Abstract
Background
For recipients of liver transplants for hepatocellular carcinoma (HCC), HCC recurrence after transplantation remains a major concern. Sirolimus, an immunosuppressant with anti-carcinogenic properties, may reduce HCC recurrence and improve survival.
Methods
The U.S. Scientific Registry of Transplant Recipients was linked to pharmacy claims. For liver recipients transplanted for HCC, Cox regression was used to estimate associations of early sirolimus use with recurrence, cancer-specific mortality, and all-cause mortality adjusting for recipient ethnicity, calendar year of transplant, total tumor volume, alpha-fetoprotein, transplant center size, use of IL-2 induction therapy, and allocated and calculated model for end-stage liver disease score. We performed stratified analyses among recipients who met Milan criteria, among those without renal failure, among those with deceased liver donors, by age at transplantation, and by tumor size.
Results
Among the 3,936 included HCC liver transplants, 234 (6%) were sirolimus users. In total, there were 242 recurrences and 879 deaths, including 261 cancer-related deaths. All-cause mortality was similar in sirolimus users and non-users (adjusted hazard ratio [HR] =1.01, 95%CI=0.73–1.39). HCC recurrence and cancer-specific mortality rates appeared lower in sirolimus users, but associations were not statistically significant (recurrence HR=0.86, 95%CI=0.45–1.65; cancer-specific mortality HR=0.80, 95%CI=0.43–1.50). Among recipients >55 years old, associations were suggestive of better outcomes for sirolimus users (all-cause mortality HR=0.62, 95%CI=0.38–1.01; recurrence HR=0.52, 95%CI=0.19–1.44; cancer-specific mortality HR=0.34, 95%CI=0.11–1.09), while among recipients ≤55 years old, sirolimus users had worse outcomes (all-cause mortality HR=1.76, 95%CI=1.12–2.75; recurrence HR=1.49, 95%CI=0.62–3.61; cancer-specific mortality HR=1.54, 95%CI=0.71–3.32).
Conclusions
Among HCC liver recipients overall, sirolimus did not appear beneficial in reducing all-cause mortality. However, there were suggestions of reductions in recurrence and cancer-specific mortality, and effects appeared to be modified by age at transplantation.
Keywords: mTOR inhibitor, liver cancer, cancer recurrence, transplant outcomes, survival
INTRODUCTION
A growing fraction of all liver transplants are performed for the indication of hepatocellular carcinoma (HCC) (1). Among these transplant recipients, approximately 10% experience a recurrence of the primary HCC following transplantation, making recurrence a leading cause of morbidity and mortality (2–5). Mammalian target of rapamycin (mTOR) is a protein that plays a key role in a signaling pathway that controls cellular growth and proliferation (6). Because this pathway is often hyperactivated in malignant cells, including HCC (6–9), use of medications that inhibit mTOR might hinder HCC recurrence.
Sirolimus is an mTOR inhibitor with immunosuppressant properties and is used to prevent graft rejection among solid organ recipients, but use of this medication in liver transplantation (LT) remains controversial. In 2002, a randomized trial showed higher rates of graft loss, hepatic artery thrombosis, and death among liver recipients randomized to sirolimus (10). However, these trial results have not since been replicated, and retrospective cohort studies have found lower or similar rates of adverse outcomes among liver recipients treated with sirolimus (10, 11). Additionally, the benefits of mTOR inhibitors may outweigh the risks among transplant recipients with high cancer risk, such as those who receive a liver transplant as treatment for HCC (11).
Along these lines, observational studies of liver recipients with HCC have reported lower cancer recurrence rates and better survival among sirolimus users (12–14). However, most of these studies came from single institutions, which have a restricted range of practice patterns and experience systematic changes in immunosuppression protocols over time. In particular, sirolimus use was typically restricted to the most recent calendar years in these studies and is therefore potentially correlated with improvements in HCC staging and surgical practice over time (13, 15, 16). Using the Scientific Registry of Transplant Recipients (SRTR, the U.S. national transplant registry), one prior study demonstrated better survival associated with sirolimus use among liver recipients with HCC (12). However, the study relied upon immunosuppression data provided by the transplant centers at the time of initial hospital discharge, which may be unreliable. Additionally, this study did not consider associations with HCC recurrence or cancer-specific death.
To address the limitations of these prior studies, we conducted a large observational study linking the SRTR to pharmacy claims data to estimate the effect of sirolimus-based immunosuppression on HCC recurrence, cancer-specific mortality, and overall mortality.
METHODS
We identified liver recipients through the SRTR, a database of all U.S. solid organ transplant recipients which includes information on organ recipients’ demographic characteristics, indications for transplant, donor organ characteristics, and transplant outcomes including death (17). We included liver recipients in the SRTR who had received waitlist exception points for HCC and for whom the SRTR had information on tumor size. The SRTR was linked with national pharmacy claims obtained from IMS Health (www.imshealth.com) to capture immunosuppressant medication use after hospital discharge. The analysis was restricted to HCC liver recipients transplanted during 2002–2012 with IMS claims for immunosuppressant medications in the first 3 months after transplantation.
Recipients with sirolimus use reported in both their SRTR discharge regimen and IMS Health claims in the first three months after transplantation were considered sirolimus users. Recipients with no sirolimus reported in their SRTR discharge regimen and no sirolimus claims in the first three months were considered sirolimus non-users. Recipients with discrepant sirolimus information (sirolimus reported only in SRTR or only in IMS) were excluded from analyses. We conducted an intention-to-treat analysis, in which recipients treated with sirolimus in the first three months after transplantation were considered sirolimus exposed for the duration of subsequent follow-up regardless of subsequent claims, while recipients not using sirolimus in the first three months were considered sirolimus unexposed. While sirolimus use may have changed over time after transplantation, these changes might have been triggered by clinical events related to an incipient recurrence. We chose not to account for these changes in order to avoid introducing bias into the analyses. However, we did find that among the sirolimus users in our study with pharmacy claims beyond 3 months post-transplant, the majority (74%) continued to have sirolimus claims beyond 3 months.
The outcomes of interest were death from any cause, cancer-related death, and HCC recurrence, as ascertained through SRTR. Cancer-related death was defined as any death classified as caused by a cancer, excluding post-transplant lymphoproliferative disorder. At-risk time for the outcomes began at three months following transplantation and ended at the first of: HCC recurrence (in analyses in which that was the outcome of interest), death, or last date of recipient follow-up information. Recipients who experienced HCC recurrence in the first three months after transplantation were excluded from all analyses, as these recurrences could have influenced early sirolimus use. We also assessed associations of sirolimus with non-cancer cause-specific deaths as secondary outcomes of interest, specifically deaths from graft failure, infection, and cardiovascular disease.
Rates of all-cause mortality, cancer-related mortality, and recurrence were calculated among the sirolimus-exposed and unexposed. For each group, we constructed Kaplan-Meier curves to estimate overall survival proportions and cumulative incidence curves accounting for the competing risk of death to evaluate the proportion of recipients with an HCC recurrence across time (18). Associations with sirolimus were estimated using Cox regression adjusting for variables selected based on correlations with sirolimus use in our data and a priori knowledge of potential predictors of mortality and recurrence: ethnicity, calendar year of transplant, total tumor volume, alpha-fetoprotein (AFP) level, allocated Model for End-Stage Liver Disease (MELD) score based on exception points, calculated MELD score based on laboratory values, use of IL-2 induction therapy, and transplant center volume. For characteristics that could change over time, such as total tumor volume and AFP level, we used the most proximal values collected prior to transplant. The time scale for Cox regression was time since LT. We evaluated potential changes in the effect of sirolimus over time by testing interaction terms of sirolimus use with the log of time since transplantation in the multivariable model.
Sirolimus users and non-users had some characteristics that appeared to differ between the two groups but that included categories too infrequent to include in a parsimonious multivariable model. We therefore conducted sensitivity analyses after excluding these infrequent categories, in order to evaluate the effects on sirolimus associations. Specifically, we estimated sirolimus associations among: 1) recipients who met Milan criteria (19), 2) recipients without a history of renal failure, and 3) recipients with a deceased liver donor. We decided a priori to evaluate potential effect modification by recipient age and total tumor volume at transplant, and we present stratified analyses using cut-offs near the median values for these characteristics (≤55 or >55 years for recipients age, and <7 cm3 or ≥7 cm3 for total tumor volume).
In a sensitivity analysis, we adjusted for the variables included in the initial multivariable regression model by using them to calculate propensity scores. We then ran the proportional hazards models assessing the associations of sirolimus with post-transplant outcomes stratifying on deciles of the propensity score.
All statistical analyses were done using SAS version 9.3. Ethical approval or exemption from review was given by institutional review boards at the National Cancer Institute and HRSA.
RESULTS
Between 2002 and 2012, there were 13,991 U.S. liver transplants performed in recipients who had received HCC exception points while on the waitlist. Of these, 4,480 liver recipients had claims data on immunosuppressant medications in the first three months after transplantation. Among these recipients, 75 were excluded because they had an HCC recurrence, died, or had their last follow-up information before 3 months post-transplantation. Additionally, 241 recipients had discrepant sirolimus information in SRTR and IMS Health, including 38 recipients for whom sirolimus use was only reported in SRTR and 203 recipients for whom sirolimus use was only reported in IMS Health claims. Tumor size information was not available for 228 recipients.
After these exclusions, 3,936 HCC liver recipients remained in our study population with a median follow-up of 2.8 years starting 3 months post-transplantation (maximum follow-up of 11 years). Among these recipients, 234 (5.9%) received sirolimus in their initial immunosuppressant regimen (Table 1). For the majority of these recipients (62%), sirolimus claims were identified within a week following transplantation.
Table 1.
Characteristics of liver recipients with hepatocellular carcinoma, and outcomes following transplantation, stratified by sirolimus use
| Characteristic | Sirolimus Exposed Recipients (N=234) |
Sirolimus Unexposed Recipients (N=3702) |
p-value |
|---|---|---|---|
| Sex, N (%) | 0.97 | ||
| Female | 54 (23%) | 858 (23%) | |
| Male | 180 (77%) | 2844 (77%) | |
| Race, N (%) | 0.73 | ||
| White | 190 (81%) | 3026 (82%) | |
| Black | 25 (11%) | 344 (9%) | |
| Other | 19 (8%) | 332 (9%) | |
| Ethnicity, N (%) | 0.02 | ||
| Hispanic | 40 (17%) | 446 (12%) | |
| Non-Hispanic | 194 (83%) | 3256 (88%) | |
| Age at transplantation in years, median (IQR) | 57 (53–62) | 57 (53–62) | 0.96 |
| Prior renal failure, N (%) | 0.16 | ||
| Yes | 1 (0%) | 59 (2%) | |
| No | 233 (100%) | 3643 (98%) | |
| Type of donor, N (%) | 0.17 | ||
| Living | 1 (0%) | 57 (2%) | |
| Deceased | 233 (100%) | 3645 (98%) | |
| Calendar year at transplantation, median (IQR) | 2009 (2007–2011) | 2008 (2005–2011) | <0.001 |
| Transplant center volumea | <0.001 | ||
| <150 | 70 (30%) | 1078 (29%) | |
| 299-150 | 20 (9%) | 1737 (47%) | |
| ≥300 | 144 (62%) | 887 (24%) | |
| Total tumor volume, median (IQR) | 7.7 (4.2–15.7) | 7.2 (2.9–15.6) | 0.13 |
| Met Milan criteria, N (%) | <0.001 | ||
| Yes | 209 (89%) | 3526 (95%) | |
| No | 25 (11%) | 176 (5%) | |
| AFP in ng/mL, median (IQR)b | 13 (5–55) | 11 (5–40) | 0.33 |
| AFP <500 ng/mL, N (%)b | 0.54 | ||
| Yes | 210 (94%) | 3219 (95%) | |
| No | 13 (6%) | 166 (5%) | |
| Allocation MELD score, median (IQR)c | 25 (22–25) | 24 (22–28) | 0.12 |
| Calculated MELD score, median (IQR)c | 13 (10–16) | 12 (9–16) | 0.04 |
| Serum creatinine in mg/dL, median (IQR)c | 0.9 (0.7–1.1) | 0.9 (0.7–1.1) | 0.95 |
| Bilirubin in mg/dL, median (IQR)c | 1.8 (1.2–2.8) | 1.6 (1.0–2.7) | 0.08 |
| INR, median (IQR)c | 1.4 (1.2–1.5) | 1.3 (1.1–1.5) | 0.002 |
| Anti-IL2 induction therapy | |||
| Yes | 168 (72%) | 1821 (49%) | |
| No | 66 (28%) | 1881 (51%) | <0.001 |
| Polyclonal antibody induction therapy | |||
| Yes | 16 (7%) | 294 (8%) | |
| No | 218 (93%) | 3408 (92%) | 0.54 |
| Days from transplant to first sirolimus claim, median (IQR) | 6 (5–15) | - | |
| Outcomes following transplantation | |||
| Deaths, N | 47 | 832 | |
| Rate per 100 person-years (95% CI) | 6.43 (4.73–8.56) | 6.03 (5.63–6.45) | |
| Cancer-Specific Deaths, N | 13 | 271 | |
| Rate per 100 person-years (95% CI) | 1.78 (0.95–3.04) | 1.96 (1.74–2.21) | |
| Recurrences, N | 11 | 231 | |
| Rate per 100 person-years (95% CI) | 1.54 (0.77–2.75) | 1.71 (1.50–1.95) | |
Chi-squared tests were used to calculate p-values for categorical variables. Wilcoxon rank-sum tests were used to calculate p-values for continuous variables.
Transplant center volume was measured as the number of liver transplants done for recipients with a hepatocellular carcinoma diagnosis between 2002 and 2012
328 recipients (8.3%) did not have information on AFP.
Values were based on most recent measurement/score prior to liver transplantation.
IQR=interquartile range, AFP=alpha-fetoprotein, MELD=Model for End-Stage Liver Disease, INR=international normalized ratio
Recipients using sirolimus were similar to those not using sirolimus with regard to sex, race, and age (Table 1). Prior to transplantation, sirolimus users and non-users had similar total tumor volumes, AFP levels, and allocation MELD scores. Liver recipients on sirolimus were transplanted in more recent calendar years than non-users (median 2009 vs. 2008), and they were more frequently transplanted at large centers. Also, sirolimus users were more frequently Hispanic, had higher calculated MELD scores, more frequently had tumors that did not meet Milan criteria (11% vs. 5%), and more frequently received anti-IL-2 induction therapy at transplantation (Table 1).
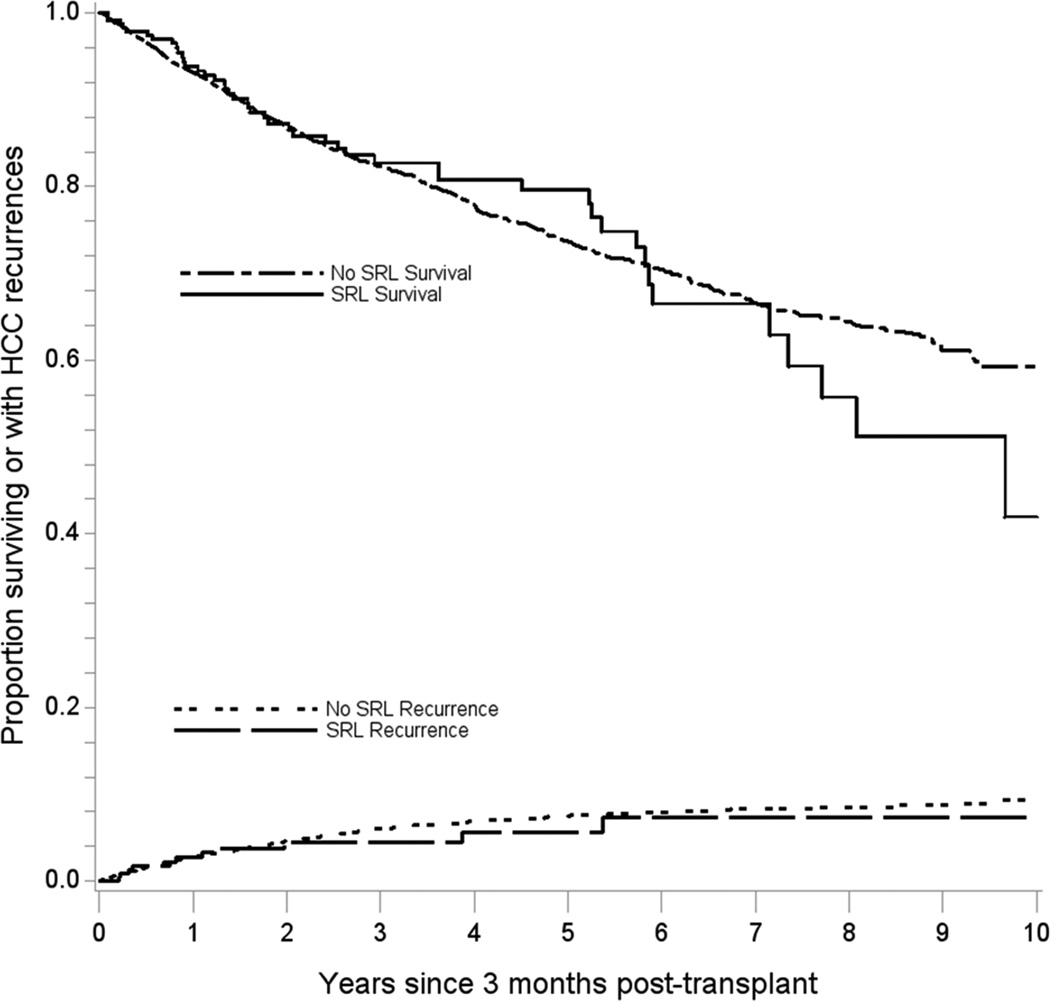
Among recipients who lived at least three months post-LT, there were 879 subsequent deaths, including 261 deaths attributable to cancer. Sirolimus users did not differ from non-users in terms of all-cause mortality (adjusted hazard ratio [aHR]: 1.01, 95%CI: 0.73–1.39; Figure 1). While cancer-specific mortality appeared lower in sirolimus users, the association was not significant (aHR: 0.80, 95%CI: 0.43–1.50) (see Table 1 and Table 2 for supporting details). The estimates of association with all-cause mortality and cancer-specific mortality did not vary over time since transplantation (p-values for interaction= 0.37 and 0.14, respectively). When other causes of death were assessed, sirolimus was associated with a non-significant increase in death from graft failure (Table 3).
Figure 1. Overall survival and cumulative incidence of hepatocellular carcinoma recurrence among sirolimus users and non-users.
The upper curves depict the overall survival probability across time after 3 months post-transplant for sirolimus users and non-users. The lower curves depict the cumulative incidence of HCC recurrence after accounting for the competing risk of death.
SRL=sirolimus
Table 2.
Associations of sirolimus use with all-cause mortality, cancer-specific mortality, and hepatocellular carcinoma recurrence, for all recipients and within subgroups
| All-cause mortality | Cancer-specific mortality | HCC recurrence | |||||
|---|---|---|---|---|---|---|---|
| Hazard Ratio (95% Confidence Interval) |
Hazard Ratio (95% Confidence Interval) |
Hazard Ratio (95% Confidence Interval) |
|||||
| N | Unadjusted | Adjusteda | Unadjusted | Adjusteda | Unadjusted | Adjusteda | |
| Overall | 3936 | 1.05 (0.78–1.40) |
1.01 (0.73–1.39) |
0.88 (0.51–1.54) |
0.80 (0.43–1.50) |
0.83 (0.45–1.51) |
0.86 (0.45–1.65) |
| Recipients who met Milan criteria | 3735 | 1.00 (0.73–1.37) |
0.98 (0.70–1.39) |
0.77 (0.41–1.44) |
0.77 (0.39–1.51) |
0.77 (0.40–1.51) |
0.91 (0.46–1.80) |
| Recipients without renal failure | 3876 | 1.05 (0.78–1.41) |
1.01 (0.73–1.40) |
0.90 (0.51–1.56) |
0.80 (0.43–1.49) |
0.84 (0.46–1.54) |
0.87 (0.45–1.67) |
| Recipients with deceased donors | 3878 | 1.05 (0.78–1.41) |
1.00 (0.72–1.38) |
0.89 (0.51–1.55) |
0.81 (0.43–1.50) |
0.76 (0.40–1.43) |
0.78 (0.39–1.56) |
| Recipients with total tumor volume<7 cm3 | 1917 | 0.92 (0.55–1.55) |
0.83 (0.47–1.47) |
1.18 (0.48–2.90) |
1.11 (0.44–2.81) |
0.84 (0.26–2.67) |
0.96 (0.29–3.12) |
| Recipients with total tumor volume≥7 cm3 | 2019 | 1.09 (0.77–1.56) |
1.09 (0.73–1.63) |
0.74 (0.36–1.50) |
0.66 (0.28–1.52) |
0.79 (0.39–1.60) |
0.78 (0.35–1.72) |
| Recipients ≤55 years of age at transplant | 1576 | 1.71 (1.15–2.54) |
1.76 (1.12–2.75) |
1.63 (0.83–3.22) |
1.54 (0.71–3.32) |
1.39 (0.64–3.00) |
1.49 (0.62–3.61) |
| Recipients >55 years of age at transplant | 2360 | 0.67 (0.43–1.04) |
0.62 (0.38–1.01) |
0.43 (0.16–1.15) |
0.34 (0.11–1.09) |
0.48 (0.18–1.31) |
0.52 (0.19–1.44) |
Models are adjusted for ethnicity, calendar year of transplant, total tumor volume, AFP, allocated MELD score, calculated MELD score, transplant center size, and use of IL-2 induction therapy.
HCC=hepatocellular carcinoma, AFP= alpha-fetoprotein, MELD=Model for End-Stage Liver Disease
Table 3.
Associations of sirolimus with non-cancer causes of death
| Hazard ratio comparing sirolimus users to non-users (95% confidence interval) |
||
|---|---|---|
| Cause of death | Unadjusted | Adjusteda |
| Graft failure | 1.78 (0.98–3.23) | 1.80 (0.93–3.48) |
| Infection | 0.39 (0.10–1.57) | 0.41 (0.10–1.70) |
| Cardiovascular disease | 1.00 (0.31–3.20) | 1.18 (0.35–3.95) |
Models are adjusted for ethnicity, calendar year of transplant, total tumor volume, AFP, allocated MELD score, calculated MELD score, transplant center size, and use of IL-2 induction therapy.
There were a total of 242 HCC recurrences observed across follow-up, of which 11 occurred in sirolimus users (Table 1). The HCC recurrence rate in sirolimus users appeared lower, but again the reduction was not statistically significant (aHR: 0.86, 95%CI: 0.45–1.65); the magnitude of the association with sirolimus did not change over time since transplantation (p-value for interaction=0.83, Figure 1).
In a sensitivity analysis using propensity scores to adjust for potential differences in sirolimus users and non-users, the associations of sirolimus with post-transplant outcomes were similar to the main analysis (all-cause mortality aHR: 1.02, 95%CI: 0.74–1.40; cancer-specific mortality aHR: 0.85, 95%CI: 0.45–1.62; HCC recurrence aHR: 0.76, 95%CI: 0.39–1.49).
Similarly, there were no clear differences in all-cause mortality after limiting to recipients who met Milan criteria (aHR: 0.98, 95%CI: 0.70–1.39), recipients without a history of renal failure (aHR: 1.01, 95%CI: 0.73–1.40), and recipients with a deceased liver donor (aHR: 1.00, 95%CI: 0.72–1.38; Table 2). Nonetheless, cancer-specific mortality and recurrence rates were non-significantly lower among sirolimus users in these subgroups, mirroring the primary analyses. Total tumor volume prior to transplantation did not appear to modify the effect of sirolimus (p-value for interaction >0.20 for all outcomes, Table 2).
Results differed by recipient age at transplantation, particularly for sirolimus associations with all-cause mortality (p-value for interaction=0.004). For recipients 55 years of age or younger at transplantation, sirolimus was associated with higher all-cause mortality (aHR: 1.76, 95%CI: 1.12–2.75). Among these younger recipients, sirolimus users also had higher recurrence and cancer-specific mortality rates, though these associations were not statistically significant (Table 2). For recipients older than 55 years of age, sirolimus had a borderline association with lower all-cause mortality (aHR: 0.62, 95%CI: 0.38–1.01). In this older age group, sirolimus was also associated with non-significant reductions in cancer-specific mortality (aHR: 0.34, 95%CI: 0.11–1.09) and recurrence (aHR: 0.52, 95%CI: 0.19–1.44).
DISCUSSION
In this large U.S. study in which we utilized two independent sources of data on use of immunosuppressant medications, we did not observe clear evidence for a benefit of sirolimus use for recipients of liver transplants for HCC. All-cause mortality rates were the same for sirolimus users and non-users. While recurrence rates and cancer-specific mortality rates tended to be lower among sirolimus users, these differences did not achieve statistical significance. Among younger recipients, sirolimus users had higher mortality and recurrence rates, while there was a suggestion that sirolimus may reduce recurrence and mortality among older recipients. However, no statistically significant reductions were seen in any subgroup of recipients.
Although mTOR inhibitors have anti-carcinogenic properties, use of sirolimus may lack meaningful benefit in this population for a number of reasons. While mutations leading to hyperactivation of the mTOR pathway have been characterized in HCC (8), hyperactivation is not uniformly present in all HCC tumors (9, 20) and was likely absent in some of the HCC cases in our study. Inhibiting mTOR would have no effect on tumors that are not dependent on the mTOR pathway, thus diluting the overall effect observed among liver recipients with HCC. It is also possible that sirolimus doses were too low to be effective for cancer prevention, as doses of mTOR inhibitors used to prevent graft rejection are typically lower than doses used in cancer treatment. In addition, HCC tumors are known to have high levels of within-tumor heterogeneity (21), which may allow the tumor to evolve resistance in response to mTOR inhibition.
Providing further evidence of the limits of HCC control through mTOR inhibition, a recently completed randomized trial of sirolimus-based immunosuppression in liver recipients with HCC did not find evidence of improved recurrence-free survival (22). Additionally, a randomized trial of patients with advanced HCC treated outside the transplant setting found no benefit associated with use of everolimus (an mTOR inhibitor that is molecularly similar to sirolimus) (23). While the prior observational study using SRTR data reported a survival benefit associated with sirolimus use in HCC liver recipients (12), we were unable to reproduce this result, even when we attempted to replicate their study design and methods. Specifically, when we limited our study population to fit the inclusion criteria of the prior observational study and only used SRTR data on immunosuppressant medications to define sirolimus use, we estimated that 5-year survival was 75% among both sirolimus users and non-users, in contrast to the previously reported results showing 83% survival among sirolimus users and 69% survival among sirolimus non-users (12).
The lack of a reduction in all-cause mortality may also be partly due to adverse effects of sirolimus use, which might negate any improvements in survival due to reduced HCC recurrence. Such adverse events might include hepatic artery thrombosis, as observed in the prior randomized trial of liver recipients (10). Although we could not directly assess these events in our study, we did observe a non-significant increase in deaths from graft failure in sirolimus users, some of which could potentially be attributable to hepatic artery thrombosis. Higher rates of hyperlipidemia and hyperglycemia have also been observed in liver recipients treated with mTOR inhibitors (24), and among kidney recipients, nephrotoxicity has been observed when sirolimus is used in conjunction with calcineurin inhibitors (25). Of note, another study that used SRTR data to assess outcomes related to sirolimus use in liver recipients, but was not restricted to HCC patients, found that sirolimus was associated with significantly higher mortality among recipients with hepatitis C infection (26).
It is more difficult to interpret the associations that we observed for cancer-specific mortality and HCC recurrence, for which there was suggestive but inconclusive evidence of a benefit. While the effect was marginal and nonsignificant for the overall group of liver recipients, mTOR inhibitors may still be useful in particular subgroups. In our study, among people older than 55 years of age at transplantation, sirolimus users had lower recurrence, cancer-specific mortality, and all-cause mortality rates, though these associations were still not statistically significant. Importantly, among the younger recipients, all-cause mortality was higher with sirolimus use, indicating that sirolimus use may actually be detrimental in this subgroup. This difference may reflect varying molecular characteristics of tumors in different age groups, or differences in recipients’ susceptibility to side effects of the various immunosuppressant medication options. Associations in stratified analyses could also be due to chance and should be interpreted with caution. In contrast to our study, the recent trial in liver recipients with HCC found that sirolimus use appeared most beneficial among younger recipients, specifically those less than 60 years of age (22).
Although beyond the scope of our study, we speculate that molecular subtyping of tumors might best identify a subset of recipients who would benefit from the use of sirolimus or everolimus, given that mTOR inhibitors would be expected to be most effective against tumors with mTOR pathway hyperactivation. Another group of recipients that may be more likely to benefit from mTOR inhibitors are those who have already experienced a recurrence after HCC LT. Once a recurrence occurs, it is far more likely that the recipient will die from cancer than from other causes, and so switching to an mTOR inhibitor-based immunosuppressant regimen may be beneficial.
Our large study of HCC liver recipients has a number of strengths. Unlike most prior observational studies of sirolimus among HCC liver recipients (14), our study drew from numerous transplant centers across the United States, and so we were able to evaluate sirolimus users and non-users transplanted during the same calendar periods. We capitalized on the availability of pharmacy claims data in addition to the information from the SRTR in order to confidently categorize recipients as sirolimus users or non-users. The identification of a number of discrepancies between SRTR information and pharmacy claims confirmed that a single source of information may not always be reliable. We evaluated multiple outcomes, including recurrence and cancer-specific mortality, in order to more clearly assess whether sirolimus had an effect on the course of HCC. We were also able to evaluate these outcomes over an extended follow-up period after transplantation, which was longer than in typical randomized trials.
We acknowledge that there were also several limitations. While measures such as AFP and total tumor volume did not differ notably between sirolimus users and non-users, we lacked information on other measures of HCC severity, such as HCC tumor grade. Clinicians may have disproportionately prescribed sirolimus to recipients with more severe HCC, thus leading to a greater risk of recurrence and mortality among sirolimus users. We also did not have information on ablative treatment prior to transplantation, which could affect outcomes after transplantation. While our study was one of the largest to date evaluating sirolimus use among HCC liver recipients, we still had a small number of sirolimus users. Given our sample size and length of follow-up, we only had 80% power to detect a 42% decrease in all-cause mortality and a 75% decrease in HCC recurrence among sirolimus users. Thus, our ability to detect more modest effects, which might still be clinically meaningful, was limited. The available sample also limited our ability to assess the effects of sirolimus in some subgroups of recipients. For example, sirolimus may be more useful in recipients who are transplanted when their tumors do not meet Milan criteria at transplantation, but in our study there were only 25 sirolimus users who met this description.
In conclusion, our study did not indicate a clear benefit of sirolimus use among liver recipients with HCC in terms of overall survival, although there was a suggestive reduction in cancer-specific mortality and HCC recurrence. Of interest, effects appeared to vary by age at transplant, and sirolimus use in our study was associated with significantly worse overall survival in younger recipients. Currently, randomized clinical trials evaluating sirolimus and everolimus use among liver recipients with HCC are ongoing (clinicaltrials.gov: NCT00355862, NCT02081755) (27). Completion of these trials will provide further evidence to inform the use of mTOR inhibitors in this population. Even if these trials fail to demonstrate benefit for recipients with HCC overall, mTOR inhibitors could still play a role in treating subsets of recipients, such as recipients with molecularly characterized tumors indicating mTOR pathway hyperactivation or those who have already experienced a recurrence after transplantation.
Acknowledgments
The authors gratefully acknowledge the support and assistance by individuals at the Scientific Registry of Transplant Recipients.
Funding
ELY and EAE were supported by the Intramural Research Program of the National Cancer Institute. The Scientific Registry of Transplant Recipients was managed by Minneapolis Medical Research Foundation in Minneapolis, MN (contract HHSH250201000018C). Research support for the linkage between the Scientific Registry of Transplant Recipients and IMS Health pharmacy claims was provided by Pfizer.
The data reported here have been supplied by the Minneapolis Medical Research Foundation (MMRF) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government.
List of Abbreviations
- AFP
alpha-fetoprotein
- aHR
adjusted hazard ratio
- HCC
hepatocellular carcinoma
- LT
liver transplantation
- MELD
Model for End-Stage Liver Disease
- mTOR
mammalian target of rapamycin
- SRTR
Scientific Registry of Transplant Recipients
Footnotes
Disclosures
The authors of this manuscript have no conflicts of interest to disclose as described by Liver Transplantation.
REFERENCES
- 1.United States Organ Transplantation: OPTN & SRTR Annual Data Report 2011. Liver. Rockville, MD: U.S. Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation; 2012. Organ Procurement and Transplantation Network and Scientific Registry of Transplant Recipients. [Google Scholar]
- 2.Cabrera R, Nelson DR. Review article: the management of hepatocellular carcinoma. Aliment Pharmacol Ther. 2010;31(4):461–476. doi: 10.1111/j.1365-2036.2009.04200.x. [DOI] [PubMed] [Google Scholar]
- 3.Toso C, Mentha G, Majno P. Liver transplantation for hepatocellular carcinoma: five steps to prevent recurrence. Am J Transplant. 2011;11(10):2031–2035. doi: 10.1111/j.1600-6143.2011.03689.x. [DOI] [PubMed] [Google Scholar]
- 4.Schlitt HJ, Neipp M, Weimann A, Oldhafer KJ, Schmoll E, Boeker K, et al. Recurrence patterns of hepatocellular and fibrolamellar carcinoma after liver transplantation. J Clin Oncol. 1999;17(1):324–331. doi: 10.1200/JCO.1999.17.1.324. [DOI] [PubMed] [Google Scholar]
- 5.Castroagudin JF, Molina-Perez E, Ferreiro-Iglesias R, Abdulkader I, Otero-Anton E, Tome S, et al. Late recurrence of hepatocellular carcinoma after liver transplantation: is an active surveillance for recurrence needed? Transplant Proc. 2012;44(6):1565–1567. doi: 10.1016/j.transproceed.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Faivre S, Kroemer G, Raymond E. Current development of mTOR inhibitors as anticancer agents. Nat Rev Drug Discov. 2006;5(8):671–688. doi: 10.1038/nrd2062. [DOI] [PubMed] [Google Scholar]
- 7.Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer. 2004;4(5):335–348. doi: 10.1038/nrc1362. [DOI] [PubMed] [Google Scholar]
- 8.Zhou Q, Lui VWY, Yeo W. Targeting the PI3K/Akt/mTOR pathway in hepatocellular carcinoma. Future Oncol. 2011;7(10):1149–1167. doi: 10.2217/fon.11.95. [DOI] [PubMed] [Google Scholar]
- 9.Zhou L, Huang Y, Li J, Wang Z. The mTOR pathway is associated with the poor prognosis of human hepatocellular carcinoma. Med Oncol. 2010;27(2):255–261. doi: 10.1007/s12032-009-9201-4. [DOI] [PubMed] [Google Scholar]
- 10.Massoud O, Wiesner RH. The use of sirolimus should be restricted in liver transplantation. J Hepatol. 2012;56(1):288–290. doi: 10.1016/j.jhep.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 11.McKenna GJ, Trotter JF. Sirolimus--it doesn't deserve its bad Rap(a) J Hepatol. 2012;56(1):285–287. doi: 10.1016/j.jhep.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 12.Toso C, Merani S, Bigam DL, Shapiro AM, Kneteman NM. Sirolimus-based immunosuppression is associated with increased survival after liver transplantation for hepatocellular carcinoma. Hepatology. 2010;51(4):1237–1243. doi: 10.1002/hep.23437. [DOI] [PubMed] [Google Scholar]
- 13.Chinnakotla S, Davis GL, Vasani S, Kim P, Tomiyama K, Sanchez E, et al. Impact of sirolimus on the recurrence of hepatocellular carcinoma after liver transplantation. Liver Transpl. 2009;15(12):1834–1842. doi: 10.1002/lt.21953. [DOI] [PubMed] [Google Scholar]
- 14.Menon KV, Hakeem AR, Heaton ND. Meta-analysis: recurrence and survival following the use of sirolimus in liver transplantation for hepatocellular carcinoma. Aliment Pharmacol Ther. 2013;37(4):411–419. doi: 10.1111/apt.12185. [DOI] [PubMed] [Google Scholar]
- 15.Zhou J, Wang Z, Wu ZQ, Qiu SJ, Yu Y, Huang XW, et al. Sirolimus-based immunosuppression therapy in liver transplantation for patients with hepatocellular carcinoma exceeding the Milan criteria. Transplant Proc. 2008;40(10):3548–3553. doi: 10.1016/j.transproceed.2008.03.165. [DOI] [PubMed] [Google Scholar]
- 16.Zimmerman MA, Trotter JF, Wachs M, Bak T, Campsen J, Skibba A, et al. Sirolimus-based immunosuppression following liver transplantation for hepatocellular carcinoma. Liver Transpl. 2008;14(5):633–638. doi: 10.1002/lt.21420. [DOI] [PubMed] [Google Scholar]
- 17.Leppke S, Leighton T, Zaun D, Chen SC, Skeans M, Israni AK, et al. Scientific Registry of Transplant Recipients: collecting, analyzing, and reporting data on transplantation in the United States. Transplant Rev. 2013;27(2):50–56. doi: 10.1016/j.trre.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Lin G, So Y, Johnston G. Analyzing survival data with competing risks using SAS Software. SAS Global Forum 2012 Conference; SAS Institute Inc; Cary, NC. 2012. [Google Scholar]
- 19.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334(11):693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 20.Sieghart W, Fuereder T, Schmid K, Cejka D, Werzowa J, Wrba F, et al. Mammalian target of rapamycin pathway activity in hepatocellular carcinomas of patients undergoing liver transplantation. Transplantation. 2007;83(4):425–432. doi: 10.1097/01.tp.0000252780.42104.95. [DOI] [PubMed] [Google Scholar]
- 21.Friemel J, Rechsteiner M, Frick L, Bohm F, Struckmann K, Egger M, et al. Intratumor heterogeneity in hepatocellular carcinoma. Clin Cancer Res. 2015;21(8):1951–1961. doi: 10.1158/1078-0432.CCR-14-0122. [DOI] [PubMed] [Google Scholar]
- 22.Geissler EK, Schnitzbauer AA, Zulke C, Lamby PE, Proneth A, Duvoux C, et al. Sirolimus use in liver transplant recipients with hepatocellular carcinoma: a randomized, multicenter, open-label phase 3 trial. Transplantation. 2015 doi: 10.1097/TP.0000000000000965. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu AX, Kudo M, Assenat E, Cattan S, Kang YK, Lim HY, et al. Effect of everolimus on survival in advanced hepatocellular carcinoma after failure of sorafenib: the EVOLVE-1 randomized clinical trial. JAMA. 2014;312(1):57–67. doi: 10.1001/jama.2014.7189. [DOI] [PubMed] [Google Scholar]
- 24.Klintmalm GB, Nashan B. The Role of mTOR Inhibitors in Liver Transplantation: Reviewing the Evidence. J Transplant. 2014;2014:845438. doi: 10.1155/2014/845438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Halloran PF. Immunosuppressive drugs for kidney transplantation. N Engl J Med. 2004;351(26):2715–2729. doi: 10.1056/NEJMra033540. [DOI] [PubMed] [Google Scholar]
- 26.Watt KD, Dierkhising R, Heimbach JK, Charlton MR. Impact of sirolimus and tacrolimus on mortality and graft loss in liver transplant recipients with or without hepatitis C virus: an analysis of the Scientific Registry of Transplant Recipients Database. Liver Transpl. 2012;18(9):1029–1036. doi: 10.1002/lt.23479. [DOI] [PubMed] [Google Scholar]
- 27.Schnitzbauer AA, Zuelke C, Graeb C, Rochon J, Bilbao I, Burra P, et al. A prospective randomised, open-labeled, trial comparing sirolimus-containing versus mTOR-inhibitor-free immunosuppression in patients undergoing liver transplantation for hepatocellular carcinoma. BMC Cancer. 2010;10:190. doi: 10.1186/1471-2407-10-190. [DOI] [PMC free article] [PubMed] [Google Scholar]