Abstract
Background
No methodological standards are available for researchers and clinicians to examine medication discrepancies between health care settings. Systematic methods of examining medication discrepancies will allow researchers and clinicians to better understand factors driving medication discrepancies, to better measure effects of medication reconciliation interventions, and to compare findings across studies.
Objective
This article proposes a four-phase approach for systematically collecting medication data and measuring medication discrepancies between a hospital and community pharmacies. Methodologic considerations related to studying medication discrepancies in health services research are also discussed.
Methods
A multi-disciplinary study team developed a four-phase systematic approach to improve quality of data and study rigor: 1) operationalization of a medication discrepancy, 2) acquiring medication data, 3) abstraction of medication data and creation of dataset, and 4) measuring and reporting medication discrepancies.
Results
Using this phase-based approach, the study team successfully identified and reported medication discrepancies between a hospital and community pharmacies at the patient, medication, and community pharmacy units of analyses.
Conclusions
Systematically measuring medication discrepancies that occur in the care transitions process is a critical step as researchers, clinicians, and other stakeholders work to improve health care quality and patient outcomes. This article detailed how a phase-based approach can be used in research to examine medication discrepancies as well as address the complexity of collecting medication data and analyzing medication discrepancies. Such methods should be considered when developing, conducting, and reporting research on medication discrepancies.
Keywords: Community pharmacy, Medication discrepancies, Transitions of care, Adverse drug events
Introduction
The Institute of Medicine (IOM) report, To Err is Human, highlighted the prevalence and devastation caused by medication errors in the US health care system.1 The 2000 Report declared that the rates of medication errors and subsequent adverse drug events (ADEs) are unacceptable and immediate action to decrease these rates should be a national priority. In a later Report, the IOM committee estimated that nearly 1.5 million ADEs result from preventable medication errors annually, contributing to over $3.5 billion in avoidable health care costs.1,2
The majority of medication errors are thought to be preventable,2 and multiple interventions may be required to significantly decrease medication errors, particularly when patients transition between health care settings. As such, The Joint Commission and the Institute for Healthcare Improvement identified medication reconciliation as a key intervention for decreasing medication errors and improving patient safety.3,4 Medication reconciliation is the formal, comprehensive process of bringing patient medication records into agreement between the patient and their health care providers.3 This complex process has been recommended at every patient transition point to prevent medication discrepancies and other medication-related issues.5 Medication discrepancies are the mismatch, or inconsistency, of information between a patient's medications lists across health care settings.6–9 Fragmented, inconsistent medication information between settings can jeopardize patient safety by placing the patient at risk of taking incorrect medications and complicating the provider's role of assessing and treating patients based on imperfect information.
Despite being the focal point of seminal IOM Reports and many research endeavors, no methodological standards are available for researchers and clinicians to examine medication discrepancies between health care settings. By understanding where medications discrepancies are occurring, interventions to strengthen medication reconciliation processes may be implemented. Thus, there is a critical need for a systematic method of examining medication discrepancies in health services research. This particularly is relevant given new mandates under the Affordable Care Act of 201010 which outlines payment reforms that will financially penalize hospitals with higher than expected readmission rates or other adverse events. This article addresses this need by proposing a four-phase approach for systematically collecting medication data and measuring medication discrepancies as well as considerations to be taken when examining medication discrepancies in health services research.
Background of the four-phase approach development
The four-phase approach was developed as part of an Agency for Healthcare Research and Quality (AHRQ)-funded study to examine the consistency of medication lists between the hospital and community pharmacies when older adult patients (65 years and older) transition from the hospital and into community care. Community pharmacy was broadly defined as licensed pharmaceutical dispensaries primarily operating on an outpatient basis. Briefly, medication records for 100 patients from a large academic Midwest hospital and over 40 community pharmacies were abstracted and compared retrospectively. Patients were identified for this study by the hospital's existing transitional care team. The transitional care team provides care to older adult patients who were admitted to the hospital for conditions such as pneumonia, congestive heart failure, or myocardial infarction and are discharged back into the community. As part of the transitional care program, patients and caregivers receive additional support during their early post-hospital period from a transitional care nurse via phone calls and/or home visits. Approval by the institution's Health Sciences Institutional Review Board (IRB) was obtained prior to implementing this study.
Upon study initiation in 2012, no standard data collection or measuring procedures were available when examining medication discrepancies between hospitals and community pharmacies, or between any settings for that matter. Therefore, to improve the quality of data, the study team focused on developing procedures that systematically informed: 1) the operationalization of a medication discrepancy, 2) acquiring medication data, 3) abstraction of medication data and creation of dataset, and 4) measuring and reporting medication discrepancies.
Description of the four-phase approach
Phase 1: operationalization of medication discrepancies
The term, medication discrepancy, is used widely in the literature, often as an outcome measure related to health care quality.7,11 However, there is wide variation in the extent to which studies detail their operationalization of medication discrepancies and no widely accepted operationalization exists. The first step was to operationalize medication discrepancies based on the seminal works by Smith et al. 2004 and Orrico 2008,12,13 as well as the clinical experiences of the team's physicians and pharmacists. Three subcomponents were deemed necessary: 1) determining which medications to include in the analysis, 2) identifying the reference medication list, and 3) defining the medication discrepancy categories.
Determining which medications to include in the analysis
To determine which medications to include in the analysis, two main decisions were made. The first decision was whether to include over-the-counter (OTC) medications. The challenge with including OTC medications in the analysis related to the limitations of record keeping among each organization's electronic medical record (EMR) systems. The hospital system allowed for the inclusion of all types of prescription and OTC medications, which routinely are collected upon patient admission. Community pharmacies, on the other hand, commonly do not incorporate OTC medication information into their patient profiles and dispensing records as these records are generally reserved for dispensed medications that require pharmacy labeling and third-party payment. Even when OTC medication information is collected at the hospital or community pharmacy, studies suggest information about OTC medications contained in EMRs also has shortcomings.13,14 Therefore, the study team decided to exclude OTC medications and focus on prescription-only medications.
The next decision was to determine whether to include all prescription-only medications or if some should be excluded. The study team agreed that topical skin, eye, and ear medications (e.g., creams, gels) should be excluded because of the anticipated ambiguity in instructions (e.g., apply a small amount to affected areas as needed). All other types of oral (i.e., solid, liquid, inhalation), injectable, and transdermal patch prescription-only medications were included. These medications also were targeted because of a higher propensity for these medications to cause increased harm if taken incorrectly.15
Identifying the reference medication list
Patient medication regimens are constantly changing, particularly for those experiencing a hospitalization. The study patients were on multiple medications that were obtained from multiple prescribers across different settings, making it difficult to establish a “gold standard” medication list. A “gold standard” medication list implies that the medication list is the most accurate reflection of what the patient actively is taking and any deviation from the “gold standard” would be an error.7 Initially, the hospital's discharge medication list was proposed as the “gold standard list” because this medication list reflected the most recent interaction the patient had with a provider. However, the study team recognized that a patient may have an encounter with a provider who may change a prescription between the time of hospital discharge and the time the patient first fills a prescription at a community pharmacy post-discharge. Therefore, a “reference list” was constructed based on the hospital discharge medication list and any subsequent medical visits that occurred prior to the patient visiting their community pharmacy. The goal was to have all medication changes during hospital discharges and follow-up provider encounters reflected at the community pharmacy. Since all study patients received their health care through one health system that shared an EMR, the study team had access to all the inpatient and outpatient medical notes which made the creation of the reference medication list possible.
Defining medication discrepancy categories
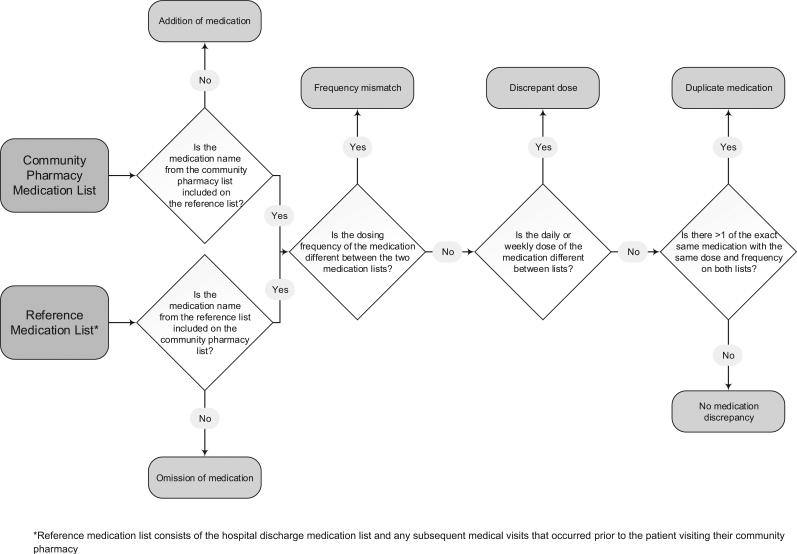
Drawing upon previous research,12,13,16,17 five medication discrepancy categories were examined between the reference medication list and the community pharmacy medication list. Each medication discrepancy category was from the perspective of the reference medication list. As listed in Table 1, a description and example of each type of medication discrepancy examined is illustrated. The flowchart (Fig. 1) allows for a uniform assignment of discrepancies, and medication discrepancies were mutually exclusive. For example, medications where the medication is both frequency and dose discrepant would only be assigned a dose discrepancy based on the flowchart.
Table 1.
Description and examples of medication discrepancy categories
| Medication discrepancy category | Description | Examples |
|
|---|---|---|---|
| Reference medication list | Community pharmacy medication list | ||
| Omission of medication | Medication is included on the reference medication list but excluded on the community pharmacy medication list | A prescription for lisinopril is included | A prescription for lisinopril is not included |
| Addition of medication | Medication is included on the community pharmacy medication list but excluded on the reference medication list | A prescription for metoprolol is not included | A prescription for metoprolol is included |
| Discrepant dose | Mismatch of the medication dose during administration between the reference and community pharmacy medication list | A prescription for gabapentin 600 mg taken three times daily | A prescription for gabapentin 300 mg taken three times daily |
| Discrepant frequency | Medication between lists has a different dosing frequency for the same medication | A prescription for furosemide 40 mg taken twice daily | A prescription for furosemide 40 mg taken once daily |
| Duplicate medicationa | Medication name, dose and frequency is listed on the reference medication list and two or more of the same prescription are available to the patient on the community pharmacy medication list | One prescription for methotrexate 2.5 mg tablets, take five tablets by mouth once weekly | Three prescriptions for methotrexate 2.5 mg tablets, take five tablets by mouth once weekly |
This excludes stored or “on-hold” prescriptions on the patient's community pharmacy list that are refills to replace soon-to-be expired prescriptions.
Fig. 1.
Flowchart depicting assignment of medication discrepancy categories.
Other considerations for researchers and clinicians
Acute medications (e.g., antibiotics) and “as needed” medications can be problematic during the analysis and should be discussed during the study conception. Drawbacks of excluding OTC medications include that a small number of high risk medications,18 such as aspirin and diphenhydramine, were not captured. Further, certain medications, such as proton-pump inhibitors or other medications that commonly are dispensed as a prescription but also available OTC, may be omitted from the analysis or inconsistently included. While some OTCs are evidence of self-treatment, others may be associated with a physician's order for achieving a therapeutic goal. A multi-disciplinary study team is critical for deciding what medications are practical to include in the analysis. Researchers and clinicians should document and report their decisions when excluding certain medications from the analyses.
Phase 2: acquiring medication data
In the early conceptual stage of the present study, the team consulted with hospital leadership and two community pharmacy managers to solicit feedback on the feasibility of obtaining medication lists and other study procedures, including a special focus on the patient consent process and privacy issues related to Health Insurance Portability and Accountability Act (HIPAA) compliance. Through these meetings, it was concluded that a patient would be required to complete two consent forms to participate in the study. The first consent form gave the study team permission to access the patient's medication list and post-discharge notes in the EMR. The second consent form gave the team permission to request prescription information from the patient's community pharmacy. The entire recruitment process took between five and 10 min to complete, and patients were given copies of the forms and could notify the researchers at any time if they wanted to discontinue participation.
Collecting medication information from the hospital
Medical notes and hospital discharge summaries were accessed by coordinating with the hospital's records office and the hospital's Senior Data Security Analyst. Patient consent forms were copied and submitted to the hospital's records office every other week. The consent form requested the patient's discharge summary as well as all inpatient and outpatient medical notes up to 60 days post-hospitalization. Patient medical records were then copied to a DVD by the hospital records office, and the DVD was released to the study team with a security code for opening and accessing the contents. Once the DVD was obtained from the records office, the researcher opened the DVD and printed off hard copies of all the patient medical records.
Collecting medication information from community pharmacies
Community pharmacies were contacted by email, post mail, or both, three to four weeks after patients were discharged to allow enough time for patients to visit their community pharmacies. Letters addressed to pharmacy managers briefly explained that one of their patients chose to enroll in the study. Letters also contained the date and time when someone from the study team would call to answer any questions regarding the study. During the phone call, a member of the study team provided more details about the study, medication information needed for analysis, and determined a mutual time to collect data. All medications from the past year were requested from the patient's community pharmacy medication profile including the following information: medication name, strength, directions, prescriber, date written, quantity, medication fill dates, if the prescription is inactive, date of prescription inactivation, or if the prescription is active or available for the patient to fill (e.g., “on-hold” in the patient's medication profile).
Each community pharmacy received a copy of the patient's consent form. In return, community pharmacies provided paper copies of the patient's medication record to the project leader who visited each pharmacy. Early in the study, pharmacist burden for collating medication information was assessed by contacting two pharmacists who chose to participate in the study. Both pharmacists reported the time to access and collate the medication information was minimal and also stated they did not experience any problems. One pharmacist delegated this task to their pharmacy student intern.
Not all pharmacies contacted agreed to participate in the study. Despite having consent forms from patients, community pharmacies were not obligated to provide information to the project leader for research purposes. Two large retail pharmacies would not provide the necessary patient medication information, and subsequently these patients were omitted from the study.
Other considerations for researchers and clinicians
There are many potential sources for medication data including patient self-report, patient medication bottles, medication histories taken by a health care personnel, insurance medication claims, hospital medication administration records, nursing home medication administration records, clinic medication lists, and pharmacy dispensing records. All have their own unique limitations and access issues and should be considered before study implementation. Soliciting feedback from key stakeholders was essential for understanding what medication data would be available to the researchers and how to access that data.
Phase 3: medical record abstraction and creation of dataset
Data were abstracted from hard-copy medical documents acquired from the hospital and community pharmacies and directly entered into an electronic dataset for analysis. Each medical note from the time the patient was discharged to the first community pharmacy prescription fill was reviewed, and the corresponding medication list was entered into the electronic dataset. Medication information was requested from each pharmacy so that the first fill since the patient's discharge was identified. Subsequently, all of the medications that would have been available to fill when the patient first filled a prescription post-discharge were entered electronically. Overall, more than 1000 medications were entered into the electronic dataset.
Creation of the electronic dataset
Medications from the patient's hospital discharge and community pharmacy were entered by two medical abstractors into an electronic database using the program, EpiData Software®.19 EpiData Software® is a free program, developed by The EpiData Association, to help researchers enter and document data. EpiData Software® provides a user-friendly electronic data entry form that saves the data in a dataset that can be uploaded to another program for statistical analysis.
An abstraction tool was developed to help standardize the process of entering medication information into the electronic database. The abstraction tool was organized by origin of the medication list (hospital or community pharmacy), route of medication (oral, injection, and transdermal), and characteristics of the prescription (strength, dose, and frequency). Additionally, an abstraction training manual was developed to serve as a reference with instructions on how to use the abstraction tool, define and describe each data element, and provide examples commonly encountered during the abstraction process. To enter the medication name, abstractors used an Excel spreadsheet of medication names and national drug codes obtained from the U.S. Food and Drug Administration Website.20 Each unique medication substance name (i.e., active ingredient) had a unique four digit medication code that was available to be inputted by the abstractors. For example, the code for metoprolol tartrate was “0695.”
To help protect patient privacy and confidentiality, patient identifiers were omitted from the electronic dataset. Also, all patient identifiers were marked out on the printed hospital discharge summaries, medical notes, and other medication information obtained from the hospital and community pharmacies. To keep patient information consistent between documents and the data set, all patients were assigned a unique code identifier which was placed on all medical notes, medication lists, and the data set. Only one patient name and unique code identifier crosswalk was kept in a separate IRB-approved electronic format.
Inter-rater reliability assessments
Re-abstraction was performed to assess abstractor inter-rater reliability (IRR). The electronic data entry form through EpiData Software® provided a platform for double entry of abstracted data by allowing a separate dataset to be stored and analyzed in conjunction with the main dataset. Overall, approximately 20% of all medications entered into the database were re-abstracted by a second abstractor. IRR between the primary and secondary abstractor was determined through calculation of Cohen κ statistic and percent agreements.21–24
The first 10% of medications were abstracted by both abstractors to test the abstraction tool and the EpiData Software® user interface. This initial assessment revealed only a few differences between the abstracted medication names and doses of each scheduled frequency. Revisions were made to both the abstraction tool and the user interface based on the initial re-abstractions. After the first 10% of medications were re-abstracted, abstractors blindly were assigned batches of 10 randomized patients with each batch containing approximately 100 medications. With each abstraction batch, a second abstractor was assigned a 10% random abstraction of medications to assess “abstractor drift.” Abstractor drifts are any unintentional deviations from the original abstraction protocol.23,25 If IRR statistics were < 0.90, the study team met with abstractors to identify any issues the abstractors experienced. After each meeting, the abstraction tool and user interface was updated to meet the needs of the abstractors. Abstractors were not instructed to continue abstracting until all IRR statistics were reviewed by the study team.
Other considerations for researchers and clinicians
The above reliabilities followed considerable work to identify and address coding challenges. For example, medications with range doses were difficult to code consistently so that medications lists could be compared. At hospital discharge, many pain medications are prescribed with a range dose such as, “Take one to two tablets by mouth every four to 6 hours as needed for pain.” Additional variables were added to the abstraction manual to record if the medication was dosed within a range. Then the maximum daily dose and frequency was included in the dataset similar to the practice in the community pharmacy when calculating the days supply of a medication. Other medications, such as warfarin, also may have varying weekly doses so special abstraction methods were developed to ensure continuity between abstractors. Any special considerations or reliability challenges should be reported so other researchers and clinicians may learn from previous studies and increase their data quality and integrity.
As another consideration, researchers and clinicians may classify medications discrepancies based on their potential to cause harm, such as potential adverse drug events (PADEs) or narrow therapeutic index. Previous studies have estimated potential harm, and methods used to determine potential harm varied across studies. Methods included: individual review and rating by clinicians of each medication discrepancy using clinical experience and pre-constructed taxonomies,8,26 applying the National Coordinating Counsel for Medication Error Reporting and Prevention Index for Categorizing Medication Errors (NCC MERP 2001),27,28 using medication lists derived from expert consensus panels such as the Institute for Safe Medication Practices high-alert list,29 and abstracting medical records to record patient lab values and medical notes to determine if a medication discrepancy was associated with worsening medical incidents.30
Phase 4: measuring and reporting medication discrepancies
There are numerous options for reporting and analyzing medication discrepancies. Data presented should be consistent with the study objectives and hypotheses. In the current study, medication discrepancies were reported at the level of patient, medication, and community pharmacy. Frequencies were calculated for each outcome, and descriptions of each calculation are presented in Table 2. Focusing on the patient as the unit of analysis, an overall percent of patients experiencing at least one medication discrepancy was reported. At the medication-level, the proportion of all medications discrepant was calculated. Medications also were organized by drug classes and the top five discrepant drug classes were reported. Then the proportion of medications discrepant by discrepancy category (e.g., omission, addition) was reported. At the community pharmacy-level, three calculations were performed to reflect each community pharmacy category. Community pharmacies were placed into one of the three categories: community pharmacies of ≥10 stores under the same ownership (i.e., large retail), community pharmacies of fewer than 10 pharmacies under the same ownership (i.e., independent), and clinic pharmacies (i.e., outpatient pharmacies part of the hospital system). These delineations were made to examine medication discrepancy variation by pharmacy type.
Table 2.
Reporting of medication discrepancies
| Unit of analysis | Calculationsa |
|---|---|
| Patient-level | |
| Overall | Number of patients who experienced at least one medication discrepancy/Total number of patients |
| Medication-level | |
| Overall | Total number of discrepant medications/Total number of unique medications between both medication lists |
| Medication class | Number of discrepant medications by medication class/Total number of discrepant medications |
| Medication discrepancy categoriesb | Number of discrepant medications by medication discrepancy category/Total number of discrepant medications |
| Community pharmacy-level | |
| Community pharmacy typec | Number of discrepant medications by pharmacy type/Total number of unique medications for that respective pharmacy type |
Reported percentages (%) by multiplying each calculation by 100.
Addition, omission, discrepant dose, discrepant frequency, and duplication.
Large retail, independent, clinic.
Before the study analysis, the study team examined all study patient records to examine cases that warranted exclusion. Overall, four patients were excluded from the analysis. One patient transitioned to a nursing home after hospital discharge and no longer received their prescriptions from a community pharmacy. Another patient entered hospice upon hospital discharge. Two patients never filled a medication at the community pharmacy they consented for the study team to contact after hospital discharge.
Other considerations for researchers and clinicians
It was not part of the inclusion criteria that patients could only use one community pharmacy while in the outpatient setting. However, the patients included in the analysis reported to only use one community pharmacy. This was not surprising as previous studies have reported that older patients tend to remain loyal to one pharmacy compared to their younger counterparts.31 To mitigate issues during analysis, deciding whether to include patients who use more than one community pharmacy should be decided during the study conception. Including patients who use multiple pharmacies would have different study implications than a study limited to patients who only use one community pharmacy in the outpatient setting.
Depending on the research question, other calculations may be appropriate. For example, the average number of medications discrepant per study patient may be reported. Further, high risk discrepant medications (e.g., insulins) may be reported to examine the potential of a medication discrepancy to cause patient harm.
Discussion
This phase-based approach was developed to assist with collecting medication data and measuring medication discrepancies when patients transition from the hospital to community care. Using this method, the study team successfully identified and reported medication discrepancies between the hospital and community pharmacy. Ultimately, this approach allowed the study team to meet the study's pre-defined goals.
Medication discrepancies are common when patients transition between health care settings and jeopardize patient safety by placing the patient at risk of an ADE.32 Multiple studies have documented medication discrepancies between health care settings, particularly between the hospital and community care settings. Tjia et al (2009) reported that at least one medication discrepancy was identified in over 70% of sampled skilled nursing facility admissions (n = 199) from the hospital. High risk medications such as cardiovascular agents, opioid analgesics, neuropsychiatric agents, hypoglycemic agents, antibiotics, and anticoagulants accounted for over 50% of all discrepant medications.33 Similarly, Kind et al (2012) found that 47% of all study patients discharged from the hospital and back into their home (n = 708) had at least one medication discrepancy between the hospital discharge medication list and what the patient reported taking within 48–72 hours of hospital discharge.11 Gastrointestinal agents, pain control medications, and cardiovascular agents were among the most common discrepant medications. Multiple other studies have examined medication discrepancies between health care settings; however, the operationalization of medication discrepancies and detailed analytic methods often is not reported within the published study making the methods difficult to replicate.
To our knowledge, this is the first publication to detail how a phase-based approach may be used to examine medication discrepancies, and the first to address the complexity of collecting medication data and analyzing medication discrepancies. There is a significant need to evaluate and improve medication reconciliation processes when patients transition between health care settings,34–36 and rigorously examining medication discrepancies will help achieve this goal. Systematically measuring medication discrepancies that occur in the care transitions process is a critical step as researchers, clinicians, and other stakeholders work to improve health care quality and patient outcome.9 Ideally, all health care settings and providers should have updated, consistent medication information on file for their patients throughout the patient's health care delivery. Unfortunately, a universal system for updating and sharing medication and medical records across the patient's care continuum is unavailable. Often health care settings will operate as a “silo,” and providers will not having access to the patient's health care information from a previous care setting.35 As a result, patients frequently are the only link between sites of care35,36 which may complicate the health care decisions between the provider, patient, and caregiver. Therefore, health care work systems should focus on the updated, successful transfer and acknowledgment of medication and medical information between health care settings, patient, and caregiver.
Several considerations related to time and effort should be considered when applying this approach to other studies and health care settings. Data collection and creation of the electronic dataset were time intensive and may not be feasible for all studies. Medication data collection and the dataset creation was an iterative process and revisions to create the final four-phase approach added four weeks to the study. In research, there is always a compromise between limited resources and data quality.24,37 However, the phase-based approach provided a systematic method for ensuring data integrity and increasing overall study rigor. Study teams should consider the limitations of the medication data sources to ensure that conclusions made during the final reporting are appropriately interpreted to inform implications and future interventions. Although this method should be considered when examining medication discrepancies in other health care settings, the study team encourages other researchers and clinicians to further build upon and test these methods. This study was possible due, in part, to the participating hospital's proactive safety culture and transitional care initiatives. The hospital had an existing transitional care program which provided access to patients who were discharged from the hospital and back into their home. Such culture and transitional programs may not be present in other settings making data obtainment and analyses unfeasible.
Conclusion
Consistent reporting of discrepancies will allow researchers and clinicians to focus on explaining why medication discrepancies exist and better measure interventions seeking to decrease potentially harmful medication discrepancies. This likely will help decrease the excessive health care costs related to avoidable ADEs experienced by patients transitioning between health care settings.
Acknowledgments
Funding support: This material is the result of work supported with resources at the William S. Middleton Memorial Veterans Hospital, Madison, WI (Geriatrics Research, Education, and Clinical Center, Manuscript No. 2015-18). The contents do not represent views of the Dept. of Veterans Affairs or the United States Government.
Research reported in this manuscript was supported by the Agency for Healthcare Research and Quality (AHRQ) Health Services Research Dissertation Grant of the National Institutes of Health under award number R36HS021984 [PI: Kennelty]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Research reported in this dissertation also was supported by the UW-Madison School of Pharmacy Sonderegger Research Center Dissertation Grant.
The project described also was supported by the Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Sciences (NCATS), grant UL1TR000427.
No funding source had a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript, and decision to submit the manuscript for publication.
We would like to thank research assistant, Julia Loosen, for study and manuscript support.
References
- 1.Institute of Medicine (IOM) In: To Err is Human: Building a Safer Health System. Kohn LT, Corrigan JM, Donaldson MS, editors. Washington, DC.: 2000. [PubMed] [Google Scholar]
- 2.Institute of Medicine (IOM) Preventing medication errors. In: Aspden P, Wolcott J, Bootman L, Cronenwett LR, editors. Quality Chasm Series. Washington, DC.: 2006. [Google Scholar]
- 3.Sentinel Event Alert, Issue 35: Using Medication Reconciliation to Prevent Errors. The Joint Commission; 2006. [PubMed] [Google Scholar]
- 4.Institute for Healthcare Improvement . How-to Guide: Prevent Adverse Drug Events by Implementing Medication Reconciliation. Cambridge, MA: 2011. [Google Scholar]
- 5.Barnsteiner JH. Medication reconciliation. In: Hughes RG, editor. Patient Safety and Quality: An Evidence-based Handbook for Nurses. Agency for Healthcare Research and Quality (US); Rockville, MD: 2008. [PubMed] [Google Scholar]
- 6.Cornish PL, Knowles SR, Marchesano R, et al. Unintended medication discrepancies at the time of hospital admission. Arch Intern Med. 2005;165:424–429. doi: 10.1001/archinte.165.4.424. [DOI] [PubMed] [Google Scholar]
- 7.Coleman EA, Smith JD, Raha D, Min SJ. Posthospital medication discrepancies: prevalence and contributing factors. Arch Intern Med. 2005;165:1842–1847. doi: 10.1001/archinte.165.16.1842. [DOI] [PubMed] [Google Scholar]
- 8.Pippins J, Gandhi T, Hamann C, et al. Classifying and predicting errors of inpatient medication reconciliation. J Gen Intern Med. 2008;23:1414–1422. doi: 10.1007/s11606-008-0687-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kostas T, Paquin AM, Zimmerman KM, Simone M, Skarf LM, Rudolph JL. Characterizing medication discrepancies among older adults during transitions of care: a systematic review focusing on discrepancy synonyms, data sources and classificaiton terms. Aging Health. 2013;9:497–508. [Google Scholar]
- 10.Patient Protection and Affordable Care Act. United States Code. United States Congress; United States of America: 2010. [Google Scholar]
- 11.Kind AJ, Jensen L, Barczi S, et al. Low-cost transitional care with nurse managers making mostly phone contact with patients cut rehospitalization at a VA hospital. Health Aff (Millwood) 2012;31:2659–2668. doi: 10.1377/hlthaff.2012.0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith JD, Coleman EA, Min SJ. A new tool for identifying discrepancies in postacute medications for community-dwelling older adults. Am J Geriatr Pharmacother. 2004;2:141–148. doi: 10.1016/s1543-5946(04)90019-0. [DOI] [PubMed] [Google Scholar]
- 13.Orrico K. Sources and types of discrepancies between electronic medical records and actual outpatient medication use. J Manag Care Pharm. 2008;14:626–631. doi: 10.18553/jmcp.2008.14.7.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tam VC, Knowles SR, Cornish PL, Fine N, Marchesano R, Etchells EE. Frequency, type and clinical importance of medication history errors at admission to hospital: a systematic review. Can Med Assoc J. 2005;173:510–515. doi: 10.1503/cmaj.045311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med. 2011;365:2002–2012. doi: 10.1056/NEJMsa1103053. [DOI] [PubMed] [Google Scholar]
- 16.Vira T, Colquhoun M, Etchells E. Reconcilable differences: correcting medication errors at hospital admission and discharge. Qual Saf Health Care. 2006;15:122–126. doi: 10.1136/qshc.2005.015347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gleason KM, McDaniel MR, Feinglass J, et al. Results of the Medications at Transitions and Clinical Handoffs (MATCH) study: an analysis of medication reconciliation errors and risk factors at hospital admission. J Gen Intern Med. 2010;25:441–447. doi: 10.1007/s11606-010-1256-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fick DM, Cooper JW, Wade WE, Waller JL, Maclean JR, Beers MH. Updating the beers criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts. Arch Intern Med. 2003;163:2716–2724. doi: 10.1001/archinte.163.22.2716. [DOI] [PubMed] [Google Scholar]
- 19.EpiData Software The EpiData Association. 2013 [Google Scholar]
- 20.New NDC Directory 2013. Vol. 2013. US Food and Drug Administration; 2013. [Google Scholar]
- 21.Engel L, Henderson C, Fergenbaum J, Colantonio A. Medical record review conduction model for improving interrater reliability of abstracting medical-related information. Eval Health Prof. 2009;32:281–298. doi: 10.1177/0163278709338561. [DOI] [PubMed] [Google Scholar]
- 22.Liddy C, Wiens M, Hogg W. Methods to achieve high interrater reliability in data collection from primary care medical records. Ann Fam Med. 2011;9:57–62. doi: 10.1370/afm.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reisch LM, Fosse JS, Beverly K, et al. Training, quality assurance, and assessment of medical record abstraction in a multisite study. Am J Epidemiol. 2003;157:546–551. doi: 10.1093/aje/kwg016. [DOI] [PubMed] [Google Scholar]
- 24.Polnaszek B, Gilmore-Bykovskyi A, Hovanes M, et al. Overcoming the challenges of unstructured data in multisite, electronic medical record-based abstraction. Med Care. doi: 10.1097/MLR.0000000000000108. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carroll KM, Connors GJ, Cooney NL, et al. Internal validity of Project MATCH treatments: discriminability and integrity. J Consult Clin Psychol. 1998;66:290–303. doi: 10.1037//0022-006x.66.2.290. [DOI] [PubMed] [Google Scholar]
- 26.Peterson JF, Shi Y, Denny JC, et al. Prevalence and clinical significance of discrepancies within three computerized pre-admission medication lists. AMIA Annu Symp Proc. 2010;2010:642–646. [PMC free article] [PubMed] [Google Scholar]
- 27.Lessard S, DeYoung J, Vazzana N. Medication discrepancies affecting senior patients at hospital admission. Am J Health Syst Pharm. 2006;63:740–743. doi: 10.2146/ajhp050291. [DOI] [PubMed] [Google Scholar]
- 28.Gleason KM, Groszek JM, Sullivan C, Rooney D, Barnard C, Noskin GA. Reconciliation of discrepancies in medication histories and admission orders of newly hospitalized patients. Am J Health Syst Pharm. 2004;61:1689–1695. doi: 10.1093/ajhp/61.16.1689. [DOI] [PubMed] [Google Scholar]
- 29.Unroe KT, Pfeiffenberger T, Riegelhaupt S, Jastrzembski J, Lokhnygina Y, Colon-Emeric C. Inpatient medication reconciliation at admission and discharge: a retrospective cohort study of age and other risk factors for medication discrepancies. Am J Geriatr Pharmacother. 2010;8:115–126. doi: 10.1016/j.amjopharm.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boockvar KS, Liu S, Goldstein N, Nebeker J, Siu A, Fried T. Prescribing discrepancies likely to cause adverse drug events after patient transfer. Qual Saf Health Care. 2009;18:32–36. doi: 10.1136/qshc.2007.025957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Polinski JM, Schneeweiss S, Levin R, Shrank WH. Completeness of retail pharmacy claims data: implications for pharmacoepidemiologic studies and pharmacy practice in elderly patients. Clin Ther. 2009;31:2048–2059. doi: 10.1016/j.clinthera.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pronovost P, Weast B, Schwarz M, et al. Medication reconciliation: a practical tool to reduce the risk of medication errors. J Crit Care. 2003;18:201–205. doi: 10.1016/j.jcrc.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 33.Tjia J, Bonner A, Briesacher BA, McGee S, Terrill E, Miller K. Medication discrepancies upon hospital to skilled nursing facility transitions. J Gen Intern Med. 2009;24:630–635. doi: 10.1007/s11606-009-0948-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coleman EA. Falling through the cracks: challenges and opportunities for improving transitional care for persons with continuous complex care needs. J Am Geriatr Soc. 2003;51:549–555. doi: 10.1046/j.1532-5415.2003.51185.x. [DOI] [PubMed] [Google Scholar]
- 35.Kennelty KA, Chewning B, Wise M, Kind A, Roberts T, Kreling D. Barriers and facilitators of medication reconciliation processes for recently discharged patients from community pharmacists’ perspectives. Res Social Adm Pharm. 2015;11:517–530. doi: 10.1016/j.sapharm.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kahn MG, Raebel MA, Glanz JM, Riedlinger K, Steiner JF. A pragmatic framework for single-site and multisite data quality assessment in electronic health record-based clinical research. Med Care. 2012:50. doi: 10.1097/MLR.0b013e318257dd67. http://dx.doi.org/10.1097/MLR.1090b1013 e318257dd318267. [DOI] [PMC free article] [PubMed]
- 37.Chabbra PT, Rattinger GB, Dutcher SK, Hare ME, Parsons KL, Zuckerman IH. Medication reconciliation during the transition to and from long-term care settings: a systematic review. Res Social Adm Pharm. 2012;8:60–75. doi: 10.1016/j.sapharm.2010.12.002. [DOI] [PubMed] [Google Scholar]