Abstract
Objective
To evaluate the expected cost-effectiveness and net benefit of the recent implementation of newborn screening (NBS) for severe combined immunodeficiency (SCID) in Washington State.
Study design
We constructed a decision analysis model to estimate the costs and benefits of NBS in an annual birth cohort of 86 600 infants based on projections of avoided infant deaths. Point estimates and ranges for input variables, including the birth prevalence of SCID, proportion detected asymptomatically without screening through family history, screening test characteristics, survival rates, and costs of screening, diagnosis, and treatment were derived from published estimates, expert opinion, and the Washington NBS program. We estimated treatment costs stratified by age of identification and SCID type (with or without adenosine deaminase deficiency). Economic benefit was estimated using values of $4.2 and $9.0 million per death averted. We performed sensitivity analyses to evaluate the influence of key variables on the incremental cost-effectiveness ratio (ICER) of net direct cost per life-year saved.
Results
Our model predicts an additional 1.19 newborn infants with SCID detected preclinically through screening, in addition to those who would have been detected early through family history, and 0.40 deaths averted annually. Our base-case model suggests an ICER of $35 311 per life-year saved, and a benefit-cost ratio of either 5.31 or 2.71. Sensitivity analyses found ICER values <$100 000 and positive net benefit for plausible assumptions on all variables.
Conclusions
Our model suggests that NBS for SCID in Washington is likely to be cost-effective and to show positive net economic benefit.
Infants with severe combined immunodeficiency (SCID)1,2 develop recurrent severe infections beginning around 2 months of age, and median age at diagnosis is 4.5 months.3-6 Roughly one-fifth of infants with SCID have an affected older sibling,5,7-9 and early diagnosis of such infants followed by hematopoietic cell transplantation (HCT) before 3.5 months of age is associated with 94% post-HCT survival, compared with 66%-69% survival for infants transplanted at 3.5 months or later.10-12 Infants with adenosine deaminase (ADA) deficient SCID (ADA-SCID), 10%-20% of infants with SCID, are typically initially treated with enzyme replacement therapy (ERT) with polyethylene glycol-modified bovine ADA (PEG-ADA).5,13-18
Based on evidence of improved survival with early diagnosis,19 in May 2010, the US Department of Health and Human Services added SCID to the Recommended Uniform Screening Panel.10,20 The addition of SCID followed the implementation of pilot state-based newborn screening (NBS) programs in Wisconsin21,22 and Massachusetts.23,24 SCID has been integrated into the majority of state NBS programs,6,13 but as of September 28, 2015, 14 states had not yet made a decision to screen for SCID.25 Our study was intended to provide information to other states on the cost-effectiveness and net benefit of NBS for SCID in Washington State. A cost-benefit model26 informed the decision by Washington to add SCID to the state NBS panel, consistent with a requirement that state agencies determine that the probable economic benefits of a rule exceed its probable costs.27 After legislative approval of a fee increase, screening for SCID in Washington began in January 2014.
Methods
We constructed a decision tree model of the costs and avoided deaths associated with NBS for SCID using a T-cell receptor excision circle (TREC) assay with a dried blood spot (DBS) punch compared with no screening. The cost-effectiveness analysis (CEA) version of the model reports the number of life-years saved, and the cost-benefit analysis (CBA) version places a dollar value on averted deaths.
The analytic perspective is that of the health sector, in which all costs are estimated as the resources used in providing services, regardless of who pays the costs. The model structure was modified from a CBA model developed during 2011-2012 by the Washington NBS program.26 A user-friendly version of the spreadsheet model that allows users to customize the model with state-specific estimates is available on the Association of Public Health Laboratories website.
Washington is one of 12 states that routinely screens newborns twice using DBS collected within 18-48 hours and again at about 7-14 days after birth. The initial screening test consists of TREC amplification and a reference gene, beta-actin. Results of the initial screen can be of 4 types: (1) presumptive cases with TREC copies/μL below the lower cut-off, for which an immediate confirmatory testing of flow cytometry is recommended; (2) borderline cases with TREC copies/μL between the 2 cut-offs; (3) normal results with TREC copies/μL above the higher cut-off; and (4) inconclusive results with an unsatisfactory beta-actin ≥28 quantitation cycle. This analysis ignores inconclusive cases and amplification failures; the Washington program reported 3 inconclusive results out of more than 170 000 specimens tested during 2014.
Infants with abnormal TREC screening results (presumptive result or borderline result with confirmation by abnormal screen on repeat test) are referred for confirmatory testing using flow cytometry. If the latter is abnormal, additional diagnostic tests are ordered to determine if the child has another form of immune deficiency, including non-SCID T-cell lymphopenia (TCL) (because of syndromes such as DiGeorge [22q11 deletion] or Jacobsen) or idiopathic TCL, or another medical condition such as trisomy 21 or pre-term birth, which can cause low T-cell counts.
The point estimates in the base-case scenario and ranges for input variables for sensitivity analyses were selected from published estimates, if available, or expert opinion (Table I). The time horizon is 5 years for assessing outcomes and the lifetime for assessing survival.
Table I.
Model variables and ranges
| Variables | Base-case | Range/Alternative | References |
|---|---|---|---|
| Birth prevalence of SCID | 1/58 000 | 1/46 000-1/80 000 | 13 |
| Proportion of SCID cases detected without NBS | 0.203 | 5,8 | |
| Birth prevalence of non-SCID TCL | 1/14 000 | 1/11 600-1/16 400 | 13 |
| Screening test characteristics | |||
| Sensitivity of the overall screen process | 99.50% | 99.00%-100.00% | 7,13 |
| Specificity of the overall screen process | 99.97% | 99.92%-99.98% | 23,26,28 |
| Survival rate | |||
| For early-identified SCID (pretreatment) | 94% | 13 | |
| For early-identified SCID (posttreatment) | 94% | 4,8,13 | |
| For late-identified SCID (pretreatment) | 78% | 5,7 | |
| For late-identified SCID (posttreatment) | 69% | 10,11 | |
| Cumulative survival for early-identified SCID* | 88% | 85%-94% | 10,11,13 |
| Cumulative survival for late-identified SCID† | 54% | 38%-72% | 5,7,29,30 |
| Costs of screening and diagnosis | |||
| Lab test for TREC assay per sample | $4.04 | $3.00-$6.00 | 26 |
| Short-term follow-up per positive case | $50.0 | 26 | |
| Flow cytometry per baby | $250.0 | 7,26 | |
| Additional costs for transient TCL‡ | $2360 | 26 | |
| Additional costs for idiopathic TCL | $6000 | 26 | |
| Additional costs for other non-SCID TCL | $6000 | 26 | |
| Costs of treatment | |||
| Average cost per infant with SCID who die before definitive treatment | $300 000 | Unpublished data | |
| Average cost per infant with ADA SCID who do not undergo early HCT | $450 000 | $200 000-$750 000 | Expert opinion |
| Average costs for infants with SCID who receive HCT as first-line therapy | |||
| Per early-identified baby | $100 000 | $80 000-$120 000 | 7,10,31 |
| Per late-identified baby | $450 000 | $300 000-$1 200 000 | 10,31,32 |
| VSL | $9 million | $4.2 million | 33,34 |
Cumulative survival for early-identified infants was calculated as 94% pretreatment survival multiplied by 94% posttransplant survival.
Cumulative survival for late-identified infants was calculated as 78% pretreatment survival multiplied by 69% posttransplant survival.
Additional costs: costs of additional diagnostic testing (ie, excluding initial flow cytometry testing), clinic visits, and prophylactic antibiotics for non-SCID TCL.
This study assumed an annual birth cohort of 86 600 babies in Washington.35 The US birth prevalence of SCID and non-SCID TCL are reported to be 1 in 58 000 and 1 in 14 000 live births, respectively.13
The sensitivity of the TREC assay or the ability of the screen to correctly identify babies with SCID was previously estimated at 99.5%.7 Although there have been no known missed cases in SCID NBS programs,13 false negatives are inevitable in screening programs. We have conservatively retained the previous estimate of 99.5%.
Specificity was estimated at 99.97% based on the experience in Washington state, with a range based on the literature.26,28 This variable refers to the probability that at the end of the screening process, an infant who does not have SCID will not be referred for confirmatory testing. In Washington, 10% of the first 20 referrals were confirmed as SCID cases, 40% had non-SCID TCL, and the remaining 50% had conditions other than SCID or non-SCID TCL.
Health Outcomes
Most published estimates of posttransplant survival in infants with SCID are stratified by age at transplantation before or after 3.5 months as the usual cut-off indicating a survival difference of 25%-28% in favor of early transplantation. Post-HCT survival in infants with ADA-SCID appears comparable with SCID in general and varies by type of donor (eg, matched family, matched nonrelative, or haploidentical).15,36
Our base-case model conservatively assumed 6% pretreatment mortality for early diagnosed infants based on findings on 52 newborns with SCID identified through 11 US NBS programs.13 An estimate of 22% mortality prior to definitive treatment or diagnosis of SCID for late diagnosed or undiag-nosed infants based on 138 children not tested at birth5 (J. Puck, personal communication, February 16, 2015) implies a survival difference of 16 percentage points. We assumed posttransplant mortality of 6% for early diagnosed infants8-13 and 31% for late diagnosed infants,11,12 a difference of 25 percentage points. Total cumulative survival to age 5 years was assumed to be 88% (94% pretreatment survival, times 94% posttransplant survival) for the early diagnosed group and 54% (78% pretreatment survival, times 69% posttreatment survival) for late diagnosed infants.
Costs
The incremental costs of adding SCID to a screening panel (eg, laboratory test and administrative costs), costs of diagnostic testing (eg, flow cytometry), costs to treat for early-or late-identified infants with SCID, and costs of deaths averted are specified in Table I. Costs of specimen collection and transport are not affected by the addition of SCID. Costs were adjusted to 2012 US dollars using the healthcare component of the Personal Consumption Expenditures Price Indexes.37
The cost of the laboratory test for the TREC assay was estimated at $4.04 per sample in Washington, and the cost of the short-term follow-up state program was assumed to be $50 per positive case. As a result, the costs of laboratory tests and short-term follow-up were calculated as $4.05 per sample (ie, $4.04 plus $0.01, the product of $50 multiplied by 0.02% of positive screening results), yielding $8.10 per baby as the health department cost of screening. We assumed no additional costs for screening in Washington because repeat DBS specimens are already collected.
The cost of flow cytometry, $250 per baby, includes phlebotomy and interpretation of test results. It does not include the cost of an office visit because most infants referred in Washington for flow cytometry are already in a neonatal intensive care unit for reasons such as prematurity, cardiac surgery, and suspicion of DiGeorge syndrome associated with low T-cell levels (data not reported). The cost of flow cytometry is multiplied by the number of infants tested who would not otherwise have been tested in the absence of screening, which includes testing costs for infants who screen positive but are not diagnosed with SCID and infants with SCID who we projected would die without diagnosis in the absence of screening.
The Washington pediatric immunology consultants provided expert opinion for the treatment course for non-SCID cases (beyond initial flow cytometry): an initial and final complete blood count, quarterly repeat flow cytometry analyses, quarterly clinic visits to the immunologists, and prophylactic trimethoprim-sulfamethoxazole treatment. Estimates of unit costs were obtained from Seattle Children's Hospital. Multiplying those charges by 0.7 to reflect costs yields the following cumulative cost estimates: (1) $2360 per infant with transient TCL during 1-year follow-up; (2) $6000 per infant with idiopathic TCL during 5-year follow-up; and (3) $6000 per infant with other non-SCID TCL during 5-year follow-up.
Our estimates of per-child treatment cost for infants with SCID who undergo HCT came from data on 74 infants with typical SCID treated at Duke University during 1998-2006 with average cost of hospital care of $450 000 for infants transplanted after 3.5 months and $100 000 for those transplanted before 3.5 months.10 The costs for treating late-diagnosed infants with SCID are higher because of prolonged hospitalizations, intensive care unit admissions, and the need for chemoablation.7
Treatment costs for infants with ADA-SCID are uncertain; prior analyses implicitly assumed the costs to be the same as for other SCID cases. However, a commonly used treatment option for ADA-SCID that does not carry risk of death, ERT with PEG-ADA, can cost hundreds of thousands of dollars per year.14,17 Infants who have sibling-matched donors, about 25%, typically undergo HCT because of the high success rate15; their cost is the same as for other early-transplanted infants. Other infants with ADA-SCID may undergo months to years of ERT before undergoing HCT or may continue on PEG-ADA indefinitely. We adopted a conservative assumption that the average treatment cost for infants without sibling donors who undergo treatment is $450 000, regardless of age of diagnosis (expert opinion).
In addition, we assumed a mean cost of $300 000 per infant with SCID who dies prior to definitive treatment as a result of the complications of infections, based on a review of Texas Medicaid claims data in 2009 (personal communication, Rachel Lee, November 5, 2014).
Outcome Measures
The primary outcome measures are the incremental cost-effectiveness ratio (ICER) in the CEA and net benefit and benefit-cost ratio in the CBA. The numerator of the ICER is the net direct cost, defined as the cost of screening and diagnosis minus the reduction in treatment costs associated with screening. The denominator is discounted life years calculated using a 3% annual discount rate38 (with midyear correction) and 2009 US Life Tables.39 Net benefit was calculated as total benefits (treatment cost reduction plus monetary value of deaths averted) minus costs of screening and diagnosis. The benefit-cost ratio is the ratio of total benefits to total costs.
CEAs, which predominate in the medical field, report ICER estimates that can be compared with commonly used benchmark values to assess likely cost-effectiveness. Beginning in the mid-1990s, US CEAs frequently used an arbitrary value of $50 000 per life-year or quality-adjusted life-year (QALY) as a benchmark value to assess cost-effectiveness.40 It has become common for US analysts to use $100 000 per QALY in addition to or in place of $50 000 per QALY, and some experts recommend analysts use a threshold of $100 000 or $150 000 per QALY.41 Although life-years and QALYs are not interchangeable, for most interventions the relative assessment of cost-effectiveness does not vary much.42
CBA is the most common type of economic evaluation and is often mandated by US regulatory decision makers at both the state and federal levels. Value of a statistical life (VSL) estimates that are typically used to monetize averted deaths in US regulatory CBAs are based on labor market comparisons of occupational fatalities and earnings differentials.33 One recent systematic review found a VSL range of $4.2-$13.7 million with a mid-point of $9.0 million (2013 US dollars).43 The lower-bound estimate of $4.2 million is well below VSL estimates used by federal agencies during 2011-2012 of $6.6-$9.8 million.34 The most robust US VSL estimates range from $7.6-$11.0 million.34
Sensitivity Analyses
We performed sensitivity analyses to evaluate how the ICER and net benefit change when estimates for the key variables differed. For 1-way sensitivity analyses, 1 variable at a time was changed for each of the scenarios keeping other variables at their base-case values. A 2-way sensitivity analysis was performed for the 2 variables that were most influential in the 1-way sensitivity analysis, with other variables remaining unchanged. Ranges for variables were selected based on the lower- and higher-bound values of estimates from published data and expert recommendations. Modeling was performed using Microsoft Excel 2010 (Microsoft, Redmond, Washington).
Results
For a birth cohort of 86 600 babies in Washington, the model predicts that NBS results in an average of 1.49 cases of SCID and 6.19 cases of non-SCID TCL detected among newborns. In the absence of NBS, 0.30 asymptomatic SCID cases would be detected among newborns each year as a result of a positive family history. The difference is an additional 1.19 cases detected preclinically each year through NBS. NBS is also expected to lead to 0.40 deaths averted by screening each year. The annual costs of screening and diagnosis, as well as management of non-SCID cases, are estimated to total $8.16 per infant (Table II). The reduced treatment cost with NBS for this cohort, $316 905, was estimated to offset 43% of the $741 376 added cost associated with NBS.
Table II.
Base-case results of cost-effectiveness and cost-benefit analyses
| Outcomes | Screening | No screening |
|---|---|---|
| Intermediate outcomes | ||
| Total cost of screening and diagnosis following screening | $741 376 | N/A |
| Cost of screening and diagnosis per infant screened | $8.16 | N/A |
| Treatment costs for infants receiving definitive treatment | $197 258 | $457 401 |
| Treatment costs for infants dying prior to definitive treatment | $27 234 | $83 996 |
| Treatment cost reduction with screening | $316 905 | N/A |
| Net direct cost with screening | $424 470 | N/A |
| Number of infants with SCID diagnosed preclinically | 1.49 | 0.30 |
| Number of additional SCID cases detected preclinically by screening | 1.19 | N/A |
| Number of cases of non-SCID TCL detected by screening | 6.19 | N/A |
| Number of infant deaths because of SCID | 0.18 | 0.58 |
| Number of deaths averted by screening | 0.40 | N/A |
| Discounted life-years gained by screening | 12.02 | N/A |
| Results of CEA | ||
| ICER (cost per life-y saved) | $35 311 | |
| Results of CBA (VSL = $9.0 million) | ||
| Total benefits (treatment cost reduction plus survival benefit) | 3.94 million | |
| Net benefit (benefits minus costs of screening and diagnosis) | 3.19 million | |
| Benefit-cost ratio | 5.31 | |
| Results of CBA (VSL = $4.2 million) | ||
| Total benefits (treatment cost reduction plus survival benefit) | 2.01 million | |
| Net benefit (benefits minus costs of screening and diagnosis) | 1.26 million | |
| Benefit-cost ratio | 2.71 |
N/A, not applicable.
Our base-case model suggests an ICER of $35 311 per life-year saved (ie, net direct medical costs of $424 470 divided by 12.02 discounted life-years gained). Using the midpoint VSL value of $9 million, the estimated net benefit of $3.19 million for a single year's Washington birth cohort results from subtracting costs from a total benefit of $3.94 million ($3.62 million mortality benefit plus $316 905 in reduced treatment costs); the corresponding benefit-cost ratio was 5.31. Using a lower-bound VSL value of $4.2 million reduces the mortality benefit to $1.69 million, net benefit to $1.26 million, and the benefit-cost ratio to 2.71 (Table II).
Sensitivity Analyses
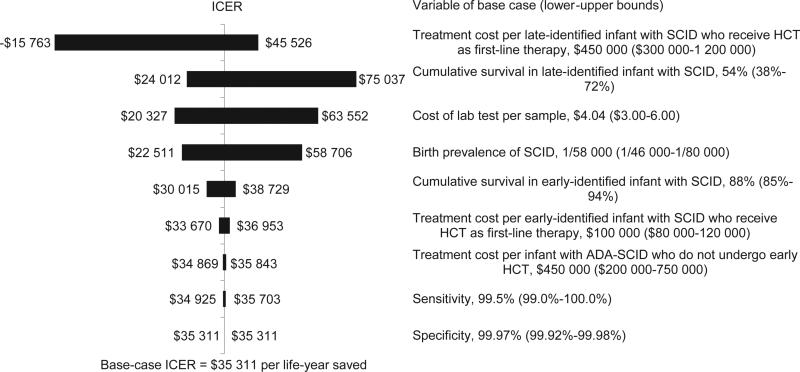
A tornado diagram displays the impacts of varying individual model variables one at a time on the ICER measure (Figure 1). One of those variables, testing cost per specimen, is known for Washington but varies across states. The ICER remained <$100 000 per life-year saved when all variables differed within predefined ranges. The upper-bound ICER estimate exceeded the traditional $50 000 benchmark value for 3 variables subject to uncertainty: the probability of survival in late-identified SCID, cost per laboratory test, and the birth prevalence of SCID. The variable that has the greatest impact on the ICER is the treatment cost per late-identified infant with SCID who receives HCT as first-line therapy. The ICER changes from $45 526 to a negative value as this variable varies from $300 000 to $1.2 million (Figure 1). NBS would be cost-saving (negative ICER) if this variable was to exceed $968 600. Net benefit was positive for all ranges (results not reported).
Figure 1.
Tornado diagram of 1-way sensitivity analyses, showing the effect of range of individual variables on the outcome measure ICER for NBS of SCID. The vertical line in the diagram represents the base-case ICER of $35 998 per life year saved. Each horizontal bar indicates the range of ICER with the lower and upper values of each variable in Table I and Table II.
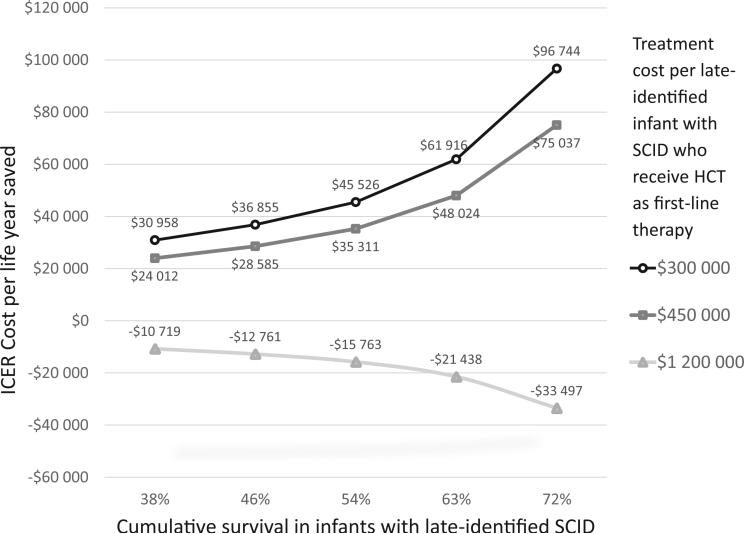
The ICER results of one 2-way sensitivity analysis are presented in Figure 2. The influence of the treatment cost per late-identified infant with SCID who receives HCT as first-line therapy increases as the cumulative survival rate for infants with late-diagnosed SCID increases. If late diagnosis of SCID has a small effect on mortality, the ICER is influenced more by the relative treatment costs of late-diagnosed vs early diagnosed cases. Additional 2-way sensitivity analyses showed positive net benefit in all cases including for a VSL value of $4.2 million (results not reported).
Figure 2.
Two-way sensitivity analyses, showing ICER for NBS of SCID as a function of treatment cost per late-identified SCID who receive HCT as first-line therapy and cumulative survival in late-identified SCID.
Discussion
Our results suggest that NBS for SCID is cost-effective and cost-beneficial despite low absolute numbers of cases detected and deaths averted. Our findings imply that approximately 1 baby with SCID every 2-3 years will not die as a result of the implementation of NBS for SCID in Washington state. The ICER estimate is roughly $35 000 per life-year saved. Screening shows net benefit at VSL estimates consistent with available evidence, even at the lowest estimate of $4.2 million. The base-case benefit-cost ratio of 5.31 is slightly larger than the ratio of 4.36 reported in the original Washington CBA.26 That in part reflects use of a more current VSL value of $9 million in place of the $7.7 million value used in the previous analysis. Substituting a highly conservative VSL value of 4.2 million, the base-case benefit-cost ratio would still be 2.71 and in sensitivity analyses it would exceed 1.0 under all pair-wise combinations of parameter estimates.
Numerous CEAs of NBS conditions have been published, but this appears to be the first published NBS CBA that uses VSL to value avoided deaths. Many CEAs have reported NBS to be cost-effective or even cost-saving.44,45 However, conflicting estimates are not uncommon, which often reflect differences in assumptions, particularly regarding child survival with and without NBS.46,47
Our model differs from previous CEAs of SCID screening by: (1) considering 2 cut-off points for the screening process in a real-world laboratory setting; (2) including the cost of short-term follow-up program staff time; (3) including costs of additional diagnostic testing, clinic visits, and prophylactic antibiotics for non-SCID TCL cases; (4) including the death rate prior to treatment as well as posttransplant mortality; and (5) estimating the cost of therapy for infants who have ADA-associated SCID.
Two previous CEAs estimated QALY gains despite an absence of information on health-related quality of life for children with SCID. One study applied published utility scores for children with cystic fibrosis, sickle cell anemia, HIV-AIDS, medium chain acyl-CoA dehydrogenase deficiency, or leukemia to children with SCID after HCT; ICER estimates were $25 429 per life-year and $27 907 per QALY gained.7 However, transferring utility scores across studies and conditions can be treacherous.48 Because the average post-HCT health utility was assumed to be 0.95,29 the difference in estimates must have been small. Extrapolating from those 2 studies, our ICER estimates would likely have been 5%-10% higher if expressed relative to QALYs.
Our ICER estimates are similar to estimates from previous US publications, which used different assumptions, including screening costs of $4-$5 per infant.7,29,49 The cost of laboratory screening per infant in this analysis, $8.08, reflects routine testing of 2 specimens for infants using a laboratory-developed test. Wisconsin, which tests just 1 specimen, has reported a testing cost of roughly $6 per infant.50 Average costs can also vary with numbers of births, with average cost decreasing with increasing volume. In France, a pilot testing program indicated a cost of $6-$9 per test.51 These cost estimates are for laboratory-developed tests; if laboratories use a newly available Food and Drug Administration-approved kit, the cost per life-year saved will likely be higher.
The analysis did not include a cost of communicating positive screening results to parents, which should be included in economic evaluations of NBS.52 However, because of the very low rate of positive TREC screening results, a $100 cost to communicate screening results raises the ICER by <1%.
The cost-effectiveness and cost-benefit findings for Washington appear robust because when variables are varied within plausible ranges the ICER remains below the $100 000 benchmark that is increasingly used in the US,40,41 and net benefit remains positive at all plausible VSL values. One of the most influential variables—cost of the laboratory screening test (TREC with beta-actin)—is not a source of uncertainty for the Washington estimates, although it is a crucial variable to assess before extrapolating the findings to other states. Another influential variable is the birth prevalence of SCID; our estimate is based on data from more than 3 million US newborns screened but did not include Washington data.13
The estimate of per-person treatment costs in the absence of NBS is an important source of uncertainty. The published estimates from Duke University10 may not capture the costs of admissions at other hospitals prior to referral to the transplant center. Four of 15 infants with SCID treated by one of the authors (L.K.) at her institution required hospitalization in a pediatric intensive care unit for 2-3 weeks prior to transplant. Our estimates of avoided costs may also be conservative because we did not model the avoided costs of treating infections that can result from administering live virus vaccinations to babies with impaired immune systems.53,54 Higher cost estimates for infants with late-diagnosed SCID would make NBS appear more cost-effective (Figures 1 and 2).
It is possible, although unlikely, that the average treatment cost for infants with late-treated SCID might exceed $1 million, in which case NBS would be cost-saving. An unpublished study from the United Kingdom estimated average treatment costs with and without NBS at approximately $120 000 and $1.2 million, respectively.32 Another modeling study assumed a cost of $2 million per infant without NBS30 based on an unpublished estimate from Wisconsin of the hospital bill for one child55; the cost may have been <$700 000. Kubiak et al31 reported mean charges for 25 infants treated at 3 referral hospitals of $1.43 million for infants treated ≤3.5 months and $365 785 for those treated <3.5 months. Using an average cost-to-charge ratio of 0.345 from Healthcare Cost and Utilization Project discharges on admissions of infants with a principal diagnosis of combined immune deficiency,56 the average cost for late-treated infants was less than $500 000. Finally, the mean cost for hospital care received by 27 late-treated French infants from birth through 1 year posttransplant (including 10 who died posttransplant) was €226 510, with median costs for late-treated (≤3 months) and early-treated (<3 months) transplanted infants of €195 776 and €86 179, respectively (median costs are lower than mean costs).51 Taking into account US healthcare prices, the US-equivalent average total treatment cost for late-treated infants might be as much as $500 000.
Other limitations include lack of information on the cost of therapy for children with ADA-SCID who do not have sibling donors. Further, the cost estimates do not include long-term medical treatment (gamma globulin therapy) or subsequent transplantation for children with SCID with failure of engraftment. Inaddition, infantswho develop complications after HCT may have highlong-term costs even withNBS, which were not considered. Each of these omissions would have the effect of lowering the estimate of treatment cost-reduction and make NBS less cost-effective or cost-beneficial.
Survival is another source of uncertainty. The base-case estimate of 88% survival to age 5 years for infants with SCID identified early, taking into account deaths both prior to treatment and posttransplant, is conservative relative to published estimates of 95%29 to 100%7 posttransplant survival. Our estimate of 54% overall survival among infants with late-diagnosed SCID is similar to the 58% survival rate5 cited in a recent fiscal analysis,31 and higher than an estimate of 38%7 used in 2 CEAs.7,30 As expected, it is lower than in 2 other CEA studies that did not take into account deaths to infants prior to transplantation, 62.5%26 and 72%.29
The 2-way sensitivity analysisshows thata lower survival rate for late-treated SCID would reduce the absolute magnitude of the ICER and make screening appear more cost-effective as long as the mean cost of treatment is less than approximately $1 million (Figure 2). The present analysis, like previous CEAs,7,29 assumes that long-term survival (>5 years posttreatment) is the same as in the general population.
The analysis did not include time costs for families seeking care or caring for an affected child, essential for a societal perspective analysis. One CEA of NBS for medium chain acyl-CoA dehydrogenase deficiency analyzed data from a parent survey to include estimates of time costs57; we did not have access to comparable information.
A final limitation is that this article does not directly address the fiscal or financial implications of SCID screening for state governments or public insurers. A budget impact analysis from the budget holder or payer perspective would need to identify which expenditures are incurred by a health-care organization, state government, or other budget holder and which of those costs would be avoided as a result of the intervention.58
Early identification of SCID is critical to the health and survival of affected infants. Mortality is greatly reduced with early treatment and medical costs are much lower than for babies treated after becoming symptomatic. We believe our study provides a useful and timely economic assessment that can inform decisions on the adoption of the federally recommended SCID screen in state NBS programs. The CBA conducted in Washington26 was a crucial step in the process of approving the adoption of SCID in that state,59 and earlier versions of the model have been shared with other states.
Acknowledgments
We thank the Washington State Department of Health and the staff of the Newborn Screening Program, in particular, Mike Glass (program director, deceased), for their contributions to the cost-benefit model of screening for SCID, which formed the foundation for the model that was developed and applied here. We also thank members of the Washington State SCID Newborn Screening Advisory Committee for their questions and input that clarified and refined the original model. Finally, we thank organizers and participants in a roundtable at the 2014 Association of Public Health Laboratories Newborn Screening and Genetic Testing Symposium in Anaheim, California, for their feedback on an earlier version of the cost-effectiveness model. In particular, we thank Ruhiyyih Degeberg, Mei Baker, John Wong, and Careema Yusuf for their helpful comments on early versions of this manuscript.
Y.D. was supported by Perkin Elmer, Inc through an unrestricted fellowship grant to the Association of Public Health Laboratories (100-210-14). Perkin Elmer, Inc sells instruments, reagents, and services to screening laboratories, but did not contribute to the study design, analysis or interpretation of data, the writing of the report, or the decision to submit the manuscript for publication. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention, the Association of Public Health Laboratories, and the Washington State Department of Health.
Glossary
- ADA
Adenosine deaminase
- ADA-SCID
ADA deficient SCID
- CBA
Cost-benefit analysis
- CEA
Cost-effectiveness analysis
- DBS
Dried blood spot
- ERT
Enzyme replacement therapy
- HCT
Hematopoietic cell transplantation
- ICER
Incremental cost-effectiveness ratio
- NBS
Newborn screening
- PEG-ADA
Polyethylene glycol-modified bovine ADA
- QALY
Quality-adjusted life-year
- SCID
Severe combined immunodeficiency
- TCL
T-cell lymphopenia
- TREC
T-cell receptor excision circle
- VSL
Value of a statistical life
Footnotes
Portions of the study were presented as a poster at the meeting of the Society for Medical Decision Making, October 20, 2015, St. Louis, MO, as well as orally at the conference of the Society for Benefit-Cost Analysis, March 20, 2015, Washington, DC.
The authors declare no conflicts of interest.
References
- 1.Puck JM. The case for newborn screening for severe combined immunodeficiency and related disorders. Ann N Y Acad Sci. 2011;1246:108–17. doi: 10.1111/j.1749-6632.2011.06346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Puck JM. Population-based newborn screening for severe combined immunodeficiency: steps toward implementation. J Allergy Clin Immunol. 2007;120:760–8. doi: 10.1016/j.jaci.2007.08.043. [DOI] [PubMed] [Google Scholar]
- 3.Adeli MM, Buckley RH. Why newborn screening for severe combined immunodeficiency is essential: a case report. Pediatrics. 2010;126:e465–9. doi: 10.1542/peds.2009-3659. [DOI] [PubMed] [Google Scholar]
- 4.Brown L, Xu-Bayford J, Allwood Z, Slatter M, Cant A, Davies EG, et al. Neonatal diagnosis of severe combined immunodeficiency leads to significantly improved survival outcome: the case for newborn screening. Blood. 2011;117:3243–6. doi: 10.1182/blood-2010-08-300384. [DOI] [PubMed] [Google Scholar]
- 5.Chan A, Scalchunes C, Boyle M, Puck JM. Early vs. delayed diagnosis of severe combined immunodeficiency: a family perspective survey. Clin Immunol. 2011;138:3–8. doi: 10.1016/j.clim.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pai SY, Cowan MJ. Stem cell transplantation for primary immunodeficiency diseases: the North American experience. Curr Opin Allergy Clin Immunol. 2014;14:521–6. doi: 10.1097/ACI.0000000000000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan K, Davis J, Pai SY, Bonilla FA, Puck JM, Apkon M. A Markov model to analyze cost-effectiveness of screening for severe combined immunodeficiency (SCID). Mol Genet Metab. 2011;104:383–9. doi: 10.1016/j.ymgme.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Myers LA, Patel DD, Puck JM, Buckley RH. Hematopoietic stem cell transplantation for severe combined immunodeficiency in the neonatal period leads to superior thymic output and improved survival. Blood. 2002;99:872–8. doi: 10.1182/blood.v99.3.872. [DOI] [PubMed] [Google Scholar]
- 9.Hague RA, Rassam S, Morgan G, Cant AJ. Early diagnosis of severe combined immunodeficiency syndrome. Arch Dis Child. 1994;70:260–3. doi: 10.1136/adc.70.4.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buckley RH. The long quest for neonatal screening for severe combined immunodeficiency. J Allergy Clin Immunol. 2012;129:597–604. doi: 10.1016/j.jaci.2011.12.964. quiz 5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pai SY, Logan BR, Griffith LM, Buckley RH, Parrott RE, Dvorak CC, et al. Transplantation outcomes for severe combined immunodeficiency, 2000-2009. N Engl J Med. 2014;371:434–46. doi: 10.1056/NEJMoa1401177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buckley RH. Transplantation of hematopoietic stem cells in human severe combined immunodeficiency: long-term outcomes. Immunol Res. 2011;49:25–43. doi: 10.1007/s12026-010-8191-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwan A, Abraham RS, Currier R, Brower A, Andruszewski K, Abbott JK, et al. Newborn screening for severe combined immunodeficiency in 11 screening programs in the United States. JAMA. 2014;312:729–38. doi: 10.1001/jama.2014.9132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Booth C, Gaspar HB. Pegademase bovine (PEG-ADA) for the treatment of infants and children with severe combined immunodeficiency (SCID). Biologics. 2009;3:349–58. [PMC free article] [PubMed] [Google Scholar]
- 15.Hassan A, Booth C, Brightwell A, Allwood Z, Veys P, Rao K, et al. Outcome of hematopoietic stem cell transplantation for adenosine deaminase-deficient severe combined immunodeficiency. Blood. 2012;120:3615–24. doi: 10.1182/blood-2011-12-396879. quiz 26. [DOI] [PubMed] [Google Scholar]
- 16.Montiel-Equihua CA, Thrasher AJ, Gaspar HB. Gene therapy for severe combined immunodeficiency due to adenosine deaminase deficiency. Curr Gene Ther. 2012;12:57–65. doi: 10.2174/156652312799789253. [DOI] [PubMed] [Google Scholar]
- 17.Chan B, Wara D, Bastian J, Hershfield MS, Bohnsack J, Azen CG, et al. Long-term efficacy of enzyme replacement therapy for adenosine deaminase (ADA)-deficient severe combined immunodeficiency (SCID). Clin Immunol. 2005;117:133–43. doi: 10.1016/j.clim.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Candotti F, Shaw KL, Muul L, Carbonaro D, Sokolic R, Choi C, et al. Gene therapy for adenosine deaminase-deficient severe combined immune deficiency: clinical comparison of retroviral vectors and treatment plans. Blood. 2012;120:3635–46. doi: 10.1182/blood-2012-02-400937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lipstein EA, Vorono S, Browning MF, Green NS, Kemper AR, Knapp AA, et al. Systematic evidence review of newborn screening and treatment of severe combined immunodeficiency. Pediatrics. 2010;125:e1226–35. doi: 10.1542/peds.2009-1567. [DOI] [PubMed] [Google Scholar]
- 20.Boyle CA, Bocchini JA, Jr, Kelly J. Reflections on 50 years of newborn screening. Pediatrics. 2014;133:961–3. doi: 10.1542/peds.2013-3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Routes JM, Grossman WJ, Verbsky J, Laessig RH, Hoffman GL, Brokopp CD, et al. Statewide newborn screening for severe T-cell lymphopenia. JAMA. 2009;302:2465–70. doi: 10.1001/jama.2009.1806. [DOI] [PubMed] [Google Scholar]
- 22.Verbsky JW, Baker MW, Grossman WJ, Hintermeyer M, Dasu T, Bonacci B, et al. Newborn screening for severe combined immunodeficiency; the Wisconsin experience (2008-2011). J Clin Immunol. 2012;32:82–8. doi: 10.1007/s10875-011-9609-4. [DOI] [PubMed] [Google Scholar]
- 23.Hale JE, Bonilla FA, Pai SY, Gerstel-Thompson JL, Notarangelo LD, Eaton RB, et al. Identification of an infant with severe combined immunodeficiencyby newborn screening. J Allergy Clin Immunol. 2010;126:1073–4. doi: 10.1016/j.jaci.2010.08.043. [DOI] [PubMed] [Google Scholar]
- 24.Comeau AM, Hale JE, Pai SY, Bonilla FA, Notarangelo LD, Pasternack MS, et al. Guidelines for implementation of population-based newborn screening for severe combined immunodeficiency. J Inherit Metab Dis. 2010;33:S273–81. doi: 10.1007/s10545-010-9103-9. [DOI] [PubMed] [Google Scholar]
- 25.NewSTEPS; Association of Public Health Laboratories [October 4, 2015];Severe Combined Immunodeficiency (SCID) https://www.newsteps.org/scid.
- 26.Thompson JD, Glass M. Guide to the Newborn Screening Cost-Benefit Model for Adding Severe Combined Immunodeficiency (SCID) Washington State Department of Health; [June 22, 2015]. http://sboh.wa.gov/Portals/7/Doc/Meetings/2013/10-09/WSBOH-10-09-13-Tab09d.pdf. [Google Scholar]
- 27.Washington State Legislature [June 24, 2015];Revised Code of Washington (RCW) 34.05.328 Section (1)(b) http://apps.leg.wa.gov/rcw/default.aspx?cite=34.05&full=true.
- 28.Kwan A, Church JA, Cowan MJ, Agarwal R, Kapoor N, Kohn DB, et al. Newborn screening for severe combined immunodeficiency and T-cell lymphopenia in California: results of the first 2 years. J Allergy Clin Immunol. 2013;132:140–50. doi: 10.1016/j.jaci.2013.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGhee SA, Stiehm ER, McCabe ER. Potential costs and benefits of newborn screening for severe combined immunodeficiency. J Pediatr. 2005;147:603–8. doi: 10.1016/j.jpeds.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 30.Modell V, Knaus M, Modell F. An analysis and decision tool to measure cost benefit of newborn screening for severe combined immunodeficiency (SCID) and related T-cell lymphopenia. Immunol Res. 2014;60:145–52. doi: 10.1007/s12026-014-8485-4. [DOI] [PubMed] [Google Scholar]
- 31.Kubiak C, Jyonouchi S, Kuo C, Garcia-Lloret M, Dorsey MJ, Sleasman J, et al. Fiscal implications of newborn screening in the diagnosis of severe combined immunodeficiency. J Allergy Clin Immunol Pract. 2014;2:697–702. doi: 10.1016/j.jaip.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chan K. A global economic evaluation simulation model of cost-savings In newborn screening for severe combined immunodeficiency.. 9th International Society for Neonatal Screening European Regional Meeting 2014.; Birmingham, United Kingdom. 2014. [Google Scholar]
- 33.Robinson LA, Hammitt JK. Skills of the trade: valuing health risk reductions in benefit-cost analysis. J Benefit Cost Anal. 2013;4:107–30. [Google Scholar]
- 34.Viscusi WK. The role of publication selection bias in estimates of the value of a statistical life. Am J Health Econ. 2015;1:27–52. [Google Scholar]
- 35.Washington State Department of Health [December 7, 2014];Vital Statistics Data for Birth Tables in 2013. http://www.doh.wa.gov/DataandStatisticalReports/VitalStatisticsData/BirthData/BirthTablesbyYear.
- 36.Dvorak CC, Hassan A, Slatter MA, Honig M, Lankester AC, Buckley RH, et al. Comparison of outcomes of hematopoietic stem cell transplantation without chemotherapy conditioning by using matched sibling and unrelated donors for treatment of severe combined immunodeficiency. J Allergy Clin Immunol. 2014;134:935–43. e15. doi: 10.1016/j.jaci.2014.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bureau of Economic Analysis [June 24, 2015];Table 2.4.4. Price Indexes for Personal Consumption Expenditures by Type of Product. 2014 http://www.bea.gov/iTable/iTable.cfm?reqid=9%26step=3%26isuri=1%26903=69#reqid=9%26step=3%26isuri=1%26903=69.
- 38.Haddix AC, Teutsch SM, Corso PS. Prevention effectiveness: A guide to decision analysis and economic evaluation. 2nd ed. Oxford University Press; New York: 2003. [Google Scholar]
- 39.Arias E. United States life tables, 2009. Natl Vital Stat Rep. 2014;62:1–63. [PubMed] [Google Scholar]
- 40.Grosse SD. Assessing cost-effectiveness in healthcare: history of the $50 000 per QALY threshold. Expert Rev Pharmacoecon Outcomes Res. 2008;8:165–78. doi: 10.1586/14737167.8.2.165. [DOI] [PubMed] [Google Scholar]
- 41.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness— the curious resilience of the $50 000-per-QALY threshold. N Engl J Med. 2014;371:796–7. doi: 10.1056/NEJMp1405158. [DOI] [PubMed] [Google Scholar]
- 42.Chapman RH, Berger M, Weinstein MC, Weeks JC, Goldie S, Neumann PJ. When does quality-adjusting life-years matter in cost-effectiveness analysis? Health Econ. 2004;13:429–36. doi: 10.1002/hec.853. [DOI] [PubMed] [Google Scholar]
- 43.Robinson LA, Hammitt JK. Valuing reductions in fatal illness risks: implications of recent research. Health Econ. 2015 Jun 30; doi: 10.1002/hec.3214. http://dx.doi.org/10.1002/hec.3214. [Epub ahead of print] [DOI] [PubMed]
- 44.Carroll AE, Downs SM. Comprehensive cost-utility analysis of newborn screening strategies. Pediatrics. 2006;117:S287–95. doi: 10.1542/peds.2005-2633H. [DOI] [PubMed] [Google Scholar]
- 45.Norman R, Haas M, Wilcken B. International perspectives on the cost-effectiveness of tandem mass spectrometry for rare metabolic conditions. Health Policy. 2009;89:252–60. doi: 10.1016/j.healthpol.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 46.Grosse SD. Cost effectiveness as a criterion for newborn screening policy decisions. In: Baily MA, Murray TH, editors. Ethics and newborn genetic screening: new technologies, new challenges. Johns Hopkins University Press; Baltimore: 2009. pp. 58–88. [Google Scholar]
- 47.Grosse SD. Economic evaluations of newborn screening interventions. In: Ungar WJ, editor. Economic evaluation in child health. Oxford University Press; New York: 2009. pp. 113–32. [Google Scholar]
- 48.Grosse SD, Prosser LA, Asakawa K, Feeny D. QALY weights for neuro-sensory impairments in pediatric economic evaluations: case studies and a critique. Expert Rev Pharmacoecon Outcomes Res. 2010;10:293–308. doi: 10.1586/erp.10.24. [DOI] [PubMed] [Google Scholar]
- 49.Lindegren ML, Kobrynski L, Rasmussen SA, Moore CA, Grosse SD, Vanderford ML, et al. Applying public health strategies to primary immunodeficiency diseases: a potential approach to genetic disorders. MMWR Recomm Rep. 2004;53:1–29. [PubMed] [Google Scholar]
- 50.Baker MW, Laessig RH, Katcher ML, Routes JM, Grossman WJ, Verbsky J, et al. Implementing routine testing for severe combined immunodeficiency within Wisconsin's newborn screening program. Public Health Rep. 2010;125(Suppl 2):88–95. doi: 10.1177/00333549101250S211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clement MC, Mahlaoui N, Mignot C, Le Bihan C, Rabetrano H, Hoang L, et al. Systematic neonatal screening for severe combined immunodeficiency and severe T-cell lymphopenia: analysis of cost-effectiveness based on French real field data. J Allergy Clin Immunol. 2015;316:1589–93. doi: 10.1016/j.jaci.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 52.Wright SJ, Jones C, Payne K, Dharni N, Ulph F. The role of information provision in economic evaluations of newborn bloodspot screening: a systematic review. Appl Health Econ Health Policy. 2015;13:615–26. doi: 10.1007/s40258-015-0177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Werther RL, Crawford NW, Boniface K, Kirkwood CD, Smart JM. Rota-virus vaccine induced diarrhea in a child with severe combined immune deficiency. J Allergy Clin Immunol. 2009;124:600. doi: 10.1016/j.jaci.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 54.Cortese MM, Parashar UD. Prevention of rotavirus gastroenteritis among infants and children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2009;58:1–25. [PubMed] [Google Scholar]
- 55.Kuehn BM. State, federal efforts under way to identify children with “bubble boy syndrome.”. JAMA. 2010;304:1771–3. doi: 10.1001/jama.2010.1485. [DOI] [PubMed] [Google Scholar]
- 56.Agency for Healthcare Research and Quality [December 7, 2014];National estimates on use of hospitals by children from the HCUP Kids’ Inpatient Database (KID) 2012 http://hcupnet.ahrq.gov/.
- 57.Prosser LA, Kong CY, Rusinak D, Waisbren SL. Projected costs, risks, and benefits of expanded newborn screening for MCADD. Pediatrics. 2010;125:e286–94. doi: 10.1542/peds.2009-0605. [DOI] [PubMed] [Google Scholar]
- 58.Sullivan SD, Mauskopf JA, Augustovski F, Caro J, Lee KM, Minchin M, et al. Budget impact analysis-principles of good practice: report of the ISPOR 2012 Budget Impact Analysis Good Practice II Task Force. Value Health. 2014;17:5–14. doi: 10.1016/j.jval.2013.08.2291. [DOI] [PubMed] [Google Scholar]
- 59.Grosse SD, Thompson JD, Ding Y, Glass M. The use of economic evaluation to inform newborn screening policy decisions: the Washington State experience. Milbank Q. doi: 10.1111/1468-0009.12196. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]