Abstract
Optogenetic and chemogenetic actuators are critical for deconstructing the neural correlates of behavior. However, these tools have several limitations, including invasive modes of stimulation or slow on/off kinetics. We have overcome these disadvantages by synthesizing a single component, magnetically sensitive actuator, “Magneto,” comprised of the cation channel, TRPV4, fused to the paramagnetic protein, ferritin. We validate non-invasive magnetic control over neuronal activity by demonstrating remote stimulation of cells using in vitro calcium imaging assays, electrophysiological recordings in brain slices, in vivo electrophysiological recordings in the brains of freely moving mice, and behavioral outputs in zebrafish and mice. As proof of concept, we used Magneto to delineate a causal role of striatal dopamine receptor 1 neurons in mediating reward behavior in mice. Together, our results present Magneto as a novel actuator capable of remotely controlling circuits associated with complex animal behaviors.
Introduction
Opto- and chemogenetic actuators have revealed critical properties of neural networks in normal and pathological states1–6. While both opto- and chemogenetics remotely control neuronal stimulation, optical strategies are limited spatially by poor light penetration into dense tissues and chemogenetic strategies suffer from slow pharmacokinetics that prevent cellular activation on a physiologically relevant timescale. Therefore, there remains a need for next generation actuators that are non-invasive and can respond rapidly and reversibly7. Several recent studies have reported transient receptor potential vanilloid 1 (TRPV1) ion channels can be engineered to become sensitive to a combination of radiowaves and magnetothermal heating through coupling to the iron storage protein, ferritin, or to inorganic paramagnetic nanoparticles8–12. While these reagents represent an important advance, they are multicomponent systems (e.g. requiring delivery of nanoparticles and a genetically encoded channel) with possible off-target heating effects. One study has employed non-thermal magnetogenetic control of somatic tissues to regulate blood glucose13, but a fully encoded, single component magnetogenetic system has yet to be applied to the nervous system. Here, we have expanded upon these strategies by engineering a magnetogenetic actuator through fusion of the non-selective cation channel, TRPV414–16, to the paramagnetic protein, ferritin17. We have successfully applied this actuator to the nervous system and validated it using in vitro calcium imaging, brain slice electrophysiology, in vivo electrophysiology, and acute modulation of behavior in freely moving zebrafish and mice.
Results
Design and screen of a novel magnetically sensitive cation channel
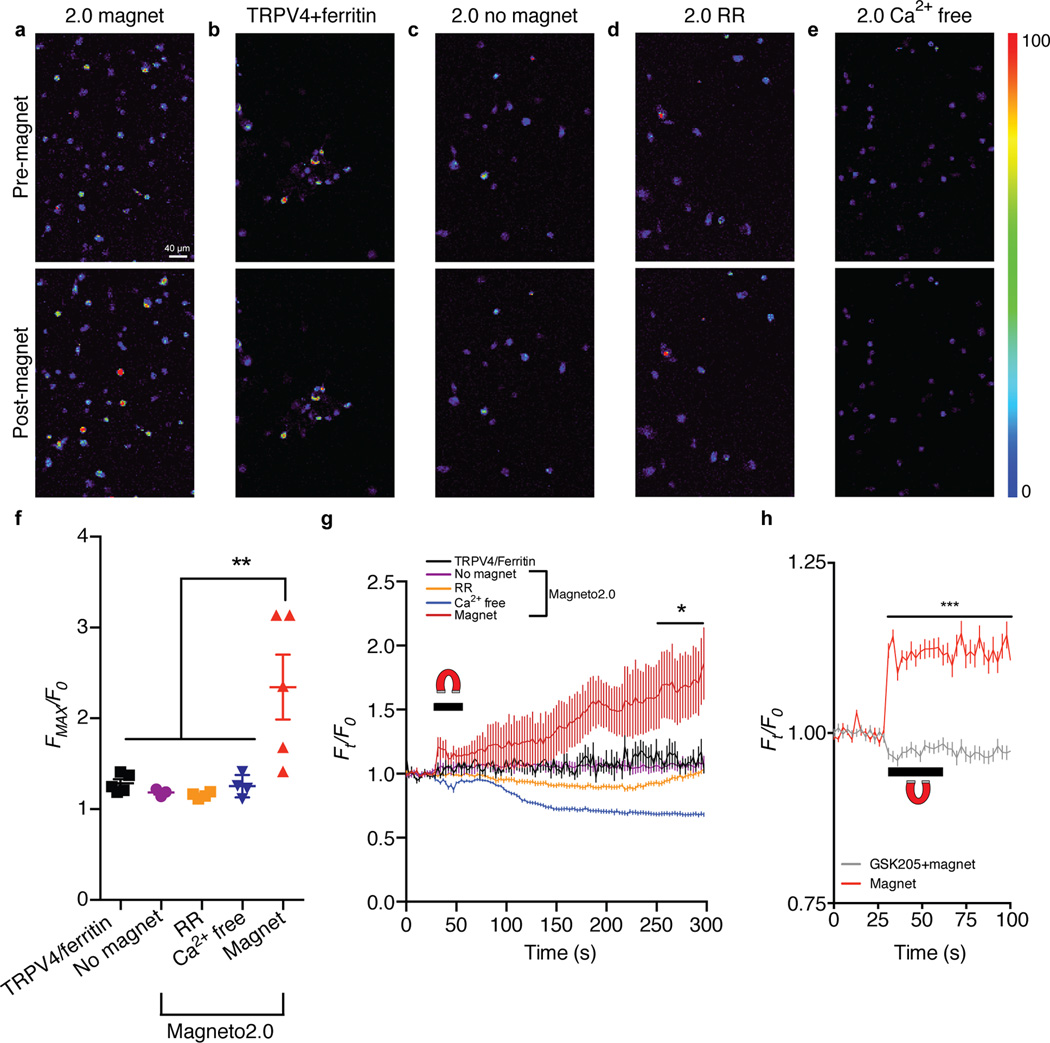
To engineer a novel single-component magnetogenetic actuator, we based our design on TRPV4 since it has been reported to respond to pressure14,15. We suspected that, when fused to TRPV4, a paramagnetic protein would enable magnetic torque to tug open the channel to depolarize cells (Supplementary Fig. 1). While we hypothesized that magnetic field dependent activation of TRPV4 would be more facile than using a non-mechanically sensitive ion channel, it may also be formally possible that application of torque to ion channels in general would achieve the same result. Therefore, we developed a small library of 21 proteins consisting of TRPV4 fused to a gene encoding two subunits of the paramagnetic ferritin protein (Supplementary Table 1)18. Human embryonic kidney (HEK) 293 cells did not express 18 of the 21 generated chimeric proteins following transient transfection, presumably due to cytotoxicity of the chimeric channels. For the three channels that did express in HEK293 cells, we performed in vitro calcium imaging to determine whether the fusion proteins responded to magnetic fields. Using the fluorescent calcium-binding dye Fluo-4, we measured calcium transients in response to a ~50 mT magnetic field delivered by an electromagnet (Supplementary Fig. 2). Of the three candidate proteins, we observed detectable calcium transients in response to magnetic stimulation with one fusion protein, consisting of ferritin tethered to a truncated TRPV4 carboxyl-terminus (Δ760–871) (Supplementary Fig. 3). Because the 17±3.5% (mean±SEM) increase in magnetically evoked calcium transients was smaller than expected TRPV4 responses16 (Supplementary Fig. 3h), we hypothesized that trafficking to the plasma membrane was disrupted19, resulting in blunted calcium signaling. We next optimized the chimeric channel’s subcellular localization by adding a series of subcellular trafficking signals to Magneto akin to the optimization of optogenetic actuators20,21. Ultimately, we determined that the addition of a plasma membrane trafficking signal enhanced the prototype channel’s membrane expression (Supplementary Fig. 4), and we dubbed this improved channel “Magneto2.0.” We confirmed that HEK293 cells were viable after Magneto2.0 expression (Supplementary Fig. 5) and then measured magnetic field dependent calcium transients produced by Magneto2.0 using the paradigm described in Supplementary Fig. 3. We observed that cells expressing Magneto2.0 (58% transfected cells, n=6 coverslips, n=539 cells) exhibited robust calcium transients approximately 2.5-fold higher than baseline after 50 mT magnetic stimulation with no change in any of the control conditions (Fig. 1a–f). Controls included: (1) cells expressing non-fused TRPV4 and ferritin moieties, (2) unstimulated Magneto2.0 expressing cells, (3) Magneto2.0 expressing cells exposed to the TRP pore blocker ruthenium red (RR), and (4) Magneto2.0 expressing cells in Ca2+ free extracellular media. We observed calcium influx immediately following magnetic stimulation but invariably, maximal calcium fluorescence was observed minutes after magnetic field stimulation of Magneto2.0 expressing cells, which was not observed in any of the above control conditions (Fig. 1g). We found that the delayed calcium response in Magneto2.0+ cells was caused by calcium release from intracellular stores following magnetically induced depolarization since this secondary response was eliminated following depletion of intracellular calcium stores by thapsigargin, a sarco-endoplasmic reticulum calcium transport ATPase pump inhibitor22 (Supplementary Fig. 6). However, we sought to determine if the increase in calcium signaling concomitant with magnetic field stimulation was GSK205 sensitive, which would suggest that the signal is TRPV4 dependent23. We thus stimulated and quantified the change in calcium fluorescence of mCherry+ Magneto2.0-p2A-mCherry transfected cells during magnetic field application both in the presence and absence of the specific TRPV4 inhibitor GSK205. We observed a magnetic field dependent calcium increase in the GSK205-untreated Magneto2.0 expressing cells compared to stimulated GSK205-treated cells (two-way ANOVA, p<0.0001) (Fig. 1h). Moreover, 70±5.1% (mean±SEM) of Magneto2.0+ cells responded to magnetic fields (n=3 coverslips, n=58 cells) with an average maximal change in calcium fluorescence of 29±9.8% (mean±SEM) during stimulation compared to only 6.5±0.9% (mean±SEM) for the GSK205-treated population (n=3 coverslips per condition, n=88 GSK205-treated cells, n=57 untreated cells, unpaired two-tailed t-test, t143=2.819, p=0.0055). Importantly, all observed changes in calcium fluorescence were noticeably improved over the poorly trafficked prototype channel (Supplementary Figs. 3, 4a). These data demonstrate that Magneto2.0 is a magnetically sensitive, genetically encoded actuator that can manipulate cellular activity in vitro.
Figure 1. Remote control of calcium signaling using Magneto2.0.
(a–e) In vitro calcium imaging micrographs of Fluo-4-loaded HEK293 cells before and after 3 pulses of 40–50 mT, 0.1 Hz, 90% duty cycle magnetic stimulation. (f) Quantification of calcium fluorescence fold change in response to the given condition. All experiments treated with magnetic fields except “no magnet” condition. Replicates are shown as individual coverslips equaling n=5 (TRPV4/ferritin), n=3 (No magnet), n=4 (Ruthenium red, (RR)), n=4 (Ca2+ free), n=5 (Magnet) coverslips per condition; total cells analyzed per condition are n=195 (TRPV4/ferritin), n=150 (No magnet), n=148 (RR), n=206 (Ca2+ free), n=396 (Magnet) with n>30 cells analyzed per coverslip. One-way ANOVA, Bonferroni post-test, (F4,16=7.268, p=0.0016). (g) Average temporal kinetics of all cells analzyed within a single coverslip per condition (n=48 (TRPV4/ferritin), n=50 (No magnet), n=45 (RR), n=45 (Ca2+ free), n=102 (Magnet)). Horizontal bar/horseshoe indicates magnetic field application. Two-way ANOVA, Bonferroni post-test, (F4,32490=199.1, p<0.0001), *p<0.05 for all time points from 250 s onward compared to “Magnet”. (h) Kinetics of calcium fluorescence fold change within mCherry+ cells in response to magnet in the presence or absence of the TRPV4 inhibitor, GSK205 (10 µM). n=3 coverslips per condition. Data represent all mCherry+ cells analyzed (n=88 GSK205-treated, n=57 untreated). Two-way ANOVA, Bonferroni post-test, (F39,5680=23.7, p<0.0001), ***p<0.0001 for all time points from 30 s onward. ***p<0.001, **p<0.01, *p<0.05. Data shown as mean±SEM.
Electrophysiological characterization of Magneto2.0 in the mammalian brain
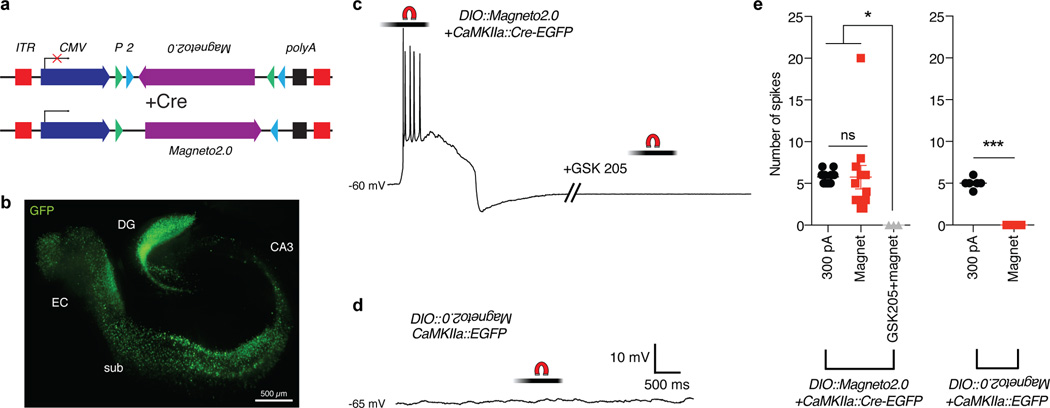
These preliminary experiments prompted us to precisely determine the temporal kinetics of Magneto2.0 activation since the future utility of Magneto2.0 is contingent on its rapid activation in response to magnetic fields in live tissues. To this end, we generated an adeno-associated virus (AAV) to express Magneto2.0 in mammalian cells under control of the cytomegalovirus (CMV) promoter using the double-floxed inverse open reading frame (DIO) approach (CMV::DIO-Magneto2.0). This strategy enables permanent Cre-dependent expression of a reversed lox site-flanked gene through Cre-lox mediated recombination (Fig. 2a)24. We bilaterally co-injected the medial entorhinal cortices (mECs) of WT mice with an AAV1 containing CMV::DIO-Magneto2.0 and an AAV9 carrying Cre recombinase fused to enhanced green fluorescent protein (EGFP) under control of the calcium/calmodulin-dependent protein kinase II alpha (CamKIIα) promoter (CamKIIα::Cre-EGFP), which will express Magneto2.0 in excitatory neurons (Fig. 2b). To test whether Magneto2.0 could elicit action potentials (APs) in neurons from brain slice preparations in response to magnetic fields, we recorded from EGFP+ neurons in the mEC of WT mice doubly transduced with AAVs carrying CMV::DIO-Magneto2.0 and CamKIIα::Cre-EGFP under whole-cell current clamp conditions. Upon application of a ~50 mT static magnetic field delivered by a NdFeB rare earth magnet, neurons in the mEC reliably fired a series of APs akin to spiking behavior evoked by injection of 300 pA of depolarizing current (Fig. 2c, Supplementary Fig. 7a). APs were elicited by both current injection and magnetic fields in 12/12 strongly EGFP+ neurons tested (n=5 mice; n=2 mice excluded due to low EGFP expression). Measurement of time to threshold and time to peak for APs evoked either by current injection or magnetic fields revealed no differences (Supplementary Fig. 7b). Membrane properties, such as resting membrane potential, AP amplitude, upstroke velocity, AP width, and firing threshold were similar between the two stimulation conditions (Supplementary Fig. 7c–g). As controls, we measured that magnetic stimulation initiated APs at a comparable rate to current injection (Supplementary Fig. 8a) and did not cause electrical interference in electrophysiology measurements (Supplementary Fig. 8b). To test if the magnetically evoked firing was due specifically to activation of TRPV4, we bathed brain slices in the selective TRPV4 antagonist GSK205 (n=3 neurons from 3 mice). After a 10-minute incubation with GSK205, magnetic stimulation failed to evoke APs (Fig. 2c, right panel), suggesting that the observed APs were due to Magneto2.0 activation. To determine whether magnetic stimulation affects mEC neurons not expressing Magneto2.0, we magnetically stimulated cells transduced with AAVs delivering CMV::DIO-Magneto2.0 and CamKIIα::EGFP, thus preventing Cre-dependent expression of Magneto2.0. We found that stimulation with magnetic fields did not evoke APs in non-Magneto2.0 expressing EGFP+ neurons of the mEC, although these neurons fired spike trains in response to injection with 300 pA of depolarizing current (n=6 neurons from 3 mice) (Fig. 2d, Supplementary Fig. 7h). In sum, we found that only Magneto2.0-expressing neurons of the mEC fired APs in response to magnetic field stimulation, and bath application of GSK205 blocked these responses (Fig. 2e). These data support the notion that activation of Magneto2.0 can rapidly and reversibly depolarize neurons leading to remote control over neural circuit dynamics.
Figure 2. Electrophysiological characterization of Magneto2.0 in mouse brain slices.
(a) Schematic of viral vector. ITR: inverted terminal repeats; CMV: cytomegalovirus promoter; P: loxP site; 2: lox2272 site. (b) EGFP immunostaining of a WT mouse brain slice showing areas of viral transduction. Hippocampus/entorhinal cortex was doubly transduced with two AAV vectors: AAV1 carrying CMV::DIO-Magneto2.0 and AAV9 carrying CaMKIIα::Cre-EGFP. DG: dentate gyrus, sub: subiculum, EC: entorhinal cortex. (c) Magnetically evoked spike train of a current-clamped mEC neuron transduced with CaMKIIα::Cre-EGFP and CMV::DIO-Magneto2.0. Neuron was stimulated with a 50 mT static magnetic field delivered by a permanent magnet. The graded bar represents the magnetic field experienced by neurons during the initiation and cessation of magnetic stimulation as the permanent magnet was brought toward the brain slice using a micromanipulator. Magnetically evoked APs were abolished by bath application of 10 µM GSK205. (d) Sample trace from an EGFP+ current-clamped mEC neuron transduced with CaMKIIα::EGFP and CMV::DIO-Magneto2.0 and thus, not expressing Magneto2.0. No action potentials are elicited in response to magnetic stimulation. (e) Quantification of the number of spikes compared between current injection (n=14 neurons, n=5 mice) and magnetic stimulation (n=12 neurons, n=5 mice) for EGFP+ cells expressing Magneto2.0. No magnetically induced APs are observed during bath application of GSK205 (n=3 neurons, n=3 mice) or when Magneto2.0 is not expressed (300 pA: n=6 neurons, Magnet: n=3 neurons, n=3 mice). All neurons examined are from a total of n=8 mice. Left panel: one-way ANOVA, Bonferroni post-test, (F2,26=4.301, p=0.0243). Right panel: unpaired two-tailed t-test, (t7=13.23, p<0.0001). ***p<0.001, *p<0.05, ns: not significant. Data shown as mean±SEM.
Genetically targeted remote magnetic control over zebrafish tactile behaviors
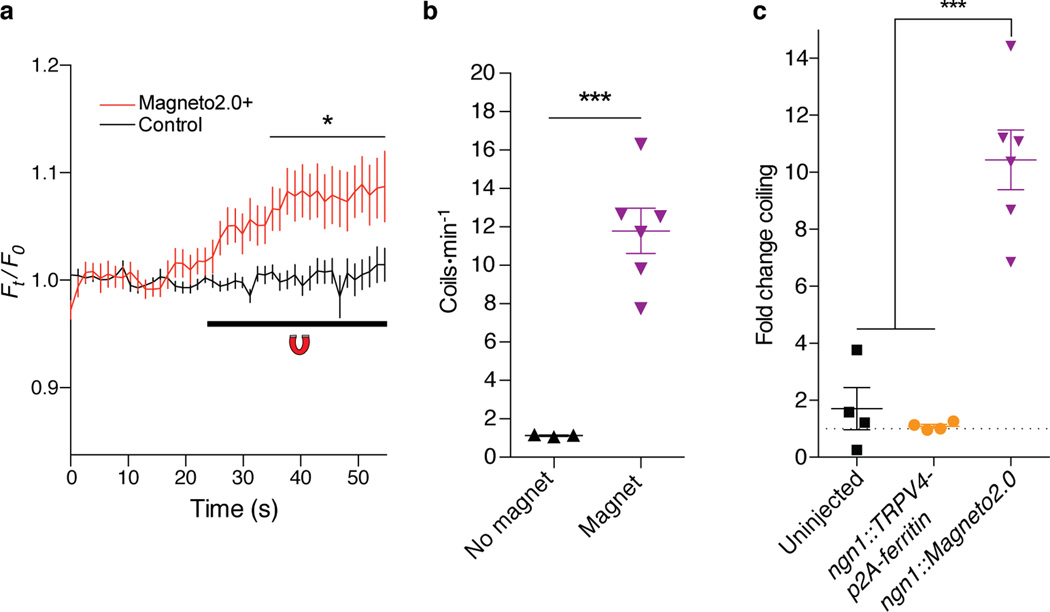
We next began validation of Magneto2.0 function in vivo. We first sought to remotely modulate a simple behavior of the zebrafish, Danio rerio. We transiently expressed Magneto2.0 in Rohon-Beard sensory neurons (5 Magneto2.0+ Rohon-Beard neurons per fish, n=9 fish), using regulatory sequences of the ngn1 promoter25,26. We identified mosaic zebrafish expressing Magneto2.0 in Rohon-Beard neurons by selecting for animals that also expressed a co-injectable fluorescent marker in the heart (Supplementary Fig. 9a). We sought to determine whether magnetic stimulation of zebrafish expressing Magneto2.0 led to an increase in calcium signaling within Rohon-Beard neurons. To this end, we performed GCaMP imaging of live, 48 hours post-fertilization (hpf) zebrafish larvae expressing Tg(s1020t::Gal4);Tg(UAS::GCaMP3);ngn1::Magneto2.0-p2A-mCherry, which enables detection of activated neurons through the genetically encoded calcium sensor, GCaMP327, which is expressed in ventral spinal cord neurons28. This transgenic combination enables direct visualization of calcium transients in response to magnetic stimulation through dual labeling of GCaMP3+ and mCherry+ Rohon-Beard neurons. We delivered a 50 mT static magnetic field via NdFeB rare earth magnets and observed an immediate increase in GCaMP3 fluorescence in stimulated Magneto2.0+, mCherry-labeled Rohon-Beard neurons but not in adjacent mCherry- neurons populating the spinal cord (Fig. 3a, Supplementary Fig. 10a). We determined that 17/20 mCherry+ neurons responded above the 6.9±0.15% (mean±SEM) average maximal fluorescence change of control, mCherry- cells (Supplementary Fig. 10a), suggesting that magnetic stimulation in vivo will reliably activate Magneto2.0+ neurons, consistent with both our calcium imaging and slice electrophysiology data. We next tested whether remote activation of Rohon-Beard neurons is sufficient to modulate the behavior of ngn1::Magneto2.0 zebrafish in the presence or absence of magnetic fields. We developed a magnetized behavioral testing arena formed by spacing two NdFeB rare earth magnets 6 mm apart (Supplementary Fig. 10b), which delivered a ten-fold greater magnetic field of ~500 mT to zebrafish larvae than the GCaMP assay. We hypothesized that even if only a few Rohon-Beard neurons were activated by Magneto2.0, the stereotypical escape response would nevertheless induce a coiling behavior, as demonstrated previously26,29. Indeed, in response to a 500 mT magnetic field, groups of 24 to 34 hours post fertilization (hpf) ngn1::Magneto2.0 expressing zebrafish larvae coiled more frequently compared to those not exposed to a field (Fig. 3b, Supplementary Movies 1–2). In contrast to ngn1::Magneto2.0 fish, which displayed an approximate ten-fold increase in coiling behavior upon magnetic field exposure, there was no observable change in this behavior for either control group—uninjected WT fish or ngn1::TRPV4-p2A-ferritin fish, which bicistronically express independent, unfused TRPV4 and ferritin moieties (Fig. 3c). Consistent with in vitro findings, fish expressing the Magneto prototype channel under control of the β-actin promoter exhibited a response that was five-fold smaller than that of fish expressing Magneto 2.0 (Supplementary Fig. 9b–d). Finally, we confirmed that Magneto2.0 expression did not disrupt normal peripheral projections of Rohon-Beard neurons by examining red fluorescent protein (RFP) expression in sensory neurons of Tg(isl1::rfp) fish and Tg(isl1::rfp);ngn1::Magneto2.0-IRES-nlsegfp chimeric fish (Supplementary Fig. 10c–f). Together, these results confirm that Magneto2.0 is a viable candidate for remotely controlling neuronal activity and animal behavior in vivo.
Figure 3. Magnetic control over zebrafish tactile behavior in vivo.
(a) Quantification of GCaMP3 fluorescence in mCherry+ Rohon-Beard sensory neurons and mCherry- spinal cord neurons in 48 hpf zebrafish larvae expressing ngn1::Magneto2.0-p2A-mCherry. n=20 mCherry+, n=33 mCherry- neurons from 8 stimulation experiments using n=5 zebrafish from 2 independent injection cohorts. Two-way ANOVA, Bonferroni post-test, (F42,2339=3.248, p<0.0001). *p<0.05 for all points from 35–55 seconds. (b) Coiling rate of 24–36 hpf ngn1::Magneto2.0 fish. Number of independent experiments for each condition is n=3 (No magnet) and n=6 (Magnet). n=26 (No magnet) and n=25 (Magnet) fish were used in respective conditions. Unpaired two-tailed t-test, (t7=6.152, p=0.0005). (c) Fold change in coiling of fish genotypes aged 24–36 hpf. Number of videos analyzing baseline coiling is n=3 per genotype, number of magnetic stimulation experiments include n=4 (Uninjected), n=4 (ngn1::TRPV4-p2A-ferritin), and n=6 (ngn1::Magneto2.0). Number of fish analyzed shown as (baseline, magnet) for each genotype is: Uninjected: (27, 18), ngn1::TRPV4-p2A-ferritin (17, 21), and ngn1::Magneto2.0 (26, 25). One-way ANOVA, Bonferroni post-test (F2,11=39.01, p<0.0001). Data pooled from 2 independent injection cohorts per genotype. ***p<0.001, *p<0.05. Data shown as mean±SEM.
Remote control of mammalian neural activity in freely behaving mice
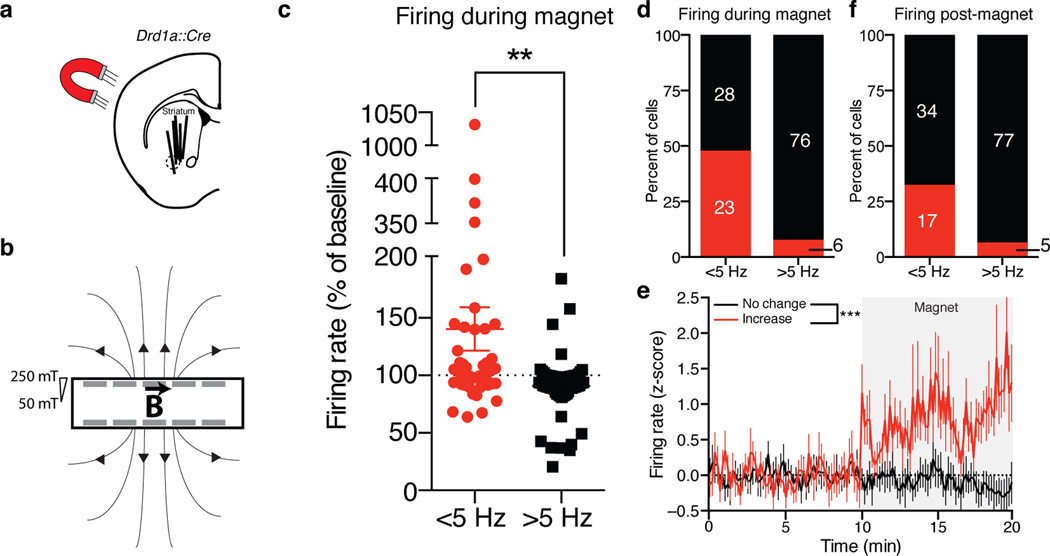
To determine if Magneto2.0 is capable of controlling mammalian neural activity in vivo, we performed electrophysiology measurements in freely behaving mice transduced with an AAV1 carrying CMV::DIO-Magneto2.0, which expresses Magneto2.0 in a Cre-dependent manner. We aimed to test if Magneto2.0 is capable of rapidly activating a large nucleus deep within the brain, which is more challenging when using optical actuators. To this end, we used mice expressing Cre recombinase under control of the dopamine receptor 1 promoter (Drd1a::Cre), which is expressed in approximately half of the medium spiny neurons (MSNs) of the striatum30. We then transduced striatal neurons of Drd1a::Cre mice with an AAV1 carrying Magneto2.0 and two weeks post-viral injection, we performed extracellular single-unit recordings with tetrode microdrives on Magneto2.0 expressing striatal cells in freely behaving mice and examined the effects of magnetic stimulation on neural firing (Fig. 4a). For this assay, we designed a magnetized chamber (23 cm×4 cm × 18 cm) consisting of NdFeB magnets embedded in the chamber walls (Fig. 4b) and quantified the firing rates of striatal neurons under three conditions: (1) at baseline without magnetic stimulation, (2) during exposure to 50–250 mT magnetic fields within the chamber, and (3) post-magnetic field exposure. We classified recorded cells into two main groups based on firing rate: slow-spiking (<5 Hz) and fast-spiking (>5 Hz) neurons with mean firing rates of 2.1±0.3 Hz (mean±SEM) and 8.6±0.6 Hz (mean±SEM), previously described as putative MSNs (either D1R+/D2R− or D1R−/D2R+) and GABAergic interneurons (D1R−), respectively31. Exposure of these mice to magnetic fields produced a 43.8±20.3% increase in the overall firing rate of slow-spiking putative MSNs (Fig. 4c–e). Importantly, the firing rate of putative GABAergic interneurons remained constant (Fig. 4c–d). Subsequent to magnetic stimulation, 66.7% of putative MSNs returned to baseline firing rates, while the putative interneuron firing rate again remained at baseline (Fig. 4f). Finally, we observed an increase in the firing rate of slow-spiking, but not fast-spiking, neurons of the striatum following systemic administration of the D1R agonist, SKF81297 (Supplementary Fig. 11a), suggesting that the D1R+ population responsive to magnetic fields are indeed slow-spiking neurons. Together, these data demonstrate that Magneto2.0 is capable of controlling neural firing in deep brain regions in response to magnetic fields.
Figure 4. Magnetogenetic control of the mammalian nervous system in vivo.
(a) Representation of magnetic stimulation and recording of D1R-expressing cells in the striatum of Drd1a::Cre mice. Solid lines indicate electrode placement from 5 mice; dashed circle indicates approximate injection area. (b) Cartoon of magnetized testing chamber, rare earth magnets (gray bars) are embedded in the walls, “B” represents magnetic field, magnetic field strength shown as gradient. (c) Quantification of single unit average firing rate during magnetic field exposure in freely behaving mice. n=51 <5 Hz neurons, n=81 >5 Hz neurons from 5 mice (n=66, n=30, n=25, n=7, n=4 cells from each mouse). Unpaired two-tailed t-test, (t130=3.210, p=0.0017). (d) Proportion of cells firing >5% over baseline during magnet exposure. (e) Standard score (z-score) over time for <5 Hz MSNs in d that fired >5% (red, n=23) vs. <5% (black, n=28). Two-way ANOVA (F1,5880=210.9, p<0.0001). Gray box represents stimulation in magnetized chamber. Dashed line shows baseline of no change. (f) Proportion of cells firing >5% over baseline post-magnet exposure. Data are shown as mean±SEM. ***p<0.001, **p<0.01.
Remote magnetogenetic control of D1R-mediated striatal reward valence
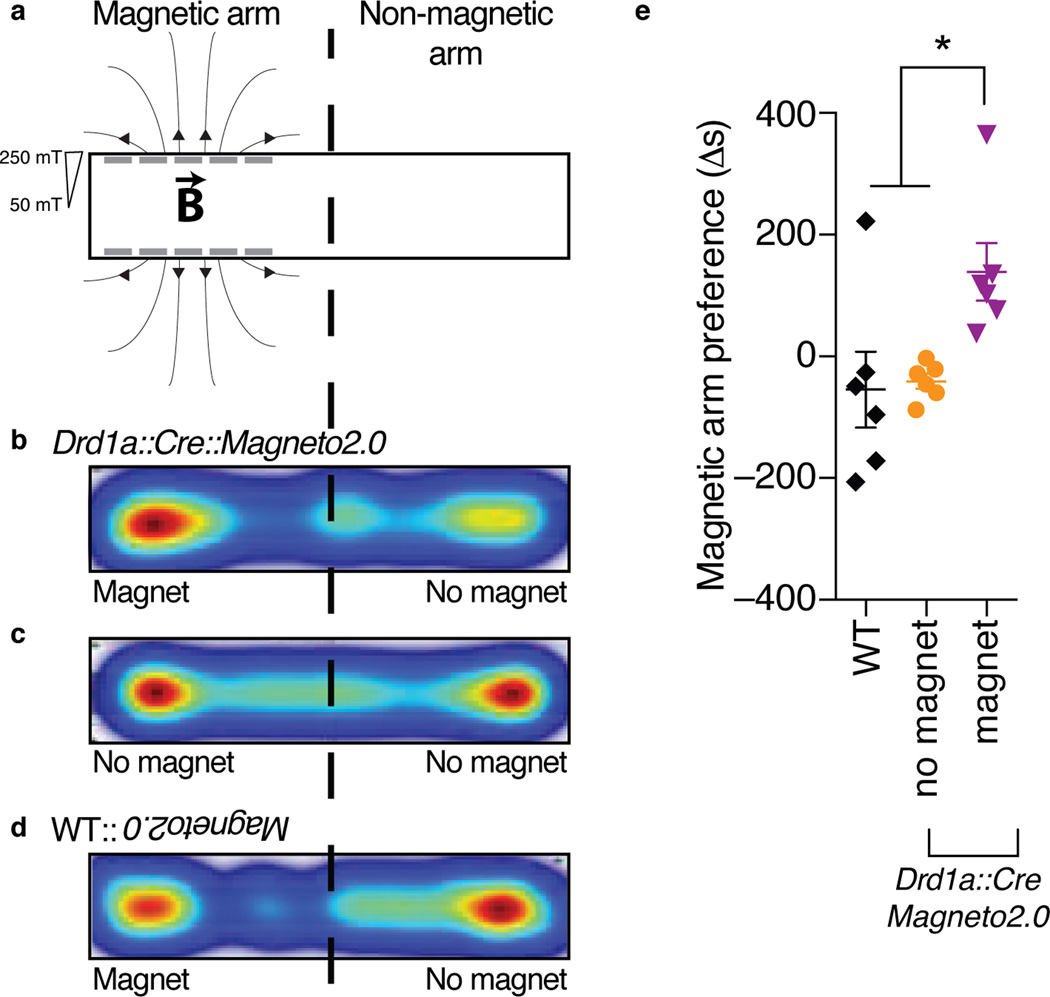
Ultimately, we sought to determine whether Magneto2.0 dependent control of neural activity in vivo could translate to control over complex mammalian reward behaviors regulated by dopamine signaling32. While optogenetic studies have implicated the dopaminergic signaling axis in causally mediating reward behavior33, it is unclear whether activation of postsynaptic D1R+ neurons is sufficient for controlling this effect. For instance, optogenetic stimulation of one subset of striatal D1R+ neurons is not causally responsible for induction of conditioned place preference (CPP)34. Conversely, studies using systemic pharmacological manipulations with D1R agonists confirm that activation of D1R+ neurons is sufficient to evoke CPP35,36, suggesting that broadly activating D1R+ neurons may cause reinforcing behaviors. Optogenetic techniques are intrinsically limited in the number of neurons that can be activated simultaneously via fiberoptic implants and pharmacological approaches lack genetic specificity. However, a magnetogenetic paradigm circumvents both obstacles simultaneously allowing resolution of this discrepancy with cell-type specificity and a real time behavioral output. We tested the sufficiency of D1R+ neurons in eliciting reward conditioning by unilaterally injecting the striata of WT and Drd1a::Cre mice with an AAV1 carrying CMV::DIO-Magneto2.0 and subjecting the mice to a real time place preference (RTPP) assay where they could choose between a magnetized arm, lined with eight permanent NdFeB magnets delivering a magnetic field gradient of 250–50 mT, and a non-magnetized arm (Fig. 5a). We observed that Magneto2.0 expressing Drd1a::Cre mice showed a significant preference for the magnetized arm of the RTPP chamber in contrast to WT mice (one-way ANOVA, p=0.0152), which exhibited no preference (Fig. 5b–e). Moreover, removal of the magnets from the chamber eliminated the preference of Magneto2.0 expressing Drd1a::Cre mice for either arm, a response identical to WT mice (Fig. 5c), demonstrating that RTPP is dependent on D1R stimulation. As a control, we measured no differences in overall locomotion between unilaterally injected WT and Drd1a::Cre mice using a modified open field assay (Supplementary Fig. 11b–c). These data show: (1) that broad activation of D1R+ neurons of the striatum is sufficient to control reward salience and (2) that Magneto2.0 can be used for remote control of complex mammalian behaviors mediated by deep brain nuclei in freely moving mice.
Figure 5. Activation of striatal D1R+ neurons is sufficient to control reward behavior.
(a) Cartoon of magnetized RTPP assay. (b–d) Representative heat maps of arm preference for each condition shown as time spent in a particular arm; mid-point of one mouse shown per map. (e) Difference in time spent in magnetic arm versus non-magnetic arm for WT and Drd1a::Cre mice (n=6 per genotype) transduced with an AAV1 carrying CMV::DIO-Magneto2.0. “No magnet” refers to non-magnetized RTPP chamber, “magnet” refers to magnetized chamber. WT mice were only tested in the magnetized chamber. One-way ANOVA, Bonferroni post-test, (F2,15=5.611, p=0.0152). Data are shown as mean±SEM. *p<0.05.
Discussion
In total, we have engineered and optimized a genetically encoded magnetogenetic actuator, Magneto2.0, and applied it to the nervous system in freely behaving animals. This is the first demonstration of bona fide magnetic control of the nervous system using engineered actuators, which we confirmed electrophysiologically and behaviorally using both zebrafish and mice. We have shown that Magneto2.0 remotely controls both neural firing rates and behavior on a rapid and physiologically relevant timescale, which is a prediction offered by the authors of an earlier study employing magnetogenetics to study insulin signaling13. Our single-component magnetogenetic system represents a significant advance in the ability to study neural circuits with relative ease as broad populations of genetically defined cells can be remotely activated in freely behaving animals. We applied Magneto2.0 to the study of reward behaviors to directly measure the behavioral consequences involved in remotely modulating large populations of cells participating in specific neural circuits37. Our findings also shed light on the sufficiency of D1R+ neurons to control reinforcing behaviors, which is consistent with the results of a recent study investigating D1R+ neuron necessity in these processes38. Magneto2.0 represents a new prototype for a novel class of magnetogenetic remote controlled actuators. While we initiated our actuator design using TRPV4 because of its small size and known pressure sensitivity14,39–41, Magneto suffers from the unique disadvantage of remaining sensitive to several endogenous stimuli known to activate TRPV415,16, a problem not encountered with many opto- or chemogenetic methods. Future studies will optimize Magneto2.0 such that it no longer responds to these stimuli and responds to magnetic fields only. In addition, it will be useful to understand if Magneto functions because of the mechanosensitive nature of TRPV4 or whether this property is immaterial to its magnetic activation. Continued optimization and utilization of this magnetogenetic actuator will position the field to better understand neural development, function, and pathology.
Online Methods
Mice information
All animal experiments were conducted in accordance with the University of Virginia Institutional Animal Care and Use Committee (IACUC). All mice were maintained on a C57Bl/6 background. Mice were housed in a vivarium on a 12-hour light/dark cycle. Mice were housed at between 1–3 mice per cage. Viral injection experiments were conducted starting at 8 weeks of age. All mice used in this study were injected between 8–10 weeks of age. Only male mice were used in this study.
Zebrafish husbandry
All animal studies were approved by the University of Virginia Institutional Animal Care and Use Committee (IACUC). Zebrafish strains used in this study were: AB*, Tg(isl:rfp), and Tg(s1020t::Gal4);Tg(UAS::GCaMP3). Embryos were raised at 28.5°C in egg water or embryo medium and staged according to hour post fertilization (hpf) or days post fertilization (dpf). Embryos of both sexes were used for experiments42.
Molecular biology
Molecular biology was performed using standard protocols. Plasmid DNA was purified using kits from Qiagen. Restriction enzymes were purchased from New England Biolabs. Amplification of template DNA was performed with Phusion Flash (Life Technologies, F-548) and sequenced by GeneWiz. For TRPV4 S4–S5 fusion proteins, site-directed mutagenesis using Quickchange II XL Site-Directed Mutagenesis (Agilent) was performed on TRPV4 to introduce a unique BamHI site, into which a successive series of DNA linkers was inserted to gradually expand the linker region flanking TRPV4 and ferritin.
Rat TRPV4 was obtained from Addgene vector: #45751, a gift from Robert Lefkowitz. To generate AAV expression vectors, we modified the Addgene vector: #35507, a gift from Karl Deisseroth. A CMV promoter was substituted in the #35507 vector, and a small pA sequence was used43 to minimize size of the vector. A human ferritin H-L fusion gene was designed according to a previous study18 and synthesized by IDT. Other than AAV vectors, mammalian expression vectors were maintained in the pcDNA3.0 backbone. Fish expression vectors were maintained in pDestTol2CG2 and all entry vector maps are freely available from (http://tol2kit.genetics.utah.edu). Relevant plasmids used in this study will be deposited in Addgene.
Magnets and magnetic field strength measurement
Electromagnets of varying sizes and strengths were purchased from Ebay (seller ID: pawnnew). Permanent N42 or N52 grade NdFeB magnets were purchased from CMS Magnetics via www.cmsmagnetics.com or http://www.amazon.com. Gaussmeters (AlphaLabs, Inc.) were used to determine the field strength of electromagnets over distance for each experiment. For the in vivo zebrafish and mice behavioral experiments using permanent NdFeB magnets, an online magnetic field calculator (K&J Magnetics) or a Gaussmeter (AlphaLabs, Inc.) was also used.
Cell transfection and cell culture
HEK293 cells were a gift from the University of Virginia tissue culture core. Cells used in this study were authenticated and checked for mycoplasma contamination. Cells were transfected using Lipofectamine 2000 (Invitrogen) according to standard protocols. Low passage (<40) HEK293 cells were transfected for 1–2 hours in well plates, trypsinized for 5 minutes using 10% trypsin, and replated onto poly-D lysine (50 µg/mL) and laminin-coated (1 µg/mL) glass coverslips in fresh DMEM:F12 media (Life Technologies) containing 1 mM non-essential amino acids (Gibco), 1 mM sodium pyruvate (Gibco), 10% FBS, and 1× penicillin/streptomycin (Gibco).
Microscopy
Imaging for calcium imaging and immunocytochemistry was performed on a Leica SP5 confocal with white light laser. Calcium imaging was performed using 10× magnification.
In vitro magnetic calcium imaging
Calcium imaging was performed largely as described previously16,44. Briefly, transfected cells were plated onto glass coverslips, incubated overnight in a humidified incubator kept at 37°C and 10% CO2. Cells were washed 3× with calcium imaging buffer (CIB) solution (105mM NaCl, 3mM KCl, 2.5mM CaCl2, 0.6mM MgCl2, 10mM HEPES, 1.2mM NaHCO3, 100mM mannitol, and 10mM glucose, adjusted to pH 7.45 with NaOH) and loaded with 3 µM Fluo-4 diluted in CIB for 30 minutes at 37°C. Cells were then washed 3× with CIB and de-esterified for 30–60 minutes at 37°C. Coverslips were then loaded into customized imaging chambers and imaged at 10× magnification for analysis. Ruthenium red (RR), a TRP channel pore blocker, (Sigma) was used at a concentration of 10 µM and cells were incubated with RR for ~2–3 minutes in the imaging chambers before imaging. For calcium-free media experiments, calcium in CIB was replaced with 10 mM EGTA, and cells were washed and incubated with calcium-free media. The TRPV4 specific antagonist, GSK205, was purchased from Calbiochem (616522) and used at a concentration of 10 µM. Cells were incubated in GSK205 for 15 minutes at 37°C before calcium imaging.
A magnetic stimulus was delivered using 3 cm electromagnets (purchased from eBay, sellerID: pawnnew) ruled for continuous duty, 12 VDC, 5 W, and 10 kg of pull-force. We situated the magnet directly above the imaging chamber during imaging. Using a Gaussmeter (AlphaLab Inc.), we calculated the magnetic field experienced by the cells (~1.25 cm away from the magnet) to be roughly 40–50 mT (Supplementary Fig. 1).
Imaging was performed by recording 30 seconds of baseline fluorescence and then turning on the magnet for 3–6 pulses of 10 seconds each (0.1 Hz, total time of 30–60 seconds, 90% duty cycle), using a standard DC powered delivery system. Coverslips were not analyzed if they significantly shifted during imaging.
Cells were randomly selected from an image field. Quantification was performed by averaging 30 seconds of baseline fluorescence measurements with no applied magnetic field followed by quantification of the largest three fluorescence values following magnetic stimulation. The three peak values were normalized to the average baseline fluorescence before magnetic stimulation to compute a relative fold change for each cell. Fold change was normalized to background by respectively scaling all values by the average fold change in the background (if applicable) during magnetic stimulation.
For time course analyses (Fig. 1g–h), fluorescence data for each cell was analyzed as a relative increase over time compared to the baseline fluorescence (30 seconds) prior to magnetic stimulation.
Thapsigargin calcium imaging
HEK cells were prepared for calcium imaging as above. Thapsigargin was purchased from Sigma (T9033) and used at a working concentration of 1 µM, diluted 1:1000 in CIB. A 30-second baseline of calcium fluorescence was recorded before direct application of 800 µL of thapsigargin into the calcium imaging chamber. Calcium fluorescence was recorded for 1 hour after thapsigargin addition (Supplementary Figure 6a).
Magneto2.0-expressing cells were treated with thapsigargin and incubated at 37°C for 30 minutes before calcium imaging as thapsigargin-induced calcium release remained steady at 30 minutes after application. Cells were stimulated with magnetic fields as above: 10-second pulses of 50 mT field for 30 seconds of total field exposure.
Immunocytochemistry
Cells plated on coverslips were washed 3× with 1× PBS, fixed in 4% PFA for 1 hour at RT, washed 3× with 1× PBS, and mounted on slides with Fluoromount-G with DAPI (Southern BioTech). Immunocytochemistry for each iteration of trafficking signals was performed on two independent populations of transfected HEK293 cells.
Fish injection
AB* or Tg(isl:rfp) embryos were injected at the one-cell stage with 1–2 nL of a working stock of 12.5 ng/µL DNA for each construct. At 24 hpf, embryos were screened for cmcl2::egfp+ transgenics. Imaging of cmcl2::egfp expression was performed on every zebrafish embryo examined (n>50 positive fish).
Zebrafish GCaMP3 live imaging
Tg(s1020t::Gal4);Tg(UAS::GCaMP3);ngn1::Magneto2.0-p2A-mCherry expressing zebrafish were mounted in 0.8–2% low melting point agarose and imaged on a Leica SP5 laser-scanning confocal microscope with a white light laser. Fish were imaged using a 40× objective with water immersion. After 30–60 seconds of baseline fluorescence readings, mounted zebrafish were stimulated by a ~50 mT magnetic field delivered by a permanent NdFeB rare earth magnet. The confocal pinhole was increased to 2 µm and the scan speed was approximately 1.3 seconds per frame. n=5 fish and n=8 stimulation experiments were analyzed from two independent pools of injections.
In vivo zebrafish imaging
Imaging was performed as described previously45. Briefly, a Quorum WaveFX-XI spinning disc confocal system (Quorum Technologies Inc.) was used, equipped with a 40× water objective (NA=1.1) on a motorized Zeiss AxioObserver ZI microscope. Images were processed with Metamorph. n=10 fish imaged per genotype.
Zebrafish behavioral tests
Injected fish were maintained on an AB* background strain. Zebrafish embryos were behaviorally tested between 24–34 hours post fertilization (hpf). Two 2”×0.5”×0.25” N52 grade NdFeB permanent magnets were oriented such that one south and one north pole were oriented towards the fish over a fixed distance of ~1 cm. Fish were maintained in egg water during the course of behavioral testing and a 30 fps video was taken using an Axio Zoom.V16 fluorescent stereo zoom microscope. Fish were randomly selected from their groups for behavioral analysis. The videos were manually scored by counting the number of coils made by each fish over the length of the video and normalized as a rate of coiling by dividing the number of coils by the length of the video. Length of original behavioral analysis is between 2–3 minutes per video and Supplementary Movies 1–2 are shown at 8× speed. Fish tested had no prior history of behavioral testing. Animals were tested once each. Exclusion criteria for analysis consisted of stereotypy such as continuous coiling during the recording of the movie. Two uninjected WT animals were excluded from the analysis given these criteria. Behavioral testing was performed during the day at consistent times (8am–4pm).
Zebrafish whole mount immunostaining
Zebrafish were fixed and immunostained according to the protocol described previously45. The antibody used was rabbit anti-GFP (Invitrogen, A-6455) at a dilution of 1:1000. The secondary antibody was donkey anti-rabbit Alexa 488 used at 1:600.
Stereotaxic injection
Striatum transduction
The AAV1, CMV::DIO-Magneto2.0-pA containing virus used in this study was produced in the University of Pennsylvania vector core. Four injections of 1 µL of ~5×1012 titer AAV1 virus were injected unilaterally into the striata of WT and D1R::Cre mice using a 30G Hamilton syringe, stereotaxic alignment system (Cartesian Research, Inc.), and automated delivery system (World Precision Instruments) while mice were under 2% isoflurane anesthesia on a heating pad. Unilateral injection was performed at (M/L: +1.6, A/P: +0.98) relative to Bregma and four 1 µL injections were performed at depths of −4.75, −3.75, −2.75, and −1.75 mm over 40 minutes at a rate of 100 nL/min. After the final injection, the syringe remained in the brain for 10 minutes, raised 0.5 mm where it remained for 5 minutes, then removed. Mice were administered 3 mg/kg ketoprofen post-injection and for 3 subsequent days and permitted to recover on a heating pad before being returned to their home cages.
mEC transduction
For expression of Magneto 2.0 into hippocampal and mEC neurons, C57Bl/6 mice (5–6 weeks old) were anesthetized with ketamine/dexmedetomidine solution and mounted on a stereotaxic apparatus. Anesthesia was maintained by inhaled isoflurane for the duration of the procedure. A small hole was opened in the skull, and a pulled glass micropipette was lowered to the target site (M/L: +/− 3.0, A/P: −3.0) at a depth of 2.0 mm. Mice were injected with an equivolume mixture of an AAV1 carrying CMV::DIO-Magneto2.0 and an AAV9 carrying CaMKIIa::EGFP-Cre (obtained from UPenn Vector Core) with titers of ~5×1012 and ~1×1013 infectious units per mL, respectively. 200 nL of virus was injected with pressure at a rate of ~50 nL/min. After injection the micropipette was maintained in place for 4 min before retraction. This procedure was repeated bilaterally. Mice were allowed to recover for at least 4 weeks following surgery before commencement of electrophysiology testing.
Brain slice electrophysiology
Horizontal brain slices were prepared as previously described46. For recordings, slices were held in a small chamber superfused with heated (32°C) oxygenated ACSF at 3 mL/min. For electrophysiology experiments, transduced mouse medial entorhinal cortex neurons were visually identified by EGFP fluorescence using a Zeiss Axioscope microscope (Zeiss, Oberkochen, Germany). Action potentials were evoked using a current injection step to 300 pA. To evoke action potentials via magnetic stimulus, a permanent NdFeB magnet (CMS Magnetics) was used delivering ~50 mT. The magnet was driven toward the EGFP+ neuron via a micromanipulator until it was approximately 1 cm from the cell. Action potential parameters were measured as previously described46.
Single unit recordings in vivo in freely moving mice
In vivo electrophysiology was performed largely as described previously6. HS-16 four-tetrode microdrives (Neuralynx) were implanted in anaesthetized mice by using stereotaxic coordinates for the striatum described above except two injections of 1 µL each were made at depths of −4.75 mm and −4.25 mm within the brain; the head stage was installed at an initial depth of −4 mm. After 2 weeks of recovery, mice were connected to a digital Lynx (10S) acquisition system through an HS-16 headstage preamplifier (Neuralynx), and signals were amplified and filtered (600–6000Hz). Data were acquired by using Cheetah acquisition software (Neuralynx). Baseline putative D1R neuron firing properties were recorded for 10 min in the non-magnetized arm of the custom-made place preference chamber, followed by 10 min in the magnetic arm of the chamber, and then 10 min of a second baseline recording period. Tetrodes were lowered 50µm daily during scanning for distinct units. Offline Sorter software (Plexon) cluster analysis was used to isolate units. Clustered waveforms were subsequently analyzed with MATLAB (MathWorks). Baseline activity recordings (10 min) were used to identify putative D1R neurons that exhibited firing rates below 5 Hz. Behavioral testing was performed at consistent times daily (9am–1pm) for 2–4 weeks. One mouse was excluded from this analysis because it failed to yield >n=3 units.
After the completion of these three recording sessions, the mice were injected with the D1R agonist SKF81297 (Cayman Chemical, diluted to 3 mg/kg in saline, injected i.p.). 15 minutes after the agonist had been administered, a final 10-minute recording period in the non-magnetized arm of the place preference chamber was completed. Drug injection experiments were performed only during a 5-day period following the triplicate recording procedure performed above (baseline, magnet, post-magnet). Data were not included in the triplicate analysis (Fig. 4) once a mouse had been injected with SKF81297. Data in Supplementary Fig. 11a using drug are from a single mouse.
Immunohistochemistry
Mice were perfused with 4% PFA in PBS. Brains were removed and post-fixed overnight at 4°C followed by dehydration in 30% sucrose for 2 days at 4°C. Brains were frozen in OCT and sectioned on a cryostat as 30 µm sections. As free-floating sections, tissue was washed 3× for 5 minutes with 0.3% PBS-T (Triton X-100), followed by blocking for 30 minutes in 5% donkey serum diluted in 0.3% PBS-T. Sections were then incubated with primary antibody diluted in blocking solution overnight at 4°C with agitation. The next day, sections were washed 3× for 5 minutes with 0.3% PBS-T followed by incubation with secondary antibody diluted in blocking solution for 2 hours at room temperature. Sections were washed 3× for 5 minutes in 0.3% PBS-T and mounted on slides.
Primary antibodies used in this study were: rabbit anti-TRPV4 (Santa Cruz, sc-98592) and rabbit anti-TRPV4 (Novus, NB110-74960). Secondary antibody used was: donkey anti-rabbit Alexa-488 (Invitrogen) at 1:500.
NOTE: The TRPV4 antibodies used showed significantly high background staining in WT and non-transduced tissue, making it difficult to distinguish between endogenous and virally-mediated TRPV4 expression.
Mouse behavioral testing
All testing was conducted was during the mouse light cycle at consistent times (9am–5pm).
Open field
A custom-built open-field chamber was constructed by A. Spano and M. Wheeler (23 cm × 23 cm), where four 10 cm diameter electromagnets fit into the floor, and were covered with a 0.5 cm wooden platform on which the mouse could walk. Each magnet was connected to an independent power supply delivering roughly 2.5A and 30V of power, and generating a magnetic field of roughly 150 mT. Mice were placed in the chamber for 5 minutes and baseline recordings of locomotion were measured. Magnets were turned on for 5 minutes to measure responses to the magnetic field. Each mouse was tested in the assay 1 time for a total of 10 minutes per mouse.
Real time place preference (RTPP)
The two arms of the assay were custom-built by A. Spano and M. Wheeler (4 cm wide (internal diameter) × 23 cm long). Five permanent NdFeB magnets (Four 2” × 0.5” × 0.25” magnets, one 1” × 0.5” × 0.25” magnet) were embedded into each wall of the magnetized arm, recessed at a depth of 1 cm. Each 2”×0.5”×0.25” magnet delivered roughly 250 mT and the magnetic field strength was roughly 50 mT in the center of the magnetized arm. The magnets were embedded at a height range of between 1.1 cm to 1.6 cm above the floor of the chamber to primarily expose the mice’s heads to the field. Mice were placed into the chamber in the center of the two arms and permitted to explore for 2 minutes before recording began. The testing session lasted a total of 10 minutes. The two arms appeared identical except for the presence/absence of magnets.
For experiments using Magneto-transduced Drd1a::Cre mice where the magnets were removed from the RTPP chamber, two cohorts of 3 mice each were used. In the first cohort, the mice were first exposed to the magnet on Day 1, then the magnets were removed and preference was assessed on Day 2. In the second cohort, Drd1a::Cre mice injected with AAV1 CMV::DIO-Magneto2.0 were trained in the chamber lacking magnets on Day 1, then tested with the magnetized chamber on Day 2. The magnetized/non-magnetized arms were transposed for each cohort to ensure that there was no preference for either side in the testing chamber.
Mouse behavioral data analysis
Mouse behaviors were measured using Ethovision XT 11 (Noldus), which is an automated tracking, recording, and measurement software package. Following each testing session of open field, linear velocity was measured (nose-point relative to center-point) with and without magnetic field for the open field assay. For RTPP, side preference was calculated as the percent of time a mouse spent in the magnetized vs. non-magnetized arm. For RTPP experiments where mice were exposed to the chamber without any magnets installed, the “magnetic arm” was chosen as the side where the magnet was placed in the testing session and numerical values were then calculated.
Statistical Methods
All statistical comparisons were performed using Prism 6 (GraphPad). No Omnibus normality test was performed for any of the data sets because sample sizes were small. Data were assumed to be normally distributed except in Fig. 4d, f and Supplementary Fig. 9c. Specific statistical tests are explicitly stated in the Figure Legends. No statistical methods were used to pre-determine sample sizes but our sample sizes are similar to those generally employed in the field6,13,33,44,47,48. No blinding was performed for data analysis or behavioral testing but automated and randomized quantification was performed where applicable.
A supplementary methods checklist is available.
Supplementary Material
Acknowledgments
We thank members of the Condron, Beenhakker, Kucenas, Patel, Deppmann, and Güler labs for helpful comments and suggestions. In particular, we are thankful for technical assistance provided by A. Morris, P. Neff, A. Rainwater, and S. Young. We are thankful for helpful comments on the manuscript from M. Caterina, I. Cheng, B. Condron, K. Gamage, and I. Provencio. We acknowledge the UVa Keck Center for Cellular Imaging staff for usage of the Leica confocal microscopy system (RR025616). This work was supported by 5T32GM008328, 5T32GM008136, a Neuroscience Center of Excellence fellowship, and a Wagner Fellowship (MAW); F32NS087791 (CJS); NIH – NINDS R01NS072212 (SK); NIH – NINDS R01NS075157 (MKP); NIH – NINDS R01NS072388 (CDD); and UVa startup funds (ADG).
Footnotes
Author Contributions
MAW and ADG designed the study. MAW, CJS, MO, BSB, AMP, RMG, RPG, AJS, and MPB performed the experiments. MAW, CJS, MO, BSB, AMP, RMG, MKP, CDD, and ADG analyzed the data. SK, MKP, CDD, and ADG supervised research. MAW and ADG wrote the manuscript with input from co-authors.
Competing Financial Interests Statement
There is no competing financial interest.
References
- 1.Zemelman BV, Lee Ga, Ng M, Miesenböck G. Selective photostimulation of genetically chARGed neurons. Neuron. 2002;33:15–22. doi: 10.1016/s0896-6273(01)00574-8. [DOI] [PubMed] [Google Scholar]
- 2.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 3.Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. Optical deconstruction of parkinsonian neural circuitry. Science. 2009;324:354–359. doi: 10.1126/science.1167093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sternson SM, Roth BL. Chemogenetic Tools to Interrogate Brain Functions. Annu. Rev. Neurosci. 2014:387–407. doi: 10.1146/annurev-neuro-071013-014048. [DOI] [PubMed] [Google Scholar]
- 5.Alexander GM, et al. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron. 2009;63:27–39. doi: 10.1016/j.neuron.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Güler AD, et al. Transient activation of specific neurons in mice by selective expression of the capsaicin receptor. Nat. Commun. 2012;3:746. doi: 10.1038/ncomms1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernstein JG, Garrity Pa, Boyden ES. Optogenetics and thermogenetics: Technologies for controlling the activity of targeted cells within intact neural circuits. Curr. Opin. Neurobiol. 2012;22:61–71. doi: 10.1016/j.conb.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hughes S, McBain S, Dobson J, El Haj AJ. Selective activation of mechanosensitive ion channels using magnetic particles. J. R. Soc. Interface. 2008;5:855–863. doi: 10.1098/rsif.2007.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang H, Delikanli S, Zeng H, Ferkey DM, Pralle A. Remote control of ion channels and neurons through magnetic-field heating of nanoparticles. Nat. Nanotechnol. 2010;5:602–606. doi: 10.1038/nnano.2010.125. [DOI] [PubMed] [Google Scholar]
- 10.Stanley Sa, et al. Radio-wave heating of iron oxide nanoparticles can regulate plasma glucose in mice. Science. 2012;336:604–608. doi: 10.1126/science.1216753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stanley Sa, Sauer J, Kane RS, Dordick JS, Friedman JM. Remote regulation of glucose homeostasis in mice using genetically encoded nanoparticles. Nat. Med. 2015;21:92–98. doi: 10.1038/nm.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen R, Romero G, Christiansen MG, Mohr A, Anikeeva P. Wireless magnetothermal deep brain stimulation. Science. 2015;347:1477–1480. doi: 10.1126/science.1261821. [DOI] [PubMed] [Google Scholar]
- 13.Stanley Sa, Sauer J, Kane RS, Dordick JS, Friedman JM. Remote regulation of glucose homeostasis in mice using genetically encoded nanoparticles. Nat. Med. 2015;21:92–98. doi: 10.1038/nm.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loukin S, Zhou X, Su Z, Saimi Y, Kung C. Wild-type and brachyolmia-causing mutant TRPV4 channels respond directly to stretch force. J. Biol. Chem. 2010;285:27176–27181. doi: 10.1074/jbc.M110.143370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liedtke W, et al. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell. 2000;103:525–535. doi: 10.1016/s0092-8674(00)00143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Güler AD, et al. Heat-evoked activation of the ion channel, TRPV4. J. Neurosci. 2002;22:6408–6414. doi: 10.1523/JNEUROSCI.22-15-06408.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stanley S. Biological nanoparticles and their influence on organisms. Curr. Opin. Biotechnol. 2014;28:69–74. doi: 10.1016/j.copbio.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 18.Iordanova B, Robison CS, Ahrens ET. Design and characterization of a chimeric ferritin with enhanced iron loading and transverse NMR relaxation rate. J. Biol. Inorg. Chem. 2010;15:957–965. doi: 10.1007/s00775-010-0657-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lei L, et al. A TRPV4 channel C-terminal folding recognition domain critical for trafficking and function. J. Biol. Chem. 2013;288:10427–10439. doi: 10.1074/jbc.M113.457291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hofherr A, Fakler B, Klöcker N. Selective Golgi export of Kir2.1 controls the stoichiometry of functional Kir2.x channel heteromers. J. Cell Sci. 2005;118:1935–1943. doi: 10.1242/jcs.02322. [DOI] [PubMed] [Google Scholar]
- 21.Gradinaru V, et al. Molecular and Cellular Approaches for Diversifying and Extending Optogenetics. Cell. 2010;141:154–165. doi: 10.1016/j.cell.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lytton J, Westlin M, Hanley MR. Thapsigargin inhibits the sarcoplasmic or endoplasmic reticulum Ca-ATPase family of calcium pumps. J. Biol. Chem. 1991;266:17067–17071. [PubMed] [Google Scholar]
- 23.Phan MN, et al. Functional characterization of TRPV4 as an osmotically sensitive ion channel in porcine articular chondrocytes. Arthritis Rheum. 2009;60:3028–3037. doi: 10.1002/art.24799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andermann P, Ungos J, Raible DW. Neurogenin1 defines zebrafish cranial sensory ganglia precursors. Dev. Biol. 2002;251:45–58. doi: 10.1006/dbio.2002.0820. [DOI] [PubMed] [Google Scholar]
- 26.Douglass AD, Kraves S, Deisseroth K, Schier AF, Engert F. Escape Behavior Elicited by Single, Channelrhodopsin-2-Evoked Spikes in Zebrafish Somatosensory Neurons. Curr. Biol. 2008;18:1133–1137. doi: 10.1016/j.cub.2008.06.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tian L, et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat. Methods. 2009;6:875–881. doi: 10.1038/nmeth.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wyart C, et al. Optogenetic dissection of a behavioural module in the vertebrate spinal cord. Nature. 2009;461:407–410. doi: 10.1038/nature08323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sagasti A, Guido MR, Raible DW, Schier AF. Repulsive Interactions Shape the Morphologies and Functional Arrangement of Zebrafish Peripheral Sensory Arbors. Curr. Biol. 2005;15:804–814. doi: 10.1016/j.cub.2005.03.048. [DOI] [PubMed] [Google Scholar]
- 30.Hersch SM, et al. Electron microscopic analysis of D1 and D2 dopamine receptor proteins in the dorsal striatum and their synaptic relationships with motor corticostriatal afferents. J. Neurosci. 1995;15:5222–5237. doi: 10.1523/JNEUROSCI.15-07-05222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berke JD, Okatan M, Skurski J, Eichenbaum HB. Oscillatory Entrainment of Striatal Neurons in Freely-Moving Rats. Neuron. 2004;43:883–896. doi: 10.1016/j.neuron.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 32.Wise Ra. Dopamine, learning and motivation. Nat. Rev. Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 33.Tsai H-C, et al. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009;324:1080–1084. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lobo MK, et al. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science. 2010;330:385–390. doi: 10.1126/science.1188472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zengin-Toktas Y, et al. Motivational properties of D2 and D3 dopamine receptors agonists and cocaine, but not with D1 dopamine receptors agonist and l-dopa, in bilateral 6-OHDA-lesioned rat. Neuropharmacology. 2013;70:74–82. doi: 10.1016/j.neuropharm.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 36.Gore BB, Zweifel LS. Genetic Reconstruction of Dopamine D1 Receptor Signaling in the Nucleus Accumbens Facilitates Natural and Drug Reward Responses. J. Neurosci. 2013;33:8640–8649. doi: 10.1523/JNEUROSCI.5532-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stuber GD, Britt JP, Bonci A. Optogenetic modulation of neural circuits that underlie reward seeking. Biol. Psychiatry. 2012;71:1061–1067. doi: 10.1016/j.biopsych.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jeong JW, et al. Wireless Optofluidic Systems for Programmable In Vivo Pharmacology and Optogenetics. Cell. 2015;162:662–674. doi: 10.1016/j.cell.2015.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O’Neil RG, Heller S. The mechanosensitive nature of TRPV channels. Pflugers Arch. 2005;451:193–203. doi: 10.1007/s00424-005-1424-4. [DOI] [PubMed] [Google Scholar]
- 40.Liedtke W, Kim C. Functionality of the TRPV subfamily of TRP ion channels: add mechano-TRP and osmo-TRP to the lexicon! Cell. Mol. Life Sci. 2005;62:2985–3001. doi: 10.1007/s00018-005-5181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matthews BD, et al. Ultra-rapid activation of TRPV4 ion channels by mechanical forces applied to cell surface beta1 integrins. Integr. Biol. (Camb) 2010;2:435–442. doi: 10.1039/c0ib00034e. [DOI] [PMC free article] [PubMed] [Google Scholar]
Methods-only References
- 42.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 43.McFarland TJ, et al. Evaluation of a novel short polyadenylation signal as an alternative to the SV40 polyadenylation signal. Plasmid. 2006;56:62–67. doi: 10.1016/j.plasmid.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 44.Wheeler Ma, et al. TNF-α/TNFR1 Signaling Is Required for the Development and Function of Primary Nociceptors. Neuron. 2014;82:587–602. doi: 10.1016/j.neuron.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith CJ, Morris AD, Welsh TG, Kucenas S. Contact-Mediated Inhibition Between Oligodendrocyte Progenitor Cells and Motor Exit Point Glia Establishes the Spinal Cord Transition Zone. PLoS Biol. 2014;12:e1001961. doi: 10.1371/journal.pbio.1001961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hargus NJ, Nigam A, Bertram EH, Patel MK. Evidence for a role of Nav1.6 in facilitating increases in neuronal hyperexcitability during epileptogenesis. J. Neurophysiol. 2013;110:1144–1157. doi: 10.1152/jn.00383.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Quintana A, et al. Lack of GPR88 enhances medium spiny neuron activity and alters motor- and cue-dependent behaviors. Nat. Neurosci. 2012;15:1547–1555. doi: 10.1038/nn.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen S, Chiu CN, McArthur KL, Fetcho JR, Prober Da. TRP channel mediated neuronal activation and ablation in freely behaving zebrafish. Nat. Methods. 2015;8:1–7. doi: 10.1038/nmeth.3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.