Abstract
The earliest and perhaps best example of an interaction between nutrition and dementia is related to thiamine (vitamin B1). Throughout the last century, research showed that thiamine deficiency is associated with neurological problems, including cognitive deficits and encephalopathy. Multiple similarities exist between classical thiamine deficiency and Alzheimer’s disease (AD) in that both are associated with cognitive deficits and reductions in brain glucose metabolism. Thiamine-dependent enzymes are critical components of glucose metabolism that are reduced in the brains of AD patients and by thiamine deficiency, and their decline could account for the reduction in glucose metabolism. In preclinical models, reduced thiamine can drive AD-like abnormalities, including memory deficits, plaques, and hyperphosphorylation of tau. Furthermore, excess thiamine diminishes AD-like pathologies. In addition to dietary deficits, drugs, or other manipulations that interfere with thiamine absorption can cause thiamine deficiency. Elucidating the reasons why the brains of AD patients are functionally thiamine deficient and determining the effects of thiamine restoration may provide critical information to help treat patients with AD.
Keywords: thiamine, vitamin B1, Alzheimer’s disease, mitochondria, glucose metabolism
Thiamine deficiency has been linked to impaired cognition for decades
Nobel Prize–winning experiments in the 19th and 20th centuries associated with the discovery of thiamine revealed that a lack of thiamine in humans caused a common “disease” called beriberi, which includes a significant neurological component. Thiamine (vitamin B1) was the first vitamin discovered, leading to the concept of vitamins. In the late 19th century, two neurological components of beriberi were carefully characterized, although the link with thiamine deficiency was not identified until the 1930s: Wernicke encephalopathy (WE; mainly affecting the central nervous system), Korsakoff syndrome (KS; amnesia with additional psychiatric manifestations), and Wernicke–Korsakoff syndrome (with both neurologic and psychiatric symptoms).1 The syndrome is often complicated by alcoholism because alcohol reduces the absorption of thiamine. However, prisoners of the Japanese in World War II became thiamine deficient and had all the symptoms of Wernicke–Korsakoff syndrome in the absence of alcoholism. By the 1930s, the criteria for the diagnosis of Korsakoff psychosis required the presence of a triad of gross memory defects for recent events, disorientation, and confabulation. Using these criteria, early experiments in 1939 treated KS patients with a high-quality diet and a thiamine supplement and showed that subjects who received excess thiamine showed a recovery approximately seven times as great as those that did not (for a review, see Ref. 1). These experiments initiated research to test the role of thiamine in memory in humans and animals. It has been shown that thiamine deficiency in humans produces many of the neurological consequences of beriberi.2
Wernicke–Korsakoff syndrome, caused by a severe thiamine deficiency from alcohol abuse or other malnourished states, is composed of two clinical stages. The acute stage, WE, refers to the classical clinical presentation of mental status changes, opthalmoplegia, and gait ataxia. In these cases the administration of thiamine not only resolves the acute symptoms but also may save the patient’s life. The chronic stage, KS, follows those who survive the acute stage when the mental confusion and the ocular findings resolve and the survivors are left with residual amnesia and confabulation. These syndromes represent the extreme consequence of thiamine deficiency, which has an essential role in CNS metabolism. We suggest that a chronic, long-term insufficiency of vitamin B1 (thiamine) may represent an upstream noxious event that later leads to the formation of plaques and tangles.
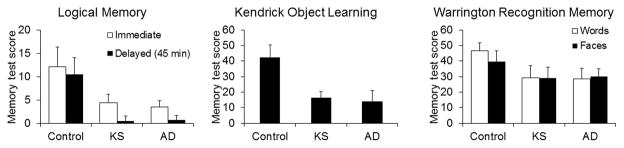
Several similarities exist between the cognitive changes in Alzheimer’s disease (AD) and KS patients in that both the types of tasks for which performance is impaired and the degree of reduction are similar; some examples are show in Figure 1.3 Both AD and KS patients performed significantly worse than healthy controls but were indistinguishable from one another.
Figure 1.
Similarities between memory deficits in Alzheimer’s disease (AD) and in thiamine deficiency in humans. Performance on a large number of cognitive tasks was compared in controls, Korsakoff syndrome (KS) patients, and AD patients.3 Performance on only three tasks are shown. Performance on each task declines similarly in patients with AD and those who are thiamine deficient (KS patients).3
Thus, understanding how disruptions in nutrition may contribute to dementia (including AD) must incorporate the rich history of research on the biochemical role of thiamine in normal and abnormal brain function. This paper discusses whether thiamine-dependent processes could play an important role in the development of AD—one of the most common disorders of the 21st century.
Perspective on Alzheimer’s disease
Alzheimer’s disease is defined by a progressive cognitive deterioration and neuropathological changes in the brain that consist of deposition of extracellular beta amyloid plaques and intracellular neurofibrillary tangles (NFTs) made of hyperphosphorylated tau proteins.4, 5 This definition is equivocal for many reasons; for example, many controls have plaques at autopsy and an emerging literature also indicates that as many as 35% of the patients diagnosed with AD by comprehensive cognitive testing do not have plaques.6, 7 This begs the question of whether the plaques and NFTs are causative of AD or just one of many downstream consequences.
Many other changes occur in the brains of patients that die with AD, including synaptic loss, changes in glucose metabolism, oxidative stress, reductions in thiamine-dependent processes, endosomal abnormalities, cholinergic dysfunction, lysosomal irregularities, and altered calcium dynamics, as well as changes in many other variables. Any one of the myriad of these pathologies could potentially lead to AD. Indeed, comparisons of immunocytochemical markers suggest that oxidative stress is more prevalent in AD than are plaques or tangles.8 Indeed, markers of oxidative stress in the urine of mice genetically engineered to make plaques precede plaque formation in the brain.9 An understanding of the relation of these variables to the disease and to the pathological hallmarks of plaques and tangles may enable development of new therapies. For most of these alterations, the temporal profiles are unknown, and defining these profiles will help to determine causal relationships.
Glucose metabolism is always diminished in AD
Extensive research has examined the relationship of cerebrospinal fluid (CSF) biomarkers of amyloid and tau and compared those to glucose metabolism, as measured by [18F]fluorodeoxyglucose ([18F]FDG) positron emission tomography (PET).10 Reduced glucose metabolism occurs in AD long before the patient demonstrates significant clinical signs of AD. The regional changes in glucose metabolism are also highly correlated to changes in cognition. The correlation of changes to amyloid as measured by PET scan is very poor.11 The common explanation for these changes is that glucose metabolism as measured by [18F]FDG PET reflects synaptic activity, and since a loss of synapses accompanies AD, the loss of glucose use merely reflects the decline in synapses. This is likely true but also raises the possibility that reductions in glucose metabolism promote AD.
Glucose metabolism is also diminished in thiamine deficiency in humans
Wernicke–Korsakoff syndrome is associated with thiamine deficiency. The percent reduction in glucose metabolism observed in this clinical syndrome is approximately the same as occurs in AD. Thus, glucose metabolism is diminished by 20–30% in many brain regions, including several temporal, frontal, and thalamic regions,12 which can be improved by the administration of thiamine.13 Thus, if the deficits leading to diminished glucose metabolism in AD are also associated with a functional thiamine deficiency, they may be reversed by thiamine as well. An understanding of these changes in brain glucose is necessary to evaluate this possibility.
Brain glucose metabolism, like that of other tissues, requires thiamine at critical regulatory steps and has unique features
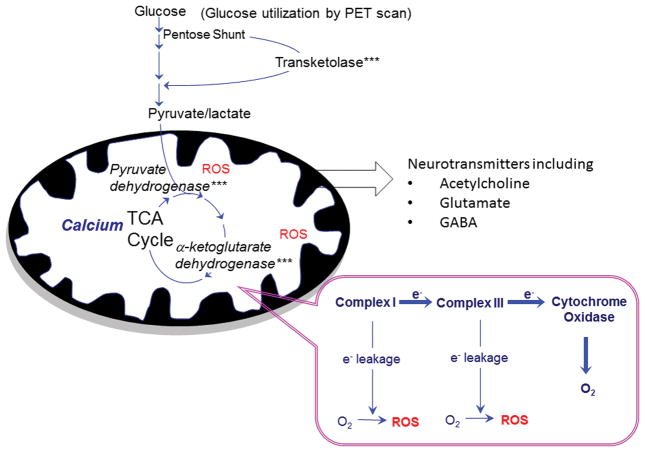
Thiamine-dependent enzymes act at key steps of glucose metabolism: transketolase in the pentose shunt, pyruvate dehydrogenase complex (PDHC) linking glycolysis and the tricarboxylic acid (TCA) cycle, and the α-ketoglutarate dehydrogenase complex (KGDHC) in the TCA cycle. Glucose metabolism in the brain is unique in that it is much higher than in other tissues. The brain represents only 2% of body mass, but it uses 20% of the glucose. The high rate of metabolism may account for the brain’s sensitivity to thiamine deficiency. Another unique feature of brain glucose is that it also provides substrates for the synthesis of multiple neurotransmitters, including both glutamate and acetylcholine. The two major types of cognitive drugs approved for the treatment of AD interact with these two neurotransmitters, as epitomized by memantine (Namenda) and cholinesterase inhibitors (e.g., donepezil). Current methods to assess brain metabolism are limited. For example, [18F]FDG PET only measures the first step of metabolism, and it is likely that other changes precede alterations measured by [18F]FDG PET.
Thiamine-dependent enzymes play key and sometimes rate-limiting roles in metabolism. For example, as mentioned above, transketolase plays a key role in the pentose shunt, PDHC links glycolysis to the TCA cycle, and KGDHC is often the rate-controlling step in the TCA cycle. Thus, a deficiency of thiamine would have a profound effect on glucose metabolism. The deficiency in glucose metabolism in Wernicke syndrome can be reversed by an addition of thiamine. This suggests that, if the deficits in AD are related to thiamine deficiency, they could also be reversed by appropriate administration of thiamine.
Thiamine-dependent processes are diminished in patients with AD
Thiamine status in the blood can be assessed by multiple methods, often indirectly by assaying the activity of the transketolase enzyme, which depends on thiamine pyrophosphate (TPP; also known as thiamine diphosphate), in erythrocyte hemolysates in the presence and absence of added TPP.14 The result, known as the TPP effect, reflects the extent of unsaturation of transketolase with TPP and gives a functional measure of thiamine deficiency. Thiamine and its esters can now also be directly assessed in small samples by very reliable methods.15 Another commonly used measure of thiamine status is urinary thiamine excretion, which provides data on dietary intakes but not tissue stores. For adults, excretion of less than 100 mcg/day thiamine in urine suggests insufficient thiamine intake, and less than 40 mcg/day indicates an extremely low intake.16 In studies that examined thiamine status in patients with AD, the TPP effect on transketolase in blood was significantly higher (12%) in AD than controls,14 and thiamine in plasma is reduced by about one-third in AD patients.17 These measures suggest that a significant portion of AD patients are thiamine deficient. Complete measures of all of the thiamine esters have not been made in blood.
The activities of thiamine-dependent enzymes in the brain have also been used as a measure of thiamine deficiency. The activity of the three thiamine-dependent enzymes shown in Figure 2—transketolase, PDHC, and KGDHC—is diminished in AD brains.14,18 The percent reduction varies (50–100%) between studies, and there are no contravening studies. Thus, a strong case can be made that thiamine-dependent processes are diminished in AD patients.
Figure 2.
Role of thiamine in brain glucose metabolism for energy utilization and neurotransmitter synthesis. Like in other tissues, glucose metabolism in the brain uses thiamine-dependent enzymes at critical steps. The brain uses ten times its body mass in glucose compared to the whole body. Thiamine-dependent enzymes (noted by ***) are situated in key steps of glucose metabolism: transketolase in the pentose shunt, pyruvate dehydrogenase as a link between glycolysis and the tricarboxylic acid (TCA) cycle, and KGDHC in the TCA cycle. Brain glucose also provides the carbon for synthesis of multiple neurotransmitters, including glutamate and acetylcholine, which are important in AD. The cognitive enhancers used in AD target the neurotransmitters acetylcholine and glutamate. Normal metabolism also results in production of reactive oxygen species (ROS), which contribute to tissue damage, including neuropathy in diabetes.
Preclinical models demonstrate that thiamine deficiency leads to memory deficits
An extensive literature documents an association between thiamine deficiency and memory impairment in animals. A few thousand papers have been published with multiple permutations in treatments and duration, with the consistent conclusion that thiamine deficiency is associated with diminished memory. For example, thiamine-deficient rats show significant impairment in performance in the passive-avoidance task,19, 20 avoidance learning,19, 20 water-maze task,21, 22 and the T-maze task.20, 23, 24 Thiamine deficiency induces impaired performance in the water-maze spatial memory task, although these animals were able to learn the task during the acquisition phase. Although the prefrontal cortex is generally considered to be invulnerable to lesions caused by thiamine deficiency, the lesions do cause neurochemical dysfunction in this brain region.19 Administration of the cholinesterase inhibitor physostigmine improves avoidance learning in thiamine-deficient rats, and thiamine deficiency results in reduced activity of the crucial acetylcholine synthetic enzyme, choline acetyltransferase (ChAT), in the cortex and hippocampus prior to actual memory impairment. As the thiamine deficiency persists and memory impairment becomes evident, thalamic ChAT activity also decreases.19 By augmenting the intrasynaptic concentration of acetylcholine, physostigmine reverses the consequences of these metabolic effects.
In addition, diminished learning in animals with thiamine deficiency has been related to reduced neurogenesis, which also occurs in AD. When mice were tested in a Y-maze, learning and memory functions declined in parallel with reduced hippocampal neurogenesis at times when the same mice did not exhibit regular pathological lesions, loss of cholinergic neurons, decrease of NeuN+ hippocampal cells, and abnormal long-term potentiation of hippocampal CA1 and CA3 cells. Re-administering thiamine reversed the weakened learning ability as well as the impaired hippocampal neurogenesis induced by thiamine deficiency at the early pre-pathological lesion stage.25
Neurochemical studies in animal models of thiamine deficiency support the concept that thiamine deficiency could be an important component of AD pathophysiology
The concept of a biochemical lesion was first coined by Sir Rudolph Peters in 1929, using thiamine-deficient pigeon brains.26 The precise substrate for these changes in thiamine deficiency has not been determined, and many of the changes mimic those in AD. The brains of thiamine-deficient mice have been shown to have reduced activities of thiamine-dependent enzymes, the consequences of which are altered by age and genetic background. For example, thiamine deficiency reduces the activity of KGDHC by about 50% in young mature mice (3 months old) and about 80% in aged mice (30 months old). With respect to the influence of genetics, it has been found that, in BalbC mice, thiamine deficiency reduces the activity of transketolase by about half, and in C57 black mice, baseline activity is less than half of that in BalbC mice. Thus, residual activity after thiamine deficiency is very small.27
Thiamine deficiency produces a cholinergic deficit, which is a well-established feature of AD. Thiamine deficiency–induced deficits in a neurological test in mice (the string test) or in an open-field test can be reversed by thiamine administration. The acetylcholinesterase inhibitor physostigmine (similar to donepezil) is as effective as thiamine, and the effect of physostigmine can be blocked by the muscarinic receptor blocker atropine. However, acetylcholinesterases that act only in the periphery are not effective. Thus, thiamine deficiency produces a central cholinergic deficit.28
Thiamine deficiency also induces excess glutamate release, and blocking glutamatergic action with an N-methyl-D-aspartate (NMDA) antagonist (similar to memantine) has a protective effect.29, 30 Memantine, which is widely used in AD, has a similar mechanism of action, suggesting that glutamatergic mechanisms are also altered in AD.
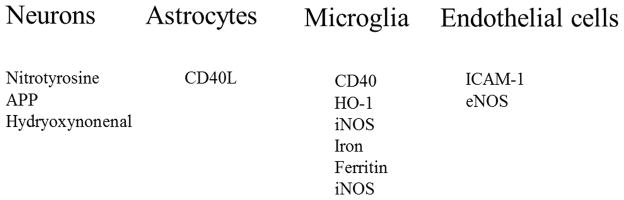
Furthermore, thiamine deficiency produces selective cell death in the brain,31 with the submedial thalamic nucleus being one of the most sensitive regions. The selective loss of neurons in this region and activation of other cell types has been studied extensively and involves both inflammation and oxidative stress. The responses in each cell type, shown in Figure 3, resemble those observed in brains from AD patients at autopsy. In thiamine-deficient mice, the temporal sequence of events can be determined; the changes in endothelial cells occur particularly early, which may lead to changes in other cell types. This is supported by the observation that blocking the early responses in endothelial cells is protective.31
Figure 3.
Cell-specific increases in markers of inflammation and oxidative stress occur in thiamine deficiency. Mice were made thiamine deficient and the brains were then analyzed for markers of inflammation and oxidative stress. Similar increases in these same variables have been observed in brains from AD patients at autopsy. In the animal model, the temporal response in each cell type was determined. Shown is the approximate order of appearance of the stressor within each cell type with thiamine deficiency, and the increases within each cell type are listed in the order of response. The endothelial cell response is first and blockade of endothelial responses protect against neuronal loss.31, 42
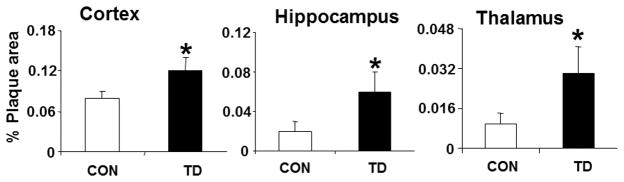
Perhaps the strongest evidence that thiamine may be involved in the etiology of AD is the relation of thiamine deficiency to plaque formation. Thiamine deficiency greatly exacerbates plaque formation in mice genetically engineered to make plaques (Fig. 4). Ten days of thiamine deficiency produces plaques throughout the brain, even in regions that do not normally have plaques. Thiamine deficiency also increase the phosphorylation of tau.32, 33 Furthermore, increasing thiamine with the compound benfotiamine reduces plaques, hyperphosphorylated tau, and memory deficits in a mouse genetically engineered to make plaques.34 Thus, the abovementioned preclinical studies provide strong evidence that thiamine deficiency produces abnormalities that are similar to those in AD, and the results support the suggestion that increasing thiamine in the brain may be beneficial to patients with AD.
Figure 4.
Thiamine deficiency accelerates deposition of thioflavin S–positive amyloid plaque. Mice were made thiamine deficient (TD) for 10 days, and the number of thioflavin S–positive plaques were determined throughout the brain.56 The graph shows the percent area occupied by plaques quantified from the cortex, hippocampus, and thalamus. Data represent the means ± SEM (control, n = 9; TD, n = 10) from 2–3 independent experiments.
Possible causes of thiamine deficiency
If the hypothesis concerning the link between thiamine deficiency and etiology of AD is true, an important question in developing a therapeutic strategy is to understand why individuals may have developed a functional thiamine deficiency in their brains. As described below, a variety of conditions give rise to thiamine deficiency; however, none of these conditions have been directly linked to AD. The disease process itself may promote thiamine deficiency, which would not negate the use of thiamine clinically. Many common foods are high in thiamine, including fish (trout), pork, nuts (macadamia), seeds (sunflower), bread (wheat), green peas, squash (acorn), asparagus (cooked), dry roasted soy beans (edamame), and beans (navy) (HealthAliciousNess.com). In advanced countries, thiamine deficiency from pure dietary deficiencies seems unlikely because foods, such as flour, are enriched with thiamine. However, conditions that lead to altered gastrointestinal function are common and can decrease absorption, including conditions that interfere with thiamine absorption from the intestine. For example, gluten sensitivity can diminish absorption, and many of the foods prepared to minimize the gluten content of food removes thiamine, which then must be enriched. Bariatric surgery can also cause thiamine deficiency.
Thiamine deficiency also occurs in many other disorders. For example, cancer patients often present with Wernicke–Korsakoff syndrome,35 and diabetes leads to an apparent thiamine deficiency, possibly because of altered kidney function.36 Many different drugs and hormones alter thiamine status, a detailed summary of which was created by Kennedy.37 Several compounds in each of the following classes of drugs have been shown to alter thiamine metabolism: antacids, antiarrythmic drugs, anticonvulsants, antidepressants, anti-infective agents, antipsychotics, barbiturates, antineoplastic drugs, cardiotonics, contraceptives, dichloroacetate, diuretics, hormones, antihypertensives, laxatives, monoamine oxidase inhibitors, proton pump inhibitors, sedatives, and stimulants (for references, see Ref. 37).
Furthermore, some individuals have a higher requirement for thiamine. For example, one study showed that only a subset of alcoholics that are thiamine deficient develop Wernicke–Korsakoff syndrome, and these individuals have a greater requirement of thiamine to activate the thiamine-dependent enzyme transketolase. More specifically, subjects who did not develop Wernicke–Korsakoff syndrome required 16 μM TPP for full activation, whereas those who did succumb to Wernicke–Korsakoff syndrome required 195 μM.38 Thus, the amount of TPP for full activation was ten times greater in the Wernicke–Korsakoff syndrome patients. These results reveal an inborn predisposition to the development of a neurological disease that is likely to be clinically silent unless the individual with the predisposition faces an appropriate stress.
Thus, a decline in thiamine-dependent processes could be due to a diminished intake of thiamine, an altered thiamine metabolism, a genetic predisposition for a higher thiamine requirement, or modification of thiamine-dependent processes by oxidative stress. Regardless, enough thiamine may overcome these deficits.
Reversal of pathology with thiamine supplements or enrichment
Although thiamine deficiency has long been linked to memory deficits, the best way to increase the concentrations of thiamine and TPP in the brain is not well established. Thiamine administration does not lead to substantial changes in brain thiamine or TPP. On the other hand, if treatment is given early enough, thiamine does reverse deficits related to thiamine deficiency. Standard practice in the Western world is to treat delirious patients with thiamine and glucose. If only glucose is given, the brain can become damaged because of acidosis, and the results suggest that thiamine enters the brain to exert protective effects. In humans, administration of thiamine diminishes the symptoms of Wernicke–Korsakoff syndrome, and in animals, thiamine administration reverses the effects of thiamine deficiency on thiamine-dependent enzymes, behavior, and neuronal death, as long as thiamine is administered before the changes are irreversible. Nevertheless, thiamine is a relatively poor therapeutic in that after administration of exogenous thiamine, thiamine and TPP levels in blood rise slightly and do not remain high for a long time. On the other hand, other compounds, such as solbutaimine, benfotiamine, and fursultiamine, have been designed to increase thiamine 5 to 10 times higher than thiamine and maintain these high levels for hours.39
The effects of benfotiamine on cognitive impairment and AD-like pathology alterations were tested in a mouse model of AD, where a chronic 8-week treatment of benfotiamine dose-dependently enhanced the spatial memory of mice in the Morris water maze test. Furthermore, benfotiamine effectively reduced both amyloid plaque numbers and phosphorylated tau levels.34 These effects were not mimicked by another lipophilic thiamine derivative, fursultiamine, although both benfotiamine and fursultiamine are effective in increasing the levels of free thiamine in the brain. Benfotiamine, but not fursultiamine, significantly elevates the phosphorylation level of glycogen synthase kinase (GSK)-3-α and -3-β, and reduces their enzymatic activities. Therefore, in animal models of AD, benfotiamine may improve cognitive function and reduce amyloid deposition via thiamine-independent mechanisms, or it may alter thiamine metabolism in a different manner than fursultiamine.34
Benfotiamine has been shown to dramatically reduce plaques in the brain.34 In this study, a comparison with the effects of furusultiamine, which does not alter plaques, suggested to the authors that the effects of benfotiamine were independent of thiamine and likely occur through benfotiamine’s action on GSK. Although the data do raise this possibility, they are limited and leave room for alternative interpretations. One important limitation of the study is that thiamine levels were measured 1 hr after one injection or after ten days of daily administration, whereas the plaques and GSK activities were measured after daily administration for 8 weeks. Brain thiamine is well known to be resistant to short-term manipulation of peripheral thiamine. Thus, both benfotiamine and furusultiamine had only minimal effects on brain thiamine and no effects on TPP or TMP. However, benfotiamine was much more effective than fursultiamine in raising blood thiamine—blood thiamine was increased two to three times more by benfotiamine than by fursultiamine (0.1 to 15 as compared to 0.1 to 7), as well as after ten days (0.1 to 6 as compared to 0.1 to 12). Thus, over 8 weeks, these higher levels may have had a larger effect on brain thiamine. Also, the authors measured whole-brain thiamine. Mammillary bodies, which are very sensitive to thiamine deficiency, have ten times the concentration of thiamine than does the cortex.40 Furthermore, endothelial cells are the cell type most sensitive to thiamine deficiency.41 Preventing these changes in endothelial cells protects against brain pathology, including neuronal loss. Benfotiamine has also been shown to benefit endothelial cells in diabetics by acting as thiamine.42 The authors suggested GSK as an alternative mechanism. However, dosages of benfotiamine that reduced plaques by 75% did not significantly alter GSK activity. Thus, although this paper is very important, the conclusion about the role of thiamine following benfotiamine requires further investigation.
Both thiamine and benfotiamine protect against the peripheral neuropathy that occurs in diabetes in humans.36, 43, 44 They both reduce advance glycation end products (AGEs) by activation of the non-oxidative portion of the pentose shunt. Benfotiamine is a better therapeutic than thiamine because it raises thiamine to much higher levels and for longer periods than does thiamine and may beneficially alter other processes as well. The pharamacokinetics of benfotiamine has been well studied and it is very safe in humans.45, 46 These studies suggest that benfotiamine may be an effective treatment for AD and have stimulated a clinical trial of benfotiamine in AD patients, supported by both the Alzheimer’s Drug Discovery Foundation and the National Institutes of Health (see ClinicalTrials.gov).
Thiamine may also have other functions in the brain
In addition, to its classical function as a cofactor in the form of TPP, thiamine has many other roles, including binding to amyloid and prions,47 altering acetylcholine release,48 and acting as an antioxidant.49 Recent studies show that thiamine binds to multiple mitochondrial enzymes in such a way that it may alter the interaction of the mitochondria with the cytosol.50 Thus, the beneficial effects of thiamine may be because of these other actions as well as on tradition thiamine-dependent enzymes.
Previous efforts to reverse Alzheimer’s disease with thiamine or its analogues
All previous studies of thiamine or thiamine analogues in AD have been underpowered; therefore, neither positive nor negative results are credible. The studies with thiamine were for short time periods and included small numbers of patients. The first was a double-blind, placebo-controlled crossover study, in which each patient received either placebo or thiamine followed by 3 months of the alternate treatment. While mean Mini Mental State Examination (MMSE) scores during treatment with thiamine were significantly better than during the placebo period, no significant difference was observed in scores on the behavioral rating scales.51 A 1-year trial of thiamine failed to replicate the changes described in this study.52 In another study that examined the effects of 3–8 g/day thiamine administered orally, thiamine at these pharmacologic dosages was suggested to have a mild beneficial effect in AD.53 Furthermore, fursultiamine had a mild beneficial effect on emotional and other mental symptoms, as well on intellectual function,54 and sulbutiamine was shown to potentiate cholinergic and glutamatergic neurotransmission, mainly in the hippocampus and prefrontal cortex.55
A multicenter, randomized, double-blind trial evaluated the effects of a cholinesterase inhibitor with or without sulbutiamine on cognitive functions (episodic and working memory, executive functions, attention) in patients with early-stage AD. Compared to pretreatment, episodic memory decreased in the group receiving anticholinesterase alone but improved in the group receiving sulbutiamine plus anticholinesterase. At the same time, both treatments produced a persistent improvement of attention. Activities of daily living also improved in the sulbutiamine group.55
Conclusions
Thiamine has been implicated in neurological problems, delirium, and dementia. Even though a role for thiamine in neurological function has long been known, we have only a limited understanding of its multiple actions. The safety of thiamine and its analogues suggests that a carefully designed trial of thiamine analogues, with appropriate power, should be conducted in AD patients.
Acknowledgments
The research was supported by grants from the Alzheimer’s Drug Discovery Foundation and the National Institutes of Health (NIH)/National Institute on Aging (R01AG043679 and PP-AG14930).
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Victor M, Adams R, Collins GH. The Wernicke-Korsakoff Syndrome. F.A. Davis; Philadelphia: 1989. [Google Scholar]
- 2.Williams RD, et al. Observations on induced thiamine (vitamin b1) deficiency in man. Archives of Internal Medicine. 1940;66:785–799. [Google Scholar]
- 3.Kopelman M. Frontal dysfunction and memory deficits in the alcoholic Korsakoff syndrome and Alzheimer-Type Dementia. Brain. 1991;114:117–137. [PubMed] [Google Scholar]
- 4.Dubois B, et al. Revising the definition of Alzheimer’s disease: a new lexicon. The Lancet Neurology. 2010;9:1118–1127. doi: 10.1016/S1474-4422(10)70223-4. [DOI] [PubMed] [Google Scholar]
- 5.Sperling RA, et al. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salloway S, Sperling R. Understanding Conflicting Neuropathological Findings in Patients Clinically Diagnosed as Having Alzheimer Dementia. Journal of the American Medical Association. 2015;72:1106–1108. doi: 10.1001/jamaneurol.2015.1804. [DOI] [PubMed] [Google Scholar]
- 7.Monsell SE, et al. Characterizing Apolipoprotein E ε4 Carriers and Noncarriers With the Clinical Diagnosis of Mild to Moderate Alzheimer Dementia and Minimal β-Amyloid Peptide Plaques. Journal of the American Medical Association. 2015:72. doi: 10.1001/jamaneurol.2015.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calingasan NY, Uchida K, Gibson GE. Protein-Bound Acrolein. Journal of Neurochemistry. 1999;72:751–756. doi: 10.1046/j.1471-4159.1999.0720751.x. [DOI] [PubMed] [Google Scholar]
- 9.Pratico D, et al. Increased lipid peroxidation precedes amyloid plaque formation in an animal model of Alzheimer amyloidosis. J Neurosci. 2001;21:4183–4187. doi: 10.1523/JNEUROSCI.21-12-04183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perrin RJ, Fagan AM, Holtzman DM. Multimodal techniques for diagnosis and prognosis of Alzheimer’s disease. Nature. 2009;461:916–922. doi: 10.1038/nature08538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furst AJ, et al. Cognition, glucose metabolism and amyloid burden in Alzheimer’s disease. Neurobiology of Aging. 2012;33:215–225. doi: 10.1016/j.neurobiolaging.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reed LJ, et al. FDG-PET Findings in the Wernicke-Korsakoff Syndrome. Cortex. 2003;39:1027–1045. doi: 10.1016/s0010-9452(08)70876-1. [DOI] [PubMed] [Google Scholar]
- 13.Hata1 T, et al. Three-Dimensional Mapping of Local Cerebral Perfusion in Alcoholic Encephalopathy With and Without Wernicke-Korsakoff Syndrome. Journal of Cerebral Blood Flow & Metabolism. 1987;7:35–44. doi: 10.1038/jcbfm.1987.6. [DOI] [PubMed] [Google Scholar]
- 14.Gibson GE, et al. Reduced activities of thiamine-dependent enzymes in the brains and peripheral tissues of patients with Alzheimer’s disease. Arch Neurol. 1988;45:836–840. doi: 10.1001/archneur.1988.00520320022009. [DOI] [PubMed] [Google Scholar]
- 15.Gangolf M, et al. Thiamine Status in Humans and Content of Phosphorylated Thiamine Derivatives in Biopsies and Cultured Cells. PLoS ONE. 2010;5:e13616. doi: 10.1371/journal.pone.0013616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mason HL, Williams RD. The urinary excretion of thiamine as an index of the nutritional level: assessment of the value of a test dose. Journal of Clinical Investigation. 1942;21:247–255. doi: 10.1172/JCI101296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gold M, Hauser R, Chen M. Plasma Thiamine Deficiency Associated with Alzheimer’s Disease but Not Parkinson’s Disease. Metabolic Brain Disease. 1998;13:43–53. doi: 10.1023/a:1020678912330. [DOI] [PubMed] [Google Scholar]
- 18.Bubber P, et al. Mitochondrial abnormalities in Alzheimer brain: mechanistic implications. Ann Neurol. 2005;57:695–703. doi: 10.1002/ana.20474. [DOI] [PubMed] [Google Scholar]
- 19.Nakagawasai O, et al. Immunohistochemical estimation of brain choline acetyltransferase and somatostatin related to the impairment of avoidance learning induced by thiamine deficiency. Brain Research Bulletin. 2000;52:189–196. doi: 10.1016/s0361-9230(00)00248-3. [DOI] [PubMed] [Google Scholar]
- 20.Langlais PJ, Savage LM. Thiamine deficiency in rats produces cognitive and memory deficits on spatial tasks that correlate with tissue loss in diencephalon, cortex and white matter. Behavioural Brain Research. 1995;68:75–89. doi: 10.1016/0166-4328(94)00162-9. [DOI] [PubMed] [Google Scholar]
- 21.Langlais PJ, Mandel RJ, Mair RG. Diencephalic lesions, learning impairments, and intact retrograde memory following acute thiamine deficiency in the rat. Behavioural Brain Research. 1992;48:177–185. doi: 10.1016/s0166-4328(05)80155-x. [DOI] [PubMed] [Google Scholar]
- 22.Carvalho FM, et al. Thiamine deficiency decreases glutamate uptake in the prefrontal cortex and impairs spatial memory performance in a water maze test. Pharmacology Biochemistry and Behavior. 2006;83:481–489. doi: 10.1016/j.pbb.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 23.Anderson CD, et al. Learning impairments after 6-OHDA treatment: A comparison with the effects of thiamine deficiency. Behavioural Brain Research. 1986;21:21–27. doi: 10.1016/0166-4328(86)90056-2. [DOI] [PubMed] [Google Scholar]
- 24.Mair RG, et al. Thiamine deficiency depletes cortical norepinephrine and impairs learning processes in the rat. Brain Research. 1985;360:273–284. doi: 10.1016/0006-8993(85)91243-0. [DOI] [PubMed] [Google Scholar]
- 25.Zhao N, et al. Impaired hippocampal neurogenesis is involved in cognitive dysfunction induced by thiamine deficiency at early pre-pathological lesion stage. Neurobiology of Disease. 2008;29:176–185. doi: 10.1016/j.nbd.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 26.Peters R. The biochemical lesion in vitamin B1 deficiency: application of modern biochemical analysis in its diagnosis. The Lancet. 1936;227:1161–1165. [Google Scholar]
- 27.Freeman GB, Nielsen PE, Gibson GE. Effect of age on behavioral and enzymatic changes during thiamin deficiency. Neurobiology of Aging. 1987;8:429–434. doi: 10.1016/0197-4580(87)90037-6. [DOI] [PubMed] [Google Scholar]
- 28.Barclay LL, Gibson GE, Blass JP. Impairment of behavior and acetylcholine metabolism in thiamine deficiency. Journal of Pharmacology and Experimental Therapeutics. 1981;217:537–543. [PubMed] [Google Scholar]
- 29.Langlais PJ, Zhang SX. Extracellular Glutamate Is Increased in Thalamus During Thiamine Deficiency-Induced Lesions and Is Blocked by MK-801. Journal of Neurochemistry. 1993;61:2175–2182. doi: 10.1111/j.1471-4159.1993.tb07457.x. [DOI] [PubMed] [Google Scholar]
- 30.Hazell AS, Butterworth RF, Hakim AM. Cerebral Vulnerability Is Associated with Selective Increase in Extracellular Glutamate Concentration in Experimental Thiamine Deficiency. Journal of Neurochemistry. 1993;61:1155–1158. doi: 10.1111/j.1471-4159.1993.tb03635.x. [DOI] [PubMed] [Google Scholar]
- 31.Ke Z-J, Gibson GE. Selective response of various brain cell types during neurodegeneration induced by mild impairment of oxidative metabolism. Neurochemistry International. 2004;45:361–369. doi: 10.1016/j.neuint.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 32.Karuppagounder SS, et al. Thiamine deficiency induces oxidative stress and exacerbates the plaque pathology in Alzheimer’s mouse model. Neurobiol Aging. 2009;30:1587–1600. doi: 10.1016/j.neurobiolaging.2007.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Q, et al. Thiamine deficiency increases beta-secretase activity and accumulation of beta-amyloid peptides. Neurobiol Aging. 2011;32:42–53. doi: 10.1016/j.neurobiolaging.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 34.Pan X, et al. Powerful beneficial effects of benfotiamine on cognitive impairment and {beta}-amyloid deposition in amyloid precursor protein/presenilin-1 transgenic mice. Brain. 2010;133:1342–1351. doi: 10.1093/brain/awq069. [DOI] [PubMed] [Google Scholar]
- 35.Elie I-G, et al. Nonalcoholic Thiamine-Related Encephalopathy (Wernicke-Korsakoff Syndrome) among Inpatients with Cancer: A series of 18 Cases. Psychosomatics. doi: 10.1016/j.psym.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Babaei-Jadidi R, et al. Prevention of Incipient Diabetic Nephropathy by High-Dose Thiamine and Benfotiamine. Diabetes. 2003;52:2110–2120. doi: 10.2337/diabetes.52.8.2110. [DOI] [PubMed] [Google Scholar]
- 37.Kennedy AR. MS dissertation. University of Manitoba; Winnipeg: 2001. The potential role of thiamine in the pathogenesis and treament of Alzheimer’s disease. [Google Scholar]
- 38.Blass JP, Gibson GE. Abnormality of a Thiamine-Requiring Enzyme in Patients with Wernicke-Korsakoff Syndrome. New England Journal of Medicine. 1977;297:1367–1370. doi: 10.1056/NEJM197712222972503. [DOI] [PubMed] [Google Scholar]
- 39.Volvert ML, et al. Benfotiamine, a synthetic S-acyl thiamine derivative, has different mechanisms of action and a different pharmacological profile than lipid-soluble thiamine disulfide derivatives. BMC Pharmacology. 2008;8:10. doi: 10.1186/1471-2210-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pincus JH, Solitare GB, Cooper JR. Thiamine triphosphate levels and histopathology: Correlation in leigh disease. Archives of Neurology. 1976;33:759–763. doi: 10.1001/archneur.1976.00500110027005. [DOI] [PubMed] [Google Scholar]
- 41.Calingasan NY, et al. Vascular factors are critical in selective neuronal loss in an animal model of impaired oxidative metabolism. J Neuropathol Exp Neurol. 2000;59:207–217. doi: 10.1093/jnen/59.3.207. [DOI] [PubMed] [Google Scholar]
- 42.Calingasan NY, et al. Induction of Nitric Oxide Synthase and Microglial Responses Precede Selective Cell Death Induced by Chronic Impairment of Oxidative Metabolism. The American Journal of Pathology. 1998;153:599–610. doi: 10.1016/S0002-9440(10)65602-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berrone E, et al. Regulation of Intracellular Glucose and Polyol Pathway by Thiamine and Benfotiamine in Vascular Cells Cultured in High Glucose. Journal of Biological Chemistry. 2006;281:9307–9313. doi: 10.1074/jbc.M600418200. [DOI] [PubMed] [Google Scholar]
- 44.Gibson GE, et al. Abnormal thiamine-dependent processes in Alzheimer’s Disease. Lessons from diabetes. Molecular and Cellular Neuroscience. 2013;55:17–25. doi: 10.1016/j.mcn.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bitsch R, et al. Bioavailability assessment of the lipophilic benfotiamine as compared to a water-soluble thiamin derivative. Ann Nutr Metab. 1991;35:292–296. doi: 10.1159/000177659. [DOI] [PubMed] [Google Scholar]
- 46.Schreeb KH, et al. Comparative bioavailability of two vitamin B1 preparations: benfotiamine and thiamine mononitrate. Eur J Clin Pharmacol. 1997;52:319–320. [PubMed] [Google Scholar]
- 47.Perez-Pineiro R, et al. The prion protein binds thiamine. FEBS Journal. 2011;278:4002–4014. doi: 10.1111/j.1742-4658.2011.08304.x. [DOI] [PubMed] [Google Scholar]
- 48.Hirsch JA, Gibson GE. Thiamin antagonists and the release of acetylcholine and norepinephrine from brain slices. Biochemical Pharmacology. 1984;33:2325–2327. doi: 10.1016/0006-2952(84)90673-7. [DOI] [PubMed] [Google Scholar]
- 49.Huang HM, Chen HL, Gibson G. Thiamine and Oxidants Interact to Modify Cellular Calcium Stores. Neurochemical Research. 2010;35:2107–2116. doi: 10.1007/s11064-010-0242-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mkrtchy; G, et al. Molecular mechanisms of the non-coenzyme action of thiamin in brain: biochemical, structural and pathway analysis. Scientific Reports. 2015:5. doi: 10.1038/srep12583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blass JP, et al. Thiamine and Alzheimer’s disease: A pilot study. Archives of Neurology. 1988;45:833–835. doi: 10.1001/archneur.1988.00520320019008. [DOI] [PubMed] [Google Scholar]
- 52.Nolan KA, et al. A trial of thiamine in Alzheimer’s disease. Archives of Neurology. 1991;48:81–83. doi: 10.1001/archneur.1991.00530130093025. [DOI] [PubMed] [Google Scholar]
- 53.Meador K, et al. Preliminary Findings of High-Dose Thiamine in Dementia of Alzheimer’s Type. Journal of Geriatric Psychiatry and Neurology. 1993;6:222–229. doi: 10.1177/089198879300600408. [DOI] [PubMed] [Google Scholar]
- 54.Mimori Y, Katsuoka H, Nakamura S. Thiamine therapy in Alzheimer’s disease. Metab Brain Dis. 1996;11:89–94. doi: 10.1007/BF02080934. [DOI] [PubMed] [Google Scholar]
- 55.Ollat H, et al. Effects of the association of sulbutiamine with an acetylcholinesterase inhibitor in early stage and moderate Alzheimer disease. Encephale. 2007;33:211–215. doi: 10.1016/s0013-7006(07)91552-3. [DOI] [PubMed] [Google Scholar]
- 56.Karuppagounder SS, et al. Dietary supplementation with resveratrol reduces plaque pathology in a transgenic model of Alzheimer’s disease. Neurochem Int. 2009;54:111–118. doi: 10.1016/j.neuint.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]