Abstract
In a recent study we explored Group-1-p21-activated kinases(GP.1-PAKs) in rat pancreatic acini. Only PAK2 was present; it was activated by gastrointestinal-hormones/neurotransmitters and growth factors in a PKC-,Src- and small-GTPases-mediated manner. PAK2 was required for enzyme-secretion and ERK/1-2-activation. In the present study we examined PAK2’s role in CCK and TPA-activation of important distal signaling cascades mediating their physiological/pathophysiological effects and analyzed its role in pathophysiological processes important in early pancreatitis. In rat pancreatic acini,PAK2-inhibition by the specific, GP.1.PAK-inhibitor, IPA-3-suppressed cholecystokinin(CCK)/TPA-stimulated activation of focal-adhesion kinases and mitogen-activated protein-kinases. PAK2-inhibition reversed the dual stimulatory/inhibitory effect of CCK/TPA on the PI3K/Akt/GSK-3β pathway. However, its inhibition did not affect PKC activation. PAK2-inhibition protected acini from CCK-induced ROS-generation; caspase/trypsin-activation, important in early pancreatitis; as well as from cell-necrosis. Furthermore, PAK2-inhibition reduced proteolytic-activation of PAK-2p34, which is involved in programmed-cell-death. To ensure that the study did not only rely in the specificity of IPA-3 as a PAK inhibitor, we used two other approaches for PAK inhibition, FRAX597 a ATP-competitive-GP.1-PAKs - inhibitor and by infection with an PAK2-dominat negative(DN)-Advirus. Those two approaches confirmed the results obtained with IPA-3. This study demonstrates that PAK2 is important in mediating CCK’s effect on the activation of signaling-pathways known to mediate its physiological/pathophysiological responses including several cellular processes linked to the onset of pancreatitis. Our results suggest that PAK2 could be a new, important therapeutic target to consider for the treatment of diseases involving deregulation of pancreatic acinar cells.
Keywords: PAK2, pancreatic acini, CCK, signaling, IPA-3, FRAX597, PAK2-DN-Advirus, cell death, pancreatitis
1. Introduction
The p21-activated kinases (PAKs) are a family of serine threonine kinases that are effectors for the small Rho GTPases, Cdc42 and Rac [1]. They consist of 6 members, which fall into 2 related subgroups of proteins: Group I, consisting of PAK 1, 2, and 3, and Group II, consisting of PAK 4, 5 and 6 [2]. Group I PAKs are the most extensively studied and in several cell types, are reported to be important for regulating and modulating signaling cascades involved in cytoskeletal organization, cell proliferation, tumorigenic processes, migration and apoptosis, protein synthesis and to play an important role in secretion, membrane recycling, generation of reactive oxygen species, apoptosis and regulation of cellular calcium levels [1,3-9]. PAK signaling roles in cell cycle regulation, neoplastic processes and inflammation have been extensively studied [2,4], as has been their activation by growth factors and growth stimulants [2,4,8]. However, increasingly, PAKs are reported to be involved in non-neoplastic responses of cells as well as responses of normal tissues. In general, little is known of the role of PAKS in mediating the action of gastrointestinal hormones/neurotransmitters. In a recent study [8] we used pancreatic acinar cells, which are responsive to many gastrointestinal (GI) hormones/neurotransmitters, to examine their ability to activate Group I PAKs. In that study [8] the only Group I PAK present in pancreatic acinar cells is PAK2. PAK2 was activated by gastrointestinal hormones/neurotransmitters activating phospholipase C, as well as by some pancreatic growth factors, and played an important role in modulating enzyme secretion and cell growth in these cells. The results of this study demonstrated Group I PAKs are important in mediating physiological effects of GI hormones/neurotransmitters. These results combined with the observation that Rac1, which is an important activator of Group I PAKs [6], is important in the mediation of pancreatitis associated lung injury in mice [10] and renal injury [11], and that Rac1 inhibition decreases the severity of pancreatitis [10], raises the possibility that PAK2, plays a significant role in the activation of signaling cascades mediating both physiological and pathophysiological processes in pancreatic acinar cells.
To assess the possible role of PAK2 in the cellular signaling cascades involved in mediating the physiological/pathophysiological changes in pancreatic acinar cells, we examined in detail its role in CCK-mediated signaling changes, because CCK is one of the principal mediators of physiological responses of the exocrine pancreas [12-17], as well as is an inductor of pathophysiological changes, such as pancreatitis [12,18,19].
Because acinar cells cannot be cultured for prolonged periods of time to allow siRNA studies, to perform the present study, we established experimental conditions that resulted in PAK2 inhibition, by using three different approaches for PAK2 inhibition. The main approach for inhibiting PAK2 was the allosteric specific inhibitor for Group I PAKs, IPA-3 [8,20,21]. Moreover, to support the results obtained with the IPA-3 we used an ATP-competitive Group I PAKs inhibitor, FRAX597 [22] and also a PAK2-dominant negative adenovirus construct (DN-Advirus) that after infecting the cells expresses an inactive copy of PAK2 that competes with the WT active PAK2 form [23]. With these experimental conditions, we determined the importance of activation of PAK2 in mediating important signaling cascades in pancreatic acini, including the focal adhesion kinase pathway, the mitogen-activated kinases (MAPKs) pathway, the PI3K/Akt/ GSK-3β pathway and the PKC pathway. In addition, using the same inhibitory conditions, we determined if PAK2 was also involved in pathophysiological processes related to the onset of pancreatitis.
2. Materials and Methods
Materials
Male Sprague-Dawley rats (150-250 g) were obtained from the Small Animals Section, Veterinary Resources Branch, National Institutes of Health (NIH), Bethesda, MD. PAK1 recombinant protein was from Abnova (Walnut, CA). Rabbit anti-PAK1, Rabbit anti-phospho-PAK1/2 (Thr402/423), rabbit anti-phospho-PYK2 (Tyr402), rabbit anti-phospho p42/44 (Tyr202/204), rabbit anti-phospho-FAK (Tyr397), rabbit anti-phospho-paxillin (Tyr118), rabbit anti-phospho-GSK3β (Ser9), rabbit anti-phospho-p70s6k (Thr389), rabbit anti-phospho-JNK (Thr183/Tyr185), mouse anti-phospho-AKT (Thr308), rabbit anti-phospho p130Cas (Tyr410), rabbit anti-phospho Src (Tyr416), rabbit anti-phospho p38 (Thr180/Tyr182), rabbit anti-phospho Mek1/2 (Ser217/221) rabbit anti-phospho-PKD (Ser744/748), rabbit anti-phospho MARCKS (Ser152/156) rabbit anti-phospho PTEN (Ser380), anti-phospho-c-Raf (Ser338), and nonfat dry milk were purchased from Cell Signaling Technology, Inc. (Beverly, MA). Stabilized goat anti-rabbit IgG peroxidase conjugated was from Pierce Biotechnology Inc. (Rockford, IL). Goat PAK2 antibody, and anti-goat-HRP-conjugate antibodies were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Tris/HCl pH 8.0 and 7.5 were from Mediatech, Inc. (Herndon, VA). 2-mercaptoethanol, protein assay solution, sodium lauryl sulfate (SDS) and Tris/Glycine/SDS (10X) were from Bio-Rad Laboratories (Hercules, CA). MgCl2, CaCl2, Tris/HCl 1M pH 7.5 and Tris/Glycine buffer (10x) were from Quality Biological, Inc. (Gaithersburg, MD). Minimal essential media (MEM) vitamin solution, amino acids 100x, Dulbecco’s phosphate buffered saline (DPBS), glutamine (200 mM), Tris-Glycine gels, L-glutamine, fetal bovine serum (FBS) and CM-H2DCFDA (for ROS generation) were from Invitrogen (Carlsbad, CA). COOH-terminal octapeptide of cholecystokinin (CCK) was from Bachem Bioscience Inc. (King of Prussia, PA). Deoxycholic acid and P21-activated kinase inhibitor 3 (IPA-3) was from Calbiochem (La Jolla, CA). FRAX597 was from APExBIO (Houston, TX). Dimethyl sulfoxide (DMSO), 12-O-tetradecanoylphobol-13-acetate (TPA), L-glutamic acid, glucose, fumaric acid, pyruvic acid, trypsin inhibitor, HEPES, TWEEN® 20, Triton X-100, trypsin, phenylmethanesulfonyl fluoride (PMSF), ethylenediaminetetraacetic acid (EDTA), ethylene glycol tetraacetic acid (EGTA), nonidet P40®, sucrose, sodium-orthovanadate, and sodium azide were from Sigma-Aldrich, Inc. (St. Louis, MO). Albumin standard and Super Signal West (Pico, Dura) chemiluminescent substrate were from Pierce (Rockford, IL). Protease inhibitor tablets were from Roche (Basel, Switzerland). Purified collagenase (type CLSPA) was from Worthington Biochemicals (Freehold, NJ). Nitrocellulose membranes were from Schleicher and Schuell Bioscience, Inc. (Keene, NH). Pir 3,5 (IPA-3 inactive control) was from Tocris Bioscience (Ellisville, MO). Albumin bovine fraction V was from MP Biomedical (Solon, OH). NaCl, KCl and NaH2PO4 were from Mallinckrodt (Paris, KY). Cytotox 96 ® Non Radioactive Cytotoxicity Assay was from Promega (Madison, WI). Caspase fluorogenic substrates, Ac-IETD-AMC, Ac-DEVD-AMC, Ac-LEHD-AMC, Trypsin fluorogenic substrate, Boc-Gln-Ala-Arg-AMC and AMC (7-Amino-4-methylcoumarin) Calibration Standard were from Enzo Life Sciences, Inc (Farmingdale, NY). Ad-PAK2, Ad-CMV Null and ViralPlus Transduction were from Applied Biological Materials (Richmond, BC). QuickTiter™ Adenovirus Quantitation Kit and ViraBind™ Adenovirus Purification Kit were from Cell Biolabs, Inc (San Diego, CA).
Methods
Pancreatic Acini Preparation
Pancreatic acini were obtained by collagenase digestion as previously described [13]. Standard incubation solution contained 25.5 mM HEPES (pH 7.45), 98 mM NaCl, 6 mM KCl, 2.5 mM NaH2PO4, 5 mM sodium pyruvate, 5 mM sodium glutamate, 5 mM sodium fumarate, 11.5 mM glucose, 0.5 mM CaCl2, 1 mM MgCl2, 1 mM glutamine, 1% (w/v) albumin, 0.01% (w/v) trypsin inhibitor, 1% (v/v) vitamin mixture and 1% (v/v) amino acid mixture.
Acini Stimulation
After collagenase digestion, dispersed acini were pre-incubated in standard incubation solution for 2 hrs. at 37 °C as described previously [13,15]. After pre-incubation 1 ml aliquots of dispersed acini were incubated at 37 °C with or without stimulants. Cells were lysed in lysis buffer (50 mM Tris/HCl pH 7.5, 150 mM NaCl, 1% Triton X-100, 1% deoxycholate, 0.1% sodium azide, 1 mM EGTA, 0.4 mM EDTA, 0.2 mM sodium orthovanadate, 1 mM PMSF, and one protease inhibitor tablet per 10 ml). After sonication, lysates were centrifuged at 10,000x g for 15 min at 4 °C and protein concentration was measured using the Bio-Rad protein assay reagent.
Inhibition experiments
Preincubation with IPA-3 was performed as previously described [8] to assess the effects of the inhibition of PAK2 on the activation of several downstream signaling kinases. Briefly, isolated acini were preincubated for 15 minutes with IPA-3, that prevents binding of CDC42 to group I PAKs [8,20,21] and its inactive analogue Pir 3,5 as a control. Cells were then treated for 3 minutes with physiological (0.3 nM) or supraphysiological (100 nM) concentrations of CCK and also with TPA (5 min, 1 μM), with untreated cells for each pretreatment used as controls. Another pharmacological approach of PAK2 inhibition was done by using the ATP-competitive inhibitor of group I PAKs FRAX 597 [22]. FRAX 597 was used at conditions of time and concentration previously indicated in other studies [24]. More specifically, cells were preincubated for 15 minutes with 40 μM FRAX597 and then incubated with CCK 100 nM for 3 minutes
Virus infection
To include a third approach, different from the pharmacological, for inhibiting PAK2 we used a dominant negative PAK2 adenovirus (Dn-PAK2-Advirus)[23] , and nulled Ad-virus (as an infection control). The viruses, were amplified and quantified as previously described [25]. Pancreatic acini were isolated as described above, infected with either Ad-CMV-Null (empty adenovirus, as infection control) or DN-PAK2-Advirus at 1×109VP/ml concentration, as described previously [25]. After 6 h, stimulants were added and cells lysed, as described below in Western Blotting.
Western Blotting
It was performed as described previously [8,26,27]. Whole cell lysates were subjected to SDS-PAGE using 4-20% Tris-Glycine gels. After electrophoresis, proteins were transferred to nitrocellulose membranes. Membranes were blocked in blocking buffer (50 mM Tris/HCl pH 8.0, 2 mM CaCl2, 80 mM NaCl, 0.05% Tween® 20, 5% non fat dry milk) at room temperature for one hour. Membranes were then incubated with primary antibody overnight at 4 °C under constant agitation at antibody dilutions suggested by the supplier. After primary antibody incubation membranes were washed twice in blocking buffer for 4 minutes and then incubated with HRP-conjugated secondary antibody (anti-mouse, anti-rabbit, anti-goat) according to the species of the first antibody for 1 hour at room temperature under constant agitation. Membranes were then washed again twice in blocking buffer for 4 minutes, twice in washing buffer (50 mM Tris/HCl pH 8.0, 2 mM CaCl2, 80 mM NaCl, 0.05% Tween® 20) for 4 minutes, incubated for 4 minutes with chemiluminescense detection reagents and finally exposed to Kodak Biomax film (XAR, MS or MR). The intensity of the protein bands was measured using Kodak ID Image Analysis, which were assessed in the linear detection range. When re-probing was necessary membranes were incubated in Stripping buffer (Pierce, Rockford, IL) for 30 minutes at room temperature, washed twice for 10 min in washing buffer, blocked for 1 hour in blocking buffer at room temperature and re-probed as described above.
Lactate dehydrogenase (LDH) assay
The assay was performed by using the commercially available Cytotox 96 non-radioactive cytotoxicity assay kit according to the manufacturer’s instructions (Promega, Madison, Wisconsin). In brief, acinar cells (50.000 per ml) were pretreated with 40 μM IPA-3, 40 μM FRAX597, or Pir 3,5 for 1 h followed by stimulation with CCK (0.3 or 100 nM) or TPA (1 μM) for a further 1 h. Samples were collected and centrifuged at 30g for 30 s. A 120 μl aliquot of medium was then removed to measure LDH release from the cells. A manufacturer’s provided lysis reagent was then added to the remaining 380 μl of cells and medium to determine total LDH. Both cell and medium samples were assayed. The results were expressed as the percent of cellular LDH, released into the medium during the incubation.
Measurement of caspase activities
Caspase activities were measured using a fluorogenic assay with substrates specific for caspase-3 (Ac-DEVD-AMC), caspase-8 (Ac-IETD-AMC), or caspase-9 (Ac-LEHD-AMC) as described previously [28,29]. Pancreatic acinar cells, preincubated 1 hour with and without inhibitors (IPA-3 and FRAX597) and incubated for 3 hours with CCK (0.3 nM and 100 nM), were lysed in buffer containing 150 mM NaCl, 50 mM Tris-HCL (pH 7.5), 0.5% Nonidet P-40, and 0.5 mM EDTA, centrifuged for 15 min at 15,000 g, and supernatants were collected. Proteolytic reactions were carried out at 37°C in a buffer containing 25 mM HEPES (pH 7.5), 10% sucrose, 0.1% CHAPS, and 10 mM dithiothreitol, using specific substrates for each caspase. Cleavage of these substrates releases 7-amino-4-methylcoumarin (AMC), which emits a fluorescence signal with excitation at 380 nm and emission at 440 nm. Fluorescence was calibrated using a standard curve for AMC. The data are expressed as picomoles of AMC per milligram of protein per minute. In the same cell lysates used for the measurement of caspases activity we also assessed for the formation of the proteolytic fragment of PAK2 p34PAK2 by Western blotting.
Trypsin Activity Assay
Trypsin activity was measured using a fluorogenic assay with a substrate specific for trypsin (Boc-Glu-Ala-Arg-AMC) as described previously [30]. After the cells were treated with the various agents, they were washed with ice-cold PBS and then lysed in morpholino propylsulphonate (MOPS) buffer, pH 7.0 (containing 250 mM sucrose, 5 mM MOPS, and 1 mM MgSO4), centrifuged for 15 min at 15,000 g, and supernatants, with the addition of the substrate, were used for the assay. Trypsin activity was measured fluorometrically, applying the same principle used for determining the activity of the caspases with excitation at 380 nm and emission at 440 nm. Fluorescence was calibrated using a standard curve with trypsin. To compare values between different treatments, the data were expressed as the percentage of maximal activity obtained when acini were incubated with 100 nM CCK for 20 min.
Determination of reactive oxygen species formation
Free radical production was measured by incubating the pancreatic acinar cells in the presence of CM-H2DCFDA (10 μM) for 1 h at 37°C. This dye has been often used for determination of ROS production [9,31]. CM-H2DCFDA is a stable non-fluorescent molecule that diffuses into the cells, where it can be cleaved producing a polar diol that is well retained within the cells and then oxidized by ROS to a fluorescent form. Cells were preincubated with 40 μM IPA-3 or Pir 3,5 for one hour, followed by stimulation for another hour with CCK (0.3 nM and 100 nM) and TPA 1 μM. Cell were then incubated with CM-H2DCFDA for one hour, and, after washing in PBS, the fluorescence signals (excitation: 488 nm/ emission:530 nm) in cell aliquots were measured.
Statistical Analysis
All experiments were performed at least 5 times. Data are presented as mean ± SEM and were analyzed with the non-parametric Kruskal-Wallis analysis using the GraphPad 5.0 software. P values <0.05 were considered significant.
3. Results
3.1. IPA-3 prevents CCK and TPA-induced activation of PAK2
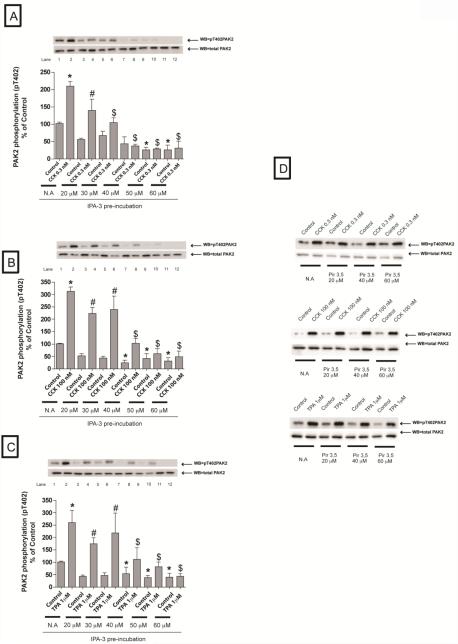
We first determined the optimal experimental conditions necessary with IPA-3 that inhibited activation of PAK2 by at least an 80%. We treated the cells with increasing concentrations of IPA-3 during 15 minutes (Fig 1A-C). using The inactive IPA-3 analogue, Pir-3, 5 as a control to ensure specificity (Fig 1D). After pre-incubation, cells were treated either with CCK (0.3 nM, 100 nM) for three minutes or with TPA (1 μM) for 5 minutes (Fig 1A-D).
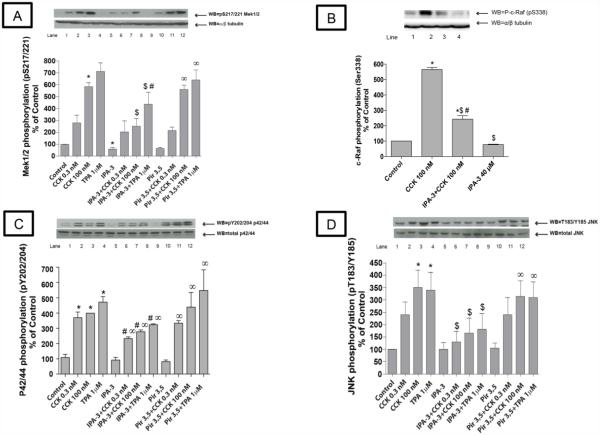
Fig. 1. Effect of increasing concentrations of the active PAK2 inhibitor, IPA-3, and its inactive control, Pir 3,5, on PAK2 activation by CCK or TPA.
Rat pancreatic acinar cells were pretreated with no additions or with IPA-3 (20, 30, 40, 50 and 60 μM) or Pir 3,5 (20, 40 and 60 μM) for 15 min and then incubated with no additions (control), with 0.3 nM CCK or 100 nM CCK for 3 min or with 1 μM TPA for 5 min and then lysed. Whole cell lysates were submitted to SDS-PAGE and transferred to nitrocellulose membranes. Membranes were analyzed using anti-pT402 PAK2 and total PAK2 was used to verify loading of equal amounts of protein. The bands were visualized using chemoluminescence and quantification of phosphorylation was assessed using scanning densitometry. Both a representative experiment of 4 others and the means of all the experiments are shown. * P< 0.05 vs. control, # P< 0.05 vs. IPA-3 alone, and $ P< 0.05 comparing stimulants (CCK or TPA) preincubated with 1% DMSO vs. stimulants pre-incubated with IPA-3.
A 20 μM IPA-3 concentration was not sufficient to induce a significant reduction of PAK2 activity in every condition (Fig. 1A-C). Preincubation with 40 μM IPA-3 completely inhibited activation of PAK2 by a physiological concentration of CCK (0.3 nM) (Fig. 1A) and inhibited stimulation by a supraphysiological concentration of CCK (100 nM) or TPA (1μM) by at least an 80% (Fig. 1B-C). The higher IPA-3 concentrations (50 and 60 μM) inhibited completely PAK2 activity at every condition (Fig. 1A-C). Because the very high dose required for complete PAK2 inhibition could lead to possible non-specific effects as well as higher expense, a 40 μM of IPA-3 preincubation for 15 minutes was used for the rest of the study. This concentration ensured > 80% inhibition of CCK-induced PAK2 activation and despite being at the upper end of the recommended dose by the manufacturer it has been used in other studies [5,8]. Preincubation with the inactive analogue of IPA-3, Pir 3,5, had no effect under any of the concentrations used (Fig. 1D).
3.2. Downstream effectors of PAK2 in pancreatic acinar cells (Fig 2-7)
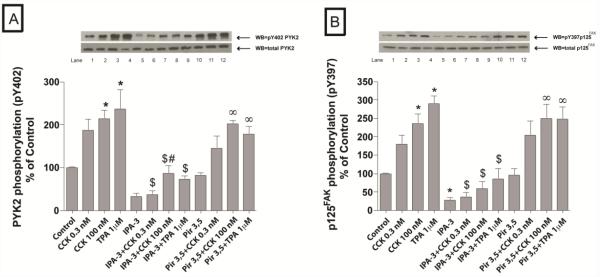
Fig. 2. Effect of IPA-3 and Pir 3,5 on the ability of physiological (0.3 nM) and supraphysiological (100 nM) concentrations of CCK and TPA (1 μM) to stimulate various kinases of the focal adhesion pathway (PYK2 and p125FAK).
Rat pancreatic acinar cells were pretreated with no additions or with IPA-3 (40 μM) or Pir 3,5 (40 μM) for 15 min and then incubated with no additions (control), with 0.3 nM CCK or 100 nM CCK for 3 min or with 1 μM TPA for 5 min and then lysed. Whole cell lysates were submitted to SDS-PAGE and transferred to nitrocellulose membranes. Membranes were analyzed using anti-pY402 PYK2 and anti-pY397 p125FAK. Antibodies detecting total amount of these kinases were used to verify loading of equal amounts of protein. The bands were visualized using chemoluminescence and quantification of phosphorylation was assessed using scanning densitometry. Both a representative experiment of 4 others and the means of all the experiments are shown. * P< 0.05 vs. control, # P< 0.05 vs. IPA-3 alone, ∞ P< 0.05 vs. Pir 3,5 alone and $ P< 0.05 comparing stimulants (CCK or TPA) preincubated with 1% DMSO vs. stimulants pre-incubated with IPA-3 or Pir 3,5, respectively.
We investigated the effect of PAK2 inhibition on the activation of kinases belonging to the focal adhesion kinase pathway (Fig. 2,3,6), the mitogen-activated kinases (MAPKs) pathway (Fig. 4,6), the PI3K/Akt/ GSK-3β pathway (Fig. 5,7) and the PKC pathway (Fig. 7) these signaling pathways after incubation with CCK for 3 minutes at physiological (0.3 nM) or a supraphysiological (100 nM) concentration or with TPA (5 min, 1 μM).
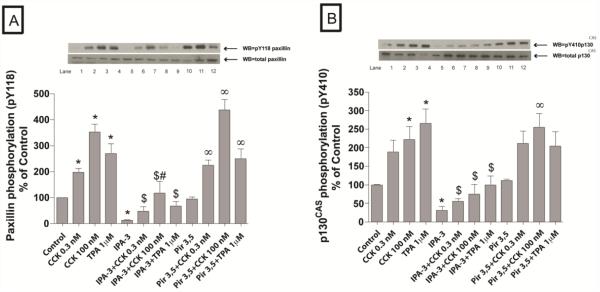
Fig. 3. Effect of IPA-3 and Pir 3,5 on the ability of physiological (0.3 nM) and supraphysiological (100 nM) concentrations of CCK and TPA (1μM) to stimulate various adapter proteins (paxillin and p130CAS).
Rat pancreatic acinar cells were processed as stated in Figure 2. Membranes were analyzed using anti-pY118 paxillin and anti-pY410 p130CAS. Antibodies detecting total amount of these kinases were used to verify loading of equal amounts of protein. The bands were visualized using chemoluminescence and quantification of phosphorylation was assessed using scanning densitometry. Both a representative experiment of 4 others and the means of all the experiments are shown. * P< 0.05 vs. control, # P< 0.05 vs. IPA-3 alone, ∞ P< 0.05 vs. Pir 3,5 alone and $ P< 0.05 comparing stimulants (CCK or TPA) preincubated with 1% DMSO vs. stimulants pre-incubated with IPA-3 or Pir 3,5, respectively.
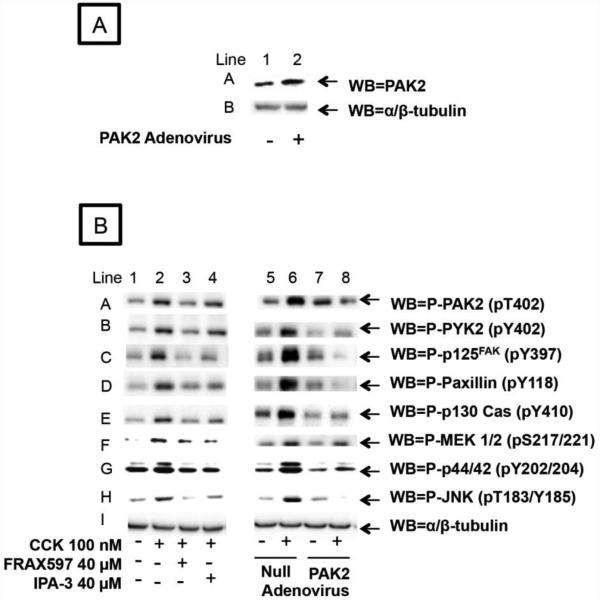
Fig. 6. Effect of pre-incubation with PAK dominant negative and control adenovirus upon the expression of PAK2, in pancreatic acinar cells, and the effect of the PAK2 inhibitors, FRAX597 and IPA-3, and the dominant negative PAK2 adenovirus on the ability of CCK 100 nM to stimulate the downstream effectors of PAK2 (Focal Adhesion Kinases and Mitogen Activated Protein Kinases pathways).
A. Rat pancreatic acinar cells were pre-incubated for 6 h without (Line 1) or with (Line 2) 109 VP/ml of DN-PAK2-Advirus or wild type adenovirus. This experiment is representative of 3 others.
B. Rat pancreatic acinar cells were processed as stated in Figure 2, except that 40 µM FRAX597 was also added during a 15 minutes pre-incubation as described in Material and Methods. Dominant negative PAK2 adenovirus or Null adenovirus were added 6 hours prior to stimulants which were added for 5 minutes. Membranes were analyzed using anti-pT402-PAK2, anti-pT402-PYK2, anti-pY397-p125FAK, anti-pY118-Paxillin, anti-pY410-p130 Cas, anti-pS217/221-MEK 1/2, anti-pY202/204-p44/42, and anti-pT183/Y185-JNK. Anti-α/β-tubulin was used to verify loading of equal amounts of protein. These results of the experiments shown are representative of 3 others.
Fig. 4. Effect of the PAK2 inhibitor, IPA-3 and its inactive analogue Pir 3,5 on the ability of physiological (0.3 nM) and supraphysiological concentrations of CCK (100 nM) and TPA to stimulate various kinases in the mitogen-activated kinases pathway (Mek 1/2, c-raf, p42/44 and JNK).
Rat pancreatic acinar cells were processed as stated in Figure 2. Membranes were analyzed using anti-pS217/221 Mek1/2, anti pS338-Raf, anti p-Y202/204 p42/44 and anti-p T183/Y185 JNK. Antibodies detecting total amount of these kinases or tubulin were used to verify loading of equal amounts of protein. The bands were visualized using chemoluminescence and quantification of phosphorylation was assessed using scanning densitometry. Both a representative experiment of 4 others and the means of all the experiments are shown. * P< 0.05 vs. control, # P< 0.05 vs. IPA-3 alone, ∞ P< 0.05 vs. Pir 3,5 alone and $ P< 0.05 comparing stimulants (CCK or TPA) preincubated with 1% DMSO vs. stimulants pre-incubated with IPA-3 or Pir 3,5, respectively.
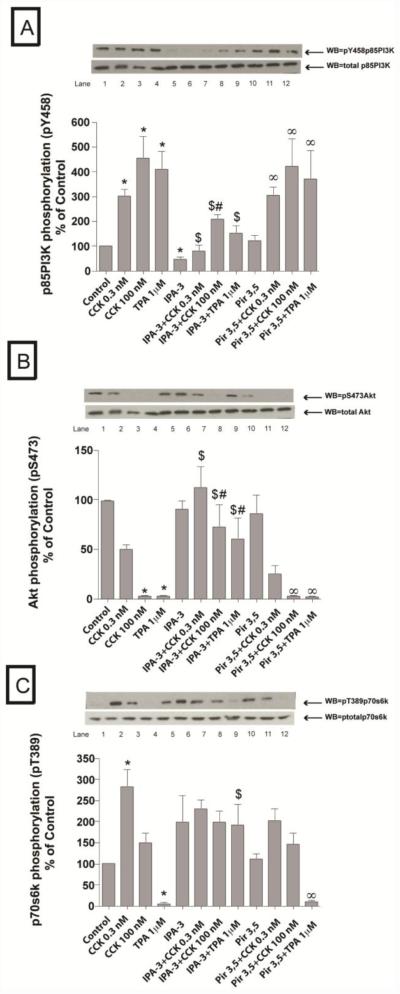
Fig. 5. Effect of the PAK2 inhibitor, IPA-3 and its inactive analogue Pir 3,5 on the ability of physiological (0.3 nM) and supraphysiological concentrations of CCK (100 nM) and TPA to stimulate various kinases in the PI3K pathway (p85, Akt and P70S6k).
Rat pancreatic acinar cells were processed as stated in Figure 2. Membranes were analyzed using anti-pY458 p85, anti-S473 Akt and anti-T389 P70S6k. Antibodies detecting total amount of these kinases or tubulin were used to verify loading of equal amounts of protein. The bands were visualized using chemoluminescence and quantification of phosphorylation was assessed using scanning densitometry. Both a representative experiment of 4 others and the means of all the experiments are shown. * P< 0.05 vs. control, # P< 0.05 vs. IPA-3 alone, ∞ P< 0.05 vs. Pir 3,5 alone and $ P< 0.05 comparing stimulants (CCK or TPA) preincubated with 1% DMSO vs. stimulants pre-incubated with IPA-3 or Pir 3,5, respectively.
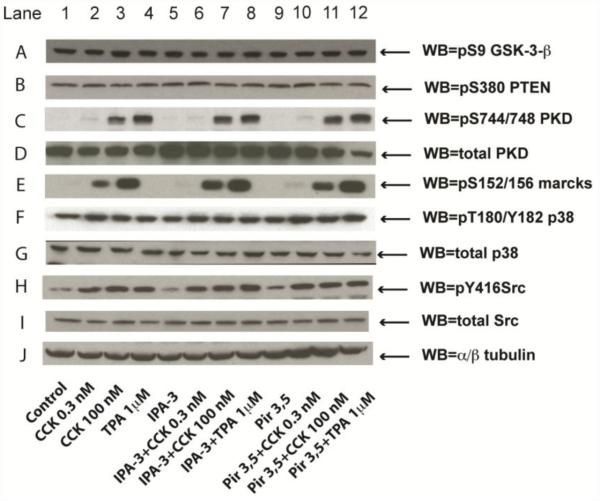
Fig. 7. Effect of the PAK2 inhibitor, IPA-3 and its inactive control, Pir 3,5 on the ability of physiological (0.3 nM) and supraphysiological concentrations of CCK (100 nM) and TPA to stimulate the PKC pathway (PKD, Marcks), PTEN, p38, Src and GSK-3β.
Rat pancreatic acinar cells were processed as stated in Figure 2. Membranes were analyzed using anti-pS9 GSK-3-β, anti-p-S380 PTEN, anti-pS744/748 PKD, pS152/156 Marcks, anti-pY183 p38 and anti-pY416 Src. Antibodies detecting total amount of these kinases or tubulin were used to verify loading of equal amounts of protein. These results of the experiments shown are representative of 4 others.
3.2.1. Focal Adhesion Kinases pathway
The basal activity of PYK2 (−68±7%Δ of basal, p<0.001), p125FAK (−72±7%Δ of basal, p<0.001), p130Cas (−68±9%Δ of basal, p<0.001) and paxillin (−89±1%Δ of basal, p<0.001) were markedly reduced by pre-incubation with 40 μM IPA-3, whereas, it were unaffected by 40 μM Pir 3,5 (Fig. 2 and Fig. 3). Moreover, IPA-3 preincubation reduced the CCK-induced phosphorylation of Y402 PYK2 (22±6% of CCK 0.1 nM alone; 41±9% of CCK 100 nM alone p<0.05) (Fig. 2A), Y397 p125FAK (21±8% of CCK 0.1 nM alone; 23±5% of CCK 100 nM alone p<0.05) (Fig. 2B), Y118 paxillin (26±10% of CCK 0.1 nM alone; 36±14% of CCK 100 nM alone p<0.001) (Fig. 3A) and Y410 p130Cas (30±3% of CCK 0.1 nM alone; 31±7% of CCK 100 nM alone p<0.05) (Fig. 3B). Inhibition of PAK2 also reduced the TPA-induced phosphorylation of Y 402 PYK2 (33±4% of TPA alone, p<0.05) (Fig. 2A), Y397 p125FAK (28±8% of TPA alone, p<0.05) (Fig. 2B), Y118 paxillin (25±7% of TPA alone, p<0.001) (Fig. 3A) and Y410 p130Cas (36±7% of TPA alone, p<0.05) (Fig. 3B). Preincubation with Pir 3,5 did not alter either basal or CCK- and TPA-induced phosphorylation of any of these kinases (Fig. 2 and Fig. 3).
3.2.2. Mitogen Activated Protein Kinases pathway
The basal activity of Mek 1/2 assessed by phosphorylation of pS217/221 (Fig. 4A; Table 1) was reduced after pre-incubation with 40 μM IPA-3 (−38±4%Δ of basal, p<0.05), whereas, it was unaffected by 40 μM Pir 3,5. However, preincubation with IPA-3 did not significantly affect the basal phosphorylation of p42/44 (0.27±0.05 of basal,)(Fig. 4C), c-raf (78±2.5 of basal) (Fig. 4B).or T183/Y185 JNK (100±26% of basal, p<0.001) (Fig. 4D). IPA-3 (40 μM) preincubation reduced significantly the CCK-induced phosphorylation of Mek 1/2 (71±12% of CCK 0.3 nM alone; 43±12% of CCK 100 nM alone, p<0.05) (Fig. 4A), c-Raf (43±3% of CCK 100 nM alone, p<0.05) (Fig. 4C), p42/44 (64±4% of CCK 0.3 nM alone; 69±3% of CCK 100 nM alone, p<0.05) (Fig. 4C) and JNK (Fig. 4D) (49±8% of CCK 0.3 nM alone; 44±8% of CCK 100 nM alone p<0.05). Inhibition of PAK2 also reduced TPA-induced phosphorylation of Mek 1/2 (64±15% of TPA alone, p<0.05) (Fig. 4A), p42/44 (70±5% of TPA alone, p<0.05) (Fig. 4C) and JNK (58±15% of TPA alone, p<0.05) (Fig. 4D). The activity of the MAPK, p38, was not affected by any of the stimulators or inhibitors used in these experiments under the experimental concentrations used (Fig. 7, row F).
Table 1.
Effects of CCK or TPA stimulation in rat pancreatic acini, with or without inhibition of the activation of PAK2 (a).
| Variable | Yes | No |
|---|---|---|
| I. Kinase stimulation | ||
| By 100 nM CCK | PAK2, PYK2, p125FAK, Paxillin, p130CAS, c- Raf, Mek1/2, p42/44, JNK, p85, Akt |
GSK-3-p, p38, p70S6k |
| By 0.3 nM CCK | PAK2, p125FAK, Paxillin, p42/44, p85, Src, PKD and MARCKS. |
PYK2, p130CAS, Mek1/2, p38, JNK, p70S6K, Akt and GSK3-p. |
| By 1 μM TPA | PAK2, PYK2, p125FAK, paxillin, p130CAS, Mek1/2, p42/44, JNK, p85, Akt (inhibited), |
P38, GSK3-p. |
| II. Kinase inhibition by 40 μM IPA-3 | ||
| Of basal | PAK2, Mek1/2, p125FAK, Paxillin and p130CAS. |
PYK2, p42/44, JNK, p38, p85, Akt, p70S6K, GSK3-P, PKD, MARCKS, Src. |
| Of 100 nM CCK kinases stimulation | PAK2, PYK2, p125FAK, paxillin, p130CAS, c- Raf, Mek1/2, p42/44, JNK, p85 and Akt |
p38, p70S6K, GSK3-P, PKD, MARCKS, Src. |
| Of 0.3 nM CCK stimulation | PAK2, PYK2, p125FAK, paxillin, p130CAS, p42/44, JNK, p85 and Akt (reversed) |
Mek1/2, p38, p70S6k, GSK3-p, PKD, MARCKS, Src. |
| Of 1 μM TPA stimulation | PAK2, PYK2, p125FAK, paxillin, p130CAS, p42/44, Mek1/2, JNK, p85, Akt (reversed |
p38, GSK3-P, PKD, MARCKS, Src. |
3.2.3. PI3K pathway
To assess the role of activation of PAK2 in the ability of CCK and TPA to alter activity of the PI3K signaling cascade we assessed the phosphorylation state of the Y458 in the p85 subunit, which has been reported to correlate with its activation of PAK2 [32]. Pre-incubation with IPA-3 reduced by half (−53±10%Δ of basal, p<0.05) the basal activity of p85PI3K (Fig. 5A). Moreover, IPA-3 (40 μM) pre-incubation reduced significantly the CCK- and TPA-induced phosphorylation of p85 PI3K (30±11% of CCK 0.3 nM alone, p<0.05; 53±15% of CCK 100 nM alone, p<0.05 and 41±11% of TPA alone, p<0.05) (Fig. 5A).
With Akt, its basal activity was not affected when PAK2 was inhibited (90±8% of control basal) (Fig. 5B). However, preincubation with 40 μM IPA-3 completely reversed the previously described CCK- and TPA-induced dephosphorylation of Akt [33], restoring the signal of pS473 Akt that was not present in the cells incubated only with CCK and TPA, while the inactive control, Pir 3,5 had no effect (Fig. 5B).
Neither CCK nor TPA stimulated the activation of PTEN in pancreatic acinar cells (Fig. 7 row B), and the suppression of activation of PAK2 had no effect on basal or the inability of CCK or TPA to stimulate changes in PTEN activity (Fig. 7 row B). The basal activity of the Akt downstream effector P70S6K was not affected by pre-incubation with 40 μM IPA-3 (90±8%Δ of control) (Fig. 5C) and neither CCK alone or with PAK suppression, altered P70S6K activity (Fig. 5C). However, TPA induced a reduction of the P70S6K activation (80±12%Δ of basal, p<0.001) (Fig. 5C). Preincubation with IPA-3 had no effect on the ability of CCK to alter P70S6K activity, however, it completely reversed the inhibitory effect of TPA on P70S6K (Fig. 5C). In addition, GSK-3-β, which is negatively regulated by Akt in many cells, was not affected by any of the stimulants or inhibitors used in this study (Fig. 7 row A).
3.2.4. PKC and Src pathways
Finally, neither phosphorylation of PKD nor the PKC substrate MARCKS (Fig. 7 row C and E), stimulated by CCK- and TPA, were affected by preincubation with IPA-3, demonstrating that CCK/TPA-stimulated activation of PKC was independent of activation of PAK2. Moreover, preincubation with IPA-3 did not modify the well-known CCK- and TPA-induced activating effect upon the Src family of kinases, demonstrating that activation of PAK2 is not necessary for Src activation by CCK/TPA (Fig. 7 row H).
3.3. PAK2 regulates cell death pathways in pancreatic acinar cells promoting cell death
Supramaximal CCK concentrations, used in vitro models of pancreatitis, are reported to activate cell death pathways stimulating apoptosis and necrosis [19]. We used our experimental conditions of >80% inhibition of PAK2 (Fig. 1) to investigate its role in mediating CCK-stimulated cell death pathways involving cell necrosis or apoptosis in pancreatic acinar cells.
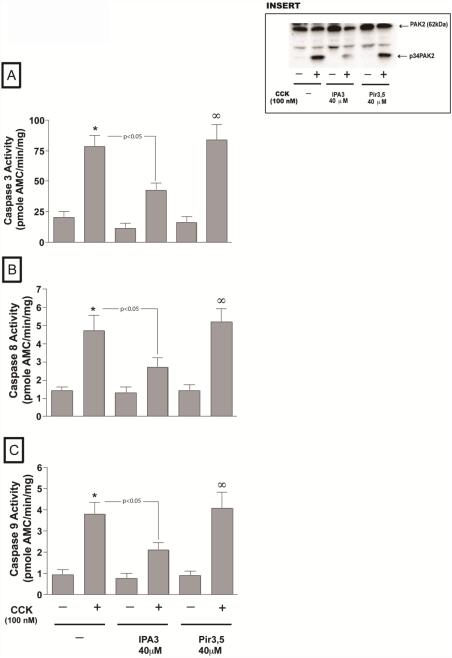
Apoptosis in pancreatic acinar cells is mediated principally by activation of caspases [28,34,35]. Treatment of pancreatic acini with 100 nM CCK stimulated activation of caspase -3 (435±95%) -8 (328±33 %) and -9 (446±89 %) in pancreatic acinar cells (Fig. 8 A-C). However, in the acini incubated with CCK, preincubation with IPA-3 resulted in a reduction of caspases activation (-3, -8 and -9) induced by a supramaximal concentration of CCK. Specifically, in the presence of IPA-3 there was a reduction of 54.7±3.3 % increase in CCK-induced caspase-3 activity, 57.6±3.6 % in caspase-8 and 55.2±3.2 % in caspase-9 (all p<0.005 vs. CCK-alone, Fig. 8 A-C). In contrast, the inactive analogue, Pir3,5 had no effect (Fig. 8A-C).
Fig. 8. Effect of inhibition of PAK2 on CCK-mediated apoptosis in pancreatic acini.
Freshly isolated rat pancreatic acini were pre-incubated with either 40 μM IPA-3 or Pir 3,5 for 3 hours. Caspase (caspase 3, 8, 9) activities were measured as described in Materials and Methods in the lysates of isolated pancreatic acini. The results are representative of 4 independent (n=4) experiments. Results shown are the means ± SEM., * P< 0.05, comparing control, $ P<0.05 vs. Pir 3,5 alone. The generation of the active p34PAK2 form by the 3 hours incubation with CCK was also analyzed by western blotting (insert).
Cells incubated with no stimulant released 3.7±0.9% of total LDH (Fig. 9B). The cells incubated with 0.3 nM CCK showed a significant increase in LDH release of 7.5±1.8% of total LDH (220±29 % over control, p<0.05). This increase was even higher in cells incubated with 100 nM CCK that released 19.3±4.0 % of total LDH (642.3±121.5 % over control, p<0.01). The cells incubated with 1 μM TPA showed a significant increase in LDH release of 8.2±1.8% (251±43 % over control, p<0.05). Neither IPA-3 (97±8% of control) (3.6±1.5% of total) nor Pir3,5 (167±34% of control) (5.3±0.5% of total) had an effect on the basal level of LDH released from the cells (Fig. 9B). Pretreatment of the acinar cells with IPA-3 significantly reduced the effect of the supramaximal dose of CCK (100 nM) on LDH release resulting in a reduction of a 42±5% of the stimulation induced by CCK alone (11.8±3.6% of total LDH release, p< 0.05 vs. 100 nM CCK-treated cells, n=4) (Fig. 9B). IPA-3 also induced significant reduction in the 0.3 nM CCK-induced LDH release (42±5% of the stimulation induced by CCK alone; 4.5±2.5% of total LDH release, p<0.05 vs. 0.3 nM CCK-treated cells, n=4) and of TPA-induced amylase release (39±10% of the stimulation induced by CCK alone 4.9±2.2% of total LDH release, p< 0.05 vs. TPA-treated cells, n=4) (Fig. 9B). In contrast, the inactive analogue Pir3,5 had no effect on CCK- or TPA-mediated stimulation of LDH release (Fig. 9B). The treatment of pancreatic acinar cells with 0.3 nM CCK did not induce any change in LDH release or caspases activity [Data not shown].
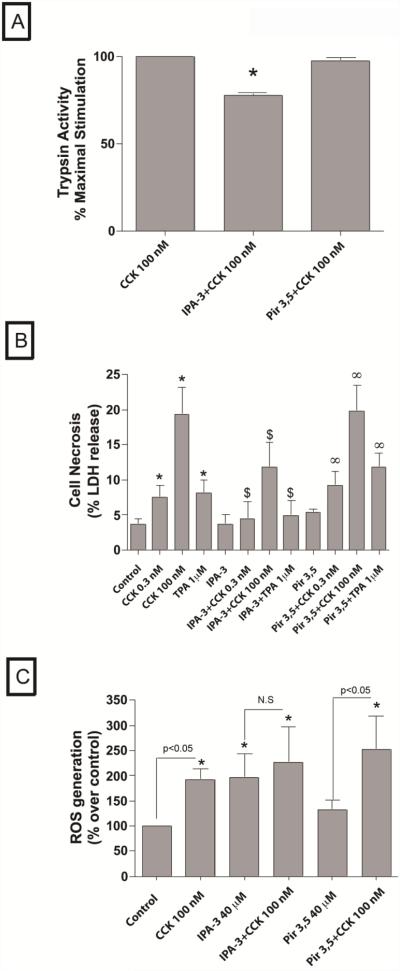
Fig. 9. Effect of inhibition of PAK2 on CCK- induced trypsin activation and CCK-mediated cell necrosis in pancreatic acini.
A. Freshly isolated rat pancreatic acini were pre-incubated with either 40 μM IPA-3 or Pir 3,5 for 1 hour followed by stimulation with 0.3 and 100 nM CCK and 1 μM TPA for 20 minutes. Trypsin activity was measured as described in Materials and Methods in the lysates of isolated pancreatic acini. The results are representative of 4 independent (n=4) experiments. The data are expressed as the percentage of maximal activity obtained when acini were incubated for 20 min with 100 nM CCK. Results shown are the means ± SEM., * P< 0.05, comparing CCK-maximal stimulation.
B. Freshly isolated rat pancreatic acini were pre-incubated with either 40 μM IPA-3 or Pir 3,5 for 1 hour followed by stimulation with 0.3 and 100 nM CCK and 1 μM TPA for 1 hour. LDH release was measured as described in Materials and Methods in the supernatants of isolated pancreatic acini. The results are representative of 4 independent (n=4) experiments. Results shown are the means ± SEM., * P< 0.05, comparing control, $ P<0.05 comparing stimulants (CCK or TPA) preincubated with 1% DMSO vs. stimulants pre-incubated with IPA-3 or Pir 3,5, respectively, ∞ P<0.05 vs. Pir 3,5 control.
C. Experimental conditions were the same as reported in Fig. Legend. ROS generation was measured as described in Materials and Methods in cell suspensions of isolated pancreatic acini. The results are representative of 6 independent (n=6) experiments. Results shown are the means ± SEM., * P< 0.05, comparing control (data on 0.3nM CCK and TPA are not shown.
3.4. IPA-3 inhibits the CCK-activated PAK2 cleavage
PAK2 is unique among the PAK isoforms because it is also activated through proteolytic cleavage by caspases or caspase-like proteases [36,37]. This proteolytic activation of PAK-2p34 is involved in programmed cell death [38,39]. To investigate this possibility we performed incubation with supramaximal CCK for 3 hours and found that it resulted in the proteolytic cleavage of PAK-2 and the generation of activated p34 subunit (Fig. 8 insert). Furthermore, IPA-3 preincubation was effective in reducing the PAK2 cleavage, but not its inactive control Pir3,5 (Fig. 8 insert).
3.5. PAK2 regulates trypsin activity in pancreatic acinar cells
The physiological dose of CCK (0.3 nM) as well as TPA (1 μM) did not stimulate trypsin activity in pancreatic acinar cells. However, the 100 nM CCK concentration stimulated trypsin activity (203±13%, p<0.05). The preincubation with 40 μM IPA-3 reduced the maximal 100 nM CCK-induced trypsin activity (−22.5±0.4%Δ of basal, p<0.05) while Pir3,5 had no any effect on trypsin activity (Fig. 9A). The treatment of pancreatic acinar cells with 0.3 nM CCK did not induce any change in trypsin activity [Data not shown].
3.6. PAK2 regulates CCK-induced reactive oxygen species generation in pancreatic acinar cells
The physiological dose of CCK (0.3 nM) as well as TPA (1 μM) did not stimulate generation of ROS in pancreatic acinar cells. However, the supraphysiological 100 nM CCK concentration stimulated ROS generation (192±22% Δ of control, p<0.05). Preincubation with 40 μM IPA-3 completely inhibited 100 nM CCK-induced ROS (116±16%Δ of IPA-3 alone, p<0.05). However, similarly to reported in other cells [40] the basal ROS generation was increased by IPA-3 incubation (196±48% Δ of control), although this number was not significant (p=0.08). The differences in the maximal stimulation of 100 nM CCK in both control and IPA-3 treated cells was significant (p<0.05). Pir3,5 had no effect on reducing CCK-induced ROS generation (176±27%Δ of Pir 3,5 alone, p<0.05) or in increasing its basal (132±19% Δ of control) (Fig. 9C). The treatment of pancreatic acinar cells with 0.3 nM CCK did not induce any change in ROS generation [Data not shown].
3.7. Alternative approaches for inhibiting PAK2 confirmed its role in activating cell signaling cascades and pathophysiological processes in rat pancreatic acini
In order to confirm the results obtained with the PAK2 inhibitor, IPA-3, we used two other approaches for inhibiting PAK2: inhibition by a dominant negative PAK2 adenovirus (DN-PAK2-Advirus) [23] and preincubation with a different class of Gp.1 PAK kinase inhbitors, a ATP-competitive inhibitor of group I PAKs, FRAX597 [22,24].
3.7.1. PAK2 inhibition with FRAX597 preincubation
We used a pharmacological approach, different than IPA-3, for PAK2 inhibition by using FRAX597 to study not only PAK-2 mediated kinases activation but also some pathophysiological processes. FRAX597 showed a similar effciency to IPA-3 in inhibiting CCK-mediated activation of Focal Adhesion Kinases and Mitogen Activated Protein Kinase pathways (Fig. 6, B Lines 1-4).
Moreover, we also used FRAX 597 to confirm the effect of PAK2 in mediating CCK-stimulated cell death pathways involving cell necrosis or apoptosis.
Treatment of pancreatic acini with 100 nM CCK stimulated activation of caspase -3 (299±74 %) -8 (471±34 %) and -9 (378±71 %) in pancreatic acinar cells (Fig. 10 A-C). However, in the acini incubated with CCK, preincubation with FRAX597 or IPA-3 (40 µM) resulted in a reduction of caspases activation (-3, -8 and -9) induced by CCK 100 nM. Specifically, in the presence of FRAX597 there was a reduction of 52±7 % in the increase by CCK of caspase-3 activity, 69.7±0.7 % reduction in CCK induced caspase-8 and 74±0.2 % in caspase-9 activity (all p<0.005 vs. CCK 100 nM, Fig. 10 A-C). In same experiment the presence of IPA-3 caused a reduction of 44±9.4 % of the increase in CCK-induced caspase-3 activity, 66±7.2 % in caspase-8 and 58±1.9 % in caspase-9 (all p<0.005 vs. CCK 100 nM, Fig. 10 A-C).
Fig. 10. Effect of the PAK2 inhibitors, FRAX597 and IPA-3, on CCK-mediated apoptosis in pancreatic acini.
Freshly isolated rat pancreatic acini were pre-incubated with either 40 μM FRAX597 or IPA-3 (40 μM ) for 1 hour and incubated for 3 hours with (CCK 100 nM). Caspase (caspase 3, 8, 9) activities were measured as described in Materials and Methods in the lysates of isolated pancreatic acini and in Fig. 8 legend. The results are representative of 3 independent (n=3) experiments. Results shown are the means ± SEM., * P< 0.05, comparing control.
Cells incubated with no stimulant released 3.1±0.8% of total LDH (Fig. 11 B). The cells incubated with 100 nM CCK showed a significant increase in LDH release of 15.2±1.1 of total LDH (395±27 % over control, p<0.05). Pretreatment of the acinar cells with FRAX597 and IPA-3 significantly reduced the effect of 100 nM CCK on LDH release resulting in a reduction of a 57±12% and 64±2% of the stimulation induced by CCK 100 nM (6.5±1.5% and 5.5±0.6 % of total LDH release, respectively, p< 0.05 vs. 100 nM CCK-treated cells, n=3) (Fig. 11 B).
Fig. 11. Effect of the PAK2 inhibitors, FRAX597 and IPA-3, on CCK- induced trypsin activation and CCK-mediated cell necrosis in pancreatic acini.
A. Freshly isolated rat pancreatic acini were pre-incubated with either 40 μM FRAX597 or IPA-3 (40 μM) for 1 hour followed by stimulation with 100 nM CCK for 20 minutes. Trypsin activity was measured as described in Materials and Methods in the lysates of isolated pancreatic acini. The results are representative of 3 independent (n=3) experiments. The data are expressed as the percentage of maximal activity obtained when acini were incubated for 20 min with 100 nM CCK. Results shown are the means ± SEM., * P< 0.05, comparing CCK-maximal stimulation.
B. Freshly isolated rat pancreatic acini were pre-incubated with either 40 μM FRAX597 or IPA-3 (40 μM) or for 1 hour followed by stimulation with 100 nM CCK for 1 hour. LDH release was measured as described in Materials and Methods in the supernatants of isolated pancreatic acini. The results are representative of 3 independent (n=3) experiments. Results shown are the means ± SEM., * P< 0.05, comparing control.
C. Freshly isolated rat pancreatic acini were pre-incubated as shown in Figure 9C. ROS generation was measured as described in Materials and Methods and in Fig. 9C legend in cell suspensions of isolated pancreatic acini. The results are representative of 4 independent (n=4) experiments. Results shown are the means ± SEM., * P< 0.05, comparing control.
Treatment of pancreatic acini with 100 nM CCK stimulated trypsin activation (185±4%, p<0.05). The preincubation with 40 μM FRAX597 and IPA-3 reduced the maximal 100 nM CCK-induced trypsin activity (−16.9±1.9 and −23.9±0.1% of CCK 100 nM, p<0.05) (Fig. 11 A).
As previous described, 100 nM CCK stimulated ROS generation (137.74±8.61%, p<0.05). Preincubation with FRAX597 and IPA-3 (40 μM) completely inhibited 100 nM CCK-induced ROS (104.65±3.81% of FRAX597 and 111.17±5.12% alone). However, similarly to reported in other cells [40] the basal ROS generation was increased by both inhibitors, FRAX597 and IPA-3 incubation (133.84±2.09 and 172.73±5.51%, p<0.05) (Fig. 11 C).
3.7.2. Preincubation with Dn-PAK2-Advirus
As a third approach for inhibiting PAK2 in pancreatic acini we infected the cells with a Dn-PAK2-Advirus that induces the cells to express an inactive copy of PAK2 blocking overall PAK2 activity and investigated its effect on CCK signaling. The incubation without or with PAK2-DN-advirus resulted in the over-expression of the dominant negative PAK2 (Fig. 6 A). Once the effectiveness of the virus infection was confirmed, new cells were infected with the virus as described above. After the 6 hour incubation the PAK2-DN-Advirus inhibited the ability of CCK to stimulate PAK2 (Fig. 6 B Line A). This resulted in the inhibition of the CCK-mediated activation of some PAK2 downstream effectors such as Focal Adhesion Kinases and their scaffolding proteins p125FAK and p130CAS (Fig. 6 B Lines B-E) or Mitogen Activated Protein Kinases (Fig. 6 B Lines F-H) pathways with respect to preincubation with the empty adenovirus (used as a control). This result confirmed the idea that the results obtained with IPA-3 preincubation responded to a specific inhibition of PAK2. Because of the prolonged pre-incubation times required to infect the cells with the DN-PAK2-Ad construct, it was not possible to use the DN-PAK2-Advirus in the pancreatitis experiments.
4. Discussion (2897/1978)
A recent study established that PAK2 is the only group I PAK present in pancreatic acini, and that pancreatic growth factors, as well as GI-hormones/neurotransmitters activated it in a PKC-, Src- and small GTPases-mediated manner [8]. In the present study we analyzed, by using three different inhibitory approaches, the role of PAK2 in activating important cellular signaling cascades and physiological/pathophysiological processes in pancreatic acinar cells. Because CCK is the main physiological stimulant of pancreatic acini, and PKC involvement is needed to activate PAK2 in pancreatic acinar cells [8] the signaling cascades activated by CCK and TPA were studied in detail that have been demonstrated to mediate physiological [12-17] and pathophysiological [12,18,19] processes in these cells.
We first established experimental conditions that enabled us to modulate PAK2 activity sufficiently to investigate PAK2's role in cellular signaling cascades. The main approach to inhibit PAK2 was to use the specific allosteric group I PAKs inhibitor, IPA-3, which is the most selective inhibitor available [2]. To confirm the specificity of the results obtained with IPA-3, we used two additional approaches: a dominant negative PAK2 adenovirus [23] and an ATP-competitive inhibitor of group I PAKs, FRAX597 [22,24]). The three inhibitory approaches gave similar results supporting the idea that the changes observed in signaling cascades and pathophysiological processes were only due to PAK inhibition and not by non-specific effects of the inhibitors. Moreover, because PAK2 is the only group I PAK present in rat pancreatic acini [8] we can conclude that the Group I PAK inhibition achieved in this study corresponded only to PAK2 inhibition. We studied the role of activation of PAK2 in four different major signaling cascades which has been shown to mediating physiological and pathophysiological effects of CCK including: focal adhesion kinases, mitogen-activated kinases, PI3K and the PKC/Src cascade [27]. In pancreatic acini, CCK, as well as other gastrointestinal hormones/neurotransmitters that stimulate the PLC cascade, activate the focal adhesion kinase pathway (p125FAK, PYK2) [13,17,26,27,41]. This pathway mediates both physiological (secretion, differentiation) and pathophysiological effects of CCK (pancreatitis, response to injury) [42,43]. Our results using the three PAK2 inhibition strategies demonstrate that activation of PAK2 induced by CCK, at both physiological and supra-physiological concentrations, or by the direct PKC activator, TPA, precedes and is required in pancreatic acini for the phosphorylation and activation of the focal adhesion kinases, p125FAK and PYK2.
In pancreatic acini and other tissues GI hormones/ neurotransmitters activating the PLC cascade, also activate the scaffolding proteins, paxillin and p130CAS [26,44-46]. These kinases have important physiological and pathophysiological roles such as cell signaling, cell motility, cell cycle control, apoptosis and oncogenic transformation [47,48] functioning as key scaffolding proteins in the cell signaling cascades. Similarly, in pancreatic acini with CCK-induced pancreatitis, paxillin redistribution plays a key role in the cellular morphology [49]. Our results, using the three PAK2 inhibition strategies, demonstrate that with CCK stimulation each of these two adapter proteins is activated in a PAK2-dependent manner in pancreatic acini. These results are consistent with several examples in the literature in which different PAK isoforms stimulate or associate with both paxillin and p130CAS [46,50-53].
We found that CCK and TPA activated the mitogen-activated protein kinases (MAPKs), p42/44, and JNK in a PAK2 dependent manner. CCK activates MEK/ERK by a Raf-dependent mechanism [54] and PAK has been previously identified as an upstream activator of this pathway [54]. Our results are consistent with the conclusion that CCK requires PAK2 stimulation to activate the RAF/MEK/ERK pathway. These results with MAPKs are consistent with many studies reporting activation of PAKs results in activation of different members of the MAPKs cascade in a number of different cells In our study we found not only CCK- but also TPA-activation of JNK was dependent on the activation of PAK2 by GTPases, demonstrating both activation of PKC and GTPases are involved in the upstream signaling for JNK activation [55]. These results are in general agreement with findings in other tissues where PAKs are usually considered as mediators of JNK activation in different cells [56]. In general our results with MAPKS demonstrate the importance of PAK2 in mediating CCK- and TPA-activation of different MAPKs in pancreatic acinar cells and suggest a participatory role of PAK2 in many of the physiological and pathophysiological processes controlled by this pathway in pancreatic acini such as proliferation, inflammation and apoptosis [57-59].
In pancreatic acini, CCK and TPA are reported to have a dual effect on the PI3K signaling cascade causing both stimulation and inhibition [33,60-62]. We observed this dual effect on the PI3K pathway because both CCK and TPA induced activation of the p85 subunit of the PI3K, while decreasing Akt activation. Our study demonstrated that the activation of PAK2 was involved in both dual actions of CCK and TPA on PI3K because, PAK2 inhibition reversed both dual actions of CCK and TPA on this pathway, by reducing their induced activation of p85 PI3K while reversing its inhibitory effect on Akt activation. All the changes in Akt were transmitted to its downstream effector P70s6k [63,64]. At present the mechanisms of this inhibitory effects of CCK on the activity of the Akt pathway in pancreatic acinar cells are unclear [33]. In pancreatic acini and other tissues, it has been proposed that these effects could be due to activation of inhibitory phosphatases [33,65]. Although PAK2 might be involved in the activation of some of these phosphatases, we excluded PTEN [66] from this list because CCK-and TPA did not affect the activation of PTEN in pancreatic acinar cells. Whether PKC, described as a PAK2 activator [8] and an Akt inactivator, in other studies [65], or phosphatases, different from PTEN, are involved in the CCK actions on Akt mediated by PAK is still unknown and requires further research. However, these results demonstrate that activation of PAK2 has a variable role on PI3K-AKT activation in various cells and that with CCK in pancreatic acini it has a number of novel features. In general our results are in contrast with the literature where PAK inhibition inhibits Akt [20] [67] and GSK-3-β activity [7,67-69]. These results demonstrate that activation of PAK2 has a variable role on PI3K-AKT activation in various cells and that with CCK in pancreatic acini it has a number of novel features.
CCK activates PKCs in pancreatic acini [62,70-74], mediating a number of cellular responses including differentiation, proliferation, apoptosis, cell death, secretion, adhesion and cell migration [73]. Our results demonstrate that CCK-stimulation of PKCs in pancreatic acinar cells is not dependent on alterations in activation of PAK2. This conclusion is supported by the fact that activation by CCK or TPA of PKD, a PKC activator in pancreatic acini [25] or MARCKS, which is a PKC substrate [75], was not altered by pre-incubation with IPA-3. This result supports the conclusion that PKC is only acting upstream of PAK2 in the signaling cascades of pancreatic acini [8]. These results are consistent with a number of studies in other cells, which demonstrated that PKC is required for the activation of PAK2 induced by various stimulants [76,77] or that PKC activation is independent of activation of PAK2 [78]. Similarly to PKC, SFKs activation was not affected by PAK2 inhibition. However, a previous study [8] demonstrated SFK activation is required in the CCK-stimulation of PAK2. This finding supports the conclusion that in pancreatic acini, SFKs are only acting upstream of PAKs in their signaling pathways.
In a recent study in pancreatic acini [8] it was demonstrated that activation of PAK2 is essential for CCK to stimulate, enzyme secretion [8]. However, it is unknown if PAK2 is involved in regulating CCK-mediated pathophysiological responses such as CCK-induced pancreatitis. A number of our results support the conclusion that activation of PAK2 plays important signaling roles in mediating early pancreatitis events induced by supraphysiological CCK concentrations. Premature intracellular activation of the digestive enzyme trypsinogen in the pancreatic acinar cells is considered to be the initiating event in pancreatitis [79]. Similar to other studies [80] we found that a supramaximal CCK concentration induced trypsin activation in pancreatic acinar cells. Moreover, we found that activation of PAK2 was required for CCK to maximally stimulate trypsin activation. This result supports a role for activation of PAK2 in initiating pancreatitis and raises the possibility of PAK2 as a therapeutic target for treating this disease. Our results are in contrast with other studies that reported that inhibition of Rac1, a PAK upstream activator [6], had no effect on pancreatitis associated trypsin activation in CCK-induced pancreatitis in dispersed acini [10]. This difference may be do to the different experimental models used in in that Rac1 has other effectors than Group-1 PAKs [3] which would not be inhibited in our study.
As previously described by others [9] supramaximal CCK, which causes pancreatitis, induced activation of ROS generation. We found that PAK2 is a mediator of the CCK-stimulated ROS production. In our study, both IPA-3 and FRAX597 increased basal ROS generation, which is a similar response reported with PAK1 in ventricular myocytes [40]. Although this result might suggest that IPA-3 exerts effects other than inhibition of PAK2, a previous study [40] demonstrated that the IPA-3-induced basal ROS production was mediated by activation of Gr 1 PAKs in myocytes isolated from mice deficient in PAK1 expression [40]. Therefore, in pancreatic acini PAK2 may be playing a dual role in ROS generation acting as a negative regulator of basal ROS generation, and as a positive mediator of CCK-induced generation of ROS. This result is in agreement to another study in pancreatic acini in which Rac1 inhibition decreased the CCK induced generation of ROS [10], but differs from studies in other tissues where PAK signaling was not involved in ROS generation [81].
In acute pancreatitis in vivo as well as in CCK-induced pancreatitis in acini [19], acinar cell death occurs and is mediated through both stimulation of apoptosis and necrosis [28,35,82]. In CCK-mediated pancreatitis, stimulation of apoptosis is mediated in part by activation of initiator and effector caspases-3, -8, and -9 [12,19,28,29,34,83]. In pancreatic acini caspases play an essential role in mediating apoptosis, but their activation also protects from necrosis as well as decreases the severity of pancreatitis induced not only by supraphysiological concentrations of CCK, as well as by other causes of pancreatitis [19,28,29,34,35,84]. Our results, with IPA-3 and FRAX 597, demonstrate that activation of PAK2 is important for activation of caspase -3, -8 and -9 by CCK. Although this conclusion is in contrast with several studies in other tissues of the effects of PAK2 on caspase activation [85-87], our results support the conclusion that PAK2 has a proapoptotic action in pancreatic acinar cells. This result has similarities with a study in which IPA-3 reduced caspase-3 level in brain injury [88]. PAK2 is unique among the PAK isoforms because it is also activated through proteolytic cleavage by caspases or caspase-like proteases [36,37]. In response to stress stimulants PAK-2 is activated as a full-length enzyme and as a proteolytic PAK-2p34 fragment. In Jurkat cells activation of full-length PAK-2 stimulates cell survival, whereas proteolytic activation of PAK-2p34 is involved in programmed cell death [38,39]. Our results support a similar proapoptotic mechanism may be occurring in pancreatic acinar cells, because we found that supramaximal CCK activates the proteolytic fraction of p34PAK2 and this cleavage is suppressed by the PAK2 inhibitor. Because an increase in apoptosis is associated with attenuation of the severity of pancreatitis, our results support the conclusion that PAK2 cleavage may play an important role in regulating the severity of this disease. This conclusion suggests that activation of PAK2 may function in a dual role (protective/non-protective) in different tissues, because with traumatic brain injury [88] inhibition of activation of PAK2 resulted in protecting the cells from secondary injury, whereas in pancreatic acinar cells its activation has a protective effect.
Our results also demonstrated that activation of PAK2 is important in mediating necrosis in pancreatic acini seen with in situ CCK-mediated pancreatitis, because CCK-mediated LDH release occurs in a PAK2-mediated manner in these cells. Consistent with previous studies [10,11], these results demonstrate that activation of PAK2 is playing a dual sentinel role in mediating the cellular cascades in pancreatitis that determine activation of apoptosis or necrosis and regulate the severity of pancreatitis.
In conclusion, as summarized in Figure 12, this study demonstrates in rat pancreatic acini the p21-activated kinase, PAK2, modulates cellular cascades that have been shown to be important in mediating numerous CCK-mediated physiological and pathophysiological effects [12,12,13,15-19]. Our results demonstrate activation of PAK2 by CCK under physiological conditions plays an important role in the activation of the focal adhesion kinases (p125FAK, PYK2); the adaptor, scaffold proteins (p130CAS, paxillin); MAPKs (ERK,JNK, p42/44), and PI3K/Akt pathways, that are all known for mediating physiological and pathophysiological changes induced by CCK in pancreatic acinar cells. Moreover, we demonstrated that PAK2 is involved in pathophysiological processes (cell death and trypsin activation) that are related to the onset of pancreatitis. These results combined with the observation that inhibition of Rac1, one of the PAKs upstream activators, decreases the severity of pancreatitis [10] raises the possibility that PAK2 could be an important therapeutic target for such pathophysiological processes as pancreatitis in pancreatic acinar cells.
Fig. 12. Schematic diagram of signaling role of PAK2 in pancreatic acinar cells.
In rat pancreatic acinar cells CCK-induced PAK2 activation is mediated by activation of CDC42/Rac1, Src and PKC [8]. Activation of PAK2 is needed to stimulate several signaling kinases including MAPKs (p42/44, JNK), FAKs (p125FAK, PYK2) and scaffolding proteins (paxillin, p130CAS) that are important in mediating numerous cellular functions. Activation of PAK2 has a unique dual role in altering activity of the PI3K-Akt pathway being required for stimulation of p85, but also mediating inhibition of AKT activity via dephosphorylation of Akt, which a previous study proposes requires activation of phosphatases in a PKC-dependent manner [33].
Research highlights.
PAK2 is a mediator of CCK/TPA action upon FAKs, MAPKs and PI3K pathways.
PAK2 mediates trypsin activation in pancreatic acini.
CCK stimulates the proteolytic fraction of PAK2, which is involved in apoptosis.
PAK2 mediates cell death processes in pancreatic acini-apoptosis and necrosis.
PAK2 mediates ROS production in pancreatic acini.
Acknowledgments
This work is partially supported by the Intramural Research Program of the NIDDK, NIH.
Abbreviations
- CCK
COOH-terminal octapeptide of cholecystokinin
- TPA
12-O-tetradecanoylphorbol-13-acetate
- PAK
p21-activated kinases SFK Src family of kinases
- PKC
protein kinase C
- PYK2
proline-rich tyrosine kinase 2
- FAK
focal adhesion kinase
- SHC
Src homology 1 domain containing transforming protein
- PKD
protein kinase D
- CAS
Crk-associated substrate
- PI3K
phosphatidylinositol-3-CAKβ, cell adhesion kinase β
- PTEN
phosphatase and tensin homolog
- Cbl
Casitas B-lineage lymphoma proto-oncogene
- RAF
proto-oncogene serine/threonine-protein kinase proto-oncogene HRP horseradish peroxidase
- HGF
hepatocyte growth factor
- PLC
phospholipase C
- ROS
reactive oxygen species
- AKT
protein kinase B
- MAPK/ERK
mitogen-activated protein kinase
- GI
gastrointestinal
- Mek
MAP kinase kinase
- JNK
c-Jun-N-terminal kinase
- GSK3-β
glycogen synthase kinase 3
- MARCKS
myristoylated alanine-rich C kinase substrate
- PKA
protein kinase A
- IPA-3
1,1'-Dithiodi-2-naphthtol
- Pir
3,5 6,6'-Dithiodi-2-naphthtol
- FRAX597
small-molecule pyridopyrimidinone-Group-1-PAK-Inhibitor
- DN
Dominant negative
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Bokoch GM. Biology of the p21-activated kinases. Annu. Rev Biochem. 2003;72:743–781. doi: 10.1146/annurev.biochem.72.121801.161742. [DOI] [PubMed] [Google Scholar]
- [2].Rane CK, Minden A. P21 activated kinases: Structure, regulation, and functions. Small GTPases. 2014;5 doi: 10.4161/sgtp.28003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ye DZ, Field J. PAK signaling in cancer. Cell Logist. 2012;2:105–116. doi: 10.4161/cl.21882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zhao ZS, Manser E. PAK family kinases: Physiological roles and regulation. Cell Logist. 2012;2:59–68. doi: 10.4161/cl.21912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wong LL, Lam IP, Wong TY, Lai WL, Liu HF, Yeung LL, Ching YP. IPA-3 inhibits the growth of liver cancer cells by suppressing PAK1 and NF-kappaB activation. PLoS. ONE. 2013;8:e68843. doi: 10.1371/journal.pone.0068843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Royal I, Lamarche-Vane N, Lamorte L, Kaibuchi K, Park M. Activation of cdc42, rac, PAK, and rho-kinase in response to hepatocyte growth factor differentially regulates epithelial cell colony spreading and dissociation. Mol Biol Cell. 2000;11:1709–1725. doi: 10.1091/mbc.11.5.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chow HY, Jubb AM, Koch JN, Jaffer ZM, Stepanova D, Campbell DA, Duron SG, O'Farrell M, Cai KQ, Klein-Szanto AJ, Gutkind JS, Hoeflich KP, Chernoff J. p21-Activated kinase 1 is required for efficient tumor formation and progression in a Ras-mediated skin cancer model. Cancer Res. 2012;72:5966–5975. doi: 10.1158/0008-5472.CAN-12-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Nuche-Berenguer B, Jensen RT. Gastrointestinal hormones/neurotransmitters and growth factors can activate P21 activated kinase 2 in pancreatic acinar cells by novel mechanisms. Biochim. Biophys Acta. 2015;1853:2371–2382. doi: 10.1016/j.bbamcr.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Granados MP, Salido GM, Pariente JA, Gonzalez A. Generation of ROS in response to CCK-8 stimulation in mouse pancreatic acinar cells. Mitochondrion. 2004;3:285–296. doi: 10.1016/j.mito.2004.02.003. [DOI] [PubMed] [Google Scholar]
- [10].Binker MG, Binker-Cosen AA, Gaisano HY, Cosen-Binker LI. Inhibition of Rac1 decreases the severity of pancreatitis and pancreatitis-associated lung injury in mice. Exp. Physiol. 2008;93:1091–1103. doi: 10.1113/expphysiol.2008.043141. [DOI] [PubMed] [Google Scholar]
- [11].Thamilselvan V, Menon M, Thamilselvan S. Selective Rac1 inhibition protects renal tubular epithelial cells from oxalate-induced NADPH oxidase-mediated oxidative cell injury. Urol. Res. 2012;40:415–423. doi: 10.1007/s00240-011-0405-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gukovskaya AS, Gukovsky I, Jung Y, Mouria M, Pandol SJ. Cholecystokinin induces caspase activation and mitochondrial dysfunction in pancreatic acinar cells. Roles in cell injury processes of pancreatitis. J. Biol. Chem. 2002;277:22595–22604. doi: 10.1074/jbc.M202929200. [DOI] [PubMed] [Google Scholar]
- [13].Tapia JA, Ferris HA, Jensen RT, Marin LJ. Cholecystokinin activates PYK2/CAKβ, by a phospholipase C-dependent mechanism, and its association with the mitogen-activated protein kinase signaling pathway in pancreatic acinar cells. J. Biol. Chem. 1999;274:31261–31271. doi: 10.1074/jbc.274.44.31261. [DOI] [PubMed] [Google Scholar]
- [14].Jensen RT. Involvement of cholecystokinin/gastrin-related peptides and their receptors in clinical gastrointestinal disorders. Pharmacol. Toxicol. 2002;91:333–350. doi: 10.1034/j.1600-0773.2002.910611.x. [DOI] [PubMed] [Google Scholar]
- [15].Pace A, Garcia-Marin LJ, Tapia JA, Bragado MJ, Jensen RT. Phosphospecific site tyrosine phosphorylation of p125FAK and proline-rich kinase 2 is differentially regulated by cholecystokinin receptor A activation in pancreatic acini. J. Biol. Chem. 2003;278:19008–19016. doi: 10.1074/jbc.M300832200. [DOI] [PubMed] [Google Scholar]
- [16].Aparicio IM, Garcia-Marin LJ, Andreolotti AG, Bodega G, Jensen RT, Bragado MJ. Hepatocyte growth factor activates several transduction pathways in rat pancreatic acini. Biochim. Biophys. Acta. 2003;1643:37–46. doi: 10.1016/j.bbamcr.2003.08.007. [DOI] [PubMed] [Google Scholar]
- [17].Rosado JA, Salido GM, Garcia LJ. A role for phosphoinositides in tyrosine phosphorylation of p125 focal adhesion kinase in rat pancreatic acini. Cell. Signal. 2000;12:173–182. doi: 10.1016/s0898-6568(99)00083-2. [DOI] [PubMed] [Google Scholar]
- [18].Yang BM, Demaine AG, Kingsnorth A. Chemokines MCP-1 and RANTES in isolated rat pancreatic acinar cells treated with CCK and ethanol in vitro. Pancreas. 2000;21:22–31. doi: 10.1097/00006676-200007000-00048. [DOI] [PubMed] [Google Scholar]
- [19].Yuan J, Liu Y, Tan T, Guha S, Gukovsky I, Gukovskaya A, Pandol SJ. Protein kinase d regulates cell death pathways in experimental pancreatitis. Front Physiol. 2012;360 doi: 10.3389/fphys.2012.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Deacon SW, Beeser A, Fukui JA, Rennefahrt UE, Myers C, Chernoff J, Peterson JR. An isoform-selective, small-molecule inhibitor targets the autoregulatory mechanism of p21-activated kinase. Chem Biol. 2008;15:322–331. doi: 10.1016/j.chembiol.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gatti A, Huang Z, Tuazon PT, Traugh JA. Multisite autophosphorylation of p21-activated protein kinase gamma-PAK as a function of activation. J Biol Chem. 1999;274:8022–8028. doi: 10.1074/jbc.274.12.8022. [DOI] [PubMed] [Google Scholar]
- [22].Rudolph J, Crawford JJ, Hoeflich KP, Wang W. Inhibitors of p21-activated kinases (PAKs) J Med. Chem. 2015;58:111–129. doi: 10.1021/jm501613q. [DOI] [PubMed] [Google Scholar]
- [23].Dechert MA, Holder JM, Gerthoffer WT. p21-activated kinase 1 participates in tracheal smooth muscle cell migration by signaling to p38 Mapk. Am J Physiol Cell Physiol. 2001;281:C123–C132. doi: 10.1152/ajpcell.2001.281.1.C123. [DOI] [PubMed] [Google Scholar]
- [24].Mortazavi F, Lu J, Phan R, Lewis M, Trinidad K, Aljilani A, Pezeshkpour G, Tamanoi F. Significance of KRAS/PAK1/Crk pathway in non-small cell lung cancer oncogenesis. BMC. Cancer. 2015;15:381. doi: 10.1186/s12885-015-1360-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Berna MJ, Hoffmann KM, Tapia JA, Thill M, Pace A, Mantey SA, Jensen RT. CCK causes PKD1 activation in pancreatic acini by signaling through PKC-delta and PKC-independent pathways. Biochim. Biophys. Acta. 2007;1773:483–501. doi: 10.1016/j.bbamcr.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Garcia LJ, Rosado JA, Gonzalez A, Jensen RT. Cholecystokinin-stimulated tyrosine phosphorylation of p125FAK and paxillin is mediated by phospholipase C-dependent and -independent mechanisms and requires the integrity of the actin cytoskeleton and participation of p21rho. Biochem. J. 1997;327:461–472. doi: 10.1042/bj3270461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Nuche-Berenguer B, Moreno P, Jensen RT. Elucidation of the Roles of the Src kinases in pancreatic acinar cell signaling. J Cell Biochem. 2015;116:22–36. doi: 10.1002/jcb.24895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Mareninova OA, Sung KF, Hong P, Lugea A, Pandol SJ, Gukovsky I, Gukovskaya AS. Cell death in pancreatitis: caspases protect from necrotizing pancreatitis. J. Biol. Chem. 2006;281:3370–3381. doi: 10.1074/jbc.M511276200. [DOI] [PubMed] [Google Scholar]
- [29].Sung KF, Odinokova IV, Mareninova OA, Rakonczay Z, Jr., Hegyi P, Pandol SJ, Gukovsky I, Gukovskaya AS. Prosurvival Bcl-2 proteins stabilize pancreatic mitochondria and protect against necrosis in experimental pancreatitis. Exp. Cell Res. 2009;315:1975–1989. doi: 10.1016/j.yexcr.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kawabata S, Miura T, Morita T, Kato H, Fujikawa K, Iwanaga S, Takada K, Kimura T, Sakakibara S. Highly sensitive peptide-4-methylcoumaryl-7-amide substrates for blood-clotting proteases and trypsin. Eur J Biochem. 1988;172:17–25. doi: 10.1111/j.1432-1033.1988.tb13849.x. [DOI] [PubMed] [Google Scholar]
- [31].Booth DM, Murphy JA, Mukherjee R, Awais M, Neoptolemos JP, Gerasimenko OV, Tepikin AV, Petersen OH, Sutton R, Criddle DN. Reactive oxygen species induced by bile acid induce apoptosis and protect against necrosis in pancreatic acinar cells. Gastroenterology. 2011;140:2116–2125. doi: 10.1053/j.gastro.2011.02.054. [DOI] [PubMed] [Google Scholar]
- [32].Pramanik KC, Kudugunti SK, Fofaria NM, Moridani MY, Srivastava SK. Caffeic acid phenethyl ester suppresses melanoma tumor growth by inhibiting PI3K/AKT/XIAP pathway. Carcinogenesis. 2013;34:2061–2070. doi: 10.1093/carcin/bgt154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Berna MJ, Tapia JA, Sancho V, Thill M, Pace A, Hoffmann KM, Gonzalez-Fernandez L, Jensen RT. Gastrointestinal growth factors and hormones have divergent effects on Akt activation. Cell Signal. 2009;21:622–638. doi: 10.1016/j.cellsig.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Bhatia M. Apoptosis of pancreatic acinar cells in acute pancreatitis: is it good or bad? J Cell Mol Med. 2004;8:402–409. doi: 10.1111/j.1582-4934.2004.tb00330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Pandol SJ, Saluja AK, Imrie C.w., Banks PA. Acute pancreatitis: bench to the bedside. Gastroenterology. 2007;132:1127–1151. doi: 10.1053/j.gastro.2007.01.055. [DOI] [PubMed] [Google Scholar]
- [36].Walter BN, Huang Z, Jakobi R, Tuazon PT, Alnemri ES, Litwack G, Traugh JA. Cleavage and activation of p21-activated protein kinase gamma-PAK by CPP32 (caspase 3). Effects of autophosphorylation on activity. J Biol Chem. 1998;273:28733–28739. doi: 10.1074/jbc.273.44.28733. [DOI] [PubMed] [Google Scholar]
- [37].Rudel T, Bokoch GM. Membrane and morphological changes in apoptotic cells regulated by caspase-mediated activation of PAK2. Science. 1997;276:1571–1574. doi: 10.1126/science.276.5318.1571. [DOI] [PubMed] [Google Scholar]
- [38].Lee N, MacDonald H, Reinhard C, Halenbeck R, Roulston A, Shi T, Williams LT. Activation of hPAK65 by caspase cleavage induces some of the morphological and biochemical changes of apoptosis. Proc Natl Acad. Sci U. S. A. 1997;94:13642–13647. doi: 10.1073/pnas.94.25.13642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Rudel T, Zenke FT, Chuang TH, Bokoch GM. p21-activated kinase (PAK) is required for Fas-induced JNK activation in Jurkat cells. J Immunol. 1998;160:7–11. [PubMed] [Google Scholar]
- [40].DeSantiago J, Bare DJ, Xiao L, Ke Y, Solaro RJ, Banach K. p21-Activated kinase1 (Pak1) is a negative regulator of NADPH-oxidase 2 in ventricular myocytes. J Mol Cell Cardiol. 2014;67:77–85. doi: 10.1016/j.yjmcc.2013.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Garcia LJ, Rosado JA, Tsuda T, Jensen RT. CCK causes rapid tyrosine phosphorylation of p125FAK focal adhesion kinase and paxillin in rat pancreatic acini. Biochim. Biophys. Acta (Mol. Cell Res. ) 1997;1358:189–199. doi: 10.1016/s0167-4889(97)00056-6. [DOI] [PubMed] [Google Scholar]
- [42].Stettner MR, Wang W, Nabors LB, Bharara S, Flynn DC, Grammer JR, Gillespie GY, Gladson CL. Lyn kinase activity is the predominant cellular SRC kinase activity in glioblastoma tumor cells. Cancer Res. 2005;65:5535–5543. doi: 10.1158/0008-5472.CAN-04-3688. [DOI] [PubMed] [Google Scholar]
- [43].Greer RL, Staley BK, Liou A, Hebrok M. Numb regulates acinar cell dedifferentiation and survival during pancreatic damage and acinar-to-ductal metaplasia. Gastroenterology. 2013;145:1088–1097. doi: 10.1053/j.gastro.2013.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Ferris HA, Tapia JA, Garcia LJ, Jensen RT. CCKA receptor activation stimulates p130cas tyrosine phosphorylation, translocation, and association with Crk in rat pancreatic acinar cells. Biochemistry (Mosc) 1999;38:1497–1508. doi: 10.1021/bi981903w. [DOI] [PubMed] [Google Scholar]
- [45].Nuche-Berenguer B, Nakamura T, Moreno P, Jensen RT. Src-Dependent Role of CCK in the Pancreatic Acinar Cell Signaling and Chemokine Release. 2013:S-322. [Google Scholar]
- [46].Hashimoto S, Tsubouchi A, Mazaki Y, Sabe H. Interaction of paxillin with p21-activated Kinase (PAK). Association of paxillin alpha with the kinase-inactive and the Cdc42-activated forms of PAK3. J Biol Chem. 2001;276:6037–6045. doi: 10.1074/jbc.M005854200. [DOI] [PubMed] [Google Scholar]
- [47].Sattler M, Pisick E, Morrison PT, Salgia R. Role of the cytoskeletal protein paxillin in oncogenesis. Crit Rev Oncog. 2000;11:63–76. [PubMed] [Google Scholar]
- [48].Barrett A, Pellet-Many C, Zachary IC, Evans IM, Frankel P. p130Cas: a key signalling node in health and disease. Cell Signal. 2013;25:766–777. doi: 10.1016/j.cellsig.2012.12.019. [DOI] [PubMed] [Google Scholar]
- [49].Leser J, Luhrs H, Beil MF, Adler G, Lutz MP. Cholecystokinin-induced redistribution of paxillin in rat pancreatic acinar cells. Biochem. Biophys. Res. Commun. 1999;254:400–405. doi: 10.1006/bbrc.1998.9413. [DOI] [PubMed] [Google Scholar]
- [50].Brown MC, West KA, Turner CE. Paxillin-dependent paxillin kinase linker and p21-activated kinase localization to focal adhesions involves a multistep activation pathway. Mol Biol Cell. 2002;13:1550–1565. doi: 10.1091/mbc.02-02-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Turner CE, Brown MC, Perrotta JA, Riedy MC, Nikolopoulos SN, McDonald AR, Bagrodia S, Thomas S, Leventhal PS. Paxillin LD4 motif binds PAK and PIX through a novel 95-kD ankyrin repeat, ARF-GAP protein: A role in cytoskeletal remodeling. J Cell Biol. 1999;145:851–863. doi: 10.1083/jcb.145.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Wang R, Li QF, Anfinogenova Y, Tang DD. Dissociation of Crk-associated substrate from the vimentin network is regulated by p21-activated kinase on ACh activation of airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2007;292:L240–L248. doi: 10.1152/ajplung.00199.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Wang R, Li QF, Anfinogenova Y, Tang DD. Dissociation of Crk-associated substrate from the vimentin network is regulated by p21-activated kinase on ACh activation of airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2007;292:L240–L248. doi: 10.1152/ajplung.00199.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Dabrowski A, Groblewski GE, Schafer C, Guan KL, Williams JA. Cholecystokinin and EGF activate a MAPK cascade by different mechanisms in rat pancreatic acinar cells. Am. J. Physiol. 1997;273:C1472–C1479. doi: 10.1152/ajpcell.1997.273.5.C1472. [DOI] [PubMed] [Google Scholar]
- [55].Bogoyevitch MA, Ngoei KR, Zhao TT, Yeap YY, Ng DC. c-Jun N-terminal kinase (JNK) signaling: recent advances and challenges. Biochim. Biophys Acta. 2010;1804:463–475. doi: 10.1016/j.bbapap.2009.11.002. [DOI] [PubMed] [Google Scholar]
- [56].Schmitz U, Ishida T, Ishida M, Surapisitchat J, Hasham MI, Pelech S, Berk BC. Angiotensin II stimulates p21-activated kinase in vascular smooth muscle cells: role in activation of JNK. Circ. Res. 1998;82:1272–1278. doi: 10.1161/01.res.82.12.1272. [DOI] [PubMed] [Google Scholar]
- [57].Dabrowski A, Tribillo I, Dabrowska MI, Wereszczynska-Siemiatkowska U, Gabryelewicz A. Activation of mitogen-activated protein kinases in different models of pancreatic acinar cell damage. Z. Gastroenterol. 2000;38:469–481. doi: 10.1055/s-2000-14885. [DOI] [PubMed] [Google Scholar]
- [58].Dabrowski A, Boguslowicz C, Dabrowska M, Tribillo I, Gabryelewicz A. Reactive oxygen species activate mitogen-activated protein kinases in pancreatic acinar cells. Pancreas. 2000;21:376–384. doi: 10.1097/00006676-200011000-00008. [DOI] [PubMed] [Google Scholar]
- [59].Ramnath RD, Sun J, Bhatia M. Involvement of SRC family kinases in substance P-induced chemokine production in mouse pancreatic acinar cells and its significance in acute pancreatitis. J Pharmacol Exp. Ther. 2009;329:418–428. doi: 10.1124/jpet.108.148684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Fischer L, Gukovskaya AS, Young SH, Gukovsky I, Lugea A, Buechler P, Penninger JM, Friess H, Pandol SJ. Phosphatidylinositol 3-kinase regulates Ca2+ signaling in pancreatic acinar cells through inhibition of sarco(endo)plasmic reticulum Ca2+-ATPase. Am. J. Physiol. (Gastrointest. Liver Physiol.) 2004;287:G1200–G1212. doi: 10.1152/ajpgi.00212.2004. [DOI] [PubMed] [Google Scholar]
- [61].Gukovsky I, Cheng JH, Nam KJ, Lee OT, Lugea A, Fischer L, Penninger JM, Pandol SJ, Gukovskaya AS. Phosphatidylinositide 3-kinase gamma regulates key pathologic responses to cholecystokinin in pancreatic acinar cells. Gastroenterology. 2004;126:554–566. doi: 10.1053/j.gastro.2003.11.017. [DOI] [PubMed] [Google Scholar]
- [62].Williams JA, Sans MD, Tashiro M, Schafer C, Bragado MJ, Dabrowski A. Cholecystokinin activates a variety of intracellular signal transduction mechanisms in rodent pancreatic acinar cells. Pharmacol. Toxicol. 2002;91:297–303. doi: 10.1034/j.1600-0773.2002.910606.x. [DOI] [PubMed] [Google Scholar]
- [63].Chen J, Fang Y. A novel pathway regulating the mammalian target of rapamycin (mTOR) signaling. Biochem Pharmacol. 2002;64:1071–1077. doi: 10.1016/s0006-2952(02)01263-7. [DOI] [PubMed] [Google Scholar]
- [64].Gingras AC, Raught B, Sonenberg N. Regulation of translation initiation by FRAP/mTOR. Genes Dev. 2001;15:807–826. doi: 10.1101/gad.887201. [DOI] [PubMed] [Google Scholar]
- [65].Li L, Sampat K, Hu N, Zakari J, Yuspa SH. Protein kinase C negatively regulates Akt activity and modifies UVC-induced apoptosis in mouse keratinocytes. J Biol Chem. 2006;281:3237–3243. doi: 10.1074/jbc.M512167200. [DOI] [PubMed] [Google Scholar]
- [66].Wan X, Helman LJ. Levels of PTEN protein modulate Akt phosphorylation on serine 473, but not on threonine 308, in IGF-II-overexpressing rhabdomyosarcomas cells. Oncogene. 2003;22:8205–8211. doi: 10.1038/sj.onc.1206878. [DOI] [PubMed] [Google Scholar]
- [67].Aslan JE, Itakura A, Haley KM, Tormoen GW, Loren CP, Baker SM, Pang J, Chernoff J, McCarty OJ. p21 activated kinase signaling coordinates glycoprotein receptor VI-mediated platelet aggregation, lamellipodia formation, and aggregate stability under shear. Arterioscler. Thromb. Vasc. Biol. 2013;33:1544–1551. doi: 10.1161/ATVBAHA.112.301165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].He H, Shulkes A, Baldwin GS. PAK1 interacts with beta-catenin and is required for the regulation of the beta-catenin signalling pathway by gastrins. Biochim. Biophys Acta. 2008;1783:1943–1954. doi: 10.1016/j.bbamcr.2008.04.016. [DOI] [PubMed] [Google Scholar]
- [69].Cho SM, Lee EO, Kim SH, Lee HJ. Essential oil of Pinus koraiensis inhibits cell proliferation and migration via inhibition of p21-activated kinase 1 pathway in HCT116 colorectal cancer cells. BMC. Complement Altern. Med. 2014;14:275. doi: 10.1186/1472-6882-14-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Tapia JA, Garcia-Marin LJ, Jensen RT. Cholecystokinin-stimulated protein kinase C-delta activation, tyrosine phosphorylation and translocation is mediated by Src tyrosine kinases in pancreatic acinar cells. J. Biol. Chem. 2003;12:35220–35230. doi: 10.1074/jbc.M303119200. [DOI] [PubMed] [Google Scholar]
- [71].Ngezahayo A, Lang F, Kolb HA. Cholecystokinin-octapeptide affects the fluorescence signal of a single pancreatic acinar cell loaded with the acrylodan-labelled MARCKS peptide, a protein kinase C substrate. Pflugers Arch. 1995;429:805–808. doi: 10.1007/BF00374804. [DOI] [PubMed] [Google Scholar]
- [72].Sancho V, Berna MJ, Thill M, Jensen RT. PKCtheta activation in pancreatic acinar cells by gastrointestinal hormones/neurotransmitters and growth factors is needed for stimulation of numerous important cellular signaling cascades. Biochim. Biophys Acta. 2011;1813:2145–2156. doi: 10.1016/j.bbamcr.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Gorelick F, Pandol S, Thrower E. Protein kinase C in the pancreatic acinar cell. J. Gastroenterol. Hepatol. 2008;23(Suppl 1):S37–S41. doi: 10.1111/j.1440-1746.2007.05282.x. [DOI] [PubMed] [Google Scholar]
- [74].Pace A, Tapia JA, Garcia-Marin LJ, Jensen RT. The Src family kinase, Lyn, is activated in pancreatic acinar cells by gastrointestinal hormones/neurotransmitters and growth factors which stimulate its association with numerous other signaling molecules. Biochim. Biophys. Acta. 2006;1763:356–365. doi: 10.1016/j.bbamcr.2006.03.004. [DOI] [PubMed] [Google Scholar]
- [75].Aderem A. The MARCKS family of protein kinase-C substrates. Biochem Soc Trans. 1995;23:587–591. doi: 10.1042/bst0230587. [DOI] [PubMed] [Google Scholar]
- [76].Woolfolk EA, Eguchi S, Ohtsu H, Nakashima H, Ueno H, Gerthoffer WT, Motley ED. Angiotensin II-induced activation of p21-activated kinase 1 requires Ca2+ and protein kinase C{delta} in vascular smooth muscle cells. Am J Physiol Cell Physiol. 2005;289:C1286–C1294. doi: 10.1152/ajpcell.00448.2004. [DOI] [PubMed] [Google Scholar]
- [77].Spratley SJ, Bastea LI, Doppler H, Mizuno K, Storz P. Protein kinase D regulates cofilin activity through p21-activated kinase 4. J Biol Chem. 2011;286:34254–34261. doi: 10.1074/jbc.M111.259424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Menard RE, Mattingly RR. Cell surface receptors activate p21-activated kinase 1 via multiple Ras and PI3-kinase-dependent pathways. Cell Signal. 2003;15:1099–1109. doi: 10.1016/s0898-6568(03)00087-1. [DOI] [PubMed] [Google Scholar]
- [79].Ji B, Gaiser S, Chen X, Ernst SA, Logsdon CD. Intracellular trypsin induces pancreatic acinar cell death but not NF-kappaB activation. J Biol Chem. 2009;284:17488–17498. doi: 10.1074/jbc.M109.005520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Han B, Ji B, Logsdon CD. CCK independently activates intracellular trypsinogen and NF-kappaB in rat pancreatic acinar cells. Am J Physiol Cell Physiol. 2001;280:C465–C472. doi: 10.1152/ajpcell.2001.280.3.C465. [DOI] [PubMed] [Google Scholar]
- [81].Orr AW, Hahn C, Blackman BR, Schwartz MA. p21-activated kinase signaling regulates oxidant-dependent NF-kappa B activation by flow. Circ Res. 2008;103:671–679. doi: 10.1161/CIRCRESAHA.108.182097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Kaiser AM, Saluja AK, Sengupta A, Saluja M, Steer ML. Relationship between severity, necrosis, and apoptosis in five models of experimental acute pancreatitis. Am. J. Physiol. 1995;269:C1295–C1304. doi: 10.1152/ajpcell.1995.269.5.C1295. [DOI] [PubMed] [Google Scholar]
- [83].Beil M, Leser J, Lutz MP, Gukovskaya A, Seufferlein T, Lynch G, Pandol SJ, Adler G. Caspase 8-mediated cleavage of plectin precedes F-actin breakdown in acinar cells during pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2002;282:G450–G460. doi: 10.1152/ajpgi.00042.2001. [DOI] [PubMed] [Google Scholar]
- [84].Gukovskaya AS, Pandol SJ. Cell death pathways in pancreatitis and pancreatic cancer. Pancreatology. 2004;4:567–586. doi: 10.1159/000082182. [DOI] [PubMed] [Google Scholar]
- [85].Jakobi R, Moertl E, Koeppel MA. p21-activated protein kinase gamma-PAK suppresses programmed cell death of BALB3T3 fibroblasts. J Biol Chem. 2001;276:16624–16634. doi: 10.1074/jbc.M007753200. [DOI] [PubMed] [Google Scholar]
- [86].Li X, Wen W, Liu K, Zhu F, Malakhova M, Peng C, Li T, Kim HG, Ma W, Cho YY, Bode AM, Dong Z, Dong Z. Phosphorylation of caspase-7 by p21-activated protein kinase (PAK) 2 inhibits chemotherapeutic drug-induced apoptosis of breast cancer cell lines. J Biol Chem. 2011;286:22291–22299. doi: 10.1074/jbc.M111.236596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Kuzelova K, Grebenova D, Holoubek A, Roselova P, Obr A. Group I PAK inhibitor IPA-3 induces cell death and affects cell adhesivity to fibronectin in human hematopoietic cells. PLoS. ONE. 2014;9:e92560. doi: 10.1371/journal.pone.0092560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Ji X, Zhang W, Zhang L, Zhang L, Zhang Y, Tang P. Inhibition of p21-activated kinase 1 by IPA-3 attenuates secondary injury after traumatic brain injury in mice. Brain Res. 2014;1585:13–22. doi: 10.1016/j.brainres.2014.08.026. [DOI] [PubMed] [Google Scholar]