Abstract
Background
Endocardial ablation of atrial ganglionated plexi (GP) has been described for treatment of atrial fibrillation (AF). Our objective in this study was to develop percutaneous epicardial GP ablation in a canine model using novel energy sources and catheters.
Methods
Phase 1: The efficacy of several modalities to ablate the GP was tested in an open chest canine model (n=10). Phase 2: Percutaneous epicardial ablation of GP was done in 6 dogs using the most efficacious modality identified in phase 1 using 2 novel catheters.
Results
Phase 1: DC in varying doses [blocking (7 -12μA), electroporation (300-500μA), ablation (3000- 7500μA)], radiofrequency ablation (25–50 W), ultrasound (1.5MHz), and alcohol (2-5ml) injection were successful at 0/8, 4/12, 5/7, 3/8, 1/5 and 5/7 GP sites. DC (500–5000μA) along with alcohol irrigation was tested in phase 2. Phase 2: Percutaneous epicardial ablation of the right atrium, oblique sinus, vein of Marshall, and transverse sinus GP was successful in 5/6 dogs. One dog died of ventricular fibrillation (VF) during DC ablation at 5000 μA. Programmed stimulation induced AF in 6 dogs pre-ablation and no atrial arrhythmia in 3, flutter in 1 and AF in 1 post-ablation. Heart rate, blood pressure, effective atrial refractory period and local atrial electrogram amplitude did not change significantly post-ablation. Microscopic examination showed elimination of GP, and minimal injury to atrial myocardium.
Conclusion
Percutaneous epicardial ablation of GP using direct current and novel catheters is safe and feasible and may be used as an adjunct to pulmonary vein isolation in the treatment of atrial fibrillation in order to minimize additional atrial myocardial ablation.
Keywords: Ganglionated plexus, autonomic innervation, atrial fibrillation, ablation, direct current, alcohol, epicardial, canine
Introduction
Radiofrequency (RF) ablation to isolate the pulmonary veins (PVs) is increasingly used to treat atrial fibrillation (AF) but is associated with modest recurrence rates.1 Hence more effective adjunctive treatments that restore sinus rhythm are needed.
The intrinsic cardiac autonomic innervation through epicardial ganglionated plexi (GP) has been implicated in the initiation and maintenance of AF.2, 3 Hence, there has been intense interest in the ablation of epicardial GP in addition to PV isolation for the treatment of AF. Procedures developed thus far to target the GP involve either epicardial ablation via cardiac surgery or endocardial ablation where the atrial myocardium between the ablation source and the epicardial GP is ablated.4-6 Minimizing damage to atrial myocardium during extensive ablation of GP may be desirable. Studies of GP ablation to date have utilized RF energy which can potentially cause thermal injury to adjacent mediastinal structures. The role of novel ablation techniques such as direct current (DC) electroporation, ultrasound, and chemical ablation in improving safety and efficacy of GP ablation has not been explored.
The primary aim of this study was to demonstrate feasibility of techniques for percutaneous epicardial ablation of cardiac GP in a canine model, for the treatment of AF, using novel energy sources and catheters.
Methods
The study was conducted in two phases: In phase 1, the efficacy and safety of different modalities and energy sources for GP ablation were tested in a canine open chest model. In phase 2, percutaneous epicardial GP ablation was performed in a closed chest canine model using two novel catheters and a novel energy source identified in phase 1.
Mongrel dogs, weighing 30 to 40 kg, were anesthetized with ketamine (10 mg/kg i.v.) and diazepam (0.5 mg/kg i.v.) for induction and isoflurane (1% to 3% continuous inhalation) for maintenance. Positive pressure ventilation was provided and heart rate (HR) and femoral arterial blood pressure (BP) were continuously monitored. Intracardiac bipolar electrograms filtered at 30 – 500 Hz were electronically recorded using Prucka (GE, Milwaukee, WI) recording system. The protocol was approved by the Mayo Clinic Institutional Animal Care and Use Committee. The experiments were performed in 3 models: 1) acute open chest model for assessment of the efficacy of different methods of GP ablation (phase 1); 2) a chronic closed chest model to assess the safety of pericardial infusion of alcohol (phase 1); and 3) an acute closed chest model to assess the feasibility and safety of percutaneous epicardial ablation (phase 2).
Phase 1
Open chest model
Ten dogs were studied under anesthesia as described above. Pain management was augmented using a continuous intravenous infusion of Fentanyl 2-5μg/kg/h. Anesthesia and pain medications were titrated based on continuous monitoring of vital signs including heart rate, blood pressure and respiratory rate and signs of pain such as piloerection. Right lateral thoracotomy at the 4th or 5th intercostal space was performed to ablate the right atrial GP4 (Figure 1A):
Figure 1.
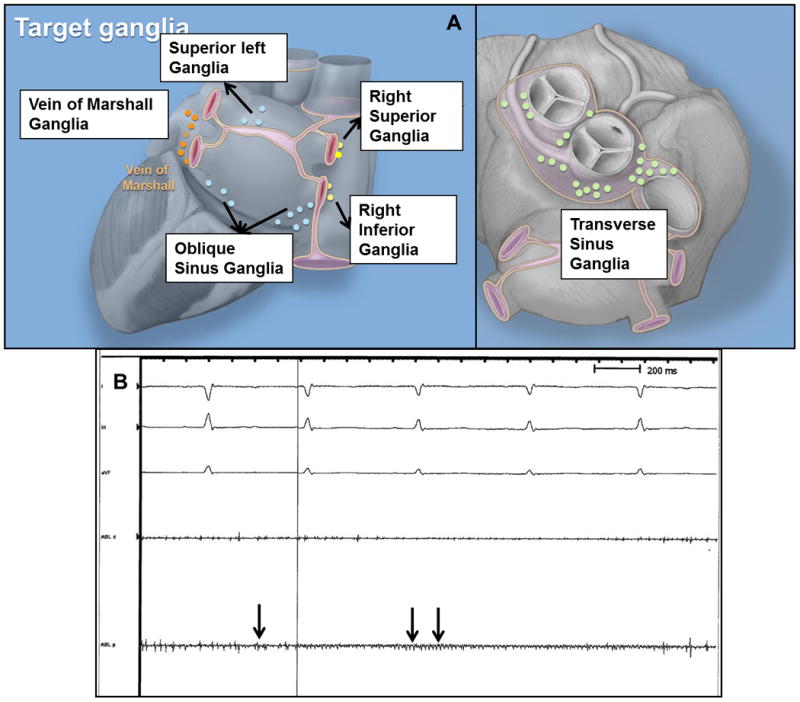
(A) Location of major ganglia targeted for ablation. (B) Ganglion potentials are seen over the transverse sinus ganglionated plexus in sinus rhythm. Ganglion potentials are characterized by high frequency signal (arrows) that are dissociated from the cardiac cycle and occur stochastically. Atrial electrograms are not seen prominently in this tracing. ABL, d and p – distal and proximal bipoles of quadripolar catheter on fat pad.
The right superior GP (RSGP) located between the superior vena cava (SVC)-right atrial (RA) junction and right superior pulmonary vein (RSPV),
The right inferior GP (RIGP) located between the inferior vena cava (IVC)-RA junction and the interatrial septum
Left lateral thoracotomy was then performed to ablate the following:7, 8
The vein of Marshall GP (VMGP) located between the anterior aspect of the left PVs and the posterior left atrial appendage,
Superior left GP located at the junction of the left superior PV with the posterior left atrium,
The great artery GP located at the base of the aorta and pulmonary artery in the transverse sinus (TSGP) and
The retro-atrial GP on the posterior surface of the LA in the oblique sinus (OSGP).
Due to a lack of consensus on terminology, we provide an anatomical description of the GP targeted and also provide correlation with terminology used in the largest human study of GP ablation to date.6 The RSGP and SLGP correspond to the anterior right GP and superior left GP described by Katritsis et al. The inferior left GP and inferior right GP described by Katritsis et al are part of the retro-atrial GP in the oblique sinus. The retro-atrial GP are located on the posterior LA often close to the junction of the PV with the LA, where they were targeted for ablation. The right inferior GP, vein of Marshall and transverse sinus GP were not targeted by Katritsis et al.
The location of the GP was identified by direct visualization of epicardial fat pads which contain clusters of GP. One to 2 GP were left unablated in each animal to serve as histological control. A quadripolar catheter (Blazer, 7 Fr, 4-mm tip, Boston Scientific) was sutured onto the epicardium at each GP site and local bipolar electrograms recorded. Ganglion potentials were characterized by high frequency signals which occur stochastically during any phase of the cardiac cycle and are dissociated from the local myocardial potential (Figure 1B). GP potentials were distinguished from myocardial signals or external noise based on the following:
A continuous fragmented signal that appeared in a stochastic fashion at the ganglia recording catheter that is dissociated from the atrial electrograms.
No evidence of electromagnetic interference or noise on any of the other intracardiac or surface electrodes at the time of the recording.
Without changing the catheter position, we noted the stopping and starting of this activity at the same site. When activation appeared fairly consistent, signals disappeared with movement of the distal electrode slightly off the ganglia.
The proximal bipole showed similar activation but of a far-field nature compared to the distal recording bipole with contact on the fat pad/ganglia.
Forty seven GP sites were modified with one of several thermal, chemical or mechanical methods applied for 60 s to 120 s to test the safety and efficacy in GP ablation: 1) Low amplitude direct current (DC) (12 μA); 2) Medium amplitude DC (300 or 500 μA); 3) High amplitude DC (3000 – 5000 μA); 4) radiofrequency (RF) (25 to 50 W, 17 ml/hr saline irrigation); 5) Ultrasound (US) at mechano-acoustic frequencies (US, 1.5 MHz, 5 cycles consisting of 20 s burst ON and 10 s OFF); or 6) 2 to 5 ml 10% ethanol in normal saline injected into the fat pad. A prototype circuit was developed for the delivery of DC and current was delivered continuously for the duration of treatment (Figure 2A). While ethanol is a known neurolytic agent, a concentration of 10% was chosen empirically for testing due to prior observation of fibrosis with use of 100% alcohol at other sites such as the pleural cavity. Each GP was modified using a single application of 1 modality. Local injection of acetylcholine into the fat pad containing GP has previously been shown to induce AF in the canine due to autonomic stimulation and GP ablation abolishes this response.9, 10 We injected acetylcholine (5 mg) into the fat pad containing the GP before and after each modification and the induction of AF was noted. If AF was not induced spontaneously, single atrial extrastimulus was delivered to induce AF. Acetylcholine injection raised a wheal in the fat pad to ensure that the drug was delivered locally and not into the myocardium or the systemic circulation. Successful ablation of a GP was defined as 1) inability to induce AF or other atrial tachyarrhythmias; 2) loss or > 50% attenuation of the HR and BP response to acetylcholine; and 3) abolition of local GP potential when seen.
Figure 2.
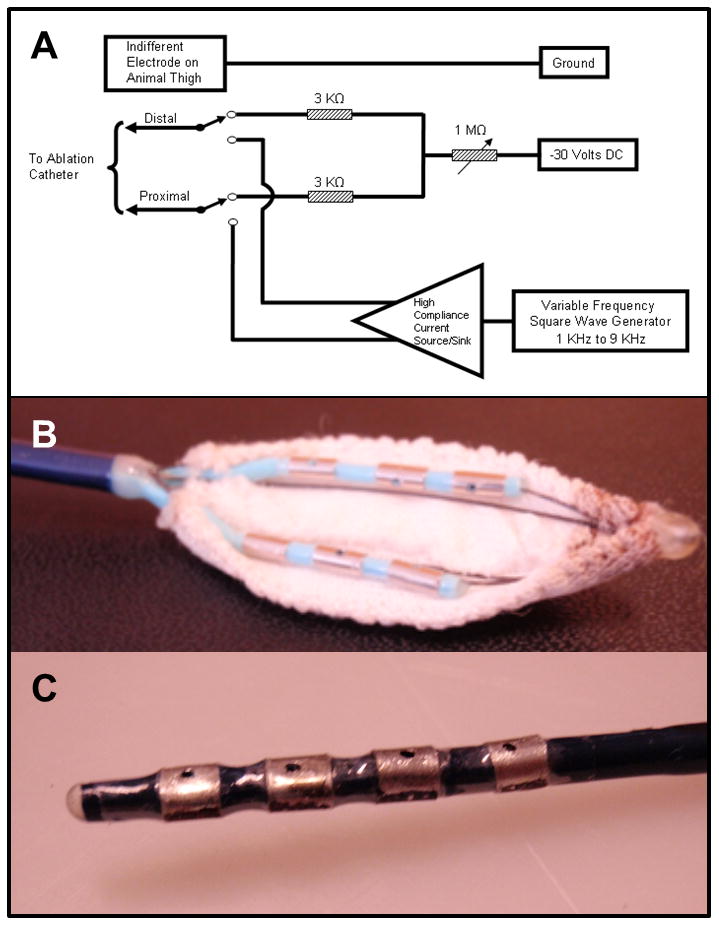
(A) Prototype direct current injection circuit. Direct current was delivered to the electrodes of the catheters described below from a 30 V regulated DC power supply with the positive terminal of the power supply connected to the grounding patch on the animal's thigh. The negative terminal of the DC power supply was connected through matched, fixed and variable resistors to the electrodes. A 6 kilo-ohms resistance was placed between the electrodes to provide adequate decoupling of the electrodes for good signal acquisition. The negative DC current injected independently to the electrodes could be varied from 10 to 7500 micro amps. (B) Prototype multi-array catheter with two rows of 3 electrodes (3.3 mm, 2mm spacing) with irrigation hole in the middle of each electrode and insulated on one side with polyester fabric to provide a wide area of ablation in the oblique sinus, and (C) Prototype quadripolar (3.2 mm electrodes, 3 mm spacing) catheter insulated on one side with polyester.
Euthanasia was performed by inducing ventricular fibrillation (VF). Under deep anesthesia, an electrophysiology catheter introduced from the femoral vein was placed in the right ventricle and DC was applied. VF was verified by electrocardiogram and absence of arterial blood pressure. Histology of ablated and unablated control GP was studied using Hematoxylin and Eosin (H&E) staining.
Chronic closed chest model
Under general anesthesia, percutaneous pericardial access was established using a 5-Fr sheath to infuse 30 ml of 20% ethanol in normal saline in 3 dogs and 30 ml of normal saline in 1 control dog. The infusate was left in place, pericardial drain removed and the dog was observed for 1 to 2 months. Recovering animals were observed every 30 minutes for the first two hours then twice daily for the remainder of the study. Buprenorphine (0.02mg/kg) was administered via intramuscular injection every 12 hours and supplemented with oral Carprofen 4mg/kg once per day for pain management. Following the period of observation, the dog was euthanized using an overdose of intravenous pentobarbital under general anesthesia and verified using ECG. The explanted heart was assessed for evidence of pericardial inflammation and adhesion.
Phase 2: Percutaneous epicardial ablation model
Percutaneous pericardial access was obtained through the subxiphoid approach using a Touhy epidural needle and an 18-Fr sheath in 6 dogs. Biplane fluoroscopy was used to guide catheter placement. An EPT catheter placed in the high RA was used to perform programmed atrial stimulation with an 8-cycle pacing train at 500 ms cycle length with one extrastimulus to induce AF/atrial flutter and to test atrial effective refractory period (AERP) before and after ablation of each GP site.
Two novel ablation catheters (Figure 2B) were designed to 1) deliver energy epicardially to the cardiac autonomic ganglia, 2) minimize injury to adjacent structures, and 3) navigate through the transverse and oblique sinuses (Figure 2B). A 9-Fr deflectable multi-array catheter was designed with six electrodes (3.3-mm electrode, 2 mm spacing) in 2 rows and irrigation ports located in the center of each electrode for ablation in the oblique sinus. Each “arm” of the catheter was built upon a nitinol frame to support the electrodes. The posterior surface of the electrode arms was covered by a polyester fabric to provide insulation of adjacent non-cardiac structures while delivering energy in a unidirectional fashion to the GP. A second 9-Fr quadripolar catheter (3.2-mm electrodes with 3 mm spacing) was built in the shape of a “finger,” in order to achieve maneuverability in smaller areas. Each electrode had an irrigation port. The posterior aspect of the electrodes was covered with polyester in order to provide insulation as noted above.
Anatomical ablation of the OSGP, TSGP, RSGP, RIGP and VMGP was performed using 3000 to 5000 μA DC and 10% alcohol in normal saline irrigation at 17 ml/h for 120 to 240s. Contact between the ablation catheter and atrium was confirmed by the presence of a near field atrial electrogram and myocardial capture during pacing. The absence of a large amplitude atrial electrogram indicated that the insulated surface of the electrode was in contact with the atrium, leading to repositioning of the catheter (Figure 3). The insulated surface in apposition to vital structures, such as the esophagus in the oblique sinus and the great arteries in the transverse sinus, minimized injury to these structures. A radio-opaque marker on the insulated side and the radio-opacity of the electrodes were used to confirm positioning of the electrodes on the myocardium using fluoroscopy. If a ventricular electrogram was noted, the catheter was repositioned to avoid ablation of ventricular myocardium and induction of VF. Finally, the dog was euthanized by induction of VF as described above and gross and microscopic examination of the GP using hematoxylin & eosin (H&E) was used to confirm ablation of GP.
Figure 3.
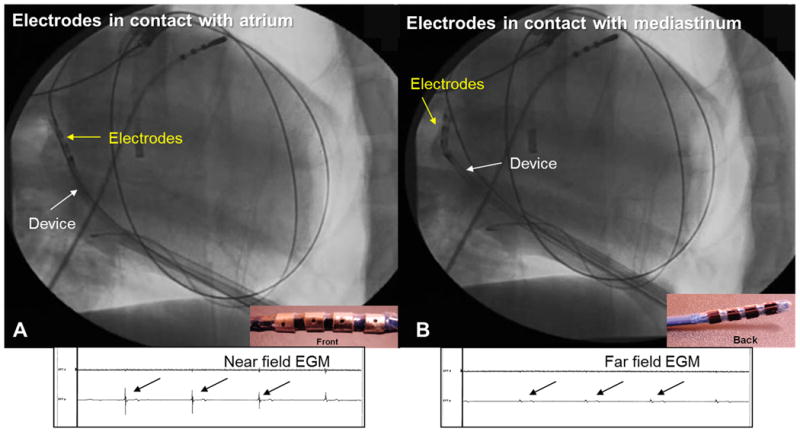
Electrogram guided ablation. The quadripolar catheter is positioned in the oblique sinus. (A) When the electrodes are in contact with the atrium, high amplitude near field atrial electrograms (EGM) is noted and ablation was safely performed. (B) When the electrodes are in contact with the pericardium in the oblique sinus, atrial electrograms are low amplitude and far field. The catheter was repositioned to avoid injury to the esophagus.
Statistical analysis
Continuous and categorical variables were summarized as mean (standard deviation) and percentages respectively and compared between groups using the t-test and Fischer exact test respectively. A two-sided p value < 0.05 was considered statistically significant. All statistical analyses were performed using JMP7 (SAS, California).
Results
Phase 1A: Efficacy of different ablation methods
Acetylcholine was successful in inducing AF spontaneously or with single atrial extrastimulus at 47/48 tested sites prior to GP ablation. Low, medium and high dose DC were effective in abolishing acetylcholine induced AF at 0/8 (0%), 4/12 (33%) and 5/7 (71%) GP sites respectively. High amplitude DC resulted in organization of AF to atrial flutter at 1/7 sites. When considering the dose response to DC, we observed that currents of 12μA (n=12) and 300 μA (n=5) were not effective at any site. DC of 500 μA was effective at 4/7 GP sites and 5000 μA ablated the GP at 5/7 sites. RF resulted in non-inducibility of AF in 3/8 (38%) and organization of AF to atrial flutter at 2/8 (25%) GP sites. Alcohol injection and ultrasound were effective at 5/7 (71%) and 1/5 (20%) sites respectively. When compared to RF, DC at ≥500 μA (p=0.4) and alcohol injection into the GP (p=0.3) were effective at a greater proportion of sites tested. The difference was not statistically significant, likely due to the small sample size.
The HR and BP (mm Hg) pre- and post-ablation at each site did not differ significantly: HR [132 ± 41 vs 129 ± 37, p=0.3], systolic BP [140 ± 30 vs142 ± 34, p= 0.5], diastolic BP [90 ± 22 vs 88 ± 23, p=0.3] and mean BP [105 ± 23 vs 102 ± 24, p=0.4] (n=46). GP potential was present at 16 of the 47 fat pads modified and was successfully abolished at 8 sites following ablation. Histology of the successfully ablated ganglia showed loss of nucleoli or complete loss of cellularity using H&E staining (Figure 4A). Histology of non-ablated control ganglia showed preserved cellular structure (Figure 4B). Examination of the atrial myocardium surrounding the GP was performed using H&E staining. RF ablation resulted in ablation of atrial myocardium in addition to ablation of GP (Figure 4C). In contrast DC ablation at doses administered in this study resulted in relative sparing of the atrial myocardium (Figure 4D).
Figure 4.
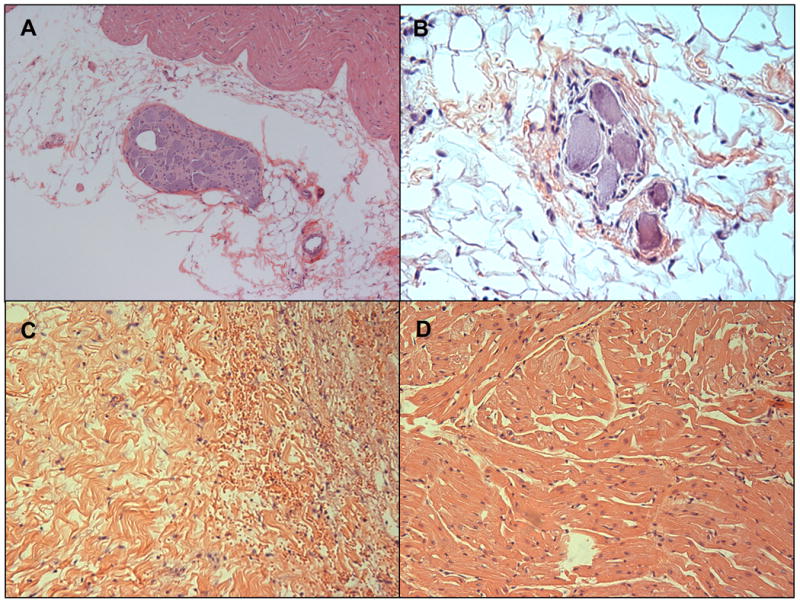
(A) Hematoxylin and Eosin (H&E) staining of an ablated epicardial ganglionated plexus (GP) with loss of neuronal nuclei and cellular integrity. (B) H&E staining of unablated control GP showing preserved nuclei and cytoplasmic granularity. (C) Radiofrequency ablation of GP resulted in ablation of underlying atrial myocardium. Coagulative necrosis, myocyte disarray and hemorrhage are noted. (D) Atrial myocardium underlying a GP ablated with direct current shows preserved myocytes.
Due to the greater success of DC at ablating GP compared to RF and US, less atrial myocardial ablation with DC and ease of delivery through a percutaneously introduced catheter, DC was tested further in the closed chest epicardial ablation model in phase 2. We also hypothesized that ethanol may potentiate DC energy delivery through fat. Hence Ethanol (10% in normal saline) was used for irrigation in phase 2, although this was not directly tested in phase 1.
Phase 1B: Safety of chronic pericardial alcohol infusion
No acute electrical or hemodynamic effects were seen following the pericardial instillation of 20% alcohol or saline. A higher concentration of ethanol was tested to ensure safety of use in the pericardial space. One to two months after the instillation, percutaneous pericardial access was again established. Gross and microscopic examination of the explanted hearts did not show pericardial inflammation or adhesions in both alcohol and saline treated dogs.
Phase 2: Percutaneous epicardial ablation of GP
Percutaneous epicardial access through the subxiphoid approach was successfully established in all 6 dogs without complications. DC ablation at 5000 μA resulted in ventricular fibrillation (VF) and early demise in one dog. Inadvertent movement of the catheter over the ventricle was noted before the induction of VF. DC ablation was performed at 3000 μA (2 applications at each site) without complications in remaining 5 dogs. The dose reduction was performed empirically based on the noted efficacy of both 500 and 5000 μA in phase 1 experiments and the reduced potential for VF induction with lower dose DC. The RSGP, RIGP, OSGP, and TSGP were ablated in all 5 dogs. In addition, the VMGP was ablated in dogs 1 and 3, and the right pulmonary artery GP was ablated in dog 1. Examples of fluoroscopic images of the novel catheters positioned at different GP sites are presented in Figure 5.
Figure 5.
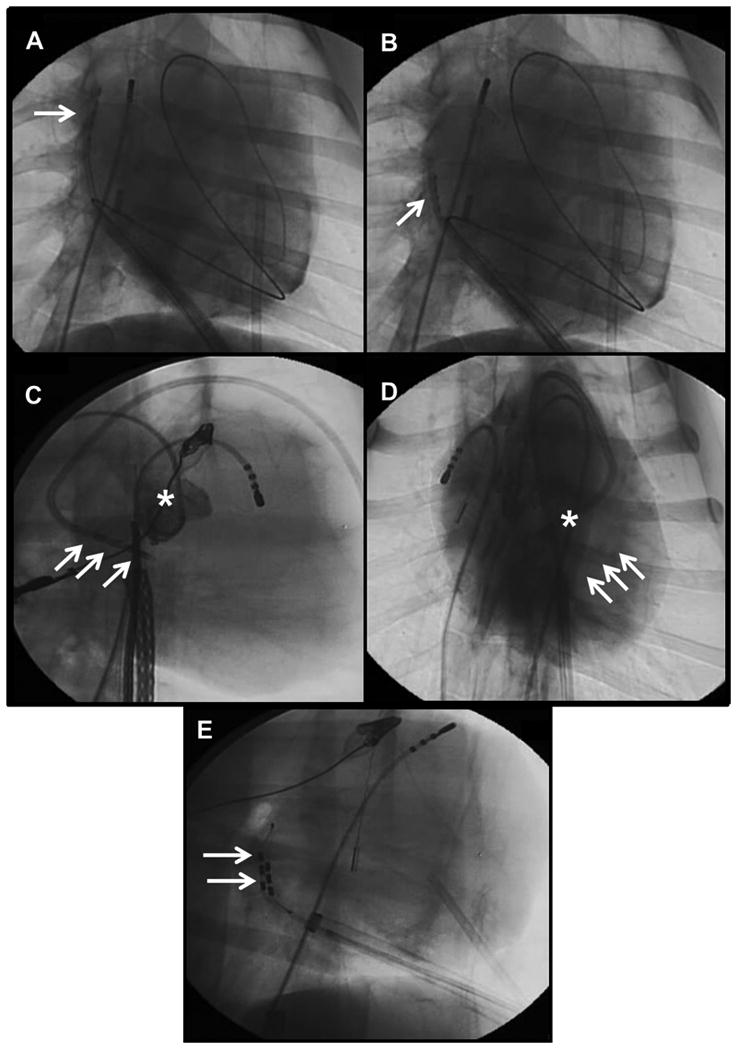
The quadripolar catheter positioned at the (A) right superior GP and (B) right inferior GP (AP view). (C) Lateral and (D) AP views showing quadripolar catheter placed in the transverse sinus. Aortic angiogram (*) shows the relationship to the aortic valve. (E) Multi-array catheter placed in the oblique sinus seen in the lateral view posterior to the atrium.
The HR, BP, AERP, and AF inducibility pre- and post-ablation in each dog are presented in the table. No significant difference was noted in the HR and BP before and after ablation. Amplitude of the local atrial electrogram after ablation was reduced by a mean of 0.3(±0.8) mV compared to before ablation (mean amplitude pre- vs post-ablation 2.3 [±1.5] vs 2.0 [±1.3] mV, [p=0.01]). Although this difference was statistically significant, the degree of reduction in electrogram amplitude was significantly less compared to that expected during endocardial RF ablation of GP. AERP prolonged from 100 to 180 ms in one dog, shortened from 260 to 80 ms in one dog and was unchanged in the rest. Sustained AF (> 30 s) was induced in all dogs before ablation using single atrial extrastimulus. AF was not inducible in 3 dogs after ablation. Atrial flutter that was easily pace-terminated was induced in dog 1, and AF was induced but at a shorter coupling interval (120 vs 100 ms) in dog 2. Continuous ECG monitoring did not show any evidence of coronary artery injury, and phrenic nerve stimulation was not observed.
Table.
Results of epicardial ablation of cardiac ganglionated plexi
| Dog 1 | Dog 2 | Dog 3 | Dog 4 | Dog 5 | |
|---|---|---|---|---|---|
| Pre-ablation | |||||
| Heart rate | 96 | 87 | 101 | 73 | 76 |
| Blood pressure* | 109/72/82 | 106/67/78 | 128/75/91 | 102/60/72 | 102/63/74 |
| Atrial effective refractory period | 500/260 | 500/100 | 500/120 | 500/30 | 500/40 |
| PAC initiating AF | 500/350 | 500/120 | 500/140 | 500/100 | 500/100 |
| Post-ablation | |||||
| Heart rate | 80 | 105 | 111 | 113 | 92 |
| Blood pressure* | 101/69/78 | 87/79/83 | 113/63/78 | 120/80/99 | 143/81/98 |
| Atrial effective refractory period | 500/80 | 500/100 | 500/120 | 500/30 | 500/180 |
| AF inducibility | Atrial flutter induced | AF induced | Not inducible | Not inducible | Not inducible |
| PAC initiating AF | N/A | 500/130 | N/A | N/A | N/A |
Systolic/diastolic/mean blood pressure
Gross pathologic examination of the explanted hearts showed clusters of 3 to 4 mm hemorrhagic lesions at sites of ablation (Figure 6A). There was no evidence of injury to the coronary arteries, great arteries, thoracic veins, and esophagus on gross examination. Microscopic examination of the ablated GP showed nuclear disarray and loss of cellular architecture with H&E staining (Figure 6B) at 17 out of 23 fat pads treated. Five sites could not be adequately examined due to poor tissue processing or absence of identifiable ganglia. GP was not ablated at 1/23 sites. No significant necrosis of the underlying atrial muscle was noted (Figure 6C).
Figure 6.
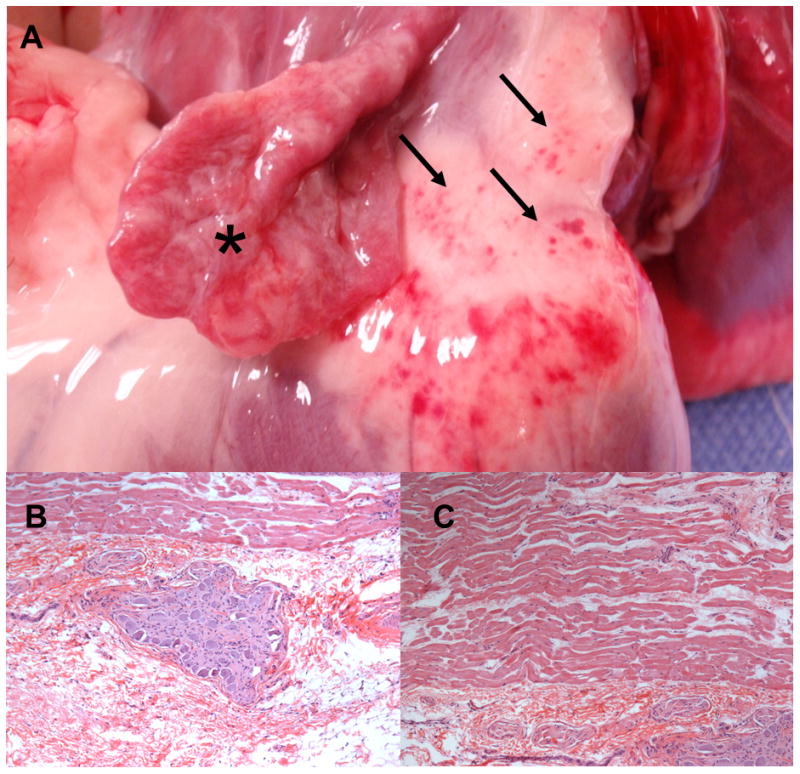
(A) Ablation of the vein of Marshal ganglionated plexi using the novel quadripolar catheter through percutaneous pericardial access. Clusters of 3 to 4 mm hemorrhagic appearing lesions are noted (arrows). The left atrial appendage (asterisk) has been reflected off to show the ligament of Marshall. (B) Histology (hematoxylin & eosin stain) of ganglionated plexus ablated through percutaneous pericardial access in the closed chest canine model. (C) Preservation of atrial myocardium underlying the GP is noted.
Discussion
We describe percutaneous epicardial ablation of cardiac GP using an anatomic approach in the canine. The results show that percutaneous epicardial ablation of cardiac GP, documented by histologic examination, using novel catheters, DC electroporation, and alcohol irrigation with minimal injury to the atrial myocardium is feasible and safe.
The occurrence of recurrences with PV isolation alone for AF has spurred a search for adjunctive targets for treatment.1 The intrinsic cardiac autonomic nervous system consisting of the cardiac GP plays an important role in the initiation and maintenance of AF.11 Stimulation of the GP can convert local PV firing into AF or induce spontaneous AF in animals.9, 10, 12 Cardiac GP ablation in the canine has been shown to abolish vagally-mediated AERP shortening, dispersion of AERP and AF induction.2, 13 Due to close proximity to the pulmonary veins, GPs are frequently ablated in the course of catheter based or surgical pulmonary vein isolation. Ablation targeting the cardiac GP with or without concurrent PV isolation in humans has shown promise in preventing AF recurrence.4-6, 14, 15
Epicardial vs endocardial ablation of cardiac ganglionated plexi
Studies of GP ablation to date have utilized percutaneous endocardial RF ablation or surgical epicardial ablation.2, 4, 5, 15-17 Epicardial GP ablation has been reported during cardiac surgery, either performed as a standalone procedure or as part of another cardiac surgical procedure.18-22 Since cardiac GP are epicardial structures, a percutaneous epicardial approach to ablation can target the GP of interest with minimal ablation of underlying atrial myocardium while avoiding cardiac surgery. Scanavacca et al. reported percutaneous epicardial RF ablation of GP in humans.23 However, using commercially available catheters, they were unable to ablate at several left atrial epicardial sites due to close proximity of the esophagus, coronary arteries, or phrenic nerve. Left atrial myocardial injury during RF ablation has been associated with pro-arrhythmia and reduction in indices of left atrial mechanical function despite restoration of sinus rhythm.24, 25 We describe the feasibility of an entirely percutaneous epicardial GP ablation approach. Using two prototype catheters insulated on one side and direct current, we were able to ablate selectively on the epicardial surface and avoid injury to the esophagus and great vessels.
Several observations from animal and human studies underscore the importance of complete autonomic denervation. The atrial GP form an interconnected neural network where stimulation of GP at one site can elicit a pro-arrhythmic response at a distant site.26 Human studies have shown greater success in maintaining sinus rhythm with more extensive anatomical ablation of GP compared to limited ablation of sites that elicit a vagal response to stimulation, potentially due to more extensive denervation.4, 27 While prior studies of GP ablation have targeted either the right or left atrial GP, this is the first study to target both. The epicardial approach allowed access to all the targeted GP and the use of prototype catheters and DC minimized injury to mediastinal structures and atrial myocardium, allowing more extensive ablation. While complete autonomic denervation is difficult to achieve, the technique described improves the potential for significant denervation.
GP ablation has been postulated to prevent AF induction by abolishing the vagal-mediated shortening of AERP.2 However, prior canine studies have shown either no change28 or a small prolongation of the AERP2 at resting autonomic tone. Consistent with these results, we did not note significant change in AERP in most canines. We however did not perform vagal stimulation to confirm abolition of the AERP response.
Direct current GP ablation
RF and high amplitude DC current can cause thermal tissue injury to surrounding atrial myocardium. DC applied at doses used in this study result in electroporation, a nonthermal source of ablation that uses electrical pulses to cause irreversible alteration in transmembrane potential and cell membrane injury, sparing the scaffold.29 We show that GP ablation using DC electroporation at 500 to 5000 μA is moderately effective in abolishing the response to local application of acetylcholine and results in less atrial myocardial injury compared to RF.
Ventricular arrhythmias can occur during DC application and are more likely with use of higher energy.30 In our experiments, one dog developed refractory VF during ablation at 5000 μA. Subsequently, limiting the delivered current to 3000 μA and utilizing an electrogram-guided approach to avoid ventricular ablation did not induce any further ventricular arrhythmia. The decision to limit DC delivery to 3000 μA was based on observed efficacy of DC between 500 and 5000 μA in phase 1 studies and the lack of pro-arrhythmia in phase 2 studies. Energy level of 3000μA was however not tested in phase 1. Synchronization of DC energy delivery with the QRS complex has also been described to prevent ventricular arrhythmias.31 Thus while ventricular arrhythmias remain a significant complication of this procedure, techniques outlined above will make this less likely.
Coronary artery injury is a dreaded complication of epicardial ablation. In this study, catheter position was monitored with fluoroscopy to avoid movement towards the annulus. Ablation in the transverse sinus was limited to the space posterior to the ascending aorta, which in the presence of normal coronary anatomy will avoid the major epicardial coronary vessels. Moreover, electroporation on or near the coronary arteries has been shown to spare the coronaries and may be safer than radiofrequency ablation.{Neven, 2014 #65}
Ethanol ablation for GP modification
Ethanol induced neurolysis is currently used for treatment of pain disorders and exploit the exquisite sensitivity of neurons to ethanol. We demonstrate the efficacy of 10% ethanol injection in ablating the cardiac GP in an open chest model. Ethanol infusion into the vein of Marshall has been used to ablate GP in canine.8 Although direct injection of ethanol into GP was not tested in the closed chest model, due to technical challenges in delivering ethanol locally, this modality warrants further investigation in the future.
Ethanol irrigation was tested as an adjunct to DC ablation in the closed chest model due to the potential for enhanced electroporation in the treated fat pad. This technique was however not tested in phase 1 experiments and comparison of ethanol to normal saline infusion during DC modification of GP warrants further investigation.
Limitations
The ablation of GP in the closed chest model was confirmed using histologic examination of ablated tissue. Functional assessment for autonomic denervation using techniques such as cervical vagal stimulation was not performed.32 The efficacy of DC with ethanol irrigation was not directly compared to normal saline irrigation in phase 1. Thus the role of ethanol irrigation in potentiating the effects of DC on GP is a proposed one and requires further study. The study was designed to provide proof of concept and was not powered to show statistically significant differences in the efficacy of each modality tested in phase 1. The small sample size may explain the lack of statistical significance in the difference between RF and DC, although DC was twice as effective in ablating GP. There was also reduced inducibility of AF following the GP ablations although a robust method to assess the internal validity of this result was not used.
We did not study the long term efficacy of epicardial GP ablation, and neural re-innervation following ablation can potentially lead to late AF recurrence. Although we did not perform long term followup, the extensive ablation targeted at neuronal cells in the GP is expected to produce long term effects. Our study however generates important hypotheses that warrant further study incorporating long term followup and functional assessment of denervation.
Conclusions
We report an entirely epicardial approach to cardiac GP ablation using DC electroporation and novel catheters. The techniques described were also shown to be safe with minimal injury to atrial myocardium and mediastinal structures. We also report for the first time, the role of local ethanol injection in ablating GP. The techniques described have the potential to improve success of AF ablation when used as an adjunct to pulmonary vein isolation and warrant further evaluation in larger preclinical followed by clinical studies.
Acknowledgments
Funding: Mayo Clinic Discovery Translational Program.
CVD was supported by an NIH training grant T32 HL007111
Glossary of abbreviations
- AF
atrial fibrillation
- BP
blood pressure
- DC
direct current
- GP
ganglionated plexi
- H&E
hematoxylin & eosin
- HR
heart rate
- IVC
inferior vena cava
- OS
oblique sinus
- PV
pulmonary vein
- RA
right atrial
- RF
radiofrequency
- RI
right inferior
- RS
right superior
- RSPV
right superior pulmonary vein
- SVC
superior vena cava
- TS
transverse sinus
- VM
vein of Marshall
- VF
ventricular fibrillation
Footnotes
Disclosures: Susan B. Johnson, receives royalties from St Jude/Navx. Samuel J. Asirvatham receives no significant honoraria and is a consultant with Abiomed, Atricure, Biotronik, Biosense Webster, Boston Scientific, Medtronic, Spectranetics, St. Jude, Sanofi-Aventis, Wolters Kluwer, and Elsevier. Dr. DeSimone is supported by a NIH Training Grant T32 #HL0070111. The other authors have no disclosures.
References
- 1.O'Neill MD, Wright M, Knecht S, Jais P, Hocini M, Takahashi Y, Jonsson A, Sacher F, Matsuo S, Lim KT, Arantes L, Derval N, Lellouche N, Nault I, Bordachar P, Clementy J, Haissaguerre M. Long-term follow-up of persistent atrial fibrillation ablation using termination as a procedural endpoint. Eur Heart J. 2009;30:1105–1112. doi: 10.1093/eurheartj/ehp063. [DOI] [PubMed] [Google Scholar]
- 2.Schauerte P, Scherlag BJ, Pitha J, Scherlag MA, Reynolds D, Lazzara R, Jackman WM. Catheter ablation of cardiac autonomic nerves for prevention of vagal atrial fibrillation. Circulation. 2000;102:2774–2780. doi: 10.1161/01.cir.102.22.2774. [DOI] [PubMed] [Google Scholar]
- 3.Scherlag BJ, Nakagawa H, Jackman WM, Yamanashi WS, Patterson E, Po S, Lazzara R. Electrical stimulation to identify neural elements on the heart: their role in atrial fibrillation. J Interv Card Electrophysiol. 2005;13(Suppl 1):37–42. doi: 10.1007/s10840-005-2492-2. [DOI] [PubMed] [Google Scholar]
- 4.Calo L, Rebecchi M, Sciarra L, De Luca L, Fagagnini A, Zuccaro LM, Pitrone P, Dottori S, Porfirio M, de Ruvo E, Lioy E. Catheter ablation of right atrial ganglionated plexi in patients with vagal paroxysmal atrial fibrillation. Circ Arrhythm Electrophysiol. 2012;5:22–31. doi: 10.1161/CIRCEP.111.964262. [DOI] [PubMed] [Google Scholar]
- 5.Pokushalov E, Romanov A, Artyomenko S, Turov A, Shugayev P, Shirokova N, Katritsis DG. Ganglionated plexi ablation for longstanding persistent atrial fibrillation. Europace. 2010;12:342–346. doi: 10.1093/europace/euq014. [DOI] [PubMed] [Google Scholar]
- 6.Katritsis DG, Pokushalov E, Romanov A, Giazitzoglou E, Siontis GC, Po SS, Camm AJ, Ioannidis JP. Autonomic denervation added to pulmonary vein isolation for paroxysmal atrial fibrillation: a randomized clinical trial. Journal of the American College of Cardiology. 2013;62:2318–2325. doi: 10.1016/j.jacc.2013.06.053. [DOI] [PubMed] [Google Scholar]
- 7.Armour JA, Murphy DA, Yuan BX, Macdonald S, Hopkins DA. Gross and microscopic anatomy of the human intrinsic cardiac nervous system. Anat Rec. 1997;247:289–298. doi: 10.1002/(SICI)1097-0185(199702)247:2<289::AID-AR15>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 8.Valderrabano M, Chen HR, Sidhu J, Rao L, Ling Y, Khoury DS. Retrograde ethanol infusion in the vein of Marshall: regional left atrial ablation, vagal denervation and feasibility in humans. Circulation Arrhythmia and electrophysiology. 2009;2:50–56. doi: 10.1161/CIRCEP.108.818427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Po SS, Scherlag BJ, Yamanashi WS, Edwards J, Zhou J, Wu R, Geng N, Lazzara R, Jackman WM. Experimental model for paroxysmal atrial fibrillation arising at the pulmonary vein-atrial junctions. Heart Rhythm. 2006;3:201–208. doi: 10.1016/j.hrthm.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 10.Zhou J, Scherlag BJ, Edwards J, Jackman WM, Lazzara R, Po SS. Gradients of atrial refractoriness and inducibility of atrial fibrillation due to stimulation of ganglionated plexi. J Cardiovasc Electrophysiol. 2007;18:83–90. doi: 10.1111/j.1540-8167.2006.00679.x. [DOI] [PubMed] [Google Scholar]
- 11.Chen YJ, Chen SA, Tai CT, Wen ZC, Feng AN, Ding YA, Chang MS. Role of atrial electrophysiology and autonomic nervous system in patients with supraventricular tachycardia and paroxysmal atrial fibrillation. Journal of the American College of Cardiology. 1998;32:732–738. doi: 10.1016/s0735-1097(98)00305-2. [DOI] [PubMed] [Google Scholar]
- 12.Scherlag BJ, Yamanashi W, Patel U, Lazzara R, Jackman WM. Autonomically induced conversion of pulmonary vein focal firing into atrial fibrillation. Journal of the American College of Cardiology. 2005;45:1878–1886. doi: 10.1016/j.jacc.2005.01.057. [DOI] [PubMed] [Google Scholar]
- 13.Zipes DP, Knope RF. Electrical properties of the thoracic veins. Am J Cardiol. 1972;29:372–376. doi: 10.1016/0002-9149(72)90533-4. [DOI] [PubMed] [Google Scholar]
- 14.Lin J, Scherlag BJ, Zhou J, Lu Z, Patterson E, Jackman WM, Lazzara R, Po SS. Autonomic mechanism to explain complex fractionated atrial electrograms (CFAE) J Cardiovasc Electrophysiol. 2007;18:1197–1205. doi: 10.1111/j.1540-8167.2007.00976.x. [DOI] [PubMed] [Google Scholar]
- 15.Pappone C, Santinelli V, Manguso F, Vicedomini G, Gugliotta F, Augello G, Mazzone P, Tortoriello V, Landoni G, Zangrillo A, Lang C, Tomita T, Mesas C, Mastella E, Alfieri O. Pulmonary vein denervation enhances long-term benefit after circumferential ablation for paroxysmal atrial fibrillation. Circulation. 2004;109:327–334. doi: 10.1161/01.CIR.0000112641.16340.C7. [DOI] [PubMed] [Google Scholar]
- 16.McClelland JH, Duke D, Reddy R. Preliminary results of a limited thoracotomy: new approach to treat atrial fibrillation. J Cardiovasc Electrophysiol. 2007;18:1289–1295. doi: 10.1111/j.1540-8167.2007.00977.x. [DOI] [PubMed] [Google Scholar]
- 17.Mehall JR, Kohut RM, Jr, Schneeberger EW, Taketani T, Merrill WH, Wolf RK. Intraoperative epicardial electrophysiologic mapping and isolation of autonomic ganglionic plexi. Ann Thorac Surg. 2007;83:538–541. doi: 10.1016/j.athoracsur.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 18.Alex J, Guvendik L. Evaluation of ventral cardiac denervation as a prophylaxis against atrial fibrillation after coronary artery bypass grafting. The Annals of thoracic surgery. 2005;79:517–520. doi: 10.1016/j.athoracsur.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 19.Bagge L, Blomstrom P, Nilsson L, Einarsson GM, Jideus L, Blomstrom-Lundqvist C. Epicardial off-pump pulmonary vein isolation and vagal denervation improve long-term outcome and quality of life in patients with atrial fibrillation. J Thorac Cardiovasc Surg. 2009;137:1265–1271. doi: 10.1016/j.jtcvs.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 20.Edgerton JR, Brinkman WT, Weaver T, Prince SL, Culica D, Herbert MA, Mack MJ. Pulmonary vein isolation and autonomic denervation for the management of paroxysmal atrial fibrillation by a minimally invasive surgical approach. J Thorac Cardiovasc Surg. 2010;140:823–828. doi: 10.1016/j.jtcvs.2009.11.065. [DOI] [PubMed] [Google Scholar]
- 21.Omran AS, Karimi A, Ahmadi H, Yazdanifard P, Sheikh Fahtollahi M, Tazik M. Prophylactic ventral cardiac denervation: does it reduce incidence of atrial fibrillation after coronary artery bypass grafting? J Thorac Cardiovasc Surg. 2010;140:1036–1039. doi: 10.1016/j.jtcvs.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 22.Ware AL, Suri RM, Stulak JM, Sundt TM, 3rd, Schaff HV. Left atrial ganglion ablation as an adjunct to atrial fibrillation surgery in valvular heart disease. The Annals of thoracic surgery. 2011;91:97–102. doi: 10.1016/j.athoracsur.2010.08.037. [DOI] [PubMed] [Google Scholar]
- 23.Scanavacca M, Pisani CF, Hachul D, Lara S, Hardy C, Darrieux F, Trombetta I, Negrao CE, Sosa E. Selective atrial vagal denervation guided by evoked vagal reflex to treat patients with paroxysmal atrial fibrillation. Circulation. 2006;114:876–885. doi: 10.1161/CIRCULATIONAHA.106.633560. [DOI] [PubMed] [Google Scholar]
- 24.Boyd AC, Schiller NB, Ross DL, Thomas L. Differential recovery of regional atrial contraction after restoration of sinus rhythm after intraoperative linear radiofrequency ablation for atrial fibrillation. Am J Cardiol. 2009;103:528–534. doi: 10.1016/j.amjcard.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 25.Thomas L, Thomas SP, Hoy M, Boyd A, Schiller NB, Ross DL. Comparison of left atrial volume and function after linear ablation and after cardioversion for chronic atrial fibrillation. Am J Cardiol. 2004;93:165–170. doi: 10.1016/j.amjcard.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 26.Hou Y, Scherlag BJ, Lin J, Zhou J, Song J, Zhang Y, Patterson E, Lazzara R, Jackman WM, Po SS. Interactive atrial neural network: Determining the connections between ganglionated plexi. Heart Rhythm. 2007;4:56–63. doi: 10.1016/j.hrthm.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 27.Pokushalov E, Romanov A, Shugayev P, Artyomenko S, Shirokova N, Turov A, Katritsis DG. Selective ganglionated plexi ablation for paroxysmal atrial fibrillation. Heart Rhythm. 2009;6:1257–1264. doi: 10.1016/j.hrthm.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 28.Oh S, Zhang Y, Bibevski S, Marrouche NF, Natale A, Mazgalev TN. Vagal denervation and atrial fibrillation inducibility: epicardial fat pad ablation does not have long-term effects. Heart Rhythm. 2006;3:701–708. doi: 10.1016/j.hrthm.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 29.Edd JF, Horowitz L, Davalos RV, Mir LM, Rubinsky B. In vivo results of a new focal tissue ablation technique: irreversible electroporation. IEEE Trans Biomed Eng. 2006;53:1409–1415. doi: 10.1109/TBME.2006.873745. [DOI] [PubMed] [Google Scholar]
- 30.Lemery R, Talajic M, Roy D, Coutu B, Lavoie L, Lavallee E, Cartier R. Success, safety, and late electrophysiological outcome of low-energy direct-current ablation in patients with the Wolff-Parkinson-White syndrome. Circulation. 1992;85:957–962. doi: 10.1161/01.cir.85.3.957. [DOI] [PubMed] [Google Scholar]
- 31.Deodhar A, Dickfeld T, Single GW, Hamilton WC, Jr, Thornton RH, Sofocleous CT, Maybody M, Gonen M, Rubinsky B, Solomon SB. Irreversible electroporation near the heart: ventricular arrhythmias can be prevented with ECG synchronization. AJR Am J Roentgenol. 2011;196:W330–335. doi: 10.2214/AJR.10.4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lemola K, Chartier D, Yeh YH, Dubuc M, Cartier R, Armour A, Ting M, Sakabe M, Shiroshita-Takeshita A, Comtois P, Nattel S. Pulmonary vein region ablation in experimental vagal atrial fibrillation: role of pulmonary veins versus autonomic ganglia. Circulation. 2008;117:470–477. doi: 10.1161/CIRCULATIONAHA.107.737023. [DOI] [PubMed] [Google Scholar]


