Abstract
Background
Non-infectious wound complications (NIWCs) following mastectomy are not routinely tracked and data are generally limited to single-center studies. Our objective was to determine the rates of NIWCs among women undergoing mastectomy and assess the impact of immediate reconstruction (IR).
Study Design
We established a retrospective cohort using commercial claims data of women aged 18–64 years with procedure codes for mastectomy from 1/2004–12/2011. NIWCs within 180 days after operation were identified by ICD-9-CM diagnosis codes and rates were compared among mastectomy with and without autologous flap and/or implant IR.
Results
18,696 procedures (10,836 [58%] with IR) among 18,085 women were identified. The overall NIWC rate was 9.2% (1,714/18,696); 56% required surgical treatment. The NIWC rates were 5.8% (455/7,860) after mastectomy-only, 10.3% (843/8,217) after mastectomy + implant, 17.4% (337/1,942) after mastectomy + flap, and 11.7% (79/677) after mastectomy + flap and implant (p<0.001). The rates of individual NIWCs varied by specific complication and procedure type, ranging from 0.5% for fat necrosis after mastectomy-only to 7.2% for dehiscence after mastectomy + flap. The percentage of NIWCs resulting in surgical wound care varied from 50% (210/416) for mastectomy + flap to 60% (507/843) for mastectomy + implant. Early implant removal within 60 days occurred after 6.2% of mastectomy + implant; 66% of the early implant removals were due to NIWCs and/or surgical site infection.
Conclusions
The rate of NIWC was approximately two-fold higher after mastectomy with IR than after mastectomy-only. NIWCs were associated with additional surgical treatment, particularly in women with implant reconstruction, and with early implant loss.
INTRODUCTION
Wound complications following breast surgical procedures, including surgical site infections (SSIs) and non-infectious wound complications (NIWCs), result in increased morbidity as well as increased healthcare utilization and costs.1 Wound complications can lead to worse outcomes after breast reconstruction including loss of implant or flap and poor cosmetic results,2–4 which often requires additional surgical procedures. Complications of breast reconstruction can result in large numbers of affected women, since over 26,000 breast reconstruction procedures were performed at the time of mastectomy during U.S. inpatient hospitalizations in 2012 (Healthcare Cost and Utilization Project National Inpatient Sample5). This number does not include subsequent reconstruction or procedures performed in ambulatory settings, and thus the total number of breast reconstruction procedures performed annually in the U.S. is much larger.
SSIs following breast procedures and healthcare facility surgical site infection rates are tracked nationally at over 14,500 facilities through the National Healthcare Safety Network (NHSN).6,7 Additionally, about 400 hospitals (most in the U.S.) participate in the American College of Surgeons National Quality Improvement Program (NSQIP), collecting data on process variables and 30-day complications, including SSIs.8 NSQIP collects NIWCs including wound dehiscence and return to the operating room, although the reason for reoperation is not specified. For breast reconstruction operations, loss of the flap or implant is also captured as a 30-day complication.9 Other than reports of wound dehiscence rates using the NSQIP database,10,11 reports of NIWC rates after breast procedures are generally limited to publications describing complications from a single surgeon or a large university-affiliated hospital. Currently available literature on breast surgery complications is problematic because many publications report only one summary measure for complications after a variety of different breast reconstruction procedures (e.g., report one combined rate for immediate and delayed implant and autologous reconstructions), and do not compare complication rates after mastectomy with breast reconstruction to complication rates after mastectomy-only. In addition, different definitions of non-infectious complications are often used with various follow-up periods which makes it difficult to compare rates from various centers.
To provide more comprehensive data concerning the rates of NIWCs, including hematoma, fat necrosis, necrosis, and wound dehiscence after mastectomy with and without immediate breast reconstruction, we identified wound complications in a large, geographically diverse cohort of women undergoing mastectomy, and assessed differences in outcome based on the type of immediate breast reconstruction performed.
METHODS
Data Source
We conducted a retrospective cohort study using the HealthCore Integrated Research Database (HIRDSM), including individuals from 12 Anthem-affiliated plans (California, Connecticut, Georgia, Indiana, Kentucky, Maine, Missouri (excluding 30 counties in the Kansas City area), Nevada, New Hampshire, New York, Ohio, Virginia (excluding the northern suburbs of Washington, DC). Data include all fully-adjudicated claims submitted from providers, facilities, and outpatient pharmacies linked to health plan enrollment information. Fully insured women with enrollment in a fee-for-service plan with medical coverage of hospital and physician services were eligible for inclusion in the study. We excluded women coded for end-stage renal disease, organ transplant, or HIV positive status due to unique risk factors for infection. Medical claims were restricted to paid claims.
The database contained up to 5 International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) diagnosis codes per claim from 2004–2008 and up to 12 diagnosis codes per claim from 2009–2011. Inpatient hospitals included up to 5 ICD-9-CM procedure codes per claim (8 in 2009–2011), while provider and ambulatory facility claims used CPT-4 (Current Procedural Terminology, 4th edition) codes.
To obtain hospital-level information, we matched the operative facility to the American Hospital Association Annual Survey of Hospitals (Health Forum, LLC, Chicago, IL) using National Provider Identifier codes, where available. Otherwise, matching to the AHA data was performed using facility name and address fields.
Identification of Mastectomy and Immediate Breast Reconstruction
We identified mastectomy operations among women aged 18–64 years from 1/1/2004–12/31/2011 using ICD-9-CM and/or CPT-4 procedure codes from inpatient and outpatient facilities and provider claims (see Appendix). We classified the mastectomy as unilateral or bilateral based on the ICD-9-CM procedure and CPT-4 codes, billed units, and CPT-4 modifier codes assigned by the provider and facility.12 Operations in members whose enrollment ended on the day of mastectomy were excluded since no follow-up was available. When CPT-4 or ICD-9-CM procedures codes for breast conserving surgery (BCS) were present during the same hospital admission or within +/− 3 days of mastectomy, we used an algorithm to determine whether BCS or mastectomy was more likely, as described previously.12 We excluded procedures that were more consistent with BCS, since concurrent BCS and mastectomy is unlikely and the incidence of SSI after BCS is lower than after mastectomy.12,13 We defined immediate reconstruction (IR) as the presence of CPT-4 and/or ICD-9-CM procedure codes for tissue expander/breast implant and/or flap reconstruction within +/− 7 days of the mastectomy (see Appendix), with prioritization of the provider classification of the type of flap when there was a discrepancy. Delayed reconstruction procedures were excluded since our focus was on NIWCs associated with mastectomy.
Identification of Wound Complications
NIWCs first coded from 2–180 days (1–180 days for hematoma) after surgery were identified using ICD-9-CM diagnosis codes from inpatient and outpatient facilities and provider claims. NIWCs included fat necrosis (ICD-9-CM diagnosis codes 567.82, 611.3), dehiscence (875.0, 875.1, 879.0, 879.1, 998.3, 998.32), hematoma (998.12), and necrosis (998.83). We excluded claims with laboratory or patient’s home locations and provider claims with laboratory CPT-4 codes (88104–88399), since these diagnosis codes may have indicated diagnostic workup and not post-surgical complications. For women with autologous flap reconstruction, the site of NIWC (donor vs. flap) could not be determined, since ICD-9-CM diagnosis codes do not distinguish the site of complication.
The date of onset of NIWCs was defined according to the timing and location of diagnosis. For NIWCs coded by an inpatient facility during the original operative admission, we assigned the date of NIWCs to the discharge date if the difference between the discharge and admission date was greater than or equal to 2 days. For NIWCs diagnosed during a subsequent inpatient admission, the date of NIWCs onset was assumed to be the date of hospital readmission. For NIWCs diagnosed initially by a provider or in an outpatient setting, the onset date was defined as the first service date coded for the NIWC.
The observation period for NIWCs was through 180 days after surgery, with earlier censoring for the end of insurance enrollment, a subsequent mastectomy, implant, flap, or nipple reconstruction. We censored one day after the subsequent surgery since we assumed that an NIWC coded on the day of or one day after a surgical procedure was preexisting and could be attributed to the previous surgery. Non-breast specific NIWC codes (e.g., 998.12, 998.3, 998.32, 998.83) were not classified as an NIWC if they were first coded after a subsequent non-breast operation, defined using the National Healthcare Safety Network procedure list.6
Preexisting NIWCs
ICD-9-CM diagnosis codes for NIWCs (other than hematoma) coded from 30 days before to 1 day after mastectomy were considered preexisting and were not used as evidence of a new-onset NIWC. For operations with a preexisting NIWC, we required a 30 day gap with no coding of an NIWC following the operation in order to identify an incident NIWC.
NIWC Requiring Surgical Wound Care
Surgical wound care within 14 days (and before applicable censoring) of an NIWC included CPT-4 codes 10140, 10160, 11000, 11001, 11040– 11044, 10060, 10061, 10180, 11005, 11008, 19020, 20000, 20005, 38300, 38305, A6550, A6551, E2402, and K0538 and ICD-9-CM procedure codes 54.0, 54.3, 83.44, 83.45, 83.49, 85.0, 86.04, 86.09, and 86.22. For mastectomy with immediate implant, we also included breast implant removal (CPT-4 11971, 19328; ICD-9-CM 85.94, 85.96), implant exchange (CPT 11970); or breast implant removal plus implant insertion (CPT-4 19325, 19340, 19342, 19357; ICD-9-CM 85.33, 85.35, 85.53, 85.54, 85.95) within 14 days (and before applicable censoring) of an NIWC as evidence of surgical wound care.
SSIs first coded from 2 to 180 days after surgery were identified using ICD-9-CM diagnosis codes from inpatient and outpatient facilities and provider claims (see Appendix), as described previously.12
Early Implant Loss, Physician and Emergency Department Encounters
We defined early implant loss as a breast implant removal, with or without an implant exchange, within 60 days of a mastectomy with implant IR. We used the CPT-4 and ICD-9-CM procedure codes described in the previous section. Codes for SSI or NIWCs in the 14 days prior to removal were considered the indication for implant removal. To compare outpatient utilization, we identified distinct dates with CPT-4 codes for physician office visits (99201–99215) and emergency department encounters (99281–99285).
Statistical Analysis
The rates of NIWCs overall and by type of complication within 180 days after surgery for mastectomy with and without IR were compared using a Chi-square test, as were other categorical variable comparisons. For the comparisons of wound complication rates by type of procedure, the alpha was adjusted for multiple testing using the Bonferroni method.14 We used the Kruskal-Wallis test to compare continuous variables. All data management and statistical analyses were performed using SAS v9.3 (SAS Institute Inc., Cary, NC). This study was approved by the Washington University Human Research Protection Office.
RESULTS
Overall Rates of NIWCs
The final analysis sample included 18,696 mastectomy procedures among 18,085 women. The median age of women was 51 years (interquartile range 45–57 years). The indication for mastectomy was cancer in 17,861 (95.5%), and prophylactic or benign conditions in 835 (4.5%). Forty-two percent of the operations were mastectomy-only, 44.0% mastectomy plus implant IR, 10.4% mastectomy plus flap IR, and 3.6% were categorized as mastectomy plus flap and implant IR (Table 1). As reported previously, 39% of procedures were bilateral and 81% were performed on during an inpatient admission.12 Among procedures performed during an inpatient hospital stay that we could link to the AHA survey (n=11,756; 63%), 50% were performed at a hospital with a residency training program and 58% at a hospital with a medical school affiliation. Thirty-nine percent of procedures were performed at a hospital with <300 beds, 33% at a hospital with 300–499 beds, and 28% at a hospital with ≥ 500 beds. Most procedures were performed at an urban hospital (94%), and the procedures were distributed across the U.S. (18% Northeast, 34% South, 26% Midwest, and 21% West).
TABLE 1.
Rates of Non-Infectious Wound Complications (NIWC) After Mastectomy With and Without Immediate Reconstruction (IR)
| Operative Category |
Total Procedures (n= 18,696), n (column %) |
NIWC Requiring Surgical Wound Care* (n= 953), n (row %) |
Any NIWC (n=1,714), n (row %) |
Relative Risk of Any NIWC (95% CI)** |
|---|---|---|---|---|
| Mastectomy-only | 7,860 (42.0) | 236 (3.0) | 455 (5.8) | 1.00 |
| Mastectomy with implant IR |
8,217 (44.0) | 507 (6.2) | 843 (10.3) | 1.77 (1.59, 1.98) |
| Mastectomy with flap IR |
1,942 (10.4) | 162 (8.3) | 337 (17.4) | 3.00 (2.63, 3.42) |
| Mastectomy with flap + implant IR |
677 (3.6) | 48 (7.1) | 79 (11.7) | 2.02 (1.61, 2.53) |
| Mastectomy with IR |
10,836 (58.0) | 717 (6.6) | 1,259 (11.6) | 2.01 (1.81, 2.23) |
Surgical wound care performed at the bedside or operating room
All comparisons significant after adjustment for 4 tests (p < 0.0125). Relative risk calculated by dividing the rate of NIWCs among mastectomy + IR by the rate of NIWCs among the mastectomy-only reference group.
CI indicates confidence interval.
The overall NIWC rate following mastectomy with and without IR was 9.2% (1,714/18,696); 56% of the NIWCs required a surgical wound care procedure. The rates of NIWCs were 5.8% after mastectomy-only, 10.3% after mastectomy with implant, 17.4% after mastectomy with flap, and 11.7% after mastectomy with flap and implant (p<0.001, Chi-square test; Table 1). The risk of an NIWC was almost 2-fold higher after mastectomy plus implant and 3-fold higher after mastectomy plus flap compared with mastectomy-only. The rates of NIWCs requiring surgical wound care ranged from 3.0% after mastectomy-only to 8.3% for mastectomy with flap reconstruction (Table 1). The proportion of NIWC diagnosed during a hospitalization (index or readmission) varied from 28% after mastectomy plus implant to 39% after mastectomy plus flap.
Effect of Uni- Versus Bilateral Procedures
Among mastectomies with IR, the NIWC rate was significantly higher after bilateral compared with unilateral procedures (12.7% (703/5,529) vs. 10.5% (556/5,307) respectively, p<0.001, Chi-square test). Similarly, among mastectomies without IR, the NIWC rate was significantly higher after bilateral compared with unilateral procedures (8.1% (141/1,739) vs. 5.1% (314/6,121), p<0.001, Chi-square test).
Complication Rates and Indication for Mastectomy
The rates of NIWCs were similar for women with cancer versus prophylaxis or benign conditions (9.2% vs. 8.9%, p=0.75, Chi-square test). NIWC after mastectomy-only occurred in 5.7% (439/7,639) of women with breast cancer (in situ or invasive) and 7.2% (16/221) of women undergoing mastectomy for prophylaxis or benign conditions (p=0.35, Chi-square test). Among mastectomy + IR, 11.7% (1,201/10,222) of procedures were complicated by NIWC in women with cancer versus 9.4% (58/614) of procedures performed for prophylaxis or benign conditions (p=0.08, Chi-square test).
Association with Surgical Site Infection
Of 1,714 mastectomy procedures complicated by an NIWC, 29% were also coded for an SSI within 180 days after surgery. Among procedures with both an NIWC and SSI, the most common NIWCs were dehiscence (60.9%) and necrosis (35.3%). Among those procedures with both an SSI and an NIWC, 36% were coded for both complications at the same time, while the NIWC preceded SSI by more than one day in 36% of procedures (Table 2). The most common timing of wound complications after mastectomy-only and mastectomy with flap was coding of NIWC before SSI, while SSI and NIWC after mastectomy with implant IR most commonly was coded simultaneously (Table 2). For this and subsequent analyses, mastectomy with immediate flap included flap with or without concurrent implant because of the small number of immediate flap plus implant procedures.
TABLE 2.
Co-Occurrence of Noninfectious Wound Complication (NIWC) and Surgical Site Infection (SSI) by Operative Category
| Operative Category (n of total NIWC) |
NIWC + SSI, n (% of total NIWC) |
NIWC + SSI, n (row %)* | ||
|---|---|---|---|---|
| NIWC Before SSI |
NIWC at Same Time as SSI** |
NIWC After SSI |
||
| Mastectomy-only (n = 455) |
105 (23.1) | 41 (39.0) | 27 (25.7) | 37 (35.2) |
| Mastectomy with implant IR (n = 843) |
265 (31.4) | 87 (32.8) | 109 (41.1) | 69 (26.0) |
| Mastectomy with flap IR† (n = 416) |
129 (31.0) | 53 (41.1) | 42 (32.6) | 34 (26.4) |
| Mastectomy with IR (n = 1,259) |
394 (31.3) | 140 (35.5) | 151 (38.3) | 103 (26.1) |
| Total NIWC (n = 1,714) |
499 (29.1) | 181 (36.3) | 178 (35.7) | 140 (28.1) |
Percentage of the total coded for both NIWC and SSI within operative category.
SSI within ± 1 day of the NIWC
Flap IR with or without concurrent implant reconstruction.
IR, immediate reconstruction.
Effect on Sustainability of Reconstruction
Among mastectomy with immediate implant reconstruction, 216 (2.6%) had implant removal within 30 days and 506 (6.2%) had implant removal within 60 days after mastectomy, with or without implant exchange. Sixty-six percent of women with an early implant removal within 60 days were coded for an SSI or NIWC in the 14 days before implant removal. Fourteen percent of the procedures with early implant removal were coded for both SSI and NIWC (n = 73), 43.1% (n = 218) were coded for SSI only, and 8.5% (n= 43) were coded for NIWC only. The remaining 34.0% (n = 172) of procedures with early implant removal were not coded for either an infectious or noninfectious wound complication in the two weeks before implant removal. Of these early implant removals without wound complication per our definition, 59.3% (n = 102) were coded for another complication (e.g., malfunction or complication of prosthetic device, deformity/disproportion of reconstructed breast, breast pain, seroma). In addition, women with NIWCs had significantly more physician visits within 180 days after operation compared to women without a wound complication (median 8 vs. 7 visits on distinct dates, p<0.001, Kruskal-Wallis test) and more emergency department (ED) encounters (median 0 for both, mean 0.4 vs. 0.2 ED encounters, p<0.001, Kruskal-Wallis test).
Timing of Incident NIWCs
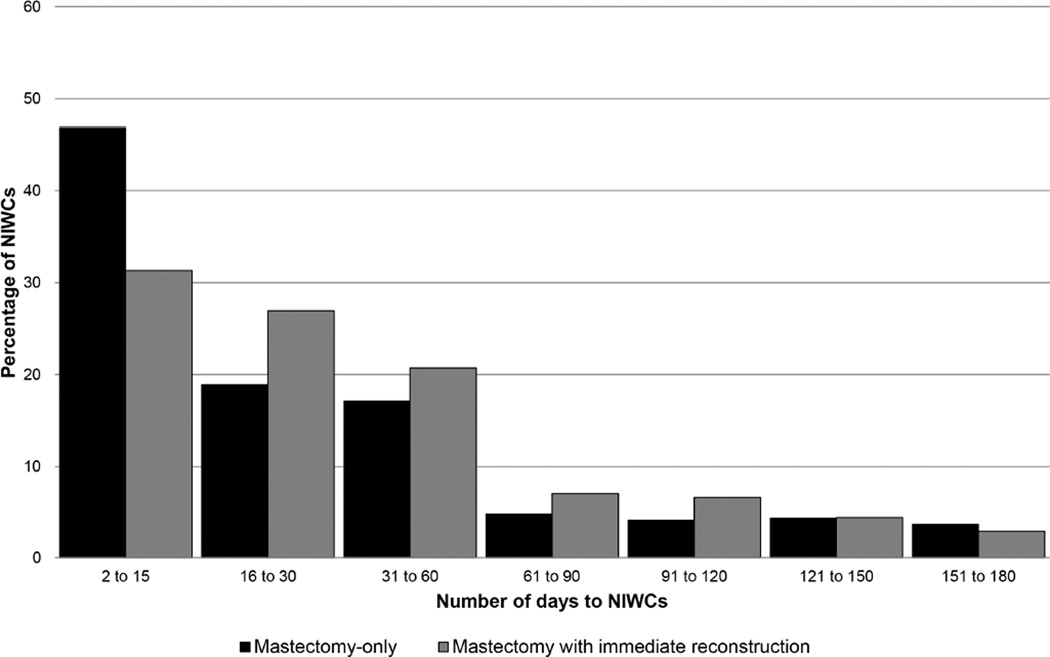
The median time to diagnosis of NIWCs was significantly shorter following mastectomy-only compared with mastectomy plus implant or mastectomy plus flap (median 17 vs. 24 and 29 days respectively; p<0.001, Kruskal-Wallis test). NIWCs were first coded within 30 days after mastectomy-only in 65.7% of procedures, with 17.1% diagnosed between 31–60 days. Among mastectomy with IR, NIWCs were first coded within 30 days in 58.2% of procedures and 20.7% were diagnosed between 31–60 days (Figure 1).
Figure 1.
Days to Non-Infectious Wound Complications (NIWC) Following Mastectomy With and Without Immediate Reconstruction (n = 1,714 NIWC).
Rates of NIWCs by Procedure Type
Individual complication rates ranged from 0.5% for fat necrosis after mastectomy-only to 7.2% for dehiscence after mastectomy with flap reconstruction (Table 3). The fat necrosis, dehiscence, and necrosis NIWC rates were all significantly higher after mastectomy plus implant and mastectomy plus flap compared with the individual NIWC rates after mastectomy-only. The proportion of operations complicated by hematoma was significantly higher after mastectomy plus flap, but not mastectomy plus implant compared to mastectomy-only. Among mastectomy with reconstruction, the rates of all NIWC categories were significantly higher after flap versus implant reconstruction. Overall the median time to NIWC diagnosis ranged from 7 days for hematoma to 35 days for fat necrosis (Table 3).
TABLE 3.
Incident Non-Infectious Wound Complications (NIWC) Following Mastectomy With and Without Immediate Reconstruction and Timing of Diagnosis after Surgery*
| Type of NIWC* |
Mastectomy-Only (n = 7,860), n (%) |
Mastectomy Plus Implant (n = 8,217), n (%) |
Mastectomy Plus Flap ± Implant (n = 2,619), n (%) |
Days to Diagnosis, Median (IQR) |
|||
|---|---|---|---|---|---|---|---|
| All | Requiring Surgical Wound Care |
All | Requiring Surgical Wound Care |
All | Requiring Surgical Wound Care |
||
| Dehiscence | 166 (2.1) | 76 (1.0) | 420 (5.1) | 241 (2.9) | 188 (7.2) | 106 (4.0) | 32 (19–57) |
| Necrosis | 96 (1.2) | 45 (0.6) | 200 (2.4) | 118 (1.4) | 122 (4.7) | 77 (2.9) | 33 (18–58) |
| Hematoma | 209 (2.7) | 139 (1.8) | 211 (2.6) | 156 (1.9) | 98 (3.7) | 61 (2.3) | 7 (2–20) |
| Fat necrosis | 42 (0.5) | 12 (0.2) | 154 (1.9) | 75 (0.9) | 138 (5.3) | 42 (1.6) | 35 (18–104) |
All comparisons of overall NIWC proportions for mastectomy plus implant and mastectomy plus flap immediate reconstruction compared to mastectomy-only p < 0.001 by Chi-square test, except for hematoma (mastectomy plus implant p = 0.72 and mastectomy plus flap ± implant (p = 0.004) compared to mastectomy-only). Comparing the overall NIWC proportions after mastectomy plus implant vs. mastectomy plus flap (± implant), all p < 0.001 except for hematoma (p = 0.002). Adjusting the alpha for a total of 12 tests, p < 0.004 considered statistically significant.
IQR, interquartile range.
DISCUSSION
We demonstrated that the overall NIWC rate after mastectomy was almost 10% in our sample of 18–64 year old women with private insurance undergoing mastectomy from 2004–2011, and complication rates were significantly higher after mastectomy with IR compared with mastectomy-only for all individual NIWCs except hematoma. Immediate flap reconstruction (with or without implant) was associated with significantly higher rates of fat necrosis, dehiscence, hematoma, and necrosis compared with immediate implant reconstruction. The increased rates of NIWCs after mastectomy with flap may be a reflection of the more prolonged surgical procedure time, longer incision and multiple surgical sites involved with performing a flap procedure compared to the time and incisions involved performing mastectomy with implant reconstruction.
The rates of several NIWCs identified in this private insurer data were at the upper end of the range of reported rates from clinical studies in the literature (Table 4). For example, we identified dehiscence/open wound within 180 days after 5.1% of mastectomy plus implant IR procedures, compared to 0.6% to 6.2% in single-center reports in the literature.15–24 Similarly, we identified hematoma in 2.6% of mastectomy plus implant and 3.7% of mastectomy plus flap IR, while the majority of studies in the literature reported lower hematoma rates for these procedures.18,21–42 Rates for dehiscence/wound disruption and implant/flap loss are also available from the NSQIP database. In the most recent analysis of complication rates after mastectomy with IR, dehiscence was recorded within 30 days after 0.6% of immediate implant and 0.9% of immediate flap reconstructive procedures.11
TABLE 4.
Summary of Noninfectious Wound Complication Rates after Immediate Breast Reconstruction in the Surgical Literature from Individual Institutions
| Implant Reconstruction | Flap Reconstruction | |||
|---|---|---|---|---|
| Wound Complication |
Literature | This Study | Literature | This Study |
| Dehiscence/ Wound Disruption/ Exposure/ Open Wound, % |
0.6–6.215–24 | 5.1 | 0.9–4.016,18 | 7.2 |
| Implant/Flap Loss, % | 1.0–21.416,17,21–23,30,31,33–36,38,40,42,44 | 6.2 | 0–4.815,18,25,26,37 | |
| Necrosis, % | 1.0–18.317,18,20–24,28–36,38,40–42,45 | 2.4 | 3.3–16.618,25–28,37,39 | 4.7 |
| Hematoma, % | 0–2.918,21–24,28–36,38,40–42 | 2.6 | 0.5–3.918,25–28,37,39 | 3.7 |
| Fat Necrosis, % | 1.9 | 2.8–24.415,18,25–27,37,43 | 5.3 | |
The complication rates we report are likely higher than rates reported in many clinical studies and from the NSQIP data for a number of reasons, including the longer time period of observation in our study (180 compared to 30 days for NSQIP). We identified complications in all settings using the medical claims, including outpatient clinic and emergency department encounters, some of which may have been missed in previous studies if patients presented to physicians other than their general or plastic surgeon. We also identified all complications regardless of severity, whereas in some clinical studies primarily severe complications were recorded.22,30,32,33,39–42 In our study, 44% of NIWCs did not have evidence of subsequent surgical wound care, so the inclusion of potentially conservatively-treated NIWCs may have increased the NIWC rates in our study by up to two-fold. Many of the publications reporting rates of complications from individual institutions reported rates per breast rather than per person, resulting in lower complication rates, particularly in studies with high proportions of bilateral procedures.15–22,24,25,27,29,31–38,41–43,45
We found that implant removal occurred in 2.6% of mastectomy plus implant within 30 days and in 6.2% within 60 days of operation. This is higher than the 0.65% implant loss rate reported recently using 2005–2013 NSQIP data.11 The rate of implant loss in our study is higher than some17,21,31,33,38,40 but not all16,22,23,30,35,36,42 studies reported in the last decade from individual U.S. institutions, although the timing of implant removal was not described in these studies. We found that 58% of operations complicated by early implant loss were associated with SSI, either alone or in combination with NIWC, and an additional 9% were associated with NIWC alone. In a recent analysis of the NSQIP data, Fischer and colleagues found that SSIs were implicated in 17.7% of implant loss within 30 days in patients who had immediate implant reconstruction, while dehiscence was present in 2.4% of the patients with implant loss.46
Nearly 30% of procedures coded for NIWCs were also coded for SSIs. The timing of SSIs and NIWCs varied slightly by procedure, with NIWCs coded most often before SSIs after mastectomy- only and after mastectomy plus flap, while SSIs and NIWCs were most often coded concurrently after mastectomy plus implant IR. Hematoma was the most common NIWC after mastectomy-only, with a median time to diagnosis of 7 days after operation. This result is also consistent with the finding of Al-Hilli et al. that bleeding was responsible for two-thirds of reoperation after mastectomy-only using the 2012 NSQIP data.47 In contrast, the most common NIWC in our study after mastectomy with implant reconstruction was dehiscence, with median time to onset of 32 days after operation, virtually the same as the median 33 days to onset of SSI after mastectomy with IR we reported previously in this population.12 Thus our finding that concurrent SSI and NIWC was most common after mastectomy with implant reconstruction is consistent with the timing and distribution of the individual NIWCs after operation.
The strengths of this study include the large number of women who underwent mastectomy and high proportion with IR in this younger privately insured population. We identified complications up to 180 days after surgery and found that 40% of complications were detected more than one month after surgery. NSQIP surveillance for non-infectious complications could be improved by increasing the duration of surveillance of complications for reconstructive procedures, and collecting the reason for return to the operating room. Limitations include the use of claims data to identify NIWCs, which by definition involves secondary analysis of data collected for billing purposes. There is potential for misclassification of diagnoses and likely undercoding of NIWCs, particularly minor complications during the 90 day global surgical reimbursement period for providers.48 Although we have previously validated the ICD-9-CM diagnosis codes for SSI,49 no validation of the diagnosis codes for the NIWCs used in this study has been reported. For mastectomy plus flap reconstruction, we were not able to determine the rates of NIWCs at the mastectomy and donor flap sites because site of complication is not available with claims data. Finally, since our analysis sample was restricted to women with private insurance <65 years of age, generalizability to women with other types or lack of health insurance and to older women is unknown.
We demonstrated that the rate of noninfectious wound complications was two-fold higher after mastectomy with IR compared to mastectomy-only, and the distribution of specific complications was different depending on the use and type of reconstruction. Over half of the NIWCs were associated with additional surgical wound care and almost one-third of NIWCs occurred in women with SSI. Further work is needed to identify risk factors for noninfectious wound complications in women undergoing these procedures to determine the best strategies to prevent these complications. Prevention of both infectious and noninfectious wound complications after mastectomy, particularly in women with IR, is important to avoid potential longer term adverse consequences, including implant/flap loss, delays in oncologic treatment, and additional surgical procedures, which may be necessary to improve cosmetic outcomes. Our findings may be helpful in guiding patients as they balance the risk of complications and surgical treatment options (e.g., breast conservation, uni- and bilateral mastectomy, immediate and delayed reconstruction) against their personal preferences, expectations, and practical considerations (e.g., support system at home, out-of-pocket costs).
Acknowledgments
We thank Cherie Hill for database and computer management support.
Support: Funding for this project was provided by the National Institutes of Health (NIH) (5R01CA149614 to MAO). Additional support was provided by the Centers for Disease Control and Prevention (CDC) Epicenters Program (U54CK000162 to VJF), grant UL1 TR000448 from the National Center for Advancing Translational Sciences (NCATS) of the NIH, and grant number R24 HS19455 from the Agency for Healthcare Research and Quality (AHRQ). The findings and conclusions in this document are those of the authors, who are responsible for its content, and do not necessarily represent the official view of NIH, CDC, or AHRQ.
Abbreviations
- BCS
breast conserving surgery
- CPT-4
Current Procedural Terminology, 4th edition
- HIRDSM
HealthCore Integrated Research Database
- ICD-9-CM
International Classification of Diseases, 9th Revision, Clinical Modification
- IR
immediate reconstruction
- NHSN
National Healthcare Safety Network
- NIWC
non-infectious wound complication
- NSQIP
National Surgical Quality Improvement Program
- SSI
surgical site infection
APPENDIX 1. Procedure Codes Used to Identify Mastectomy and Immediate Reconstruction Procedures
| Operative Category | ICD-9-CM Procedure Code | CPT-4 or HCPCS Procedure Code |
|---|---|---|
| Mastectomy* | 85.41–85.48 | 19180, 19200–19240, 19303, 19305–19307 |
| Breast implant | 85.33, 85.35, 85.53, 85.54, 85.95 | 19325, 19340, 19342, 19357 |
| Flap reconstruction | 85.7–85.79, 85.85 | 19361, 19364, 19367–19369, S2066–S2068 |
CPT-4 indicates Current Procedural Terminology, 4th edition; HCPCS, Healthcare Common Procedure Coding System; ICD-9-CM, International Classification of Diseases, 9th Revision, Clinical Modification.
For mastectomy coded by facility- or provider-only, a code for anesthesia (CPT-4 00400– 00406), pathology (CPT-4 88307, 88309), or a surgery revenue code (Uniform Billing 0201, 0360, 0361, 0369, 0370, 0379, 0490, 0499, 0963, 0964, 0975) was required as evidence for surgery.
APPENDIX 2. Procedure and Diagnosis Codes Used in Combination to Identify Surgical Site Infection Following Mastectomy With and Without Immediate Reconstruction
| ICD-9-CM Diagnosis Code |
CPT-4 or HCPCS Procedure Code |
ICD-9-CM Procedure Code |
|
|---|---|---|---|
| Breast-specific codes | |||
| Infection, lymphadenitis | 611.0, 683.0, 996.69* |
||
| Incision/drainage†, ‡ | 19020, 38300, 38305 | 85.0, 85.91 | |
| Non-infectious wound complication† |
611.3, 875.0, 875.1, 879.0, 879.1 |
||
| Breast implant removal‡ | 11971, 19328 | 85.94, 85.96 | |
| General codes§ | |||
| Postoperative infection | 998.5–998.59 | ||
| Cellulitis† | 682.2, 682.3, 682.9∥ | ||
| Staphylococcus aureus‡,¶ | 041.1–041.19 | ||
| Incision/drainage†, ‡ | 10060, 10061, 10140–10180, 11000, 11001, 11005,# 11008,# 11040–11044, 20000, 20005, A6550, A6551, E2402, K0538 |
54.0,# 54.3,# 83.44– 83.49, 86.01, 86.04, 86.09, 86.22, 86.28 |
|
| Non-infectious wound complication† |
567.82,# 998.12, 998.3, 998.32, 998.83 |
CPT-4 indicates Current Procedural Terminology, 4th edition; HCPCS, Healthcare Common Procedure Coding System; ICD-9-CM, International Classification of Diseases, 9th Revision, Clinical Modification.
ICD-9-CM diagnosis code 996.69 was excluded if it was coded before a breast implant or flap reconstruction procedure or if it was on a claim with pathology code 88300 on the same day as a catheter removal (because 996.69 could be referring to an infection found on gross examination of the catheter).
Codes were used in combination with an ICD-9-CM diagnosis code for Staphylococcus aureus.
Codes were used in combination with an ICD-9-CM diagnosis code for cellulitis.
Excluded if occurred +/− 7 days of an SSI code that was specific to another type of device (ICD-9-CM 996.61–996.68, 999.31) while a catheter was in place.
Diagnosis code 682.9 codes for cellulitis and abscess at an unspecified site; it was used only if it was on the same claim line as a breast-specific incision/drainage code, on the same day as an implant removal, or coded by the patient’s breast surgeon.
A S. aureus diagnosis code associated with an incision/drainage code was only used if the incision/drainage code was breast-specific or coded by the patient’s breast surgeon.
Excluded if coded before a non-latissimus dorsi flap reconstruction procedure.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Information: Nothing to disclose.
Disclosures outside the scope of this work: Dr Wallace is an employee of HealthCore, a wholly-owned subsidiary of Anthem, Inc., and has participated in an Anthem employee stock purchase plan.
Presented at the Pharmacoepidemiology & Therapeutic Risk Management 31st International Conference, Boston, MA, August 2015.
REFERENCES
- 1.Olsen MA, Chu-Ongsakul S, Brandt KE, et al. Hospital-associated costs due to surgical site infection after breast surgery. Arch Surg. 2008;143:53–60. doi: 10.1001/archsurg.2007.11. [DOI] [PubMed] [Google Scholar]
- 2.Andrade WN, Baxter N, Semple JL. Clinical determinants of patient satisfaction with breast reconstruction. Plast Reconstr Surg. 2001;107:46–54. doi: 10.1097/00006534-200101000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Colakoglu S, Khansa I, Curtis MS, et al. Impact of complications on patient satisfaction in breast reconstruction. Plast Reconstr Surg. 2011;127:1428–1436. doi: 10.1097/PRS.0b013e318208d0d4. [DOI] [PubMed] [Google Scholar]
- 4.Lu SM, Nelson JA, Fischer JP, et al. The impact of complications on function, health, and satisfaction following abdominally based autologous breast reconstruction: a prospective evaluation. J Plast Reconstr Aesthet Surg. 2014;67:682–692. doi: 10.1016/j.bjps.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 5.Agency for Healthcare Research and Quality. HCUPnet, Healthcare Cost and Utilization Project. [Accessed May 15, 2015];Agency for Healthcare Research and Quality. 2015 Available at: http://hcupnet.ahrq.gov.
- 6.National Healthcare Safety Network (NHSN) Procedure-Associated (PA) module: surgical site infection (SSI) event. [Accessed November 14, 2013];Centers for Disease Control and Prevention. 2013 Jul; Available at: http://www.cdc.gov/nhsn/PDFs/pscManual/9pscSSIcurrent.pdf.
- 7.Centers for Disease Control and Prevention. National Healthcare Safety Network (NHSN): About NHSN. [Accessed January 24, 2014];Centers for Disease Control and Prevention. 2013 Nov; Available at: http://www.cdc.gov/nhsn/about.html.
- 8.American College of Surgeons. [Accessed July 18, 2015];Frequently asked questions about ACS NSQIP. American College of Surgeons. 2015 Available at: https://www.facs.org/quality-programs/acs-nsqip/joinnow/joinfaq.
- 9.Ju MH, Cohen ME, Bilimoria KY, et al. Effect of wound classification on risk adjustment in American College of Surgeons NSQIP. J Am Coll Surg. 2014;219:371–381. doi: 10.1016/j.jamcollsurg.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Blacam C, Ogunleye AA, Momoh AO, et al. High body mass index and smoking predict morbidity in breast cancer surgery: a multivariate analysis of 26,988 patients from the national surgical quality improvement program database. Ann Surg. 2012;255:551–555. doi: 10.1097/SLA.0b013e318246c294. [DOI] [PubMed] [Google Scholar]
- 11.Silva AK, Lapin B, Yao KA, et al. The effect of contralateral prophylactic mastectomy on perioperative complications in women undergoing immediate breast reconstruction: a NSQIP analysis. Ann Surg Oncol. 2015;22:3474–3480. doi: 10.1245/s10434-015-4628-7. [DOI] [PubMed] [Google Scholar]
- 12.Olsen MA, Nickel KB, Fox IK, et al. Incidence of surgical site infection following mastectomy with and without immediate reconstruction using private insurer claims data. Infect Control Hosp Epidemiol. 2015;36:907–914. doi: 10.1017/ice.2015.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olsen MA, Nickel KB, Margenthaler JA, et al. Increased risk of surgical site infection among breast-conserving surgery re-excisions. Ann Surg Oncol. 2015;22:2003–2009. doi: 10.1245/s10434-014-4200-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pagano M, Gauvreau K. Principles of biostatistics. Belmont, CA: Wadsworth; 1993. [Google Scholar]
- 15.Sullivan SR, Fletcher DR, Isom CD, Isik FF. True incidence of all complications following immediate and delayed breast reconstruction. Plast Reconstr Surg. 2008;122:19–28. doi: 10.1097/PRS.0b013e3181774267. [DOI] [PubMed] [Google Scholar]
- 16.Chang DW, Barnea Y, Robb GL. Effects of an autologous flap combined with an implant for breast reconstruction: an evaluation of 1000 consecutive reconstructions of previously irradiated breasts. Plast Reconstr Surg. 2008;122:356–362. doi: 10.1097/PRS.0b013e31817d6303. [DOI] [PubMed] [Google Scholar]
- 17.McCarthy CM, Mehrara BJ, Riedel E, et al. Predicting complications following expander/implant breast reconstruction: an outcomes analysis based on preoperative clinical risk. Plast Reconstr Surg. 2008;121:1886–1892. doi: 10.1097/PRS.0b013e31817151c4. [DOI] [PubMed] [Google Scholar]
- 18.Crosby MA, Garvey PB, Selber JC, et al. Reconstructive outcomes in patients undergoing contralateral prophylactic mastectomy. Plast Reconstr Surg. 2011;128:1025–1033. doi: 10.1097/PRS.0b013e31822b6682. [DOI] [PubMed] [Google Scholar]
- 19.Vardanian AJ, Clayton JL, Roostaeian J, et al. Comparison of implant-based immediate breast reconstruction with and without acellular dermal matrix. Plast Reconstr Surg. 2011;128:403e–410e. doi: 10.1097/PRS.0b013e31822b6637. [DOI] [PubMed] [Google Scholar]
- 20.Boneti C, Yuen J, Santiago C, et al. Oncologic safety of nipple skin-sparing or total skin-sparing mastectomies with immediate reconstruction. J Am Coll Surg. 2011;212:686–693. doi: 10.1016/j.jamcollsurg.2010.12.039. [DOI] [PubMed] [Google Scholar]
- 21.Salzberg CA, Ashikari AY, Koch RM, Chabner-Thompson E. An 8-year experience of direct-to-implant immediate breast reconstruction using human acellular dermal matrix (AlloDerm) Plast Reconstr Surg. 2011;127:514–524. doi: 10.1097/PRS.0b013e318200a961. [DOI] [PubMed] [Google Scholar]
- 22.Seth AK, Hirsch EM, Fine NA, Kim JY. Utility of acellular dermis-assisted breast reconstruction in the setting of radiation: a comparative analysis. Plast Reconstr Surg. 2012;130:750–758. doi: 10.1097/PRS.0b013e318262f009. [DOI] [PubMed] [Google Scholar]
- 23.Mitchell RE. Porcine acellular dermis-assisted breast reconstruction: influence of adjuvant radiotherapy on complications and outcomes. Plast Reconstr Surg Glob Open. 2013;1:e77. doi: 10.1097/GOX.0000000000000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seth AK, Persing S, Connor CM, et al. A comparative analysis of cryopreserved versus prehydrated human acellular dermal matrices in tissue expander breast reconstruction. Ann Plast Surg. 2013;70:632–635. doi: 10.1097/SAP.0b013e318250f0b4. [DOI] [PubMed] [Google Scholar]
- 25.Selber JC, Kurichi JE, Vega SJ, et al. Risk factors and complications in free TRAM flap breast reconstruction. Ann Plast Surg. 2006;56:492–497. doi: 10.1097/01.sap.0000210180.72721.4a. [DOI] [PubMed] [Google Scholar]
- 26.Miller RB, Reece G, Kroll SS, et al. Microvascular breast reconstruction in the diabetic patient. Plast Reconstr Surg. 2007;119:38–45. doi: 10.1097/01.prs.0000244745.21562.58. [DOI] [PubMed] [Google Scholar]
- 27.Carlson GW, Page AL, Peters K, et al. Effects of radiation therapy on pedicled transverse rectus abdominis myocutaneous flap breast reconstruction. Ann Plast Surg. 2008;60:568–572. doi: 10.1097/SAP.0b013e31815b6ced. [DOI] [PubMed] [Google Scholar]
- 28.Spear SL, Newman MK, Bedford MS, et al. A retrospective analysis of outcomes using three common methods for immediate breast reconstruction. Plast Reconstr Surg. 2008;122:340–347. doi: 10.1097/PRS.0b013e31817d6009. [DOI] [PubMed] [Google Scholar]
- 29.Chun YS, Verma K, Rosen H, et al. Implant-based breast reconstruction using acellular dermal matrix and the risk of postoperative complications. Plast Reconstr Surg. 2010;125:429–436. doi: 10.1097/PRS.0b013e3181c82d90. [DOI] [PubMed] [Google Scholar]
- 30.Lanier ST, Wang ED, Chen JJ, et al. The effect of acellular dermal matrix use on complication rates in tissue expander/implant breast reconstruction. Ann Plast Surg. 2010;64:674–678. doi: 10.1097/SAP.0b013e3181dba892. [DOI] [PubMed] [Google Scholar]
- 31.de Alcantara FP, Capko D, Barry JM, et al. Nipple-sparing mastectomy for breast cancer and risk-reducing surgery: the Memorial Sloan-Kettering Cancer Center experience. Ann Surg Oncol. 2011;18:3117–3122. doi: 10.1245/s10434-011-1974-y. [DOI] [PubMed] [Google Scholar]
- 32.Liu AS, Kao HK, Reish RG, et al. Post-operative complications in prosthesis-based breast reconstruction using acellular dermal matrix. Plast Reconstr Surg. 2011;127:1755–1762. doi: 10.1097/PRS.0b013e31820cf233. [DOI] [PubMed] [Google Scholar]
- 33.Colwell AS, Damjanovic B, Zahedi B, et al. Retrospective review of 331 consecutive immediate single-stage implant reconstructions with acellular dermal matrix: indications, complications, trends, and costs. Plast Reconstr Surg. 2011;128:1170–1178. doi: 10.1097/PRS.0b013e318230c2f6. [DOI] [PubMed] [Google Scholar]
- 34.Weichman KE, Wilson SC, Weinstein AL, et al. The use of acellular dermal matrix in immediate two-stage tissue expander breast reconstruction. Plast Reconstr Surg. 2012;129:1049–1058. doi: 10.1097/PRS.0b013e31824a2acb. [DOI] [PubMed] [Google Scholar]
- 35.Peled AW, Foster RD, Garwood ER, et al. The effects of acellular dermal matrix in expander-implant breast reconstruction after total skin-sparing mastectomy: results of a prospective practice improvement study. Plast Reconstr Surg. 2012;129:901e–908e. doi: 10.1097/PRS.0b013e31824ec447. [DOI] [PubMed] [Google Scholar]
- 36.Butterfield JL. 440 Consecutive immediate, implant-based, single-surgeon breast reconstructions in 281 patients: a comparison of early outcomes and costs between SurgiMend fetal bovine and AlloDerm human cadaveric acellular dermal matrices. Plast Reconstr Surg. 2013;131:940–951. doi: 10.1097/PRS.0b013e3182865ab3. [DOI] [PubMed] [Google Scholar]
- 37.Chun YS, Verma K, Sinha I, et al. Impact of prior ipsilateral chest wall radiation on pedicled TRAM flap breast reconstruction. Ann Plast Surg. 2013;71:16–19. doi: 10.1097/SAP.0b013e318248b643. [DOI] [PubMed] [Google Scholar]
- 38.Weichman KE, Wilson SC, Saadeh PB, et al. Sterile “ready-to-use” AlloDerm decreases postoperative infectious complications in patients undergoing immediate implant-based breast reconstruction with acellular dermal matrix. Plast Reconstr Surg. 2013;132:725–736. doi: 10.1097/PRS.0b013e31829fe35b. [DOI] [PubMed] [Google Scholar]
- 39.Yezhelyev M, Duggal CS, Carlson GW, Losken A. Complications of latissimus dorsi flap breast reconstruction in overweight and obese patients. Ann Plast Surg. 2013;70:557–562. doi: 10.1097/SAP.0b013e31827a2c02. [DOI] [PubMed] [Google Scholar]
- 40.Colwell AS, Tessler O, Lin AM, et al. Breast reconstruction following nipple-sparing mastectomy: predictors of complications, reconstruction outcomes, and 5-year trends. Plast Reconstr Surg. 2014;133:496–506. doi: 10.1097/01.prs.0000438056.67375.75. [DOI] [PubMed] [Google Scholar]
- 41.Khavanin N, Fine NA, Bethke KP, et al. Tumescent technique does not increase the risk of complication following mastectomy with immediate reconstruction. Ann Surg Oncol. 2014;21:384–388. doi: 10.1245/s10434-013-3311-0. [DOI] [PubMed] [Google Scholar]
- 42.Parks JW, Hammond SE, Walsh WA, et al. Human acellular dermis versus no acellular dermis in tissue expansion breast reconstruction. Plast Reconstr Surg. 2012;130:739–746. doi: 10.1097/PRS.0b013e318262f06e. [DOI] [PubMed] [Google Scholar]
- 43.Baumann DP, Lin HY, Chevray PM. Perforator number predicts fat necrosis in a prospective analysis of breast reconstruction with free TRAM, DIEP, and SIEA flaps. Plast Reconstr Surg. 2010;125:1335–1341. doi: 10.1097/PRS.0b013e3181d4fb4a. [DOI] [PubMed] [Google Scholar]
- 44.Miller ME, Czechura T, Martz B, et al. Operative risks associated with contralateral prophylactic mastectomy: a single institution experience. Ann Surg Oncol. 2013;20:4113–4120. doi: 10.1245/s10434-013-3108-1. [DOI] [PubMed] [Google Scholar]
- 45.Matsen CB, Mehrara B, Eaton A, et al. Skin flap necrosis after mastectomy with reconstruction: a prospective study. Ann Surg Oncol. 2016;23:257–264. doi: 10.1245/s10434-015-4709-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fischer JP, Nelson JA, Serletti JM, Wu LC. Peri-operative risk factors associated with early tissue expander (TE) loss following immediate breast reconstruction (IBR): A review of 9305 patients from the 2005–2010 ACS-NSQIP datasets. J Plast Reconstr Aesthet Surg. 2013;66:1504–1512. doi: 10.1016/j.bjps.2013.06.030. [DOI] [PubMed] [Google Scholar]
- 47.Al-Hilli Z, Thomsen KM, Habermann EB, et al. Reoperation for complications after lumpectomy and mastectomy for breast cancer from the 2012 National Surgical Quality Improvement Program (ACS-NSQIP) Ann Surg Oncol. 2015;22(Suppl 3):459–469. doi: 10.1245/s10434-015-4741-7. [DOI] [PubMed] [Google Scholar]
- 48.Centers for Medicare & Medicaid Services. Global surgery fact sheet. [Accessed August 5, 2015];Centers for Medicare & Medicaid Services. 2015 Mar; Available at: http://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/GloballSurgery-ICN907166.pdf.
- 49.Olsen MA, Fraser VJ. Use of diagnosis codes and/or wound culture results for surveillance of surgical site infection after mastectomy and breast reconstruction. Infect Control Hosp Epidemiol. 2010;31:544–547. doi: 10.1086/652155. [DOI] [PMC free article] [PubMed] [Google Scholar]