Abstract
Purpose
The effect of capacity strain in an ICU on the timing of end-of-life decision making is unknown. We sought to determine how changes in strain impact timing of new DNR orders and of death.
Methods
Retrospective cohort study of 9,891 patients dying in the hospital following an ICU stay ≥ 72 hours in Project IMPACT, 2001–2008. We examined the effect of ICU capacity strain (measured by standardized census, proportion of new admissions, and average patient acuity) on time to initiation of DNR orders and time to death for all ICU decedents using fixed-effects linear regression.
Results
Increases in strain were associated with shorter time to DNR for patients with limitations in therapy (predicted time to DNR 6.11 days for highest versus 7.70 days for lowest quintile of acuity, p=0.02; 6.50 days for highest versus 7.77 days for lowest quintile of admissions, p<0.001), and shorter time to death (predicted time to death 7.64 days for highest versus 9.05 days for lowest quintile of admissions, p<0.001; 8.28 days for highest versus 9.06 days for lowest quintile of census, only in closed ICUs, p=0.006). Time to DNR order significantly mediated relationships between acuity and admissions and time to death, explaining the entire effect of acuity, and 65% of the effect of admissions. There was no association between strain and time to death for decedents without a limitation in therapy.
Conclusions
Strains in ICU capacity are associated with end-of-life decision making, with shorter times to placement of DNR orders and death for patients admitted during high-strain days.
Keywords: end-of-life care, palliative care, decision-making, critical care
Ideally, end-of-life care decision making in the intensive care unit (ICU) is governed by knowledge of how patients’ values and goals can best be promoted given the patient’s current clinical circumstances. However, growing evidence suggests that other factors, such as providers’ perceptions of prognosis or institution-specific practices regarding intensity of treatment may also play a role [1–5]. One such factor that is increasingly being studied is how the dynamic nature of the ICU may influence patient outcomes. Prior studies examining the effect of ICU strain [6] (a measure of how “busy” an ICU is) have demonstrated associations between strain and ICU length-of-stay [7] and mortality [8], suggesting that fluctuations in the surrounding ICU environment can affect clinical care. However, it is uncertain whether increases in ICU strain impact end-of-life decision-making.
Conceivably, when ICUs are strained, clinicians may have insufficient time or cognitive reserve to have in-depth discussions with families and other physicians regarding end-of-life decisions [9]. Decision-making related to the use of life-sustaining therapy is time-consuming, and competing demands on clinicians’ time has been cited as a barrier to end-of-life care delivery [10]. In one study of pediatric ICUs in France, clinicians spent a median of 11 hours on decision-making [11], and this process may be further lengthened by disagreements between members of the care team [12]. These phenomena could delay appropriate transitions in the goals of care [10], and these effects may be more marked in ICUs with closed physician staffing models, which have been shown to be less flexible to changes in strain, perhaps because the strain is borne by fewer physicians [8].
Alternatively, in settings of increased strain, physicians may have conversations regarding end-of-life care more expediently, consistent with the view that scarcity (of time, beds, or other resources) may encourage physicians to perform more efficiently [13]. Indeed, higher ICU strain has been associated with earlier ICU discharges without a concomitant increase in hospital mortality [7, 14]. Given these conflicting hypotheses, we sought to elucidate the relationships between ICU strain and end-of-life decision making for critically ill patients. Specifically, we explored the effects of strain on time to death for patients with and without limitations in life-sustaining therapy, and determined whether effects differed among ICUs employing different physician staffing models. Some of the results of this study were presented at the 2015 American Thoracic Society International Conference [15].
Methods
Patients and data collection
Data for this study came from Project IMPACT (Cerner Corporation, Kansas City, MO), a database of U.S. ICU admissions for the years 2001–2008. Further details about Project IMPACT have been published previously [16]. We included patients with a first admission to the ICU (readmission episodes were excluded) for a period of 72 hours or greater. We chose to limit the analysis to patients with ICU length of stay of at least 72 hours, as we wanted to allow for a plausible time period for decision-making, and any effect of strain on decision-making, to occur. In primary analyses, we included only patients dying in the ICU. In a secondary analysis of time to ICU discharge, we evaluated patients who survived an initial ICU stay but died during the hospitalization.
We excluded patients who were not eligible for severity of illness assessment using the MPM0III score, a scoring system that combines physiologic variables, acute and chronic diagnoses, and a variety of patient factors. This score was developed and validated within the Project IMPACT database and has similar performance to the APACHE score [17, 18]. To maintain a check on data quality, we excluded admissions from ICUs that contributed data to Project IMPACT for less than 1 year or less than 20 patients per quarter of a year in order. We also excluded patients admitted to the ICU with any limitation in therapy (including DNR orders, withholding or withdrawal of life-sustaining therapy, comfort care) already in place, as end-of-life decision making for these patients largely occurred prior to ICU admission, and time to death for these patients may not be comparable to other decedents (Figure 1 in the electronic supplementary material (ESM)). Patients discharged to hospice were not included in the analysis, as we did not have information about when these patients ultimately died. The study protocol was reviewed and approved by the institutional review board of Columbia University Medical Center (IRB-AAAJ1051 New York, NY). Written informed consent was waived.
ICU strain and end-of-life decision making
The primary exposure variables were 3 measures of ICU capacity strain that derive from a conceptual model [6] have been validated based on associations with ICU clinicians’ contemporary perceptions of strain [19]. and have been used in prior studies [7–9]. These 3 measures included ICU census, the proportion of new admissions, and the acuity of other patients in the ICU [8]. To allow for comparisons among ICUs of differing sizes, we standardized the census by taking the daily census (including all patients in the ICU for at least 2 hours), subtracting the yearly mean daily census, and dividing by the yearly standard deviation. New ICU admissions were calculated as the proportion of the day’s census that comprised new admissions that day. ICU acuity was calculated using the average predicted probability of death (using the MPM0III score) of all patients in the ICU, excluding the index patient. We chose to enter each metric separately instead of forming a combined measure of strain, as this would allow us to assess for the effect of each metric individually, while controlling for the other two. We chose to use the average of each strain metric over the first three days of the ICU stay because ICU strain may affect patient outcomes over a period of time beyond the day of admission [8].
To assess the effect of strain on end-of-life decision making, we examined the associations of strain with several key outcomes. First, we explored time to DNR among decedents who had any new limitation in life-sustaining therapy, defined as having had either a DNR order placed, or a withholding or withdrawal of life-sustaining therapy prior to death. Patients who died, but did not have a code for cardiopulmonary resuscitation (CPR) on the day of death or the day prior, were also counted as having had a limitation placed. This coding approach in the Project IMPACT data has been used to study withdrawal of life-sustaining therapy [4], and results in estimates of the percentage of ICU patients dying with a limitation in life-sustaining therapy that are similar to those previously published [20, 21]. For patients who did not have CPR prior to death but were missing a time-date stamp for the DNR order (n=3,528), we instead used the time and date of death to calculate the time to DNR, with the assumption that DNR was issued prior to death occurring. Time to placement of other limitations in life-sustaining therapy (e.g. withholding therapy, comfort care) were not available in the database. Second, we evaluated time to death among patients who died in the ICU with a limitation in life-sustaining therapy in place. Lastly, we evaluated time to ICU discharge among patients who had a limitation in life-sustaining therapy placed in the ICU, and subsequently died in the hospital.
We then performed a series of secondary analyses to help distinguish whether strain influences time to death by changing end-of-life decision making or through some other mechanism (e.g., lower-quality care). First, we examined the effect of ICU strain on time to death for patients who died without limitations on life-sustaining therapy, reasoning that time to death for these patients would not vary as a function of strain if decision-making causally explained the association with time to death. Second, we examined time to death for patients at lowest risk of death (defined as having an MPM0-III score in the lowest quartile of scores), reasoning that among such patients, an association between strain and time to death may be more likely to be due to strain-induced deficiencies in the quality of care because these patients would not be expected to die and hence would require fewer discussions of end-of-life care. Third, we performed a mediation analysis (described fully below) to determine whether the effect of strain on time to death was mediated by time to placement of a DNR order [22–24]. Mediation analysis is a statistical technique used to test a hypothesized causal pathway between an independent (here, strain), a mediating variable (time to DNR) and dependent variable (time to death). Significant mediation effects would suggest that time to DNR plays a causal role between strain and time to death.
Statistical analysis
We evaluated the effect of strain on time to death and time to DNR using linear regression with ICU-year modeled as a fixed effect to adjust for clustering of patients within ICUs and the possibility of difference in care over time. Covariates forced into the multivariable models were demographic variables (age, gender, race, type of insurance), clinical characteristics to adjust for severity of illness (type of patient - surgical, non-surgical, functional status on admission, MPM0III score, use of mechanical ventilation and vasopressors) and admission characteristics (weekend admission, location prior to admission). A priori, we chose to investigate interaction terms between census and unit acuity, census and staffing model, and number of new admissions and staffing model, given that these relationships were found to be significant in prior analyses relating strain to mortality [8].
In conducting the mediation analysis, we included only those patients who had a complete date-time stamp for a DNR order. We excluded from these analyses those patients for whom the time of DNR was coded as the time of death due to the absence of CPR provision because including such patients would bias the results toward showing significant mediation. We calculated the direct, indirect and total effect of ICU strain on time to death, where the direct effect represents the effect of ICU strain on time to death that is not mediated through time to DNR, the indirect effect represents the effect of ICU strain on time to death that is mediated, and the total effect is the sum of the direct and indirect effects (Figure 1). To conclude that mediation was present, we examined both the proportion of the total effect that was indirect, as well as bootstrapped confidence intervals around the estimate of the indirect effect [22]. Further details of regression modeling and mediation analysis are available in the ESM. Database management and statistical analysis were performed using Stata 13.1 (StataCorp LP, College Station, Tex).
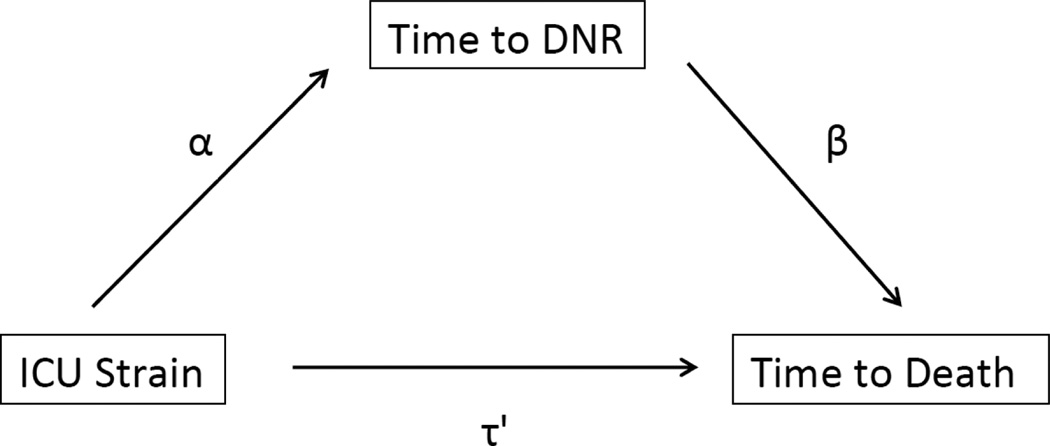
Fig. 1. Conceptual model of mediation analysis.
ICU strain affects time to death directly (τ’), as well as through an indirect path that is mediated by time to DNR. The direct effect of ICU strain on time to death is equal to the coefficient τ’, while the indirect effect of ICU strain on time to death is equal to αβ. The presence of a significant indirect effect is suggestive of mediation, or that time to DNR plays a causal role in the effect of ICU strain on time to death
Results
Characteristics of decedents and ICUs
Of 400,129 admissions during the study period, 9,891 patients died in the ICU after a stay of 72 hours or greater. These decedents had a high rate of mechanical ventilation (91.3%), vasopressor use (73.1%), and had a median predicted probability of death on ICU admission (using MPM0III) was 20.5% (interquartile range (IQR) 10.7%–38.8%). A vast majority had a DNR order in place (91.3%) prior to death, and 56.8% had other life-sustaining therapies withheld or withdrawn (Table 1). Decedents with a limitation in life-sustaining therapy were more likely to be Caucasian (79.7% vs. 62.9%), had higher predicted probability of death (21.3% vs. 14.3%) and were less likely to have received vasopressors (72.2% vs. 82.7%) compared with decedents without any limitation (Table 1). Most ICUs employed an open staffing model (149 out of 161 ICUs, 92.6%), did not have nighttime intensivist staffing (122 of 161 ICUs, 75.8%), were in community, non-profit hospitals (104 of 161 ICUs, 64.6%), and were mixed medical-surgical units (84 of 161 ICUs, 53.5%) (ESM, Table 1).
Table 1.
Characteristics of Patients who Died During an ICU Admission
| All Decedentsa n=9,891 |
With Any Limitation in Life-Sustaining Therapy n=9,066 |
Without Any Limitation in Life-Sustaining Therapy n=825 |
|
|---|---|---|---|
| Age, n (mean, SD) | 9,891 (64.3, 16.3) | 9,066 (64.7, 16.2) | 825 (59.8, 17.0) |
| Female, n (%) | 4,461 (45.1) | 4,147 (45.7) | 314 (38.1) |
| Race, n (%)* | |||
| Caucasian | 7,747 (78.3) | 7,228 (79.7) | 519 (62.9) |
| Black | 1,345 (13.6) | 1,113 (12.3) | 232 (28.1) |
| Other | 799 (8.1) | 725 (8.0) | 74 (9.0) |
| Type of admission, n (%) Surgical | 2,362 (23.9) | 2,128 (23.5) | 234 (28.4) |
| Mechanical ventilation, n (%)* | 9,032 (91.3) | 8,267 (91.2) | 765 (92.7) |
| Vasopressor use, n (%)* | 7,225 (73.1) | 6,543 (72.2) | 682 (82.7) |
| Predicted probability of death (MPM0III score), n (median, IQR)* | 9,891 (0.21, 0.11–0.39) | 9,066 (0.21, 0.11–0.40) | 825 (0.14, 0.08–0.29) |
| ICU length of stay (median, IQR)* | 9,891 (7, 5–12) | 9,066 (7, 5–12) | 825 (7, 4–12) |
| Insurance, n (%) | |||
| Private | 2,430 (24.6) | 2,206 (24.3) | 224 (27.2) |
| Medicare | 5,389 (54.5) | 4,984 (55.0) | 405 (49.1) |
| Medicaid | 769 (7.8) | 690 (7.6) | 79 (9.6) |
| Self-Pay | 686 (6.9) | 615 (6.8) | 71 (8.6) |
| Government/Other | 542 (5.5) | 506 (5.6) | 36 (4.4) |
| Source of admission, n (%) | |||
| Emergency room | 3,616 (36.6) | 3,317 (36.6) | 299 (36.2) |
| General care | 1,936 (19.6) | 1,810 (20.0) | 126 (15.3) |
| Step-down unit | 1,091 (11.0) | 993 (11.0) | 98 (11.9) |
| Procedure | 2,003 (20.3) | 1,802 (19.9) | 201 (24.4) |
| Another ICU | 541 (5.5) | 500 (5.5) | 41 (5.0) |
| Other | 702 (7.1) | 642 (7.1) | 60 (7.3) |
| Weekend admission, n (%) | 3,394 (34.3) | 3,125 (34.5) | 269 (32.6) |
| Closed ICU, n (%)* | 1,543 (15.6) | 1,444 (15.9) | 99 (12.0) |
| Nighttime intensivist staffing, n (%) | 3,456 (33.5) | 3,061 (33.8) | 283 (34.3) |
| Do-Not-Resuscitate prior to death, n (%) | 9,033 (91.3) | - | - |
| Withholding or withdrawal of life sustaining therapy prior to death, n (%) | 5,614 (56.8) | - | - |
ICU, intensive care unit; SD, standard deviation; IQR, interquartile range.
Totals may vary due to missing data.
p<0.001 for comparisons between decedents with and without changes in orders for life-sustaining therapy.
P=0.004 is used for statistical significance after performing a Bonferroni correction for multiple comparisons.
Effect of strain on time to DNR
For patients who died with a limitation in life-sustaining therapy, we analyzed the effect of ICU strain by including all three strain metrics into a fully adjusted model with ICU-year modeled as a fixed effect (ESM, Table 2). We evaluated interaction terms by including each interaction term separately. Only the interaction between census and staffing model was significant, such that increases in census were associated with shorter time to DNR only in closed ICUs (p-value for interaction=0.04) (ESM, Table 3). In the final model (including the interaction term between census and staffing model), effects for both admissions and acuity were significant, with increases in strain being associated with a decrease in time to DNR (ESM, Table 4).
When the final model was used to generate predicted outcomes, patients in the highest quintile of admissions or unit acuity were predicted to have a DNR order placed more than a day earlier than patients in the lowest quintile (6.50 days, 95% CI 6.42–6.57 for highest quintile of admissions versus 7.77 days, 95% CI 7.69–7.85 days for lowest; 6.11 days, 95% CI 6.04–6.18 days for highest quintile of acuity versus 7.70 days, 95% CI 7.62–7.77 days for lowest) (Table 2).
Table 2.
Mean Predicted Outcomes by Quintile of ICU Straina
| Quintile of ICU strain |
Time to DNR (days) (95% CI) |
Time to Death for Patients with Any Limitation in Life-Sustaining Therapy (days) (95% CI) |
|
|---|---|---|---|
| Census - closed unit | |||
| Lowest | 7.44 (7.23 – 7.64) | 9.06 (8.86 – 9.27) | |
| 2nd | 7.13 (6.96 – 7.30) | 8.61 (8.44 – 8.78) | |
| 3rd | 7.17 (6.98 – 7.35) | 8.60 (8.41 – 8.78) | |
| 4th | 7.14 (6.94 – 7.34) | 8.46 (8.27 – 8.65) | |
| Highest | 7.06 (6.90 – 7.21) | 8.28 (8.12 – 8.43) | |
| Census - open unit | |||
| Lowest | 6.94 (6.85 – 7.02) | 8.12 (8.05 – 8.21) | |
| 2nd | 7.10 (7.02 – 7.19) | 8.28 (8.20 – 8.36) | |
| 3rd | 7.08 (6.99 – 7.16) | 8.26 (8.18 – 8.34) | |
| 4th | 7.16 (7.08 – 7.24) | 8.35 (8.27 – 8.43) | |
| Highest | 7.29 (7.21 – 7.37) | 8.46 (8.39 – 8.54) | |
| Admissions per day | |||
| Lowest | 7.77 (7.69 – 7.85) | 9.05 (8.97 – 9.12) | |
| 2nd | 7.38 (7.31 – 7.46) | 8.64 (8.57 – 8.71) | |
| 3rd | 7.08 (7.01 – 7.15) | 8.31 (8.24 – 8.38) | |
| 4th | 6.87 (6.80 – 6.94) | 8.07 (8.00 – 8.14) | |
| Highest | 6.50 (6.42 – 6.57) | 7.64 (7.57 – 7.71) | |
| Unit Acuity | Lowest | 7.70 (7.62 – 7.77) | 8.77 (8.69 – 8.84) |
| 2nd | 7.47 (7.40 – 7.54) | 8.66 (8.59 – 8.73) | |
| 3rd | 7.33 (7.26 – 7.40) | 8.56 (8.49 – 8.62) | |
| 4th | 7.01 (6.95 – 7.08) | 8.30 (8.23 – 8.36) | |
| Highest | 6.11 (6.04 – 6.18) | 7.45 (7.37 – 7.52) |
ICU, intensive care unit; CI, confidence interval.
Estimates in bold are associated with statistically significant coefficients in the final models.
Effect of strain on time to death
In the fixed-effects model, the proportion of new admissions was inversely associated with time to death (p=0.02) (ESM, Table 2). Again, there was a significant interaction between census and staffing model (p-value for interaction=0.003) (ESM, Table 3), such that increases in census were associated with a decreased time to death only for patients in closed ICUs (a 0.05 decrease in log(time to death) for every unit increase in standardized census, β = −0.05, 95% CI −0.08 to −0.01, p=0.006) (ESM, Table 4) (ESM, Figure 2). Patients in the highest quintile of admissions were estimated to die more than one day earlier than patients in the lowest quintile of admissions (7.64 days, 95% CI 7.57–7.71 days for highest quintile versus 9.05 days, 95% CI 8.97–9.12 days for lowest) (Table 2). Similarly, patients cared for in closed ICUs in the highest quintile of census were predicted to die an average of half a day earlier than patients in closed ICUs in the lowest quintile (8.28 days, 95% CI 8.12–8.43 days for highest quintile versus 9.06 days, 95% CI 8.86–9.27 days for lowest) (Table 2).
Effect of strain on time to ICU discharge
To further elucidate the effect of strain on potential end-of-life decision making, we analyzed the effect of strain on time to ICU discharge among a separate cohort of patients who had limitations in life-sustaining therapy placed during an ICU stay, were subsequently discharged to the floor, and died during their hospitalization (n=5,011). For this cohort, 57.8% had a DNR order placed in the ICU, and 42.2% had life-sustaining therapy withheld or withdrawn. The median time between time to DNR and ICU discharge was 24 hours (IQR 0–48 hours); the median time between time to ICU discharge and death was 2 hours (IQR 1–4 hours). In the fixed-effect model, both census (β = −0.04, 95% CI −0.08 to −0.01, p=0.008) and admissions (β = −0.99, 95% CI −1.39 to −0.59, p<0.001) showed inverse associations with time to ICU discharge. An interaction between the proportion of new admissions and staffing model was significant, such that higher numbers of new admissions were associated with larger reductions in time to ICU discharge in closed than in open ICUs (p=0.03). Similar effects were observed in the final multivariable model (ESM, Table 5). Predicted times to discharge from this model were half a day earlier among patients in the highest versus the lowest quintile of census (4.30 days, 95% CI 4.17–4.43 days for highest quintile versus 4.79 days, 95% CI 4.65–4.94 days for lowest). Similarly, patients in the highest quintile of admissions were predicted to be discharged more than a day earlier than those in the lowest quintile in open ICUs (3.83 days, 95% CI 3.71–3.95 days for highest versus 5.25 days for lowest, 95% CI 5.09–5.42 days), and more than two and a half days earlier in closed ICUs (2.87 days, 95% CI 2.36–3.38 days for highest versus 5.68 days, 95% CI 5.38–5.98 days for lowest).
Analysis of decedents without limitations in life-sustaining therapy and decedents with lower severity of illness
For patients who died without any limitations in life-sustaining therapy (n=825), there was no significant effect of any metric of ICU strain on time to death (ESM, Table 6). Similarly, there was no significant effect of any metric of ICU strain on time to death for decedents admitted with lower severity of illness (n=2,486) (ESM, Table 6). None of the pre-specified interactions tested were significant (ESM, Table 7).
Mediation Analysis
Of all decedents who had a DNR order placed, 5,711 (57.7%) patients had complete date and time information for when the order was placed. The median time between DNR placement and death was 18 hours (IQR 3–55 hours). When examining mediation effects for ICU census, we only included patients cared for in closed ICUs (n=1,080), as the effect of census on time to death and time to DNR was not significant in open ICUs. For ICU census, there was no evidence of mediation, with only the direct effect of census being statistically significant (p=0.05). For the proportion of new admissions, direct, indirect and total effects were significant (p≤0.001 for all effects), showing an inverse relationship between admissions and time to death. For ICU acuity, indirect and total effects were both significant (p<0.001), and demonstrated an inverse relationship between an increase in acuity and time to death; the direct effect showed a positive relationship, but was not statistically significant (p=0.88). For both admissions and acuity, the majority of the effect of ICU strain on time to death was mediated by time to DNR (proportion of total effect mediated 65.8% for admissions, 101% for acuity) (Table 3).
Table 3.
Results of Mediation Analysis, with Time to DNR as a Mediator between ICU Strain and Time to Death*
| Direct effect (τ’) (95% CI) |
p- value |
Indirect effect (αβ) (95% CI) |
p- value |
Total effect τ’ + αβ) (95% CI) |
p- value |
|
|---|---|---|---|---|---|---|
| Census - closed | −0.027 (−0.053 to 0.002) | 0.05 | −0.005 (−0.037 to 0.023) | 0.76 | −0.032 (−0.070 to 0.005) | 0.11 |
| Admissions | −0.238 (−0.376 to −0.098) | 0.001 | −0.457 (−0.666 to −0.236) | <0.001 | −0.695 (−0.946 to −0.454) | <0.001 |
| Acuity | 0.006 (−0.061 to 0.086) | 0.88 | −0.538 (−0.645 to −0.426) | <0.001 | −0.532 (−0.662 to −0.375) | <0.001 |
CI, confidence interval.
N=5,711, excluding patients without complete date-time stamp on time of DNR order placement.
Discussion
Among a national sample of ICU patients, increases in several measures of ICU capacity strain during the first 3 days of ICU admission were associated with shorter time to DNR and death among patients who had limitations in life-sustaining therapy and who died in the ICU, as well as a shorter time to ICU discharge among patients who survived an initial ICU stay but died later in the same hospitalization. These associations were not statistically significant across all metrics of strain, but in no case was strain associated with longer times to death, DNR, or ICU discharge. Furthermore, in a mediation analysis, we found that the majority of the effect that ICU strain exhibited on time to death was mediated by time to placement of a DNR order, suggesting a causal relationship whereby strain hastens changes in orders to limit life-sustaining therapy, resulting in shorter time to death. Recent studies have shown that use of intensive communication strategies are associated with decreased time to implementation of decisions to withhold and withdraw life-sustaining therapy [25, 26]. Our findings provide further evidence that time to decision-making in the ICU may be modifiable, and may represent an additional factor that influences how decisions about life-sustaining therapy are made.
One potential explanation for our overall finding is that increases in strain heighten clinicians’ awareness of resource limitations, making them perform more “efficiently” in situations where patients no longer benefit from ongoing ICU care. This more efficient care could result in expedited end-of-life decision making and faster time to death among patients who will die regardless. Such observations parallel other recent studies showing that physician behaviors related to end-of-life decision-making may be influenced by resource availability and the surrounding environment. In a simulation study, ICU bed availability was strongly associated with physicians’ likelihood of initiating life-sustaining therapy for older adult patients [27]. Correspondingly, in a study of hospitalized adults assessed by a medical emergency team on the ward, patients were much more likely to have their goals of care changed when there were no ICU beds available in comparison to patients seen when two or more beds were available [28]. Furthermore, mortality did not vary with ICU bed availability, suggesting that physicians were more expedient at recognizing potentially futile situations and facilitating end-of-life decision making. How physicians approach the decision-making process also may be malleable. A simulation study found that physicians more often played a “directive” role in a time-pressured scenario, making treatment decisions independently of the patient and family [29], and differences in institutional culture have been shown to influence trainees’ willingness to make recommendations regarding placement of a DNR order [30]. Thus, our study adds evidence to strengthen the assertion that physician behaviors surrounding end-of-life decision-making for critically ill patients may be context dependent.
Another possible explanation is that increases in strain negatively impact care that is delivered, resulting in clinical deterioration (perhaps prompting faster decision-making) and a faster time to death. If the decrease in time to death was mediated by clinical deterioration, we would expect this effect to be observed among decedents with and without orders to limit life-sustaining therapy. Yet, we found no association between strain and time to death for the decedents without limitations. Similarly, for patients admitted with lower severity of illness, any decrease in time to death associated with increased strain may more likely represent deterioration due to lower quality care, and we again found no relationship between strain and time to death for these patients. Although we did not observe an effect in these subgroups, sample sizes for these analyses were smaller, increasing the possibility of type II error.
We also found that increases in strain were more pronounced for patients cared for in ICUs employing a closed staffing model. In a closed staffing system, comprised of a primary team that bears the majority of responsibility for all aspects of patients care, there may be less ability to absorb increases in strain without impacting care due to the limited personnel on the team, or a greater awareness by the team of the unit as a system and demand for ICU beds. Also, physicians working in open units may be directly responsible for patients on the floor and may be able to better anticipate need and control triage to mitigate effects of strain. However, we did observe a non-significant trend where increased strain was associated with increased time to DNR and death. If true, this effect may be secondary to difficulty coordinating among different care providers to facilitate decision-making in times of increased strain.
Our study has limitations, and thus should be interpreted cautiously. At the outset of this study we set forth to test competing hypotheses examining the impact of ICU strain on time to death. There may indeed be instances in which strain increases time to decision-making; however, we are unable to distinguish whether this effect is absent or is merely dwarfed by the opposite effect of strain decreasing time to death for the majority of individuals. Because of the retrospective nature of the study, we do not have information regarding whether conversations regarding decision-making actually occurred, time to these discussions occurring, or time to placement of orders to limit life-sustaining therapy other than DNR orders. We are also unable to assess whether any effect of strain on the timing of limitations on life-sustaining therapy influences patient-centered outcomes, such as family satisfaction or the quality of death. Moreover, given the age of the data, it is possible that the effect of ICU strain on clinicians’ behavior and practice patterns has changed over time; however, the more contemporary dataset (APACHE Outcomes) does not contain the necessary data to be able to examine these associations. Lastly, there is the possibility that our results represent a type I error.
Overall, we found evidence that increases in ICU strain are associated with a decrease in time to death for patients who have limitations in life-sustaining therapy, which may be mediated by physicians behaving more efficiently, expediting discussions regarding limitations in therapy in the face of decreased ICU resources. Our study adds to a growing body of evidence demonstrating ways in which physicians’ decision-making for critically ill patients are affected by factors unrelated to patients, families and their specific preferences. Physicians should be aware of outside influences on decision-making, as better understanding of such effects may eventually lead to improved judgement and decision-making. This suggests great need for future research aimed at elucidating the mechanisms by which these non-patient-centered factors influence communication and other aspects of care.
Supplementary Material
Take-home message.
Increases in ICU strain are associated with shorter time to placement of treatment limitations and shorter time to death for patient dying in the ICU. These data suggest that physicians are capable of performing more efficiently and expediting the delivery of end-of-life care for critically ill patients.
Tweet: When ICUs are busier, physicians may perform more efficiently and expedite end-of-life care for patients who die.
Acknowledgments
May S. Hua, MD, MSc
Support: Dr. Hua is supported by a Mentored-Training Research Grant from the Foundation in Anesthesia Education and Research.
Scott D. Halpern, MD, PhD
Support: Dr. Halpern is supported by a grant from The Otto Haas Charitable Trust.
Nicole B. Gabler, PhD, MPH, MHA
Support: None.
Hannah Wunsch, MD, MSc
Support: Dr. Wunsch is supported by Award Number K08AG038477 from the National Institute On Aging.
Footnotes
May S. Hua, MD, MSc, This author helped conceive and design the study, conduct the study, analyze and interpret the data, draft and critically revise the manuscript. This author had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Scott D. Halpern, MD, PhD, This author helped design and conduct the study, analyze and interpret the data and critically revise the manuscript.
Nicole B. Gabler, PhD, MPH, MHA, This author helped conduct the study, analyze and interpret the data and critically revise the manuscript.
Hannah Wunsch, MD, MSc, This author helped conceive and design the study, conduct the study, analyze and interpret the data, draft and critically revise the manuscript.
May Hua reported no conflicts of interest.
Scott Halpern reported no conflicts of interest.
Nicole Gabler reported no conflicts of interest.
Hannah Wunsch reported no conflicts of interest.
Contributor Information
May Hua, Department of Anesthesiology, Columbia University College of Physicians and Surgeons.
Scott D. Halpern, Division of Pulmonary, Allergy and Critical Care Medicine, Perelman School of Medicine at the University of Pennsylvania; Leonard Davis Institute of Health Economics, University of Pennsylvania; Center for Clinical Epidemiology and Biostatistics, Perelman School of Medicine at the University of Pennsylvania; Leonard Davis Institute of Health Economics, University of Pennsylvania; Fostering Improvement in End-of-Life Decision Science (FIELDS) Program, University of Pennsylvania; Department of Medical Ethics and Health Policy, Perelman School of Medicine at the University of Pennsylvania.
Nicole B. Gabler, Fostering Improvement in End-of-Life Decision Science (FIELDS) Program, University of Pennsylvania; Center for Clinical Epidemiology and Biostatistics, Perelman School of Medicine at the University of Pennsylvania.
Hannah Wunsch, Department of Critical Care Medicine, Sunnybrook Health Sciences Center; Department of Anesthesia and Interdisciplinary Department of Critical Care Medicine, University of Toronto; Department of Anesthesiology, Columbia University College of Physicians and Surgeons.
References
- 1.Garland A, Connors AF. Physicians' influence over decisions to forego life support. J Palliat Med. 2007;10:1298–1305. doi: 10.1089/jpm.2007.0061. [DOI] [PubMed] [Google Scholar]
- 2.Turnbull AE, Krall JR, Ruhl AP, Curtis JR, Halpern SD, Lau BM, Needham DM. A scenario-based, randomized trial of patient values and functional prognosis on intensivist intent to discuss withdrawing life support. Crit Care Med. 2014;42:1455–1462. doi: 10.1097/CCM.0000000000000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnato AE, Berhane Z, Weissfeld LA, Chang CC, Linde-Zwirble WT, Angus DC Robert Wood Johnson Foundation ICUE-o-LPG. Racial variation in end-of-life intensive care use: a race or hospital effect? Health Serv Res. 2006;41:2219–2237. doi: 10.1111/j.1475-6773.2006.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quill CM, Ratcliffe SJ, Harhay MO, Halpern SD. Variation in decisions to forgo life-sustaining therapies in US ICUs. Chest. 2014;146:573–582. doi: 10.1378/chest.13-2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hart JL, Harhay MO, Gabler NB, Ratcliffe SJ, Quill CM, Halpern SD. Variability Among US Intensive Care Units in Managing the Care of Patients Admitted With Preexisting Limits on Life-Sustaining Therapies. JAMA internal medicine. 2015;175:1019–1026. doi: 10.1001/jamainternmed.2015.0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halpern SD. ICU capacity strain and the quality and allocation of critical care. Curr Opin Crit Care. 2011;17:648–657. doi: 10.1097/MCC.0b013e32834c7a53. [DOI] [PubMed] [Google Scholar]
- 7.Wagner J, Gabler NB, Ratcliffe SJ, Brown SE, Strom BL, Halpern SD. Outcomes among patients discharged from busy intensive care units. Ann Intern Med. 2013;159:447–455. doi: 10.7326/0003-4819-159-7-201310010-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gabler NB, Ratcliffe SJ, Wagner J, Asch DA, Rubenfeld GD, Angus DC, Halpern SD. Mortality among patients admitted to strained intensive care units. Am J Respir Crit Care Med. 2013;188:800–806. doi: 10.1164/rccm.201304-0622OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown SE, Rey MM, Pardo D, Weinreb S, Ratcliffe SJ, Gabler NB, Halpern SD. The allocation of intensivists' rounding time under conditions of intensive care unit capacity strain. Am J Respir Crit Care Med. 2014;190:831–834. doi: 10.1164/rccm.201406-1127LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nelson JE, Angus DC, Weissfeld LA, Puntillo KA, Danis M, Deal D, Levy MM, Cook DJ Critical Care Peer Workgroup of the Promoting Excellence in End-of-Life Care P. End-of-life care for the critically ill: A national intensive care unit survey. Crit Care Med. 2006;34:2547–2553. doi: 10.1097/01.CCM.0000239233.63425.1D. [DOI] [PubMed] [Google Scholar]
- 11.Cremer R, Hubert P, Grandbastien B, Moutel G, Leclerc F treatments GFssgof. Prevalence of questioning regarding life-sustaining treatment and time utilisation by forgoing treatment in francophone PICUs. Intensive Care Med. 2011;37:1648–1655. doi: 10.1007/s00134-011-2320-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jensen HI, Ammentorp J, Erlandsen M, Ording H. Withholding or withdrawing therapy in intensive care units: an analysis of collaboration among healthcare professionals. Intensive Care Med. 2011;37:1696–1705. doi: 10.1007/s00134-011-2345-7. [DOI] [PubMed] [Google Scholar]
- 13.Wagner J, Halpern SD. Deferred admission to the intensive care unit: rationing critical care or expediting care transitions? Arch Intern Med. 2012;172:474–476. doi: 10.1001/archinternmed.2012.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strauss MJ, LoGerfo JP, Yeltatzie JA, Temkin N, Hudson LD. Rationing of intensive care unit services. An everyday occurrence. JAMA. 1986;255:1143–1146. [PubMed] [Google Scholar]
- 15.Hua M, Halpern SD, Gabler NB, Wunsch H. Effect of ICU Strain on Time to Death in the Intensive Care UnitB105 IMPROVING PATIENT EXPERIENCE IN CRITICAL CARE. American Thoracic Society. 2015:A3764–A3764. [Google Scholar]
- 16.Cook SF, Visscher WA, Hobbs CL, Williams RL Project ICIC. Project IMPACT: results from a pilot validity study of a new observational database. Crit Care Med. 2002;30:2765–2770. doi: 10.1097/00003246-200212000-00024. [DOI] [PubMed] [Google Scholar]
- 17.Higgins TL, Teres D, Copes WS, Nathanson BH, Stark M, Kramer AA. Assessing contemporary intensive care unit outcome: an updated Mortality Probability Admission Model (MPM0-III) Crit Care Med. 2007;35:827–835. doi: 10.1097/01.CCM.0000257337.63529.9F. [DOI] [PubMed] [Google Scholar]
- 18.Zimmerman JE, Kramer AA, McNair DS, Malila FM. Acute Physiology and Chronic Health Evaluation (APACHE) IV: hospital mortality assessment for today's critically ill patients. Crit Care Med. 2006;34:1297–1310. doi: 10.1097/01.CCM.0000215112.84523.F0. [DOI] [PubMed] [Google Scholar]
- 19.Kerlin MP, Harhay MO, Vranas KC, Cooney E, Ratcliffe SJ, Halpern SD. Objective factors associated with physicians' and nurses' perceptions of intensive care unit capacity strain. Annals of the American Thoracic Society. 2014;11:167–172. doi: 10.1513/AnnalsATS.201306-141OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prendergast TJ, Claessens MT, Luce JM. A national survey of end-of-life care for critically ill patients. Am J Respir Crit Care Med. 1998;158:1163–1167. doi: 10.1164/ajrccm.158.4.9801108. [DOI] [PubMed] [Google Scholar]
- 21.Sprung CL, Cohen SL, Sjokvist P, Baras M, Bulow HH, Hovilehto S, Ledoux D, Lippert A, Maia P, Phelan D, Schobersberger W, Wennberg E, Woodcock T Ethicus Study G. End-of-life practices in European intensive care units: the Ethicus Study. JAMA. 2003;290:790–797. doi: 10.1001/jama.290.6.790. [DOI] [PubMed] [Google Scholar]
- 22.Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput. 2004;36:717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- 23.Krull JL, MacKinnon DP. Multilevel modeling of individual and group level mediated effects. Multivar Behav Res. 2001;36:249–277. doi: 10.1207/S15327906MBR3602_06. [DOI] [PubMed] [Google Scholar]
- 24.Imai K, Keele L, Tingley D. A general approach to causal mediation analysis. Psychol Methods. 2010;15:309–334. doi: 10.1037/a0020761. [DOI] [PubMed] [Google Scholar]
- 25.Quenot JP, Rigaud JP, Prin S, Barbar S, Pavon A, Hamet M, Jacquiot N, Blettery B, Herve C, Charles PE, Moutel G. Impact of an intensive communication strategy on end-of-life practices in the intensive care unit. Intensive Care Med. 2012;38:145–152. doi: 10.1007/s00134-011-2405-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Curtis JR, Treece PD, Nielsen EL, Gold J, Ciechanowski PS, Shannon SE, Khandelwal N, Young JP, Engelberg RA. Randomized Trial of Communication Facilitators to Reduce Family Distress and Intensity of End-of-life Care. Am J Respir Crit Care Med. 2015 doi: 10.1164/rccm.201505-0900OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garrouste-Orgeas M, Tabah A, Vesin A, Philippart F, Kpodji A, Bruel C, Gregoire C, Max A, Timsit JF, Misset B. The ETHICA study (part II): simulation study of determinants and variability of ICU physician decisions in patients aged 80 or over. Intensive Care Med. 2013;39:1574–1583. doi: 10.1007/s00134-013-2977-x. [DOI] [PubMed] [Google Scholar]
- 28.Stelfox HT, Hemmelgarn BR, Bagshaw SM, Gao S, Doig CJ, Nijssen-Jordan C, Manns B. Intensive care unit bed availability and outcomes for hospitalized patients with sudden clinical deterioration. Arch Intern Med. 2012;172:467–474. doi: 10.1001/archinternmed.2011.2315. [DOI] [PubMed] [Google Scholar]
- 29.Uy J, White DB, Mohan D, Arnold RM, Barnato AE. Physicians' decision-making roles for an acutely unstable critically and terminally ill patient. Crit Care Med. 2013;41:1511–1517. doi: 10.1097/CCM.0b013e318287f0dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dzeng E, Colaianni A, Roland M, Chander G, Smith TJ, Kelly MP, Barclay S, Levine D. Influence of institutional culture and policies on do-not-resuscitate decision making at the end of life. JAMA internal medicine. 2015;175:812–819. doi: 10.1001/jamainternmed.2015.0295. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.