particle size (50–400 nm diameter) and targeting, which were both dependent on the molecular weight of HA. Both high and low MW HA NPs were endocytosed by MDA-MB 231 and PC-3 cells via energy-dependent pathways and demonstrated low cytotoxicity (more than 80% cell survival). High MW HA was able to inhibit NP uptake (up to 50% inhibition) unlike low MW HA possibly due to multivalent interaction with the receptors and less reversible binding. We expect future in vivo studies to demonstrate differences in biodistribution, tumor uptake, and residence times of these NPs dependent on HA MW. A rigorous in vivo study is currently underway to test efficiency of these NPs as contrast agents for image-guided surgery.
Supplementary Material
Statement of Significance.
Here, hyaluronic acid (HA), a well-studied biomacromolecule, is modified with a near infrared fluorophore and a hydrophobic moiety. The significance of this work, especially for imaging applications, is that the impact of HA molecular weight and the hydrophobic moiety conjugation degree on fluorescence and cell interaction can be predicted. With respect to existing literature, the eventual use of these HA-based NPs is image-guided surgery; thus, we focus on the dye, Cy7.5, for conjugation, which is more NIR than most existing HA literature. Furthermore, HA is a ligand for CD44, which is associated with cancer and tumor microenvironment cells. Systematic studies in this work highlight that HA can be tuned to maximize or minimize CD44 binding.
Acknowledgments
This work was supported in part by the National Institutes of Health; P30 CA012197 (Wake Forest University Comprehensive Cancer Center), P30 CA036727 (Fred and Pamela Buffett Cancer Center, UNMC), R00 CA153916 (AMM), and R01 EB019449 (AMM). We thank Dr. David Horita for his expertise and assistance with NMR, Kenneth Gyabaah for microscopy and Mary Beth Laughridge for FACS analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Sneha S. Kelkar, Email: skelkar@wakehealth.edu.
Tanner K. Hill, Email: tanner.hill@unmc.edu.
Frank C. Marini, Email: fmarini@wakehealth.edu.
References
- 1.DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, Alteri R, Robbins AS, Jemal A. Cancer treatment and survivorship statistics, 2014. CA. Cancer J. Clin. 2014;64:252–271. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 2.Yi X, Wang F, Qin W, Yang X, Yuan J. Near-infrared fluorescent probes in cancer imaging and therapy: an emerging field. Int. J. Nanomedicine. 2014;9:1347–1365. doi: 10.2147/IJN.S60206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo Z, Park S, Yoon J, Shin I. Recent progress in the development of near-infrared fluorescent probes for bioimaging applications. Chem. Soc. Rev. 2014;43:16–29. doi: 10.1039/c3cs60271k. [DOI] [PubMed] [Google Scholar]
- 4.Ntziachristos V, Bremer C, Weissleder R. Fluorescence imaging with near-infrared light: new technological advances that enable in vivo molecular imaging. Eur. Radiol. 2003;13:195–208. doi: 10.1007/s00330-002-1524-x. [DOI] [PubMed] [Google Scholar]
- 5.Vahrmeijer AL, Hutteman M, van der Vorst JR, van de Velde CJH, Frangioni JV. Image-guided cancer surgery using near-infrared fluorescence. Nat. Rev. Clin. Oncol. 2013;10:507–518. doi: 10.1038/nrclinonc.2013.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnes KD, Shafirstein G, Webber JS, Koonce NA, Harris Z, Griffin RJ. Hyperthermia-enhanced indocyanine green delivery for laser-induced thermal ablation of carcinomas. Int. J. Hyperth. 2014;29:474–479. doi: 10.3109/02656736.2013.817615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schaafsma BE, Mieog JS, Hutteman M, van der Vorst JR, Kuppen PJ, Löwik CW, Frangioni JV, van de Velde CJ, Vahrmeijer AL. The clinical use of indocyanine green as a near-infrared fluorescent contrast agent for image-guided oncologic surgery. J. Surg. Oncol. 2011;104:323–332. doi: 10.1002/jso.21943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Terwisscha van Scheltinga AG, van Dam GM, Nagengast WB, Ntziachristos V, Hollema H, Herek JL, Schröder CP, Kosterink JG, Lub-de Hoog MN, de Vries EG. Intraoperative Near-Infrared Fluorescence Tumor Imaging with Vascular Endothelial Growth Factor and Human Epidermal Growth Factor Receptor 2 Targeting Antibodies. J. Nucl. Med. 2011;52:1778–1785. doi: 10.2967/jnumed.111.092833. [DOI] [PubMed] [Google Scholar]
- 9.Chen M, Yin M. Design and development of fluorescent nanostructures for bioimaging. Prog. Polym. Sci. 2014;39:365–395. [Google Scholar]
- 10.Zhang X, Zhang X, Tao L, Chi Z, Xu J, Wei Y. Aggregation induced emission-based fluorescent nanoparticles: fabrication methodologies and biomedical applications. J. Mater. Chem. B. 2014;2:4398–4414. doi: 10.1039/c4tb00291a. [DOI] [PubMed] [Google Scholar]
- 11.Peng HS, Chiu DT. Soft fluorescent nanomaterials for biological and biomedical imaging. Chem. Soc. Rev. 2015;44:4699–4722. doi: 10.1039/c4cs00294f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and t h e antitumor agents smancs. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 13.Wang AZ, Langer R, Farokhzad OC. Nanoparticle delivery of cancer drugs. Annu. Rev. Med. 2012;63:185–198. doi: 10.1146/annurev-med-040210-162544. [DOI] [PubMed] [Google Scholar]
- 14.Livney YD, Assaraf YG. Rationally designed nanovehicles to overcome cancer chemoresistance. Adv. Drug Deliv. Rev. 2013;65:1716–1730. doi: 10.1016/j.addr.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Kogan G, Soltés L, Stern R, Gemeiner P. Hyaluronic acid: a natural biopolymer with a broad range of biomedical and industrial applications. Biotechnol. Lett. 2007;29:17–25. doi: 10.1007/s10529-006-9219-z. [DOI] [PubMed] [Google Scholar]
- 16.Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat. Rev. Cancer. 2004;4:528–539. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]
- 17.Stern R, Jedrzejas MJ. Hyaluronidases: their genomics, structures, and mechanisms of action. Chem. Rev. 2006;106:818–839. doi: 10.1021/cr050247k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zöller M. CD44: can a cancer-initiating cell profit from an abundantly expressed molecule? Nat. Rev. Cancer. 2011;11:254–67. doi: 10.1038/nrc3023. [DOI] [PubMed] [Google Scholar]
- 19.Gires O. Lessons from common markers of tumor-initiating cells in solid cancers. Cell Mol Life Sci. 2011;68:4009–4022. doi: 10.1007/s00018-011-0772-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoon HY, Koo H, Choi KY, Chan Kwon I, Choi K, Park JH, Kim K. Photo-crosslinked hyaluronic acid nanoparticles with improved stability for in vivo tumor-targeted drug delivery. Biomaterials. 2013;34:5273–5280. doi: 10.1016/j.biomaterials.2013.03.050. [DOI] [PubMed] [Google Scholar]
- 21.Lee H, Ahn CH, Park TG. Poly[lactic-co-(glycolic acid)]-grafted hyaluronic acid copolymer micelle nanoparticles for target-specific delivery of doxorubicin. Macromol. Biosci. 2009;9:336–342. doi: 10.1002/mabi.200800229. [DOI] [PubMed] [Google Scholar]
- 22.Choi KY, Min KH, Na JH, Choi K, Kim K, Park JH, Kwon IC, Jeong SY. Self-assembled hyaluronic acid nanoparticles as a potential drug carrier for cancer therapy: synthesis, characterization, and in vivo biodistribution. J. Mater. Chem. 2009;19:4102–4107. [Google Scholar]
- 23.Jin YJ, Termsarasab U, Ko SH, Shim JS, Chong S, Chung SJ, Shim CK, Cho HJ, Kim DD. Hyaluronic acid derivative-based self-assembled nanoparticles for the treatment of melanoma. Pharm. Res. 2012;29:3443–3454. doi: 10.1007/s11095-012-0839-9. [DOI] [PubMed] [Google Scholar]
- 24.Park JH, Cho HJ, Yoon HY, Yoon IS, Ko SH, Shim JS, Cho JH, Park JH, Kim K, Kwon IC, Kim DD. Hyaluronic acid derivative-coated nanohybrid liposomes for cancer imaging and drug delivery. J. Control. Release. 2014;174:98–108. doi: 10.1016/j.jconrel.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 25.Choi KY, Chung H, Min KH, Yoon HY, Kim K, Park JH, Kwon IC, Jeong SY. Self-assembled hyaluronic acid nanoparticles for active tumor targeting. Biomaterials. 2010;31:106–114. doi: 10.1016/j.biomaterials.2009.09.030. [DOI] [PubMed] [Google Scholar]
- 26.Coradini D, Pellizzaro C, Miglierini G, Daidone MG, Perbellini A. Hyaluronic acid as drug delivery for sodium butyrate: improvement of the anti-proliferative activity on a breast-cancer cell line. Int. J. Cancer. 1999;81:411–416. doi: 10.1002/(sici)1097-0215(19990505)81:3<411::aid-ijc15>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 27.Ganesh S, Iyer AK, V Morrissey D, Amiji MM. Hyaluronic acid based self-assembling nanosystems for CD44 target mediated siRNA delivery to solid tumors. Biomaterials. 2013;34:3489–3502. doi: 10.1016/j.biomaterials.2013.01.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hill TK, Abdulahad A, Kelkar SS, Marini FC, Long TE, Provenzale JM, Mohs AM. Indocyanine Green-Loaded Nanoparticles for Image-Guided Tumor Surgery. Bioconjug. Chem. 2015;26:294–303. doi: 10.1021/bc5005679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohs AM, Mancini MC, Provenzale JM, Saba CF, Cornell KK, Howerth EW, Nie S. An Integrated Widefield Imaging and Spectroscopy System for Contrast-Enhanced, Image-guided Resection of Tumors. IEEE Trans. Biomed. Eng. 2015;62:1416–1424. doi: 10.1109/TBME.2015.2389626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park K, Kim JH, Nam YS, Lee S, Nam HY, Kim K, Park JH, Kim IS, Choi K, Kim SY, Kwon IC. Effect of polymer molecular weight on the tumor targeting characteristics of self-assembled glycol chitosan nanoparticles. J. Control. Release. 2007;122:305–314. doi: 10.1016/j.jconrel.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 31.Takeuchi H, Kojima H, Yamamoto H, Kawashima Y. Evaluation of circulation profiles of liposomes coated with hydrophilic polymers having different molecular weights in rats. J. Control. Release. 2001;75:83–91. doi: 10.1016/s0168-3659(01)00368-6. [DOI] [PubMed] [Google Scholar]
- 32.Sizovs A, Xue L, Tolstyka ZP, Ingle NP, Wu Y, Cortez M, Reineke TM. Poly(trehalose): Sugar-coated nanocomplexes promote stabilization and effective polyplex-mediated siRNA delivery. J. Am. Chem. Soc. 2013;135:15417–15424. doi: 10.1021/ja404941p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li X, Mya KY, Ni X, He C, Leong KW, Li J. Dynamic and Static Light Scattering Studies on Self-Aggregation Behavior of Biodegradable Amphiphilic Poly (ethylene oxide) -Poly [(R) −3-hydroxybutyrate ] -Poly (ethylene oxide) Triblock Copolymers in Aqueous Solution. J. Phys. Chem. B. 2006;110:5920–5926. doi: 10.1021/jp057004g. [DOI] [PubMed] [Google Scholar]
- 34.Mok H, Jeong H, Kim S-J, Chung BH. Indocyanine green encapsulated nanogels for hyaluronidase activatable and selective near infrared imaging of tumors and lymph nodes. Chem. Commun. 2012;48:8628–8630. doi: 10.1039/c2cc33555g. [DOI] [PubMed] [Google Scholar]
- 35.Wang W, Cameron AG, Ke S. Developing fluorescent hyaluronan analogs for hyaluronan studies. Molecules. 2012;17:1520–1534. doi: 10.3390/molecules17021520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hong G, Tabakman SM, Welsher K, Chen Z, Robinson JT, Wang H, Zhang B, Dai H. Near-Infrared Fluorescence Enhanced (NIR-FE) Molecular Imaging of Live Cells on Gold Substrates. Angew. Chem. Int. Ed. Engl. 2011;50:4644–4648. doi: 10.1002/anie.201100934. [DOI] [PubMed] [Google Scholar]
- 37.Bardhan R, Grady NK, Cole JR, Joshi A, Halas NJ. Fluorescence enhancement by au nanostructures: Nanoshells and nanorods. ACS Nano. 2009;3:744–752. doi: 10.1021/nn900001q. [DOI] [PubMed] [Google Scholar]
- 38.Li J, Huo M, Wang J, Zhou J, Mohammad JM, Zhang Y, Zhu Q, Waddad AY, Zhang Q. Redox-sensitive micelles self-assembled from amphiphilic hyaluronic acid-deoxycholic acid conjugates for targeted intracellular delivery of paclitaxel. Biomaterials. 2012;33:2310–2320. doi: 10.1016/j.biomaterials.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 39.Mizrahy S, Raz SR, Hasgaard M, Liu H, Soffer-Tsur N, Cohen K, Dvash R, Landsman-Milo D, Bremer MG, Moghimi SM, Peer D. Hyaluronan-coated nanoparticles: the influence of the molecular weight on CD44-hyaluronan interactions and on the immune response. J. Control. Release. 2011;156:231–238. doi: 10.1016/j.jconrel.2011.06.031. [DOI] [PubMed] [Google Scholar]
- 40.Wolny PM, Banerji S, Gounou C, Brisson AR, Day AJ, Jackson DG, Richter RP. Analysis of CD44-hyaluronan interactions in an artificial membrane system: Insights into the distinct binding properties of high and low molecular weight hyaluronan. J. Biol. Chem. 2010;285:30170–30180. doi: 10.1074/jbc.M110.137562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee MY, Yang Ja, Jung HS, Beack S, Choi JE, Hur W, Koo H, Kim K, Yoon SK, Hahn SK. Hyaluronic acid-gold nanoparticle/interferon alpha complex for targeted treatment of hepatitis C virus infection. ACS Nano. 2012;6:9522–9531. doi: 10.1021/nn302538y. [DOI] [PubMed] [Google Scholar]
- 42.Oh N, Park JH. Endocytosis and exocytosis of nanoparticles in mammalian cells. Int. J. Nanomedicine. 2014;9:51–63. doi: 10.2147/IJN.S26592. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.