Abstract
Although fibrosis is an essential response to acute cardiac tissue injury, prolonged myofibroblast activation and progressive fibrosis lead to further distortion of tissue architecture and worsened cardiac function. Thus, optimal tissue repair following injury requires tight control over myofibroblast activation. It is now recognized that inflammation plays a critical role in regulating fibrosis. In this review we will highlight how advances in the field of innate immunity have led to a better understanding of the role of inflammation in cardiovascular disease and, in particular, in the regulation of fibrosis. Specifically, we will discuss how the innate immune system recognizes tissue damage in settings of acute injury and chronic cardiovascular disease. We will also review the role of different cell populations in this response, particularly the unique role of different macrophage subsets and mast cells.
Keywords: Fibrosis, inflammation, innate immunity, macrophage, mast cell
Introduction
Recovery from tissue injury requires the coordinated activation of a variety of different reparative pathways. In the context of healing of damaged tissue, the term “repair” refers to the restoration of tissue architecture and function resulting either from tissue regeneration and/or tissue replacement. Regeneration refers to the type of wound healing wherein new tissue growth completely restores portions of damaged tissue to their normal state, whereas replacement refers to a type of wound healing in which severely damaged non-regenerable tissues are replaced by the creation of new connective tissue (i.e. tissue scarring), that is essential for maintaining the structural integrity of the injured tissue. Given that the adult mammalian heart has negligible regenerative capacity, the repair process following tissue injury requires a coordinated process that allows for the removal of the dead cells, followed by the replacement of the dead cells with new connective tissue. Here we will review the literature which suggests that the inflammation that occurs following tissue injury is required for proper tissue repair, as well as review the literature which suggests that if the inflammatory response becomes hyperactive following tissue injury, that this homeostatic repair process can lead to unwanted collateral damage and pathological tissue fibrosis. Finally, we will highlight the central role of fibroblast activation in tissue injury-induced inflammation and myocardial fibrosis.
Recognition of acute tissue injury by the immune system
Work over the last two decades has led to a greater understanding of the molecular mechanisms by which the innate and adaptive immune systems recognize infection and tissue injury. A wide variety of immune and non-immune cell types residing in the myocardium express germ line encoded pattern recognition receptors (PRR) that are capable of recognizing conserved molecular motifs shared by pathogenic bacteria and/or viruses, referred to as pathogen-associated molecular patterns (PAMPs). PAMPs are structures conserved among microbial species such as lipopolysaccharide of gram negative bacteria, teichoic acids of gram positive organisms, and double-stranded RNAs of viruses. The PRRs that have been implicated in sensing PAMPs include the canonical PRRs such as Toll-like receptors (TLRs), nucleotide-binding oligomerization domain (NOD)-like receptors and retinoic acid-inducible gene I (RIG-I) receptors, or atypical PRRs such as the RAGE receptor [1]. Germane to this discussion, activation of PRRs leads to activation of a variety of inflammatory mediators in the heart (reviewed in [2]). More recently it has become apparent that PRRs not only recognize PAMPs, but are also capable of recognizing molecular motifs residing on endogenous molecules that are released from damaged tissues, which have been referred to as damage associated molecular patterns (DAMPs) [1, 3]. When cells die by accidental necrosis, regulated necrosis (necroptosis), and/or secondary necrosis (late apoptosis), they release their cytosolic contents into the extracellular space, thereby initiating a brisk inflammatory response that mimics the inflammatory response triggered by pathogenic bacteria and/or viruses [4] [3]. Because the inflammatory response that ensues following tissue injury occurs in the absence of a known pathogenic infection, it is referred to as “sterile inflammation.”
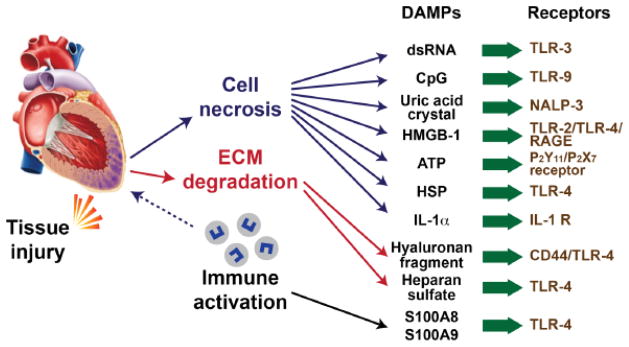
Ischemic injury is one of the best characterized models of acute cardiac injury [5, 6]. Infarction leads to necrotic cell death, with the subsequent release of DAMPs (Figure 1). The biological activity of DAMPs depends upon the overall extent of tissue injury, the type of cell death (necrosis vs apoptosis) and the type of cells dying (epithelial vs mesenchymal). DAMPs have been subdivided into 3 major categories, although some molecules can be included in multiple groups depending on the situation: leaderless proteins secreted by professional immune cells, also referred to as “alarmins” (e.g. high mobility box group 1 protein (HMGB1), interleukin IL-1β, galectin-3, uric acid); intracellular molecules released by dying cells (S100 proteins, HMGB1, IL-1α, galectin-3, heat shock protein (HSP) 60, HSP 70, HSP 72[7]) and/or molecules that are expressed on the cell surface membranes of stressed of dying cells (e.g. phosphatidylserine); and components of the extracellular matrix (hyaluronan, heparan sulphate, fibronectin and degraded matrix constituents). The precise biochemical moieties that distinguish whether an intracellular protein is immunogenic or non-immunogenic are unclear; however, it has been proposed that many known DAMPs contain hydrophobic regions that are ordinarily hidden in healthy living cells, that then become immunogenic when released into the extracellular space [8]. Importantly, DAMPs are capable of triggering the activation of NF-κB, AP1, CREB, c/EBP, and IRF transcription factors, which activate genes encoding chemokines, cytokines, and adhesion molecules that promote the recruitment and activation of leukocytes [9].
Figure 1.
Damage associated molecular patterns (DAMPs) are derived from dying cells that release their cytosolic content following myocardial injury, or from degradation of the extracellular matrix, as well as by immune cells that become activated following tissue injury. (Key: ATP = adenosine triphosphate, HSP = heat shock protein; HMGB1 = high mobility box group 1 protein, IL-1α - interleukin-1α; IL-1R– interleukin receptor NALP = NACHT, LRR and PYD domains-containing protein 3 (cryopyrin); RAGE = receptor for advanced glycation end products, TLR = Toll-like receptor) (Reproduced with permission Mann DL. In Blankesteijn WM and Altara R, eds. Inflammation in Heart Failure, Elsevier/Associated Press; 2015. p. 191–201)
Although a variety of different DAMPs have been implicated in initiating inflammation following myocardial infarction, the relative importance of individual molecules in the heart is unknown. While it is intuitively obvious that DAMPs play an important role in the setting of myocardial infarction, it is less clear what role DAMPs play, if any, in more chronic forms of tissue injury, such as sustained hemodynamic overloading, wherein the burden of ongoing cell death is much lower. Further, assessing the importance of DAMPs in vivo has been complicated by the redundancy of the system and the essential role that many DAMPs play in normal biology. A recent paper was able to dissect an important role for HMGB1 (a non-histone nuclear protein) in various forms of liver injury [10]. Mice with hepatocyte specific deficiency of HMGB1 survived doses of acetaminophen that are normally lethal. However, HMGB1 deficiency did not have an effect on inflammation or lethality in response to tumor necrosis factor (TNF), Fas-mediated apoptosis or LPS induced shock, suggesting that HMGB1 was not required for mediating classical inflammatory signaling pathways. As will be discussed below, following myocyte death that results from any form of tissue injury, DAMPs released by the dying cells are responsible for initiating myocardial inflammation and myocardial fibrosis.
Myofibroblasts
Myofibroblasts are mesenchymal cells that reside within the connective tissue of the heart, and are responsible for laying down extracellular matrix and generating scar tissue following tissue injury [11, 12]. These cells express α-smooth muscle actin (α-SMA), and share characteristics of both fibroblasts and smooth muscle cells. Fibroblast activation is determined by the increased α-SMA expression on fibroblasts, increased fibroblast proliferation, and increased fibroblast production of extracellular matrix components [13]. Although fibroblasts are abundant in the healthy heart, few if any myofibroblasts are present in the naïve heart. The ultimate source of myofibroblasts in the heart has been studied extensively, and is reviewed elsewhere in this series (see review by Davis). Myofibroblast activation and accumulation are controlled by a complex interaction of mechanical, neurohormonal and inflammatory signals [11, 14, 15]. Germane to this discussion, a variety of cytokines produced by immune cells, including transforming growth factor- β (TGF-β), platelet-derived growth factor (PDGF), and endothelin-1 play critical roles in activating resident interstitial fibroblasts to become myofibroblasts, as well as promote the persistence of myofibroblasts in areas of injured tissue, where they are capable of inducing the expression of a wide variety of extracellular matrix components [16]. In addition, fibroblasts express a variety of PRR and can directly recognize DAMPs, which can have important effects on the activation status of these cells. This topic is discussed below briefly, as well as addressed at length in an accompanying review in the series (see review by Turner). Interestingly, although sustained expression of classical pro-inflammatory cytokines such as TNF and IL-1 are associated with the development of myocardial fibrosis[17, 18], both IL-1 and TNF inhibit collagen gene expression and/or collagen synthesis in cardiac fibroblasts[19, 20], and therefore are not directly pro-fibrotic, suggesting that the effects of these canonical pro-inflammatory mediators on myocardial fibrosis are likely mediated indirectly, as will be discussed below.
Role of different immune cell types in tissue injury
A wide variety of immune cell types including mast cells, neutrophils, monocytes and macrophages, dendritic cells, T and B cells all play a role in response to cardiac injury and are activated in a stereotypical pattern following injury [21, 22]. Dissecting the functions of individual cell types can help to unravel the complex interrelationship between inflammation and the development of cardiovascular disease. The role of monocytes in myocardial fibrosis is reviewed elsewhere in this series (see review by Hulsmans). Here we will focus on the role of embryonic and bone marrow derived macrophages in the heart, as well as mast cells.
Macrophages have a wide variety of functions including roles in growth, regeneration, tissue repair, and defense against infections [23, 24]. Macrophages have long been believed to derive from circulating bone marrow-derived monocytes. However, it has recently become clear that in many tissues resident macrophages are not derived from circulating monocytes, but originate during embryonic development [25]. Recent studies have shown that macrophages in the heart are not a homogenous cell population, but can be divided into subtypes with unique origins and functions [26]. CC chemokine receptor 2 (CCR2) expression can be used to distinguish between macrophages that are derived from embryonic progenitors or adult monocytes. CCR2− macrophages are largely derived from yolk-sac and fetal monocyte progenitors, and are established during embryonic development. These cells are the most abundant macrophage subtype in the uninjured adult heart. CCR2+ macrophages are derived from adult monocytes. Following necrotic cardiac myocyte cell death there is recruitment of CCR2+ monocytes and monocyte-derived macrophages, resulting in increased numbers of these cells in the injured heart [27]. These macrophage subsets have unique functional properties; CCR2+ macrophages are pro-inflammatory, while CCR− macrophages are more reparative [27]. CCR2+ cells express significantly higher levels of IL-1, TNF, IL-6 and pro-inflammatory chemokines in response to cardiac injury or LPS treatment. In contrast, CCR2− macrophages, but not CCR2+ cells, are able to support angiogenesis and neonatal cardiomyocyte proliferation in vitro and in vivo [27].
The distinct functional properties of these macrophage subsets may explain the different outcomes observed following neonatal and adult cardiac injury [27]. Following cardiac injury neonatal mice regenerate myocardium and recover left ventricular function. In these mice, embryonic-derived CCR2− macrophages expand following injury and support the repair process. Depletion of macrophages in the neonatal mouse leads to defective repair and enhanced fibrosis. Conversely, in adult mice bone marrow derived monocytes are recruited to the heart following tissue injury. This leads to the accumulation of pro-inflammatory CCR2+ monocytes and monocyte-derived macrophages, and a suppression of the embryonic-derived CCR2− macrophage population, resulting in collateral tissue damage and progressive left ventricle remodeling. These unique functional characteristics also help explain why in a number of experimental settings that non-selective elimination of monocytes and macrophages leads to defective wound healing, whereas limiting monocyte influx into the heart is cytoprotective [15, 28–30]. The existence of tissue resident macrophages with reparative functions explains why non-selective elimination of these embryonic cell types results in worsened outcomes. In contrast, preventing bone marrow derived monocyte influx prevents the accumulation of pro-inflammatory macrophages that can further cause collateral damage, while allowing the populations of embryonic derived macrophages to be preserved. Given that minimizing fibrosis relies on optimal tissue repair it is likely that these macrophage subtypes play critical roles in determining the extent of fibrosis in a wide variety of cardiovascular diseases, although more evidence is needed to clarify their roles in different settings.
Another innate immune cell type that plays an important role in fibroblast activation and myocardial fibrosis is the tissue resident mast cell, which has been implicated in chronic inflammatory conditions that lead to fibrosis in the liver, lung and skin [31]. Multiple studies have demonstrated a direct relationship between mast cell density and collagen volume fraction in experimental [32, 33] and human heart failure[34–36]. Mast cells that home to sites of tissue injury express pattern recognition receptors that have been implicated in mast cell activation, as well as elaboration of pro-inflammatory cytokines by mast cells. Mast cells likely orchestrate the early recruitment of immune cells, including neutrophils, to the sites of tissue damage. This co-localization promotes cellular crosstalk and activation and results in the amplification of the local inflammatory response thereby promoting and sustaining tissue damage [37]. A recent study using mice with cardiac-restricted overexpression of TNF, as a model of para-inflammation, showed that sustained inflammatory signaling in the heart leads to progressive myocardial fibrosis and diastolic cardiac dysfunction through a mechanism that requires increased myocardial mast cell density, with a resultant increase in TGF-β mediated pro-fibrotic signaling between cardiac mast cells and resident cardiac fibroblasts [38]. In this study, mast cell and fibroblast co-culture experiments demonstrated that mast cell secretory products provoked a pro-fibrotic phenotype in wild-type fibroblasts, and that fibroblasts isolated from transgenic mouse model that had sustained myocardial inflammatory signaling exhibited an enhanced repertoire of pro-fibrotic phenotypic responses to mast cell mediators when compared to wild-type fibroblasts, suggesting that sustained inflammation promotes fibrosis by making fibroblasts more sensitive to TGF-β stimulation.
Linking tissue injury, inflammation and myocardial fibrosis
Recent studies have demonstrated a clear link between the release of DAMPs from necrotic myocardial tissue, and the development of myocardial inflammation and fibrosis [39]. As mentioned above, DAMPs released by injured cells are recognized by tissue resident immune and non-immune cells that are capable of elaborating a variety of pro-inflammatory cytokines and chemokines [39]. These molecules play an important role with respect to myofibroblast activation and tissue fibrosis. DAMPs are capable of activating a suite of transcription factors that upregulate genes that encode for cytokines and chemokines that stimulate the recruitment of neutrophils and pro-inflammatory macrophages to the site of tissue injury. Macrophages and neutrophils that are recruited to the site of tissue injury have proteolytic activity and phagocytic activity, which is essential for removal of necrotic material and is critical to the initiation of the repair process [28, 29, 40].
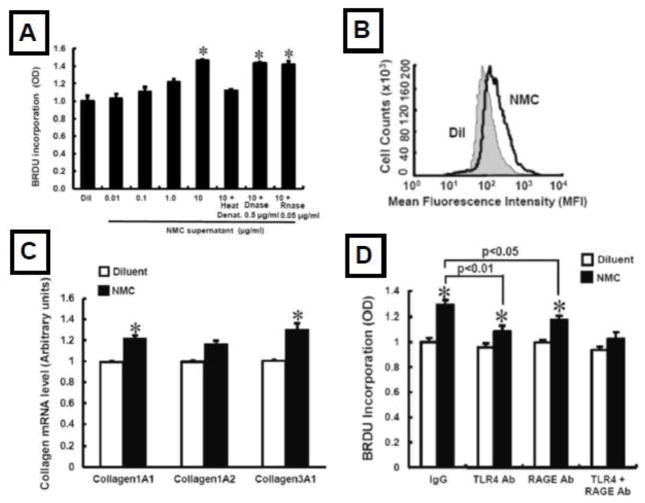
The “proliferative phase” of repair in response to myocyte death follows the acute inflammatory phase [6], and is characterized by the accumulation of myofibroblasts that synthesize extracellular matrix proteins and contribute to the neovascularization of injured tissue. DAMPs from necrotic myocardium lead to increased fibroblast α-SMA expression, increased fibroblast proliferation and migration, and increased collagen gene expression. DAMP-induced fibroblast responses require activation of TLR4 (Figure 2), and are dependent upon Akt and ERK signaling [39]. This same study showed that HMGB1, which has been referred to as the “master regulator of innate immunity [41], serves a non-redundant role with respect to fibroblast activation in vitro, and that HMGB1 was sufficient to provoke myocardial inflammation and fibrosis in vivo [39].
Figure 2.
Effect of DAMPs on fibroblast activation. A) Necrotic myocardial cell extracts provoke increased fibroblast proliferation; B) increased α-SMA expression by fibroblasts; C) increased collagen gene expression; D) the increase in DAMP-induced fibroblast proliferation was sensitive to inhibition with an anti-TLR4 antibody as well as an anti-RAGE antibody. (Key: RAGE = receptor for advanced glycation end products; TLR4 = Toll-like receptor 4). (Reproduced with permission from Zhang W, Lavine KJ, Epelman S et al. Necrotic myocardial cells release damage-associated molecular patterns that provoke fibroblast activation in vitro and trigger myocardial inflammation and fibrosis in vivo. J Am Heart Assoc 2015;4(6):e001993)
Macrophages also play a key role in mediating the transition from acute inflammation to fibrosis. Neutrophils that infiltrate the heart undergo apoptosis, where they are subsequently phagocytosed by macrophages. Recognition of phosphatidylserine motifs on apoptotic neutrophils, and engulfment of these apoptotic cells by macrophages, results in the downregulation of inflammatory cytokines such as TNF and IL-1 and the coordinated upregulation of anti-inflammatory cytokines such as IL-10 and TGF-β [42]. TGF-β plays a central role in driving myofibroblast accumulation and production of extracellular matrix [11, 15]. Thus, the same signals that suppress tissue injury induced inflammation are also responsible for triggering the activation of pro-fibrotic responses in cardiac fibroblasts. Pertinent to this review, depleting monocytes and macrophages after cardiac injury impairs myofibroblast accumulation, tissue angiogenesis, and collagen production [28], suggesting that these professional immune cells are required for fibroblast activation and proper tissue repair.
Chronic inflammation leads to chronic fibrosis
Multiple lines of evidence from experimental models of myocardial infarction and/or ischemia reperfusion injury suggest that defects in the pathways that are involved in timely resolution of the inflammatory responses in the heart result in adverse left ventricular remodeling. While the fibrosis that occurs following acute tissue injury is often critical for tissue repair and structural integrity of the heart, myocardial fibrosis can also result in excessive muscle fiber entrapment, myocyte loss, myocyte atrophy, electrical anisotropy and reentrant arrhythmias and/or abnormal diastolic and systolic stiffness of the myocardium, each of which is sufficient to contribute to the development and progression of left ventricular dysfunction [43]. Although it is clear that progressive myocardial fibrosis is deleterious to the heart, and although previous studies have identified a number of molecules that are sufficient to provoke increased collagen synthesis in isolated cardiac fibroblasts in vitro and in experimental models in vivo (e.g. PDGF, granulocyte colony-stimulating factor, angiotensin II, aldosterone, endothelin, connective tissue growth factor (CCN2/CTGF), and TGF-β)[44, 45] it is not at all clear from existing studies exactly how or why wound healing becomes dysregulated in the adult mammalian heart.
Although tremendous progress has been made with respect to determining the physiologic role of acute inflammation in response to tissue injury, far less is known about chronic inflammation, which is important in a wide variety of chronic cardiovascular diseases [46, 47]. There are multiple circumstances which can lead to chronic inflammation [46]. One important mechanism is the failure or inability to resolve the inflammatory response within the proper time frame following tissue injury. In this situation, the sustained inflammation can be related either to a persistent stimulus and/or failure of the molecular mechanisms that are responsible for resolution of the inflammatory response to complete the tissue repair process. This idea is well-illustrated by the chronic low grade inflammation that ensues following a myocardial infarction [2, 5]. Inflammation may continue because tissue homeostasis is not restored, and the innate and adaptive immune systems continue to recognize molecular motifs associated with ongoing tissue damage. As one example, the ongoing tissue damage that occurs secondary to the neurohormonal activation following an acute myocardial infarction may contribute to sustained myocardial inflammation and adverse left ventricular remodeling. Indeed, both angiotensin II and aldosterone are sufficient to provoke myocyte cell necrosis, apoptosis, activation of pro-inflammatory cytokines and chemokines, and directly activate fibroblasts (reviewed in [48]). Norepinephrine has been show to trigger myocyte necrosis and apoptosis, and thus may contribute to ongoing tissue damage (reviewed in [48]). Alternatively, the inflammatory reaction may never switch off because of a failure of the molecular mechanisms that are responsible for completing the tissue repair process remain persistently activated, as will be discussed below.
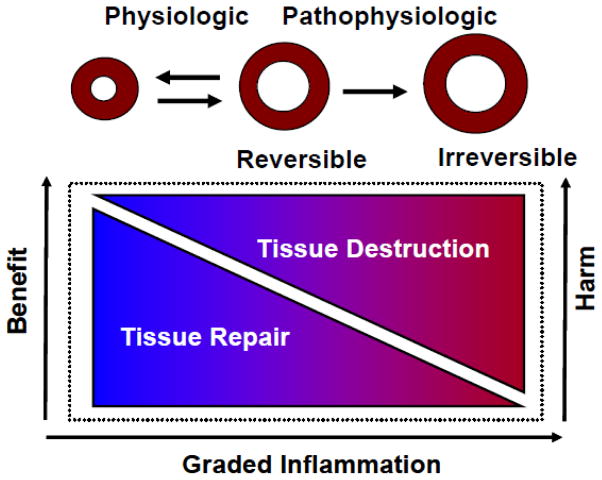
An intriguing concept that has arisen very recently is the notion that persistent low grade inflammation may develop within tissues that cannot restore their homeostatic balance, which has been termed “para-inflammation” [47]. Para-inflammation represents a graded sustained inflammatory response that remains “switched on” in dysfunctional tissue, in an attempt to restore homeostasis and tissue functionality (see Figure 3). The persistent low grade inflammation can contribute to further tissue dysfunction by virtue of the deleterious effects of sustained inflammatory signaling, including the development of pathological tissue fibrosis. Para-inflammation can arise from a variety of stresses at either the cellular or tissue level depending on the extent of the tissue dysfunction. For example, stresses that have been linked to para-inflammation include hypoxia, ER stress, and oxidative stress [49–51]. Although speculative at this time, it is possible that para-inflammation is an important mechanism that contributes to the myocardial fibrosis that occurs in heart failure. Several potential mechanisms that may contribute to para-inflammation in the failing heart include neurohormonal activation, as well as ongoing myocyte necrosis and/or apoptosis. However, although the level of cell death that occurs in the failing heart likely plays a role in disease progression [52], at the time of this writing it is unclear whether this low level of cell death is sufficient to drive an inflammatory response through DAMP-induced activation of innate immune responses. Another interesting possibility is that impaired autophagic flux might also contribute to sustained inflammation, as has been demonstrated experimentally, following hemodynamic overloading [53].
Figure 3.
Para-inflammation. The primary purpose of the inflammatory response in the heart is to resolve tissue injury, thereby allowing the heart to adapt to the abnormal conditions in the short-term, and ultimately to restore homeostasis and cardiovascular function in the long-term. If the abnormal condition is sustained, then a chronic inflammatory state persists in a tissue, which is referred to as para-inflammation. Para-inflammation is a graded response that can restore tissue homeostasis, or if sustained can contribute to further disease progression, by virtue of the deleterious effects of sustained inflammation on cardiac myocytes and the extracellular matrix. (Reproduced with permission from Mann DL. Innate Immunity and the Failing Heart: The Cytokine Hypothesis Revisited. Circ Res 2015 March 27;116:1254–68).
Conclusion
In the foregoing review we have highlighted the literature which supports the thesis that activation of the innate and adaptive immune systems play a central role in mediating tissue repair in the heart. Although the inflammatory response that occurs during tissue injury is necessary for proper tissue repair, it can lead to pathological tissue fibrosis if it becomes dysregulated. The observation that inflammation-induced tissue fibrosis is essential for proper tissue repair, but is also capable of causing tissue dysfunction, emphasizes the importance of a obtaining a deeper understanding the regulation of this critical homeostatic process, particularly as it relates to the concept of para-inflammation and pathological tissue fibrosis.
Highlights.
Fibrosis is an essential response to acute cardiac tissue injury
Optimal tissue repair following injury requires tight control over myofibroblast activation to prevent pathological fibrosis
Advances in the field of innate immunity have led to a better understanding of the role of inflammation and the regulation of fibrosis in the heart.
A variety of different cell types participate in the development of fibrosis including fibroblasts, subsets of macrophages and mast cells
Acknowledgments
This research was supported by research funds from the N.I.H. (RO1 HL89543 R01 HL58081, RO1 111094, T32 HL007081)
Reference List
- 1.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–20. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 2.Mann DL. Innate Immunity and the Failing Heart: The Cytokine Hypothesis Revisited. Circ Res. 2015;116:1254–68. doi: 10.1161/CIRCRESAHA.116.302317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rock KL, Latz E, Ontiveros F, Kono H. The sterile inflammatory response. Annu Rev Immunol. 2010;28:321–42. doi: 10.1146/annurev-immunol-030409-101311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8:279–89. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frangogiannis NG. The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol. 2014;11:255–65. doi: 10.1038/nrcardio.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shinde AV, Frangogiannis NG. Fibroblasts in myocardial infarction: a role in inflammation and repair. J Mol Cell Cardiol. 2014;70:74–82. doi: 10.1016/j.yjmcc.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin L, Knowlton AA. Innate immunity and cardiomyocytes in ischemic heart disease. Life Sci. 2014;100:1–8. doi: 10.1016/j.lfs.2014.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seong SY, Matzinger P. Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat Rev Immunol. 2004;4:469–78. doi: 10.1038/nri1372. [DOI] [PubMed] [Google Scholar]
- 9.Newton K, Dixit VM. Signaling in innate immunity and inflammation. Cold Spring Harb Perspect Biol. 2012:4. doi: 10.1101/cshperspect.a006049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huebener P, Pradere JP, Hernandez C, Gwak GY, Caviglia JM, Mu X, et al. The HMGB1/RAGE axis triggers neutrophil-mediated injury amplification following necrosis. J Clin Invest. 2015;125:539–50. doi: 10.1172/JCI76887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis J, Molkentin JD. Myofibroblasts: trust your heart and let fate decide. J Mol Cell Cardiol. 2014;70:9–18. doi: 10.1016/j.yjmcc.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weber KT, Sun Y, Bhattacharya SK, Ahokas RA, Gerling IC. Myofibroblast-mediated mechanisms of pathological remodelling of the heart. Nat Rev Cardiol. 2013;10:15–26. doi: 10.1038/nrcardio.2012.158. [DOI] [PubMed] [Google Scholar]
- 13.Bechtel W, McGoohan S, Zeisberg EM, Muller GA, Kalbacher H, Salant DJ, et al. Methylation determines fibroblast activation and fibrogenesis in the kidney. Nat Med. 2010;16:544–50. doi: 10.1038/nm.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duffield JS, Lupher M, Thannickal VJ, Wynn TA. Host responses in tissue repair and fibrosis. Annu Rev Pathol. 2013;8:241–76. doi: 10.1146/annurev-pathol-020712-163930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18:1028–40. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leask A. Getting to the heart of the matter: new insights into cardiac fibrosis. Circ Res. 2015;116:1269–76. doi: 10.1161/CIRCRESAHA.116.305381. [DOI] [PubMed] [Google Scholar]
- 17.Sivasubramanian N, Coker ML, Kurrelmeyer K, DeMayo F, Spinale FG, Mann DL. Left ventricular remodeling in transgenic mice with cardiac restricted overexpression of tumor necrosis factor. Circulation. 2001;2001:826–31. doi: 10.1161/hc3401.093154. [DOI] [PubMed] [Google Scholar]
- 18.Kubota T, McTiernan CF, Frye CS, Slawson SE, Koretsky AP, Demetris AJ, et al. Dilated cardiomyopathy in transgenic mice with cardiac specific overexpression of tumor necrosis factor-alpha. Circ Res. 1997;81:627–35. doi: 10.1161/01.res.81.4.627. [DOI] [PubMed] [Google Scholar]
- 19.Siwik DA, Chang DL, Colucci WS. Interleukin-1beta and tumor necrosis factor-alpha decrease collagen synthesis and increase matrix metalloproteinase activity in cardiac fibroblasts in vitro. Circ Res. 2000;86:1259–65. doi: 10.1161/01.res.86.12.1259. [DOI] [PubMed] [Google Scholar]
- 20.Peng J, Gurantz D, Tran V, Cowling RT, Greenberg BH. Tumor necrosis factor-alpha-induced AT1 receptor upregulation enhances angiotensin II-mediated cardiac fibroblast responses that favor fibrosis. Circ Res. 2002;91:1119–26. doi: 10.1161/01.res.0000047090.08299.d5. [DOI] [PubMed] [Google Scholar]
- 21.Epelman S, Mann DL. Communication in the heart: the role of the innate immune system in coordinating cellular responses to ischemic injury. J Cardiovasc Transl Res. 2012;5:827–36. doi: 10.1007/s12265-012-9410-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Epelman S, Liu PP, Mann DL. Role of innate and adaptive immune mechanisms in cardiac injury and repair. Nat Rev Immunol. 2015;15:117–29. doi: 10.1038/nri3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–37. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–55. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Epelman S, Lavine KJ, Randolph GJ. Origin and functions of tissue macrophages. Immunity. 2014;41:21–35. doi: 10.1016/j.immuni.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Epelman S, Lavine KJ, Beaudin AE, Sojka DK, Carrero JA, Calderon B, et al. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during Inflammation. Immunity. 2014;40:91–104. doi: 10.1016/j.immuni.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lavine KJ, Epelman S, Uchida K, Weber KJ, Nichols CG, Schilling JD, et al. Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling. doi: 10.1073/pnas.1406508111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–47. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Amerongen MJ, Harmsen MC, van RN, Petersen AH, van Luyn MJ. Macrophage depletion impairs wound healing and increases left ventricular remodeling after myocardial injury in mice. Am J Pathol. 2007;170:818–29. doi: 10.2353/ajpath.2007.060547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Panizzi P, Swirski FK, Figueiredo JL, Waterman P, Sosnovik DE, Aikawa E, et al. Impaired infarct healing in atherosclerotic mice with Ly-6C(hi) monocytosis. J Am Coll Cardiol. 2010;55:1629–38. doi: 10.1016/j.jacc.2009.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Metz M, Grimbaldeston MA, Nakae S, Piliponsky AM, Tsai M, Galli SJ. Mast cells in the promotion and limitation of chronic inflammation. Immunol Rev. 2007;217:304–28. doi: 10.1111/j.1600-065X.2007.00520.x. [DOI] [PubMed] [Google Scholar]
- 32.De Jongste MJ, Haaksma J, Hautvast RW, Hillege HL, Meyler PW, Staal MJ, et al. Effects of spinal cord stimulation on myocardial ischaemia during daily life in patients with severe coronary artery disease. A prospective ambulatory electrocardiographic study. Br Heart J. 1994;71:413–8. doi: 10.1136/hrt.71.5.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patella V, Marino I, Arbustini E, Lamparter-Schummert B, Verga L, Adt M, et al. Stem cell factor in mast cells and increased mast cell density in idiopathic and ischemic cardiomyopathy. Circulation. 1998;97:971–8. doi: 10.1161/01.cir.97.10.971. [DOI] [PubMed] [Google Scholar]
- 34.Akgul A, Skrabal CA, Thompson LO, Loebe M, Lafuente JA, Noon GP, et al. Role of mast cells and their mediators in failing myocardium under mechanical ventricular support. J Heart Lung Transplant. 2004;23:709–15. doi: 10.1016/j.healun.2003.06.006. [DOI] [PubMed] [Google Scholar]
- 35.Batlle M, Perez-Villa F, Lazaro A, Garcia-Pras E, Ramirez J, Ortiz J, et al. Correlation between mast cell density and myocardial fibrosis in congestive heart failure patients. Transplant Proc. 2007;39:2347–9. doi: 10.1016/j.transproceed.2007.06.047. [DOI] [PubMed] [Google Scholar]
- 36.Akgul A, Youker KA, Noon GP, Loebe M. Quantitative changes in mast cell populations after left ventricular assist device implantation. ASAIO J. 2005;51:275–80. doi: 10.1097/01.mat.0000150507.61120.00. [DOI] [PubMed] [Google Scholar]
- 37.Brown MA, Hatfield JK. Mast Cells are Important Modifiers of Autoimmune Disease: With so Much Evidence, Why is There Still Controversy? Front Immunol. 2012;3:147. doi: 10.3389/fimmu.2012.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang W, Chancey AL, Tzeng HP, Zhou Z, Lavine KJ, Gao F, et al. The Development of Myocardial Fibrosis in Transgenic Mice With Targeted Overexpression of Tumor Necrosis Factor Requires Mast Cell-Fibroblast Interactions. Circulation. 2011;124:2106–16. doi: 10.1161/CIRCULATIONAHA.111.052399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang W, Lavine KJ, Epelman S, Evans SA, Weinheimer CJ, Barger PM, et al. Necrotic myocardial cells release damage-associated molecular patterns that provoke fibroblast activation in vitro and trigger myocardial inflammation and fibrosis in vivo. J Am Heart Assoc. 2015;4:e001993. doi: 10.1161/JAHA.115.001993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frantz S, Hofmann U, Fraccarollo D, Schafer A, Kranepuhl S, Hagedorn I, et al. Monocytes/macrophages prevent healing defects and left ventricular thrombus formation after myocardial infarction. FASEB J. 2013;27:871–81. doi: 10.1096/fj.12-214049. [DOI] [PubMed] [Google Scholar]
- 41.Vezzoli M, Castellani P, Corna G, Castiglioni A, Bosurgi L, Monno A, et al. High-mobility group box 1 release and redox regulation accompany regeneration and remodeling of skeletal muscle. Antioxid Redox Signal. 2011;15:2161–74. doi: 10.1089/ars.2010.3341. [DOI] [PubMed] [Google Scholar]
- 42.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–8. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weber KT, Brilla CG, Janicki JS. Myocardial Fibrosis - Functional Significance and Regulatory Factors. Cardiovasc Res. 1993;27:341–8. doi: 10.1093/cvr/27.3.341. [DOI] [PubMed] [Google Scholar]
- 44.Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med. 2000;342:1350–8. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- 45.Reisdorf P, Lawrence DA, Sivan V, Klising E, Martin MT. Alteration of transforming growth factor-beta1 response involves down-regulation of Smad3 signaling in myofibroblasts from skin fibrosis. Am J Pathol. 2001;159:263–72. doi: 10.1016/s0002-9440(10)61692-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140:871–82. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 47.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–35. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 48.Mann DL, Bristow MR. Mechanisms and models in heart failure: the biomechanical model and beyond. Circulation. 2005;111:2837–49. doi: 10.1161/CIRCULATIONAHA.104.500546. [DOI] [PubMed] [Google Scholar]
- 49.Chovatiya R, Medzhitov R. Stress, inflammation, and defense of homeostasis. Mol Cell. 2014;54:281–8. doi: 10.1016/j.molcel.2014.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ip WK, Medzhitov R. Macrophages monitor tissue osmolarity and induce inflammatory response through NLRP3 and NLRC4 inflammasome activation. Nat Commun. 2015;6:6931. doi: 10.1038/ncomms7931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–17. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Whelan RS, Kaplinskiy V, Kitsis RN. Cell death in the pathogenesis of heart disease: mechanisms and significance. Annu Rev Physiol. 2010;72:19–44. doi: 10.1146/annurev.physiol.010908.163111. [DOI] [PubMed] [Google Scholar]
- 53.Oka T, Hikoso S, Yamaguchi O, Taneike M, Takeda T, Tamai T, et al. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature. 2012;485:251–5. doi: 10.1038/nature10992. [DOI] [PMC free article] [PubMed] [Google Scholar]