Abstract
Rationale
Establishing a behavioral model for the effect of social environment on nicotine intake in rodents facilitates the investigation of molecular mechanisms critical for the interaction between social environment and cigarette smoking.
Objectives
Our main objective was to test the hypothesis that nicotine is the primary reinforcer in the socially acquired nicotine intravenous self-administration (IVSA) model by using an aversive flavor cue.
Methods
Adolescent female rats were placed in operant conditioning chambers equipped with two lickometers. Operant licking triggered concurrent deliveries of a flavor (i.e., taste and odor) cue containing either quinine or saccharin and an i.v. infusion (30 μg/kg nicotine or saline). An audiovisual cue was provided for some groups of rats. A second rat that did not receive nicotine was placed in the operant conditioning chambers to provide either a neutral or an inducing (i.e., by consuming the flavored solution) social environment. These two rats were separated by a divider that allowed orofacial interactions.
Results
Rats acquired stable nicotine IVSA with either the aversive or the appetitive flavor cue in the inducing social environment, and obtained similar amounts of infusions. The neutral social environment did not support nicotine IVSA with either cue. The audiovisual cue per se did not support nicotine IVSA but enhanced nicotine intake. Nicotine increased the number of concurrent nose pokes by the two rats into the center divider, a measure of social interaction.
Conclusions
Despite its aversive effects, nicotine is the primary reinforcer for the operant responses in the socially acquired nicotine IVSA model.
Keywords: Social learning, nicotine, self-administration, adolescence, aversion, reward, olfactogustatory, cue
Introduction
There is a paradox in the initiation of cigarette smoking. Most smokers’ initial experience with cigarettes is aversive (e.g. nausea, coughing, dizziness, etc.) (Eissenberg and Balster 2000). Although these unpleasant effects decrease across the first few smoking episodes, there is no increase in the pleasant effects (Hahn et al. 1990). Further, nicotine, the principal psychoactive agent of cigarettes (Stolerman and Jarvis 1995), induces measures of drug high only in significantly nicotine-deprived smokers and has primarily adverse effects in non-smokers and former smokers (Kalman 2002). Despite these adverse effects, many teenagers continue to smoke and eventually become addicted to tobacco products.
A key factor for teenagers to overcome these aversive responses is likely the social environment. The majority of first-time cigarette use occurs in the presence of friends (Friedman et al. 1985; Hahn et al. 1990), and peer smoking is one of the strongest predictors of smoking initiation (Greenlund et al. 1997; White et al. 2008). Further, being friends of smokers increases the likelihood of becoming a smoker (O'Loughlin et al. 1998; Powell et al. 2005), while discouragement from the social network is negatively associated with smoking status (Hofstetter et al. 2007).
Using operant licking responses, we have shown that rats developed conditioned aversion to an appetitive flavor (i.e., taste and odor) cue for intravenous self-administration (IVSA) of nicotine (Chen et al. 2011), suggesting that self-administered nicotine is aversive. We then modeled the effect of social learning by placing a second rat (i.e., a demonstrator) in the operant conditioning chamber during the self-administration sessions. Social interaction, in the form of orofacial interaction, was allowed through a series of holes in the divider that separated the two rats. The demonstrator rat had free access to the same flavor cue but did not receive nicotine. We found that social learning reversed the conditioned aversion and supported stable nicotine intake. We further showed that socially acquired nicotine IVSA required the concurrent transmission of a nicotine-associated odor cue and carbon disulfide, a component of rodent breath (Wang and Chen 2014).
These studies demonstrated the strong effect of social learning in promoting nicotine self-administration. However, the relationships between the various sensory cues and nicotine intake are yet to be defined in this model. For example, because we used an appetitive flavor cue, the reinforcing effects of nicotine and the cue were confounded. It was also unknown whether the flavor cue must have a positive subjective value to support nicotine intake. Thirdly, the role of the audiovisual (AV) cue used in the original model was unknown. In this study, we hypothesized that nicotine, and not the flavor cue, is the primary reinforcer of operant responding in this model. We also hypothesize that a flavor cue does not need to be appetitive to support operant responding for nicotine, and that the AV cue facilitates nicotine intake. We tested all three hypotheses by using quinine, which is bitter, as the taste component of the flavor cue either with or without the AV cue. Our results supported these hypotheses.
Methods
Drugs
Nicotine hydrogen tartrate was purchased from Sigma (N5260, St. Louis, MO) and was prepared in sterile saline (pH = 7.0-7.2). Quinine hemisulfate salt, an aversive tastant, and saccharin, an appetitive tastant, were also purchased from Sigma. A working solution for quinine (0.006%, in distilled water) was prepared daily from a stock solution (0.06%). Heparin and methohexital were purchased from Butler Schein Animal Health (Dublin, OH).
Animals and surgeries
Adolescent Sprague-Dawley rats (postnatal days 29-31) (Harlan Laboratories, Madison, WI) were given seven days to acclimate to a reversed 12:12 h light/dark cycle (lights off at 9:00 AM). Although we found that female heterogeneous stock rats self-administered more nicotine than males (Wang et al. 2014a), we did not find a sex difference in nicotine intake in adolescent Sprague-Dawley rats using either the socially acquired (Chen et al. 2011) or the lever press (Chen et al. 2006) models. Therefore, we only used one sex (female) in this study. We did not record the endocrine status of these rats because previous studies have shown that it had little effect on nicotine intake in humans or animals (Perkins et al. 1999; Donny et al. 2000). Jugular catheters constructed from PE-60 and silastic tubing were implanted between postnatal days 36 - 38 as previously described (Wang et al. 2014b). Rats were group housed after the surgery to avoid social isolation. A 3D printed implant and a metal spring were used to prevent cage mates from damaging the catheter (Wang et al. 2014b). Rats received three days of post-surgery recovery before the commencement of IVSA. All procedures were conducted in accordance with the NIH Guidelines Concerning the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of Tennessee Health Science Center.
Operant conditioning chambers
An illustration of the operant conditioning chamber was provided in a previous publication (Wang et al. 2014a). In brief, two contact lickometers, a white cue light (Med Associates, Model ENV-221M) and a tone generator (Sonalert Mallory, Model SC628) were fitted on the same wall of these chambers (Med Associates, St. Albans, VT). A custom-made divider (25 W × 205 H × 230 L mm), having six evenly-spaced holes (35 mm apart, diameter = 20 mm but can be reduced) located 30 mm above the floor, was placed in the middle of the chamber. Two sets of infrared emitters and sensors were embedded in the divider to record the nose pokes from either side.
Testing the subjective value of the grape flavor
Naïve rats were placed in the operant conditioning chambers for 3 h per day. Licking on the active spout triggered the delivery of a grape-flavored, unsweetened Kool-Aid solution (60 μl, 0.1% in distilled water), controlled by a fixed ratio 10 (FR 10) reinforcement schedule with a 20 s timeout period. Rats were not deprived of food or water before the test.
Nicotine IVSA in two different social contexts
All IVSA sessions were conducted in the dark-phase of the light cycle and lasted 3 h. Standard rat chow and water were provided ad libitum throughout the experiments, but neither was available during the IVSA sessions. An FR10 (20 s time-out) schedule was used. Licking on the inactive spout or on the active spout during the timeout period had no programmed consequence. No cue was available during the timeout period. Ten licks on the active spout triggered the concurrent delivery of 60 μl flavor cue to the spout and 50 μl of nicotine (30 μg/kg, free base, pH = 7.2) or saline through the jugular catheter. Two flavor cues were used. The aversive cue contained 0.006% quinine solution, while the appetitive cue contained 0.4% saccharin. Both cues contained 0.1% unsweetened grape Kool-Aid.
IVSA was conducted in two social contexts. In both contexts, a same sex demonstrator rat, randomly selected from a pool of demonstrator rats , was placed on the side of the operant conditioning chamber opposite to the self-administration rat. Infrared sensors recorded orofacial interactions between the rats through the divider. In the Inducing Social Environment (ISE), the demonstrator rat had free access to the appetitive flavor cue. This ensured the demonstrator rats consume large amounts of the flavored solution, which was critical for social learning. The demonstrator rat did not have access to any solution in the Neutral Social Environment (NSE).
Rats were assigned to treatment groups as shown in Table 1. Rats in groups I - IV (ISE vs NSE × nicotine vs saline) received the aversive flavor cue and the AV cue. These groups allow us to study the effect of social environment on nicotine IVSA with an aversive cue, using saline i.v. infusion as controls. Groups V and VI received nicotine IVSA in either ISE or NSE with the aversive flavor cue but without the AV cue. When compared to groups III and IV, respectively, these two groups allowed us to study the effect of the AV cue, which were used in our original model (Chen et al. 2011). Lastly, rats in groups VII and VIII self-administered nicotine in the ISE with the appetitive flavor cue, one with and one without the AV cue. When compared with groups III and V, these groups allowed us to investigate the effect of the subjective values of flavor cues on nicotine intake. The patency of the jugular catheters was tested using Methohexital (0.1 ml, 10 mg/ml). Data from rats that failed the patency test were excluded from the analysis.
Table 1.
Experimental groups.
| Group (animal number, n) | Social Environment | Olfactogustatory Cue | Audiovisual Cue | Drug |
|---|---|---|---|---|
| I (n= 10) | ISE | Aversive | Yes | Saline |
| II (n= 9) | NSE | Aversive | Yes | Saline |
| III (n= 11) | ISE | Aversive | Yes | Nicotine |
| IV (n=9) | NSE | Aversive | Yes | Nicotine |
| V (n= 11) | ISE | Aversive | No | Nicotine |
| VI (n= 9) | NSE | Aversive | No | Nicotine |
| VII (n= 6) | ISE | Appetitive | Yes | Nicotine |
| VIII (n= 6) | ISE | Appetitive | No | Nicotine |
Statistics
Data are presented as mean ± SEM. All statistical analyses were conducted using the R statistical package. The number of licks were converted to log scale in order to fit a normal distribution. A paired t-test was used to analyze the preference for the grape flavor. All lick and injection data were analyzed by repeated measures ANOVA, with spout and session treated as within subject variables. Post-hoc analyses were conducted using the Tukey HSD procedure.
A Perl script extracted the timing of nose pokes from the raw data. The IRanges package of the R statistical language was then used to find the number of overlaps in the timing of nose pokes between the two rats. A social interaction episode was defined as two rats poking their noses within 0.5 s of each other. The total amount of time the two rats poking their noses into the divider was also calculated.
Results
Subjective value of the flavor cue
The flavor cues we used were composed of a taste (quinine or saccharin) and an odor (grape). We first tested the subjective value of a solution containing only the grape odor. We have previously shown that similar to inter-lick intervals, the ratio of licks on the active vs the inactive spouts reflected the subjective value of the oral stimuli (Wang et al. 2014b). The number of licks produced by naïve rats on the active (459.1 ± 140.3) and inactive (619.1 ± 171.2) spouts were not statistically different (paired t-test, p > 0.05, n = 6). These data indicated that the grape component of the flavor cue had a neutral subjective value. We then tested the subjective value of the bitter quinine-containing cue. Rats that received i.v. saline with the quinine cue in the NSE (AV+ group) licked significantly fewer times on the active spout compared to the inactive spout (Fig. 1a, F1,8 = 9.1, p < 0.05). The number of licks and infusions reduced significantly across the sessions (F9,72 = 3.0, p < 0.01 and F9,72 = 2.0, p < 0.05, respectively). These data confirmed that the quinine-grape cue was aversive.
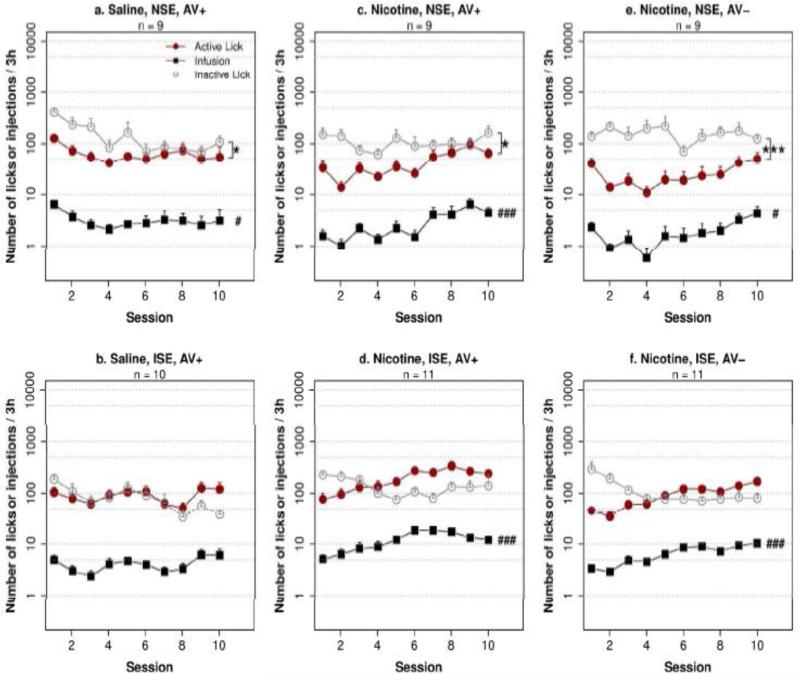
Fig. 1. Socially acquired nicotine IVSA with an aversive flavor cue.
Adolescent rats were given access to i.v. infusions (saline or 30 μg/kg nicotine) with a contingent flavor cue containing quinine. ISE: inducing social environment. NSE: neutral social environment. AV+: with a contingent audiovisual cue. AV−: without an audiovisual cue. Statistical significance were designated as the following: active vs. inactive spout, * p < 0.05; *** p < 0.001; main effect of session: # p < 0.05, ### p < 0.001
Social learning promotes nicotine IVSA with an aversive flavor cue
We tested the effect of social learning on nicotine infusions with the aversive cue using a 2 × 2 design (i.e. social environment × i.v. treatment). Consistent with our prior study (Chen et al. 2011), an AV cue was provided for all four groups (Fig. 1 a-d). Repeated measures ANOVA found that the effect of social environment (F1,34 = 20.8, p < 0.001), i.v. treatment (F1,34 = 15.7, p < 0.001) and session (F9,34 = 7.4, p < 0.001) were all statistically significant. There was also a significant interaction between social environment and i.v. treatment (F1,34 = 14.1, p < 0.001). Tukey HSD post hoc analysis showed that rats in the nicotine ISE group obtained significantly greater number of infusions than the nicotine NSE, saline ISE, or the saline NSE groups (p < 0.001 for all three comparisons). These results indicated that social learning supported nicotine IVSA with an aversive flavor cue.
The behavioral data from each group were also analyzed in detail. Rats that received i.v. saline with the aversive cue in the ISE (AV+ group, Fig. 1b) did not change the number of licks significantly across the sessions (F9,81 = 1.3, p > 0.05), nor did the number of infusions change significantly (F9,81 = 1.8, p > 0.05). The number of licks was not significantly different between the two spouts (F1,9 = 0.1, p > 0.05). However, there was a trend for the number of licks on the active spout to be higher than that of the inactive spout during the last three sessions (99.4 ± 27.1 for the active spout and 42.7 ± 7.3 for the inactive spout, F1,9 = 4.4, p = 0.06). These data indicated that the aversive flavor cue gained a neutral subjective value after social learning.
Rats received nicotine IVSA with the aversive flavor cue in the NSE (AV+ group, Fig. 1c) licked significantly fewer times on the active spout compared to the inactive spout (F1,8 = 10.2, p < 0.05). Although the number of licks did not change significantly across the sessions (F9,71 = 1.2, p > 0.05), the number of nicotine infusions significantly increased during the sessions (F9,71 = 3.7, p < 0.001). This seeming discrepancy between the number of licks and the number of infusions was caused by reduced number of licks in the timeout period during later sessions.
Rats in the nicotine ISE AV+ group (Fig. 1d) showed a significant interaction between spout and session (F9,90 = 9.8, p < 0.001). The licks on the active spout increased significantly across the sessions (F9,90 = 7.1, p < 0.001), which resulted in significant increases in the number of nicotine infusions (F9,90 = 9.5, p < 0.001). The main effect of session on the number of licks on the inactive spout also reached statistical significance (F9,90 = 2.2, p < 0.05). However, the change was bidirectional. There were fewer licks on the active compared to the inactive spout before session four (109.3 ± 29.6 on the active spout and 180.3 ± 29.2 on the inactive spout), and the number of active licks became significantly greater than that of the inactive ones thereafter (F1,10 = 15.1, p < 0.01), with 281.7 ± 50.2 on the active spout and 135 ± 42.0 on the inactive spout for the last three sessions. These data indicated that despite providing an initial aversive subjective value, i.v. nicotine with contingent aversive flavor cue became appetitive after social learning, and facilitated the acquisition of nicotine IVSA.
An AV cue facilitates nicotine intake
The effect of an AV cue on nicotine intake was examined using a repeated measures ANOVA that compared the effect of social environment × AV cue condition (i.e. Fig. 1 c-f). The analysis found a significant main effect by the AV cue (F1,35 = 12.7, p < 0.01) and social environment (F1,35 = 47.5, p < 0.001). The interaction between the AV cue and the social environment was also significant (F1,35 = 5.2, p < 0.05). Post hoc Tukey HSD tests showed that the AV cue significantly enhanced nicotine intake in both the ISE and the NSE groups (p < 0.001 for both). These results supported the hypothesis that the AV cue enhanced nicotine intake.
The number of active licks were significantly fewer than those of the inactive ones in the nicotine NSE AV− group (Fig. 1e, F1,8 = 38.5, p < 0.001) and the number of infusions also increased across sessions (F9,72 = 2.2, p < 0.05). In the nicotine ISE AV− group (Fig. 1f), the number of infusions also increased significantly (F9,90 = 7.6, p < 0.001). However, unlike the group with the AV cue, the number of licks on the active spout was not significantly higher than those on the inactive spout after session four (F1,10 = 1.9, p > 0.05). Together, these data suggested that the AV cue enhanced the subjective value of the stimuli.
Interaction between social environment and sensory cues on stable nicotine intake
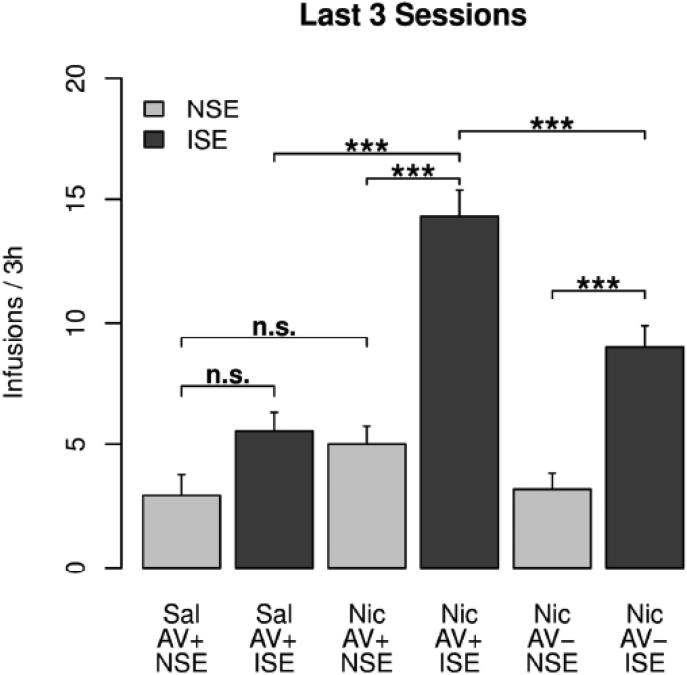
Fig. 2 summarizes the amount of nicotine intake during the last three sessions for the six treatment groups with the aversive flavor cue. Repeated measures ANOVA found the main effects of the social environment (F1,60 = 26.2, p < 0.001) and nicotine (F1,60 = 10.9, p < 0.01) to be significant. There was also a significant interaction between them (F1,60 = 6.9, p < 0.05). Tukey HSD post hoc tests showed that 1) the number of nicotine infusions were significantly greater than that of saline infusions in the ISE (p < 0.001) but not in the NSE (p > 0.05), indicating that nicotine was a strong reinforcer of the operant behavior but only in the inducing social environment and 2) the number of infusions in the ISE were significantly greater than that of the NSE for nicotine (p < 0.001) but not for saline (p > 0.05) infusions. These results again indicated a strong synergy between social learning and nicotine. 3) Also, AV cue enhanced nicotine intake in the ISE (p < 0.001) but not in the NSE (p > 0.05), indicating that the AV cue per se was not sufficient to support nicotine IVSA in this model.
Fig. 2. Effect of social environment and audiovisual cue on nicotine IVSA.
The average number of i.v. infusions obtained during the last three sessions were compared. Post hoc Tukey HSD test: ***: p < 0.001. n.s.: not significant (p > 0.05)
Subjective value of the flavor cue does not affect nicotine intake
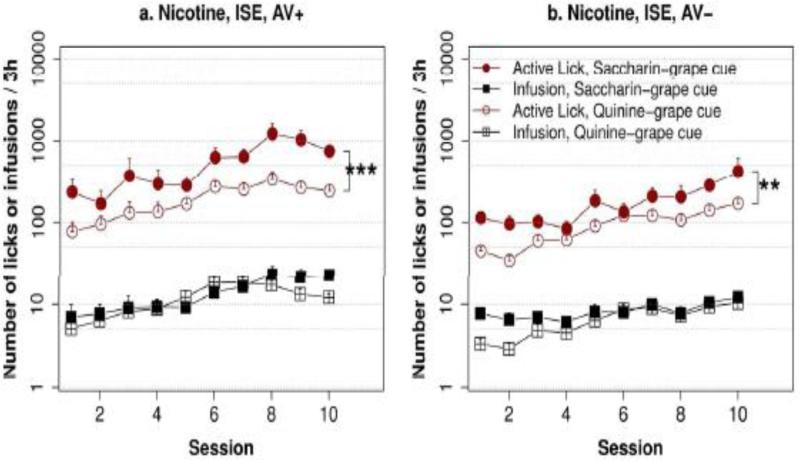
We compared the effect of appetitive vs. aversive cue on nicotine intake by testing two group of rats in the ISE (Fig. 3) using an appetitive cue, one with and the other one without the AV cue. The data from these groups were compared with their equivalent groups shown in Figs. 1c and 1e, where an aversive cue was used. Repeated measures ANOVA showed that the subjective value of the flavor cue did not have a significant effect on nicotine intake (F1,33 = 0.01, p > 0.05). However, the effect of the AV cue was significant (F1,33 = 16.5, p < 0.001). Post hoc Tukey HSD tests confirmed that the amount of nicotine infusion did not differ by flavored cues between groups with the same AV cues. These results indicated that nicotine, rather than the flavor cue, is the primary reinforcer of operant behavior in this model.
Fig. 3. The effect of an appetitive vs an aversive flavor cue on nicotine IVSA.
Rats self-administered i.v. nicotine (30 μg/kg/infusion) with a contingent flavor cue that was either appetitive (i.e., saccharin-containing) or aversive (i.e., quinine-containing) for 10 sessions in an inducing social environment. Although the number of licks on the active spouts were greater with the appetitive cue compared to the aversive cue, the number of nicotine infusions was similar, regardless of the condition of the audiovisual cue (AV+ vs. AV−). ** p < 0.01, *** p < 0.001
A significant effect of flavor cue was found (F1,33 = 23.4, p < 0.001) when the number of active licks were analyzed. This is most likely caused by continued licking during the timeout period after the appetitive but not the aversive flavor cue was delivered. The effect of AV cue was also significant (F1,33 = 21.5, p < 0.001). In addition, there was also a significant interaction between the AV cue and the flavor cue (F1,33 = 9.2, p < 0.01). Tukey HSD analysis showed the number of active licks was significantly greater in the appetitive cue group than in the aversive cue group when the AV cue was used (p < 0.001). These results further support the idea that the AV cue enhanced the subjective value of the stimuli.
Nicotine enhances social interaction
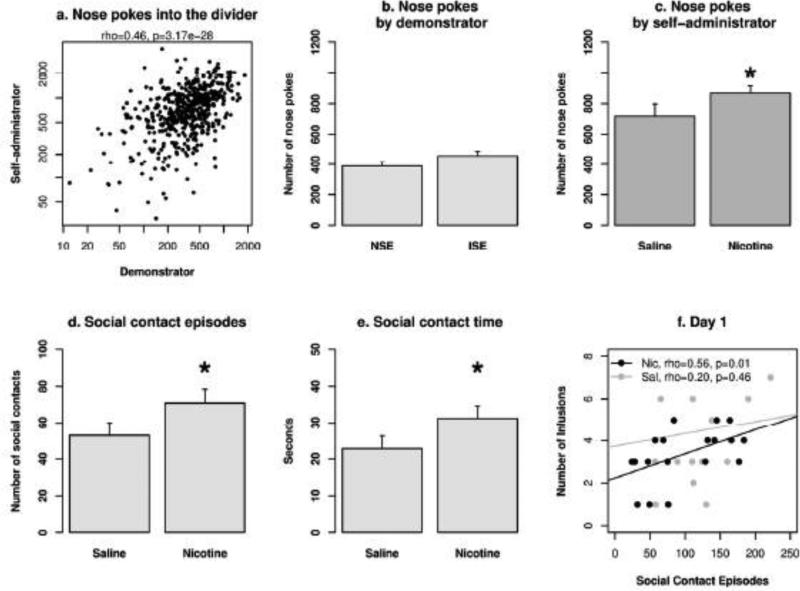
The number of nose pokes into the divider by each rat was recorded by embedded infrared sensors. There was a significant correlation between the nose pokes by the nicotine self-administration rats and the demonstrator rats (Fig. 4a. rho = 0.46, p < 0.001). This indicates that the nose pokes were social interactions in nature. The number of nose pokes produced by the demonstrator rats was not significantly affected by session (p > 0.05) or the AV cue (p > 0.05). There was a trend toward a greater number of nose pokes in the ISE than the NSE (Fig. 4b. F1, 39 = 3.2, p = 0.08). The number of nose pokes produced by the self-administration rats were significantly greater in the nicotine groups compared to the saline groups (Fig. 4c. F1,39 = 4.6, p < 0.05) but the social environment did not change the number of nose pokes by the self-administration rats (F1,39 = 2.5, p > 0.05). The AV cue did not have a significant effect on the number of nose pokes by either the demonstrator rats or self-administration rats (F1,43 = 0.01, p > 0.05 and F1,43 = 2.4, p > 0.05, respectively).
Fig. 4. Social interaction during nicotine IVSA sessions.
a) The highly significant correlation on the number of nose pokes per session between the two rats indicated the social nature of this behavior in our model. b) The social environment did not have a significant effect on the number of nose pokes produced by the demonstrator rats. c) Rats that self-administered i.v. nicotine produced a greater number of nose pokes compared to those that self-administered i.v. saline. d) and e) The effect of nicotine on social interaction was confirmed by analyzing the number of social contact episodes and the amount of time spend on social interaction via the holes in the divider. f) The number of social contact episodes for ISE groups was positively correlated with the number of infusions on the first day of IVSA for the nicotine but not the saline group. ISE: inducing social environment. NSE: neutral social environment. *: p < 0.05 compared to the saline group.
The number of social contact episodes (i.e., two rats poking their noses into the divider within 0.5 s of each other) was significantly greater in the nicotine groups compared to the saline groups (Fig. 4d. F1,38 = 5.3, p < 0.05). Social environment did not have a significant effect on the number of social contact episodes (F1,38 = 0.17, p > 0.05). Similarly, the total amount of social interaction time was significantly greater in the nicotine groups compared to the saline groups (Fig. 4e. F1,38 = 5.2, p < 0.05) but was not different between social environments (F1,38 = 0.07, p > 0.05). Lastly, there was a significant correlation between the number of nicotine infusions and the number of social contact episodes on the first day of nicotine IVSA (Fig 4f, rho = 0.56, p = 0.01), but no such correlation was found in the saline group (rho = 0.20, p = 0.46). These data indicate that nose pokes into the divider were a social behavior in this model. Further, social interaction was correlated with and enhanced by nicotine intake.
Discussion
Our main finding was that social learning supported nicotine, but not saline, IVSA with an aversive flavor cue in adolescent rats. Further, almost identical amounts of nicotine was obtained when either an aversive or an appetitive flavor cue was used, indicating that nicotine is the primary reinforcer in the socially acquired nicotine IVSA model, and that the flavor cue does not need to be appetitive to support nicotine IVSA. Additionally, our data showed that an AV cue enhanced nicotine intake in the ISE. Lastly, not only did social learning enable nicotine IVSA with the aversive flavor cue, but nicotine also increased the number of social interaction episodes.
Unlike most drugs of abuse, altered subjective experience is not the main reason people start smoking cigarettes, because the initial experience of cigarette smoking is usually aversive and lacks euphoria (Eissenberg and Balster 2000; Kalman 2002). Some have suggested that a cognitive and performance enhancing effect of nicotine may be the reason. However, there is no direct evidence to support this claim (Heishman et al. 2010). In contrast, many lines of evidence support the role of social environment. For example, peer smoking is one of the strongest predictors of smoking initiation (Greenlund et al. 1997; White et al. 2008). The social learning theory (Akers 1977), which emphasizes that the definition of rewards/punishments associated with the behavior is transferred between individuals, is well supported by data on smoking initiation (Spear and Akers 1988; Flay et al. 1994). Therefore, our finding that nicotine intake with contingent flavor cues requires the individuals to learn the subjective value of the stimuli from the social environment is in agreement with both theoretical and empirical studies of human smoking initiation.
Appetitive flavor cues were used in our previous studies of the socially acquired nicotine IVSA model. One consequence of this design was that the amount of infusions was greater in the control groups than in the nicotine groups. The current study used an aversive flavor cue. As expected, the number of infusions obtained by the nicotine group was significantly greater than that of the control group (Fig. 2). Interestingly, social learning did not significantly enhance i.v. infusions of saline (Fig. 1b). These data provide strong support for our hypothesis that nicotine is the primary reinforcer in this model.
We also compared the number of nicotine infusions between the aversive and appetitive cues. We found that similar amounts of nicotine were obtained by rats that received either one of the cues (Fig. 3). One of the potential contributors to this lack of difference was that the aversive cue was only mildly aversive, as shown by the 50 to 100 licks on the active spout per session by rats in the saline NSE group, and that nicotine caused further reduction in the number of licks on the active spout in the NSE groups (Fig. 1a vs 1c). These data unequivocally demonstrated that nicotine, and not the flavor cue, was the primary reinforcer for the operant behavior in the socially acquired nicotine IVSA model.
AV cues are commonly used in nicotine IVSA models. We have found that, in the absence of flavor cues, operant licking supported nicotine IVSA with a visual cue, but not when the visual cue was omitted (Wang et al. 2014b). An AV cue was also used in one of our previous studies (Chen et al. 2011). Fig 2. shows that the AV cue increased the number of nicotine infusions in the ISE, potentially because it enhanced the subjective value of nicotine, as indicated by the increased ratio of licks on the active vs. inactive spouts (Fig. 1d vs Fig. 1f). However, the AV cue per se was not sufficient to support nicotine IVSA with the quinine-containing flavor cue (Fig. 2). These data supported the idea that the AV cue is not the primary reinforcer for the operant behavior in the socially acquired nicotine IVSA model.
Social interaction is rewarding in both humans (Walter et al. 2005) and rodents (Zernig et al. 2013). Thiel et al. (2009) reported a synergistic effect between a subthreshold dose of nicotine and social interaction in producing conditioned place preference. Similar findings were reported for cocaine (Thiel et al. 2008). We measured the number of nose pokes into the central divider by both rats during the operant tests. The highly significant correlation on the number of nose pokes between the dyads (Fig. 4a) indicated the social nature of this behavior in our model. Most interestingly, rats that received i.v. nicotine had a significantly greater number of nose pokes than those that received i.v. saline, regardless of the social environment (Fig. 4c). Analyzing the timing of nose poke further clarified that nicotine not only enhanced the number of social contact episodes (i.e. two rats poke their nose into the divider within 0.5 s of each other), but also increased the total time of social contact. This analysis also suggested that the increased nose poke counts were unlikely to have been caused by increased locomotor response. These results were in agreement with those reported by Trezza et al. (2009) that nicotine increased social play, without affecting locomotion or social exploration. One caveat of our analysis was that our setup can distinguish the nose pokes by each rat but can not separate nose pokes for the different holes in the divider. However, our anecdotal observation confirmed that when two rats poke their noses into the divider at the same time, they were always at the same hole. Nicotine is known to enhance the effect of nonpharmacological rewards (Palmatier et al. 2005; Palmatier et al. 2013). Our data suggest that nicotine enhances social reward. Given the lack of strong positive subjective experience provided by cigarettes (Kalman 2002), enhanced social reward by nicotine is a potential reason for smoking among adolescents. In summary, the data presented herein provide strong evidence demonstrating that nicotine, rather than the flavor cue or the AV cue, was the primary reinforcer for the operant behavior in the socially acquired nicotine IVSA model. These data also demonstrated a complex interaction between nicotine and social learning: while social learning was required for nicotine IVSA, nicotine also enhanced social interaction in this model.
Acknowledgments
This work was supported by NIH DA-026894 (HC) and DA-037844 (HC). The authors thank Ms. Qingling Wu and Ms. Hongxiao Song for their excellent technical assistance, and Dr. Jeffery Steketee for comments on the manuscript.
Footnotes
Conflict of Interest: The authors have no conflict of interest to declare.
References
- Akers RL. Deviant behavior: A social learning approach. 2nd edition Wadsworth Pub. Co; 1977. [Google Scholar]
- Chen H, Matta SG, Sharp BM. Acquisition of nicotine self-administration in adolescent rats given prolonged access to the drug. Neuropsychopharmacology. 2006;32:700–709. doi: 10.1038/sj.npp.1301135. [DOI] [PubMed] [Google Scholar]
- Chen H, Sharp BM, Matta SG, Wu Q. Social interaction promotes nicotine self-administration with olfactogustatory cues in adolescent rats. Neuropsychopharmacology. 2011;36:2629–2638. doi: 10.1038/npp.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Rowell PP, et al. Nicotine self-administration in rats: estrous cycle effects, sex differences and nicotinic receptor binding. Psychopharmacology. 2000;151:392–405. doi: 10.1007/s002130000497. [DOI] [PubMed] [Google Scholar]
- Eissenberg T, Balster RL. Initial tobacco use episodes in children and adolescents: current knowledge, future directions. Drug Alcohol Depend 59 Suppl. 2000;1:S41–60. doi: 10.1016/s0376-8716(99)00164-7. [DOI] [PubMed] [Google Scholar]
- Flay BR, Hu FB, Siddiqui O, et al. Differential influence of parental smoking and friends’ smoking on adolescent initiation and escalation of smoking. J Health Soc Behav. 1994;35:248–265. [PubMed] [Google Scholar]
- Friedman LS, Lichtenstein E, Biglan A. Smoking onset among teens: an empirical analysis of initial situations. Addict Behav. 1985;10:1–13. doi: 10.1016/0306-4603(85)90048-6. [DOI] [PubMed] [Google Scholar]
- Greenlund KJ, Johnson CC, Webber LS, Berenson GS. Cigarette smoking attitudes and first use among third-through sixth-grade students: the Bogalusa Heart Study. Am J Public Health. 1997;87:1345–1348. doi: 10.2105/ajph.87.8.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn G, Charlin VL, Sussman S, et al. Adolescents’ first and most recent use situations of smokeless tobacco and cigarettes: similarities and differences. Addict Behav. 1990;15:439–448. doi: 10.1016/0306-4603(90)90030-2. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Kleykamp BA, Singleton EG. Meta-analysis of the acute effects of nicotine and smoking on human performance. Psychopharmacology. 2010;210:453–469. doi: 10.1007/s00213-010-1848-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofstetter CR, Hovell MF, Jung K-R, et al. The first puff: forces in smoking initiation among Californians of Korean descent. Nicotine Tob Res. 2007;9:1277–1286. doi: 10.1080/14622200701704863. [DOI] [PubMed] [Google Scholar]
- Kalman D. The subjective effects of nicotine: methodological issues, a review of experimental studies, and recommendations for future research. Nicotine Tob Res. 2002;4:25–70. doi: 10.1080/14622200110098437. [DOI] [PubMed] [Google Scholar]
- O'Loughlin J, Paradis G, Renaud L, Sanchez Gomez L. One-year predictors of smoking initiation and of continued smoking among elementary schoolchildren in multiethnic, low-income, inner-city neighbourhoods. Tob Control. 1998;7:268–275. doi: 10.1136/tc.7.3.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmatier MI, Evans-Martin FF, Hoffman A, et al. Dissociating the primary reinforcing and reinforcement-enhancing effects of nicotine using a rat self-administration paradigm with concurrently available drug and environmental reinforcers. Psychopharmacology. 2005;184:391–400. doi: 10.1007/s00213-005-0183-4. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Lantz JE, O'Brien LC, Metz SP. Effects of nicotine on olfactogustatory incentives: preference, palatability, and operant choice tests. Nicotine Tob Res. 2013;15:1545–1554. doi: 10.1093/ntr/ntt016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Donny E, Caggiula AR. Sex differences in nicotine effects and self-administration: review of human and animal evidence. Nicotine Tob Res. 1999;1:301–315. doi: 10.1080/14622299050011431. [DOI] [PubMed] [Google Scholar]
- Powell LM, Tauras JA, Ross H. The importance of peer effects, cigarette prices and tobacco control policies for youth smoking behavior. J Health Econ. 2005;24:950–968. doi: 10.1016/j.jhealeco.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Spear SF, Akers RL. Social learning variables and the risk of habitual smoking among adolescents: the Muscatine study. Am J Prev Med. 1988;4:336–342. [PubMed] [Google Scholar]
- Stolerman IP, Jarvis MJ. The scientific case that nicotine is addictive. Psychopharmacology. 1995;117:2–10. doi: 10.1007/BF02245088. discussion 14–20. [DOI] [PubMed] [Google Scholar]
- Thiel KJ, Okun AC, Neisewander JL. Social reward-conditioned place preference: a model revealing an interaction between cocaine and social context rewards in rats. Drug Alcohol Depend. 2008;96:202–212. doi: 10.1016/j.drugalcdep.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel KJ, Sanabria F, Neisewander JL. Synergistic interaction between nicotine and social rewards in adolescent male rats. Psychopharmacology. 2009;204:391–402. doi: 10.1007/s00213-009-1470-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V, Baarendse PJJ, Vanderschuren LJMJ. Prosocial effects of nicotine and ethanol in adolescent rats through partially dissociable neurobehavioral mechanisms. Neuropsychopharmacology. 2009;34:2560–2573. doi: 10.1038/npp.2009.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter H, Abler B, Ciaramidaro A, Erk S. Motivating forces of human actions. Neuroimaging reward and social interaction. Brain Res Bull. 2005;67:368–381. doi: 10.1016/j.brainresbull.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Wang T, Chen H. Carbon disulfide mediates socially-acquired nicotine self-administration. PLoS One. 2014;9:e115222. doi: 10.1371/journal.pone.0115222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Han W, Wang B, et al. Propensity for social interaction predicts nicotine-reinforced behaviors in outbred rats. Genes Brain Behav. 2014a;13:202–212. doi: 10.1111/gbb.12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Wang B, Chen H. Menthol facilitates the intravenous self-administration of nicotine in rats. Front Behav Neurosci. 2014b;8:437. doi: 10.3389/fnbeh.2014.00437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White VM, Byrnes GB, Webster B, Hopper JL. Does smoking among friends explain apparent genetic effects on current smoking in adolescence and young adulthood? Br J Cancer. 2008;98:1475–1481. doi: 10.1038/sj.bjc.6604250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zernig G, Kummer KK, Prast JM. Dyadic social interaction as an alternative reward to cocaine. Front Psychiatry. 2013;4:100. doi: 10.3389/fpsyt.2013.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]