Abstract
Objective
To assess whether individual obesity risk factors, present during gestation and the first 6 months of life, can be combined into a simple prognostic model that has the ability to accurately predict childhood obesity at age 5 years in a high-risk cohort.
Study design
201 Latina women were recruited during pregnancy and their infants followed longitudinally. Ten risk factors for childhood obesity were included in an initial logistic model; a second reduced model was created via stepwise deletion (confirmed with nonparametric conditional random forest classifier), after which 5 risk factors remained. From each model, an obesity risk equation was derived and an obesity risk score was generated for each patient. Derived algorithms were assessed using discrimination, calibration and via predictive statistics.
Results
56 of 166 children (32%) followed through age 5 years met criteria for childhood obesity. Discrimination accuracy for both derivation models was excellent, and after optimism-corrected bootstrapping, both models showed meaningful clinical performance. Both models were adequately calibrated, showed strong sensitivity and negative predictive value at conservatively set obesity risk thresholds, and displayed excellent specificity amongst those classified as highest risk. Birth weight z-score and change in weight-for-age z-score between birth and 6 months were the risk factors with the strongest contribution to the obesity risk score.
Conclusions
Obesity risk algorithms are reliable in their prediction of childhood obesity and have the potential to be integrated into the electronic medical record. These models could provide a filter for directing early prevention resources to children with high obesity risk, but should be evaluated in a larger external dataset.
Keywords: childhood obesity, Latinos, prevention, risk factors, algorithms
Despite the medical and financial severity of childhood obesity, it has proven difficult to treat. Longitudinal data show that once childhood obesity is present, it is likely to persist into adolescence and adulthood(8). Pediatric health care practitioners (HCPs) have thus turned their focus to obesity prevention. Many recent studies have focused on single risk factors that are highly associated childhood obesity and are present during gestation or early infancy, such as maternal smoking and rapid early infant weight gain, as potential prevention targets(9). However, as the development of childhood obesity is influenced by genetic, environmental, and socioeconomic factors, in a complex interaction, targeting single obesity risk factors for intervention may be ineffective.
Prognostic modeling, whereby influence weights from multiple risk factors are combined to estimate an individual’s risk of a medical outcome(10), may be useful. An accurate childhood obesity risk score, derived from the presence of known pre-natal and early post-natal obesity risk factors, could provide a simple identifying infants at low risk of obesity and directing them to standard weight monitoring(11), while reserving intensive obesity prevention resources for those at high risk. This would be particularly useful in medical centers serving the urban poor, where the prevalence of obesity is often high(12), yet resources are low. The purpose of this study was to examine whether a prognostic model for childhood obesity could be derived from data gathered among an urban, Latino cohort, using only objective measures available from the medical record in a low resource setting.
Methods
Latina women living in the San Francisco area were recruited during their pregnancy for a prospective cohort study. The full recruitment protocol has been described previously(13). Demographic and general health characteristics of maternal participants were collected and maternal body mass index (BMI) was calculated from self-reported pre-pregnancy weight and height on the intake questionnaire. Mothers with pre-existing diabetes, polycystic ovary syndrome, insulin dependent gestational diabetes, and those with health issues or beliefs that would prevent breastfeeding were excluded from the cohort. Infants were excluded at delivery if they had any special care needs or an Apgar score ≤7 at 5 minutes of life. 201 mothers were enrolled in the study, 196 infants met criteria for participation, and 166 mother-child pairs (83 and 85%, respectively) remained in follow-up at 5 years.
At birth, 6 months, 1 year and subsequent annual visits, anthropometric measures were obtained on child participants, using standard digital scales for weight and tape measurements for length. Weight and height were calculated based on sex-specific Centers for Disease Control (CDC) growth references. A small percentage of the participants (<5% at each time point) could not attend study visits, so weight and height measures were extracted from the medical record. Data were obtained on the child’s nutritional intake via maternal interviews.
The Committee on Human Research at University of California, San Francisco approved all study procedures. Written informed consent was obtained from all participants at study entry and follow-up visits. Data collection ran from 2006–2012 and statistical analysis was conducted from 2014–2015.
For our predictive modeling, we included only risk factors that were clearly defined, reliably measurable, and available in standard clinical settings. We only considered predictors that have been previously described as affecting infant or childhood weight status(13,14). Whenever possible, we avoided dichotomization or categorization of linear predictors(15). All continuous predictors were checked for non-linearity. The primary outcome was childhood obesity at age 5 years, defined as BMI ≥95th percentile, using CDC growth references(16).
In reviewing our longitudinal data, we identified 19 candidate predictors of childhood obesity, including 11 prenatal/maternal and 8 early post-natal risk factors (Table I; available at www.jpeds.com). We applied our guiding principles in creating the predictive model, removing several maternal variables, including maternal depression, employment status and years in the United State, due to concerns over infrequent inclusion in the standard medical record and/or time burden on the HCP to document. Maternal smoking was excluded due to low prevalence of smoking in the cohort—both during pregnancy and in the first year of follow up, reported smoking prevalence was <3%. Gestational age was excluded due to co-linearity with birth weight and little independent predictive value.
Table 1.
Candidate Predictors of Childhood Obesity Within the Dataset
| Infant |
| Delivery Type (dichotomous, cesarean-section or vaginal) |
| Gestational Age (continuous, in years) |
| Sex (dichotomous, male or female) |
| Birth weight (continuous, in z-scores, plotted on both CDC and WHO references) |
| Infant weight gain (continuous, in z-score change between birth and 6 months, plotted on both CDC and WHO references) |
| Exclusively breastfed at 4–6 weeks, no formula or solids (dichotomous, yes or no) |
| Any Breastfeeding at 6 months (dichotomous, yes or no) |
| Timing of Introduction of Solids (dichotomous, <6 months or >6 months) |
| Maternal |
| Pre-Pregnancy BMI (continuous, in kg/m2) |
| First Child (dichotomous, yes or no) |
| Age (continuous, in years) |
| Employment (dichotomous, prior to or during pregnancy, yes or no) |
| Education (dichotomous, beyond high school, yes or no) |
| Depression (dichotomous, clinical depression at intake or immediately post-partum, yes or no) |
| Cohabitation Status (dichotomous, living with partner, yes or no) |
| Years Living in the US (dichotomous, <5 years, yes or no) |
| English Language Proficiency (dichotomous, proficient in English, yes or no) |
| Maternal Smoking (dichotomous, smoking at intake or post-partum period, yes or no) |
| Mexican Ethnicity (dichotomous, yes or no) |
Statistical Analyses
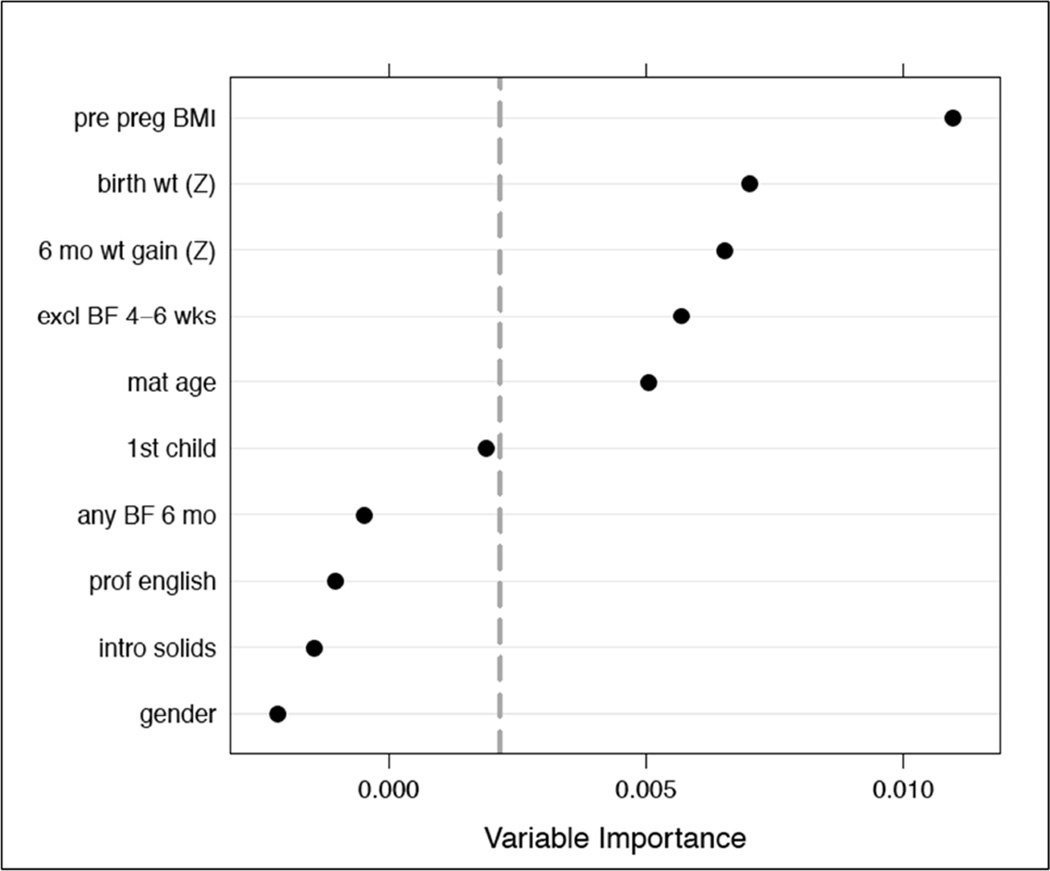
The 10 remaining candidate predictors were placed into a logistic regression model, referred to as the “full model.” An obesity risk score was generated for each participant, with the regression constant serving as the intercept and the beta-coefficient indicating the adjusted contribution of each predictor to the obesity risk (Table II; available at www.jpeds.com). The predicted risk of obesity was calculated using the formula 1/(1+e^−risk score)(17). We also developed an alternate predictive regression model using two variable selection strategies: stepwise backward deletion using the 20% significance level as a criterion for variable retention, and based on variable importance rankings from a nonparametric conditional random forest classifier (Figure 1; available at www.jpeds.com) (18). Both approaches resulted in the same group of five predictors, which were used to fit a “reduced model.” Risk scores were also created for each patient using this model. Regression diagnostics for each model included identification of observations with high leverage, assessment for pairwise interactions and nonlinearity.
Table 2.
Childhood Obesity Risk Score Algorithms
| Full Model: |
| −1.58 + (1.35*CDC WFA Z-Score Change between Birth and 6 Months) + (1.7*CDC Birth Weight Z-Score) + (0.11*Maternal Pre-Pregnancy BMI) − (0.72*Exclusive Breast Feeding at 4–6 Weeks) − (0.33*Any Breastfeeding at 6 Months) + (0.57*gender) − (0.09*Maternal Age) − (0.7*Introduction of Solids After 6 Months) − (0.5*First Child) + (0.55*English Language Proficiency) |
| Reduced Model: |
| −1.14 + (1.14*CDC WFA Z-Score Change between Birth and 6 Months) + (1.39*CDC Birth Weight Z-Score) + (0.1*Maternal Pre-Pregnancy BMI) − (0.1*Maternal Age) − (0.76*Exclusive Breast Feeding at 4–6 Weeks) |
Figure 1.
Variable Importance Rankings from a Nonparametric Conditional Random Forest Classifier
Both the full model and the reduced model were evaluated for discrimination and calibration performance. Discrimination was assessed by creating a receiver operating characteristic curve, with concordance index (area under the ROC curve) ≥0.8 considered to be excellent accuracy and ≥0.75 clinically meaningful(19). Model calibration was analyzed using the Hosmer-Lemeshow goodness of fit test, with p<0.05 defined as failure of adequate agreement between estimated and observed values(19). To test predictive accuracy, an arbitrary risk threshold (e.g. obesity risk score >50th percentile) was established and those above the threshold were labeled as positive on the prognostic test for childhood obesity. Sensitivity, specificity, positive and negative predictive values for the prognostic test at several different risk thresholds were calculated, as were likelihood ratios.
Due to the small sample size in this specific cohort, we elected not to split the dataset into derivation and validation sets. Rather, we used the entire sample to develop the prediction models and estimated internal measures of prediction performance using the bootstrap with 1000 samples(20). Statistics were performed with Stata 13 (College Station, Texas) and R, version 3.2.2.
Because 17% and 12% of individuals had missing observations for at least one predictor in the full and reduced models, respectively, we used chained multiple imputation to assess the sensitivity of our estimated scores to missing information(21). In both cases, estimates from imputed data were quite similar to those obtained using observed data, so we present results from the latter only.
Results
Our cohort had relatively low prevalence of higher education, employment and English proficiency, comparable with the underserved, recent immigrant populations typically seen at urban safety net hospitals (Table III). Maternal pre-pregnancy BMI was reflective of national obesity trends amongst Latina women(22), with 33% of mothers in the overweight range and an additional 18% obese.
Table 3.
Maternal and Early Infant Candidate Predictors of Childhood Obesity by Child’s Obesity Status at Age 5 Years
| Total | Obese N=53 |
Not Obese N=113 |
|
|---|---|---|---|
| Maternal Predictors | N=201 | ||
| Mexican Ethnicity (%) | 123 (61) | 32 (60) | 67 (59) |
| English Lang Proficiency (%) | 46 (23) | 14 (26) | 26 (23) |
| Maternal Age, years (SD) | 26.3 (5.2) | 25.6 (5.9) | 26.8 (4.9) |
| Maternal Pre-Pregnancy BMI, kg/m2 (SD) | 25.8 (5.5) | 27.4 (6.9) | 25.2 (4.6)** |
| Depression at Intake or Peripartum (%) | 67 (33) | 15 (28) | 41 (36) |
| First Child (%) | 95 (47) | 26 (49) | 52 (46) |
| > High School Education (%) | 44 (22) | 9 (18) | 23 (21) |
| In US <5 Years (%) | 99 (49) | 27 (51) | 52 (46) |
| Cohabitation with Partner (%) | 166 (83) | 44 (85) | 94 (83) |
| Maternal Smoking (%) | 4 (2) | 1 (2) | 3 (3) |
| Employed (%) | 57 (28) | 14 (26) | 32 (28) |
| Infant Predictors, Measured At Birth | N=196 | ||
| Male sex (%) | 98 (50) | 25 (47) | 55 (49) |
| Gestation Age, weeks (SD) | 39.3 (1.5) | 39.6 (1.4) | 39.1 (1.6)* |
| Vaginal Delivery (%) | 166 (85) | 44 (83) | 99 (88) |
| Birth Weight Z-Score, CDC (SD) | −0.14 (0.9) | 0.16 (0.9) | −0.24 (0.9)** |
| Infant Predictors, Measured Over First 6 Months | |||
| Weight-for-Age Z-Score Change between Birth and 6 Months, CDC (N=173, SD) | 0.62 (1.1) | 0.91 (1.2) | 0.46 (1.1)* |
| Exclusively Breastfed at 4–6 Weeks (N=190, %) | 71 (37) | 16 (30) | 48 (42) |
| Any Breastfeeding at 6 Months (N=180, %) | 120 (66) | 30 (57) | 75 (66) |
| Introduction of Solids (N=169, %) | |||
| <6 Months | 98 (58) | 31 (66) | 61 (58) |
| >6 Months | 71 (42) | 16 (34) | 45 (42) |
Boldface indicates statistical significance by t-test, (*p<0.05, **p<0.01)
Only 7% of included children were born preterm (<37 weeks GA). The mean birth weight (3368 grams, SD: 476) was similar to 2005 data for term babies born to Hispanic mothers (mean 3369 grams, SD: 455) in the US and slightly below the overall US birth weight average (mean 3389 grams, SD: 466)(23). Ninety-one percent of infants were breast fed at 4–6 weeks after birth, with 37% receiving breast milk as their only source of nutrition. Sixty-six percent of infants continued to breastfeed through the first 6 months of life, though over one-half of breastfeeding babies (57%) were supplemented with some formula. Children who remained in follow-up through the 3, 4 and 5-year old visits had obesity point prevalence of 28%, 25% and 32%, respectively.
Candidate Predictor Analysis
Maternal pre-pregnancy BMI, infant birth weight z-score, and weight-for-age (WFA) z-score change between birth and 6 months were independent risk factors for childhood obesity in unadjusted analysis and remained significant predictors after adjustment in both regression models (Tables III and IV). Maternal age was a significant protector against childhood obesity in the reduced model but not the full model.
Table 4.
Multivariable Logistic Regression Models for Prediction of Childhood Obesity.
| Predictor | Full Model | Reduced Model | ||
|---|---|---|---|---|
| Beta- Coefficient (SE)A |
OR (95% CI) |
Beta- Coefficient (SE)A |
OR (95% CI) |
|
| Weight-for-Age Z-Score Change between Birth and 6 Months, CDC | 1.35 (0.32) | 3.85 (2.04–7.28)*** | 1.14 (0.28) | 3.15 (1.82–5.46)*** |
| Birth Weight Z-score, CDC | 1.7 (0.41) | 5.47 (2.47–12.1)*** | 1.39 (0.35) | 4.02 (2.01–8.03)*** |
| Maternal Pre-Pregnancy BMI | 0.11 (0.05) | 1.12 (1.02–1.22)* | 0.1 (0.04) | 1.11 (1.02–1.2)* |
| Maternal Age | −0.09 (0.05) | 0.92 (0.83–1.01) | −0.1 (0.05) | 0.9 (0.82–0.99)* |
| Exclusively Breastfed at 4–6 Weeks | −0.72 (0.48) | 0.48 (0.18–1.26) | −0.76 (0.46) | 0.47 (0.19–1.15) |
| Introduction of Solids After 6 Months | −0.7 (0.51) | 0.5 (0.18–1.37) | ||
| Sex | 0.57 (0.49) | 1.76 (0.67–4.6) | ||
| Any Breastfeeding at 6 Months | −0.33 (0.51) | 0.72 (0.27–1.94) | ||
| First Child | −0.5 (0.54) | 0.61 (0.23–1.62) | ||
| English Language Proficiency | 0.55 (0.54) | 1.73 (0.6–5) | ||
| Intercept (SE) | −1.58 (1.7) | −1.14 (1.50) | ||
| Concordance Index | 0.84 | 0.82 | ||
Boldface indicates statistical significance by t-test, (*p<0.05, **p<0.01, ***p<0.001)
SE: Standard Error
Model Performance
The regression output for the full model and the reduced model are displayed in Table IV. Both models contained predictors that conferred increased childhood obesity risk as they increased in value, marked by a positive beta-coefficient (e.g. maternal pre-pregnancy BMI). They also had variables with negative beta-coefficients—such that increase (e.g. maternal age) was associated with a decrease in obesity risk. Discrimination accuracy for both models was excellent in the derivation cohort (full model concordance index=0.84, 95% CI: 0.77–0.91; reduced model concordance index=0.82, 95% CI: 0.74–0.89; Figure 2) and clinically meaningful in the optimism-corrected bootstrap-based estimates (full model concordance index=0.78; reduced model concordance index=0.76). Both models displayed adequate calibration (p-values for Hosmer-Lemeshow test >0.5).
Figure 2.
Receiver Operating Characteristic Curve for Obesity Prediction Models
Upper legend: Prediction Algorithms Applied to Derivation Cohort
To illustrate how risk scores were calculated, consider the following patient from the cohort: female sex, birth weight Z-score 0.33, WFA z-score change between birth and 6 months 2.44, solids introduced at <6 months, not exclusively breastfed at 4–6 weeks, and no breast feeding at 6 months. This child was born to an 18 year-old mother, who had no previous children, no English language proficiency and a pre-pregnancy BMI of 28.4. Using the prediction algorithms in Table II, we arrive at a risk score of 3.89 using the full model algorithm (3.22 with reduced model), with a predicted obesity risk of 98% (96% with reduced model). This patient was obese at age 5 years, with a BMI >99.9th percentile.
Birth weight z-score and change in WFA z-score between birth and 6 months were consistently the strongest contributors, across both models, to the obesity risk score. There were modest protective effects of breastfeeding and later introduction of solid foods in the full model, but only exclusive breastfeeding at 4–6 weeks met criteria for inclusion in the reduced model.
Predictive Statistics
The predictive metrics of the algorithms are displayed in Table V. The sensitivity and NPV were high (>90%) when the obesity risk score threshold was set at the 25th percentile, yet remain strong when the threshold is raised to the 50th percentile, especially in the full model algorithm (86% sensitivity, 91% NPV). In setting the risk threshold higher (e.g. at the 75th percentile and above), we found that patients above the threshold reliably went on to childhood obesity (high specificity and positive likelihood ratio), but this came at the cost of lowering sensitivity and NPV significantly.
Table 5.
Predictive Accuracy of Childhood Obesity Algorithms Based on Placement of Risk Threshold
| Sensitivity (%) | Specificity (%) | Negative Predictive Value (%) |
Positive Predictive Value (%) |
Positive Likelihood Ratio |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Risk Threshold |
Population Above Threshold (%) |
Full Model |
Reduced Model |
Full Model |
Reduced Model |
Full Model |
Reduced Model |
Full Model |
Reduced Model |
Full Model |
Reduced Model |
| 25th Percentile | 75 | 95 | 96 | 35 | 37 | 94 | 95 | 40 | 41 | 1.46 | 1.52 |
| 50th Percentile | 50 | 86 | 80 | 66 | 64 | 91 | 88 | 54 | 51 | 2.55 | 2.23 |
| 75th Percentile | 25 | 51 | 46 | 85 | 84 | 79 | 77 | 61 | 57 | 3.47 | 2.85 |
| 90th Percentile | 10 | 30 | 24 | 99 | 97 | 76 | 73 | 93 | 79 | 28.72 | 7.97 |
Discussion
Previously published childhood obesity prediction algorithms(24–29) were most often derived from European cohorts, with childhood obesity rates as low as 3%(24). While adhering to predefined guiding principles in candidate predictor selection, we were able to derive two prognostic algorithms for childhood obesity with adequate discrimination and calibration and predictive capabilities with potential for meaningful clinical applications and cost savings.
Current recommendations for the universal assessment of childhood obesity risk includes monitoring BMI at well child visits and considering medical, behavioral and attitude risks(11), but there is no method by which the clinician can combine individual risk factors in a quantifiable risk assessment. Prognostic modeling has the potential to aid childhood obesity prediction tremendously—the weighted algorithm allows infant HCPs to quickly obtain a risk score that puts their patient’s obesity risk in contrast against population risk. Further, as all measures in the described models are either taken in the pre-natal period or in the infant’s first 6 months, there is potential for early intervention. Applying the obesity risk algorithm at the 6-month visit would allow infant HCPs to present tangible concern about obesity risk, starting the discussion around healthy weight and nutrition, while gauging the family’s readiness for change(30). Risk score data may be particularly useful in counseling Latino families, where cultural practices lead to perceptions of healthy weight that are skewed towards higher weight ranges(31). Although consensus continues to build that early obesity prevention efforts are effective(32), best timing, setting and methodology of intervention continues to be a point of debate. Studies in obesity prevention during infancy are few and although some have shown promise(33), additional study is necessary to show which interventions lead to sustained effects(34).
Applying risk thresholds across the spectrum of obesity risk could lead to an innovative, multi-tiered, prevention strategy. Using our full model prediction equation and risk score threshold at the 25th percentile, 94 percent of patients below this risk threshold were in the normal weight range at age 5 years (this is the NPV of the model using this risk threshold, true negatives divided by all testing negative). In contrast, 61% of those above the 75th percentile risk score (and 93% of those above the 90th percentile) were obese at age 5 years (this corresponds to the PPV, true positives divided by all testing positive). HCPs can use this type of data to allocate prevention resources within their own patient populations, safely directing those at low obesity risk to standard monitoring, while reserving high-value prevention resources, such as gym memberships, dietician visits and in-home behavioral counseling, for patients at high risk of actually developing the disease outcome. Even though there is a vast literature on effectiveness of childhood obesity prevention efforts, there is an opportunity to study how intervention efficacy may differ across obesity risk strata. Identifying those at low risk and minimizing resources applied to this group could confer cost savings over efforts that prescribe obesity prevention resources to the entire population.
As electronic medical records (EMRs) become a standard of practice across the US(35), childhood obesity risk scoring has the potential for immediate and impactful applications. The models presented here lend themselves to the development of an EMR-based risk calculator. Most EMRs have the capability to generate a risk score automatically from risk factor data entered into the medical record and/or a clinic visit note. A pop-up message could alert providers to their patient’s obesity risk score and whether they are above a set risk threshold where intervention is indicated. Risk score alerts could serve as an important tool for busy HCPs(28,36), who frequently fail to classify patients in the appropriate weight category(37) and may not appreciate rapid infant growth if contained within the normal weight range (e.g. 15th% at birth to 75th% at 6 months).
Compared with other recently published predictive risk equations for childhood obesity, the models described in this study have several relative methodological advantages. First, the candidate predictors based on anthropometric measures are continuous, allowing for relative contribution of values across the spectrum of the variable. The beta-coefficients for birth weight z-score and WFA z-score change are stronger than those presented previously for dichotomized or categorized versions of these same variables. Second, other than self-reported maternal prepregnancy BMI, we only included candidate predictors with reliable, objective measures, minimizing measurement error. Lastly, we measured the obesity outcome at age 5 years, a time where children often display issues with portion control(38) and are prone to choosing heavily advertised sugary beverages and snacks(39). Further, it has been shown that those who are obese entering school have higher risk of obesity into adulthood than obese toddlers(8).
One important limitation to this analysis is that the models derived have yet to be validated and evaluated for predictive accuracy in a larger, external dataset with similar demographics. Validation in an external dataset is critical in confirming the discrimination, calibration and predictive accuracy of the models and will allow for further efficacy comparisons between the full and reduced models, as well as the establishment of the most clinically useful obesity risk thresholds in considering resource allocation. Further, because of the relatively small size of this dataset and some missing data across variables, several predictors that have been shown to be protective against obesity, such as breastfeeding and timing of solids introduction, may have been underpowered to reach statistical significance and meet inclusion criteria in the reduced model. Lastly, this is a ‘locally’ derived model(24). Although it is likely to display high predictive accuracy amongst Latinos in urban areas, it may not generalize as well to mixed ethnicity and socioeconomic status cohorts. However, based on obesity risk data for other underserved minorities (African Americans, Native Americans) living in metropolitan areas, there is reason to believe that this model may still be useful for obesity prediction in these groups(7,12).
Further study is needed to show which prevention measures are most clinically effective (and cost effetive) in preventing obesity when directed used for infants and toddlers with a high obesity risk.
Acknowledgments
Data collection was funded to J.W. by the National Institutes of Health (NIH; K01 DK 080825) and Nestle and Hellman Family Foundation (CDHNF <grant number>). J.R. and S.V.’s analysis and writing were funded by NIH (T32 DK007762). No study funding sources had any role in study design, analysis, writing or decision to submit for publication.
We thank Joel Simon, MD, MPH, Michael Kohn, MD, MPP, David Glidden, PhD, Saunak Sen, PhD, MS, and Emily Perito, MD, MAS, for mentorship in this work as part of the UCSF Master’s Program in Advanced Science and Clinical Research.
Abbreviations
- BMI
Body mass index
- CDC
Centers for Disease Control
- CI
Confidence interval
- EMRs
Electronic medical records
- HCPs
Health care practitioners
- NPV
Negative predictive value
- PPV
Positive predictive value
- WFA
Weight for age
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References
- 1.Ebbeling CB, Pawlak DB, Ludwig DS. Childhood obesity: public-health crisis, common sense cure. Lancet. 2002;360:473–482. doi: 10.1016/S0140-6736(02)09678-2. [DOI] [PubMed] [Google Scholar]
- 2.Han JC, Lawlor DA, Kimm SY. Childhood obesity. Lancet. 2010;375:1737–1748. doi: 10.1016/S0140-6736(10)60171-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franks PW, Hanson RL, Knowler WC, Sievers ML, Bennett PH, Looker HC. Childhood obesity, other cardiovascular risk factors, and premature death. N Engl J Med. 2010;362:485–493. doi: 10.1056/NEJMoa0904130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang YC, McPherson K, Marsh T, Gortmaker S, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet. 2011;378:815–825. doi: 10.1016/S0140-6736(11)60814-3. [DOI] [PubMed] [Google Scholar]
- 5.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skinner AC, Skelton JA. Prevalence and trends in obesity and severe obesity among children in the United States, 1999–2012. JAMA Pediatr. 2014;168:561–566. doi: 10.1001/jamapediatrics.2014.21. [DOI] [PubMed] [Google Scholar]
- 7.Kimbro RT, Brooks-Gunn J, McLanahan S. Racial and ethnic differentials in overweight and obesity among 3-year-old children. Am J Public Health. 2007;97:298–305. doi: 10.2105/AJPH.2005.080812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo SS, Wu W, Chumlea WC, Roche AF. Predicting overweight and obesity in adulthood from body mass index values in childhood and adolescence. Am J Clin Nutr. 2002;76:653–658. doi: 10.1093/ajcn/76.3.653. [DOI] [PubMed] [Google Scholar]
- 9.Taveras EM, Rifas-Shiman SL, Belfort MB, Kleinman KP, Oken E, Gillman MW. Weight status in the first 6 months of life and obesity at 3 years of age. PEDIATRICS. 2009;123:1177–1183. doi: 10.1542/peds.2008-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moons KGM, Royston P, Vergouwe Y, Grobbee DE, Altman DG. Prognosis and prognostic research: what, why, how? BMJ. 2009;338:b375. doi: 10.1136/bmj.b375. [DOI] [PubMed] [Google Scholar]
- 11.Barlow SE the Expert Committee. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. PEDIATRICS. 2007;120:S164–S192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 12.Goodell LS, Wakefield DB, Ferris AM. Rapid weight gain during the first year of life predicts obesity in 2–3 year olds from a low-income, minority population. J Community Health. 2009;34:370–375. doi: 10.1007/s10900-009-9164-6. [DOI] [PubMed] [Google Scholar]
- 13.Wojcicki JM, Holbrook K, Lustig RH, Epel E, Caughey AB, Muñoz RF, et al. Chronic maternal depression is associated with reduced weight gain in Latino infants from birth to 2 years of age. PLoS ONE. 2011;6:e16737. doi: 10.1371/journal.pone.0016737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weng SF, Redsell SA, Swift JA, Yang M, Glazebrook CP. Systematic review and meta-analyses of risk factors for childhood overweight identifiable during infancy. Arch Dis Child. 2012;97:1019–1026. doi: 10.1136/archdischild-2012-302263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Royston P, Altman DG, Sauerbrei W. Dichotomizing continuous predictors in multiple regression: a bad idea. Statist Med. 2005;25:127–141. doi: 10.1002/sim.2331. [DOI] [PubMed] [Google Scholar]
- 16.Ogden CL, Flegal KM. Changes in terminology for childhood overweight and obesity. Natl Health Stat Report. 2010;22:1–6. [PubMed] [Google Scholar]
- 17.Royston P, Moons KGM, Altman DG, Vergouwe Y. Prognosis and prognostic research: developing a prognostic model. BMJ. 2009;338:b604. doi: 10.1136/bmj.b604. [DOI] [PubMed] [Google Scholar]
- 18.Strobl C, Boulesteix AL, Kneib T, Augustin T, Zeileis A. Conditional variable importance for random forests. BMC Bioinformatics. 2008;9:307. doi: 10.1186/1471-2105-9-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hosmer DW, Lemeshow S. Applied logistic regression. 2nd. New York, NY: John Wiley & Sons; 2000. [Google Scholar]
- 20.Harrell FE, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Statist Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 21.van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16:219–242. doi: 10.1177/0962280206074463. [DOI] [PubMed] [Google Scholar]
- 22.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA. 2004;291:2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 23.Donahue SMA, Kleinman KP, Gillman MW, Oken E. Trends in Birth Weight and Gestational Length Among Singleton Term Births in the United States. Obstet Gyn. 2010;115:357–364. doi: 10.1097/AOG.0b013e3181cbd5f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morandi A, Meyre D, Lobbens S, Kleinman K, Kaakinen M, Rifas-Shiman SL, et al. Estimation of newborn risk for child or adolescent obesity: lessons from longitudinal birth cohorts. PLoS ONE. 2012;7:e49919. doi: 10.1371/journal.pone.0049919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weng SF, Redsell SA, Nathan D, Swift JA, Yang M, Glazebrook C. Estimating overweight risk in childhood from predictors during infancy. PEDIATRICS. 2013;132:e414–e421. doi: 10.1542/peds.2012-3858. [DOI] [PubMed] [Google Scholar]
- 26.Druet C, Stettler N, Sharp S, Simmons RK, Cooper C, Davey Smith G, et al. Prediction of childhood obesity by infancy weight gain: an individual-level meta-analysis. Paediatr Perinat Epidemiol. 2011;26:19–26. doi: 10.1111/j.1365-3016.2011.01213.x. [DOI] [PubMed] [Google Scholar]
- 27.Levine RS, Dahly DL, Rudolf MCJ. Identifying infants at risk of becoming obese: Can we and should we? Public Health. 2012;126:123–128. doi: 10.1016/j.puhe.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 28.Santorelli G, Petherick ES, Wright J, Wilson B, Samiei H, Cameron N, et al. Developing prediction equations and a mobile phone application to identify infants at risk of obesity. PLoS ONE. 2013;8:e71183. doi: 10.1371/journal.pone.0071183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Graversen L, Sørensen TIA, Gerds TA, Petersen L, Sovio U, Kaakinen M, et al. Prediction of adolescent and adult adiposity outcomes from early life anthropometrics. Obesity. 2014;23:162–169. doi: 10.1002/oby.20921. [DOI] [PubMed] [Google Scholar]
- 30.Rhee KE, DeLago CW, Arscott-Mills T, Mehta SD, Krysko Davis R. Factors associated with parental readiness to make changes for overweight children. PEDIATRICS. 2005;116:e94–e101. doi: 10.1542/peds.2004-2479. [DOI] [PubMed] [Google Scholar]
- 31.Rich SS, DiMarco NM, Huettig C, Essery EV, Andersson E, Sanborn CF. Perceptions of health status and play activities in parents of overweight Hispanic toddlers and preschoolers. Fam Community Health. 2005;28:130–141. doi: 10.1097/00003727-200504000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Waters E, de Silva-Sanigorski A, Hall BJ, Brown T, Campbell KJ, Gao Y, et al. Interventions for preventing obesity in children. Cochrane Database Syst Rev. 2011;12:CD001871. doi: 10.1002/14651858.CD001871.pub3. [DOI] [PubMed] [Google Scholar]
- 33.Paul IM, Savage JS, Anzman SL, Beiler JS, Marini ME, Stokes JL, et al. Preventing obesity during infancy: a pilot study. Obesity. 2010;19:353–361. doi: 10.1038/oby.2010.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wen LM, Baur LA, Simpson JM, Rissel C, Wardle K, Flood VM. Effectiveness of home based early intervention on children's BMI at age 2: randomised controlled trial. BMJ. 2012;344:e3732. doi: 10.1136/bmj.e3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adler-Milstein J, DesRoches CM, Furukawa MF, Worzala C, Charles D, Kralovec P, et al. More than half of US hospitals have at least a basic EHR, but stage 2 criteria remain challenging for most. Health Affairs. 2014;33:1664–1671. doi: 10.1377/hlthaff.2014.0453. [DOI] [PubMed] [Google Scholar]
- 36.Rattay KT, Ramakrishnan M, Atkinson A, Gilson M, Drayton V. Use of an electronic medical record system to support primary care recommendations to prevent, identify, and manage childhood obesity. PEDIATRICS. 2008;123:S100–S107. doi: 10.1542/peds.2008-1755J. [DOI] [PubMed] [Google Scholar]
- 37.Riley MR, Bass NM, Rosenthal P, Merriman RB. Underdiagnosis of pediatric obesity and underscreening for fatty liver disease and metabolic syndrome by pediatricians and pediatric subspecialists. J Pediatr. 2005;147:839–842. doi: 10.1016/j.jpeds.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 38.Rolls BJ, Engell D, Birch LL. Serving Portion Size Influences 5-Year-Old but Not 3-Year-Old Children's Food Intakes. J Am Diet Assoc. 2000;100:232–234. doi: 10.1016/S0002-8223(00)00070-5. [DOI] [PubMed] [Google Scholar]
- 39.Chamberlain LJ, Wang Y, Robinson TN. Does children's screen time predict requests for advertised products? Cross-sectional and prospective analyses. Arch Pediatr Adolesc Med. 2006;160:363–368. doi: 10.1001/archpedi.160.4.363. [DOI] [PubMed] [Google Scholar]