Abstract
Growth hormone (GH) promotes postnatal human growth primarily by regulating insulin-like growth factor (IGF)-I production through activation of the GH receptor (GHR)-signal transducer and activator of transcription (STAT)-5B signaling cascade. The critical importance of STAT5B in human IGF-I production was confirmed with the identification of the first homozygous, autosomal recessive, STAT5B mutation in a young female patient who phenotypically resembled patients with classical growth hormone insensitivity (GHI) syndrome (Laron syndrome) due to mutations in the GHR gene, presenting with severe postnatal growth failure and marked IGF-I deficiency. Of note, the closely related STAT5A, which share >95% amino acid identity with STAT5B, could not compensate for loss of functional STAT5B. To date, 7 homozygous, inactivating, STAT5B mutations in 10 patients have been reported. STAT5B deficient patients, unlike patients deficient in GHR, can also present with a novel, potentially fatal, primary immunodeficiency, which can manifest as chronic pulmonary disease. STAT5B deficiency may be underestimated in endocrine, immunology and pulmonary clinics.
Keywords: STAT5B mutations, IGF-I deficiency, growth hormone insensitivity, T regulatory cell deficiency
Introduction
The 7 human STAT (signal transducer and activator of transcription) family of proteins (STAT1, -2, -3, -4, -5a, -5b and -6) are expressed in multiple cell types, activated by multiple growth factors and cytokines, participating in a diverse set of biological activities(1). With increased accessibility and application of next-generation whole exome sequencing in clinical settings, genetic defects have now been identified for all, but the STAT5A, genes, germ-line as well as somatic (2–5). To date, each of the described STAT deficiencies is associated with distinct immuno-deficiencies (2–5). Only STAT5B deficiency (MIM245590) present co-morbidities of growth hormone insensitivity (GHI) syndrome (MIM262500), with severe insulin-like growth factor (IGF)-1 deficiency (IGFD) and profound postnatal growth failure(6). Of note, recent reports describe activating, heterozygous, STAT3 mutations associated with growth failure and IGFD, but these features, in contrast to STAT5B defects, was proposed to be secondary to the lymphoproliferation and autoimmunity phenotype(7–9). The present review will focus on the impacts of germ-line STAT5B mutations in human growth and immunity.
STAT5B protein
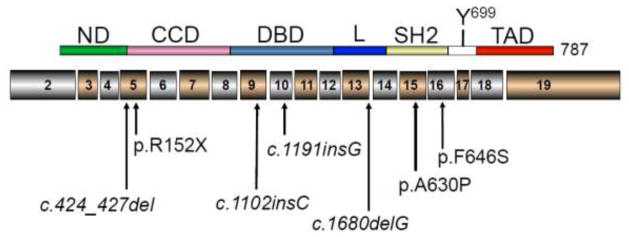
Human STAT5B, typical of members of the STAT family (1), consist of discrete protein modules (Figure 1) of which the modular src-homology 2 (SH2) domain permits STAT5B to bind phosphorylated tyrosines, including those on activated receptors such as the GH receptor (GHR) or interleukin (IL) receptors. Recruited STAT5B itself is activated upon phosphorylation of tyrosine residue, Y699, downstream of the SH2 domain (Figure 1), by kinases such as cytosolic Janus kinase 2, JAK2. The phosphorylation of two serines, S128 and S193, and acetylation of L701, have been reported as other mechanisms for regulating STAT5B activities (10–13).
Figure 1.
Homozygous human STAT5B mutations identified. Schematic of the human STAT5B peptide (upper schematic) and encoding exons (lower schematic). Mutations identified are indicated. Tyrosine 699 (Y699) that can be phosphorylated by JAK2 and other kinases is shown. The domains indicated: ND, N-terminal domain; CCD, coil-coiled domain; DBD, DNA binding domain; L, linker domain; SH2, Src-homology 2 domain; TAD, transactivation domain.
STAT5B is most closely related to STAT5A, sharing a striking 96% identity at the amino acid residue level. By contrast, STAT5B shares only 24% similarity with STAT3. Interestingly, the genes encoding these 3 STAT proteins lie within ~204 kilobases (kb) region of each other on chromosome 17q11.2, with STAT5A and STAT5B genes (77.23 kb and 24.4 kb, respectively) only 11 kb apart(14), suggesting the possibility of a gene duplication. The divergence between the translated STAT5B and STAT5A proteins is primarily at the C-terminus of the TAD region, where a 20 amino acid sequence in STAT5A distinguishes it from STAT5B. Indeed, the inability to readily differentiate between these two closely-related proteins, for many years, led them to be considered interchangeable, redundant, entities, designated STAT5. The identification of STAT5B mutations associated with the complex clinical syndrome of GHI and immune deficiency firmly established, in humans, that STAT5B and STAT5A have certain distinct and non-redundant roles, despite their high degree of identity. Human mutations in STAT5A, as noted above, have yet to be identified.
The Human Growth Hormone Receptor – STAT5B Signaling Pathway
Pituitary-derived growth hormone (GH) promote normal human postnatal growth by regulating the expression of insulin-like growth factor (IGF)-I, both circulating (liver derived) and peripheral. The importance of IGF-I for human growth is supported by clinical conditions of primary IGFD due to GH deficiency (GHD), mutations in the GH receptor (GHR), and rare homozygous inactivating IGF1 mutations identified in patients characterized by in utero and severe postnatal growth failure (height SDS, HtSDS, below −4.9), microcephaly, intellectual impairment, and sensorineural deafness(15–17).
The binding of GH to cell surface homo-dimeric GHR(18) activates the associated JAK2, which initiates signaling cascades including four STAT pathways (STAT1, STAT3, STAT5A and STAT5B), the MAPK (mitogen-activated protein kinase) and the PI3K (phosphoinositide-3 kinase) pathways. Recent studies indicate that three of the seven intracellular tyrosines in the human GHR (mature peptide: Y516, Y548, Y609) are necessary, individually or in combination, for STAT5B signaling(19). This redundancy in tyrosine utilization by STAT5B may explain why, of the more than 80 GHR mutations associated with GHI syndrome(20,21), only a handful are located within the intracellular domain of the GHR(22) and why damaging mutations frequently involve frameshifts that abrogate the JAK2 binding site and/or the three critical GHR tyrosines(23).
Surprisingly little is known regarding the DNA elements recognized by STAT5B in the transcriptional regulation of the human IGF1 gene. In the rat Igf1 gene locus, 7 Gh-induced Stat5b response elements were recently reported (24). Interestingly, 3 of these Gh response elements (GHRE) were located well upstream of the Igf1 gene (63 kb, 73kb and 86 kb from the Igf1 start site), and 4 were intronic (one in intron 2 and three in intron 3). These rat GHREs did not align with established canonical response elements (GAS, γ-interferon-activated sequences) that are frequently recognized by members of the STAT family. Although both human STAT5B and STAT5A associated with these rat elements in in vitro gel-shift binding assays (25), it remains unclear whether variations of these rat elements, found in the human IGF1 gene (24,26), are utilized by STAT5B for regulating IGF1 expression.
The Stat5b−/− mouse model for growth
The experimental disruption of each of the seven Stat proteins in rodent models implicated Stat5b, in particular, as important for postnatal growth. Stat5b−/− mice displayed loss of sexually dimorphic growth, with a concomitant reduction of serum Igf-I concentrations by 30–50% (27,28). While male Stat5b−/− mice were reduced in size to wild-type female mice, no differences were observed between Stat5b−/− and Stat5b+/+ female mice, suggesting a critical role for Stat5b in male/female differential growth in mice. Disruption of both Stat5b and the Stat5a genes imparted a more pronounced effect on growth, affecting both male and female mice, with growth phenotypes similar to those observed in mice deficient for either Gh or the Ghr (28,29). This growth restriction, together with observed profound immune deficiency, recapitulated the human STAT5B deficient condition (see below) but suggested that, in mice, Stat5a can partially compensate for loss of Stat5b.
Molecular defects in the human STAT5B gene
Molecular defects along the human GH-IGF-I axis are extremely rare. The identification of STAT5B mutations associated with severe growth failure, marked IGF-I deficiency, and insensitivity to GH, provided the first definitive demonstrations that the STAT5B signaling pathway is critical for GH-induced IGF-I production and normal growth in humans (Table 1; (30,31)). The first STAT5B mutation, reported in 2003, was identified in a 16 year old female from a consanguineous pedigree who presented biochemistries, a growth profile, and facial features, similar to Laron syndrome (classical GHI), but had wild-type GHR and additional presentations of chronic pulmonary disease (Table 1, (32)). An autosomal recessive, homozygous missense mutation, p.Ala630Pro located in the SH2 domain of STAT5B, was subsequently identified. The p.Ala630Pro substitution disrupted the core of anti-parallel β-sheets that forms the pocket for binding phosphate groups(33), causing loss of thermodynamic stability, as well as aberrant folding and aggregation of the mutant STAT5B protein(34,35). Quantitative real-time-PCR (polymerase chain reaction) amplification analysis of primary dermal fibroblast cells derived from the patient, furthermore, demonstrated that the loss of functional STAT5B correlated to loss of GH-induced IGF1 expression(32) which was not restored by activation of endogenous STAT5A(36). Altogether, these in vitro analyses supported in vivo presentations of IGF deficiency and GH resistance in the patient.
Table 1.
Phenotype of patients carrying homozygous STAT5B mutations. Phenotype was as described in reports. The nonsense mutation, p.Arg152*, was identified in two unrelated subjects.
| STAT5B Mutation Homozygous |
Gender | Age, yr |
Height SDS |
Birth | GHI | IGFD | Prolactin Elevated |
Hypergamma- globulinemia |
T-cell lymphopenia |
Pulmonary Disease |
Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| p.Ala630Pro1 | F | 16.5 | −7.5 | AGA | +++ | +++ | +++ | +++ | +++ | +++ | 28, 43, 44 |
| c.1191insG1 | F | 16.4 | −7.8 | AGA | +++ | +++ | ND | ND | ND | +++ | 33 |
| p.Arg152* | F | 15.3 | −9.9 | unknown | ND | ND | +++ | +++ | +++ | +++ | 35 |
| p.Arg152*1 | F | 12 | −5.3 | SGA | +++ | +++ | Normal | No | No | +++ | 36 |
| c.1102insC | M | 31 | −5.9 | AGA | +++ | +++ | +++ | No | No | No | 34, 41 |
| c.1680delG2 | F | 2 | −5.8 | AGA | +++ | +++ | ND | ND | ND | + | 38 |
| c.1680delG2 | F | 4 | −5.6 | AGA | +++ | +++ | ND | ND | ND | + | 38 |
| c.424_427del3 | M | 6 | −5.6 | AGA | +++ | +++ | +++ | + | +++ | +++ | 39 |
| c.424_427del3 | M | 2 | −3.0 | AGA | +++ | +++ | +++ | No | +++ | +++ | 39 |
| p.Phe646Ser | F | 14.8 | −5.95 | unknown | +++ | +++ | +++ | +++ | +++ | No | 37 |
Deceased as of this review;
Siblings;
Siblings.
AGA, appropriate for gestational age; ND, not determined
+ to +++, increasing severity of indications
Six other homozygous STAT5B mutations have been described to date, identified in 9 subjects(37–41), including 2 sets of siblings(42,43). All the mutations were autosomal recessive, suggesting that haploinsufficiency of STAT5B has modest, or minimal, effects on IGF-I expression, growth, and immune complications (30). The mutations, located in different domains of the STAT5B protein (Figure 1), include one nonsense mutation and 4 frameshifts (deletions or insertions), all of which is predicted to result in early protein termination. Only one other missense mutation, p.Phe646Ser, has been identified. Similar to p.Ala630P, p.Phe646Ser is located within the SH2 domain, but In contrast to p.Ala630Pro, loss of function was due to an inability to drive transcription(41).
Impact of STAT5B deficiency on Growth
Auxology
Birth size, where documented for the STAT5B deficient subjects, was normal for gestation (Table 1), similar to patients who carry GHR mutations. Postnatal growth failure was significant and consistent with the degree of IGF deficiency. Growth profiles were indistinguishable from those with GHI (or Laron) syndrome (44). At first report, height SDS ranged from −3.0 SDS to −9.9 (Table 1). Bone age, when measured, was considerably delayed (39,43). Puberty was also consistently delayed (Table 1), reflecting the low levels of circulating IGF-I (Table 1) and a state of chronic illness (see below). Mild facial dysmorphic features, such as a prominent forehead, depressed nasal bridge and high-pitched voice, were noted for some of the STAT5B deficient subjects (32,39,43).
Hormonal Evaluations
The severe post-natal growth failure of STAT5B deficient patients correlated with the clinical endocrine profile (Table 1). Basal GH levels were normal, and when stimulated, GH concentrations were frequently elevated. Serum IGF-I, IGFBP-3 and ALS concentrations in all cases were abnormally low (Table 1), and remained low after GH treatment in an IGF-I generation test (32,37,45) or during GH therapy (38,43). Some of the subjects underwent growth hormone therapy (1yr to 4yr), but growth response was uniformly poor (32,43). Interestingly, serum prolactin levels, when recorded, were abnormally high (Table 1). Pugliese-Pires et al determined that the hyperprolactinemia state for Cases 9 and 10 was not a result of macroPRL or of pituitary tumor (43). It is likely that the STAT5B mutations disrupted the negative feedback loop for PRL production, although the mechanisms involved remain to be clarified.
Impact of STAT5B deficiency on Immunity
Immune deficiency phenotype
A distinguishing feature in the patients carrying STAT5B mutations from those carrying GHR, IGFI, IGF1R or IGFALS mutations was symptoms of immune dysfunction. Shared symptoms in 8 of the 10 patients include severe eczema, chronic pulmonary disease manifesting as early as the first year of life (39,40,43), and confirmed lung fibrosis and/or lymphoid interstitial pneumonia (LIP), a condition of unknown etiology that is rare in children and often associated with autoimmune disease (46). Corticosteroid and oxygen treatments temporarily stabilize worsening pulmonary functions, but three of the patients, including the first described case of STAT5B deficiency (carrying p.Ala630Pro)(47), succumbed and died as consequences of progressive pulmonary fibrosis and respiratory failure (40) (Dr Merih Berberoglu, personal communication). Only one of the patients has undergone a lung transplantation at age 17.5 years that appeared to have successful alleviated impaired pulmonary function and the requirement for oxygen (43).
Interestingly, two of the patients lacked severe pulmonary problems, although both had symptoms of mild immune dysfunction: the patient carrying STAT5B c.1102insC was reported to have contracted haemorrhagic varicella at 16 years of age and had congenital ichthyosis and erythema, but, otherwise, appeared relatively healthy(38), and the patient carrying STAT5B p.Phe646Ser(41) had autoimmune thyroiditis, psoriasis, atopecia and was diagnosed with Celiac disease at age 20 yr(47). The explanation for the lack of chronic pulmonary disease in these two patients remains to be elucidated.
Immunological evaluations
Unlike the endocrine profiles that showed an absolute association between STAT5B mutations and IGF deficiency, the abnormalities in the immunological profile, where evaluated, was variable, even between siblings who carried the same homozygous STAT5B mutation(43). Nevertheless, hypergammaglobulinemia and T-cell lymphopenia were common observations, with both CD4+ and CD8+ T-cells often below normal ranges(39,41,43,47,48). In particular, a subset of CD4+ cells, the CD4+CD25high cells or T regulatory cells (Treg), is significantly diminished. CD25high is the α-subunit of the heterotrimeric IL-2 receptor (IL2R) complex and its expression permits IL2 to bind IL2R complex with high affinity. Perturbations of Tregs, which are essential for the propagation and homeostasis of T-cell populations (49), most likely lead to an abnormal accumulation and proliferation of lymphocytes in extra-lymphoid tissues. The increased susceptibility to opportunistic infections could also be related to decreased CD25 on all T cells, as has been reported in humans with severe CD25 deficiency (50). The forkhead-box family of transcription factor, FOXP3, shown to be regulated by STAT5 (51,52), is highly expressed in Treg, but was significantly reduced in STAT5B deficient Treg (48,53). Altogether, the reduced CD25high and FOXP3 expression associated with homozygous STAT5B mutations appears likely to have contributed to the novel immunodeficiency observed in these patients.
Summary
The identification of patients with unequivocal defects in the GH-induced STAT5B signaling has furthered our understanding of the molecular basis of growth failure associated with primary IGF deficiency. The STAT5B mutations identified to date were autosomal recessive, suggesting that haploinsufficiency of STAT5B has minimal effects on IGF-I expression and growth. Significantly, in humans, unlike in rodent models, the presence of STAT5A cannot compensate for the loss of STAT5B, thus supporting the hypothesis that GH-induced regulation of IGF-I production is mediated predominantly by STAT5B. In addition to severe IGFD and profound growth failure, mutation in the STAT5B gene cause a novel form of primary immune deficiency involving Treg dysfunction and associated pulmonary diseases. The lack of chronic pulmonary diseases in two of the patients supported the hypothesis STAT5B deficiency may be underestimated in endocrine, immunology and pulmonary clinics.
At present, therapeutic options to improve both poor statural growth and immunodeficiency are non-existent. A lung transplantation appeared to have alleviated problems caused by the chronic pulmonary disease (43), although prognosis remains unclear for the long-term. Bone marrow transplants and its use in clinical settings of autoimmunity and alloimmunity have been intensely studied (54), and may be a therapeutic option for deficiencies in T cells related to STAT5B deficiency. For statural growth, the resistance to GH therapy suggested that recombinant human IGF-I treatment could be a therapeutic option, although response was poor in the one report where the proband was on rhIGF-I therapy for one year(43). The poor response to rhIGF-I was attributed to complications associated with the immuno-compromised status of the subject. Continued clinical evaluations of patients carrying these rare STAT5B mutations and elucidating the impact of these mutations on structure and function of the STAT5B protein, is important to understanding the pathophysiology of this complex disease.
Highlights.
Rare autosomal recessive STAT5B mutations are associated with severe growth failure, marked IGF-I deficiency and growth hormone insensitivity (GHI) syndrome.
STAT5B deficiency can lead to potentially fatal primary immunodeficiency.
The closely related STAT5A cannot compensate for loss of STAT5B in humans.
Acknowledgments
My thanks to Dr Mirta Miras, and Dr Merih Berberoglu, and their colleagues, for sharing unpublished information. This work is supported by funding from NIH, R01HD078592.
Footnotes
Conflict of Interest
Vivian Hwa has received lecture fees from EMD Serono, and sponsored travel from Pfizer, Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Levy DE, Darnell JE., Jr STATs: transcriptional control and biological impact. Nat Rev Mol Cell Biol. 2002;3:651–662. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- 2.Casanova J-L, Holland SM, Notarangelo LD. Inborn Errors of Human JAKs and STATs. Immunity. 2012;36:515–528. doi: 10.1016/j.immuni.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Shea JJ, Holland SM, Staudt LM. JAKs and STATs in immunity, immunodeficiency, and cancer. N Engl J Med. 2013;368(2):161–170. doi: 10.1056/NEJMra1202117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hambleton S, Goodbourn S, Young DF, Dickinson P, Mohamad SM, Valappil M, McGovern N, Cant AJ, Hackett SJ, Ghazal P, Morgan NV, Randall RE. STAT2 deficiency and susceptibility to viral illness in humans. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(8):3053–3058. doi: 10.1073/pnas.1220098110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yildiz M, Li H, Bernard D, Amin NA, Ouillette P, Jones S, Saiya-Cork K, Parkin B, Jacobi K, Shedden K, Wang S, Chang AE, Kaminski MS, Malek SN. Activating STAT6 mutations in follicular lymphoma. Blood. 2015;125(4):668–679. doi: 10.1182/blood-2014-06-582650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hwa V, Nadeau K, Wit JM, Rosenfeld RG. STAT5b deficiency: lessons from STAT5b gene mutations. Best Pract Res Clin Endocrinol Metab. 2011;25:61–75. doi: 10.1016/j.beem.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Flanagan SE, Haapaniemi E, Russell MA, Caswell R, Lango Allen H, De Franco E, McDonald TJ, Rajala H, Ramelius A, Barton J, Heiskanen K, Heiskanen-Kosma T, Kajosaari M, Murphy NP, Milenkovic T, Seppanen M, Lernmark A, Mustjoki S, Otonkoski T, Kere J, Morgan NG, Ellard S, Hattersley AT. Activating germline mutations in STAT3 cause early-onset multi-organ autoimmune disease. Nature genetics. 2014;46(8):812–814. doi: 10.1038/ng.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Milner JD, Vogel TP, Forbes L, Ma CA, Stray-Pedersen A, Niemela JE, Lyons JJ, Engelhardt KR, Zhang Y, Topcagic N, Roberson ED, Matthews H, Verbsky JW, Dasu T, Vargas-Hernandez A, Varghese N, McClain KL, Karam LB, Nahmod K, Makedonas G, Mace EM, Sorte HS, Perminow G, Rao VK, O’Connell MP, Price S, Su HC, Butrick M, McElwee J, Hughes JD, Willet J, Swan D, Xu Y, Santibanez-Koref M, Slowik V, Dinwiddie DL, Ciaccio CE, Saunders CJ, Septer S, Kingsmore SF, White AJ, Cant AJ, Hambleton S, Cooper MA. Early-onset lymphoproliferation and autoimmunity caused by germline STAT3 gain-of-function mutations. Blood. 2015;125(4):591–599. doi: 10.1182/blood-2014-09-602763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haapaniemi EM, Kaustio M, Rajala HL, van Adrichem AJ, Kainulainen L, Glumoff V, Doffinger R, Kuusanmaki H, Heiskanen-Kosma T, Trotta L, Chiang S, Kulmala P, Eldfors S, Katainen R, Siitonen S, Karjalainen-Lindsberg ML, Kovanen PE, Otonkoski T, Porkka K, Heiskanen K, Hanninen A, Bryceson YT, Uusitalo-Seppala R, Saarela J, Seppanen M, Mustjoki S, Kere J. Autoimmunity, hypogammaglobulinemia, lymphoproliferation, and mycobacterial disease in patients with activating mutations in STAT3. Blood. 2015;125(4):639–648. doi: 10.1182/blood-2014-04-570101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beausoleil SA, Villen J, Gerber SA, Rush J, Gygi SP. A probability-based approach for high-throughput protein phosphorylation analysis and site localization. Nat Biotechnol. 2006;24:1285–1292. doi: 10.1038/nbt1240. [DOI] [PubMed] [Google Scholar]
- 11.Daub H, Olsen JV, Bairlein M, Gnad F, Oppermann FS, Korner R, Greff Z, Keri G, Stemmann O, Mann M. Kinase-selective enrichment enables quantitative phosphoproteomics of the kinome across the cell cycle. Mol Cell. 2008;31:438–448. doi: 10.1016/j.molcel.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 12.Mayya V, Lundgren DH, Hwang S-J, Rezaul K, Wu L, Eng JK, Rodionov V, Han DK. Quantitative phosphoproteomic analysis of T cell receptor signaling reveals system-wide modulation of protein-protein interactions. Sci Signal. 2009;2:RA46–RA46. doi: 10.1126/scisignal.2000007. [DOI] [PubMed] [Google Scholar]
- 13.Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther T, Olsen JV, Mann M. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325:834–840. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- 14.Ambrosio R, Fimiani G, Monfregola J, Sanzari E, De Felice N, Salerno MC, Pignata C, D’Urso M, Ursini MV. The structure of human STAT5A and B genes reveals two regions of nearly identical sequence and an alternative tissue specific STAT5B promoter. Gene. 2002;285(1–2):311–318. doi: 10.1016/s0378-1119(02)00421-3. [DOI] [PubMed] [Google Scholar]
- 15.Woods KA, Camacho-Hubner C, Savage MO, Clark AJ. Intrauterine growth retardation and postnatal growth failure associated with deletion of the insulin-like growth factor I gene. New Engl J Med. 1996;335:1363–1367. doi: 10.1056/NEJM199610313351805. [DOI] [PubMed] [Google Scholar]
- 16.Walenkamp MJE, Karperien M, Pereira AM, Hilhorst-Hofstee Y, van Doorn J, Chen JW, Mohan S, Denley A, Forbes BE, van Duyvenvoorde H, van Thiel SW, Sluimers CA, Bax JJ, de Laat JAPM, Breuning MB, Romijn JA, Wit JM. Homozygous and heterozygous expression of a novel insulin-like growth factor-I mutation. J Clin Endocrinol & Metab. 2005;90:2855–2864. doi: 10.1210/jc.2004-1254. [DOI] [PubMed] [Google Scholar]
- 17.Netchine I, Azzi S, Houang M, Seurin D, Perin L, Ricot J-M, Daubas C, Legay C, Mester J, Herich R, Godeau F, Le Bouc Y. Partial primary deficiency of insulin-like growth factor (IGF)-I activity associated with IGF-1 mutation demonstrates its critical role in growth and brain development. J Clin Endocrinol & Metab. 2009;94(10):3913–3921. doi: 10.1210/jc.2009-0452. [DOI] [PubMed] [Google Scholar]
- 18.Brown RJ, Adams JJ, Pelekanos RA, Wan Y, Mckinstry WJ, Palethorpe K, Seeber RM, Monks TA, Eidne KA, Parker MW, Waters MJ. Model for growth hormone receptor activation based on subunit rotation within a receptor dimer. Nat Struct Mol Biol. 2005;12(9):814–821. doi: 10.1038/nsmb977. [DOI] [PubMed] [Google Scholar]
- 19.Derr MA, Fang P, Sinha SK, Ten S, Hwa V, Rosenfeld RG. A novel Y332C missense mutation in the intracellular domain of the human growth hormone receptor (GHR) does not alter STAT5b signaling: redundancy of GHR intracellular tyrosines involved in STAT5b signaling. Horm Res. 2010 doi: 10.1159/000320461. In press. [DOI] [PubMed] [Google Scholar]
- 20.David A, Hwa V, Metherell LA, Netchine I, Camacho-Hubner C, Clark AJ, Rosenfeld RG, Savage MO. Evidence for a continuum of genetic, phenotypic, and biochemical abnormalities in children with growth hormone insensitivity. Endo Rev. 2011;32(4):472–497. doi: 10.1210/er.2010-0023. [DOI] [PubMed] [Google Scholar]
- 21.Hwa V. Growth Hormone Receptor in Growth. In: Ho K, editor. Growth Hormone Related Diseases and Therapy: a molecular and physiological perspective for the clinician. New York: Humana Press; 2011. pp. 3–16. [Google Scholar]
- 22.Savage MO, Attie KM, David A, Metherell LA, Clark AJ, Camacho-Hubner C. Endocrine assessment, molecular characterization and treatment of growth hormone insensitivity disorders. Nat Clin Pract Endocrinol Metab. 2006;2:395–407. doi: 10.1038/ncpendmet0195. [DOI] [PubMed] [Google Scholar]
- 23.Milward A, Metherell L, Maamra M, Barahona MJ, Wilkinson IR, Camacho-Hubner C, Savage MO, Bidlingmaier CM, Clark AJL, Ross RJM, Webb SM. Growth hormone (GH) insensitivity syndrome due to a GH receptor truncated after Box1, resulting in isolated failure of STAT5 signal transduction. J Clin Endocrinol Metab. 2004;89:1259–1266. doi: 10.1210/jc.2003-031418. [DOI] [PubMed] [Google Scholar]
- 24.Chia DJ, Varco-Merth B, Rotwein P. Dispersed chromosomal Stat5b-binding elements mediate growth hormone-activated insulin-like growth factor-I gene transcription. J Biol Chem. 2010;285:17636–17646. doi: 10.1074/jbc.M110.117697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varco-Merth B, Rotwein P. Differential effects of STAT proteins on growth hormone-mediated IGF-I gene expression. Am J Physiol Endocrinol Metab. 2014;307(9):E847–855. doi: 10.1152/ajpendo.00324.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, Jiang H. Identification of a distal STAT-binding DNA region that may mediate growth hormone regulation of insulin-like growth factor-I gene expression. J Biol Chem. 2005;280(12):10955–10963. doi: 10.1074/jbc.M412808200. [DOI] [PubMed] [Google Scholar]
- 27.Udy GB, Towers RP, Snell RG, Wilkins RJ, Park SH, Ram PA, Waxman DJ, Davey HW. Requirement of STAT5b for sexual dimorphism of body growth rates and liver gene expression. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(14):7239–7244. doi: 10.1073/pnas.94.14.7239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teglund S, McKay C, Schuetz E, van Deursen JM, Stravopodis D, Wang D, Brown M, Bodner S, Grosveld G, Ihle JN. Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell. 1998;93(5):841–850. doi: 10.1016/s0092-8674(00)81444-0. [DOI] [PubMed] [Google Scholar]
- 29.Zhou Y, Xu BC, Maheshwari HG, He L, Reed M, Lozykowski M, Okada S, Cataldo L, Coschigamo K, Wagner TE, Baumann G, Kopchick JJ. A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse) Proc Natl Acad Sci USA. 1997;94(24):13215–13220. doi: 10.1073/pnas.94.24.13215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenfeld RG, Belgorosky A, Camacho-Hubner C, Savage MO, Wit JM, Hwa V. Defects in growth hormone receptor signaling. Trends Endocrinol Metab. 2007;18(4):134–141. doi: 10.1016/j.tem.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 31.Rosenfeld RG, Hwa V. The growth hormone cascade and its role in mammalian growth. Horm Res. 2009;71(Suppl 2):36–40. doi: 10.1159/000192434. [DOI] [PubMed] [Google Scholar]
- 32.Kofoed EM, Hwa V, Little B, Woods KA, Buckway CK, Tsubaki J, Pratt KL, Bezrodnik L, Jasper H, Tepper A, Heinrich J, Rosenfeld RG. Growth-hormone insensitivity (GHI) associated with a STAT-5b mutation. N Engl J Med. 2003;349:1139–1147. doi: 10.1056/NEJMoa022926. [DOI] [PubMed] [Google Scholar]
- 33.Chen X, Vinkemeier U, Zhao Y, Jerzalmi D, James E, Darnell J, Kuriyan J. Crystal Structure of a tyrosine phosphorylated STAT-1 dimer bound to DNA. Cell. 1998;93:827–839. doi: 10.1016/s0092-8674(00)81443-9. [DOI] [PubMed] [Google Scholar]
- 34.Chia DJ, Subbian E, Buck TM, Hwa V, Rosenfeld RG, Skach WR, Shinde U, Rotwein P. Aberrant folding of a mutant STAT5b causes growth hormone insensitivity and proteasomal dysfunction. J Biol Chem. 2006;281:6552–6558. doi: 10.1074/jbc.M510903200. [DOI] [PubMed] [Google Scholar]
- 35.Fang P, Kofoed EM, Little BM, Wang X, Ross RJM, Frank SJ, Hwa V, Rosenfeld RG. A mutant signal transducer and activator of transcription 5B, associated with growth hormone insensitivity and insulin-like growth factor-I deficiecny, cannot function as a signal transducer or transcription factor. J Clin Endocrinol & Metab. 2006;91:1526–1534. doi: 10.1210/jc.2005-2558. [DOI] [PubMed] [Google Scholar]
- 36.Hwa V, Little B, Kofoed EM, Rosenfeld RG. Transcriptional regulation of insulin-like growth factor-I (IGF-I) by interferon-gamma (IFN-g) requires Stat-5b. J Biol Chem. 2004;279:2728–2736. doi: 10.1074/jbc.M310495200. [DOI] [PubMed] [Google Scholar]
- 37.Hwa V, Little B, Adiyaman P, Kofoed EM, Pratt KL, Ocal G, Berberoglu M, Rosenfeld RG. Severe growth hormone insensivity resulting from total absence of signal transducer and activator of transcription 5b. J Clin Endocrinol & Metab. 2005;90:4260–4266. doi: 10.1210/jc.2005-0515. [DOI] [PubMed] [Google Scholar]
- 38.Vidarsdottir S, Walenkamp MJE, Pereira AM, Karperien M, van Doorn J, van Duyvenvoorde HA, White S, Breuning MH, Roelfsema F, Femke Kruithof M, van Dissel J, Janssen R, Wit JM, Romijn JA. Clinical and biochemical characteristics of a male patient with a novel homozygous STAT5b mutation. J Clin Endocrinol & Metab. 2006;91:3482–3485. doi: 10.1210/jc.2006-0368. [DOI] [PubMed] [Google Scholar]
- 39.Bernasconi A, Marino R, Ribas A, Rossi J, Ciaccio M, Oleastro M, Ornani A, Paz R, Rivarola M, Zelazko M, Belgorosky A. Characterization of immunodeficiency in a patient with growth hormone insensitivity secondary to a novel STAT5b gene mutation. Pediatrics. 2006;118:e1584–e1592. doi: 10.1542/peds.2005-2882. [DOI] [PubMed] [Google Scholar]
- 40.Boyanovsky A, Lozano A, Testa G, Munoz L, Marino R, Bernasconi A, Belgorosky A, Miras M. Growth hormone insensitivity and immunodeficiency: mutation in the STAT5B gene. 8th Joint Meeting of the Lawson Wilkins Pediatric Endocrine Society/European Society for Paediatric Endocrinology; 2009; P01–067. [Google Scholar]
- 41.Scaglia PA, Martinez AS, Feigerlová E, Bezrodnik L, Gaillard MI, Di Giovanni D, Ballerini MG, Jasper HG, Heinrich JJ, Fang P, Domené HM, Rosenfeld RG, Hwa V. A novel missense mutation in the SH2 domain of the STAT5B gene results in a transcriptionally inactive STAT5b associated with severe IGF-I deficiency, immune dysfunction, and lack of pulmonary disease. J Clin Endocrinol & Metab. 2012;97:E830–E839. doi: 10.1210/jc.2011-2554. [DOI] [PubMed] [Google Scholar]
- 42.Hwa V, Camacho-Hubner C, Little BM, David A, Metherell LA, El-Khatib N, Savage MO, Rosenfeld RG. Growth hormone insensitivity and severe short stature in siblings: a novel mutation at the exon13-intron 13 junction of the STAT5b gene. Horm Res. 2007;68(5):218–224. doi: 10.1159/000101334. [DOI] [PubMed] [Google Scholar]
- 43.Pugliese-Pires PN, Tonelli CA, Dora JM, Silva PCA, Czepielewski M, Simoni G, Arnhold IJ, Jorge AAL. A novel STAT5B mutation causing GH insenstivity syndrome associated with hyperprolactinemia and immune dysfunction in two male siblings. Eur J Endocrinol. 2010;163:349–355. doi: 10.1530/EJE-10-0272. [DOI] [PubMed] [Google Scholar]
- 44.Laron Z, Lilos P, Klinger B. Growth curves for Laron syndrome. Arch Dis Child. 1993;68:768–770. doi: 10.1136/adc.68.6.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walenkamp MJE, Vidarsdottir S, Pereira AM, Karperien M, van Doorn J, van Duyvenvoorde HA, Breuning MH, Roelfsema F, Kruithof MF, van Dissel J, Janssen R, Wit JM, Romijn JA. Growth hormone secretion and immunological function of a male patient with a homozygous STAT5b mutation. Eur J Endocrinol. 2007;156:155–165. doi: 10.1530/eje.1.02327. [DOI] [PubMed] [Google Scholar]
- 46.Rubinstein A, Bernstein LJ, Charytan M, Krieger BZ, Ziprkowski M. Corticosteroid treatment for pulmonary lymphoid hyperplasia in children with the acquired immunoe deficiency syndrome. Pediatr Pulmonol. 1988;4:13–17. doi: 10.1002/ppul.1950040105. [DOI] [PubMed] [Google Scholar]
- 47.Bezrodnik L, Di Giovanni D, Caldirola MS, Azcoiti ME, Torgerson T, Gaillard MI. Long-term follow-up of STAT5B deficiency in three argentinian patients: clinical and immunological features. Journal of clinical immunology. 2015;35(3):264–272. doi: 10.1007/s10875-015-0145-5. [DOI] [PubMed] [Google Scholar]
- 48.Cohen AC, Nadeau KC, Tu W, Hwa V, Dionis K, Bezrodnik L, Teper A, Gaillard M, Heinrich J, Krensky AM, Rosenfeld RG, Lewis DB. Cutting Edge: decreased accumulation and regulatory function of CD4+CD25high T cells in human STAT5b deficiency. J Immunol. 2006;177:2770–2774. doi: 10.4049/jimmunol.177.5.2770. [DOI] [PubMed] [Google Scholar]
- 49.Burchill MA, Yang J, Vang KB, Farrar MA. Interleukin-2 receptor signaling in regulatory T cell development and homeostasis. Immunol Lett. 2007;114(1):1–8. doi: 10.1016/j.imlet.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Torgerson TR. Immune dysregulation in primary immunodeficiency disorders. Immunol Allergy Clin N Am. 2008;28:315–327. doi: 10.1016/j.iac.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 51.Zorn E, Nerlson EA, Mohseni M, Porcheray F, Kim H, Litsa D, Bellucci R, Raderschall E, Canning C, Soiffer RG, Frank DA, Ritz J. IL-2 regulates FOXP3 expression in human CD4+CD25+ regulatory T cells through a STAT-dependent mechanism and induces the expansion of these cells in vivo. Blood. 2006;108:1571–1579. doi: 10.1182/blood-2006-02-004747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yao Z, Kanno Y, Kerenyi M, Stephens G, Durant L, Watford WT, Laurence A, Robinson GW, Shevach EM, Moriggl R, Hennighausen L, Wu C, O’Shea JJ. Nonredundant roles for STAT5a/b in directly regulating Foxp3. Blood. 2007;109:4368–4375. doi: 10.1182/blood-2006-11-055756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jenks JA, Seki S, Kanai T, Huang J, Morgan AA, Scalco RC, Nath R, Bucayu R, Wit JM, Al-Herz W, Ramadan D, Jorge AA, Bacchetta R, Hwa V, Rosenfeld R, Nadeau KC. Differentiating the roles of STAT5B and STAT5A in human CD4+ T cells. Clin Immunol. 2013;148:227–236. doi: 10.1016/j.clim.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Riley JL, June CH, Blazar BR. Human T regulatory cell therapy: take a billion or so and call me in the morning. Immunity. 2009;30(5):656–665. doi: 10.1016/j.immuni.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]