Abstract
Introduction
Outpatient treatments for adolescent substance use demonstrate clinically meaningful reductions in substance use, but effect sizes are often low, relapse rates are high, and response to treatment is heterogeneous across participants. The present study utilized cluster analysis to identify subgroups of treatment response among adolescents from three randomized clinical trials evaluating behavioral treatments for substance use.
Methods
Analyses were performed on a sample of 194 adolescents (average age=15.8, 81.4% male) who reported cannabis use during the past 30 days or had a cannabis-positive urine test. Clustering was based on percent days cannabis use at 5 time periods (intake, end of treatment, 3, 6, and 9 months post-treatment). Participants in the identified subgroups were then compared across a number of variables not involved in the clustering (e.g., substance use, demographics, and psychopathology) to test for predictors of cluster membership.
Results
Four clusters were identified based on statistical indices and visual inspection of the resulting cluster profiles: Low Use Responders (n=109, low baseline level, sustained decrease); High Use Responders (n=45, high baseline level, sustained decrease); Relapsers (n=25, medium baseline level, decrease, rapid increase post-treatment); and Non-Responders (n=15; consistently high level of use). Cannabis dependence, mean cannabis uses per day, and socioeconomic status were predictive of cluster membership.
Conclusions
Cluster analysis empirically identified different patterns of treatment response over time for adolescent outpatients. Investigating homogenous subgroups of participants provides insight into study outcomes, and variables associated with clusters have potential utility to identify participants that may benefit from more intensive treatment.
Keywords: Cluster analysis, subgroup analysis, adolescents, cannabis use, substance abuse
1. Identifying Treatment Response Subgroups for Adolescent Cannabis Use
Among adolescents admitted to substance use treatment, 76% report cannabis as the primary substance (Substance Abuse and Mental Health Services Administration (SAMHSA), 2014). Outpatient treatments for cannabis use disorders have demonstrated effectiveness at reducing substance use (Tanner-Smith, Wilson, & Lipsey, 2013; Waldron & Turner, 2008; Williams & Chang, 2000). Although treatments demonstrate clinically meaningful reductions in substance use, treatment effect sizes are often low in magnitude. Rates of relapse in the year following treatment are also high (Brown, Vik, & Creamer, 1989; Williams & Chang, 2000), with an average of about one-third of adolescents demonstrating sustained post-treatment abstinence, and about one-half of adolescents sustaining a level of reduced substance use compared to pre-treatment levels.
One step towards improving substance use treatment is to better understand relations among participant characteristics and treatment response. Response to treatment across participants is heterogeneous, but although each individual may respond differently, patterns of similar responses are likely also present (Maisto, Pollock, Lynch, Martin, & Ammerman, 2001; Spear, Ciesla, & Skala, 1999; Waldron, Turner, & Ozechoswski, 2005). Demographic, psychopathological, and treatment-related variables have been extensively explored in past studies as predictors of adolescent substance use treatment outcome. Variables such as age, gender, race, substance use severity, treatment length, conduct disorder, oppositional defiant disorder, depression, anxiety, family conflict, and school attendance have predicted outcomes (Crowley, Mikulich, MacDonald, Young, & Zerbe, 1998; Dakof, Tejeda, & Liddle, 2001; Friedman, Terras, & Kreisher, 1995; Hendricks, van der Schee, & Blanken, 2012; Latimer, Newcomb, Winters, & Stinchfield, 2000; Latimer, Winters, Stinchfield, & Traver, 2000; Rowe, Liddle, Greenbaum, & Henderson, 2004; Williams & Chang, 2000).
Exploratory statistical techniques, such as cluster analysis, can be used to empirically identify homogenous subgroups; this creates new opportunities to test predictors of treatment response, particularly when treatment response is on a continuum, such as percent days of substance use, rather than categorical, such as abstinent versus not abstinent. Waldron and colleagues (2005) utilized cluster analysis to identify subgroups of adolescents that received outpatient treatment for substance use. Four clusters, based on percent days cannabis use across treatment assessment points, were identified: Improvers (rapid improvement and continued low use), Slow Improvers (gradual improvement), Relapsers (rapid improvement followed by increasing use), and Resistant (continuous heavy use). Trajectories of post-treatment continued low use, continued high use, and relapse, based on measures of cannabis use severity, have been identified in additional studies (Brown, Vik, & Creamer, 1989; Godley, Dennis, Godley, & Funk, 2004; Henderson, Dakof, Greenbaum, & Liddle, 2010) and suggest these may be common long-term responses to substance use treatment. The present study was conducted to complement the findings of Waldron et al. (2005), in a new sample involving different types of treatment, to contribute to evidence for common treatment response patterns of cannabis use.
The goals of the present study were to identify treatment response subgroups in a combined sample of adolescents that received outpatient therapy for substance use and to identify predictors of these subgroups. Cluster analysis, based on percent days cannabis use measured at five assessment points, was utilized to identify homogenous subgroups of treatment response. Baseline variables, including other substance use variables, demographics, and psychopathology, were tested as predictors of the identified clusters. We hypothesized that empirically distinct patterns of cannabis use over time would emerge from cluster analyses, and that these patterns would be comparable to those reported by Waldron et al. (2005).
2. Method
2.1. Participants
Data were combined from three randomized clinical trials that evaluated outpatient behavioral treatments for adolescent substance use (Stanger, Budney, Kamon, & Thostensen, 2009; Stanger, Ryan, Scherer, Norton, & Budney, 2015; Stanger, Scherer, Babbin, Ryan, Norton, & Budney, 2016). In these trials, all adolescents received individual Motivational Enhancement Therapy/Cognitive Behavioral Therapy (MET/CBT; Sampl & Kadden, 2001; Webb, Scudder, Kaminer, & Kadden, 2002). At least one treatment arm in each study also included an abstinence-based contingency management (CM) intervention.
Two studies, Arkansas 1 (AR-1) and Arkansas 2 (AR-2), were completed at the University of Arkansas for Medical Sciences, and one (Vermont; VT) was completed at the University of Vermont; each study was conducted in compliance with the Institutional Review Board of the corresponding university. Youth were enrolled in VT between April 2003 and April 2005, with follow-up assessments completed by April 2006; youth were enrolled in AR-1 between December 2007 and October 2011, with follow-up assessments completed by June 2012; and youth in AR-2 were enrolled between December 2007 and March 2011, with follow-up assessments completed by July 2012. Inclusion criteria consistent across all studies included: age 12–18 (if 18, in high school) and living with a parent or guardian who agreed to participate. Additional inclusion criteria for AR-2 (N=153) and VT (N=69) involved cannabis use in the past 30 days or a cannabis-positive urine test. Criteria for AR-2 also included a diagnosis of cannabis abuse or dependence. Additional details about these two studies are available elsewhere (Stanger et al., 2009; Stanger et al., 2015). Additional inclusion criteria for AR-1 (N=75) included alcohol use during the past 30 days or an alcohol-positive urine test and meeting criteria for alcohol abuse or dependence or reporting one or more binging episodes (5 or more drinks) in the past 90 days. Cannabis use disorders were not exclusion criteria. Additional details about this study are available elsewhere (Stanger et al., 2016).
For the present analyses, we included only participants who, at intake, had used cannabis during the past 30 days or had a cannabis-positive urine test, and had data at all assessment time points. This resulted in a sample size of N=194 (out of a possible N=297), with 12, 49, and 20 participants excluded for missing data at any follow-up assessments from AR-1, AR-2, and VT, respectively. An additional 22 participants were excluded from AR-1 due to no cannabis use at intake. Baseline characteristics are included in Table 1. Included participants (N=194) did not significantly differ from excluded participants (n=103) across these baseline characteristics.
Table 1.
Sample characteristics at baseline for each study and for the combined analytic sample.
| AR 1 (n=41) | AR 2 (n=104) | VT (n=49) | χ2 or F | Combined (N=194) | |
|---|---|---|---|---|---|
| Mean age | 16.0 (SD=1.2) | 15.7 (SD=1.4) | 15.9 (SD=1.1) | 1.2 | 15.8 (SD=1.3) |
| Hollingshead 9-step SES | 6.8 (SD=2.1) | 5.0 (SD=2.5) | 6.9 (SD = 1.8) | 16.0* | 5.9 (SD=2.1) |
| Gender (Male) | 68.3% | 85.6% | 83.7% | 6.0* | 81.4% |
| Ethnicity (Hispanic) | 2.4% | 1.9% | 0% | 0.1 | 2.1% |
| Race (White) | 85.4% | 33.7% | 89.8% | 58.5* | 60.3% |
| Cannabis Dependencea | 51.2% | 26.0% | 42.9% | 9.7* | 35.6% |
| Cannabis Abusea | 39.0% | 74.0% | 44.9% | 20.6* | 59.3% |
| % Days Cannabis Use | 43.2% | 37.5% | 50.3% | 2.6 | 41.9% |
| Mean Cannabis Uses per Day | 2.0 (SD=1.4) | 1.7 (SD=1.0) | 1.8 (SD=1.4) | 0.8 | 1.8 (SD=1.2) |
| Alcohol Dependencea | 19.5% | 0% | 0% | 31.1* | 4.1% |
| Alcohol Abusea | 39.0% | 4.8% | 20.4% | 26.6* | 16.0% |
| Mean Drinks per Day | 6.0 (SD=4.2) | 1.9 (SD=3.1) | 3.8 (SD=4.4) | 18.2* | 3.2 (SD=4.1) |
p<0.05
Based on youth interview.
2.2 Procedure
Study procedures were similar across the three studies. Adolescents received 14 weeks of individual MET/CBT. Additional treatment components included a CM intervention, which involved a combination of clinic and home-based incentives for abstinence from all substances, and a parent training (PT) intervention which targeted conduct problems. Therapists included master’s level and postdoctoral level clinicians for AR-1 and VT, and master’s level clinicians for AR-2. In each study, treatment integrity was assessed by videotaping sessions and discussing each session in weekly supervision. In AR-1 and AR-2, adherence to the Family Management Curriculum was assessed using the Fidelity of Implementation (FIMP; Forgatch, Patterson, & GeGarmo, 2005), and adherence to MET/CBT was assessed using the Yale Adherence Competence Scale (YACS; Carrol et al., 2000). Fidelity scores were in acceptable ranges and were similar across studies (Stanger et al., 2015; 2016).
Adolescents attended an intake to complete an assessment battery, and eligible adolescents were then assigned to treatment condition. Across studies adolescents were randomized to one of three conditions: MET/CBT (n=77), MET/CBT+CM (n=34), or MET/CBT+CM+PT (n=83). Overall, 117/194 received treatment that included CM. Those in AR-1 received once-weekly urine drug testing while those in AR-2 and VT received twice-weekly drug testing; other drug testing details were identical. Parents/guardians were informed of drug toxicology results across all conditions. All families were offered an additional 12 weeks of once-weekly urine drug testing after treatment. At the end of treatment (ETx) and at 3, 6, and 9 months post-treatment, adolescent and parent(s) completed a follow-up assessment.
Each of these trials demonstrated statistically significant decreases in cannabis use during treatment and demonstrated stronger effects for MET/CBT+CM than MET/CBT alone (Stanger et al., 2009; 2015; 2016). The addition of the PT intervention was not associated with additional change in cannabis use compared to MET/CBT+CM (Stanger et al., 2015). Thus, the treatment group predictor variable is the present study was defined as CM vs. no CM. Post-treatment maintenance of these decreases in cannabis use was poor, with increases in cannabis use at follow-up time points.
2.3 Measures
Substance use, including cannabis, alcohol, and other substances was assessed at each time point using the timeline follow back method (TLFB; Sobell & Sobell, 1992). Percent days cannabis use was calculated as the number of reported days of use divided by the number of days for which data were available. Mean cannabis uses per day was calculated as the total number of uses (each “use” reported was separated by at least an hour in the day) divided by the number of reported days of cannabis use. Mean drinks per day was calculated as the number of standard drinks (approximately 12oz beer, 4oz wine, or 1oz liquor) divided by the number of reported days of alcohol use. For cannabis and drinks per day, 0 days of use was coded with a mean of 0, and incomplete data (fewer than 85% of possible days) were coded as missing. The Vermont Structured Diagnostic Interview (VSDI; Hudziak, Copeland, Stanger, & Wadsworth, 2004) was used to assess DSM-IV substance use disorders and mental health diagnoses. The VSDI was administered separately to adolescents and parents, with parents reporting about their teen. Substance use diagnoses were based on adolescent report. ADHD, anxiety, depression, conduct disorder, and oppositional defiant disorder diagnoses were based on adolescent and parent reports; a positive diagnosis from either (or both) was counted as a positive diagnosis in the analyses. Discrepancies are common when comparing self-reports and parent reports of psychopathy, and considering both informants is necessary to prevent under-reporting (Comer & Kendall, 2004; Salbach-Andrae, Klinkowski, Lenz, & Lehmkuhl, 2009).
2.4 Cluster analysis
Cluster analyses were based on the percent days cannabis use reported during five time periods assessed via TLFB (intake, ETx, 3, 6, and 9 months post-treatment). Individuals were grouped into categories based on the level (means), scatter (variances), and shape of the patterns over these time periods. Clustering was performed using a squared Euclidean distance metric (Cronbach & Gleser, 1953) and Ward’s minimum variance clustering (Ward, 1963). Multiple methods were utilized to determine the number of clusters, including statistical indices such as the inverse scree test (Lathrop & Williams, 1987), the pseudo-F test (Calinski & Harabasz, 1974), and the cubic clustering criterion (Sarle, 1983), as well as visual inspections of the resulting cluster profiles. Descriptions of the subgroups were based primarily on shape of the profiles (pattern of changes in level across time). Discrimination among the clusters, including effects of cluster and the cluster*time interaction, was tested with repeated measures analysis of variance (ANOVA) with cluster membership as the independent variable and percent days cannabis use at the five time points as dependent variables. A series of post-hoc paired t-tests were conducted for each cluster to test for differences in percent days cannabis use between assessment points.
2.5 Predictor variables
A total of twelve variables were tested as predictors. Demographic and study-related variables tested were: age, race (white vs. other), sex (male vs. female), socioeconomic status based on the Hollingshead 9-step occupation scale (SES; Hollingshead, 1975), and treatment (CM vs. no CM). Substance use variables tested were: DSM cannabis diagnosis (cannabis dependence vs. cannabis abuse or no abuse/dependence), mean cannabis uses per day, DSM alcohol diagnosis (alcohol abuse or dependence vs. no alcohol abuse or dependence), and mean drinks per day. For cannabis diagnosis, youth with cannabis abuse or no/abuse dependence were grouped together due to the small number without a use disorder (n=10). Psychopathology variables tested were: internalizing (DSM diagnosis of depression or anxiety vs. none), externalizing (DSM diagnosis of conduct disorder or oppositional defiant disorder vs. none), and ADHD (diagnosis vs. none). Data were available for other predictors, including parenting factors, but a limited number of adolescent-focused variables were selected to reduce experimentwise error. Parents were involved in all three studies, but the parenting intervention was only administered to the families that received CM. As parenting was integrated with the CM condition, differences in parenting factors were controlled by testing CM as a predictor variable. The predictor variables were tested as predictors at the univariate level with a series of chi-square and one-way ANOVAs. Significant predictors were then tested with multinomial logistic regression.
3. Results
3.1 Cluster subgroups
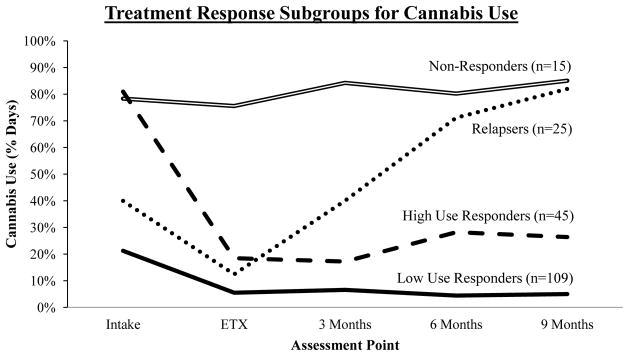
Statistical indices and visual inspections of different cluster solutions suggested that a four-cluster solution provided the best representation of the data; cluster means for percent days cannabis use are displayed in Figure 1. Cluster sizes, means, and standard deviations are summarized in Table 2. There was a significant effect for cluster, F(3,190)=346.72, p<.001, η2=.85; time, F(4,760)=43.85, p<.001, η2=.19; and for the cluster*time interaction, F(12,760)=39.69, p<.001, η2=.39.
Figure 1.
Four treatment response subgroups for percent days cannabis use across five assessment points.
Table 2.
Means and standard deviations of percent days cannabis use (converted to decimals) for the four clusters across the five assessment points.
| Low Use Responders (n=109) | High Use Responders (n=45) | Relapsers (n=25) | Non-Responders (n=15) | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Mean | S.D. | Mean | S.D. | Mean | S.D. | Mean | S.D. | |
| Intake | 0.21 | 0.17 | 0.81 | 0.19 | 0.40 | 0.32 | 0.78 | 0.25 |
| ETx | 0.05 | 0.11 | 0.18 | 0.26 | 0.12 | 0.17 | 0.76 | 0.20 |
| 3 Months | 0.07 | 0.14 | 0.17 | 0.22 | 0.40 | 0.31 | 0.84 | 0.24 |
| 6 Months | 0.04 | 0.08 | 0.28 | 0.25 | 0.71 | 0.25 | 0.80 | 0.32 |
| 9 Months | 0.05 | 0.09 | 0.26 | 0.24 | 0.82 | 0.17 | 0.85 | 0.16 |
Cluster 1 was labeled Low Use Responders (n=109, 56.2%). This subgroup was characterized by the lowest percent days cannabis use at intake (21%) followed by a statistically significant decrease by the end of treatment and then non-significant changes (Table 3). The Low Use Responders were the largest cluster and generally had the lowest amount of variability (scatter) across all assessment points. Cluster 2 was labeled High Use Responders (n=45, 23.2%). This subgroup was characterized by a very high percent days cannabis use at intake (81%) followed by a statistically significant decrease by the end of treatment and then non-significant changes (Table 3). The High Use Responders were the second-largest cluster. Cluster 3 was labeled Relapsers (n=25, 12.9%). This subgroup was characterized by a medium percent days cannabis use at intake (40%), a statistically significant decrease by the end of treatment, a statistically significant increase after treatment to six months, and then non-significant changes (Table 3). The Relapsers were the third-largest cluster. Cluster 4 was labeled Non-Responders (n=15, 7.7%). This subgroup was characterized by a very high percent days cannabis use at intake (78%) with non-significant changes over time (Table 3). The Non-Responders were the smallest cluster.
Table 3.
Paired t-tests of mean percent of days used by subgroup across assessment points.
| Assessment point comparison | Mean difference | S.E. | p-valuea | 95% CI |
|---|---|---|---|---|
| Low Use Responders (n=109) | ||||
| ETx - Intake | −0.16 | 0.02 | <0.01 | (−0.21, −0.11) |
| 3 Months – ETx | 0.01 | 0.02 | 1.00 | (0.03, 0.06) |
| 6 Months – 3 Months | −0.02 | 0.01 | 1.00 | (−0.06, 0.02) |
| 9 Months – 6 Months | 0.01 | 0.01 | 1.00 | (−0.02, 0.03) |
| High Use Responders (n=45) | ||||
| ETx – Intake | −0.63 | 0.05 | <0.01 | (−0.77, −0.48) |
| 3 Months – ETx | −0.01 | 0.04 | 1.00 | (−0.14, 0.12) |
| 6 Months – 3 Months | 0.11 | 0.04 | 0.10 | (−0.01, 0.23) |
| 9 Months – 6 Months | −0.02 | 0.05 | 1.00 | (−0.15, 0.12) |
| Relapsers (n=25) | ||||
| ETx – Intake | −0.28 | 0.08 | 0.02 | (−0.51, −0.04) |
| 3 Months – ETx | 0.28 | 0.08 | 0.02 | (0.03, 0.53) |
| 6 Months – 3 Months | 0.31 | 0.06 | <0.01 | (0.12, 0.51) |
| 9 Months – 6 Months | 0.11 | 0.04 | 0.11 | (−0.01, 0.02) |
| Non-Responders (n=15) | ||||
| ETx – Intake | −0.03 | 0.08 | 1.00 | (−0.28, 0.23) |
| 3 Months – ETx | 0.09 | 0.08 | 1.00 | (−0.19, 0.37) |
| 6 Months – 3 Months | −0.04 | 0.09 | 1.00 | (−0.34, 0.25) |
| 9 Months – 6 Months | 0.05 | 0.09 | 1.00 | (−0.24, 0.34) |
p-values are Bonferroni adjusted for multiple comparisons
3.2 Predictors of cluster membership
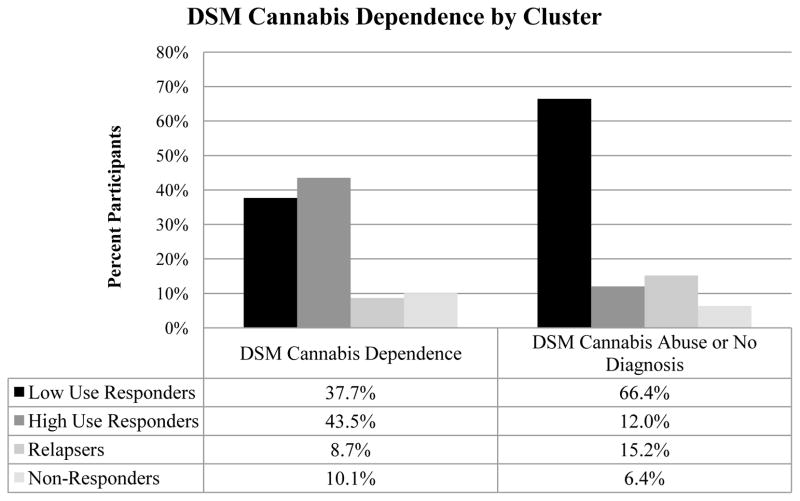
Among the twelve variables tested (Table 4), five variables were found to significantly predict subgroup membership: age (F(3,190)=3.21, p=.024, η2=.093); DSM cannabis diagnosis (χ2(3)=27.78, p<.001, Cramer’s V=.378, Figure 2); externalizing (χ2(3)=12.93, p=.005, Cramer’s V=.258); mean cannabis use per day (F(3,190)=10.76, p<.001, η2=.144, Figure 3); and SES (F(3, 190)=3.02, p=.031, η2=.054, Figure 4). The significant predictors were then entered into a multinomial logistic regression along with clinical trial (AR-1, AR-2, VT) to adjust for differences in sample characteristics. Three of the five variables remained significant: DSM cannabis diagnosis (Wald χ2(3)=13.27, p=.004), mean cannabis use per day (Wald χ2(3)=13.20, p=.004), and SES (Wald χ2(3)=8.51, p=.037) (see Table 5). Adolescents that were cannabis dependent (compared to cannabis abuse or no diagnosis) were about 4.4 times as likely to be High Use Responders than Low Use Responders (OR=4.42) and were about .2 times as likely to be Relapsers than High Use Responders (OR=.16). Adolescents that used more cannabis per day (compared to those that used less cannabis per day) were about 2.2 times as likely to be in High Use Responders (OR=2.22), 2.7 times as likely to be Relapsers (OR=2.69), and about 3 times as likely to be Non-Responders (OR=3.0) than Low Use Responders. Adolescents that had a higher SES (compared to those with lower SES) were about .7 times as likely to be Non-Responders than Low Use Responders (OR=.69) or Relapsers (OR=.73).
Table 4.
Univariate comparisons of baseline characteristics by cluster. For continuous variables, tabled values are means (standard deviations); for categorical variables, tabled values are counts (within cluster percentages).
| Low Use Responders (n=109) | High Use Responders (n=45) | Relapsers (n=25) | Non-Responders (n=15) | χ2 or F | |
|---|---|---|---|---|---|
| Demographic and study related | |||||
| Age | 15.59 (1.26) | 15.89 (1.25) | 16.16 (1.21) | 16.47 (1.30) | 3.21* |
| Race (white) | 65 (59.6%) | 29 (64.4%) | 14 (56.0%) | 6 (40.0%) | 2.89 |
| Sex (male) | 88 (80.7%) | 35 (77.8%) | 21 (84.0%) | 14 (93.3%) | 1.95 |
| Hollingshead 9-step SES | 6.12 (2.30) | 5.87 (2.32) | 5.74 (2.61) | 4.17 (2.62) | 3.02* |
| Contingency Management | 69 (63.3%) | 23 (51.1%) | 17 (68.0%) | 8 (53.3%) | 2.92 |
| Substance use | |||||
| Cannabis Dependencea | 26 (23.9%) | 30 (66.7%) | 6 (24.0%) | 7 (46.7%) | 27.78* |
| Mean cannabis uses per day | 1.43 (.66) | 2.33 (1.59) | 2.08 (1.44) | 2.65 (1.50) | 10.76* |
| Alcohol Abuse/Dependencea | 25 (22.9%) | 11 (28.2%) | 2 (8.0%) | 1 (6.7%) | 5.04 |
| Mean drinks per day | 3.32 (4.12) | 3.47 (4.07) | 3.58 (4.69) | 1.77 (1.84) | .78 |
| Psychopathology | |||||
| ADHDb | 55 (50.5%) | 18 (40.0%) | 12 (48.0%) | 3 (20.0%) | 5.63 |
| Internalizingb | 30 (27.5%) | 20 (44.4%) | 4 (16.0%) | 5 (33.3%) | 7.13 |
| Externalizingb | 48 (44.0%) | 34 (75.6%) | 13 (52.0%) | 7 (46.7%) | 12.93* |
p<0.05
Based on youth interview.
Based on youth or parent/guardian interview
Figure 2.
Baseline differences across clusters on cannabis use disorders.
Figure 3.
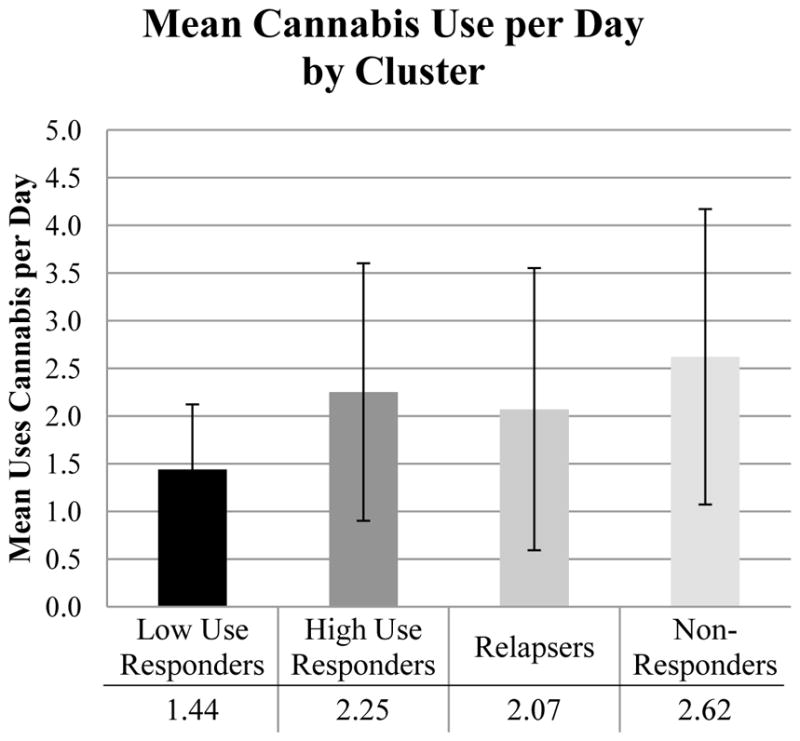
Baseline differences across clusters on mean cannabis use per day.
Figure 4.
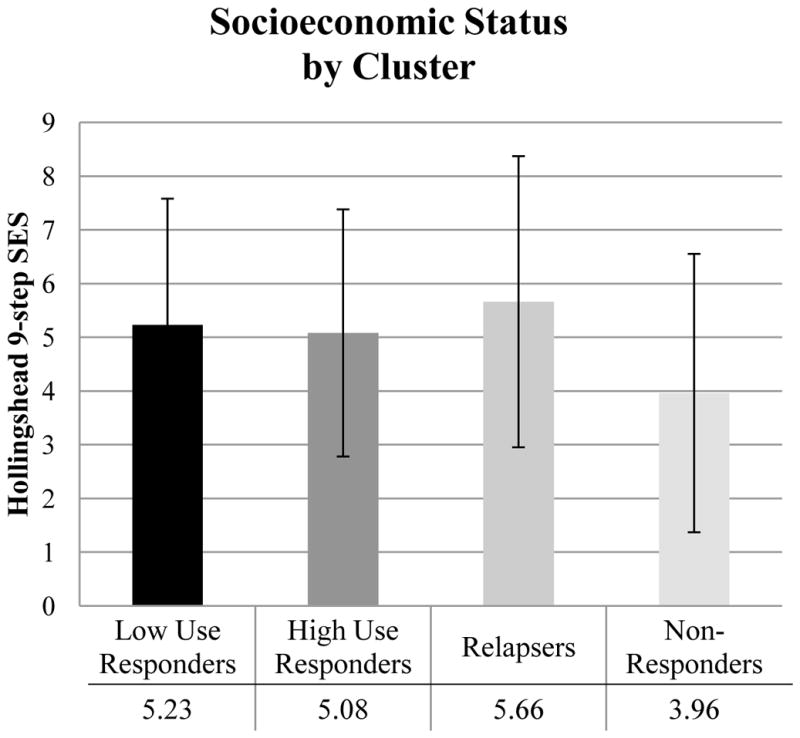
Baseline differences across clusters on SES.
Table 5.
Multinomial logistic regression results testing relations among clusters and intake variables.
| Low Use Responders (n=109) | High Use Responders (n=45) | Relapsers (n=25) | Non-Responders (n=15) | p-value | |
|---|---|---|---|---|---|
|
| |||||
| Variable | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | |
| Cannabis Dependencea (vs. cannabis abuse or no abuse/dependence) | 1.0 | 4.42 (1.84, 10.65) | 0.69 (0.20, 2.38) | 1.34 (0.31, 5.84) | <0.01 |
| 1.0 | 0.16 (0.04, 0.58) | 0.30 (0.07, 1.42) | |||
| 1.0 | 1.94 (0.35, 10.91) | ||||
| Externalizingb (vs. no externalizing diagnosis) | 1.0 | 2.77 (1.14, 6.72) | 1.35 (0.51, 3.58) | 0.81 (0.21, 3.15) | 0.13 |
| 1.0 | 0.49 (0.15, 1.57) | 0.29 (0.07, 1.27) | |||
| 1.0 | 0.60 (0.13, 2.72) | ||||
| SES | 1.0 | 0.88 (0.73, 1.07) | 0.95 (0.77, 1.18) | 0.69 (0.53, 0.89) | 0.04 |
| 1.0 | 1.08 (0.85, 1.37) | 0.78 (0.59, 1.03) | |||
| 1.0 | 0.73 (0.54, 0.97) | ||||
| Age | 1.0 | 1.18 (0.83, 1.69) | 1.47 (0.98, 2.21) | 1.60 (0.95, 2.70) | 0.14 |
| 1.0 | 1.24 (0.78, 1.98) | 1.36 (0.78, 2.35) | |||
| 1.0 | 1.09 (0.60, 1.97) | ||||
| Mean Cannabis Uses per Day | 1.0 | 2.22 (1.27, 3.89) | 2.69 (1.48, 4.91) | 3.03 (1.58, 5.78) | <0.01 |
| 1.0 | 1.21 (0.77, 1.90) | 1.36 (0.85, 2.18) | |||
| 1.0 | 1.13 (0.66, 1.91) | ||||
Analyses are adjusted for clinical trial.
1.0 indicates reference group.
Based on youth interview.
Based on youth or parent/guardian interview.
4. Discussion
Cluster analyses provided evidence for four robust, interpretable patterns of response to treatment for adolescent substance use based on percent days cannabis use: Low Use Responders, High Use Responders, Relapsers, and Non-Responders. Relations among these clusters and baseline variables revealed predictors of cluster membership.
Two response subgroups represented positive treatment outcomes. The Low Use Responders represented an ideal trajectory; these adolescents decreased their cannabis use by the end of treatment and then maintained low levels of use. At intake, their cannabis use severity was low; they had the lowest percent days cannabis use, were less likely to be cannabis dependent than High Use Responders, and used cannabis fewer times per day than any of the other three subgroups. Adolescents in the second-largest cluster, the High Use Responders, were also successful in treatment. The decrease in use for the High Use Responders was much larger than for the Low Use Responders; on average, they decreased from using cannabis 81% of days to 18% during treatment. Visual inspections of the trajectory suggested there was some relapse after 3 months post-treatment, but this increase in use was not statistically significant. Ultimately these adolescents were using far less cannabis 9 months post-treatment than they were at intake.
The other two subgroups did not achieve long-term reductions in cannabis use. The Relapsers had an intermediate level of cannabis use at intake and decreased their use by the end of treatment to levels comparable to Low Use Responders and High Use Responders. Unfortunately, this decrease was followed by a steady and large relapse to levels of cannabis use that exceeded their intake cannabis use. Adolescents in the smallest subgroup, the Non-Responders, were using cannabis very frequently, and the high rates of use remained consistent through all assessment points. Just as the Low Use Responders had some characteristics that predicted success, the Non-Responders had some characteristics that predicted poor response. These adolescents had the highest cannabis use severity, based on their percent days use and their high mean cannabis use per day at baseline. They also had the lowest SES.
The present study complements the analysis of Waldron and colleagues’ (2005) clustering. The Waldron et al. sample consisted of N=232 adolescents from two randomized clinical trials of substance use treatment. Participants were randomly assigned to either Functional Family Therapy, individual cognitive behavioral therapy, a combination of Functional Family Therapy and cognitive behavioral therapy, or group psychoeducation. In their analysis, adolescents were clustered across four time points (intake, ETx, 8, and 18 months post-treatment) based on percent days cannabis use. As with the present study, TLFB data were used for the clustering variable. Four clusters were identified: Improvers (36.8%), Slow Improvers (13.7%), Relapsers (24.8%), and Resistant (24.8%). Three of these clusters were very closely replicated in the present study: Low Use Responders (Improvers); Relapsers, and Non-Responders (Resistant). High Use Responders had a different trajectory than Slow Improvers. In the present study, these adolescents decreased their use by the end of treatment; in Waldron et al. (2005), these adolescents did not decrease their use until after treatment ended, and then they kept decreasing. There could be various reasons for this difference, including differences in treatments and timing of assessments. Although not identical, these clusters were similar, as they involved adolescents that began treatment with a high percent days cannabis use and demonstrated a substantial decrease by the final assessment point. This trajectory highlights how a proportion of high severity users can have positive treatment outcomes. Ultimately, post-treatment trajectories identified in both studies included continued low use, continued high use, and relapse trajectories, and these findings contribute to evidence that these trajectories represent common long-term responses to substance use treatment (Brown, Vik, & Creamer, 1989; Godley et al., 2004; Henderson et al., 2010).
The present study also replicated predictors of substance use treatment outcomes found in past studies. Cannabis dependence, mean cannabis uses per day, and SES were the three most important predictors of cluster membership. Indicators of high substance use severity, including a diagnosis of dependence and mean cannabis use per day, have frequently been found to predict poor treatment outcome (Crowley et al., 1998; Latimer et al., 2000; Williams & Chang, 2000). The importance of SES as a predictor fits with some past research on substance use treatment and socioeconomic status (Friedman, Glickman, & Morrissey, 1986; McCaul, Svikis, & Moore, 2001; Tomlinson, Brown, & Abrantes, 2004), with lower SES associated with poorer treatment outcomes. Cannabis dependence and mean cannabis uses per day are directly related to individual cannabis use, but SES is different, as it represents an environmental predictor. This finding emphasizes the importance of considering contextual factors as predictors of treatment outcome. Identifying predictors of treatment trajectory is key to identifying adolescents that are at high-risk for poor outcomes. Such predictors can be utilized as tailoring variables to better help these individuals. For example, adolescents with high cannabis use severity or low SES could be initially assigned to a more intensive or alternative treatment.
Similarities across clusters emphasized future directions for investigation. High Use Responders and Non-Responders had very different trajectories over time, but at intake they were very similar across a wide range of variables. Relapsers also had a number of similarities to the High Use Responders and Non-Responders at intake. If baseline characteristics do not discriminate among these subgroups, then different experimental designs may be essential to addressing subgroup differences that develop over time. Trials aimed at designing adaptive interventions represent an approach to treatment where intervention components and sequence of these components vary based on pre-defined criteria, such as abstinence from substances at a specified point in time (Collins, Murphy, & Bierman, 2004; Collins, Murphy, & Strecher, 2007; Murphy, 2005). Since some subgroups did not start to differentiate until post-treatment, sequential randomization could be utilized to better address the needs of these subgroups. For example, participants could be randomly assigned to treatments at baseline, and participants that are not abstinent by mid-treatment could be randomly assigned to a different or more intense treatment. This example design would help tease apart how to best help Non-Responders. Adaptive interventions have potential to identify which components (e.g., therapy, CM) are most crucial to addressing high severity and preventing relapse.
The three trials that comprised the combined sample demonstrated improved outcomes during treatment for groups that had a CM component. In the present study, CM was not a significant predictor of treatment response. There are at least two possible explanations for these null findings. First, the advantage for CM was largest in these trials during treatment, not post-treatment (Stanger et al., 2009; Stanger et al., 2015; 2016). Since three out of five time points used for clustering were post-treatment, the advantage for CM may have been lost in the overall trajectories. Second, CM may not have been a significant predictor because of the strength of the control condition (MET/CBT plus urine drug testing). This individual therapy alone was effective at reducing cannabis use, and the improvement in outcomes related to the addition of CM may not have been detectable in the present analyses due to the low sample sizes.
Several limitations of this study were related to details of the sample. Data were combined from three randomized clinical trials, and these trials had some minor differences in treatment conditions and study procedures. More importantly, the participants had baseline differences across a variety of demographics and substance use variables (Table 1). Differences across trials could have resulted in different trajectories than if any of the three trials had a large enough sample to cluster individually. The similarity of the identified clusters to those found in Waldron et al. (2005), however, suggests that the trajectories identified in each individual trial would be similar. Trial differences were statistically controlled for in the multinomial logistic regression analyses, but between-trial heterogeneity could have masked some predictive associations at the univariate level; this could have prematurely excluded variables from the multivariate analysis. The somewhat small combined sample size may have also limited power for predictive analyses.
Diagnoses for substance use disorders were based on the DSM-IV-TR, and the finding that a diagnosis of cannabis dependence predicts treatment outcome does not directly translate to the DSM-5. Instead of abuse and dependence (American Psychiatric Association, 2000), the DSM-5 defines cannabis use disorder as mild, moderate, or severe (American Psychiatric Association, 2013). These diagnoses are based on numbers of symptoms; in addition to the categories, the symptom count could be explored as a predictor variable in a future study. We hypothesize that severity would predict cluster membership based on the present results.
Multiple variables tested that did not discriminate across clusters were significant predictors of treatment outcome in past studies including age (Friedman et al., 1995; Hendricks et al., 2012), and internalizing and externalizing psychopathology (Dakof et al., 2001; Hendricks et al., 2012). Some of these variables, including age and externalizing, were significant predictors at the univariate level, but not at the multivariate level. These variables lost some predictive power because of correlations with other, stronger predictors. In this sample, cannabis dependence and mean cannabis uses per day were strongly correlated to the clustering variable, percent days cannabis use (r=.46 and .r=49, respectively, at intake). Both age and externalizing were positively correlated with a cannabis dependence (r=.13 and r=.19, respectively) and mean cannabis use per day (r=.25 and r=.23, respectively). The present sample was over 80% male, which reflects the higher prevalence of males seeking treatment for cannabis use (SAMHSA, 2014). However, sex may relate to treatment outcome trajectory in ways that could not be tested in the present study due to the small subsample of females; the identified clusters are likely more representative of males than females. Some psychopathology variables more common among adolescent males, including ADHD and externalizing, may have been inflated due to the high percentage of males. SES was a significant predictor in the multinomial models, but the odds ratios were small. The small effect size for SES limits the clinical implications of the finding. The interpretation of SES would be clearer with a more diverse sample. Replicating the clusters in a larger sample would both strengthen evidence for the patterns and could more clearly highlight predictor variables with clinical value.
Participants with missing data were not included in the cluster analyses. Complete case analysis was utilized because the excluded versus included participants did not vary across baseline measures. The use of complete case analysis was a limitation because participants that left the study and refused to participate in follow-ups were not represented in the cluster subgroups. These participants may have may have represented a different trajectory entirely.
Another limitation involved what has been labeled the “cat’s cradle” (Sher, Jackson, & Steinley, 2011) pattern. Sher and colleagues (2011) have argued that trajectory approaches often produce four groups: low, increasing, decreasing, and high. These trajectories seem to appear regardless of sample or measurement details. Although four trajectories were produced in the present study, post-hoc pairwise comparisons over time provided evidence against the cat’s cradle; two groups decreased, one group decreased and then increased, and one group remained high. Although the cat’s cradle pattern is very common, the interpretability and complexity of the subgroups in the present study, as well as the evidence for replication, provide evidence of the meaningfulness of these subgroups.
4.1 Conclusions
Response to substance use treatment is heterogeneous across individuals, but subgroups with similar patterns over time likely exist. The four subgroups identified in the present study represented four distinct patterns of response to adolescent substance use treatment. These patterns were more related to pre-treatment cannabis use patterns than most demographics or psychopathology. Better understanding these subgroups is an important challenge for improving substance use treatment outcomes.
Highlights.
Response to outpatient adolescent substance use treatment is heterogeneous.
Cluster analysis was utilized to identify response subgroups for cannabis use.
Low Use Responders, High Use Responders, Relapsers, and Non-Responders were found.
Cannabis dependence, cannabis uses per day, and SES predicted cluster membership.
These clusters provide insight into study outcomes.
Acknowledgments
Role of Funding Sources
This study was supported by National Institutes of Health (NIH) Grants T32 DA037202 (NIDA), R01 DA015186 (NIDA), and R01 AA016917 (NIAAA). NIH had no role in study design, analysis, interpretation of the data, writing of the manuscript, or the decision to submit the paper for publication.
Footnotes
Contributors
Authors: Steven F. Babbin, Ph.D., Catherine Stanger, Ph.D., Emily A. Scherer, Ph.D., & Alan J. Budney, Ph.D.
Dr. Babbin conducted statistical analyses and wrote drafts of the manuscript. Dr. Stanger contributed to the development of the study and to drafts. Dr. Scherer assisted with statistical analyses and contributed to drafts. Dr. Budney contributed to drafts. All authors have contributed to and have approved the final manuscript.
Conflict of Interest
All authors declare that they have no conflicts of interest.
Preliminary results from this research were presented as a poster at the 77th Annual Meeting of the College on Problems of Drug Dependence, Phoenix, Arizona, USA, June 2015.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achenbach TM, Rescorla LA. Manual for ASEBA School-age Forms and Profiles. University of Vermont, Research Center for Children, Youth, and Families; Burlington, VT: 2001. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 2000. text rev. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Washington, DC: Author; 2013. [Google Scholar]
- Brown SA, Vik PW, Creamer VA. Characteristics of relapse following adolescent substance abuse treatment. Addictive Behaviors. 1989;14(3):291–300. doi: 10.1016/0306-4603(89)90060-9. [DOI] [PubMed] [Google Scholar]
- Brown SA, D’Amico EJ, McCarthy DM, Tapert SF. Four-year outcomes from adolescent alcohol and drug treatment. Journal of Studies on Alcohol. 2001;62(3):381–388. doi: 10.15288/jsa.2001.62.381. [DOI] [PubMed] [Google Scholar]
- Caldeira KM, O’Grady KE, Vincent KB, Arria AM. Marijuana use trajectories during the post-college transition: Health outcomes in young adulthood. Drug and Alcohol Dependence. 2012;125(3):267–275. doi: 10.1016/j.drugalcdep.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caliński T, Harabasz J. A dendrite method for cluster analysis. Communications in Statistics-Theory and Methods. 1974;3(1):1–27. [Google Scholar]
- Carroll KM, Nich C, Sifry RL, Nuro KF, Frankforter TL, Ball SA, et al. A general system for evaluating therapist adherence and competence in psychotherapy research in the addictions. Drug and Alcohol Dependence. 2000;57:225–238. doi: 10.1016/s0376-8716(99)00049-6. [DOI] [PubMed] [Google Scholar]
- Collins LM, Murphy SA, Bierman KL. A conceptual framework for adaptive preventive interventions. Prevention Science. 2004;5(3):185–196. doi: 10.1023/b:prev.0000037641.26017.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins LM, Murphy SA, Strecher V. The multiphase optimization strategy (MOST) and the sequential multiple assignment randomized trial (SMART): new methods for more potent eHealth interventions. American Journal of Preventive Medicine. 2007;32(5):S112–S118. doi: 10.1016/j.amepre.2007.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer JS, Kendall PC. A symptom-level examination of parent–child agreement in the diagnosis of anxious youths. Journal of the American Academy of Child & Adolescent Psychiatry. 2004;43(7):878–886. doi: 10.1097/01.chi.0000125092.35109.c5. [DOI] [PubMed] [Google Scholar]
- Cronbach LJ, Gleser G. Assessing similarities between profiles. Psychological Bulletin. 1953;50:456–473. doi: 10.1037/h0057173. [DOI] [PubMed] [Google Scholar]
- Crowley TJ, Mikulich SK, MacDonald M, Young SE, Zerbe GO. Substance-dependent, conduct-disordered adolescent males: severity of diagnosis predicts 2-year outcome. Drug and alcohol dependence. 1998;49(3):225–237. doi: 10.1016/s0376-8716(98)00016-7. [DOI] [PubMed] [Google Scholar]
- Dakof GA, Tejeda M, Liddle HA. Predictors of engagement in adolescent drug abuse treatment. Journal of the American Academy of Child & Adolescent Psychiatry. 2001;40(3):274–281. doi: 10.1097/00004583-200103000-00006. [DOI] [PubMed] [Google Scholar]
- Everitt BS, Landau S, Leese M, Stahl D. Cluster analysis. 5. Padstow, Cornwall, UK: Wiley; 2011. [Google Scholar]
- Forgatch MS, Patterson GR, DeGarmo DS. Evaluating fidelity: Predictive validity for a measure of competent adherence to the Oregon model of parent management training. Behavior Therapy. 2005;36:3–13. doi: 10.1016/s0005-7894(05)80049-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman AS, Terras A, Kreisher C. Family and client characteristics as predictors of outpatient treatment outcome for adolescent drug abusers. Journal of Substance Abuse. 1995;7(3):345–356. doi: 10.1016/0899-3289(95)90027-6. [DOI] [PubMed] [Google Scholar]
- Friedman AS, Glickman NW, Morrissey MR. Prediction to successful treatment outcome by client characteristics and retention in treatment in adolescent drug treatment programs: A large-scale cross validation study. Journal of Drug Education. 1986;16(2):149–165. doi: 10.2190/98EM-3TNT-5066-7QHP. [DOI] [PubMed] [Google Scholar]
- Godley SH, Dennis ML, Godley MD, Funk RR. Thirty-month relapse trajectory cluster groups among adolescents discharged from out-patient treatment. Addiction. 2004;99(s2):129–139. doi: 10.1111/j.1360-0443.2004.00860.x. [DOI] [PubMed] [Google Scholar]
- Henderson CE, Dakof GA, Greenbaum PE, Liddle HA. Effectiveness of multidimensional family therapy with higher severity substance-abusing adolescents: Report from two randomized controlled trials. Journal of Consulting and Clinical Psychology. 2010;78(6):885–897. doi: 10.1037/a0020620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriks V, van der Schee E, Blanken P. Matching adolescents with a cannabis use disorder to multidimensional family therapy or cognitive behavioral therapy: Treatment effect moderators in a randomized controlled trial. Drug and Alcohol Dependence. 2012;125(1):119–126. doi: 10.1016/j.drugalcdep.2012.03.023. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four Factor Index of Social Status. Department of Sociology, Yale University; 1975. [Google Scholar]
- Hudziak JJ, Copeland W, Stanger C, Wadsworth M. Screening for DSM-IV externalizing disorders with the Child Behavior Checklist: A receiver-operating characteristic analysis. The Journal of Child Psychology and Psychiatry. 2004;45(7):1299–1307. doi: 10.1111/j.1469-7610.2004.00314.x. [DOI] [PubMed] [Google Scholar]
- Jackson KM, Sher KJ, Schulenberg JE. Conjoint developmental trajectories of young adult substance use. Alcoholism: Clinical and Experimental Research. 2008;32(5):723–737. doi: 10.1111/j.1530-0277.2008.00643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain AK, Murty MN, Flynn PJ. Data clustering: A review. ACM Computing Surveys. 1999;31(3):264–323. [Google Scholar]
- Latimer WW, Newcomb M, Winters KC, Stinchfield RD. Adolescent substance abuse treatment outcome: The role of substance abuse problem severity, psychosocial, and treatment factors. Journal of Consulting and Clinical Psychology. 2000;68(4):684. [PubMed] [Google Scholar]
- Latimer WW, Winters KC, Stinchfield R, Traver RE. Demographic, individual, and interpersonal predictors of adolescent alcohol and marijuana use following treatment. Psychology of Addictive Behaviors. 2000;14(2):162. doi: 10.1037//0893-164x.14.2.162. [DOI] [PubMed] [Google Scholar]
- Lathrop RG, Williams JE. The reliability of inverse scree tests for cluster analysis. Educational and Psychological Measurement. 1987;47(4):953–959. [Google Scholar]
- Maisto SA, Pollock NK, Lynch KG, Martin CS, Ammerman R. Course of functioning in adolescents 1 year after alcohol and other drug treatment. Psychology of Addictive Behaviors. 2001;15(1):68. doi: 10.1037/0893-164x.15.1.68. [DOI] [PubMed] [Google Scholar]
- McCaul ME, Svikis DS, Moore RD. Predictors of outpatient treatment retention: patient versus substance use characteristics. Drug and Alcohol Dependence. 2001;62(1):9–17. doi: 10.1016/s0376-8716(00)00155-1. [DOI] [PubMed] [Google Scholar]
- Murphy SA. An experimental design for the development of adaptive treatment strategies. Statis Med. 2005;24:1455–1481. doi: 10.1002/sim.2022. [DOI] [PubMed] [Google Scholar]
- Prendergast M, Podus D, Finney J, Greenwell L, Roll J. Contingency management for treatment of substance use disorders: A meta-analysis. Addiction. 2006;101(11):1546–1560. doi: 10.1111/j.1360-0443.2006.01581.x. [DOI] [PubMed] [Google Scholar]
- Rowe CL, Liddle HA, Greenbaum PE, Henderson CE. Impact of psychiatric comorbidity on treatment of adolescent drug abusers. Journal of Substance Abuse Treatment. 2004;26(2):129–140. doi: 10.1016/S0740-5472(03)00166-1. [DOI] [PubMed] [Google Scholar]
- Salbach-Andrae H, Klinkowski N, Lenz K, Lehmkuhl U. Agreement between youth-reported and parent-reported psychopathology in a referred sample. European Child & Adolescent Psychiatry. 2009;18(3):136–143. doi: 10.1007/s00787-008-0710-z. [DOI] [PubMed] [Google Scholar]
- Sampl S, Kadden R. Cannabis Youth Treatment (CYT) Series. Vol. 1. Rockville, MD: Center for Substance Abuse Treatment, Substance Abuse and Mental Health Services Administration; 2001. Motivational Enhancement Therapy and Cognitive Behavioral Therapy for Adolescent Cannabis Users: 5 Sessions. [Google Scholar]
- Sarle WS. Cubic clustering criterion. Cary, NC: SAS Institute; 1983. SAS Tech. Rep. A-108. [Google Scholar]
- Sher KJ, Jackson KM, Steinley D. Alcohol use trajectories and the ubiquitous cat’s cradle: Cause for concern? Journal of Abnormal Psychology. 2011;120(2):322–335. doi: 10.1037/a0021813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back. In: Litten R, Allen J, editors. Measuring alcohol consumption. Totowa, NJ7: Humana Press; 1992. pp. 41–72. [Google Scholar]
- Spear SF, Ciesla JR, Skala SY. Relapse patterns among adolescents treated for chemical dependency. Substance use & Misuse. 1999;34(13):1795–1815. doi: 10.3109/10826089909039427. [DOI] [PubMed] [Google Scholar]
- Stanger C, Budney AJ, Kamon JL, Thostensen J. A randomized trial of contingency management for adolescent marijuana abuse and dependence. Drug and Alcohol Dependence. 2009;105(3):240–247. doi: 10.1016/j.drugalcdep.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanger C, Ryan SR, Scherer EA, Norton GE, Budney AJ. Clinic- and home-based contingency management plus parent training for adolescent cannabis abuse. Journal of the American Academy of Child & Adolescent Psychiatry. 2015;54(6):445–453. doi: 10.1016/j.jaac.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanger C, Scherer EA, Babbin SF, Ryan SR, Norton GE, Budney AJ. A Randomized Trial of Abstinence Based Incentives for Adolescent Alcohol Abuse. 2016 Manuscript submitted for publication. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Treatment Episode Data Set (TEDS): 2002–2012. National Admissions to Substance Abuse Treatment Services. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014. BHSIS Series S-71, HHS Publication No. (SMA) 14–4850. [Google Scholar]
- Tanner-Smith EE, Wilson SJ, Lipsey MW. The comparative effectiveness of outpatient treatment for adolescent substance abuse: A meta-analysis. Journal of Substance Abuse Treatment. 2013;44(2):145–158. doi: 10.1016/j.jsat.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson KL, Brown SA, Abrantes A. Psychiatric comorbidity and substance use treatment outcomes of adolescents. Psychology of Addictive Behaviors. 2004;18(2):160–169. doi: 10.1037/0893-164X.18.2.160. [DOI] [PubMed] [Google Scholar]
- Waldron HB, Turner CW. Evidence-based psychosocial treatments for adolescent substance abuse. Journal of Clinical Child & Adolescent Psychology. 2008;37(1):238–261. doi: 10.1080/15374410701820133. [DOI] [PubMed] [Google Scholar]
- Waldron HB, Turner CW, Ozechowski TJ. Profiles of drug use behavior change for adolescents in treatment. Addictive Behaviors. 2005;30(9):1775–1796. doi: 10.1016/j.addbeh.2005.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JH. Hierarchical grouping to optimize an objective function. Journal of the American Statistical Association. 1963;58(301):236–244. [Google Scholar]
- Webb C, Scudder M, Kaminer Y, Kadden R. The Motivational Enhancement Therapy and Cognitive Behavioral Therapy Supplement: 7 Sessions of Cognitive Behavioral Therapy for Adolescent Cannabis Users, Cannabis Youth Treatment (CYT) Series. Vol. 2. Rockville, MD: Center for Substance Abuse Treatment, Substance Abuse and Mental Health Services Administration; 2002. [Google Scholar]
- Williams RJ, Chang SY. A comprehensive and comparative review of adolescent substance abuse treatment outcome. Clinical Psychology: Science and Practice. 2000;7(2):138–166. [Google Scholar]
- Winters KC, Lee CYS. Likelihood of developing an alcohol and cannabis use disorder during youth: Association with recent use and age. Drug and Alcohol Dependence. 2008;92(1):239–247. doi: 10.1016/j.drugalcdep.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]