Summary
Hypoxic-ischemic (HI) brain injury in newborns results in serious damage. Magnesium sulfate has been clinically used as a cyto-protective agent against HI brain injury in newborns in some countries, including Japan. However, it is not clear how magnesium exerts this effect and how it acts on the individual types of cells within the newborn brain. In this study, we exposed cultured rat oligodendrocyte precursor cells to magnesium sulfate during the period when they differentiate into oligodendrocytes, and showed that magnesium-exposed oligodendrocytes exhibited more resistance to hypoxic-ischemic injury. Our data may support the use of magnesium sulfate in the clinical setting.
Keywords: oligodendrocyte, magnesium sulfate, white matter, hypoxic-ischemic injury
White matter injury after hypoxic-ischemic (HI) injury results in serious damage to the developing brain. Many affected infants suffer from severe neurological deficits and lifelong handicaps. Therefore, therapy to protect the white matter and reduce the injury is urgently needed. In seeking therapeutic agents that can provide protection to the vulnerable developing brain, magnesium sulfate has been examined for its potential. Although still controversial, magnesium is one of the supportive drug used in newborn hypoxic-ischemic injury, often in conjunction with therapeutic hypothermia (Shea and Palanisamy, 2015). However, effects of magnesium in newborn hypoxia and/or ischemia are not fully understood, and it is important to evaluate whether and how magnesium can protect developing brains against hypoxic stress. In this study, therefore, we examined the effects of magnesium sulfate on oligodendrocyte lineage cells (oligodendrocytes and oligodendrocyte precursor cells), which constitute the major cell types in cerebral white matter.
All experiments were performed following institutionally approved protocol by Massachusetts General Hospital Subcommittee on Research Animal Care, and in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Primary oligodendrocyte precursor cells (OPCs) were isolated from neonatal rat brain cortex as previously described (Arai and Lo, 2009). For proliferation, OPCs were cultured in Neurobasal media containing 1% penicillin/streptomycin, 2 mM L-glutamine, 10 ng/ml basic fibroblast growth factor, 10 ng/ml platelet-derived growth factor-AA, and 2% B27 supplement. For differentiation of OPCs into mature oligodendrocytes, culture media was switched to differentiation media (Dulbecco's Modified Eagle's Medium (DMEM) containing 1% penicillin/streptomycin, 10 ng/ml ciliary neurotrophic factor, 15 nM triiodo-L-thyronine, and 2% B27 supplement).
Maturation of OPCs was assessed on the seventh day after the start of differentiation. Western blots using anti-myelin basic protein (MBP) and glutathione-S-transferase (GST) pi, which are markers for mature oligodendrocytes, and PDGF receptor-α (PDGFR-α), a marker for OPCs, were done to confirm the maturation state of the cells. Western blot of cell lysates was carried out using mouse monoclonal antibodies to MBP (1:500 dilution, Thermo scientific), rabbit polyclonal antibodies to GST-pi (1:1000 dilution, MBL international), rabbit polyclonal antibodies to PDGFR-α (1:1000 dilution, Santa Cruz) and mouse monoclonal antibodies to β-actin (1:10000 dilution, Sigma Aldrich). OPC maturation was also assessed by quantitative real-time PCR (QRT-PCR) on the third day after switching the culture media. RNA was isolated with RNeasy Plus Mini kit (QIAGEN, Hilden, Germany), and first-strand cDNA was synthesized with PrimeScript RT reagent (Takara-Clonetech). QRT-PCR was performed using SYBR Premix Ex Taq II (Takara-Clonetech) and analyzed with Fast real time system 7500 (Applied Biosystems). Expression levels were measured relative to GAPDH as internal control. The sequences of primers used in this study are as follows - 5’- ttgactccatcgggcgcttcttta -3’ for rat MBP forward; 5’- ttcatcttgggtc ctctgcgactt -3’ for rat MBP reverse. These markers (PDGFR-α for OPCs, MBP/GST-pi for mature oligodendrocytes) are now relatively well accepted in the literature (Galvao et al., 2014; Han et al., 2015), and we have been using them consistently in our previous studies (Maki et al., 2015; Miyamoto et al., 2015).
Oligodendrocytes matured in the presence of magnesium sulfate or vehicle (control oligodendrocytes) both underwent oxygen/glucose deprivation (OGD) in a rat cell-culture model of hypoxic-ischemic injury. OGD experiments were conducted as previously described (Arai and Lo, 2009). During the OGD period (2-hr), culture media was changed to DMEM that contained no glucose, and switched back to OPC differentiation media with added magnesium sulfate or vehicle after OGD. After 24 hours of re-oxygenation, cell viability was evaluated by WST assay (Cell counting kit, Dojindo) or direct cell counting in a blinded manner. All results presented in this study were expressed as mean ± S.D. Statistical significance was evaluated using Mann-Whitney U test to compare between two groups, and Kruskal-Wallis test for multiple comparisons. A p value of < 0.05 was considered to be statistically significant.
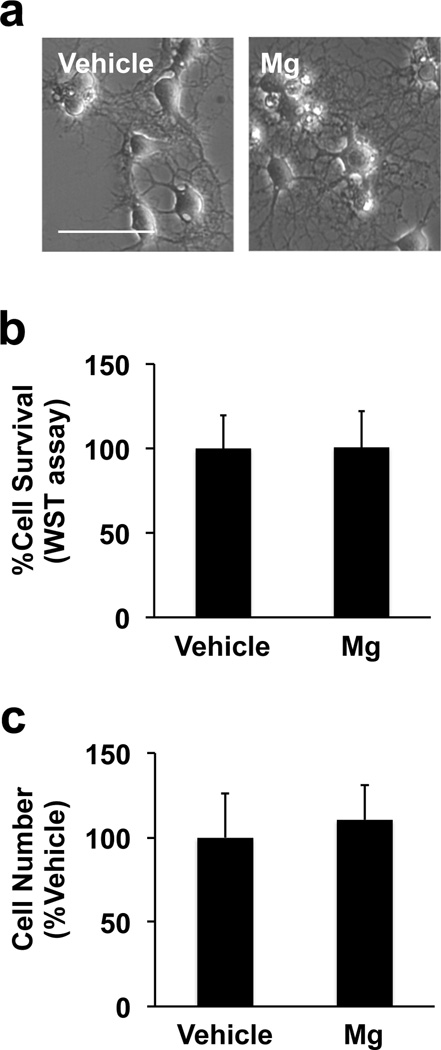
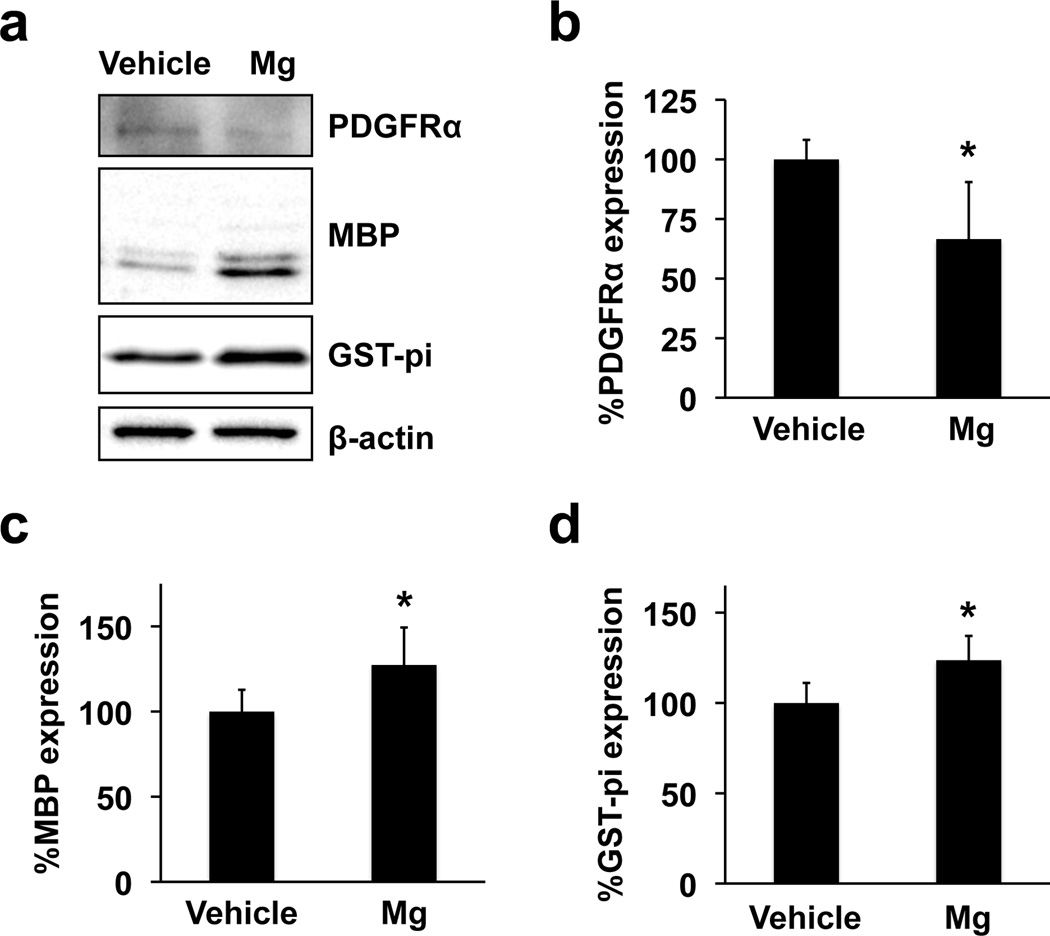
During the period of OPC maturation in vitro, primary rat OPC cultures were exposed to 5 mM magnesium sulfate. This magnesium dose was compatible with previous studies, which showed beneficial effects of magnesium for neural stem cells and endothelial cells in vitro (Lapidos et al., 2001; Vennemeyer et al., 2014). In the clinical setting, the physiological serum magnesium concentration is around 1 mM, but may increase to 3mM after magnesium administration (Westermaier et al., 2013). In addition, the serum magnesium concentration in asphyxiated newborns who received magnesium therapy was reported to be 1.4 - 2.8 mM (Bhat et al., 2009). In our cell culture system, OPCs started their differentiation process once their culture media was switched to the differentiation media. In fact, even in the vehicle group, cells showed oligodendrocyte-like shape on day 7 after starting OPC differentiation (Figure 1a - left). However, when OPCs were exposed to 5 mM magnesium sulfate for 7 days during the time period of differentiation, cells exhibited more matured oligodendrocytes with spider’s web-like processes (Figure 1a - right). On the other hand, magnesium sulfate did not affect cell survival (Figure 1b) and cell number (Figure 1c). Since magnesium sulfate changed cell morphology, we then assessed whether magnesium sulfate promoted the maturation process of OPCs. As expected, protein expression of GST-pi and MBP (oligodendrocyte markers) increased in the magnesium sulfate-treated group (Figure 2a-c). Correspondingly, the level of PDGFR-α expression (OPC marker) in the magnesium-sulfateexposed oligodendrocytes was lower compared to vehicle-treated oligodendrocytes (Figure 2a and 2d). In addition, magnesium-sulfate-treated cells exhibited a higher level of MBP mRNA compared to vehicletreated OPCs (Supplementary Figure S1).
Figure 1. Effects of magnesium sulfate on OPC number.
(a) Primary rat OPCs were exposed to vehicle or 5 mM magnesium sulfate (Mg) for 7 days during the time period in which OPCs differentiate into oligodendrocytes. Magnesium-sulfate-treated cells exhibited more maturated oligodendrocytes. Scale bar = 50 μm. (b-c) After 7 days of vehicle or magnesium sulfate incubation, cells were subjected to the WST assay or direct cell count. Magnesium sulfate did not affect cell viability of our cultured rat oligodendrocyte lineage cells. Data are represented as Mean ± SD of 3 independent experiments.
Figure 2. Effects of magnesium sulfate on OPC differentiation.
Primary rat OPCs were exposed to vehicle or 5 mM magnesium sulfate (Mg) for 7 days during the time period in which OPCs differentiate into oligodendrocytes. Then cell lysates were subjected to western blotting. (a) Representative western blot images. PDGFR-α was used as a marker for OPCs, and MBP/GST-pi as a marker for oligodendrocytes. β-actin was used as a loading control. (b-d) Western blots showed that Mg increased oligodendrocyte marker proteins MBP and GST-pi, but decreased an OPC marker protein PDGFR-α. Data are represented as Mean ± SD of 4 independent experiments. *P<0.05.
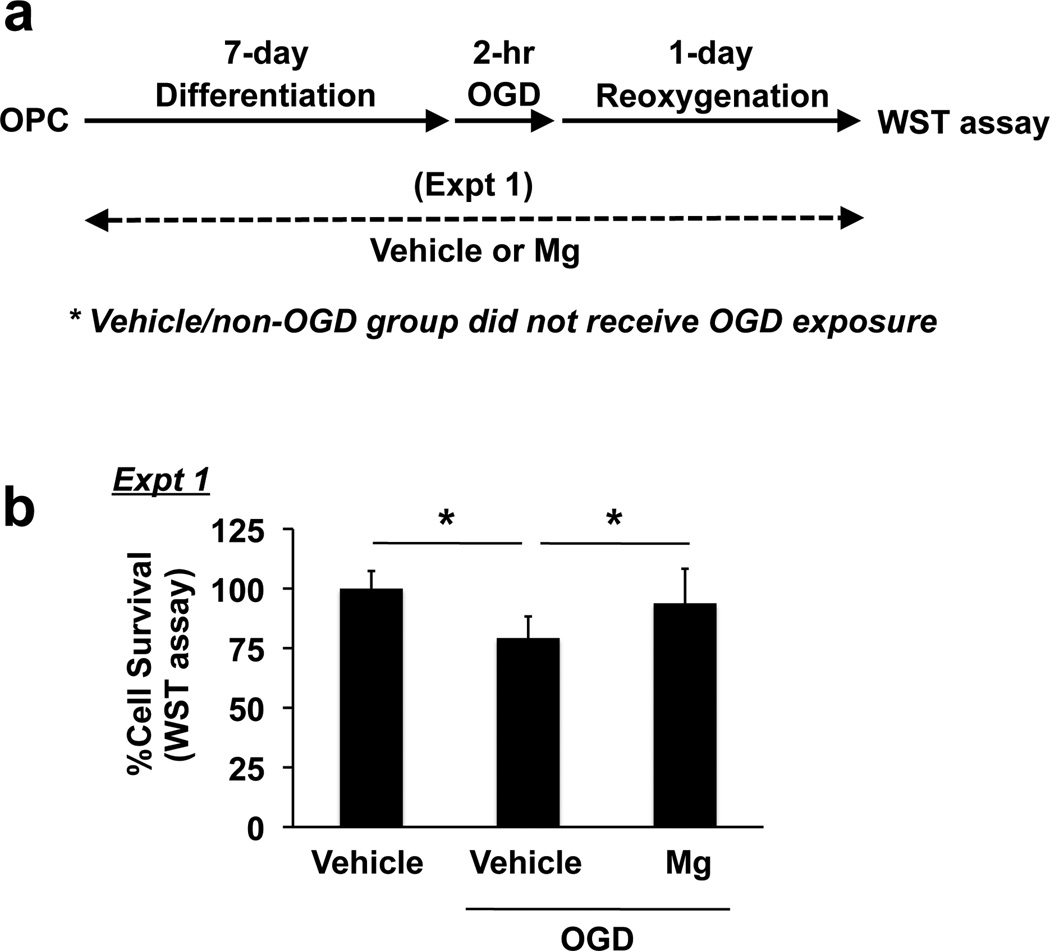
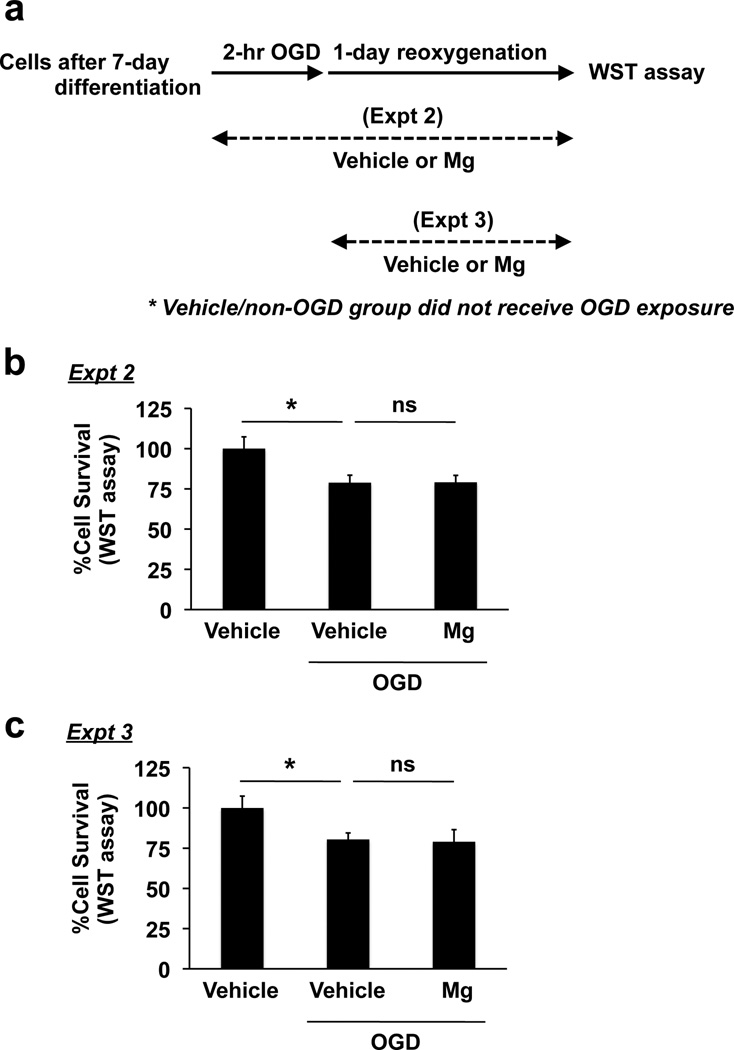
In general, mature oligodendrocytes are less vulnerable to ischemic damage compared to OPCs (Volpe et al., 2011). In fact, in our cell culture system, mature cells showed more resistance to OGD stress (Supplementary Figure S2). Therefore, we examined if magnesium-sulfate-treated cells would be more tolerant of hypoxia/ischemic exposure. After 7 days of magnesium sulfate incubation, differentiated oligodendrocytes were subjected to 2-hr OGD followed by 24-hr re-oxygenation (Figure 3a). Compared to untreated cells, OGD caused a decrease in cell viability in oliogodendrocytes (Figure 3b). On the other hand, magnesium-sulfate-exposed oligodendrocytes had improved cell viability compared to vehicleexposed oligodendrocytes after OGD (Figure 3b). Notably, when magnesium sulfate was added to differentiated oligodendrocytes just before OGD (i.e. during OGD/reoxygenation) or after OGD (i.e. during reoxygenation) (Figure 4a), magnesium sulfate did not show oligo-protection effects against OGD (Figure 4b-c), suggesting that magnesium sulfate does not directly protect matuare oligodendrocytes from OGD, but that it supports the progression of OPCS into mature oligodendrocytes, which are more resistant to OGD-induced injury.
Figure 3. Protective effects of prolonged exposure of magnesium sulfate on oligodendrocyte survival under in vitro ischemic conditions.
(a) Schematics for experiments. (b) Primary rat OPCs were exposed to 5 mM magnesium sulfate (Mg) or vehicle for 7 days during the time of differentiation into oligodendrocytes. Then the oligodendrocyte cultures were subjected to 2-hr OGD followed by 24-hr reoxygenation. Cell viability was assessed by WST assay, and the cell viability was calculated as a comparison to the control group (no magnesium sulfate treatment, no OGD exposure). Data are represented as Mean ± SD of 4 independent experiments. *P<0.05.
Figure 4. Effects of short-term exposure of magnesium sulfate on oligodendrocyte survival under in vitro ischemic conditions.
(a) Schematics for experiments. (b-c) Primary rat oligodendrocytes were incubated with 5 mM magnesium sulfate (Mg) or vehicle during 2-hr OGD + 24-hr reoxygenation (Expt 1) or 24-hr reoxygenation (Expt 2). Cell viability was assessed by WST assay, and the cell viability was as a comparison to the control group (no magnesium sulfate treatment, no OGD exposure). Data are represented as Mean ± SD of 3 independent experiments. *P<0.05. n.s. stands for “not significant”.
Taken together, our current study demonstrates that oligodendrocyte lineage cells with prolonged exposure to magnesium sulfate may acquire resistance to hypoxic-ischemic stress. Magnesium sulfate may accelerate the differentiation step of oligodendrocyte lineage cells, which contributes to the protection of developing white matter against hypoxic/ischemic stress. Although future deeper studies would be helpful to further dissect the mechanisms of magnesium-sulfate-induced OPC differentiation, our finding is compatible with previous studies that showed that preterm infants who were exposed to magnesium antenatally had a reduced incidence of cerebral palsy (Bozkurt et al., 2015; Rouse et al., 2008). We speculate that infants exposed to magnesium during fetal life, before their preterm delivery, would have an increased number of mature oligodendrocytes and increased myelination in the white matter, which make the white matter less vulnerable to the hypoxic-ischemic injury that infants occasionally suffer at the time of their delivery. Our data suggest that exposure to magnesium sulfate increases OPC maturation, and support the use of antenatal magnesium sulfate for prevention of adverse neurodevelopmental outcome due to hypoxic-ischemic injury in preterm infants.
Although the precise mechanisms of neuroprotection remain to be fully defined, current knowledge points to several steps in various pathophysiologic pathways where magnesium may modify signals involved in apoptosis and inflammation after hypoxic-ischemic injury. The putative neuroprotective effects of magnesium include competitive reduction of intracellular calcium entry (Mami et al., 2006), blocking glutamatergic N-methyl-D-aspartate (NMDA) receptors (Nowak et al., 1984) and modulating the actions of pro-inflammatory cytokines and oxygen free radicals (Laires et al., 2004). With these postulated mechanisms of action, magnesium has been shown to be neuroprotective in animal models of stroke, trauma, and subarachnoid hemorrhage. Clinical trials have already been conducted for the use of magnesium sulfate in ischemic stroke. The IMAGES trial which investigated the effect of magnesium given within 12 hours of stroke onset, failed to prove efficacy of magnesium for acute stroke but showed a benefit in lacunar strokes only (Intravenous Magnesium Efficacy in Stroke (IMAGES) Study Investigators, 2004). Similarly, the FAST-Mag trial, which had a shorter time window of 2 hours, also failed to show the benefit of magnesium (Saver et al., 2015). Based on the results of those two large trials, magnesium is now not recommended for use as a neuroprotective drug for adult stroke. Our current data are consistent with the results of these clinical trials. Magnesium did not directly protect mature oligodendrocytes against hypoxic-ischemic damage. Instead, magnesium's positive effects appear to be due to its ability to amplify OPC differentiation, thus making the entire pool of oligodendrocyte-lineage cells more resistant to stress and able to recover from injury. Therefore, the use of magnesium sulfate may be most effective in preterm infants but not recommended for the prevention of hypoxic-ischemic developmental impairment in term infants.
In summary, our proof-of-concept study supports the idea that fetal exposure to magnesium may be beneficial to developing brains during the OPC maturation period. Oligodendrocyte lineage cells play critical roles in cerebral white matter, and magnesium sulfate may enhance their maturation, which decreases their vulnerability to hypoxic-ischemic injury. This beneficial action of magnesium is a novel finding and further research to elucidate its mechanism of action will be important in the search for new treatments of white matter injury in preterm infants.
Supplementary Material
Highlights.
Magnesium-sulfate-exposed oligodendrocytes exhibited more resistance to hypoxic-ischemic injury.
Magnesium sulfate accelerated the differentiation step of oligodendrocyte lineage cells.
Magnesium sulfate did not directly protect mature oligodendrocytes against hypoxic-ischemic damage.
Acknowledgments
Supported in part by National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
References
- Muir KW, Lees KR, Ford I, Davis S Intravenous Magnesium Efficacy in Stroke (IMAGES) Study Investigators. Magnesium for acute stroke (Intravenous Magnesium Efficacy in Stroke trial): randomised controlled trial. The Lancet. 2004;363:439–445. doi: 10.1016/S0140-6736(04)15490-1. [DOI] [PubMed] [Google Scholar]
- Arai K, Lo EH. An Oligovascular Niche: Cerebral Endothelial Cells Promote the Survival and Proliferation of Oligodendrocyte Precursor Cells. The Journal of Neuroscience. 2009;29:4351–4355. doi: 10.1523/JNEUROSCI.0035-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat MA, Charoo BA, Bhat JI, Ahmad SM, Ali SW, Mufti MU. Magnesium sulfate in severe perinatal asphyxia: a randomized, placebo-controlled trial. Pediatrics. 2009;123:e764–e769. doi: 10.1542/peds.2007-3642. [DOI] [PubMed] [Google Scholar]
- Bozkurt O, Eras Z, Canpolat FE, Oguz SS, Uras N, Dilmen U. Antenatal magnesium sulfate and neurodevelopmental outcome of preterm infants born to preeclamptic mothers. J Matern Fetal Neonatal Med. 2015;20:1–4. doi: 10.3109/14767058.2015.1035641. [DOI] [PubMed] [Google Scholar]
- Galvao RP, Kasina A, McNeill RS, Harbin JE, Foreman O, Verhaak RG, Nishiyama A, Miller CR, Zong H. Transformation of quiescent adult oligodendrocyte precursor cells into malignant glioma through a multistep reactivation process. Proc Natl Acad Sci U S A. 2014;111:E4214–E4223. doi: 10.1073/pnas.1414389111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L, Cai W, Mao L, Liu J, Li P, Leak RK, Xu Y, Hu X, Chen J. Rosiglitazone Promotes White Matter Integrity and Long-Term Functional Recovery After Focal Cerebral Ischemia. Stroke. 2015;46:2628–2636. doi: 10.1161/STROKEAHA.115.010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laires MJ, Monteiro CP, Bicho M. Role of cellular magnesium in health and human disease. Front Biosci. 2004;9:262–276. doi: 10.2741/1223. [DOI] [PubMed] [Google Scholar]
- Lapidos KA, Woodhouse EC, Kohn EC, Masiero L. Mg(++)-induced endothelial cell migration: substratum selectivity and receptor-involvement. Angiogenesis. 2001;4:21–28. doi: 10.1023/a:1016619414817. [DOI] [PubMed] [Google Scholar]
- Mami A, Ballesteros J, Mishra O, Delivoria-Papadopoulos M. Effects of Magnesium Sulfate Administration During Hypoxia on Ca2+ Influx and IP3 Receptor Modification in Cerebral Cortical Neuronal Nuclei of Newborn Piglets. Neurochem Res. 2006;31:63–70. doi: 10.1007/s11064-005-9076-5. [DOI] [PubMed] [Google Scholar]
- Maki T, Takahashi Y, Miyamoto N, Liang AC, Ihara M, Lo EH, Arai K. Adrenomedullin promotes differentiation of oligodendrocyte precursor cells into myelin-basic-protein expressing oligodendrocytes under pathological conditions in vitro. Stem Cell Research. 2015;15:68–74. doi: 10.1016/j.scr.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto N, Maki T, Shindo A, Liang AC, Maeda M, Egawa N, Itoh K, Lo EK, Lok J, Ihara M, Arai K. Astrocytes Promote Oligodendrogenesis after White Matter Damage via Brain-Derived Neurotrophic Factor. The Journal of Neuroscience. 2015;35:14002–14008. doi: 10.1523/JNEUROSCI.1592-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Rouse DJ, Hirtz DG, Thom E, Varner MW, Spong CY, Mercer BM, Iams JD, Wapner RJ, Sorokin Y, Alexander JM, Harper M, Thorp JM, Ramin SM, Malone FD, Carpenter M, Miodovnik M, Moawad A, O'Sullivan MJ, Peaceman AM, Hankins GDV, Langer O, Caritis SN, Roberts JM the Eunice Kennedy Shriver, N.M.F.M.U.N. A Randomized, Controlled Trial of Magnesium Sulfate for the Prevention of Cerebral Palsy. The New England journal of medicine. 2008;359:895–905. doi: 10.1056/NEJMoa0801187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saver JL, Starkman S, Eckstein M, Stratton SJ, Pratt FD, Hamilton S, Conwit R, Liebeskind DS, Sung G, Kramer I, Moreau G, Goldweber R, Sanossian N. Prehospital Use of Magnesium Sulfate as Neuroprotection in Acute Stroke. New England Journal of Medicine. 2015;372:528–536. doi: 10.1056/NEJMoa1408827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea KL, Palanisamy A. What can you do to protect the newborn brain? Current Opinion in Anesthesiology. 2015;28:261–266. doi: 10.1097/ACO.0000000000000184. [DOI] [PubMed] [Google Scholar]
- Vennemeyer JJ, Hopkins T, Kuhlmann J, Heineman WR, Pixley SK. Effects of elevated magnesium and substrate on neuronal numbers and neurite outgrowth of neural stem/progenitor cells in vitro. Neurosci Res. 2014;84:72–78. doi: 10.1016/j.neures.2014.05.001. [DOI] [PubMed] [Google Scholar]
- Volpe JJ, Kinney HC, Jensen FE, Rosenberg PA. The developing oligodendrocyte: key cellular target in brain injury in the premature infant. International Journal of Developmental Neuroscience. 2011;29:423–440. doi: 10.1016/j.ijdevneu.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermaier T, Stetter C, Kunze E, Willner N, Raslan F, Vince GH, Ernestus RI. Magnesium treatment for neuroprotection in ischemic diseases of the brain. Experimental & translational stroke medicine. 2013;5:6. doi: 10.1186/2040-7378-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.