Abstract
In this study, methacrylamide chitosan modified with perfluorocarbon chains (MACF) is used as the base material to construct hydrogel dressings for treating dermal wounds. MACF hydrogels saturated with oxygen (+ O2) are examined for their ability to deliver and sustain oxygen, degrade in a biological environment, and promote wound healing in an animal model. The emerging technique of metabolomics is used to understand how MACF + O2 hydrogel dressings improve wound healing. Results indicate that MACF treatment facilitates oxygen transport rate that is two orders of magnitude greater than base MAC hydrogels. MACF hydrogel dressings are next tested in an in vivo splinted rat excisional wound healing model. Histological analysis reveals that MACF + O2 dressings improve re-epithelialization (p < 0.0001) and synthesis of collagen over controls (p < 0.01). Analysis of endogenous metabolites in the wounds using global metabolomics demonstrates that MACF + O2 dressings promotes a regenerative metabolic process directed toward hydroxyproline and collagen synthesis, with confirmation of metabolite levels within this pathway. The results of this study confirm that increased oxygen delivery through the application of MACF + O2 hydrogels enhances wound healing and metabolomics analyses provides a powerful tool to assess wound healing physiology.
Keywords: chitosan, perfluorocarbons, oxygen delivery, metabolomics, wound healing
1. Introduction
Successful wound healing is a process of events that begins with hemostasis followed by overlapping phases of inflammation, proliferation, and reorganization, resulting in newly formed tissue [1]. Wounds with impaired healing are often mired in a vicious cycle of hypoxia and prolonged inflammation and do not proceed through the normal healing process [2]. It is well established that oxygen availability has a profound influence on repair processes [3]. During the early stages of wound healing, reduced nicotinamide adenine dinucleotide (NADH) oxidase in the presence of molecular oxygen produces superoxide radicals, which help to prevent bacterial infections [4]. Oxygen has also been shown to regulate angiogenesis and encourage cell proliferation and cell motility [5]. Further, oxygen is a prerequisite for hydroxyproline synthesis, which is used as one of the building blocks of collagen that allows fibrillogenesis, increased tensile strength, and improved wound remodeling [6, 7]. Although hypoxia initiates neovascularization, it cannot sustain it [7–9]. Thus, supplemental oxygen cycling is required in many cases to facilitate accelerated wound healing [8].
Several studies have shown that wound oxygenation is closely tied to the rate of wound healing [10]. Hyperbaric oxygen therapy is a clinically effective treatment for hypoxic chronic wounds, but the associated costs to build and maintain facilities, overall availability to patients, and the willingness of insurers to reimburse treatments, are major obstacles to widespread adoption of the technology [11]. Various attempts are being pursued to deliver oxygen to the wound via cheaper and less cumbersome methods [12]. One strategy is the in situ formation of oxygen within a biomaterial scaffold via chemical reaction or enzymatic transformation. Unfortunately, this strategy has a number of shortcomings including the possibility of systemic side-effects from the locally applied reactive chemicals or enzymes [13], local pH changes, and heating via exothermic reactions. Specific biomaterial strategies have been developed to blunt these potentially negative effects [9, 12, 14, 15], or avoid their need altogether including work using methacrylamide chitosan modified with perfluorocarbons (MACF) [16, 17].
Perfluorocarbons (PFCs) were originally introduced as blood substitutes because of their impressive abilities to carry respiratory gasses, especially oxygen, in biological conditions [18]. PFCs are created by complete fluorination of hydrocarbons (replacement of hydrogen with fluorine), which generates a biologically non-reactive moiety [19]. The presence of a dense electron cloud, higher ionization potential, and greater electron affinity give rise to the highly hydrophobic and fluorophilic structure of PFCs [20]. The hydrophobic nature of PFCs is typically problematic in biologic aqueous environments if not conjugated to a more hydrophilic structure. PFCs are often combined with excipients and formed into short-lived colloidal suspensions [21]. To overcome these PFC limitations, MACF can form the basis of aqueous hydrogels that can sustain the benefit of PFCs and be used to enhance local oxygenation [17]. Chitosan is the base biopolymer of choice, because it is naturally abundant, biodegradable, and acceptable for use in many biomedical applications [22]. Chitosan is characterized as a polysaccharide polymer of β-(1, 4) linked D-glucosamine that is similar in structure to hyaluronic acid (HA), a predominant constituent of the mammalian extracellular matrix (ECM) [23]. The free amino group on a primary sugar ring in chitosan is advantageous, as it allows various bioconjugate substitutions and can serve as a ligand for complex formation [24]. We have demonstrated that nucleophilic substitution targeting chitosan’s primary amine groups is a straightforward way to add methacrylamide and PFCs through amide linkage. Chitosan itself acts as an antimicrobial agent against fungi, bacteria, and viruses [25], and has been shown to have beneficial hemostatic properties [26].
A plethora of FDA-approved clinical wound dressings focus on maintaining wound hydration, antimicrobial properties, and supportive ECM structure for new granulation tissue [17, 27]. Many wound dressings used in standard wound care are synthetic such as polyester pads, polyurethane foams, and various synthetic blends [17]. Other materials based on collagen, alginate, cellulose, and gelatin have been formatted into dressings, and a variety of further improvements to enhance healing are being tested in preclinical studies [28]. Hydrogels have been shown to be especially beneficial for treating light to non-exudating wounds, since they provide a moist healing environment and promote the wound healing process [29]. Unfortunately, there are few dressing-based technologies available on the market to address the problem of hypoxia [30]. Thus, there exists a need for easy and affordable treatment that can provide oxygen to the wound and provide the benefits of a hydrogel dressing.
Metabolomics is the study of small molecules involved in metabolic processes in an organism [31]. Metabolomics is a powerful tool to assay end points of the genome and proteome and reveal the underlying biochemistry and physiologic state [31]. Recently, the need has been highlighted for high throughput biological techniques such as metabolomics, proteomics, and genomics to reveal new understanding of wound healing [32]. In a recent study, wound fluid samples were analyzed with proteomics and metabolomics to improve the understanding of bone defects and its connection to the biochemical mechanisms in the wound environment [33]. Another study used metabolomics profiling of diabetic and non-diabetic wounds in mice and identified key metabolites that were differentially regulated and could serve as future biomarkers [34]. Notably, only a few researchers have so far used metabolomics to understand interactions of biomaterials with biological environments [35, 36].
Given the importance of oxygen in wound healing and tissue regeneration, the main objectives of this study were to create an oxygenating MACF hydrogel dressing and to evaluate this dressing in a rat excisional wound model. Metabolomics was used to reveal the physiologic state and confirm regenerative processes tied to wound treatment with MACF dressings. The MACF hydrogel studied here provides a dressing that can deliver oxygen to the wound and sustain enhanced local levels of oxygen to overcome hypoxia while providing a beneficial moist environment for enhancing reparative processes. Using MACF eliminates the need of complex oxygenating set-ups (e.g., hyperbaric oxygen, oxygen generators), colloidal suspensions, chemical reactions, and enzymatic conversions.
2. Materials and Methods
2.1. Preparation of pentadecafluorooctanoyl methacrylamide chitosan hydrogels
Fluorinated and non-fluorinated methacrylamide chitosan hydrogels, MACF and MAC, respectively, were created and characterized as previously described [15]. Briefly, chitosan, average molecular weight 200,000 g/mol and degree of deacetylation 82 %, (Novamatrix, Drammen, Norway) was used in a two-step reaction using methacrylic anhydride (Sigma-Aldrich, Saint Louis, MO, USA) to add methacrylate groups, followed by reaction with pentadecafluorooctanoyl chloride (Sigma-Aldrich) to obtain MACF. The resulting material was freeze-dried (Labconco, Kansas City, MO) for storage. For NMR quantification chitosan, MAC and MACFs were dissolved in 2 vol % deuterated acetic acid/D2O. Methacrylation and fluorine substitution were confirmed by 19F and 1H NMR (Varian 500 MHz). The corresponding peak areas were used to calculate % degree of substitution [16]. To form hydrogels, MACF was first dissolved in ultrapure water (18 MΩ resistance, Millipore, Billerica, MA, USA) and sterilized by autoclaving. Photoinitiator solution, 1-hydroxycyclohexyl phenyl ketone (Sigma-Aldrich) dissolved in 1-vinyl-2-pyrrolidinone (Sigma-Aldrich), was added at (0.9 mg/g) to MACF/MAC polymer solutions (2 % wt/wt in ultrapure water). The resulting solution was mixed and degassed (Speed Mixer DAC 150 FVZ, Hauschild Engineering, Hamm, Germany) at 3000 RPM for 2 min at room temperature (RT). Hydrogels were then washed extensively with phosphate buffer saline (PBS, pH 7.4) to remove unreacted polymer and crosslinker.
2.2. Oxygen permeability/diffusion study
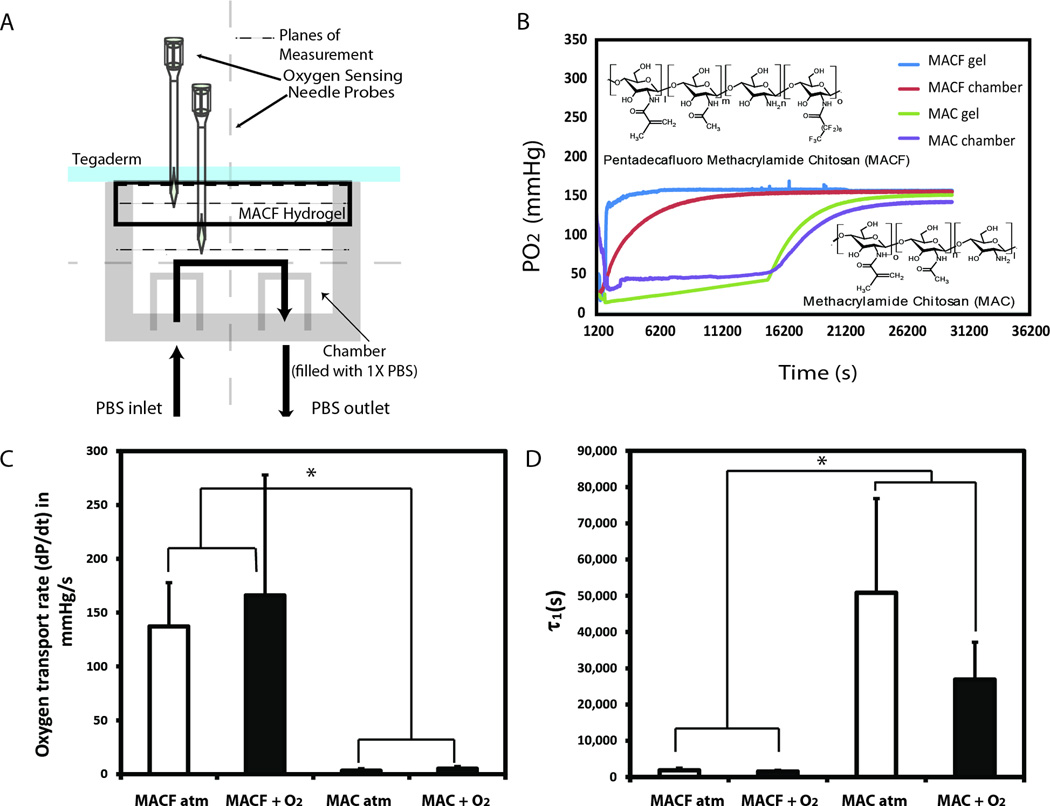
A glass chamber was designed to perform the oxygen permeability studies and fabricated by the University of Akron Glass Shop (Figure 1A). A surgical grade membrane, Tegaderm™ (3M, Saint Paul, MN, USA) was placed on the hydrogel (MACF + O2, MACF atm, MAC + O2, or MAC atm). The construct was then placed over the top of the glass chamber. Static conditions were imposed and the PBS solution was pumped in until the chamber was full. Clamps were then used to seal off the inlet and outlet ports. The experiments began with purging the chamber with nitrogen (Praxair, Danbury, CT, USA). Oxygen removal was verified using needle-type dissolved oxygen sensors (FireSting O2, Pyro Science, Aachen, Germany), which recorded continuously during set-up and experiments in several locations (Figure. 1A). The oxygen sensor measures oxygen using red light excitable materials that generate oxygen-dependent luminescence in the near infrared [37, 38]. Oxygen measurement within the gel was performed at 5 mm depth (hydrogel center). Data were logged using the FireSting O2 software (OxyView PST6-V5.41). The experiment was considered complete when both the chamber and hydrogel systems had reached an oxygenated equilibrium such that the change was less than 1% partial pressure of oxygen (PO2) per minute. The maximum oxygen transport rate was calculated by taking maximum slope of each PO2 versus time curve in the exponential region.
Figure 1.
A) Oxygen permeability/diffusion study chamber with hydrogel placed on top covered with Tegaderm™ membrane. PO2 is measured inside the hydrogel and inside the chamber. B) Representative PO2 vs. time data for MACF initially saturated with oxygen (+O2) and MAC +O2 at RT. C) Oxygen transport rate measurements taken at their maximum show there is a significant difference between MACF and MAC gels (p <0.0001). D) Euler’s 1st time constant (τ1) calculations demonstrate time required for MACF is significantly shorter than MAC (p = 0.0011). Mean ± SD, n = 3.
The time to equilibrium was calculated using Euler’s method, and the first time constant (τ1) was calculated for each saturation curve [39]. The final 1000 points of each data set were averaged to determine the concentration of oxygen at equilibrium (CEq). Then τ1 in terms of Euler’s constant (e) is:
| (3) |
2.3. Oxygen release study
MACF hydrogels, each (0.3 g), were prepared for oxygen release studies by placing hydrogels in PBS, then saturating with 100% oxygen for 10 min. The hydrogels were then placed on dissolved oxygen sensors (FireSting O2), 0.5 mm in diameter, in a static vented well plate (Greiner Bio-One, Kremsmünster, Austria) and covered with a lid. The oxygen concentrations in the hydrogels were measured at 1, 24, and 48 h after saturation.
2.4. Hydrogel degradation study
Chitosan is degraded by lysozyme through hydrolysis of glycosidic bonds [40]. Lysozyme is a common hydrolytic enzyme in body fluids and an essential part of the immune system [41]. A lysozyme activity assay (Enzcheck Lysozyme Assay kit, Life Technologies, Carlsbad, CA, USA) was performed with hydrogel samples before beginning the degradation study (Supplementary Figure. 1). MAC and MACF hydrogels (0.5 g each) were degraded for 8 d in lysozyme (1000 U mL−1) (Sigma-Aldrich) in PBS (pH 7.4). The hydrogels were submerged in lysozyme solution and stored in an incubator at 37°C. At each time point (t = 0, 2, 4, 6 d), supernatant was removed from each sample and analyzed using the lysozyme activity assay. On day four, after the standard activity assay was completed, a concentrated lysozyme solution (20 µL at 80,000 UmL−1) was added to each sample to account for reduced activity of lysozyme (Supplementary Figure. 1) and the assay was performed a second time to confirm activity change.
For the main degradation study, MACF and MAC (0.5 g) hydrogels were prepared then freeze-dried. The initial dry mass (m0) was obtained, then the hydrogels were swollen in lysozyme solution (2 mL at 1000 U mL−1) (Sigma-Aldrich) for 4, 7, 11, 18, and 21 d (with n=4). Every four days, the samples were supplemented with concentrated lysozyme solution (20 µL at 80,000 U mL−1). At each time point, samples were washed three times with ultrapure water, and then freeze dried to obtain dry mass (mt). The mass loss (mloss) was then calculated:
| (5) |
2.5. Animal wound model
All experiments were approved by the Institutional Animal Care and Use Committee (IACUC) guidelines at the University of Akron before any animal testing was conducted. A splinted excisional wound model was used with 10-week old male Wistar rats at the time of wounding (Harlan, Indianapolis, IN, USA). 8 animals were used in a study. Rats were anesthetized, then splinted using 0.5 mm thick silicone sutured to the back of animal in five locations. A 6 mm biopsy punch was used to make a uniform full-thickness wound in the middle of the exposed area inside the splint. All three layers of the skin, epidermis, dermis, and subcutaneous tissue, were removed along with the first layer of fascia.
Hydrogel treatments were arranged in five rotations on the back of each animal: MAC + O2, MACF + O2, MAC atm, MACF atm, and no gel. The treatments were prepared beforehand and consisted of hydrogels (0.2 g) that were cross-linked for 4 min in a 96-well plate and thoroughly washed with sterile PBS. Oxygen treatments were oxygenated with 100% pure oxygen for 10 min just before application. The hydrogels were placed directly into the wounds and sealed to the splint with a Tegaderm membrane (2.5 by 2.5 cm). A second Tegaderm bandage (5 by 10 cm) was placed over all the splints. Every two days, a wound hydrogel dressing change was performed under anesthesia with the same treatments. Hydrogels were present and did not degrade qualitatively. The hydrogels were removed from the wounds, which were then gently debrided with sterile saline. Hydrogels were replaced with new hydrogels with appropriate treatments including oxygenation and atmospheric. On day 8, the rats were sacrificed in a CO2 chamber. A 12 mm biopsy punch was then used to remove samples from the back of each rat. A 1 mm biopsy punch was used to take a small sample from near the center of each wound and placed in a small centrifuge tube, which was immediately frozen in a −80°C freezer for metabolomics analyses. The remaining tissue samples were fixed for histology and immunohistochemistry (IHC) analyses.
2.6. Metabolomics
Wound biopsies were lysed by the addition of high performance liquid chromatography (HPLC) grade water (180 µL) and methanol (20 µL) followed by three cycles of freezing in liquid nitrogen, thawing, and sonication. (750 µL 1:2 (v:v)) chloroform:methanol and (125 µL) chloroform were added to each sample. The samples were vortexed and (250 µL) of water was added, then incubated at −20°C for one hour and centrifuged at 1000×g for 10 min at 4°C to form a two-phase system. The aqueous and organic phases were separated into tubes (1.5 mL). Extracted metabolites from the aqueous phase were dried in a CentriVap Concentrator (Labconco) and then stored at −80°C until analysis. Protein pellets were used to normalize extracted metabolites quantities by using a bicinchoninic acid assay (BCA) for protein (G-Biosciences, St. Louis. MO, USA).
Chromatographic separation was performed on a Micro200 LC (Eksigent, Redwood, CA, USA) equipped with hydrophilic interaction liquid chromatography (HILIC) column (Luna 3µ NH2 100Å, 150mm by 1.0mm, Phenomenex, Torrance, CA, USA). Polar metabolites were re-suspended in 35: 65 (v:v) acetonitrile:water solution (200 µL), and the sample (5 µL) were injected into the column. Mobile phase A was water and mobile phase B was acetonitrile, each with the addition of (5 mM) ammonium acetate and (5 mM) ammonium hydroxide. The flow rate was 30 µLmin−1. The gradient consisted of the following linear changes in mobile phase B over time: 0 min 98%, 0.5 min 98%, 1 min 95%, 5 min 80%, 6 min 46%, 13 min 14.7%, 17 min 0%, 17.1 min 100%, 23 min 100%.
Samples were analyzed on a 5600+ TripleTOF Mass Spectrometer (AB SCIEX, Framingham, MA, USA) in both positive and negative mode and were processed with Information Dependent Acquisition (IDA). The ion source nebulizer gas (GS1) was set at 15 psi, the heater gas (GS2) was 20 psi, and the curtain gas (CUR) was 25 psi. In the IDA experiment, a time of flight mass spectroscopy (TOF MS) scan was performed over the mass range of 60–1,000 Da. A 250 ms accumulation time was used for precursor ions acquisition. In the positive mode, +5000 V ionspray voltage was used and a +100 V declustering potential (DP) was selected. The background threshold for candidate ion selection was set to 10 counts per second to eliminate peaks with a low signal to noise (S/N) ratio. Fragmentation data was subsequently collected using a collision energy spread (CES) of + (25–40) V. In negative mode, the same GS1, GS2, and CUR in IDA criterion were used. A −4500 V ionspray voltage and a −100 V declustering potential (DP) was selected. Fragmentation data was subsequently collected using a collision energy spread (CES) of (− (40–25) Volts).
Initial processing of the data was performed using MarkerView (version 1.2.1.1) by (AB SCIEX, USA). Isotopic ion peaks were excluded before analysis. Principal component analysis (PCA) was performed with the Pareto Scaling method to compare groups. Features were identified by comparing accurate mass and fragmentation data to standards in the METLIN and the Human Metabolome Database (HMDB) [42].
2.7. Histology
Histology was performed to directly visualize and assess healing responses. Samples were first paraffin embedded and sectioned at 16 µm. Next, the sections were deparaffinized using xylene and then dehydrated in alcohol solutions. Hematoxylin and eosin (H&E) staining was performed on one set of sections for each wound sample using the manufacturer’s protocol (EMD Millipore, Billerica, MA, USA). Masson’s Trichrome (Richard-Allan Scientific, Kalamazoo, MI, USA) was used on a second set of sections. A final set of sections were used for immunostaining for CD86 and CD163 positive cells. Endogenous peroxidase activity was inactivated with 3% hydrogen peroxide for 10 min. Mouse primary antibodies for anti-CD86 (MCA2874) and anti-CD163 (MCA342R) (both AbD Serotec, Oxford, UK) were used at (1:250 µl:µl) dilution and incubated with sections at 4°C overnight. Sections were incubated with horseradish peroxidase (HRP) conjugated secondary antibody donkey-anti-mouse (Millipore, Temecula, CA, USA), which was used at 1:400 dilution for 2 h. A DAB staining kit (Sigma-Aldrich) was used to develop peroxidase staining for 5 min. After the slides were washed with water, the sections were counterstained with hematoxylin for 5 min and washed with PBS and xylene. All slides were then mounted and imaged on a microscope (IX-81, Olympus, Tokyo, Japan) and Image J (National Institute of Health, Bethesda, Maryland) software was used for analyses [43].
3. Results
MACF was successfully synthesized and purified. 1H NMR showed 22 % degree of substitution for methacrylation and 19F NMR showed 42 % PFC substitution, as previously reported [16].
3.1. Oxygen transport rate and release measurements
Oxygen transport properties of the dressings were characterized using a new experimental set-up (Figure 1A). As expected, PFC addition significantly enhanced both oxygen transport and delivery through dressings (Figure 1B–D, Figure 2A). MACF outperformed MAC in all metrics. MACF hydrogels (oxygenated (MACF + O2) and non-oxygenated/ atmospheric, (MACF atm)) showed significantly higher oxygen transport rates that were two orders of magnitude greater than non-flourinated MAC hydrogels (p < 0.0001) (Figure 1C). Calculation of Euler’s first time constant (τ1) revealed similar enhancement of oxygen transport in both atmospheric and oxygenated MACF hydrogels. MAC hydrogels had greater values for τ1 as compared to MACF hydrogels (Figure 1D) and, thus, took significantly longer to equilibrate (p = 0.0011).
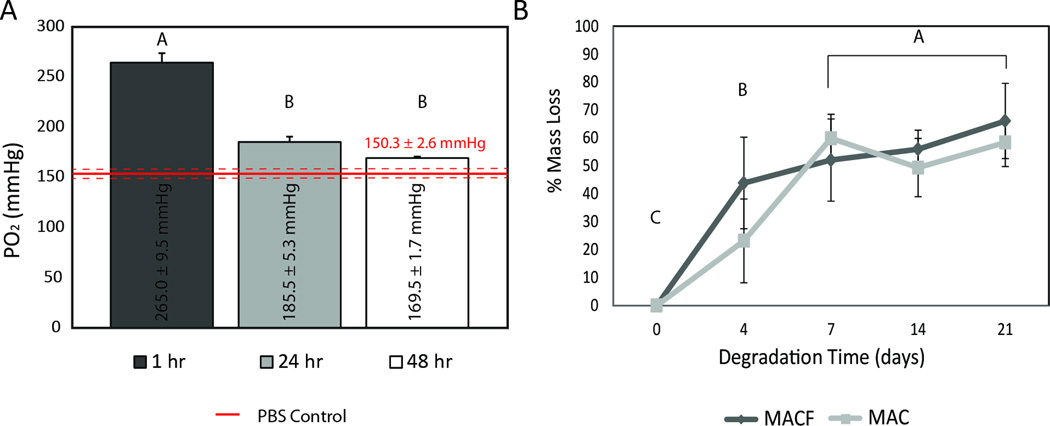
Figure 2.
A) Oxygen release from MACF + O2 hydrogels in a static well-plate maintained at atmosphere and 37°C. Different letters denote statistical significance by one factor ANOVA (p < 0.0001) Mean ± SD, n = 3. B) Degradation testing of MAC and MACF hydrogels in PBS + 1000 U/mL lysozyme maintained at 37°C. Different letters denote statistical significance by one ANOVA with student’s t test (p < 0.0001). Mean ± SD, n = 4.
Next, oxygen release was studied over 48 h (Figure 2A). It was found that MACF hydrogels saturated for 10 min with 100% oxygen gas and immersed in PBS in a covered well-plate started at 265.0 ± 9.5 mmHg partial pressure of oxygen (PO2), which exceeds atmospheric oxygen tension (159 mmHg PO2 at STP). The MACF hydrogels at 48 h resulted in an average PO2 value that remained above atmospheric oxygen tensions by over 10 mmHg in an enclosed 37°C environment.
3.2. Degradation testing
Degradation characteristics were next determined since mass of the hydrogel directly correlates to the amount of PFCs, and thus the ability to enhance oxygenation. An initial study to assess activity levels of lysozyme found that re-supplementation was required (Supplementary Figure 1), and a 4 d time period was chosen in an attempt to maintain high lysozyme activity, acknowledging that chitosan degradation byproducts inhibit activity [44]. The degradation data show that PFC modification did not change degradation kinetics of the chitosan-based hydrogels (Figure 2B). Most importantly, less than 20% mass loss was observed for all groups by day 2, the target exposure time for dressings.
3.3. Excisional wound model testing
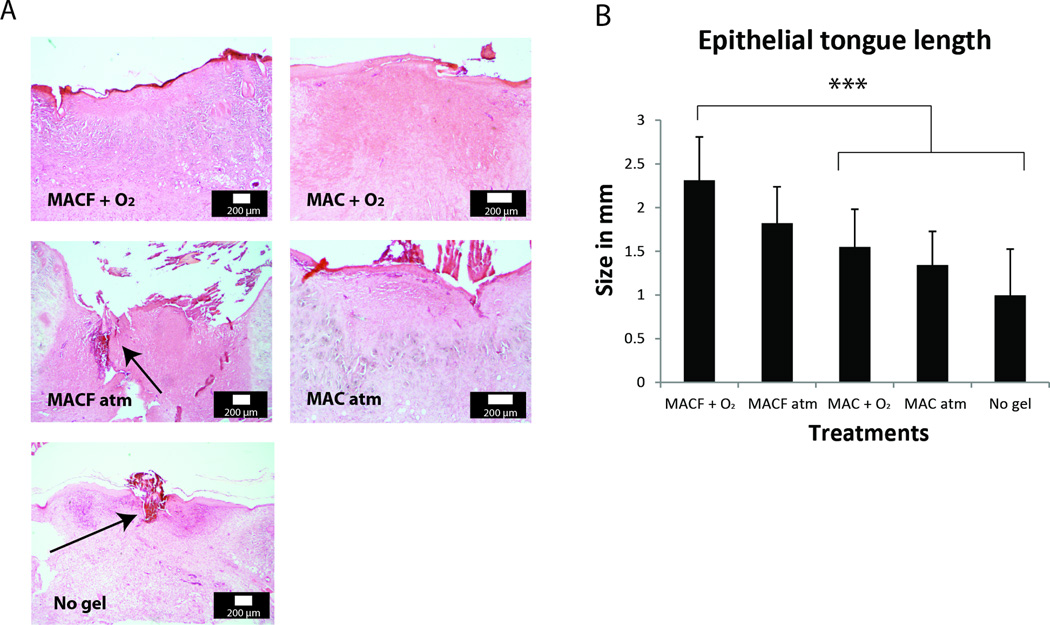
An in vivo splinted excisional wound rat model [45] was implemented to test the safety and efficacy of the hydrogels. The study primarily aimed to assess healing benefits of MACF + O2 as compared to controls (MACF atm, MAC + O2, MAC atm, no treatment (no gel), and healthy skin (for metabolomics)). Re-epithelialization studies in rodent models are difficult to perform, because rodent wounds heal primarily by contraction [46]. To overcome this problem, circular silicone splints were sutured around the wound [47]. The rat study was conducted for 8 d, with dressing changes every 2 d, after which animals were sacrificed and samples were collected for analyses. H&E staining of tissue sections (Figure 3) revealed comparative differences. In tissue sections taken from wounds treated with MACF + O2 hydrogels, granulation tissue was dense (Figure 3A) and showed the best re-epithelialization (Figure 3B).
Figure 3.
A) H & E histology representative images for all experimental groups. Arrows show presence of fibrin clots in granulation tissue. B) Epithelial tongue measurements obtained from analyzing H & E stained wound sections. *** denotes significant difference by one-factor ANOVA with Tukey’s post hoc analysis (p < 0.0001). Mean+/−SD, n = 5.
3.4. Metabolomics analyses of wound healing
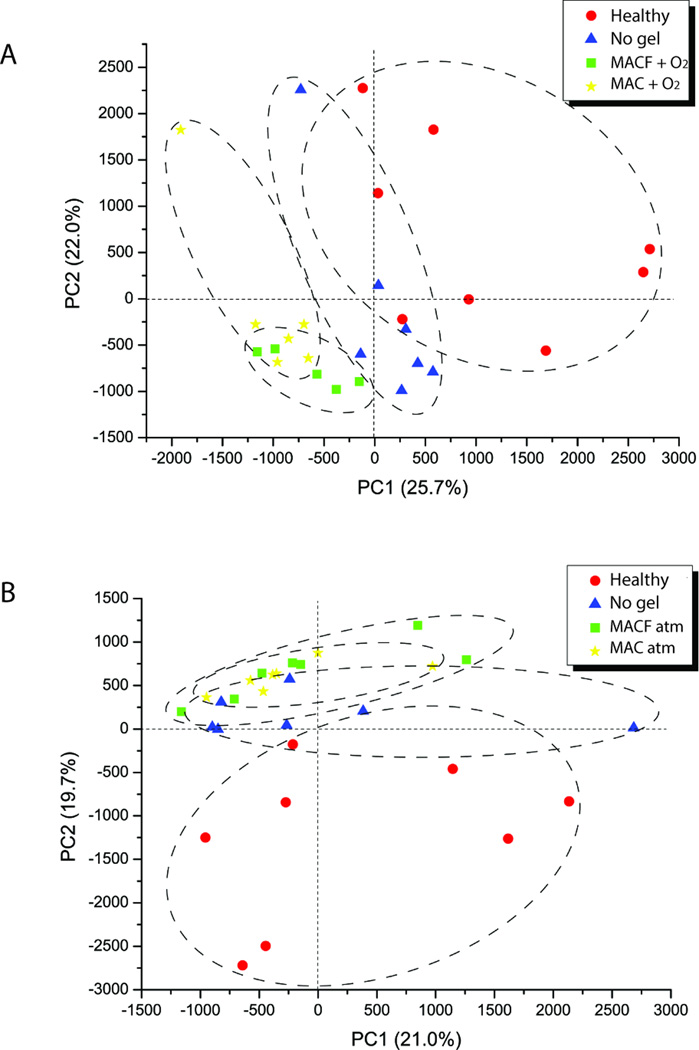
To better understand the underlying molecular mechanisms of wound healing responses to the MACF + O2 hydrogel treatment, samples obtained from the wounds were immediately processed and analyzed for metabolites using tandem-mass spectrometry (LC MS/MS). In this study, PCA scores with a global untargeted approach were used to provide an overall assessment of wound healing responses; with specific focus on the effects of the MACF treatments. Both MACF + O2 and MACF atm treatments show separation compared to no gel treatments based on principal components PC1 and PC2. Based on the standardized variable PC1, MACF + O2 shows a larger difference than no gel (Figure 4A). However, MACF atm and no gels were not resolved by PC2 as much as MACF + O2 compared to no gel (Figure 4B); suggesting similar metabolic profiles. The number of differentially expressed significant metabolites was counted for treatment vs. no gel and treatment vs. healthy skin to further illustrate differences in treatments (Table 1). A list of the most altered or affected metabolites provides an understanding of changes on a global level and this approach is most commonly used in the field of metabolomics [48]. Lists are provided (Supplementary Tables 1–8).
Figure 4.
A) PCA charts showing differences between oxygenated treatments, no gel and healthy skin groups based on PC1 (25.7%) and PC2 (22%). B) PCA charts showing differences between non-oxygenated treatments, no gel and healthy skin groups based on PC1 (21%) and PC2 (19.7%).
Table 1.
Differentially expressed significant metabolites from wound samples
| Differentially Expressed Significant Metabolites | |||
|---|---|---|---|
| Treatment (vs No Gel) |
# of Metabolites |
Treatment (vs Healthy Skin) |
# of Metabolites |
| MACF atm | 563 | MACF atm | 3345 |
| MACF + O2 | 1113 | MACF + O2 | 3347 |
| MAC atm | 1073 | MAC atm | 3594 |
| MAC + O2 | 2011 | MAC + O2 | 2525 |
3.5. Assessment of key wound healing pathways via metabolic and histological analyses
An applied metabolomics approach was used to assess and better understand the effects of the MACF materials on wound healing. The goal was to reveal the underlying mechanism(s) behind the material-wound interactions by studying alterations in individual metabolites and directly relating this to healing outcomes in the wound.
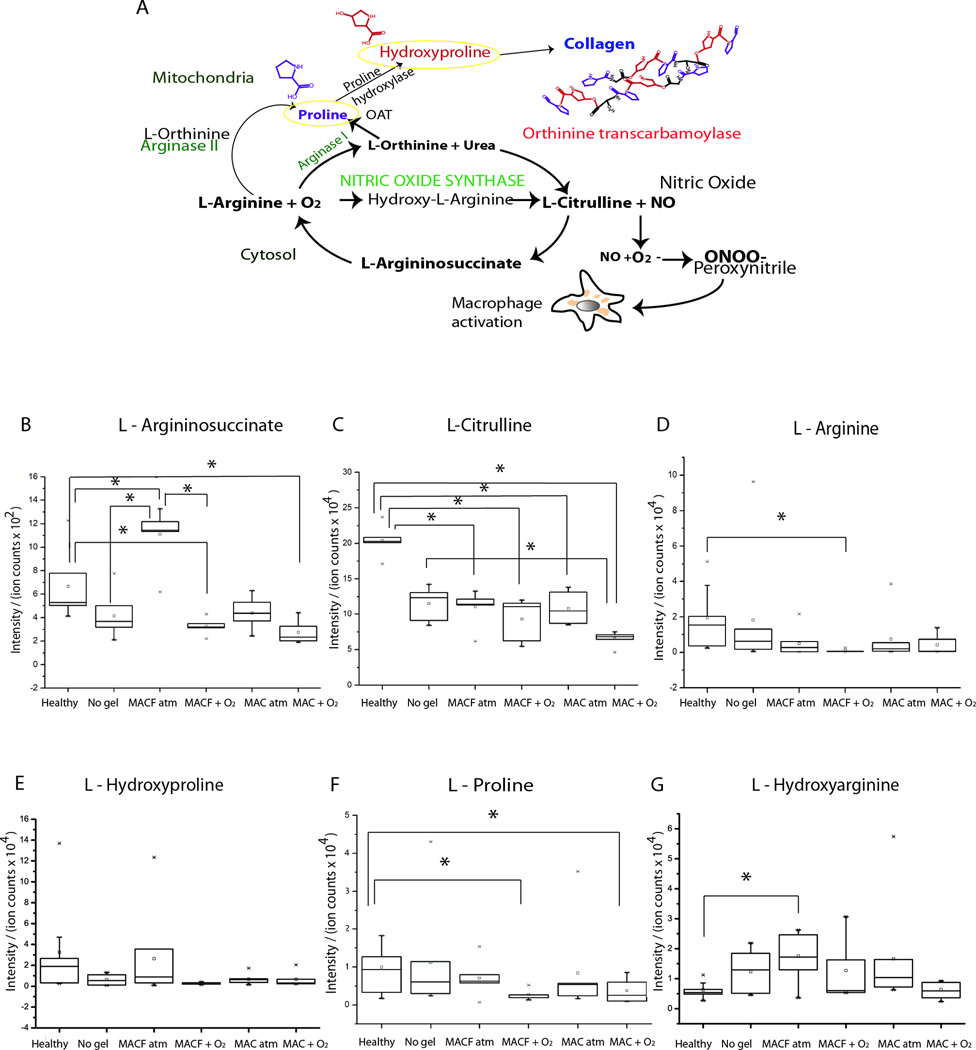
Analyses of individual metabolites (Figure 5), as confirmed with the fingerprint MS/MS spectra of standards (Supplementary Figure 2) uncovered important alterations in arginine and proline metabolic pathways. Down-regulation of arginine, proline, and hydroxyarginine was seen in the MACF + O2 treatment group compared to untreated and healthy skin controls after wounding, further confirming a metabolic bias toward collagen synthesis (Figure 5).
Figure 5.
A) Arginine and proline metabolism pathway supplies fibrillary collagen synthesis. Normalized intensity of metabolites B) L-Argininosuccinate, C) L-Citruline, D) L-Arginine, E) L-Hydroxyproline, F) L-Proline, and G) L-Hydroxyarginine for all groups. * denotes significance by one-way ANOVA with Tukey’s post hoc analysis (p < 0.05). Mean ± SD, n = 5
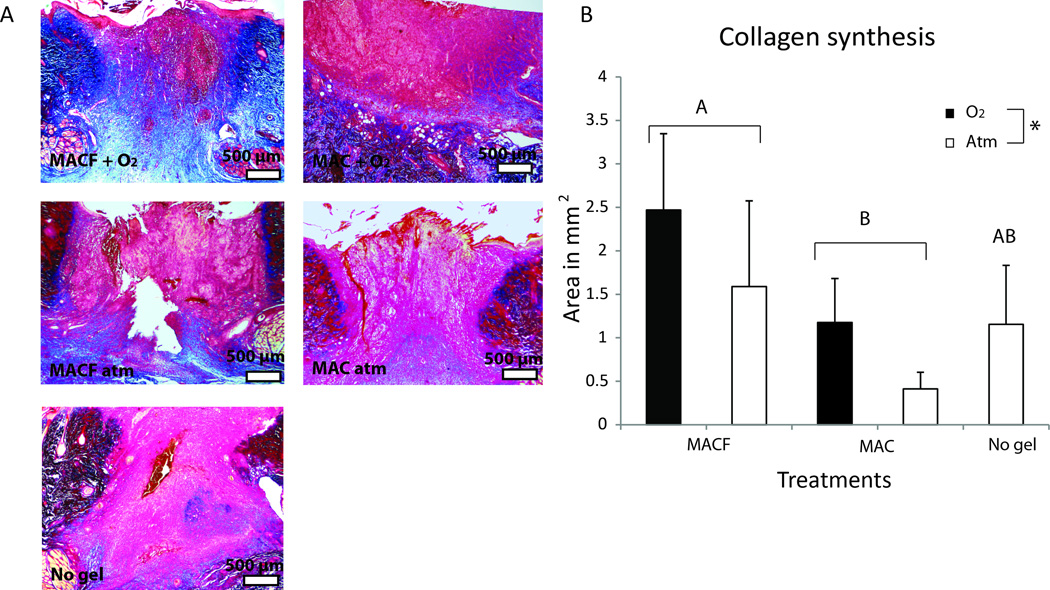
As a confirmation of the key metabolomics findings, collagen synthesis was assayed at the protein level to test the hypothesis that MACF + O2 positively influence metabolic regulation of collagen. Masson’s trichrome histology was used to directly confirm collagen synthesis for wound tissue sections and collagen amounts were quantified with image quantification (Figure 6). MACF + O2 treated wounds resulted in the greatest area of newly formed collagen in the granulation bed followed by MACF atm. Interestingly, the base material MAC atm group was seen to have the least amount of new collagen at 8 d (Figure 6B; p < 0.05). Supplemental oxygen benefits (+ O2) surpassed all other treatments in terms of total collagen in the maturing granulation bed.
Figure 6.
A) Representative Masson’s trichrome histology images for all groups, collagen appears blue. B) Collagen synthesis measured in terms of total blue area from Masson’s trichrome staining. Letters denotes significant difference by two factor ANOVA, (p < 0.01) and * denotes significant difference by two factor ANOVA, (p < 0.05). Mean+/−SD, n = 5.
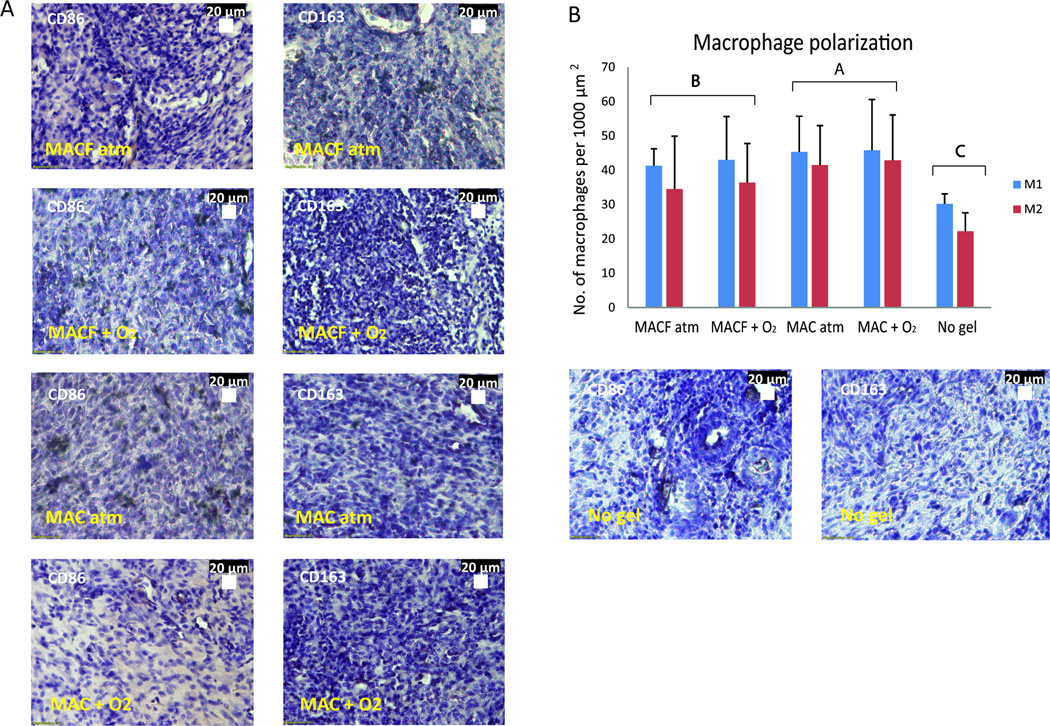
M1 and M2 macrophages (Figure 7) were stained to better understand both presence and the polarization of macrophages, while attempting to connect this to arginine and proline metabolism analyses. Interestingly, there was no polarization of M1 and M2 macrophages observed in any treatment (Figure 7B). However, a significant difference was observed in the total number of macrophages in response to each treatment type. The wounds with no treatment had less total number of macrophages in wounds (p < 0.001, Figure 7B). This also corresponded to relative accumulation of metabolites in the pathway as shown by higher fold changes for L-arginine, proline and hydroxyarginine (Figure 5D, F, G).
Figure 7.
A) Representative images of CD86 (M1 inflammatory macrophage) immunostaining on wound sections (left) and CD163 (M2 anti-inflammatory macrophage) immunostaining (right). Positive staining is brown in color, purple are nuclei via hematoxylin. B) Number of macrophages per 1000 µm2 area for M1 and M2 macrophages inside the wound area of sections. Different letters denote statistical significance by two factor ANOVA (p < 0.0001) using Tukey’s post hoc analysis. Mean ± SD, n = 3.
4. Discussion
Previously reported in vitro results indicate that MACF can uptake and deliver oxygen to enhance fibroblast cell proliferation and metabolism [16]. Long aliphatic PFC chains immobilized to chitosan provide maximal oxygen affinity and biological benefit, which has also been reported in colloidal suspensions [49]. This foundational work also studied varied PFC modifications (aromatic and aliphatic). In the present study, the most promising formulation was used, integrating 15 fluorines per PFC substitution (Ali15F), to create the MACF hydrogel dressings.
Overall, the data supports the hypothesis that MACF hydrogels can sequester and deliver oxygen (Figures 1–2) via fluorine modifications, thus offering the ability to supply significant oxygen to a local environment in the short term, while sustaining it long term. Healthy skin oxygen tensions are typically reported as 40 mmHg (interstitial) and chronic wound oxygen levels have been measured as low as 5 mmHg just below the epidermal surface [50]. Excitingly, the in vitro release results (Figure 2A) demonstrate that MAC + O2 can sustain significant oxygen levels to reverse such deficits, and by 48 h PO2 levels were nearly 20 mmHg above PBS control in an enclosed environment. It is important to highlight that fluorines have high affinity towards other gases such as nitric oxide and carbon dioxide. Theoretically, PFCs not only transport oxygen but can also help respiration, much like the action of hemoglobin, by removing gases at high concentration such as CO2 from tissue [51], thus stabilized PFCs potentially improve biological oxygen transport in a bidirectional manner.
Comparing MACF to other oxygenating biomaterial strategies, poly(d,l-lactide–co–glycolide) (PLGA) and calcium peroxide based oxygen generating scaffolds have been shown to elevate PO2 by 7 mmHg in media for up to 10 d [12]. Another microsphere system using PLGA and H2O2/poly(2-vinlypyrridione) liberated approximately 150 mmHg PO2 into a sealed container over several days [52]. Clinical hyperbaric oxygen therapy maintains oxygen pressure at 2.5 times that of atmospheric oxygen for 30 to 120 min, and systemic oxygen levels in the patient are elevated afterward for some time [53]. This exposure must be closely administered since hyperbaric oxygen therapy can lead to complications such as ruptured ear drums, sinus and lung damage, vision changes, and most seriously, oxygen poisoning, which can permanently damage the lungs and the central nervous system [54].
As release studies in vitro confirmed MACF hydrogels could deliver and sustain oxygen for 48 h (Figure 2A), the next step before in vivo testing was to assess degradation of the MAC and MACF hydrogels to a simulated wound environment with an initial concentration of 1000 U mL−1 lysozyme [55] (Figure 2B). The resulting degradation half-lives of MAC and MACF hydrogels were 6.21 and 6.24 d, respectively. The exposure of a hydrogel on the surface of a real wound is different than when fully immersed in a solution in vitro in many key ways, including fluctuating enzyme concentrations and the surface area of the gel exposed to lysozyme. Thus, it is likely that the degradation observed in vitro was greater than what the hydrogel would experience in vivo when used as a wound dressing [56]. Combining the degradation results (Figure 2B) with the release results (Figure 2A), dressing changes every 2 d were chosen for proceeding to the animal study, to maximize benefits of the MACF hydrogel treatment. This is in line with clinical wound care practice, where dressings are often changed daily or even twice a day [45].
The main animal study proceeded to evaluate the MACF + O2 treatment versus controls in a splined rat excisional wound model; to understand if the benefits reported in vitro both previously [16] and in this study (Figures 1, 2A) would translate in vivo. After 8 d, H&E evaluation of wounds (Figure 3) demonstrated that MACF + O2 treatment provided the best wound healing responses over controls, including dense granulation tissue and the best re-epithelization Conversely, in untreated wounds, 8 d after wounding it was common to observe poor granulation with the presence of lasting fibrin clots (Figure 3A). Improved re-epithelialization in MACF + O2 treated wounds (Figure 3B) is likely due to the combined benefits of oxygenation and the hydrogel, as supplemental oxygen from MACF provides a required nutrient for migration and synthesis, and a moist environment improves directed cell migration.[57] Interestingly, MACF + O2 and non-oxygenated (MACF atm) treatments resulted in epithelial tongue lengths that were similar (p > 0.05). This may suggest that super-physiologic oxygen levels do not provide additional benefit for closure as compared to elevated oxygenation (PO2 5–20 mmHg over ambient, Figure 2A).
Metabolomics analysis afforded a new tool for global assessment of tissue metabolic state and specific levels of metabolites vital to the processes of wound healing in this study, and most notably PCA revealed important clustering differences between treatment and control groups (Figure 4). As discussed in the introduction, targeted and untargeted metabolomics approaches have only recently been applied to wound healing studies, but never in conjugation with a wound healing treatment [33, 34]. For this study, focus was placed on dysregulated metabolites that were classified based on their functional pathways, then these individual metabolites were targeted based on their connected wound healing processes and direct impact on the tissue of interest (Figure 5). Further, from these analyses we chose to focus on the two primary endpoints: hydroxyproline and nitric oxide synthesis (Figures 6, 7).
One of the most significant findings of this study is the fact that MACF treatment accelerates collagen syntheses during wound healing (Figure 6). Fibrillary collagen synthesis, which is an important process in the proliferative phase of wound healing, requires the formation of proline and hydroxyproline [58]. Several other studies have shown that oxygen accelerates collagen synthesis and improves proline hydroxylase enzyme activity [59]. Data presented here suggest a mechanism where these intermediates are being rapidly consumed (Figure 5) to synthesize fibrillary collagen to promote enhanced maturation of the wound granulation bed (Figure 6). Similar changes associated with these amino acids have been shown in other studies to be influenced by oxygen consumption during collagen synthesis and proliferation [60]. It remains to be determined by more in-depth analyses whether flux through these pathways is increased or if these amino acids are being used for other metabolic pathways important for wound healing.
Another important outcome of the arginine and proline pathway (Figure 5A) is the nitric oxide (NO) synthase pathway. NO converts hydroxyarginine into L-citruline to supply NO radicals, which are important in early phases of wound healing to enhance macrophage recruitment and activation [60]. Further, hydroxyarginine is a known inhibitor of arginase and increases the availability of L-arginine for NO synthesis in macrophages [61]. In the presence of cytokines, L-arginine and other intermediate metabolites are utilized in NO synthesis and activated in inflammatory macrophages (M1) [62]. Macrophage polarization M1 (pro-inflammatory) and M2 (anti-inflammatory) markers (CD86 and CD163, respectively) were probed using IHC in all treatment group samples from day 8 (Figure 7). Activation of M1 and M2 macrophages is regulated by arginine and proline metabolism to some extent through arginase or NO synthase, which are two competing pathways [63]. Polarization of macrophages, or preference to one phenotype, was not observed in wound tissue samples (Figure 7B). However, the total number of macrophages was significantly lower in no gel control treated wounds as compared to MAC and MACF treated wounds (p < 0.0001), suggesting some recruitment due to the presence of the base hydrogel at 8 d. Also, MACF wounds contained less total number of macrophages as compared to MAC (p < 0.0001). Metabolomics results suggest proline, L-arginine, and hydroxyarginine relative consumption in the MACF + O2 treatment (Figure 5A), which is also complicated by regulation by both collagen synthesis and the iNOS pathways. It’s hard to make many definitive conclusions in regard to macrophage presence and phenotype since only a single time point was probed in all analyses. Overall, results suggest that the balance of macrophage phenotypes, slightly elevated macrophage levels in the MAC + O2 treatment combined with elevated oxygen and a moist environment leads to an accelerated regenerative program as compared to the other treatments.
5. Conclusions
This study demonstrated that the chitosan-based material MACF can be formulated into an oxygenating hydrogel wound dressing, which married benefits of oxygen therapy with moist wound healing treatments. MACF + O2 treatment was shown to enhance wound healing as evidenced by greater re-epithelialization and faster collagen synthesis when compared to controls. Metabolomics revealed and connected mechanisms that depend on available oxygen and contribute to successful and enhanced wound healing. The combined results (histology, metabolomics, etc.) of the in vivo rat study confirmed treatment safety and efficacy in a simple wound model. The MACF hydrogel dressings should be further refined in more complex wound models to provide further evidence for establishing a clinically beneficial hydrogel oxygenation therapy.
Supplementary Material
Supplementary Figure 1. Lysozyme activity assay performed to assess changes in activity. Lysozyme supplemented on day 4 (after). Mean+/−SD, n = 4.
Supplementary Figure 2. Relative intensity profiles from fragmentation of key metabolites demonstrate putative hits and confirms identification of metabolites. Grey spectra is obtained from experimental samples and blue spectra shows respective standards.
Statement of Significance.
This work presents the first application of a novel class of oxygen delivering biomaterials (methacrylamide chitosan modified with perfluorocarbon chains (MACF)) as a hydrogel wound dressing. This manuscript also contains strong focus on the biochemical benefits of MACF dressings on underlying mechanisms vital to successful wound healing. In this vein, this manuscript presents the application of applied metabolomics (tandem mass spectroscopy) to uncover biomaterial interactions with wound healing mechanisms. We believe the approaches described in this manuscript will be of great interest to biomedical scientists and particularly to researchers studying wound healing, metabolomics, applied biomaterials and regenerative medicine.
Acknowledgments
We would like to acknowledge funding from the National Institute of General Medical Sciences of the National Institutes of Health under award number R15GM104851 to PSP, NFD, JF and NDL. We would also like to acknowledge support from AB SCIEX Young Investigator Award, to LPS. Parag Joshi is acknowledged for assistance with histology and accompanying analyses. We want to thank Jack Gillespie for glasswork and Frank Pelc for regular troubleshooting and setup, Bethany Noble for her help and expertise in animal care, Karen Day at the University of Akron Vivarium for her expertise and guidance, and Kelly Bondor for assistance with dermal fibroblast isolation and culture. Also, we want to thank Carla Giai and Nick Callow for help with the oxygen sensors and experimental setup and Dr. William Landis for use of histology equipment. Finally, we would like to thank group members Hang Li, Aleesha McCormick, Trevor Ham, and Ashley Wilkinson for assistance with analyses.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Guo S, DiPietro LA. Factors Affecting Wound Healing. Journal of Dental Research. 2010;89:219–229. doi: 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mirza RE, Fang MM, Novak ML, Urao N, Sui A, Ennis WJ, Koh TJ. Macrophage PPAR-gamma and Impaired Wound Healing in Type 2 Diabetes. J Pathol. 2015;236:433–444. doi: 10.1002/path.4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Igwegbe I, Onojobi G, Fadojutimi-Akinsiku MO, Hirsh AM, Park NJ, Yao M, Driver VR. Case studies evaluating transdermal continuous oxygen for the treatment of chronic sickle cell ulcers. Adv Skin Wound Care. 2015;28:206–210. doi: 10.1097/01.ASW.0000462327.30245.ae. [DOI] [PubMed] [Google Scholar]
- 4.Schaper W SJ. Heart Physiology and Pathophysiology. Fourth. Elsevier; 2007. [Google Scholar]
- 5.Sen CK. Wound healing essentials: let there be oxygen. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2009;17:1–18. doi: 10.1111/j.1524-475X.2008.00436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gorres KL, Raines RT. Prolyl 4-hydroxylase. Critical Reviews in Biochemistry and Molecular Biology. 2010;45:106–124. doi: 10.3109/10409231003627991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gordillo GM, Sen CK. Evidence-based recommendations for the use of topical oxygen therapy in the treatment of lower extremity wounds. Int J Low Extrem Wounds. 2009;8:105–111. doi: 10.1177/1534734609335149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gordillo GM, Sen CK. Revisiting the essential role of oxygen in wound healing. American journal of surgery. 2003;186:259–263. doi: 10.1016/s0002-9610(03)00211-3. [DOI] [PubMed] [Google Scholar]
- 9.Harrison BS, Eberli D, Lee SJ, Atala A, Yoo JJ. Oxygen producing biomaterials for tissue regeneration. Biomaterials. 2007;28:4628–4634. doi: 10.1016/j.biomaterials.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Wu D, Yotnda P. Induction and Testing of Hypoxia in Cell Culture. Journal of Visualized Experiments : JoVE. 2011:2899. doi: 10.3791/2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gajendrareddy PK, Sen CK, Horan MP, Marucha PT. Hyperbaric oxygen therapy ameliorates stress-impaired dermal wound healing. Brain Behav Immun. 2005;19:217–222. doi: 10.1016/j.bbi.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Oh SH, Ward CL, Atala A, Yoo JJ, Harrison BS. Oxygen generating scaffolds for enhancing engineered tissue survival. Biomaterials. 2009;30:757–762. doi: 10.1016/j.biomaterials.2008.09.065. [DOI] [PubMed] [Google Scholar]
- 13.Ward CL, Corona BT, Yoo JJ, Harrison BS, Christ GJ. Oxygen Generating Biomaterials Preserve Skeletal Muscle Homeostasis under Hypoxic and Ischemic Conditions. PLoS ONE. 2013;8:e72485. doi: 10.1371/journal.pone.0072485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oh SH, Ward CL, Atala A, Yoo JJ, Harrison BS. Oxygen generating scaffolds for enhancing engineered tissue survival. Biomaterials. 2009;30:757–762. doi: 10.1016/j.biomaterials.2008.09.065. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Zhu Y, Bawa HK, Ng G, Wu Y, Libera M, van der Mei HC, Busscher HJ, Yu X. Oxygengenerating nanofiber cell scaffolds with antimicrobial properties. ACS Appl Mater Interfaces. 2011;3:67–73. doi: 10.1021/am100862h. [DOI] [PubMed] [Google Scholar]
- 16.Wijekoon A, Fountas-Davis N, Leipzig ND. Fluorinated methacrylamide chitosan hydrogel systems as adaptable oxygen carriers for wound healing. Acta biomaterialia. 2013;9:5653–5664. doi: 10.1016/j.actbio.2012.10.034. [DOI] [PubMed] [Google Scholar]
- 17.Sood A, Granick MS, Tomaselli NL. Wound Dressings and Comparative Effectiveness Data. Adv Wound Care (New Rochelle) 2014;3:511–529. doi: 10.1089/wound.2012.0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lowe KC, Davey MR, Power JB. Perfluorochemicals: their applications and benefits to cell culture. Trends in biotechnology. 1998;16:272–277. doi: 10.1016/s0167-7799(98)01205-0. [DOI] [PubMed] [Google Scholar]
- 19.Meyer A, Condon RG, Keil G, Jhaveri N, Liu Z, Tsao YS. Fluorinert, an oxygen carrier, improves cell culture performance in deep square 96-well plates by facilitating oxygen transfer. Biotechnol Prog. 2012;28:171–178. doi: 10.1002/btpr.712. [DOI] [PubMed] [Google Scholar]
- 20.Riess JG. Perfluorocarbon-based oxygen delivery. Artificial cells, blood substitutes, and biotechnology. 2006;34:567–580. doi: 10.1080/10731190600973824. [DOI] [PubMed] [Google Scholar]
- 21.Lattes A, Rico-Lattes I. Microemulsions of perfluorinated and semi-fluorinated compounds. Artif Cells Blood Substit Immobil Biotechnol. 1994;22:1007–1018. doi: 10.3109/10731199409138798. [DOI] [PubMed] [Google Scholar]
- 22.Singh DK, Ray AR. Biomedical applications of chitin, chitosan, and their derivatives. Journal of Macromolecular Science, Part C: Polymer Reviews. 2000;40:69–83. [Google Scholar]
- 23.Quan T, Wang F, Shao Y, Rittié L, Xia W, Orringer JS, Voorhees JJ, Fisher GJ. Enhancing structural support of the dermal microenvironment activates fibroblasts, endothelial cells and keratinocytes in aged human skin in vivo. J Invest Dermatol. 2013;133:658–667. doi: 10.1038/jid.2012.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poon L, Wilson LD, Headley JV. Chitosan-glutaraldehyde copolymers and their sorption properties. Carbohydrate polymers. 2014;109:92–101. doi: 10.1016/j.carbpol.2014.02.086. [DOI] [PubMed] [Google Scholar]
- 25.Ruiz Matute AI, Cardelle-Cobas A, García-Bermejo AB, Montilla A, Olano A, Corzo N. Synthesis, characterization and functional properties of galactosylated derivatives of chitosan through amide formation. Food Hydrocolloids. 2013;33:245–255. [Google Scholar]
- 26.Pusateri AE, McCarthy SJ, Gregory KW, Harris RA, Cardenas L, McManus AT, Goodwin CW., Jr Effect of a chitosan-based hemostatic dressing on blood loss and survival in a model of severe venous hemorrhage and hepatic injury in swine. J Trauma. 2003;54:177–182. doi: 10.1097/00005373-200301000-00023. [DOI] [PubMed] [Google Scholar]
- 27.Dreifke MB, Jayasuriya AA, Jayasuriya AC. Current wound healing procedures and potential care. Mater Sci Eng C Mater Biol Appl. 2015;48:651–662. doi: 10.1016/j.msec.2014.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao X, Lang Q, Yildirimer L, Lin ZY, Cui W, Annabi N, Ng KW, Dokmeci MR, Ghaemmaghami AM, Khademhosseini A. Photocrosslinkable Gelatin Hydrogel for Epidermal Tissue Engineering. Adv Healthc Mater. 2015 doi: 10.1002/adhm.201500005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fonder MA, Lazarus GS, Cowan DA, Aronson-Cook B, Kohli AR, Mamelak AJ. Treating the chronic wound: A practical approach to the care of nonhealing wounds and wound care dressings. J Am Acad Dermatol. 2008;58:185–206. doi: 10.1016/j.jaad.2007.08.048. [DOI] [PubMed] [Google Scholar]
- 30.Ladizinsky D, Roe D. New insights into oxygen therapy for wound healing. Wounds. 2010;22:294–300. [PubMed] [Google Scholar]
- 31.Dettmer K, Aronov PA, Hammock BD. Mass spectrometry-based metabolomics. Mass spectrometry reviews. 2007;26:51–78. doi: 10.1002/mas.20108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gordillo GM, Bernatchez SF, Diegelmann R, Di Pietro LA, Eriksson E, Hinz B, Hopf HW, Kirsner R, Liu P, Parnell LK, Sandusky GE, Sen CK, Tomic-Canic M, Volk SW, Baird A. Preclinical Models of Wound Healing: Is Man the Model? Proceedings of the Wound Healing Society Symposium, Adv Wound Care (New Rochelle) 2013;2:1–4. doi: 10.1089/wound.2012.0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalkhof S, Forster Y, Schmidt J, Schulz MC, Baumann S, Weissflog A, Gao W, Hempel U, Eckelt U, Rammelt S, von Bergen M. Proteomics and metabolomics for in situ monitoring of wound healing. BioMed research international. 2014;2014:934848. doi: 10.1155/2014/934848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sood RF, Gu H, Djukovic D, Deng L, Ga M, Muffley LA, Raftery D, Hocking AM. Targeted metabolic profiling of wounds in diabetic and nondiabetic mice. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2015;23:423–434. doi: 10.1111/wrr.12299. [DOI] [PubMed] [Google Scholar]
- 35.Bo Y, Jin C, Liu Y, Yu W, Kang H. Metabolomic analysis on the toxicological effects of TiO(2) nanoparticles in mouse fibroblast cells: from the perspective of perturbations in amino acid metabolism. Toxicology mechanisms and methods. 2014;24:461–469. doi: 10.3109/15376516.2014.939321. [DOI] [PubMed] [Google Scholar]
- 36.Li H, Shi X, Qiao L, Lu X, Xu G. Synthesis of a new type of echinus-like Fe3O4@TiO2 core-shellstructured microspheres and their applications in selectively enriching phosphopeptides and removing phospholipids. Journal of chromatography A. 2013;1275:9–16. doi: 10.1016/j.chroma.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 37.Shavaleev NM, Adams H, Best J, Edge R, Navaratnam S, Weinstein JA. Deep-red luminescence and efficient singlet oxygen generation by cyclometalated platinum(II) complexes with 8-hydroxyquinolines and quinoline-8-thiol. Inorganic chemistry. 2006;45:9410–9415. doi: 10.1021/ic061283k. [DOI] [PubMed] [Google Scholar]
- 38.Borisov SM, Nuss G, Klimant I. Red light-excitable oxygen sensing materials based on platinum(II) and palladium(II) benzoporphyrins. Analytical chemistry. 2008;80:9435–9442. doi: 10.1021/ac801521v. [DOI] [PubMed] [Google Scholar]
- 39.Tzafriri AR, Lerner EI, Flashner-Barak M, Hinchcliffe M, Ratner E, Parnas H. Mathematical modeling and optimization of drug delivery from intratumorally injected microspheres. Clinical Cancer Research. 2005;11:826–834. [PubMed] [Google Scholar]
- 40.Davies G, Henrissat B. Structures and mechanisms of glycosyl hydrolases. Structure. 1995;3:853–859. doi: 10.1016/S0969-2126(01)00220-9. [DOI] [PubMed] [Google Scholar]
- 41.Hankiewicz J, Swierczek E. Lysozyme in human body fluids. Clinica chimica acta. 1974;57:205–209. doi: 10.1016/0009-8981(74)90398-2. [DOI] [PubMed] [Google Scholar]
- 42.Benton HP, Ivanisevic J, Mahieu NG, Kurczy ME, Johnson CH, Franco L, Rinehart D, Valentine E, Gowda H, Ubhi BK, Tautenhahn R, Gieschen A, Fields MW, Patti GJ, Siuzdak G. Autonomous metabolomics for rapid metabolite identification in global profiling. Analytical chemistry. 2015;87:884–891. doi: 10.1021/ac5025649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Meth. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hamaguchi K, Rokkaku K, Funatsu M, Hayashi K. Studies on the structure and enzymatic fuction of lysozyme: I. Enzymatic action of lysozyme on glycol chitin. Journal of Biochemistry. 1960;48:351–357. [Google Scholar]
- 45.Benner P. From novice to expert. Menlo Park; 1984. [Google Scholar]
- 46.Abercrombie M, Flint M, James D. Collagen formation and wound contraction during repair of small excised wounds in the skin of rats. Journal of Embryology and Experimental Morphology. 1954;2:264–274. [Google Scholar]
- 47.Carlson MA, Longaker MT, Thompson JS. Wound splinting regulates granulation tissue survival. Journal of Surgical Research. 2003;110:304–309. doi: 10.1016/s0022-4804(02)00098-7. [DOI] [PubMed] [Google Scholar]
- 48.German JB, Hammock BD, Watkins SM. Metabolomics: building on a century of biochemistry to guide human health. Metabolomics. 2005;1:3–9. doi: 10.1007/s11306-005-1102-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Castro CI, Briceno JC. Perfluorocarbon-based oxygen carriers: review of products and trials. Artif Organs. 2010;34:622–634. doi: 10.1111/j.1525-1594.2009.00944.x. [DOI] [PubMed] [Google Scholar]
- 50.Schreml S, Szeimies R, Prantl L, Karrer S, Landthaler M, Babilas P. Oxygen in acute and chronic wound healing. British Journal of Dermatology. 2010;163:257–268. doi: 10.1111/j.1365-2133.2010.09804.x. [DOI] [PubMed] [Google Scholar]
- 51.Pilarek M, Glazyrina J, Neubauer P. Enhanced growth and recombinant protein production of Escherichia coli by a perfluorinated oxygen carrier in miniaturized fed-batch cultures. Microbial Cell Factories. 2011;10:50. doi: 10.1186/1475-2859-10-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li Z, Guo X, Guan J. An oxygen release system to augment cardiac progenitor cell survival and differentiation under hypoxic condition. Biomaterials. 2012;33:5914–5923. doi: 10.1016/j.biomaterials.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 53.Lin LC, Yau G, Lin TF, Lin TK, Tang YY, Wang KY. The efficacy of hyperbaric oxygen therapy in improving the quality of life in patients with problem wounds. The journal of nursing research : JNR. 2006;14:219–227. doi: 10.1097/01.jnr.0000387580.97092.95. [DOI] [PubMed] [Google Scholar]
- 54.Arieli Y, Kotler D, Eynan M, Hochman A. Hyperbaric oxygen preconditioning protects rats against CNS oxygen toxicity. Respiratory physiology & neurobiology. 2014;197:29–35. doi: 10.1016/j.resp.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 55.Chen S-C, Wu Y-C, Mi F-L, Lin Y-H, Yu L-C, Sung H-W. A novel pH-sensitive hydrogel composed of N,O-carboxymethyl chitosan and alginate cross-linked by genipin for protein drug delivery. Journal of Controlled Release. 2004;96:285–300. doi: 10.1016/j.jconrel.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 56.Lu L, Peter SJ, Lyman MD, Lai H-L, Leite SM, Tamada JA, Uyama S, Vacanti JP, Langer R, Mikos AG. In vitro and in vivo degradation of porous poly (DL-lactic-co-glycolic acid) foams. Biomaterials. 2000;21:1837–1845. doi: 10.1016/s0142-9612(00)00047-8. [DOI] [PubMed] [Google Scholar]
- 57.Ghosh K, Ren XD, Shu XZ, Prestwich GD, Clark RA. Fibronectin functional domains coupled to hyaluronan stimulate adult human dermal fibroblast responses critical for wound healing. Tissue engineering. 2006;12:601–613. doi: 10.1089/ten.2006.12.601. [DOI] [PubMed] [Google Scholar]
- 58.Phang JM, Liu W, Hancock CN, Fischer JW. Proline metabolism and cancer: emerging links to glutamine and collagen. Current opinion in clinical nutrition and metabolic care. 2015;18:71–77. doi: 10.1097/MCO.0000000000000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Togami K, Miyao A, Miyakoshi K, Kanehira Y, Tada H, Chono S. Efficient delivery to human lung fibroblasts (WI-38) of pirfenidone incorporated into liposomes modified with truncated basic fibroblast growth factor and its inhibitory effect on collagen synthesis in idiopathic pulmonary fibrosis. Biological & pharmaceutical bulletin. 2015;38:270–276. doi: 10.1248/bpb.b14-00659. [DOI] [PubMed] [Google Scholar]
- 60.Otto GP, Neugebauer S, Claus RA, Sossdorf M. Arginine metabolism is markedly impaired in polymicrobial infected mice. Crit Care. 2012;16:412. doi: 10.1186/cc11205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hecker M, Nematollahi H, Hey C, Busse R, Racke K. Inhibition of arginase by NG-hydroxy-Larginine in alveolar macrophages: implications for the utilization of L-arginine for nitric oxide synthesis. FEBS Lett. 1995;359:251–254. doi: 10.1016/0014-5793(95)00039-c. [DOI] [PubMed] [Google Scholar]
- 62.El-Gayar S, Thuring-Nahler H, Pfeilschifter J, Rollinghoff M, Bogdan C. Translational control of inducible nitric oxide synthase by IL-13 and arginine availability in inflammatory macrophages. J Immunol. 2003;171:4561–4568. doi: 10.4049/jimmunol.171.9.4561. [DOI] [PubMed] [Google Scholar]
- 63.Rath M, Muller I, Kropf P, Closs EI, Munder M. Metabolism via Arginase or Nitric Oxide Synthase: Two Competing Arginine Pathways in Macrophages. Front Immunol. 2014;5:532. doi: 10.3389/fimmu.2014.00532. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Lysozyme activity assay performed to assess changes in activity. Lysozyme supplemented on day 4 (after). Mean+/−SD, n = 4.
Supplementary Figure 2. Relative intensity profiles from fragmentation of key metabolites demonstrate putative hits and confirms identification of metabolites. Grey spectra is obtained from experimental samples and blue spectra shows respective standards.