Abstract
Mesenchymal stem cells (MSC) hold promise in promoting vascular regeneration of ischemic tissue in conditions like critical limb ischemia of the leg. However, this approach has been limited in part by poor cell retention and survival after delivery. New biomaterials offer an opportunity to localize cells to the desired tissue after delivery, but also to improve cell survival after delivery. Here we characterize the mechanical and microstructural properties of a novel hydrogel composed of pooled human platelet lysate (PL) and test its ability to promote MSC angiogenic activity using clinically relevant in vitro and in vivo models. This PL hydrogel had comparable storage and loss modulus and behaved as a viscoelastic solid similar to fibrin hydrogels despite having 1/4-1/10th the fibrin content of standard fibrin gels. Additionally, PL hydrogels enabled sustained release of endogenous PDGF-BB for up to 20 days and were resistant to protease degradation. PL hydrogel stimulated pro-angiogenic activity by promoting human MSC growth and invasion in a 3D environment, and enhancing endothelial cell sprouting alone and in co-culture with MSCs. When delivered in vivo, the combination of PL and human MSCs improved local tissue perfusion after 8 days compared to controls when assessed with laser Doppler perfusion imaging in a murine model of hind limb ischemia. These results support the use of a PL hydrogel as a scaffold for MSC delivery to promote vascular regeneration.
Keywords: Platelet lysate, Mesenchymal Stem Cell, Angiogenesis, Cellular therapy, Cell Scaffold
Graphical abstract
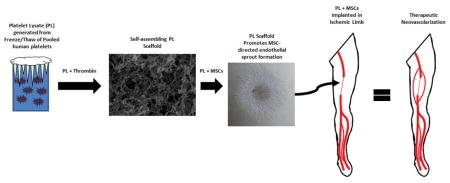
1.0 Introduction
Regenerative therapies hold great promise for improving the treatment of patients with disabling chronic diseases from tissue ischemia, such as critical limb ischemia (CLI). CLI is the most severe form of peripheral arterial disease (PAD), whereby the tissues of the leg do not have sufficient perfusion to meet resting tissue demands. This causes rest pain, tissue loss, or gangrene. These patients require timely revascularization in order to limit their risk of major amputation [1–4]. However, approximately half of CLI patients do not have traditional revascularization options [5]. Thus novel approaches to promote vascular regeneration, such as cellular therapy, are critically needed [6]. Mesenchymal stem cells (MSCs) are a particularly attractive cell to stimulate vascular regeneration as they promote neovascularization [7,8] and have robust paracrine activity on surrounding cells, including endothelial cells (EC) [9].
Despite generally optimistic summaries of cellular and regenerative therapy trials in PAD [10,11], the effectiveness of cellular therapies in preventing amputations remains to be proven [11]. This is likely due in part to poor cellular retention after intravascular delivery (~1% 2 hours after arterial injection) [12] or direct injection into the muscular tissue (11% 1 hour after injection into pig hearts) [13]. Limited retention of cells after delivery provides opportunities for the use of biomaterials to help localize these cells and retain them in the desired tissue. Advancing technologies enable biomaterials that may be designed to promote cell survival and engraftment into these tissues for sustained function.
In order to meet the clinical needs of cellular therapy with biomaterial solutions, we have developed a novel hydrogel from human platelet lysate (PL). PL is a promising non-xenogenic serum supplement designed for the expansion of human MSCs that replaces fetal bovine serum [14]. We have shown that PL significantly increases human MSC expansion capacity compared to fetal bovine serum and can re-stimulate senescing MSCs [15]. In addition to PL’s nutritive effect on MSCs, PL is rich in a variety of endogenous growth factors [16], including platelet derived growth factor-BB (PDGF-BB) [17]. PDGF-BB has recently been shown to improve MSC engraftment into tissues [18], therefore PL is a nearly ideal supplement that when incorporated into a gel could encourage MSC retention, viability, and sustained neovascular activity.
The objective of this study was to develop and characterize the mechanical properties of a PL scaffold and assess the biologic effect of PL gel on MSCs and ECs in vitro and in vivo. Here we test the hypotheses that PL gel has desirable mechanical properties for cell delivery and supports MSC ingrowth. Additionally we examine the ability of PL to enhance the pro-angiogenic activity of MSCs on ECs in 3D culture. Finally, we test whether MSCs in PL gel will promote neovascularization in vivo in an immunocompromised murine model of hind limb ischemia (HLI).
2.0 Materials and Methods
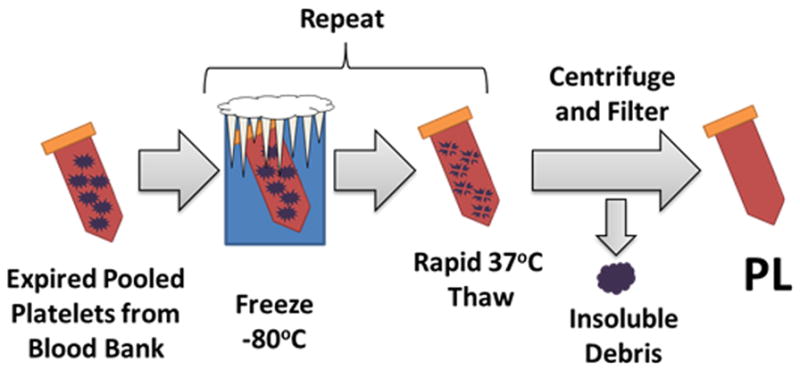
2.1 Production of Fibrinogen-rich Platelet Lysate Hydrogels
Two units of expired human platelets were obtained from the Emory University blood bank through an IRB approved research protocol. The platelets were pooled and exposed to two sequential freeze/thaw cycles [freezing at −80 ° C for 48 hours, rapidly thawing at 37 ° C for 8 hours] followed by centrifugation at 1500xg for 10 minutes. The supernatant was collected and stored at −20 ° C. Prior to use, the platelet lysate was thawed at 37 ° C, centrifuged at 10,000g for 10 minutes in 1.5 mL microcentrifuge tubes, and sequentially filtered through 0.45 and 0.2-micron syringe tip filters. Fibrinogen content was determined using an ELISA kit for human fibrinogen (Molecular Innovations) [17].
For hydrogel production, an activating solution was prepared containing αMEM (Corning), bovine thrombin (Sigma), and CaCl2 (Sigma). Cells were suspended in αMEM at pre-specified concentrations and added to the activating solution. Hydrogels were polymerized by adding PL to the activating solution in a 1:1 ratio with a final concentration of CaCl2 and thrombin at 5 mM and 2 U/mL in a 50% PL gel, respectively. For the control fibrin hydrogel, fibrinogen from human plasma (Sigma) was dissolved in αMEM then mixed with activating solution [final fibrinogen concentration was 2.5 mg/mL and 1.0 mg/mL for high and low concentration fibrin gels, respectively]. The 2.5 mg/mL fibrin-only hydrogel was chosen as a control because it represents a physiologically relevant concentration of fibrinogen that is equivalent to that found in human plasma. Additionally, the use of 2.5 mg/ml fibrin gels has been used extensively in the hydrogel invasion assay described below [19]. The 1.0 mg/mL fibrin gels were selected as an additional control to more closely represent the concentration of fibrinogen found in PL solution. The 1.0 mg/ml fibrin hydrogels were mechanically equivalent to the 50% PL hydrogels, and hydrogels containing less than 1.0 mg/mL fibrinogen either would not form hydrogels or formed hydrogels that lacked the durability to be utilized in our assays. The addition of CaCl2 to fibrin-only hydrogels caused precipitation of calcium phosphate, so for generation of fibrin-only hydrogels an activating solution was prepared containing only thrombin in αMEM (which contains of CaCl2 at a concentration of 1.80 mM).
2.2 Rheology
A Physica MCR 501 cone and plate rheometer (Anton-Paar, Graz, Austria) was used (2.014° cone angle and 24.960 mm tool diameter) to measure the viscoelastic properties of fibrin and PL gels. Gels were formulated as described above. Strain sweeps from 0.1–100% strain at 1Hz were performed to establish the linear regime. Frequency sweeps from 0.01–1Hz in the linear regime at 0.5% strain were then performed to obtain the frequency-dependence of the storage modulus (G’) and loss modulus (G”). Average storage moduli were calculated from the average value over the frequency range for three different gel constructs.
2.3 Confocal Imaging
PL and fibrin hydrogels were formed as described above at a volume of 0.5 mL and polymerized in 24-well 1.5 glass bottom tissue culture plates[20,21]. Laser scanning confocal microscopy (LSM 700, Carl Zeiss, Inc.) with a 63X oil immersion objective was performed for visualization of the fibrin network using 5% FITC-labeled fibrinogen (Sigma). At least 3 gels per group were imaged at a distance at least 25 μm above the glass interface to avoid heterogeneities in the network near contact with the glass surface.
2.4 Scanning Electron Microscopy
40 μL hydrogels were formed as described above and allowed to polymerize at 37 ° C for 1 hour, then fixed overnight in 2% glutaraldehyde. After fixation, samples were dehydrated with an ethanol gradient, critically point dried, and sputter coated with gold-palladium. SEM images were acquired at 20,000X magnification using a Topcon DS-130F Field Emission Scanning Electron Microscope.
2.5 FITC-Dextran Diffusion
Solute diffusion through PL and fibrin hydrogels was assessed as previously described [22]. Briefly, 0.5 mg/mL 70 kDa FITC-dextran (Sigma) was added to 0.5 mL hydrogels in microcentrifuge tubes prior to polymerization. The hydrogels were then allowed to polymerize and covered with 0.5 mL of phosphate buffered saline (PBS, pH 7.4) with or without 10 μg/mL of aprotinin. The PBS was collected off of each hydrogel and replaced with fresh PBS at predetermined time points for up to 20 days. Each time point was performed in triplicate. The concentration of FITC-dextran released into the supernatant was determined by comparing sample fluorescence to a standard curve using a Synergy-HT microplate leader with 485/20 Ex, 528/20 Em filter set.
2.6 Protein Release
0.5 mL PL hydrogels were prepared in 2.0 mL microcentrifuge tubes and covered with 0.5 mL of PBS (pH 7.4) with or without 10 μg/mL of aprotinin and stored at 37 ° C. At predetermined time points for up to 20 days the PBS was collected off of each hydrogel and replaced with fresh PBS. Total protein concentration from collected samples was determined using protein assay reagent dye (Biorad). Absorbance at 490 nm was measured in collected samples using a Synergy-HT microplate reader (Biorad). To quantify PDGF-BB release from PL hydrogels, total PDGF-BB in the collected samples was quantified using a colorimetric ELISA (Ray Biotech). Three independent replicates were performed for all conditions.
2.7 Hydrogel Degradation
0.5 mL 50% PL or fibrin hydrogels were prepared with the addition of 100 μg Alexa Fluor 488 conjugated fibrinogen. Cell free hydrogels were cast in 24-well tissue culture plates and covered with PBS (with or without 10 μg/mL of aprotinin) At predetermined time points for up to 7 days the supernatant was collected off of each hydrogel and replaced with PBS. At completion of the time course the scaffold was digested with Dispase solution (Stem Cell Technologies). The concentration of Alexa fluor 488 conjugated fibrin released in each sample was determined by comparing sample fluorescence to a standard curve using a Synergy-HT microplate leader with 485/20 Ex, 528/20 Em filter set. Three independent replicates were performed for all conditions.
2.8 Cell Culture
Human MSCs obtained through an IRB approved protocol. Human MSCs were subcultured under standard conditions with αMEM (Corning) containing 1%L-glutamine (Gibco), 1% pen/strep (Gibco), and standard serum supplementation with 10% fetal bovine serum (FBS; Atlanta Biological Inc). MSCs were used at passage 5–8 for all experiments. Pooled Human Umbilical Vein Endothelial Cells (HUVECs) were obtained from Genlantis and subcultured with full endothelial cell media per the distributors recommendations. HUVECs were used at passage 3–5 for all experiments.
2.9 Proliferation Assay
100 μL hydrogels containing 2x103 cells were cast in flat-bottom tissue culture treated 96-well microplates. Hydrogels were prepared containing 50% PL, 1.0 or 2.5 mg/mL fibrinogen and either MSCs or HUVECs. Hydrogels were covered with 150 μL of serum free αMEM. Control wells were prepared by plating 2x103 cells per well in a monolayer in serum free media, media with 5% PL or EC media. All media conditions were supplemented with 10 μg/mL of aprotinin, which is required for our fibrin gels. Samples were incubated at 37 ° C for 3, 5, and 7 days. At each time point, 50 μL of Celltiter 96 Aqueous reagent (Promega) was added to each well and allowed to incubate for 4 hours. A Synergy-HT microplate reader was used to measure absorbance at 490 nm to quantify metabolic activity. Each treatment group was normalized to MSCs or HUVECs grown in a monolayer under serum free conditions on the day the assay was performed, i.e. day 3 MSCs were normalized to MSCs grown in quiescent media for 3 days. For each group, 8 independent replicates were performed twice.
2.10 3-D Angiogenesis Assay
A previously described EC sprouting assay [19] was used to assess outgrowth of cell pellets into hydrogels. Briefly, cell pellets were created with a 1:1 ratio (total 2x105 cells) of MSCs and HUVECs in wells of a 96-well suspension culture microplate pre-blocked with a solution of 2% HSA. Pellets were allowed to form overnight, and then embedded in 400 μL fibrin or PL hydrogels using a nylon ring support. To allow for positioning of the cell pellet within the fibrin hydrogel a final thrombin concentration of 0.5 U/mL was used to slow polymerization times. Hydrogels were covered with 1.0 mL of serum free αMEM. In order to delineate EC invasion in co-culture, HUVECs were labeled with PKH26 (Sigma) and imaged under fluorescent microscopy. Bright field and fluorescent images were taken at 24 hour intervals for 3 days using an Olympus inverted fluorescent microscope. Total cell invasion, which is predominantly MSC invasion in this assay, was quantified under bright field microscopy. EC sprouting was quantified under fluorescent imaging. To quantify sprout length, a 12-segment radial grid overlay was generated for each image, and the distance from pellet center to furthest sprout border was quantified in each segment then averaged for the image. All image analyses were performed using a custom Matlab program. In order to test the effect of PL gel on either MSCs or ECs, single cell pellets (2x105 MSCs or 2x105 HUVECs) were formed and tested in the same manner. At least 4 replicates were tested per condition, and each experiment was performed twice.
2.11 Transwell Migration Assay
PL or fibrin hydrogels (600 μL) were cast in the bottom of 24-well cell culture plates either without or embedded with 20,000 human MSCs/mL . Pre-hydrated transwell inserts (Corning) with a 6.5 μm thickness and 8.0 μm pore size were then placed onto the top of the gels, and 100 μL of serum free media containing 50,000 HUVECs was placed on top of the insert. After 24 hours, the inserts were removed and stained with crystal violet Images were obtained of the bottom of the inserts at 10x magnification and the number of stained cells was counted. Each group was performed in quadruplicate and repeated twice. Values were reported as number of cells migrated per high power field.
2.12 Hind Limb Ischemia Model
Murine work was done under an IACUC approved protocol in an AALAAC accredited institution. Briefly, 12 week old male NOD-SCID mice underwent right sided hind limb ischemia via ligation and removal of their femoral artery as described prior [23] . Prior to surgery all fur was removed on bilateral hind limbs with depilatory cream. Mice were segregated into groups of PL + MSCs; Pl alone; saline + MSCs, and saline alone; N=4 in all groups. The saline vector was chosen because this is used in clinical trialing of intramuscular injections for peripheral arterial disease (PAD). Treatment volumes were 200 μL for all groups. There were 1,000,000 human MSCs delivered with a 23 gauge needle into each limb treated with PL + MSCs, Saline +MSCs, PL alone, or saline alone. Animals then underwent laser Doppler perfusion imaging (LDPI) on postoperative days 1 and 8. An LDPI with an 810 nm LASER (MooreLDI, Moore Instruments) was used to assess perfusion in the ischemic and nonischemic legs following arterial ligation. Scanning distance was 21 cm with a scan speed of 4 ms/pixel and a resolution of 256x256 pixels was used. Mean perfusion was quantified on each limb by setting an area of interest over the entire leg and foot, and then a separate are of interest over the gastrocnemius muscle (the ischemic portion of the leg). The results were reported as mean perfusion ration of the ischemic limb (IL) to the nonischemic limb (NIL).
2.12 Statistical Analysis
A one and two-way Analysis of variance (ANOVA) with Tukey’s test was used for multiple comparisons. A P value less than 0.05 was considered statistically significant. All statistical analyses were performed using Graphpad Prism statistical software package.
3.0 Results
3.1 PL rapidly self assembles into 3D hydrogels with a dense fibrin network
PL was generated by exposing human platelets to sequential freeze-thaw cycles with a rapid warming phase, which prevents the formation of cryoprecipitate (Figure 1A). The fibrinogen concentration in the resultant PL solution determined with ELISA was 454 (+/− 75) μg/mL. We chose a solution of 50% PL for generation of our scaffold, so fibrin content in the gels was ~225 μg/mL. The gel was polymerized by the addition of thrombin and calcium chloride to a 50% PL solution at 37 C°.
Figure 1.
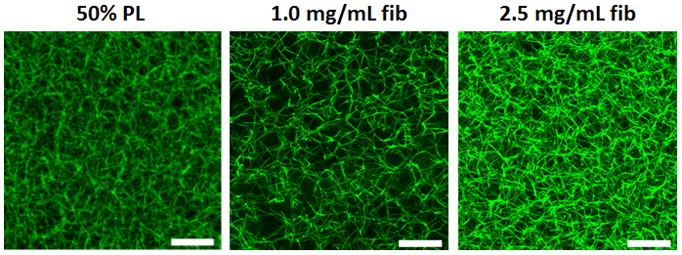
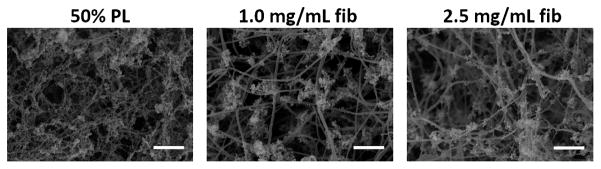
PL hydrogels self-assemble with thrombin activation. A) Fibrinogen rich platelet lysate (PL) was generated by exposing human to sequential rounds of freeze thaw cycles with a rapid warming phase. Immediately prior to hydrogel formation, frozen PL aliquots were rapidly warmed to 37 C°, centrifuged and filtered. The addition of thrombin led to self-assembly of 3D hydrogels. B) Alexa Fluor 488 conjugated fibrinogen was incorporated into 50% PL and fibrin hydrogels (5% labeled fibrinogen by weight) and imaged with confocal microscopy. Representative maximum intensity projections from a 10 μm stack are shown for each condition at 63X. Scale bars = 20 μm. C) Scanning electron microscopy performed on 50% PL and 1.0 mg/mL and 2.5 mg/ mL fibrin hydrogels. Representative images are shown for each condition at 20,000X. Scale bar = 1μm. Despite a fibrin concentration of ~0.250 mg/mL, PL gel has an intermediary appearance between the 1 and 2.5 mg/mL fibrin gels in both confocal and electron microscopy.
Confocal imaging of PL hydrogels loaded with 5% alexafluor-488 labeled fibrinogen revealed an organized fibrin network more dense than the 1.0 mg/mL fibrin comparison gel, and similar to that seen in control 2.5 mg/ml fibrin hydrogels (Figure 1B). The labeled fibrinogen was incorporated randomly into the polymerizing fibrin fibers along with the native fibers. This was confirmed in a separate experiment as labeled fibrinogen alone did not form networks at low concentrations (Supplementary figure S1). Microstructural analysis with scanning electron microscopy revealed that the morphology of the PL consisted of thin, highly interconnected branched networks that were distinct from the fibrin hydrogels, which formed more elongated fibrils (Figure 1C).
3.2 PL hydrogel enables sustained release of growth factors
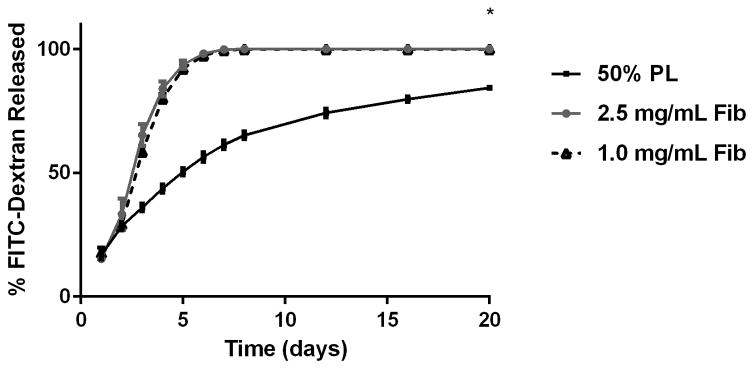
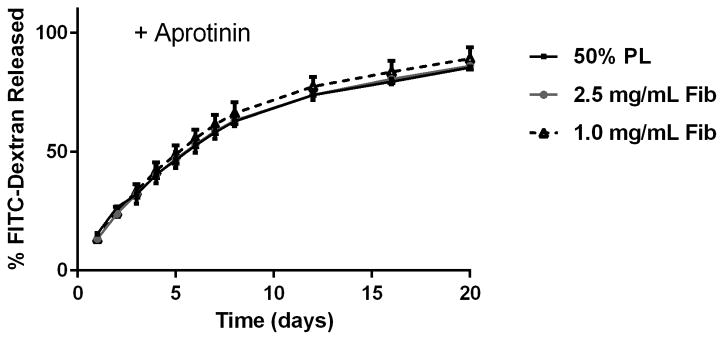
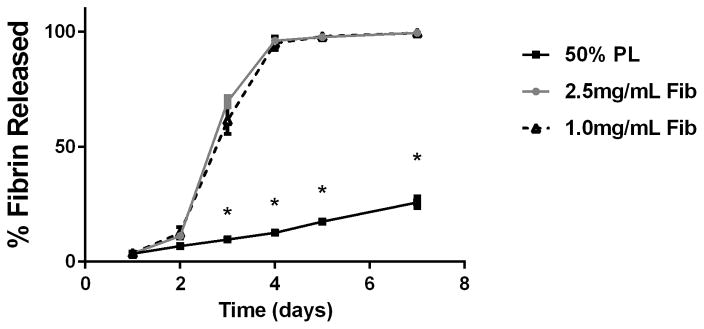
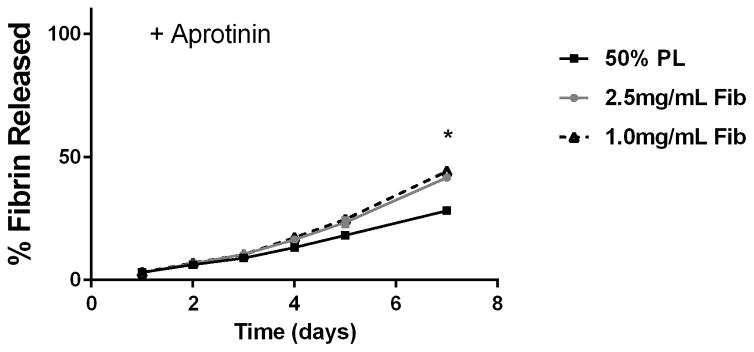
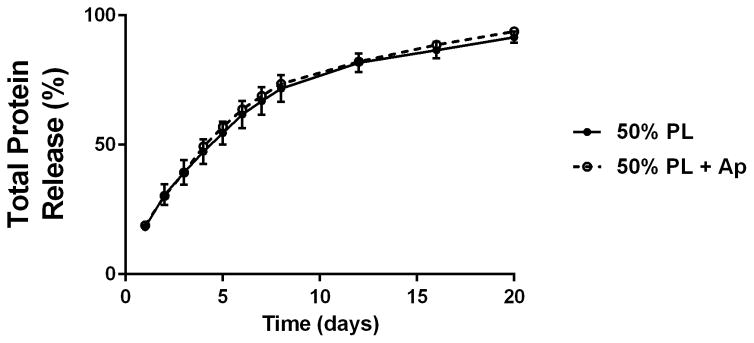
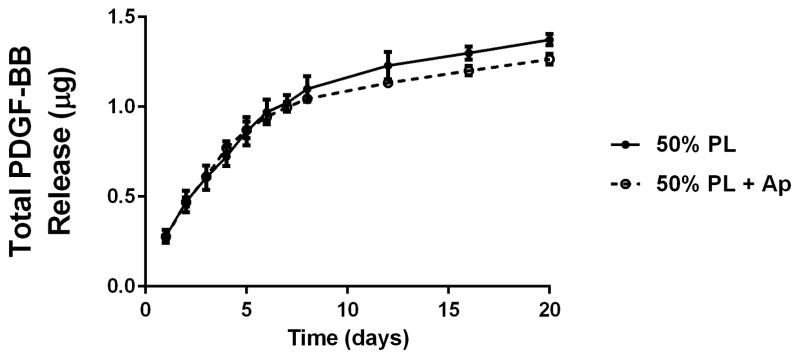
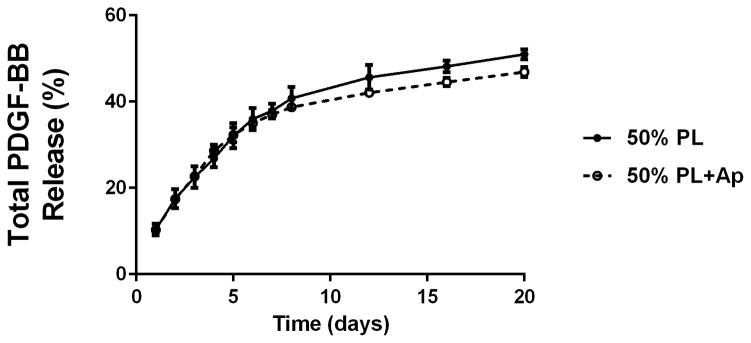
To examine the diffusion of soluble mediators from within the PL hydrogels, we embedded scaffolds with 70 kDa FITC-dextran. When incubated in PBS, 50% PL hydrogels had slow and sustained release of FITC-dextran over 20 days. Conversely, fibrin hydrogels rapidly released FITC-dextran from the scaffolds (Figure 2A). The addition of the protease inhibitor aprotinin to PBS improved retention of FITC-dextran in the fibrin hydrogels but had no effect on the PL gel (Figure 2B). To characterize the degradation of the PL scaffold, alexaflour-488 labeled fibrinogen was incorporated directly into PL and fibrin hydrogels. The hydrogels were incubated in PBS and the amount of fluorescent fibrin released into the PBS was quantified over 7 days. The fibrin hydrogels rapidly degraded after 48 hours, but the PL hydrogels retained over 70% of the labeled fibrin over this time (Figure 2C). The addition of aprotinin delayed degradation of the fibrin hydrogels, but the release of incorporated fibrin was still lower in the PL group (Figure 2D).
Figure 2.
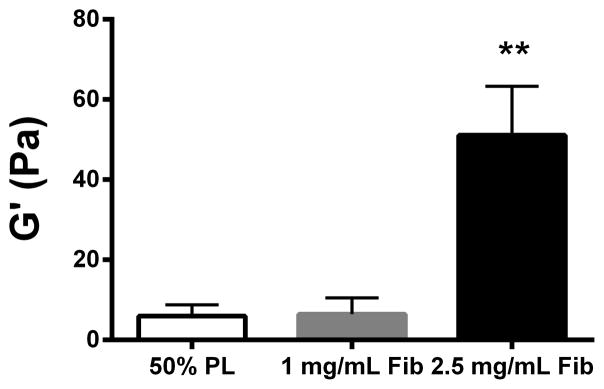
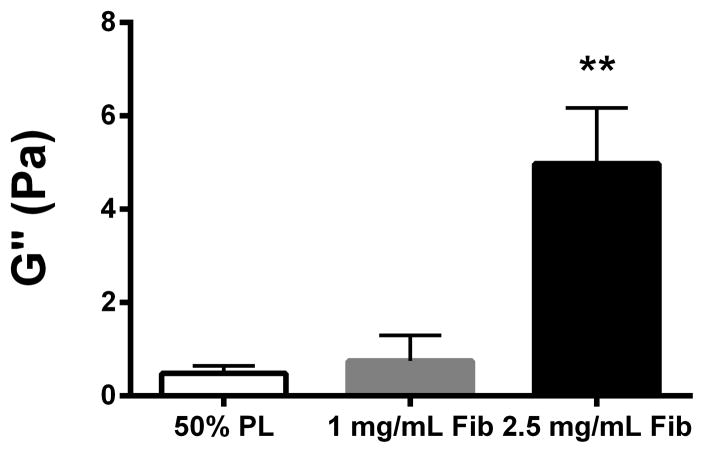
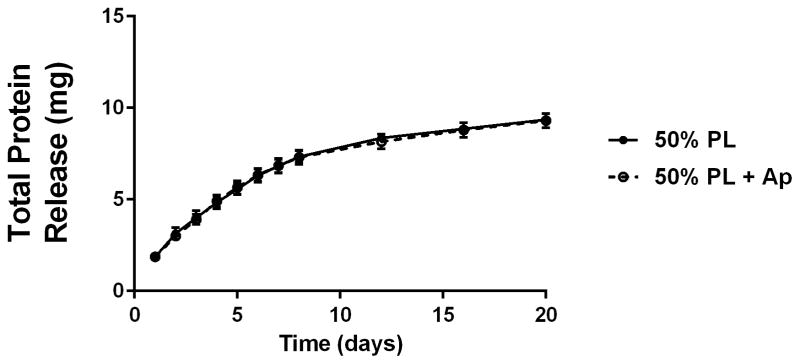
Structural properties of PL hydrogels. 70 kDa FITC-dextran was incorporated into 50% PL fibrin hydrogels. Percent of FITC-dextran release was quantified over 20 days in the absence (A) and presence of (B) aprotinin. Here PL gel had sustained release of dextran over 20 days that was superior over the fibrin gels. Aprotinin abrogated this benefit. Alexafluor-488 conjugated fibrinogen was incorporated in 50% PL and fibrin hydrogels. Scaffold degradation was analyzed by quantifying release of labeled fibrinogen over 7 days in the absence (C) and presence (D) of aprotinin. Here PL gel had superior integrity over fibrin gels that persisted, although to a lesser degree, in the presence of aprotinin. Oscillatory rheology was used to assess mechanical properties of the 50% PL and fibrin hydrogels. Storage modulus (E) and loss modulus (F) were calculated from an average G’ or G” at 0.5% strain over a frequency sweep from 0.01–1 Hz. Total protein released from 50% PL hydrogels over 20 days was calculated using a modified Bradford assay over time in the presence and absence of aprotinin. Protein release is shown both as cumulative protein released (G) and the percent of total protein released from the hydrogels (H). PDGF-BB released from 50% PL hydrogels over 20 days was measured with ELISA in the presence and absence of aprotinin. Total PDGF-BB release is shown both as cumulative protein (I) and percent of total protein released from the hydrogels (J). Aprotinin was not necessary to improve gel integrity or to sustain the protein release or PDGF-BB release in PL gels. * = p<0.005. G’ = Storage modulus. G” = Loss modulus.
To evaluate the mechanical properties of the PL scaffold, oscillatory rheology was performed on 50% PL and control fibrin hydrogels. The PL behaved as a soft viscoelastic solid. The storage and loss modulus of the high-fibrin (2.5 mg/mL) scaffold was higher than both the PL and low fibrin (1.0 mg/mL) hydrogels (P>0.005). However, there was no difference between the 50% PL and the 1.0 mg/ml fibrin scaffolds in either the storage modulus or loss modulus (Figure 2E and F), despite an approximately 4-fold lower fibrin content in the PL hydrogel.
To examine the release of endogenous growth factors from within the PL hydrogels, we first quantified total protein released into PBS over 20 days using a modified Bradford assay. There was no difference in the percentage of total protein released from the gels over the 20 day time course with the addition of aprotinin to the PBS (Figure 2G and H). To directly evaluate the release of pro-angiogenic growth factors from the PL scaffold, we quantified the amount of PDGF-BB released into PBS with and without aprotinin from 50% PL scaffolds over a 20-day time course using ELISA. The PL scaffold continuously released PDGF-BB for 20 days, and retained more than 45% of the of growth factor (Figure 2I and J). The addition of exogenous aprotinin to incubation media did not impact the growth factor release from the PL hydrogel.
3.3 PL scaffold promotes cell sprouting in an in vitro angiogenesis co-culture assay
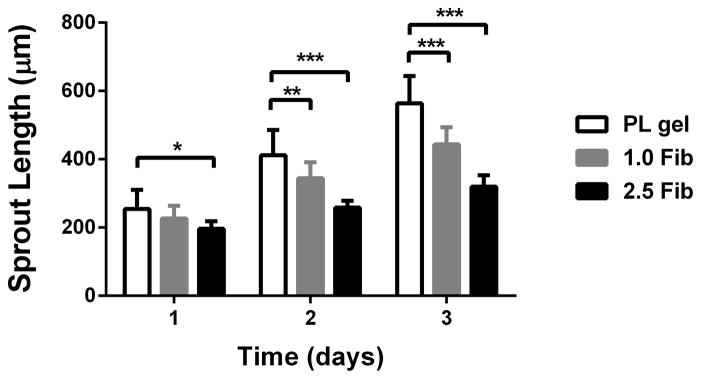
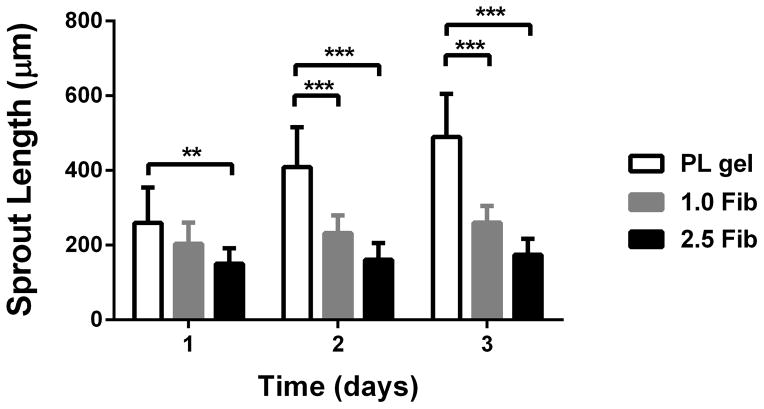
An in vitro co-culture assay was used to characterize the PL gel on MSC invasion and EC sprouting in a co-culture assay. To mimic an austere environment, we assessed MSC invasion and EC sprouting under serum free media conditions. Cell pellets containing a 1:1 ratio of human MSCs and PKH26 labeled HUVECs were embedded in 50% PL or fibrin hydrogels. Unlabeled MSC invasion was quantified under bright field microscopy and PKH26 labeled ECs were quantified under fluorescent microscopy. Both invasion (MSC) and sprout (EC) lengths were quantified over 3 days. We used both our standard 2.5 mg/mL [19] and a “low” concentration (1 mg/mL) fibrin gel as controls. Using bright field microscopy, which signified mainly MSC invasion when contrasted with fluorescent HUVECs, invasion was increased in the PL hydrogels compared to both the high and low concentration fibrin hydrogels over 3 days (Figure 3A and B). Endothelial cell sprouting within the co-culture assay was quantified by fluorescent imaging of the gel. At 3 days there was increased HUVEC sprouting in the PL hydrogel compared to high concentration fibrin hydrogels (Figure 3C and D), but there was no measurable difference in endothelial cell sprout length between the PL gel and the low concentration fibrin group at 3 days (p = 0.30).
Figure 3.
Pro-angiogenic effect of PL on MSCs in an in vitro co-culture assay under serum free conditions. Cell pellets containing MSCs and HUVECs labeled with PKH26 were embedded in PL and fibrin hydrogels. A) Representative bright field images of cell pellets within different scaffolds at 3 days captured combined MSC and EC invasion. B) Average cell invasion length was quantified over time in hydrogels cultured under serum free conditions. Total cell invasion was significantly improved in the PL gel compared to both fibrin gels. C) Endothelial specific sprouting was determined using fluorescent microscopy to capture only the PKH26 labeled HUVECs specifically from the co-culture assay. D) Representative fluorescent images of HUVEC sprouting from co-culture at 3 days. Average EC sprout length was quantified over time (E). Here MSC’s angiogenic and stromal activity on ECs was significantly greater in PL gel than in the 2.5 mg/mL fibrin gel. *=p<0.05, **=p<0.005, ***=p<0.0001. Scale bars = 500 μm.
3.4 PL gel selectively promotes MSC proliferation
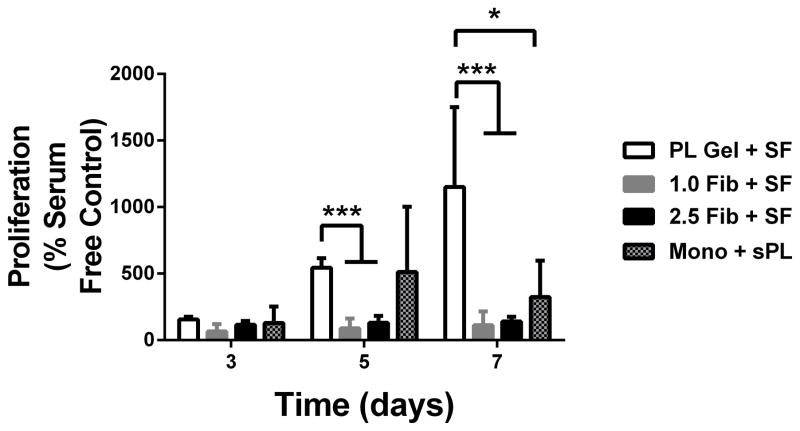
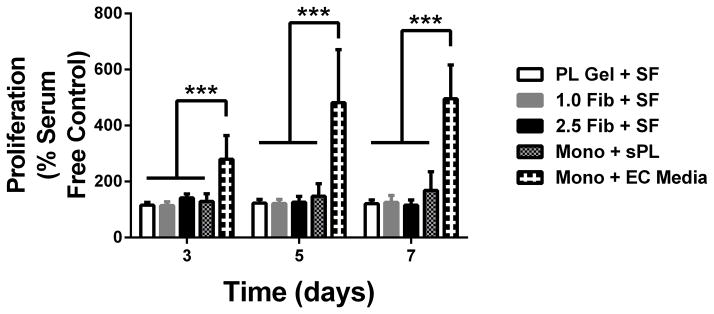
To evaluate the effect of the PL scaffold on cellular proliferation, human MSCs and HUVECs were cultured separately in PL and fibrin hydrogels under serum free conditions. Mitotic activity was measured using an MTS assay over 7 days. Growth in the PL hydrogel led to an increased proliferative response in human MSCs compared to the fibrin control hydrogels at 5 days. After 7 days, the metabolic activity of human MSCs grown in PL hydrogels under serum free conditions was significantly higher than the fibrin controls. This was also seen when MSCs grown in a monolayer were supplemented with soluble PL (Figure 4A). There was no detectable increase in metabolic activity in HUVECs grown in PL compared to HUVECs grown in fibrin hydrogels or in a monolayer in media supplement with soluble PL (Figure 4B). However, proliferation of HUVECs grown in a monolayer with full endothelial cell media was significantly higher than all other culture conditions (Figure 4B), indicating that the mitogenic effect of PL preferentially stimulates MSCs over HUVECs.
Figure 4.
Effect of PL scaffold on MSC and HUVEC proliferation. A) Proliferation of MSCs in PL and fibrin gels determined by MTS assay. All groups were normalized to MSCs grown in a monolayer under serum free conditions. B) Proliferation of HUVECs in PL and fibrin gels. All groups are normalized to HUVECS grown in a monolayer under serum free conditions. *=p<0.05, ***p<0.0001
3.5 PL scaffold promotes invasion of both MSCs and ECs independently
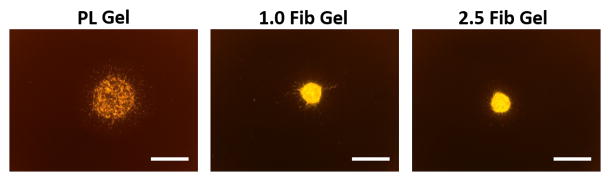
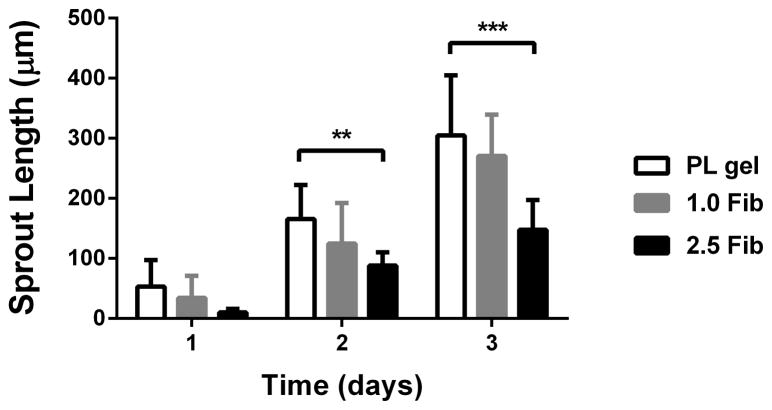
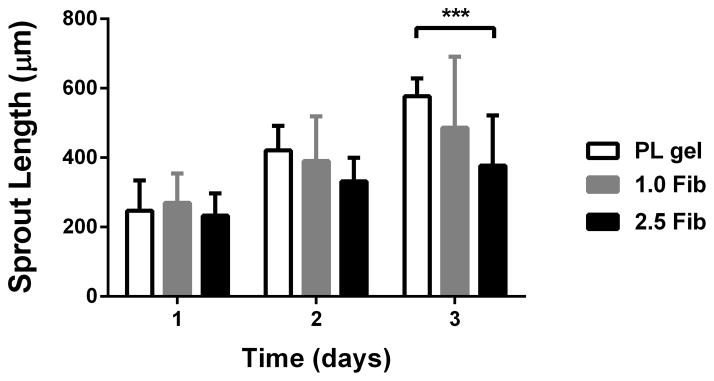
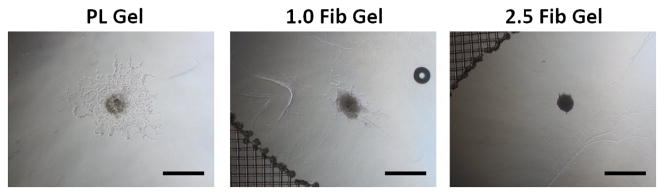
To assess the effect of the PL scaffold on MSC invasion or HUVEC sprouting in 3D, cell pellets containing only MSCs or HUVECs were embedded in PL or fibrin hydrogels, and their respective invasion/sprout length was quantified over 3 days. At the 3-day time point sprout length from MSC pellets invading the PL hydrogel was increased compared to pellets in high concentration fibrin gels with serum free media or media with soluble PL (Figure 5A and B). There was no difference in sprout length between the PL and low concentration fibrin hydrogels under serum free conditions or when supplemented with soluble PL. There was also an increase in sprout length from HUVEC pellets embedded in PL compared to both fibrin hydrogels with serum free media at both 2 and 3 days (Figure 5C and D).
Figure 5.
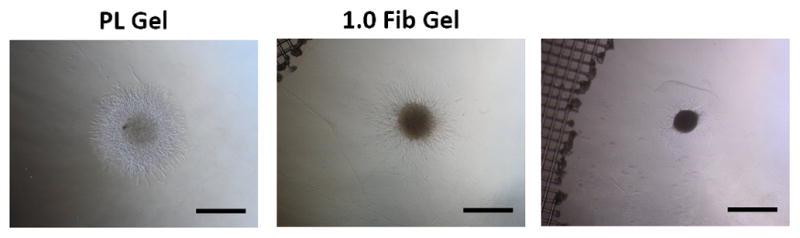
MSC and EC invasion of PL scaffolds. Cell pellets containing single cell type of either MSCs or HUVECs embedded in PL and fibrin hydrogels. A) Representative images of MSC sprout formation in PL, low and high concentration fibrin hydrogels at 3 days. B) Average invasion length from MSC pellets over 3 days. C) Representative images of HUVEC sprout formation in PL, low and high concentration fibrin hydrogels at 3 days. D) Average sprout length from HUVEC pellets over 3 days **=p<0.005, ***=p<0.001, ****=p<0.0001. Scale bars = 500 μm.
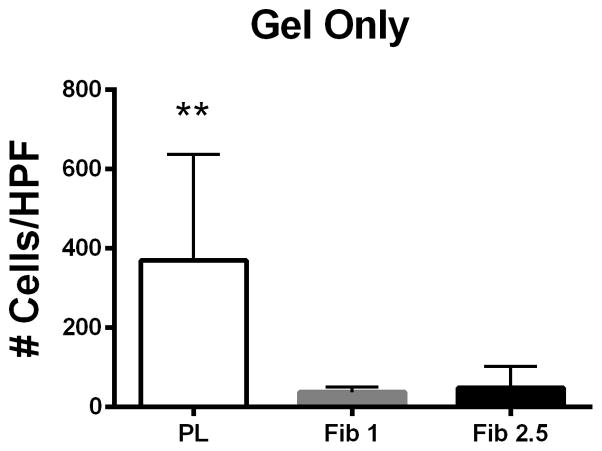
For cell therapy applications in a clinical setting, the PL would serve as a scaffold for MSC delivery, which would then recruit endogenous host endothelial cells. To assess whether the PL scaffold promotes migration of remote HUVECs toward the hydrogel, a transwell migration assay was modified so that HUVECs could migrate through a transwell insert toward the PL or a control hydrogel. HUVECs preferentially migrated toward the PL gel when compared to fibrin only gels (Figure 6A). When MSCs were embedded within the hydrogels, the PL gel also led to a significant increase in the number of recruited HUVECs when compared to the control fibrin gels containing MSCs.
Figure 6.
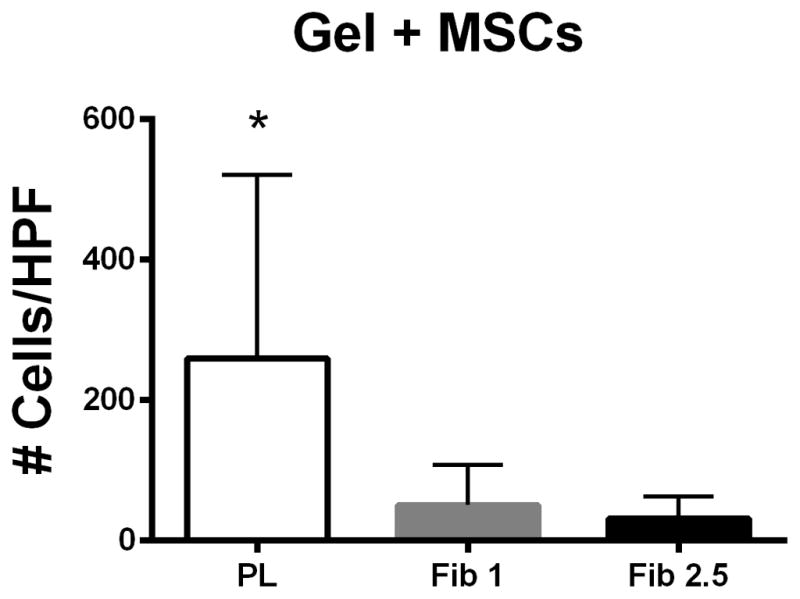
PL hydrogel recruits remote endothelial cells. Figure 6A) Cell free PL promotes migration of HUVECs when compared to fibrin only in a transwell migration assay. Figure 6B) MSCs embedded in PL promote migration of HUVECs when compared to MSCs embedded in fibrin hydrogels. * = p < 0.05, ** = p < 0.005. HPF = High Powered Field.
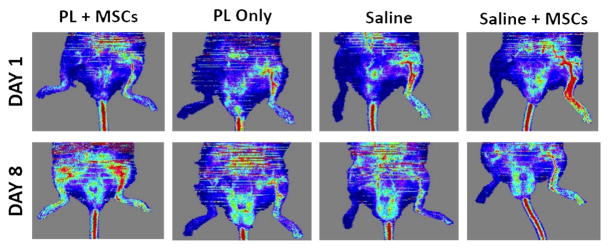
3.6 MSCs delivered in PL scaffolds lead to rapid neovascularization in vivo
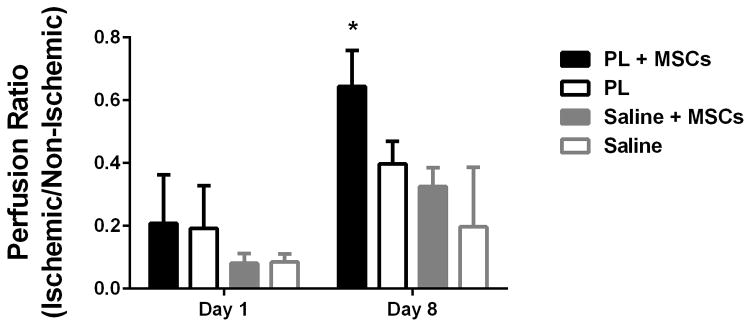
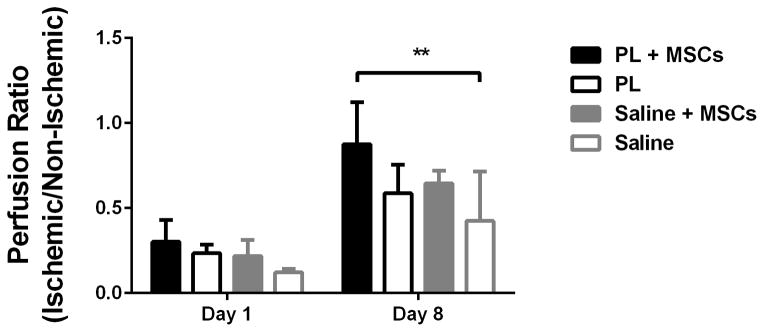
Implantation of MSCs embedded in PL into ischemic limbs in a mouse model of HLI led to rapid neovascularization of ischemic tissues by 8 days when assessed with LDPI. Perfusion ratios in the gastrocnemius muscle of mice that received PL embedded with MSCs were significantly higher than control groups containing PL only, MSCs in saline, or a saline only vehicle at 8 days (Figure 7A and B). LDPI of the entire surgical leg revealed complete restoration of perfusion after 8 days (signified by a ratio of 1 compared to the contralateral limb). This was significant in comparison with the saline control groups. (Figure 7C).
Figure 7.
MSCs in PL Gel Restore Perfusion Rapidly in NOD-SCID mice treated with MSCs in PL gel compared to control groups (saline and PL gel alone, and MSCs in saline). Figure 7A Representative laser Doppler images showing limb perfusion in each group at 1 and 8 days. Figure 7B Quantification of limb perfusion at 1 and 8 days in the ischemic are of the leg pertaining to the calf muscle. Here the ischemic right leg perfusion normalizes (right to left ratio of 1) in the PL + MSCs group by day 8 after HLI. Comparison of PL + MSCs to all groups was significant. Figure 7C Quantification of limb perfusion of the ischemic leg including the foot showed a significant difference between the PL + MSCs and saline only group. * = p < 0.05, ** = p < 0.01.
4.0 Discussion
The number of recent clinical trials using MSCs for CLI demonstrate the growing interest in the use of MSC therapy for therapeutic neovascularization [24–26], yet there remain barriers to clinical success. These barriers include the generation/availability of adequate cell numbers for therapy and the retention of these cells in the desired tissues with sufficient survivability to complete revascularization or tissue regeneration [27,28]. Here we demonstrate promising results of a novel PL hydrogel that has many of the desired characteristics needed to promote MSC retention and survival for cellular therapy including favorable gel density, sustained gel integrity, and a superior growth factor retention and release profile. In vitro, PL gel promotes MSC invasion and stimulation of EC sprouting. The proangiogenic effect of a MSC seeded PL scaffold was demonstrated in vivo, where PL gel with human MSCs rapidly (8 days) regenerated vascular perfusion in a NOD-SCID HLI model. Thus the use of MSCs in PL gel is an exciting and novel MSC delivery strategy for targeted vascular regeneration.
In its soluble form, PL increases the expansion capacity of MSCs [17,29–31]. PL is superior to bovine serum for culture of MSCs [15,32] and can be derived from cross matched blood products to reduce the risk of immunogenicity in cell therapy. Also, it avoids the use of xenogenic cell culture supplements and subsequent xenogenic contamination [14,33]. In its hydrogel form, PL can be used as a cell delivery vehicle that also retains the nutritive functions of soluble PL, thereby enabling MSC survival and engraftment. This may be particularly important to clinical outcomes when using cells from patients with impaired or depleted stem cell populations, such as those with coronary artery disease [34], stroke [35], diabetes mellitus [36,37], and tobacco smokers [38]. Overcoming impaired stem cell function may be particularly true for patients with CLI, as they commonly have several of these risk factors. Excitingly, we have recently published the benefit of soluble PL on the expansion of MSCs from patients with CLI [32], and most importantly, we and others have shown that MSCs from CLI patients cultured in PL-supplemented media retain angiogenic activity similar to that of healthy donor MSCs [39,40]. In these patients, the use of soluble PL is critical to the successful expansion of MSCs in a clinically useful timeframe. In this study, we observed that the biologic effects of PL gel are consistent with that seen with soluble PL, allowing for sustained nutritive support after delivery in vivo.
The number of functioning cells in the desired tissue appears to be critical for therapeutic impact of cell therapy, therefore it is imperative that cell delivery strategies must overcome the poor cell retention and viability that has impaired advancement of cell therapies [41–43]. Recently a number of biologic materials have been developed to promote cell retention and viability. Examples of these scaffolds include encapsulation with alginate [44,45], generation of tissue engineered patches [46,47], cell seeding of fibrin sutures [48], and embedding cells within a hydrogel. Following alginate encapsulation, sustained paracrine secretion from MSCs is enabled by the capsule’s active role in preventing cellular rejection by the immune system. This approach has been shown to improve neovascularization in a mouse HLI model when compared to non-encapsulated controls [44,49]. An alternative encapsulation strategy has been used to generate preconditioned cellular microniches. Implantation of adipose tissue derived stem cell (ASC) loaded gelatin microgels in the ischemic limbs of mice led to improved revascularization that was comparable to mice treated with ASCs alone but with 1/10th of the total cell number. Clearly microgel priming of ASCs in vitro led to improved angiogenic function and retention when transplanted into ischemic tissue beds [50]. While these pre-clinical studies clearly demonstrate the potential benefit of scaffold mediated cell delivery, they lack the nutritive benefit to MSCs that is intrinsic to PL hydrogels. This feature of the PL gel may be critical not only to the initial MSC retention and viability, but also to the survivability of MSCs over time. In support of this hypothesis, we tested PL gel’s effect on MSC invasion in an austere serum free environment, and MSCs in PL gel had significantly greater invasion than in fibrin gel.
The PL scaffold has unique structural properties that allow it to behave as a viscoelastic solid with improved mechanical properties compared to fibrin only hydrogels. The relatively soft nature of PL gel likely contributes to the favorable MSC invasion and endothelial sprouting paradigms discovered here. These results are consistent with published reports indicating that MSC invasion is dependent on matrix stiffness with preferential growth in softer substrates [51–53]. Since the site of MSC injection in CLI is known to be stiff and fibrotic, the use of a softer substrate for cell delivery may theoretically improve the function of transplanted MSCs in the ischemic limb. MSC growth and proliferation, on the other hand, appears independent of matrix stiffness and instead is regulated by the growth factor milieu contained within the PL scaffold. In contrast to the effect of PL on MSCs, the PL scaffold led to superior invasion of HUVECs compared to both high and low fibrin controls, but had no detectable effect on endothelial cell proliferation. These data indicate that PL gel contains a targeted growth factor milieu that induces sprouting of ECs with minimal effect on mitogenic activity. In contrast, MSCs in PL gel had increased invasion and proliferation. The PL gel native form and embedded with MSCs has the ability to recruit remote endothelial cells, as demonstrated in the transwell migration assay. These data support the proposed clinical treatment strategy, whereby PL gel embedded with MSCs recruits host ECs for neovascularization following implantation in ischemic tissues. The coordinated neovascular activity of PL gel and MSCs was further supported in vivo, where rapid and complete neovascularization occurred in 8 days, which was increased significantly when compared to PL gel alone and MSCs alone. This combinatorial benefit enables single cell type (MSC) delivery in the PL hydrogel, which greatly simplifies the regulatory barriers to clinical translation. We also identified differences between the 1 and 2.5 mg/mL fibrin gels. Here the 1 mg/mL gel acted more similar to PL gel in both mechanical and biologic testing. Taken together, these data suggest that the mechanical properties of the PL hydrogel (i.e. stiffness, porosity) selectively promote MSC invasion, while the growth factor milieu contained within the PL promotes MSC over EC proliferation.
Mechanically, the PL scaffold behaved as a viscoelastic solid. An organized network of fluorescently labeled fibrin was clearly visible within in the PL scaffold, and the storage and loss modulus of PL were comparable to that of the 1 mg/mL fibrin gel, which had four times the fibrin content. SEM imaging of the PL hydrogel revealed a microstructure distinct from that seen in the fibrin gels, indicating that other structural components are likely involved in PL gel formation and its favorable growth factor release kinetics. It is well established that composite structures with fibrin and additional components such as collagen can improve the mechanical properties of hydrogels without increasing protein content [54,55]. The superior performance of PL may be the result of reaction conditions distinct from fibrin-only gels during thrombin induced polymerization. Macromolecules within the PL may alter fibrin binding sites or serve as molecular crowders [56,57], improving the structural properties of the scaffold. The presence of an enriched milieu of proteins within PL, including Factor XIII, could also lead to enhanced fibrin crosslinking and strengthening of the PL gel. Additionally, extracellular proteins (i.e. fibronectin, collagen), proteoglycans, and adhesion proteins such as Von Willebrand Factor within the PL may incorporate into and reinforce the fibrin network as well as provide sustained release of PL’s robust growth factors and angiogens.
Here we demonstrate that PL gel has sustained release of endogenous PDGF-BB over 20 days in vitro, and that ~45% of PDGF-BB was still present in the PL gel at that distant time point. This excellent retention is far superior to that seen in optimized formulations of fibrin-only hydrogels in vitro [58,59], whereby there is approximately 6% of growth factor retained after 7 days [60]. The desirable sustained release of growth factors from PL hydrogels identified here may be due to sequestration by the microstructural networks as well as the result of resistance of PL to autolysis compared to fibrin. Specific angiogens in PL include PDGF, VEGF, EGF, and BDNF [17]. These angiogens may contribute to PLs’ superior performance augmenting neovessel formation compared to fibrin gel. In addition to serving as a proangiogenic growth factor[61], PDGF-BB has recently been established as a critical mediator of MSC engraftment into tissue [18]. Thus delivery of MSCs in PL gel may improve MSC therapy by both providing a scaffold with nutritive growth factors and sustained delivery of these growth factors over time.
While not discounting the value of other biomaterials in the advancement of stem cell therapies, regulatory hurdles for these technologies can restrict clinical translation for combined cellular and biomaterial applications. PL is human-derived and already approved in soluble form for the expansion of human MSCs for clinical therapies in our cell therapy center [NCT 01659762], and therefore as a hydrogel PL is a fairly straight forward biomaterial. In addition, PL may have an advantage over other human platelet products, including platelet rich plasma (PRP). PRP has had limitations in clinical effectiveness in wound healing [62,63], dental repair [64] and orthopedic tissue engineering [65], which is thought to be due in part to the extensive variability in the quality of PRP across donors [66,67]. Our strategy of generating platelet lysate through pooled, healthy donors minimizes this variability and provides a more consistent and reproducible clinical benefit [17]. Furthermore, through standard cross matching of blood products, we can substantially reduce the risk of a deleterious immunogenic response to PL while allowing for a more reproducible clinical effect. When combined with sufficient cell numbers and the ability for repeated delivery due to decreased immunogenic response, we have strong motivation for further testing of MSCs from patients with CLI in PL gel to promote prolonged survivability in the pro-inflammatory environment of critical limb ischemia.
There are a number of limitations to this work that must be addressed. We utilized two techniques in this laboratory project that are a potential source of xenogen contamination that would require modification in clinical trialing: polymerization of hydrogels was achieved with bovine thrombin, and MSCs were cultured in media supplemented with FBS prior to embedding in PL gels. While the bovine thrombin was diluted into a 1:100 V/V ratio in the PL gel, it could certainly introduce xenogens into the delivery system. We have utilized both human and bovine sources of thrombin in the past and have chosen bovine thrombin for preclinical work because of the similar biochemical activity for these assays and the cost benefit of bovine thrombin is significant. Also, the healthy MSCs used in this study were cultured in standard FBS instead of PL supplementation. We have recently demonstrated that PL supplementation outperforms FBS even in healthy MSCs [32]; thus future work will utilize PL supplementation for MSC expansion in addition to the PL gel delivery system.
We found rapid and complete neovascularization of NOD-SCID mice after HLI with human MSCs in PL gel, and significantly improved neovascularization of ischemic tissue compared to control groups. This early time point plus the testing in immunocompromised NOD-SCID mice limited our ability to test in vivo the biologic role of PL gel alone. Extending the time points for the saline and PL gel alone groups may allow us to stratify a benefit of the PL gel alone over saline control. In fact, we would expect some benefit from PL gel over saline from our in vitro testing. However the combination of MSCs in PL gel seems to drive the in vivo benefit. Finally, the unique mechanical behavior of PL gel required us to test in vitro fibrin gels in two different concentrations. The higher concentration represents a physiologically relevant fibrinogen level representative of normal human serum that more precisely mimicked the micro network of PL gel, while the lower concentration fibrin gels mimicked the PL gel’s actual fibrin concentration. Not surprisingly, these two fibrin gels had different effects on the MSC and EC behaviors in the gel with PL gel and the 1 mg/mL fibrin gel being most similar.
In summary, the use of a PL scaffold for delivery of MSCs in a setting of therapeutic angiogenesis offers several advantages over existing cell therapy platforms. First, PL is derived entirely from human blood products, and therefore it does not pose a risk for xenogenic contamination. PL enables delivery of autologous cells with a scaffold that could be cross matched to further reduce the potential immunogenic risk of cell therapy. PL can provide both sustained delivery of growth factors and structural support for localized delivery of MSCs to desired tissue beds. The combination of these properties may be helpful in overcoming current clinical limitations to cell therapy, and PL effectively meets these requirements in a single biomaterial. In addition, the robust proliferative response of MSCs to the PL hydrogel may also permit in vivo expansion of MSCs, thus enabling the use of a lower initial cell dose to achieve a therapeutic benefit. Finally, in vivo testing of MSCs in PL gel demonstrated robust and complete neovascularization by day 8 in a NOD-SCID HLI model, confirming the translational potential of this technology.
5.0 Conclusions
Innovative strategies are needed for improved delivery and retention of MSCs for cell based therapy. Here we detail the generation of a rapidly assembling PL hydrogel with desirable structural properties and biological activity on MSC and ECs. PL hydrogel is advantageous over existing biomaterials because it augments the pro-angiogenic qualities of MSCs and is readily derived from human source materials that have been tested for safe delivery to patients. As a result of these unique traits, PL hydrogel is ideally suited to serve as a vector for delivery of MSCs to ischemic tissues as was demonstrated in a murine model of HLI.
Supplementary Material
Figure S1: Labeled fibrinogen does not form hydrogels at low concentrations. Confocal microscopy after the addition of thrombin to Alexafluor labeled fibrinogen at 150 μg/ml did not form hydrogels. Scale bar = 500 μm.
Statement of Significance.
Innovative strategies for improved retention and viability of mesenchymal stem cells (MSCs) are needed for cellular therapies. Human platelet lysate is a potent serum supplement that improves the expansion of MSCs. Here we characterize our novel PL hydrogel’s desirable structural and biologic properties for human MSCs and endothelial cells. PL hydrogel can localize cells for retention in the desired tissue, improves cell viability, and augments MSCs’ angiogenic activity. As a result of these unique traits, PL hydrogel is ideally suited to serve as a cell delivery vehicle for MSCs injected into ischemic tissues to promote vascular regeneration, as demonstrated here in a murine model of hindlimb ischemia.
Acknowledgments
Scanning Electron Microscopy was performed by the Robert P. Apkarian Integrated Electron Microscopy Core at Emory University. This work was supported in part by American Medical Association Seed Grant (STR), Society for Vascular Surgery Medical Student Research Fellowship (STR), American Heart Association (AMD), and NIH R01EB011566 (THB), Emory/Georgia Institute of Technology Regenerative Engineering and Medicine (LB), which is supported in part by PHS Grant UL1TR000454 from the Clinical and Translational Science Award Program, National Institutes of Health, National Center for Advancing Translational Sciences. NHLBI KO8HL119592 & Society for Vascular Surgery/American College of Surgeons Scientific Development Grant (LB), American Heart Award Innovative Research Grant IRG14740001 (LB/IC), Emory Department of Surgery Startup Funds (LB).
Footnotes
7.0 Conflict of Interest:
The authors confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
8.0 References
- 1.Fowkes FG, Housley E, Cawood EH, Macintyre CC, Ruckley CV, Prescott RJ. Edinburgh Artery Study: prevalence of asymptomatic and symptomatic peripheral arterial disease in the general population. [accessed November 16, 2014];Int J Epidemiol. 1991 20:384–92. doi: 10.1093/ije/20.2.384. http://www.ncbi.nlm.nih.gov/pubmed/1917239. [DOI] [PubMed] [Google Scholar]
- 2.Novo S, Avellone G, Di Garbo V, Abrignani MG, Liquori M, Panno AV, Strano A. Prevalence of risk factors in patients with peripheral arterial disease. A clinical and epidemiological evaluation. [accessed November 16, 2014];Int Angiol. 11:218–29. http://www.ncbi.nlm.nih.gov/pubmed/1460357. [PubMed] [Google Scholar]
- 3.Critical limb ischaemia: management and outcome. Report of a national survey. The Vascular Surgical Society of Great Britain and Ireland. [accessed November 16, 2014];Eur J Vasc Endovasc Surg. 1995 10:108–13. doi: 10.1016/s1078-5884(05)80206-0. http://www.ncbi.nlm.nih.gov/pubmed/7633958. [DOI] [PubMed] [Google Scholar]
- 4.Barshes NR, Menard MT, Nguyen LL, Bafford R, Ozaki CK, Belkin M. Infrainguinal bypass is associated with lower perioperative mortality than major amputation in high-risk surgical candidates. J Vasc Surg. 2011;53:1251–1259.e1. doi: 10.1016/j.jvs.2010.11.099. [DOI] [PubMed] [Google Scholar]
- 5.Powell RJ, Simons M, Mendelsohn FO, Daniel G, Henry TD, Koga M, Morishita R, Annex BH. Results of a double-blind placebo-controlled study to assess the safety of intramuscular injection of hepatocyte growth factor plasmid to improve limb perfusion in patients with critical limb ischemia. Circulation. 2008;118:58–65. doi: 10.1161/CIRCULATIONAHA.107.727347. [DOI] [PubMed] [Google Scholar]
- 6.Lawall H, Bramlage P, Amann B. Treatment of peripheral arterial disease using stem and progenitor cell therapy. J Vasc Surg. 2011;53:445–53. doi: 10.1016/j.jvs.2010.08.060. [DOI] [PubMed] [Google Scholar]
- 7.Amin AH, Abd Elmageed ZY, Nair D, Partyka MI, Kadowitz PJ, Belmadani S, Matrougui K. Modified multipotent stromal cells with epidermal growth factor restore vasculogenesis and blood flow in ischemic hind-limb of type II diabetic mice. Lab Invest. 2010;90:985–96. doi: 10.1038/labinvest.2010.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuo YR, Wang CT, Cheng JT, Wang FS, Chiang YC, Wang CJ. Bone marrow-derived mesenchymal stem cells enhanced diabetic wound healing through recruitment of tissue regeneration in a rat model of streptozotocin-induced diabetes. Plast Reconstr Surg. 2011;128:872–80. doi: 10.1097/PRS.0b013e3182174329. [DOI] [PubMed] [Google Scholar]
- 9.Wang CY, Yang HB, Hsu HS, Chen LL, Tsai CC, Tsai KS, Yew TL, Kao YH, Hung SC. Mesenchymal stem cell-conditioned medium facilitates angiogenesis and fracture healing in diabetic rats. J Tissue Eng Regen Med. 2012;6:559–69. doi: 10.1002/term.461. [DOI] [PubMed] [Google Scholar]
- 10.Giordano A, Galderisi U, Marino IR. From the laboratory bench to the patient’s bedside: an update on clinical trials with mesenchymal stem cells. J Cell Physiol. 2007;211:27–35. doi: 10.1002/jcp.20959. [DOI] [PubMed] [Google Scholar]
- 11.Powell RJ. Update on clinical trials evaluating the effect of biologic therapy in patients with critical limb ischemia. J Vasc Surg. 2012;56:264–6. doi: 10.1016/j.jvs.2012.03.255. [DOI] [PubMed] [Google Scholar]
- 12.Kang WJ, Kang H-J, Kim H-S, Chung J-K, Lee MC, Lee DS. Tissue distribution of 18F-FDG-labeled peripheral hematopoietic stem cells after intracoronary administration in patients with myocardial infarction. [accessed November 16, 2014];J Nucl Med. 2006 47:1295–301. http://www.ncbi.nlm.nih.gov/pubmed/16883008. [PubMed] [Google Scholar]
- 13.Hou D, Youssef EAS, Brinton TJ, Zhang P, Rogers P, Price ET, Yeung AC, Johnstone BH, Yock PG, March KL. Radiolabeled cell distribution after intramyocardial, intracoronary, and interstitial retrograde coronary venous delivery: Implications for current clinical trials. Circulation. 2005;112 doi: 10.1161/CIRCULATIONAHA.104.526749. [DOI] [PubMed] [Google Scholar]
- 14.Spees JL, Gregory Ca, Singh H, Tucker HA, Peister A, Lynch PJ, Hsu S-C, Smith J, Prockop DJ. Internalized antigens must be removed to prepare hypoimmunogenic mesenchymal stem cells for cell and gene therapy. Mol Ther. 2004;9:747–56. doi: 10.1016/j.ymthe.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 15.Griffiths S, Baraniak PR, Copland IB, Nerem RM, McDevitt TC. Human platelet lysate stimulates high-passage and senescent human multipotent mesenchymal stromal cell growth and rejuvenation in vitro. Cytotherapy. 2013;15:1469–83. doi: 10.1016/j.jcyt.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 16.Chase LG, Lakshmipathy U, Solchaga LA, Rao MS, Vemuri MC. A novel serum-free medium for the expansion of human mesenchymal stem cells. Stem Cell Res Ther. 2010;1:8. doi: 10.1186/scrt8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Copland IB, Garcia MA, Waller EK, Roback JD, Galipeau J. The effect of platelet lysate fibrinogen on the functionality of MSCs in immunotherapy. Biomaterials. 2013;34:7840–50. doi: 10.1016/j.biomaterials.2013.06.050. [DOI] [PubMed] [Google Scholar]
- 18.Lin RZ, Moreno-Luna R, Li D, Jaminet SC, Greene AK, Melero-Martin JM. Human endothelial colony-forming cells serve as trophic mediators for mesenchymal stem cell engraftment via paracrine signaling. Proc Natl Acad Sci U S A. 2014;111:10137–42. doi: 10.1073/pnas.1405388111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xue L, Greisler HP. Angiogenic effect of fibroblast growth factor-1 and vascular endothelial growth factor and their synergism in a novel in vitro quantitative fibrin-based 3-dimensional angiogenesis system. [accessed June 12, 2013];Surgery. 2002 132:259–67. doi: 10.1067/msy.2002.125720. http://www.ncbi.nlm.nih.gov/pubmed/12219021. [DOI] [PubMed] [Google Scholar]
- 20.Stabenfeldt SE, Aboujamous NM, Soon ASC, Barker TH. A new direction for anticoagulants: inhibiting fibrin assembly with PEGylated fibrin knob mimics. Biotechnol Bioeng. 2011;108:2424–33. doi: 10.1002/bit.23184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soon ASC, Lee CS, Barker TH. Modulation of fibrin matrix properties via knob:hole affinity interactions using peptide-PEG conjugates. Biomaterials. 2011;32:4406–14. doi: 10.1016/j.biomaterials.2011.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Branco MC, Pochan DJ, Wagner NJ, Schneider JP. Macromolecular diffusion and release from self-assembled beta-hairpin peptide hydrogels. Biomaterials. 2009;30:1339–47. doi: 10.1016/j.biomaterials.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Landazuri N, Joseph G, Guldberg RE, Taylor WR. Growth and regression of vasculature in healthy and diabetic mice after hindlimb ischemia. AJP Regul Integr Comp Physiol. 2012;303:R48–R56. doi: 10.1152/ajpregu.00002.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta PK, Chullikana A, Parakh R, Desai S, Das A, Gottipamula S, Krishnamurthy S, Anthony N, Pherwani A, Majumdar AS. A double blind randomized placebo controlled phase I/II study assessing the safety and efficacy of allogeneic bone marrow derived mesenchymal stem cell in critical limb ischemia. J Transl Med. 2013;11:143. doi: 10.1186/1479-5876-11-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu D, Chen B, Liang Z, Deng W, Jiang Y, Li S, Xu J, Wu Q, Zhang Z, Xie B, Chen S. Comparison of bone marrow mesenchymal stem cells with bone marrow-derived mononuclear cells for treatment of diabetic critical limb ischemia and foot ulcer: a double-blind, randomized, controlled trial. Diabetes Res Clin Pract. 2011;92:26–36. doi: 10.1016/j.diabres.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 26.Powell RJ, Marston WA, Berceli SA, Guzman R, Henry TD, Longcore AT, Stern TP, Watling S, Bartel RL. Cellular therapy with Ixmyelocel-T to treat critical limb ischemia: the randomized, double-blind, placebo-controlled RESTORE-CLI trial. Mol Ther. 2012;20:1280–6. doi: 10.1038/mt.2012.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richardson JD, Bertaso AG, Psaltis PJ, Frost L, Carbone A, Paton S, Nelson AJ, Wong DTL, Worthley MI, Gronthos S, Zannettino ACW, Worthley SG. Impact of timing and dose of mesenchymal stromal cell therapy in a preclinical model of acute myocardial infarction. J Card Fail. 2013;19:342–353. doi: 10.1016/j.cardfail.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 28.Shen D, Cheng K, Marbán E. Dose-dependent functional benefit of human cardiosphere transplantation in mice with acute myocardial infarction. J Cell Mol Med. 2012;16:2112–2116. doi: 10.1111/j.1582-4934.2011.01512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schallmoser K, Bartmann C, Rohde E, Reinisch A, Kashofer K, Stadelmeyer E, Drexler C, Lanzer G, Linkesch W, Strunk D. Human platelet lysate can replace fetal bovine serum for clinical-scale expansion of functional mesenchymal stromal cells. Transfusion. 2007;47:1436–46. doi: 10.1111/j.1537-2995.2007.01220.x. [DOI] [PubMed] [Google Scholar]
- 30.Iudicone P, Fioravanti D, Bonanno G, Miceli M, Lavorino C, Totta P, Frati L, Nuti M, Pierelli L. Pathogen-free, plasma-poor platelet lysate and expansion of human mesenchymal stem cells. J Transl Med. 2014;12:28. doi: 10.1186/1479-5876-12-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doucet C, Ernou I, Zhang Y, Llense JR, Begot L, Holy X, Lataillade JJ. Platelet lysates promote mesenchymal stem cell expansion: a safety substitute for animal serum in cell-based therapy applications. J Cell Physiol. 2005;205:228–36. doi: 10.1002/jcp.20391. [DOI] [PubMed] [Google Scholar]
- 32.Brewster L, Robinson S, Wang R, Griffiths S, Li H, Peister A, Copland I, McDevitt T. Expansion and Angiogenic Potential of Mesenchymal Stem Cells from Patients with Critical Limb Ischemia. J Vasc Surg. n.d doi: 10.1016/j.jvs.2015.02.061. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Selvaggi TA, Walker RE, Fleisher TA, Selvaggi BTA. Development of Antibodies to Fetal Calf Serum With Arthus-Like Reactions in Human Immunodeficiency Virus. Infected Patients Given Syngeneic Lymphocyte Infusions. 2014:776–779. [PubMed] [Google Scholar]
- 34.Heeschen C, Lehmann R, Honold J, Assmus B, Aicher A, Walter DH, Martin H, Zeiher AM, Dimmeler S. Profoundly reduced neovascularization capacity of bone marrow mononuclear cells derived from patients with chronic ischemic heart disease. Circulation. 2004;109:1615–22. doi: 10.1161/01.CIR.0000124476.32871.E3. [DOI] [PubMed] [Google Scholar]
- 35.Ghani U, Shuaib A, Salam A, Nasir A, Shuaib U, Jeerakathil T, Sher F, O’Rourke F, Nasser AM, Schwindt B, Todd K. Endothelial progenitor cells during cerebrovascular disease. Stroke. 2005;36:151–3. doi: 10.1161/01.STR.0000149944.15406.16. [DOI] [PubMed] [Google Scholar]
- 36.Loomans CJM, de Koning EJP, Staal FJT, Rookmaaker MB, Verseyden C, de Boer HC, Verhaar MC, Braam B, Rabelink TJ, van Zonneveld A-J. Endothelial progenitor cell dysfunction: a novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes. 2004;53:195–9. doi: 10.2337/diabetes.53.1.195. http://www.ncbi.nlm.nih.gov/pubmed/14693715. [DOI] [PubMed] [Google Scholar]
- 37.Tepper OM. Human Endothelial Progenitor Cells From Type II Diabetics Exhibit Impaired Proliferation, Adhesion, and Incorporation Into Vascular Structures. Circulation. 2002;106:2781–2786. doi: 10.1161/01.CIR.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- 38.Kondo T, Hayashi M, Takeshita K, Numaguchi Y, Kobayashi K, Iino S, Inden Y, Murohara T. Smoking cessation rapidly increases circulating progenitor cells in peripheral blood in chronic smokers. Arterioscler Thromb Vasc Biol. 2004;24:1442–7. doi: 10.1161/01.ATV.0000135655.52088.c5. [DOI] [PubMed] [Google Scholar]
- 39.Gremmels H, Teraa M, Quax PH, den Ouden K, Fledderus JO, Verhaar MC. Neovascularization capacity of mesenchymal stromal cells from critical limb ischemia patients is equivalent to healthy controls. Mol Ther. 2014;22:1960–70. doi: 10.1038/mt.2014.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smadja DM, d’Audigier C, Guerin CL, Mauge L, Dizier B, Silvestre J-S, Dal Cortivo L, Gaussem P, Emmerich J. Angiogenic potential of BM MSCs derived from patients with critical leg ischemia. Bone Marrow Transplant. 2012;47:997–1000. doi: 10.1038/bmt.2011.196. [DOI] [PubMed] [Google Scholar]
- 41.Swijnenburg RJ, Govaert JA, van der Bogt KEA, Pearl JI, Huang M, Stein W, Hoyt G, Vogel H, Contag CH, Robbins RC, Wu JC. Timing of bone marrow cell delivery has minimal effects on cell viability and cardiac recovery after myocardial infarction. Circ Cardiovasc Imaging. 2010;3:77–85. doi: 10.1161/CIRCIMAGING.109.872085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hong KU, Guo Y, Li QH, Cao P, Al-Maqtari T, Vajravelu BN, Du J, Book MJ, Zhu X, Nong Y, Bhatnagar A, Bolli R. C-Kit+ Cardiac Stem Cells Alleviate Post-Myocardial Infarction Left Ventricular Dysfunction Despite Poor Engraftment and Negligible Retention in the Recipient Heart. PLoS One. 2014;9:1–7. doi: 10.1371/journal.pone.0096725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sheikh AY, Huber BC, Narsinh KH, Spin JM, van der Bogt K, de Almeida PE, Ransohoff KJ, Kraft DL, Fajardo G, Ardigo D, Ransohoff J, Bernstein D, Fischbein MP, Robbins RC, Wu JC. In vivo functional and transcriptional profiling of bone marrow stem cells after transplantation into ischemic myocardium. Arterioscler Thromb Vasc Biol. 2012;32:92–102. doi: 10.1161/ATVBAHA.111.238618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Landázuri N, Levit R. Alginate microencapsulation of human mesenchymal stem cells as a strategy to enhance paracrine-mediated vascular recovery after hindlimb ischaemia. J Tissue. 2012 doi: 10.1002/term. …. epub ahead. [DOI] [PubMed] [Google Scholar]
- 45.Levit RD, Landázuri N, Phelps Ea, Brown ME, García AJ, Davis ME, Joseph G, Long R, Safley Sa, Suever JD, Lyle AN, Weber CJ, Taylor WR. Cellular encapsulation enhances cardiac repair. J Am Heart Assoc. 2013;2:e000367. doi: 10.1161/JAHA.113.000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simpson DL, Boyd NL, Kaushal S, Stice SL, Dudley SC. Use of human embryonic stem cell derived-mesenchymal cells for cardiac repair. Biotechnol Bioeng. 2012;109:274–83. doi: 10.1002/bit.23301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wei HJ, Chen CH, Lee WY, Chiu I, Hwang SM, Lin WW, Huang CC, Yeh YC, Chang Y, Sung HW. Bioengineered cardiac patch constructed from multilayered mesenchymal stem cells for myocardial repair. Biomaterials. 2008;29:3547–3556. doi: 10.1016/j.biomaterials.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 48.Guyette JP, Fakharzadeh M, Burford EJ, Tao ZW, Pins GD, Rolle MW, Gaudette GR. A novel suture-based method for efficient transplantation of stem cells. J Biomed Mater Res - Part A. 2013;101 A:809–818. doi: 10.1002/jbm.a.34386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Katare R, Riu F, Rowlinson J, Lewis A, Holden R, Meloni M, Reni C, Wallrapp C, Emanueli C, Madeddu P. Perivascular delivery of encapsulated mesenchymal stem cells improves postischemic angiogenesis via paracrine activation of VEGF-A. Arterioscler Thromb Vasc Biol. 2013;33:1872–1880. doi: 10.1161/ATVBAHA.113.301217. [DOI] [PubMed] [Google Scholar]
- 50.Li Y, Liu W, Liu F, Zeng Y, Zuo S, Feng S, Qi C, Wang B, Yan X, Khademhosseini A, Bai J, Du Y. Primed 3D injectable microniches enabling low-dosage cell therapy for critical limb ischemia. Proc Natl Acad Sci. 2014;111:13511–13516. doi: 10.1073/pnas.1411295111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hanson AJ, Quinn MT. Effect of fibrin sealant composition on human neutrophil chemotaxis. J Biomed Mater Res. 2002;61:474–81. doi: 10.1002/jbm.10196. [DOI] [PubMed] [Google Scholar]
- 52.Ho W, Tawil B, Dunn JCY, Wu BM. The behavior of human mesenchymal stem cells in 3D fibrin clots: dependence on fibrinogen concentration and clot structure. Tissue Eng. 2006;12:1587–95. doi: 10.1089/ten.2006.12.1587. [DOI] [PubMed] [Google Scholar]
- 53.Stolzing A, Colley H, Scutt A. Effect of age and diabetes on the response of mesenchymal progenitor cells to fibrin matrices. Int J Biomater. 2011;2011:378034. doi: 10.1155/2011/378034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rowe SL, Stegemann JP. Microstructure and mechanics of collagen-fibrin matrices polymerized using ancrod snake venom enzyme. J Biomech Eng. 2009;131:061012. doi: 10.1115/1.3128673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rowe SL, Stegemann JP. Interpenetrating collagen-fibrin composite matrices with varying protein contents and ratios. Biomacromolecules. 2006;7:2942–8. doi: 10.1021/bm0602233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stabenfeldt SE, Gourley M, Krishnan L, Hoying JB, Barker TH. Engineering fibrin polymers through engagement of alternative polymerization mechanisms. Biomaterials. 2012;33:535–44. doi: 10.1016/j.biomaterials.2011.09.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barker TH, Fuller GM, Klinger MM, Feldman DS, Hagood JS. Modification of fibrinogen with poly(ethylene glycol) and its effects on fibrin clot characteristics. [accessed February 4, 2015];J Biomed Mater Res. 2001 56:529–35. doi: 10.1002/1097-4636(20010915)56:4<529::aid-jbm1124>3.0.co;2-2. http://www.ncbi.nlm.nih.gov/pubmed/11400130. [DOI] [PubMed] [Google Scholar]
- 58.Thomopoulos S, Zaegel M. PDGF-BB released in tendon repair using a novel delivery system promotes cell proliferation and collagen remodeling. J …. 2007:1358–1368. doi: 10.1002/jor. [DOI] [PubMed] [Google Scholar]
- 59.Sakiyama-Elbert S, Das R. Controlled-release kinetics and biologic activity of platelet-derived growth factor-BB for use in flexor tendon repair. J Hand …. 2008;33:1548–1557. doi: 10.1016/j.jhsa.2008.05.030.Controlled. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Greisler HP, Cziperle DJ, Kim DU, Garfield JD, Petsikas D, Murchan PM, Applegren EO, Drohan W, Burgess WH. Enhanced endothelialization of expanded polytetrafluoroethylene grafts by fibroblast growth factor type 1 pretreatment. [accessed February 12, 2015];Surgery. 1992 112:244–54. discussion 254–5. http://www.ncbi.nlm.nih.gov/pubmed/1641764. [PubMed] [Google Scholar]
- 61.Battegay EJ, Rupp J, Iruela-Arispe L, Sage EH, Pech M. PDGF-BB modulates endothelial proliferation and angiogenesis in vitro via PDGF beta-receptors. [accessed January 24, 2016];J Cell Biol. 1994 125:917–28. doi: 10.1083/jcb.125.4.917. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2120083&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shan GQ, Zhang YN, Ma J, Li YH, Zuo DM, Qiu JL, Cheng B, Chen ZL. Evaluation of the effects of homologous platelet gel on healing lower extremity wounds in patients with diabetes. Int J Low Extrem Wounds. 2013;12:22–9. doi: 10.1177/1534734613477113. [DOI] [PubMed] [Google Scholar]
- 63.de Leon JM, Driver VR, Fylling CP, Carter MJ, Anderson C, Wilson J, Dougherty RM, Fuston D, Trigilia D, Valenski V, Rappl LM. The clinical relevance of treating chronic wounds with an enhanced near-physiological concentration of platelet-rich plasma gel. Adv Skin Wound Care. 2011;24:357–68. doi: 10.1097/01.ASW.0000403249.85131.6f. [DOI] [PubMed] [Google Scholar]
- 64.Forni F, Marzagalli M, Tesei P, Grassi A. Platelet gel: applications in dental regenerative surgery. Blood Transfus. 2013;11:102–7. doi: 10.2450/2012.0007-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cervellin M, de Girolamo L, Bait C, Denti M, Volpi P. Autologous platelet-rich plasma gel to reduce donor-site morbidity after patellar tendon graft harvesting for anterior cruciate ligament reconstruction: a randomized, controlled clinical study. Knee Surg Sports Traumatol Arthrosc. 2012;20:114–20. doi: 10.1007/s00167-011-1570-5. [DOI] [PubMed] [Google Scholar]
- 66.Russell RP, Apostolakos J, Hirose T, Cote MP, Mazzocca AD. Variability of platelet-rich plasma preparations. Sports Med Arthrosc. 2013;21:186–90. doi: 10.1097/JSA.0000000000000007. [DOI] [PubMed] [Google Scholar]
- 67.Eppley BL, Woodell JE, Higgins J. Platelet quantification and growth factor analysis from platelet-rich plasma: implications for wound healing. [accessed February 12, 2015];Plast Reconstr Surg. 2004 114:1502–8. doi: 10.1097/01.prs.0000138251.07040.51. http://www.ncbi.nlm.nih.gov/pubmed/15509939. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Labeled fibrinogen does not form hydrogels at low concentrations. Confocal microscopy after the addition of thrombin to Alexafluor labeled fibrinogen at 150 μg/ml did not form hydrogels. Scale bar = 500 μm.