Abstract
Background
Timothy grass (TG) pollen is a common seasonal airborne allergen associated with symptoms ranging from mild rhinitis to severe asthma.
Objective
The aim of this study was to characterize changes in TG-specific T cell responses as a function of seasonality.
Methods
Peripheral blood mononuclear cells (PBMC) obtained from allergic individuals and non-allergic controls, either during the pollen season or out-of-season, were stimulated either with TG extract or a pool of previously identified immunodominant antigenic regions.
Results
PBMC from allergic subjects exhibit higher IL-5 and IL-10 responses in-season than when collected out of season. In the case of non-allergic subjects, as expected we observed lower IL-5 responses and robust production of IFNγ compared to allergic individuals. Strikingly, non-allergic donors exhibited an opposing pattern, with decreased immune reactivity in-season. The broad downregulation in non-allergic donors indicates that healthy individuals are not oblivious to allergen exposure but rather react with an active modulation of responses following the antigenic stimulus provided during the pollen season. Transcriptomic analysis of allergen-specific T cells defined genes modulated in concomitance with allergen exposure and inhibition of responses in non-allergic donors.
Conclusion and Clinical Relevance
Magnitude and functionality of T-helper cell responses differ substantially in-season versus out-of-season in allergic and non-allergic subjects. The results indicate specific and opposing modulation of immune responses following the antigenic stimulation during the pollen season. This seasonal modulation reflects the enactment of specific molecular programs associated with health and allergic disease.
INTRODUCTION
The prevalence of IgE-mediated allergic diseases has steadily increased over the last decades [1–4]. An estimated 25–40% of the western civilization suffers from allergic rhinitis and/or asthma [5]. Phleum pratense (Timothy grass, TG) is one the most prevalent outdoor allergens [6] that elicit IgE responses and trigger symptoms in subjects with rhinitis and asthma.
Allergen-specific CD4+ T cells exist in both allergic and non-allergic individuals, exhibiting distinct cytokine profiles in the respective cohorts [7, 8]. Allergic immune responses are characterized by excessive production of Th2-related cytokines by allergen-specific CD4+ T cells [9, 10] whereas it has been proposed that IFNγ-producing Th1 cells or IL-10 production by regulatory T cells are associated with the establishment of a healthy immune response and tolerance induction in non-allergic individuals [7–9, 11, 12].
The impact of seasonality on immune reactivity has been studied in allergic subjects [13, 14], but little is known about T cell reactivity in non-allergic subjects during natural allergen exposure. In the present study, we analyzed the impact of grass pollen season on Timothy grass (TG)-specific T cell responses in allergic and non-allergic subjects. T cell responses in allergic individuals were enhanced. However, unexpectedly, T cell responses of non-allergic individuals were generally downregulated following antigenic stimulation during TG pollen season. Transcriptomic analysis of antigen-specific T cells indicated that this seasonal modulation is associated with specific molecular programs in healthy versus allergic immune responses, respectively.
MATERIAL AND METHODS
Characteristics of the study population and PBMC isolation
Donor recruitment followed Institutional Review Board (La Jolla Institute for Allergy and Immunology, La Jolla, CA) approval (Federal Wide Assurance no. FWA00000032). Each individual gave informed consent, was assigned a study identification number, and information on clinical case histories was recorded. Immediate hypersensitivity skin-test reactivity to a panel of 32 common allergen extracts was determined by standard methods using a cut-off of 3 mm wheal diameter [15]. The panel was purchased from Greer Laboratories, Inc. (Lenoir, NC, USA) and included black walnut pollen, alder, hazel, English plantain, orchard, wheat, western ragweed, giant ragweed, spring birch, olive, timothy grass, dog epithelia, white oak, sweet vernal grass, mite (Dermatophagoides pteronyssinus, D. farinae), bermuda grass, common mugwort, fungi (Aspergillus fumigatus, Penicillium chrysogenum, Cladosporium herbarum, Alternaria alternate), perennial rye, Kentucky blue grass, cypress, Russian thistle, American cockroach, German cockroach, cat hair, canary grass, palm and juniper.
Most donations were from a cross-sectional donor cohort, and a more limited number of samples were available from a longitudinal donor cohort. Donors were chosen for different assays based on availability of longitudinal versus single (cross-sectional) donations and cell number availability. A summary of the characteristics of the cohort is provided in Supplementary Table S1A. Supplementary tables S1B–D present all available information for all donors regarding their atopic/allergic status, symptom severity, date of blood draws (and associated in- versus out-of-season classification). This table also includes information regarding polysensitization, wheal, gender, and age. The non-allergic donors were both asymptomatic and non-atopic, having no skin-prick test reactivity (reactions of zero millimeter diameter) to any of the tested allergen extracts listed above including timothy, Bermuda, juniper, canary, Kentucky blue and sweet vernal grass. These individuals could have allergic sensitivities to other aeroallergens that were not included in the skin-prick test panel. PBMCs were isolated by density gradient centrifugation according to manufacturer instructions (Ficoll-Hypaque, Amersham Biosciences, Uppsala, Sweden) [16] and cryopreserved for further analysis.
Definition of seasonality
We noticed that T cell reactivity was slightly delayed relative to the reported timothy grass season in California; the seasonal increase was maintained throughout August, September, and October and inclusion of samples collected in May and June decreased the overall effect of season. These samples were therefore excluded from analysis. As a result of these findings, the period between July 1st and October 15th was considered in-season.
HLA typing
Genomic DNA was isolated from PBMC by standard techniques (QIAmp, Qiagen, Valencia, CA) used for HLA Class II typing. High resolution Luminex-based Sequence-Specific Oligonucleotide (SSO) typing and PCR-based Sequence-Specific Primer (SSP) subtyping methods (if necessary) were utilized according to manufacturer instructions (One Lambda, Canoga Park, CA).
In vitro expansion of TG-specific T cells and dual ELISPOT assays
PBMCs of allergic and non-allergic individuals were stimulated with TG extract (Greer, Lenoir, NC) or a pool of 20 predominant TG epitopes (P20, supplementary Table S2) at a concentration of 50 µg/mL or 5 µg/mL, respectively. Cells were cultured in RPMI1640 supplemented with 5% human AB serum in 24 well plates (BD Bioscience, San Diego, CA) at a density of 4 × 106/mL and incubated at 37 °C. IL-2 was added every 3 days after initial stimulation. Depending on the experiment, cells were harvested on day 0, 4, 7, 11, 14, 17 or 21 and screened for reactivity following re-stimulation with TG extract or P20 by dual ELISPOT assay as described previously [16]. Each assay was performed in triplicate. Three independent criteria had to apply for a response to be considered positive: p < 0.05 in a Student’s t-test using the mean of triplicate values of the response against the extract, pool or individual peptides, compared to the response against the negative control (PBMC in medium without stimulus), stimulation index (SI) exceeded 2-fold the mean negative control wells and net SFC were above the threshold of 20 SFCs/106 PBMC.
Capture assay for antigen-specific IL-5- or IFNγ-producing cells, flow cytometry and tetramer staining assays
Cytokine-producing cells were captured using a cytokine secretion assay (Miltenyi Biotech, Bergisch Gladbach, Germany) according to manufacturer instructions. Following overnight rest in 6-well plates (Costar Corp.), 4 × 106/mL PBMCs from allergic and non-allergic individuals were stimulated by adding 2.5 µg/mL of a pool of 100 immunodominant peptides (Tg megapool, Supplementary Table S2 and S3) for 3 h. Thereafter, cells were harvested, washed and labeled with 20 µL of anti-IFNγ/CD45- or anti-IL5/CD45- antibody conjugates (Miltenyi Biotech). After capturing the secreted cytokine, cells were stained with 20 µL/107 cells of PE-labeled detection antibody. Thereafter, PBMCs were washed and co-stained with antibodies for CD3, CD4, CD45RA and CCR7. INFγ- or IL5-producing cells from effector and central memory compartment were sorted on a BD ARIA cell sorter.
Tetramer staining and tetramer enrichment assays were performed as previously described [17]. Magnetically enriched cells were co-stained with antibodies specific for CD3 (anti-CD3-AF700, clone UCHT1, BD Bioscience) and CD4 (anti-CD4-APCeF780, clone RPA-T4, Ebioscience). Tetramer-positive cells were analyzed on a BD LSRII.
Micro-scaled RNA sequencing
Total RNA was purified using a miRNAeasy kit (Qiagen, USA) as described previously [18]. Purified total RNA (0.2–5 ng) was amplified following the smart-seq2 protocol [19]. cDNA was purified using AMPure XP beads (Beckman Coulter). From this step, 1 ng of cDNA was used to prepare a standard Nextera XT sequencing library (Nextera XT DNA sample preparation and index kit, Illumina). Samples were sequenced using HiSeq2500 (Illumina) to obtain 50-bp single-end reads. Both whole-transcriptome amplification and sequencing library preparations were performed in a 96-well format to reduce assay-to-assay variability. Quality control steps were included to determine total RNA quality and quantity, optimal number of PCR amplification cycles, and fragment size selection
Bioinformatic RNA sequencing analysis
Single-end reads that passed Illumina filters were filtered for reads aligning to tRNA, rRNA, adapter sequences, and spike-in controls. Reads were then aligned to the UCSC hg19 reference genome using TopHat (v 1.4.1) [20]. DUST scores were calculated with PRINSEQ Lite (v 0.20.3) [21] and low-complexity reads (DUST > 4) were removed from the BAM files. Alignment results were parsed via SAMtools [22] to generate SAM files. Read counts to each genomic feature were obtained using the htseq-count program (v 0.6.0) [23] using the “union” option. After removing absent features, raw counts were imported to R/Bioconductor package DESeq2 [24] to identify differentially expressed genes. DESeq2 normalizes counts by dividing each column of the count table (samples) by the size factor of this column, calculated by dividing the samples by geometric means of the genes. P-values for differential expression were calculated using the binomial test for differences between the base means of two conditions and adjusted for multiple tests using the Benjamini Hochberg algorithm [25]. Genes were considered differentially expressed if DESeq2 analysis resulted in a p-value of < 0.05 and the change in expression was >two-fold. Hierarchical clustering was performed using ward linkage with Euclidean metric. The sequences have been submitted to the Gene Expression Omnibus under accession number GSE70050 (http://www.ncbi.nlm.nih.gov/geo/).
Statistical analysis
Statistical tests were performed using Graphpad Prism Version 6 (La Jolla, CA) or Microsoft Excel (Microsoft, Redmond, WA). In general, non-parametric tests were applied because the data could not be assumed to be normally distributed. The unpaired Mann-Whitney U-test was used to calculate P values in the cross sectional cohort. For analysis of significance in the longitudinal cohort where samples from the same donor were compared in- and out-of-season, a paired Wilcoxon test was applied. All P values <0.05 were considered to be significant. In the case of ELISPOT assays, as in previous studies [26–30], Student’s t-test was used to determine significance of the mean of triplicate values of the response against negative control. This analysis is also supported by an independent Poisson distribution to allow for a non-normalized distribution if the number of cytokine-producing cells was low.
RESULTS
Seasonality of cytokine production in allergic and non-allergic donors
Previous studies [16, 30, 31] identified twenty epitopes (Supplementary Table S2) that account for 79.5% of the total response to a set of Timothy grass (TG) -derived pollen antigens (Phl p allergens) in TG-allergic individuals. Here, PBMCs were expanded in vitro with either a pool of those epitopes, (referred to as P20 throughout the paper) or TG extract, and cytokine production was assessed at different time points after culture using ELISPOT assays (Figure 1A, B).
Figure 1. Kinetics and magnitude of cytokine production in TG-allergic and non-allergic donors.
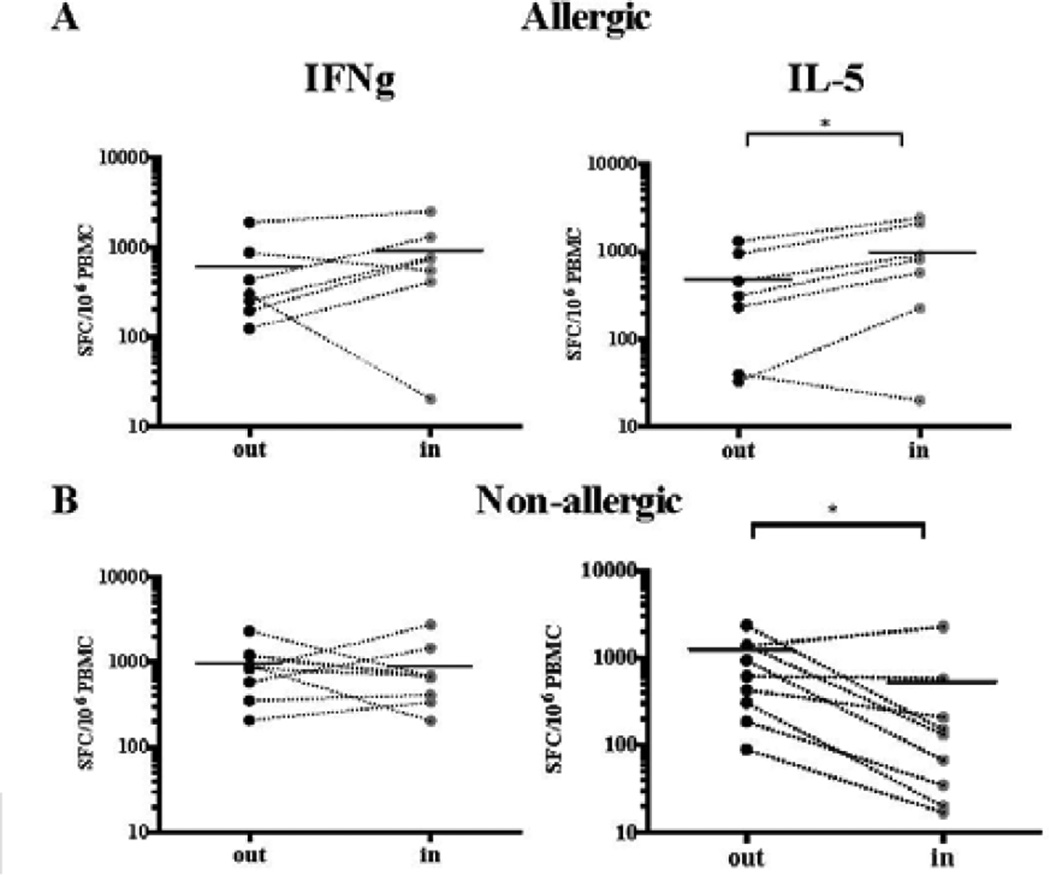
A–B: Cytokine production over 21 days following restimulation with TG extract (A) or a pool of 20 predominant TG epitopes (B). Means of 10 donors are shown, respectively. Dashed line, allergics; continuous line, non-allergics. Error bars indicate SD. C–D: Seasonal cytokine responses in a cross-sectional cohort of TG allergic (C) and non-allergic (D) individuals. IFNγ, IL-5, IL-10 and IL-17 reponses elicited by P20 in a 14-day in vitro restimulation assay. Samples obtained between Jul 1st to Oct 15th are considered in-season. *P < 0.05, ** P < 0.01, Mann-Whitney U-test.
In allergic individuals, considerable IL-5 and IFNγ responses were observed (specifically, in the case of P20-expanded cultures, average 2387 and 614 SFC/106 PBMC). Lower responses were noted for IL-17 and IL-10 (330 and 146 SFC/106PBMC) as compared to IFNγ and IL-5. In non-allergic individuals, as expected, lower IL-5 and IL-10 responses were observed. Surprisingly, IFNγ and IL-17 responses in allergic and non-allergic individuals were essentially superimposable (Figure 1A, B).
Based on these results, 14–17 days stimulation with P20 was selected for use in subsequent experiments analyzing cytokine production as a function of seasonality. For this analysis, cytokine responses of allergic and non-allergic donors were segregated and analyzed as a function of TG season, with the July to mid-October period considered “in-season”, and any other time point “out-of-season”. Overall, a total of 37 in-season and 39 out-of-season donations were tested for IFNγ, IL-5, IL-10 and IL-17 production (Figure 1C, D).
As expected, in-season PBMC donations from allergic individuals exhibited significantly higher IL-5 and IL-10 responses. IFNγ levels did not change depending on seasonality (Figure 1C). Strikingly, in non-allergic donors both IFNγ and IL-5 were significantly lower in-season compared to out-of-season (Figure 1D).
The ratios between IL-5/IFNγ and IL-5/IL-10 production were also determined (Supplementary Figure S1). No significant differences regarding T cell polarization were observed when in- and out-of-season ratios in allergic and non-allergic individuals were compared.
Seasonal modulation of cytokine reactivity is allergen-specific
Next, we investigated whether this inverse pattern of seasonality in allergic versus non-allergic individuals was TG-specific, or rather reflective of a general modulation of all T cell reactivity. TG-allergic individuals were classified as allergic or not allergic to other common seasonal or perennial allergens, based on skin-prick test reactivity. In- and out-of-season PBMC donations were stimulated with various allergen extracts for 14 days and the ratio of in- and out-of-season IFNγ or IL-5 production was calculated. Ratios greater than one reflect increased cytokine production in season compared to out of season. For example, TG-sensitized donor D78_1/D78_2, additionally sensitized to mugwort but not to juniper or canary grass, was stimulated with mugwort, but also juniper- or canary grass extract; and TG-sensitized donor U102_1/U102_2, additionally sensitized to mugwort, was stimulated with mugwort and also bermuda grass and black walnut extracts to which the donor was not allergic.
IL-5 reactivity to seasonal allergens was increased in-season for allergens to which the donors were sensitized (Figure 2). However, when donors were not sensitized to the tested allergen, in-season IL-5 reactivity was lower. The difference in the in- and out-of-season ratios between those two groups was significant (p = 0.039).
Figure 2. Seasonal modulation of cytokine reactivity is allergen-specific.
Ratio of in- and out-of-season cytokine reactivity in TG-allergic donors tested against (A) a set of other common seasonal (trees: date palm, olive, black walnut, prickly juniper; grasses: rye grass, bermuda grass, canary grass, wheat, kentucky blue; weeds: giant ragweed, mugwort) and (B) perennial allergens (american cockroach, german cockroach, house dust mite (Der p 1), cat, dog, Alternaria sp.). All donors tested were TG-allergic and were further classified as allergic or non-allergic for other allergens on the basis of skin test reactivity (All, allergic; Ctr, not allergic to the tested seasonal or perennial allergen). PBMC, obtained in and out-of-season, were stimulated with allergen extracts for 14 days and IFNγ and IL-5 production was analyzed longitudinally (in the same donor in- and out-of-season). Donors were chosen for representative assays based on availability of longitudinal samples and type of assay. P values were determined using Mann-Whitney U-test.
Similarly, IFNγ reactivity to seasonal allergens did not change with the season (Figure 2, supplementary Table S4) for allergens to which the donors were sensitized, on the basis of skin test reactivity. However, IFNγ production in response to allergens that did not trigger positive skin reactivity in these donors was significantly decreased in season and the in- and out-of-season ratio was significantly lower compared to allergens to which donors were allergic (Figure 2; p = 0.023; one tailed Mann-Whitney U-test). In the same donors, IFNγ/IL-5 reactivity to perennial allergens was not significantly changed regardless of whether donors were sensitized to the particular allergen or not (Figure 2, supplementary Table S5).
Longitudinal analysis of the impact of seasonality on T cell reactivity
The initial observation of inverse patterns of immune reactivity depicted in Figure 1C–D was made in a cross-sectional cohort where samples analyzed in and out-of-season were from different donors. We next analyzed IL-5- and IFNγ-producing T cells from longitudinal PBMC samples, in which the same allergic or non-allergic donors provided in-season and out-of-season blood donations (Figure 3). Confirming the results from the cross-sectional donors, in allergics the IL-5 response was upregulated in-season, whereas IFNγ responses were not significantly changed. In non-allergics, a different pattern was observed: as in the cross-sectional donors, the IL-5 response was downregulated in-season. IFNγ responses, which were significantly downregulated in the more numerous cross-sectional cohort, were not significantly decreased. Overall, despite the minor discrepancy in IFNγ responses in non-allergics, the longitudinal analysis confirms differential seasonal modulation of cytokine responses between allergic and non-allergic individuals.
Figure 3. Seasonal cytokine response in longitudinal samples from TG-allergic and non-allergic individuals.
Cytokine responses (IFNγ, IL-5) elicited by P20 in a 14-day in vitro restimulation assay in allergic (A) and non-allergic donors (B). *P < 0.05, paired Wilcoxon one-tailed test.
Ex vivo tetramer analysis reveals no seasonal difference in cell numbers in allergic and non-allergic donors
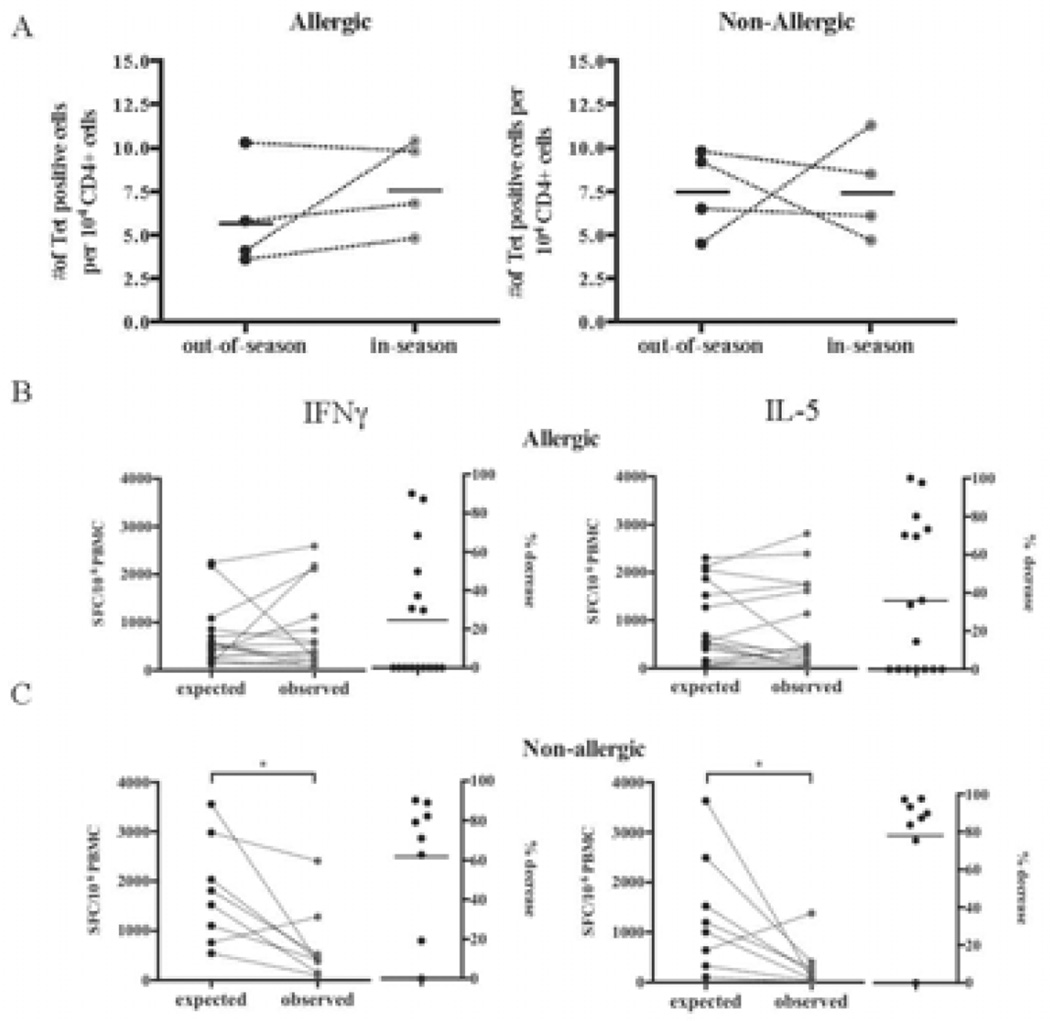
To further characterize the mechanisms associated with the phenomena described above we utilized longitudinal samples and tetrameric staining reagents. The use of tetramer staining addressed whether the lower reactivity observed as a function of seasonality is due to a decrease in the absolute number of reactive cells, or alternatively, if the decrease of reactivity might be due to lower reactivity of cells. In these experiments we determined the frequency of tetramer-positive cells ex vivo in longitudinal blood donations from 4 non-allergic and 4 allergic donors. PBMCs were stained with a pool of 4 tetramer reagents matching the donors’ HLA types (Supplementary Table S6), enriched and co-stained with antibodies specific for CD3 and CD4. Longitudinal IFNγ and IL-5 reactivity against P20 and HLA class II tetramer-stained PBMCs from a representative non-allergic donor are shown in Supplementary Figure S2A–B. A compilation of the results for all eight donors is shown in Figure 4A. No significant changes were observed. Interestingly, in allergic donors higher reactivity was paralleled by a subtle increase in the number of tetramer-positive cells detected in season. By contrast, in non-allergic donors, despite lower reactivity, no change in the number of allergen-specific cells was detected. Allergen-specific CD4+ T cells of non-allergic donors might therefore become anergic/hyporesponsive following the antigen stimulation provided by the pollen season.
Figure 4. A Ex vivo tetramer analysis.
Numbers of TG-specific CD4+ T cells in- and out-of-season in allergic and non-allergic donors. B–C. Co-culture of longitudinal samples. PBMC from in and out-of-season donations were stimulated in vitro with P20 separately as described before. Immune reactivity in 1:1 co-cultures of in and out-of-season donations from the respective allergic (B) or non-allergic (C) donors were analyzed. The expected reactivity in absence of any inhibitory mechanisms, calculated as the average of the in and out-of-season reactivity (in terms of SFC per million PBMC) is plotted and compared to the actual observed reactivity by the end of the in vitro co-culture. *P < 0.05, as calculated by paired Wilcoxon one-tailed test.
To assess whether this stimulation is associated with development of regulatory CD4+ cells, the expression of several Treg- and/or Tr1-associated markers, such as CD25, CD127 and FoxP3 (Treg), as well as CD49b and LAG3 (Tr1), was analyzed in non-allergic donors longitudinally using flow cytometry [32]. However, no change in the bulk frequency of those cells was detected (data not shown).
Co-culture of longitudinal samples reveals suppressive activity in-season for non-allergic donors
The decrease in reactivity of non-allergic donors observed in-season could be due to elicitation or actual suppressive/regulatory activity by seasonal pollen exposure. To address this point, in- and out-of-season PBMC donations were separately stimulated in vitro with P20 as described before. In parallel, we assayed co-cultures of equal numbers of PBMCs from in- and out-of-season donations from the same donors. The reactivity detected after 14 days is shown in Figure 4B–C. More specifically, for each donor, the expected reactivity, calculated as the average reactivity of the in- and out-of-season separate cell cultures (expressed in terms of SFC per million PBMC), is plotted, along with the actual observed reactivity in the in vitro co-culture. In addition, the percentage decrease of the immune responses detected in the co-cultures (calculated as 100 minus the ratio of co-culture vs. expected reactivity based on separate cultures) is shown. In allergic individuals, immune responses were not significantly modulated (Figure 4B). In contrast, in non-allergic individuals, IFNγ and IL-5 responses were observed as decreased, on average, by 60 and 80%, respectively (Figure 4C).
Isolation of TG-specific T cell memory subsets for mRNA profiling
To further characterize seasonal changes in TG-specific memory subsets, we utilized an ex vivo capture assay to isolate IL-5- and IFNγ-producing T cells. To maximize the number of cell captures for analysis, we supplemented the P20 pool with an additional 80 previously described TG-derived epitopes (Supplementary Table S2, S3; [15, 26]). The megapool of 100 peptides was used to increase the ex vivo signal. This decision was based on results of parallel studies revealing that the use of peptide megapools comprised of a large number of epitopes and prepared by sequential lyophilization allow detection of responses directly ex vivo [33], in particular in the case of allergic diseases where the frequency of progenitor cells is generally very low [34]. After 3h stimulation with this pool of TG epitopes, PBMCs were incubated with an anti-CD45/IL-5 (or IFNγ) capture antibody and co-stained with an antibody cocktail as described in the methods section prior to sorting of cytokine-producing cells.
We, and others, have successfully used the classification scheme based on CD45 and CCR7 to distinguish TCM and TEM cells [35, 36]. To demonstrate that seasonal modulation reflects activation of particular memory T cell subsets, cells were further separated into naïve (TN; CD45RA+CCR7+), effector memory (TEM; CD45RA−CCR7−), central memory (TCM; CD45RA−CCR7+), and terminally differentiated effector memory T cells (TEMRA, CD45RA+CCR7−) by FACS sorting (Supplementary Figure S3A). Cytokine production was associated with TEM and TCM, with a general trend toward TEM being decreased and TCM increased in-season. The data from 5 allergic and 6 non-allergic donors is shown in Supplementary Figure S3B–C.
Transcriptional profiles revealed differential patterns of gene regulation in IFNγ- and IL-5- producing cells
We determined the transcriptional profile of allergen-specific T cells from 5 allergic and 6 non-allergic individuals by sorting IFNγ- or IL-5-producing T cells from TEM and TCM-compartment following ex vivo stimulation and using the capture assay described above. When compared to the control CD4+ cells not releasing IFNγ or IL-5, cytokine-producing cells from the majority of the donors had a transcriptional profile suggestive of antigen-specific activation (Supplemental Figure S4). For example, expression of HLA-DR, IL-2, TNF, CCL3, miR155HG were significantly upregulated in both IFNγ- and IL-5-producing cells Figure S4 top panel), with greatest increases in TEM subsets compared to TCM subsets. Expression of signature Th1 genes like IFNγ, GZMB, TBX21 (encoding for transcription factor T-bet) and other relevant genes like IL-17A, RORC, and IL-10 were upregulated in IFNγ captured cells (Figure S4 middle panel). Expression of signature Th2 genes like IL-5, IL-4, IL-13, IL1RL1 (encoding for IL-33 receptor), was upregulated in IFNγ producing cells Figure S4 bottom panel). Overall, these results validate the cytokine capture assay as an effective means to isolate T cells of defined subsets.
Transcriptional profiles reveal seasonal gene regulation in IFNγ- and IL-5- producing cells
Next, differentially regulated genes between samples obtained in-season versus out-of-season in either IFNγ- or IL-5-producing cells were identified based on an adjusted p-value of < 0.05 and as well as a two-fold change in gene expression (Figure 5A, red circles). Comparing the number of significantly modulated genes in general, in allergic subjects, more genes were downregulated as a function of seasonality in Th1-like cells (IFNγ capture) than in IL-5-producing cells (1002 vs. 437 genes, Figure 5B) while in non-allergic donors nine times more genes were downregulated in IL-5- than in IFNγ- producing cells (360 vs. 40 genes). Similarly, in allergic individuals more genes were upregulated (650 vs. 373) in IL-5-than in IFNγ-producing cells, while in non-allergic donors more genes were upregulated in IFNγ - than in IL-5-producing cells (214 vs. 270, Figure 5B).
Figure 5. Gene expression patterns of IFNγ- and IL-5-producing antigen-specific cells.
A. MA plots comparing gene expression patterns of IFNγ- or IL-5-producing cells in allergic and non-allergic donors as a function of pollen season. Geometric mean of gene expression (x-axis) is compared with fold change in expression between in- and out-of-season samples. Differentially regulated genes were identified based on an adjusted P-value of < 0.05 and as well as a two-fold change in gene expression (red circles). B. Histogram of the number of genes differentially expressed between in- and out-of-season samples categorized by expression direction (black, upregulation; grey, downregulation) and by allergic status and type of molecule captured. C. Heat map gene expression of IL-5-producing cells from TCM (green) and TEM (blue) in non-allergic donors in- and out-of-season (green and red, respectively). Data from 6 non-allergic individuals (3 cross-sectional and 3 longitudinal samples) is shown. Each row indicates gene expression levels of each individual donor represented in the respective column. Shades of red are reflective of the level of downregulation; blue, upregulation; white, no change in expression with season. The heat map was generated by taking into account the distance for similarity between genes and samples using the Euclidean distance. Genes and samples were clustered using the Ward linkage method. Micro-scaled RNA-seq was performed on a total of 76 samples: 64 samples (11 donors) for each allergen-specific CD4+ subset (IFNγ-TEM, IFNγ-TCM IL-5-TEM, IL-5-TCM) and 12 samples (5 donors) for each CD4+ memory subset not producing IFNγ or IL-5 (TEM, TCM).
Pattern of seasonal modulation in transcriptional profiles in non-allergic individuals
We next examined genes differentially regulated in non-allergic donors in more detail, focusing on IL-5 responses, which were most consistently modulated in the experiments presented in Figures 1, 3 and 4 above. From the list of 3,382 genes in Figure 5B, we identified 543 genes that had a significant seasonal up- or downregulation in IL-5-producing cells in non-allergic individuals, and showed discordant patterns in IL-5-producing cells in allergic donors (Table 1A). Thus, the genes listed in Table 1A are a subset of those presented in Figure 5B, selected on the basis of being differentially expressed in non-allergic as compared to allergic donors. The rationale for examining genes from both allergic and non-allergic donors in both IL-5- and IFNγ-producing cells was to identify genes that are not only modulated by seasonality, but actually modulated in different directions when allergic and non-allergic donors are considered.
Table 1. Differentially regulated genes in IL-5- and IFNγ-producing T cells in allergic compared to non-allergic donors.
Table 1A, 543 genes in category 1 to 4 were identified based on an adjusted p-value of < 0.05 as well as a two-fold change in gene expression. To compare specific molecular signatures of IL-5-producing TG-specific T cells in allergic and non-allergic individuals, a total of 20,000 genes were analyzed. Of those, about 3,000 were differentially modulated. To identify unique transcriptional features and molecular signatures from allergic and non-allergic individuals as a function of seasonality, genes were categorized as significantly up- or downregulated or neutral in IL-5-producing cells in allergic donors in-season but oppositely regulated in non-allergic individuals. Table 1B lists 85 differentially regulated genes by function.
| Table 1A | |||||
|---|---|---|---|---|---|
| Differentially regulated genes in IL-5- and IFNγ-producing T cells in allergic compared to non-allergic donors | |||||
| Category | Non-allergic | Allergic | Non-allergic | Allergic | Number of diff. regul genes |
| IL-5 | IFNγ | ||||
| 1 | up | down/neutral | down/neutral | down/neutral | 234 |
| 2 | up | down/neutral | up | down/neutral | 6 |
| 3 | down | up/neutral | up/neutral | up/neutral | 302 |
| 4 | down | up/neutral | down/neutral | neutral | 1 |
| Table 1B | ||||
|---|---|---|---|---|
| Category | Short name | Allergics: IL-5 | Non-allergics: IL-5 | Biological significance |
| Adhesion | ||||
| ICAM3 | Neutral | Up | Intracellular adhesion molecule | |
| IGBP1 | Neutral | Up | Integrins | |
| ITFG3 | Neutral | Up | Integrins | |
| ITGB1 | Neutral | Up | Integrins | |
| THBS1 | Neutral | Up | Integrins | |
| LGALS3 | Neutral | Up | Integrins | |
| AMICA1 | Neutral | Down | Integrins | |
| IFN-related | ||||
| PRDM1 | Down | Up | Interferon | |
| IRF7 | Neutral | Up | Interferon | |
| TRIM22 | Neutral | Up | Interferon | |
| IFI16 | Up | Down | Interferon | |
| BTN3A2 | Neutral | Down | Interferon | |
| IFI6 | Neutral | Down | Interferon | |
| IRF9 | Neutral | Down | Interferon | |
| Chemokines | ||||
| CCL5 | Neutral | Up | Chemokines | |
| CXCL5 | Neutral | Up | Chemokines | |
| CXCR3 | Neutral | Down | Chemokines | |
| CXCR4 | Neutral | Down | Chemokines | |
| ISG15 | Neutral | Down | Chemokines | |
| CMTM6 | Neutral | Down | Chemokines | |
| Kinases | ||||
| MAP2K1 | Neutral | Up | Kinases | |
| PTP4A2 | Neutral | Up | Kinases | |
| PTPN2 | Neutral | Up | Kinases | |
| ITK | Neutral | Up | Kinases | |
| JAK3 | Neutral | Up | Kinases | |
| LAX1 | Neutral | Up | Kinases | |
| LCK | Neutral | Up | Kinases | |
| DUSP4 | Down | Up | Kinases | |
| LAT | Neutral | Down | Kinases | |
| SHARPIN | Neutral | Down | Kinases | |
| RICTOR | Neutral | Down | Kinases | |
| GRAP2 | Neutral | Down | Kinases | |
| CCM2 | Neutral | Down | Kinases | |
| TNF-related | ||||
| LTB | Neutral | Up | TNF | |
| LY6E | Neutral | Up | TNF | |
| ZMYND8 | Neutral | Up | TNF | |
| TNFRSF10C | Neutral | Up | TNF | |
| APOL3 | Neutral | Up | TNF | |
| TRAF7 | Neutral | Up | TNF | |
| T cell markers | ||||
| CD2 | Neutral | Down | T cells | |
| CD69 | Neutral | Down | T cells | |
| IL2RG | Neutral | Down | T cells | |
| IL32 | Neutral | Down | T cells | |
| CYTIP | Neutral | Up | T cells | |
| CD247 | Up | Down | T cells | |
| GNLY | Neutral | Up | T cells | |
| BATF | Neutral | Up | T cells | |
| Retinoic/Heme | ||||
| HBB | Neutral | Down | Retinoic | |
| UROD | Neutral | Down | Retinoic | |
| MDK | Neutral | Up | Retinoic | |
| SNW1 | Neutral | Up | Retinoic | |
| PDCD5 | Up | Down | Retinoic | |
| RARRES3 | Up | Down | Retinoic | |
| SP110 | Up | Down | Retinoic | |
|
Heat shock, proteosome and HLA- related |
||||
| DNAJC19 | Neutral | Down | HSP | |
| HPS3 | Neutral | Up | HSP | |
| STIP1 | Neutral | Up | HSP | |
| PSMB9 | Neutral | Up | Proteasome | |
| PSMG2 | Neutral | Up | Proteasome | |
| PSMB8 | Down | Up | Proteasome | |
| PACS1 | Neutral | Up | HLA class I | |
| HLA-DPB1 | Neutral | Down | HLA | |
| HLA-F | Up | Down | HLA class I | |
| TAP1 | Neutral | Down | Proteasome | |
| HSPA9 | Up | Down | HSP | |
| Other | ||||
| ENO1 | Neutral | Down | Allergy | |
| PIK3AP1 | Neutral | Down | B cells | |
| STIM2 | Neutral | Down | Calcium | |
| CAPN2 | Neutral | Down | Calpain | |
| CAPN5 | Neutral | Down | Calpain | |
| CASP1 | Neutral | Up | Caspase | |
| DAPK2 | Neutral | Up | Caspase | |
| CTSD | Neutral | Up | Cathepsin | |
| BAX | Neutral | Up | BCL2 | |
| BNIP2 | Up | Down | BCL2 | |
| DPP8 | Neutral | Down | Immune regulation | |
| ADRB2 | Neutral | Up | Neutrophils | |
| SERPINB1 | Neutral | Down | Neutrophils | |
| SERPINB2 | Neutral | Up | Serpin | |
| COPS5 | Neutral | Up | Signalosome | |
| GPS1 | Neutral | Up | Signalosome | |
| CNPY3 | Neutral | Up | TLR4 | |
| OFD1 | Up | Down | WNT | |
| CSNK2B | Neutral | Down | WNT | |
| ANP32A | Up | Down | WNT | |
up/down, significant up- or downregulation of gene expression in-season based on an adjusted P-value of < 0.05 as well as a 2-fold change in gene expression
neutral, no change in gene expression in-season compared to out-of-season
Expression of the majority of genes upregulated in IL-5-producing cells in non-allergic donors (but not in allergic subjects) did not change in IFNγ -producing cells (234 vs. 6). Similarly, the vast majority of genes downregulated in IL-5-producing cells were unchanged in IFNγ-producing cells in both allergic and non-allergic subjects (302 vs. 1, Table 1A). These findings suggest an IL-5-specific modulation, since IFNγ-producing cells are not modulated in opposite direction for those genes, but rather left unperturbed. Given that these two cell types are quite distinct, it is not surprising that there is little overlap of common gene changes between them.
Heat maps of IL-5-captured cells from non-allergic donors as a function of seasonality for the 543 genes are shown in Figure 5C, where shades of red reflect the degree of downregulation, while blue indicates the level of upregulation and genes with unchanged expression levels according to seasonality are plotted in white. The expression patterns clustered in three broad categories. A first group of five in-season samples clustered together and showed mostly opposite patterns of regulation compared to a group of five out-of-season samples. A third cluster was associated with intermediate and less pronounced patterns and corresponded to a mixed group of 3 in-season and 5 out-of-season samples.
A specific molecular program associated with seasonal down-modulation of IL-5-producing cells in non-allergic individuals
Within the subset of genes, we observed several genes of interest that are significantly up- or downregulated in IL-5-producing cells in-season compared to out-of-season in non-allergic donors (Table 1B). These included a number of genes encoding TNF- and IFN-related genes; several heat shock- and proteasome-related proteins, were also modulated (Table 1B). In parallel, several genes encoding chemokines and adhesion proteins, such as CCL5, ICAM3, IGBP1 or ITFG3, were significantly upregulated in non-allergic donors but remained unchanged in allergic subjects. The list of differentially modulated genes also included genes encoding kinases regulating cellular location, activation pathways and signal transduction.
A number of T cell-related surface antigens essential for activation of T cell responses and immune reactivity were significantly downregulated in IL-5-producing cells in-season in non-allergic individuals, while no change in expression was observed in allergic donors. Examples include CD2, an early and widely expressed T cell marker, which interacts with LFA-3 and CD48/BCM1 [37] as well as CD69 and IL2RG. The expression of RICTOR, encoding for a T cell surface antigen particularly critical for Th2 differentiation, and the inflammatory cytokine IL-32, were also significantly downregulated in non-allergic donors and unchanged in allergic subjects in-season compared to out-of-season. In contrast, CD247, a part of the TCR complex, was upregulated in allergic individuals in-season and downregulated in non-allergic individuals.
Conversely, a number of genes related to the retinoic acid and heme pathways, known to regulate immunosuppressive pathways [38, 39] were differentially expressed. These include HBB and UROD, encoding for heme and a uroporphyrinogen decarboxylase involved in the production of heme, respectively (Table 1B).
DISCUSSION
In the present study, the impact of timothy grass (TG) pollen season on TG-specific T cell responses was analyzed in allergic and non-allergic individuals. In line with previous studies [13, 14], responses in allergic subjects were polarized towards a Th2 type, significantly increased during TG pollen season. Strikingly, in non-allergic donors an opposing pattern with broadly decreased immune reactivity in-season was observed.
The broad downregulation observed in non-allergic donors indicates that healthy individuals are not oblivious to allergen exposure but rather react with an active modulation of responses following the antigenic stimulus provided by the pollen season. A significant reduction in IFNγ and IL-5 is also reflected in trends towards decreased IL-10 and IL-17 responses. To make sure that any difference we saw was not due simply to modulation of the relative composition of memory subsets as a function of seasonality, we separately characterized different memory subsets. Further, memory cells were studied to exclude the possibility that the patterns observed are an artifact of in vitro stimulation of naïve T cells. The fact that similar patterns of modulation are observed in the different T cell subsets indicates that it does not result from changes in the relative composition of memory subsets as a function of seasonality.
Previous studies have shown that non-allergic donors have detectable allergen-specific responses, mostly polarized towards a Th1 or Tr1 phenotype [7, 8] [13]. The current study is novel in that it suggests that in addition to a different polarization, non-allergic individuals respond to increased antigen exposure during the pollen season with a generalized downregulation of responses.
Downregulation of T cell responses was allergen-specific, because it was specifically associated with seasonal allergens to which donors were not sensitized, and was not observed in perennial allergens to which they were sensitized. One hypothesis to explain this declined reactivity is an actual decline in numbers of allergen-specific T cells due to induction of apoptosis or deletion of responding cells [40, 41]. Selected Th1 cell apoptosis was previously reported to be more prominent in atopic subjects compared to non-allergic individuals as a mechanism of Th2 predominance [41].
Here we used allergen-specific tetramers to assess whether the declined reactivity in-season was due to a decrease in frequency of antigen-specific cells. In line with other studies, we detected TG-specific CD4+ T cells at frequencies in the range of 1–6/10,000 in those experiments in both allergic and healthy individuals [17, 42]. Since there were no significant changes in frequencies of allergen-specific T cells despite lower in-season reactivity, these data rule out apoptosis or deletion of responding cells as a potential mechanism.
The lack of changes in the frequency of allergen-specific T cells also seems to rule out that the seasonal modulation is due to their migration to airway-associated tissues. This possibility is also unlikely based on the observation that allergen-specific T cells are actually slightly increased in frequency in allergic individuals in-season – the opposite of what would be expected if allergen-specific T cells would be depleted by migration to the airway.
The seasonal decrease in T cell reactivity of non-allergic individuals seems to be associated with a regulatory mechanism [43, 44]. We could support this hypothesis through co-culture of PBMCs from longitudinal in- and out-of-season donations. It is possible that this regulatory mechanism might involve allergen-specific regulatory T cells (Treg) [45]. Overtvelt et al. analyzed birch pollen-specific CD4+ T cells and showed that in healthy subjects, circulating T cells exhibited an IL-10-producing FOXP3-positive phenotype [8]. However, in our experiments we did not detect increases in IL-10 production, but rather a general decrease of cytokine production, including IL-10.
The present study highlights changes in reactivity as a function of seasonality at the level of allergen-specific T cells. As such, it should be noted that these changes are associated with the “regulated” cells rather than the regulatory cells. In preliminary analysis we did not detect differences in the bulk non-allergen-specific T cells at the level of elements associated with Treg and/or Tr1 cells, such as FoxP3, CD49b or LAG3, at either the mRNA or protein (as assayed by FACS) level (data not shown). Future experiments will address these issues in more detail, and the alternative possibility that the regulatory interaction might be mediated by other cell types, such as B cells or antigen-presenting cells [46–51].
We focused our investigation on the transcriptional profiles of allergen-specific T cells. Studies to characterize allergen-specific T cells are limited by low progenitor frequencies within peripheral blood mononuclear cells [42, 52]. To enable ex vivo characterization of responding cells, we utilized a cytokine capture assay and a pool of 100 TG-specific epitopes that were previously identified [16, 30]. This experimental strategy, coupled with micro-scaled methods to profile global mRNA levels in a few hundred cells, uncovered differential patterns of seasonal gene regulation in IL-5-producing cells in healthy compared to allergic donors. Strikingly, in non-allergic donors, we observed upregulation of a number of genes encoding for TNF- and IFN-related genes. This could reflect a molecular program opposing Th2 activation [53]. Moreover, several heat shock- and proteasome-related proteins were also modulated, potentially as a consequence of the modulation of IFN- and TNF-related pathways [54]. In parallel, several genes encoding chemokines and adhesion proteins, such as CXCR4, were modulated, suggesting changes in cell-cell interactions and homing characteristics [55]. Active down-modulation of genes encoding for T cell markers like CD2, CD69, CD247 or IL-2R might also contribute to confining exaggerated immune responses in non-allergic subjects upon allergen exposure [56–58]. Since CD2 plays an important role in mediating adhesion between T cells and other cell types [56–58], its selective downregulation might be associated with decreased immune reactivity. Likewise, lower expression of CD247, part of the CD3-TCR complex, may limit reactivity in non-allergic individuals [57].
When we clustered the gene expression patterns in IL-5-producing cells from non-allergic donors, three main groups were revealed, associated with in-season or out-of-season changes or with intermediate profiles, respectively, and populated by both in-season and out-of-season donations. We hypothesize that this last “mixed group” heat map profile might reflect variability in terms of season onset and actual donor exposure. Future longitudinal studies with more frequent sampling will address this possibility. Furthermore, it is important to note that a lack of grass pollen skin-prick reactivity and grass pollen allergy symptoms does not make a subject non-atopic but rather non-grass pollen allergic. Even though these individuals were tested for sensitivity to a variety of allergens, allergic sensitivities to other aeroallergens that were not included in the skin-prick test panel cannot be excluded. While the present study was not designed or powered to longitudinally examine seasonality changes as a function of disease severity, additional studies addressing these issues will be key to provide valuable stratification of true allergic phenotypes and strengthen findings suggesting cellular and molecular mechanisms of seasonal allergy. These studies may also be expanded to include analysis of responding T cells before and after nasal challenge with allergen extracts [59].
This study identifies a number of genes whose modulation might contribute to maintaining a healthy immune response upon allergen exposure. The fact that we detected a robust IL-10 signal in IFNγ-producing TEM cells suggests a double IFNγ/IL-10 producing cell population, which may be crucial for distinguishing the regulatory mechanism governing tolerance to allergens from that responsible for an allergy phenotype. Meiler et al. demonstrated that high-dose bee venom allergen tolerance is associated with clonal expansion of specific IL-10-secreting Tr1 cells, which may switch from existing Th1- and Th2-like allergen-specific cells [60]. In this context it is important to underline that here we characterized mRNA profiles associated with cytokine-producing allergen-specific T cells, and thus associated with “regulated” rather than “regulatory” cells.
Results from this study suggest that the down regulation of T cell responses may be due to either an actual decline in numbers of allergen-specific T cells or inhibited functionality of the cells following allergen exposure. The lack of change in specific T cell frequency as detected using a tetramer-based approach seems to rule out apoptosis or deletion of responding cells as a potential mechanism for the lower in-season reactivity. Indeed, consistent with this notion, co-culture of PBMCs from longitudinal in- and out-of-season donations revealed suppressive activity in season in non-allergic donors. In this study, we focused on the changes detected at the level of allergen specific, memory CD4 T cells, i.e. associated with the “regulated” cells rather than the regulatory cells. While our preliminary analysis did not suggest a role for Tregs, other cell types such as B cells or other antigen-presenting cells might mediate the regulatory interaction [48–51]. Our transcriptomic analysis revealed changes in reactivity as a function of seasonality at the level of allergen-specific T cells. In non-allergic donors, genes encoding for TNF- and IFN-related genes were upregulated, while T cell markers such as CD2, CD69, CD247 and IL-2R were downregulated, suggesting the potential for a specific transcriptional program being associated with seasonal downregulation of allergen-specific T cells in non-allergic individuals. Future experiments will address these issues in more detail.
In conclusion, the mechanism of suppression in healthy individuals remains unclear, and may reflect different mechanisms from those operating in the context of high-dose allergen tolerance. It would be of significant interest to characterize distinct transcriptional programs in allergic and non-allergic donors in- and out-of-season by PCR in more detail in future studies and to assess the functional role of specific genes by RNAi approaches [61, 62]. Indeed, understanding the mechanisms associated with this seasonal downregulation may be key to recapitulate natural tolerance upon allergen exposure and enable more effective interventions. The identification of specific molecular signatures and target molecules that are modulated in healthy donors during allergen season may be used as potential biomarkers in a diagnostic setting, and also suggest potential targets for therapeutic intervention.
Supplementary Material
Acknowledgments
We thank Mr. J. Day for assistance with high-throughput sequencing at the La Jolla Institute for Allergy and Immunology. This project was funded by the National Institute of Allergy and Infectious Diseases, National Institutes of Health, under contract number HHSN272200700048C and grant number U19 AI100275.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Maziak W, Behrens T, Brasky TM, Duhme H, Rzehak P, Weiland SK, Keil U. Are asthma and allergies in children and adolescents increasing? Results from ISAAC phase I and phase III surveys in Munster, Germany. Allergy. 2003;58:572–579. doi: 10.1034/j.1398-9995.2003.00161.x. [DOI] [PubMed] [Google Scholar]
- 2.Kay AB. Allergy and allergic diseases. Second of two parts. New Engl J Med. 2001;344:109–113. doi: 10.1056/NEJM200101113440206. [DOI] [PubMed] [Google Scholar]
- 3.Kay AB. Allergy and allergic diseases. First of two parts. New Engl J Med. 2001;344:30–37. doi: 10.1056/NEJM200101043440106. [DOI] [PubMed] [Google Scholar]
- 4.Umetsu DT, McIntire JJ, Akbari O, Macaubas C, DeKruyff RH. Asthma: an epidemic of dysregulated immunity. Nat Immunol. 2002;3:715–720. doi: 10.1038/ni0802-715. [DOI] [PubMed] [Google Scholar]
- 5.Larche M, Akdis CA, Valenta R. Immunological mechanisms of allergen-specific immunotherapy. Nat Rev Immunol. 2006;6:761–771. doi: 10.1038/nri1934. [DOI] [PubMed] [Google Scholar]
- 6.Singh MB, Bhalla PL. Hypoallergenic derivatives of major grass pollen allergens for allergy vaccination. Immunol Cell Biol. 2003;81:86–91. doi: 10.1046/j.0818-9641.2002.01144.x. [DOI] [PubMed] [Google Scholar]
- 7.Akdis M, Verhagen J, Taylor A, Karamloo F, Karagiannidis C, Crameri R, Thunberg S, Deniz G, Valenta R, Fiebig H, Kegel C, Disch R, Schmidt-Weber CB, Blaser K, Akdis CA. Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific T regulatory 1 and T helper 2 cells. J Exp Med. 2004;199:1567–1575. doi: 10.1084/jem.20032058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Overtvelt L, Wambre E, Maillere B, von Hofe E, Louise A, Balazuc AM, Bohle B, Ebo D, Leboulaire C, Garcia G, Moingeon P. Assessment of Bet v 1-specific CD4+ T cell responses in allergic and nonallergic individuals using MHC class II peptide tetramers. J Immunol. 2008;180:4514–4522. doi: 10.4049/jimmunol.180.7.4514. [DOI] [PubMed] [Google Scholar]
- 9.Till S, Dickason R, Huston D, Humbert M, Robinson D, Larche M, Durham S, Kay AB, Corrigan C. IL-5 secretion by allergen-stimulated CD4+ T cells in primary culture: relationship to expression of allergic disease. J Allergy Clin Immunol. 1997;99:563–569. doi: 10.1016/s0091-6749(97)70085-x. [DOI] [PubMed] [Google Scholar]
- 10.El Biaze M, Boniface S, Koscher V, Mamessier E, Dupuy P, Milhe F, Ramadour M, Vervloet D, Magnan A. T cell activation from atopy to asthma: more a paradox than a paradigm. Allergy. 2003;58:844–853. doi: 10.1034/j.1398-9995.2003.00282.x. [DOI] [PubMed] [Google Scholar]
- 11.Ebner C, Schenk S, Najafian N, Siemann U, Steiner R, Fischer GW, Hoffmann K, Szepfalusi Z, Scheiner O, Kraft D. Nonallergic individuals recognize the same T cell epitopes of Bet v 1, the major birch pollen allergen as atopic patients. J Immunol. 1995;154:1932–1940. [PubMed] [Google Scholar]
- 12.Mavroleon G. Restoration of cytokine imbalance by immunotherapy. Clin Exp Allergy. 1998;28:917–920. doi: 10.1046/j.1365-2222.1998.00336.x. [DOI] [PubMed] [Google Scholar]
- 13.Majori M, Rossi G, Caminati A, Bosoni A, Corradi M, Pesci A. Seasonal variations of T-cell cytokine pattern in peripheral blood from atopic subjects. J Asthma. 2001;38:469–476. doi: 10.1081/jas-100105867. [DOI] [PubMed] [Google Scholar]
- 14.Lagier B, Pons N, Rivier A, Chanal I, Chanez P, Bousquet J, Pene J. Seasonal variations of interleukin-4 and interferon-gamma release by peripheral blood mononuclear cells from atopic subjects stimulated by polyclonal activators. J Allergy Clin Immunol. 1995;96:932–940. doi: 10.1016/s0091-6749(95)70231-8. [DOI] [PubMed] [Google Scholar]
- 15.Bousquet PJ, Burbach G, Heinzerling LM, Edenharter G, Bachert C, Bindslev-Jensen C, Bonini S, Bousquet-Rouanet L, Demoly P, Bresciani M, Bruno A, Gjomarkaj M, Canonica GW, Darsow U, Durham S, Fokkens WJ, Giavi S, Gramiccioni C, Papadopoulos NG, Haahtela T, Kowalski ML, Magyar P, Murakozi G, Orosz M, Rohnelt C, Stingl G, Todo-Bom A, von Mutius E, Wiesner A, Wohrl S, Bousquet J, Zuberbier T. GA2LEN skin test study III: minimum battery of test inhalent allergens needed in epidemiological studies in patients. Allergy. 2009;64:1656–1662. doi: 10.1111/j.1398-9995.2009.02169.x. [DOI] [PubMed] [Google Scholar]
- 16.Oseroff C, Sidney J, Kotturi MF, Kolla R, Alam R, Broide DH, Wasserman SI, Weiskopf D, McKinney DM, Chung JL, Petersen A, Grey H, Peters B, Sette A. Molecular determinants of T cell epitope recognition to the common Timothy grass allergen. J Immunol. 2010;185:943–955. doi: 10.4049/jimmunol.1000405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wambre E, James EA, Kwok WW. Characterization of CD4+ T cell subsets in allergy. Curr Opin Immunol. 2012;24:700–706. doi: 10.1016/j.coi.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seumois G, Vijayanand P, Eisley CJ, Omran N, Kalinke L, North M, Ganesan AP, Simpson LJ, Hunkapiller N, Moltzahn F, Woodruff PG, Fahy JV, Erle DJ, Djukanovic R, Blelloch R, Ansel KM. An integrated nano-scale approach to profile miRNAs in limited clinical samples. Am J Clin Exp Immunol. 2012;1:70–89. [PMC free article] [PubMed] [Google Scholar]
- 19.Picelli S, Faridani OR, Bjorklund AK, Winberg G, Sagasser S, Sandberg R. Full-length RNA-seq from single cells using Smart-seq2. Nature protocols. 2014;9:171–181. doi: 10.1038/nprot.2014.006. [DOI] [PubMed] [Google Scholar]
- 20.Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25:1105–1111. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmieder R, Edwards R. Quality control and preprocessing of metagenomic datasets. Bioinformatics. 2011;27:863–864. doi: 10.1093/bioinformatics/btr026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R. Genome Project Data Processing S, The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anders S, Pyl PT, Huber W. HTSeq-a Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31:166–169. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Beha Brain Res. 2001;125:279–284. doi: 10.1016/s0166-4328(01)00297-2. [DOI] [PubMed] [Google Scholar]
- 26.Moutaftsi M, Peters B, Pasquetto V, Tscharke DC, Sidney J, Bui HH, Grey H, Sette A. A consensus epitope prediction approach identifies the breadth of murine T(CD8+)-cell responses to vaccinia virus. Nat Biotechnol. 2006;24:817–819. doi: 10.1038/nbt1215. [DOI] [PubMed] [Google Scholar]
- 27.Weiskopf D, Yauch LE, Angelo MA, John DV, Greenbaum JA, Sidney J, Kolla RV, De Silva AD, de Silva AM, Grey H, Peters B, Shresta S, Sette A. Insights into HLA-restricted T cell responses in a novel mouse model of dengue virus infection point toward new implications for vaccine design. J Immunol. 2011;187:4268–4279. doi: 10.4049/jimmunol.1101970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arlehamn CS, Sidney J, Henderson R, Greenbaum JA, James EA, Moutaftsi M, Coler R, McKinney DM, Park D, Taplitz R, Kwok WW, Grey H, Peters B, Sette A. Dissecting mechanisms of immunodominance to the common tuberculosis antigens ESAT-6, CFP10, Rv2031c, (hspX), Rv2654c (TB7.7) and Rv1038c (EsxJ) J Immunol. 2012;188:5020–5031. doi: 10.4049/jimmunol.1103556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oseroff C, Sidney J, Vita R, Tripple V, McKinney DM, Southwood S, Brodie TM, Sallusto F, Grey H, Alam R, Broide D, Greenbaum JA, Kolla R, Peters B, Sette A. T cell responses to known allergen proteins are differently polarized and account for a variable fraction of total response to allergen extracts. J Immunol. 2012;189:1800–1811. doi: 10.4049/jimmunol.1200850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schulten V, Greenbaum JA, Hauser M, McKinney DM, Sidney J, Kolla R, Lindestam Arlehamn CS, Oseroff C, Alam R, Broide DH, Ferreira F, Grey HM, Sette A, Peters B. Previously undescribed grass pollen antigens are the major inducers of T helper 2 cytokine-producing T cells in allergic individuals. Proc Nat Acad Sci U S A. 2013;110:3459–3464. doi: 10.1073/pnas.1300512110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKinney DM, Southwood S, Hinz D, Oseroff C, Arlehamn CS, Schulten V, Taplitz R, Broide D, Hanekom WA, Scriba TJ, Wood R, Alam R, Peters B, Sidney J, Sette A. A strategy to determine HLA class II restriction broadly covering the DR, DP and DQ allelic variants most commonly expressed in the general population. Immunogenetics. 2013;65:357–370. doi: 10.1007/s00251-013-0684-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gagliani N, Magnani CF, Huber S, Gianolini ME, Pala M, Licona-Limon P, Guo B, Herbert DR, Bulfone A, Trentini F, Di Serio C, Bacchetta R, Andreani M, Brockmann L, Gregori S, Flavell RA, Roncarolo MG. Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nat Med. 2013;19:739–746. doi: 10.1038/nm.3179. [DOI] [PubMed] [Google Scholar]
- 33.Carrasco Pro S, Sidney J, Paul S, Lindestam Arlehamn C, Weiskopf D, Peters B, Sette A. Automatic Generation of Validated Specific Epitope Sets. J Immunol Res. 2015;2015:11. doi: 10.1155/2015/763461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hinz D, Oseroff C, Pham J, Sidney J, Peters B, Sette A. Definition of a pool of epitopes that recapitulates the T cell reactivity against major house dust mite allergens. Clin Exp Allergy. 2015;45:1601–1612. doi: 10.1111/cea.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sallusto F, Geginat J, Lanzavecchia A. Central memory effector memory T cell subsets: function generation and maintenance. Ann Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 36.Appay V, van Lier RA, Sallusto F, Roederer M. Phenotype and function of human T lymphocyte subsets: consensus and issues. Cytometry Part A. 2008;73:975–983. doi: 10.1002/cyto.a.20643. [DOI] [PubMed] [Google Scholar]
- 37.Tang F, Wu Q, Ikenoue T, Guan KL, Liu Y, Zheng P. A critical role for Rictor in T lymphopoiesis. J Immunol. 2012;189:1850–1857. doi: 10.4049/jimmunol.1201057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lehmann JC, Listopad JJ, Rentzsch CU, Igney FH, von Bonin A, Hennekes HH, Asadullah K, Docke WD. Dimethylfumarate induces immunosuppression via glutathione depletion and subsequent induction of heme oxygenase 1. J Invest Dermatol. 2007;127:835–845. doi: 10.1038/sj.jid.5700686. [DOI] [PubMed] [Google Scholar]
- 39.Pae HO, Oh GS, Choi BM, Chae SC, Kim YM, Chung KR, Chung HT. Carbon monoxide produced by heme oxygenase-1 suppresses T cell proliferation via inhibition of IL-2 production. J Immunol. 2004;172:4744–4751. doi: 10.4049/jimmunol.172.8.4744. [DOI] [PubMed] [Google Scholar]
- 40.Macaubas C, Wahlstrom J, Galvao da Silva AP, Forsthuber TG, Sonderstrup G, Kwok WW, DeKruyff RH, Umetsu DT. Allergen-specific MHC class II tetramer+ cells are detectable in allergic, but not in nonallergic individuals. J Immunol. 2006;176:5069–5077. doi: 10.4049/jimmunol.176.8.5069. [DOI] [PubMed] [Google Scholar]
- 41.Akkoc T, de Koning PJ, Ruckert B, Barlan I, Akdis M, Akdis CA. Increased activation-induced cell death of high IFN-gamma-producing T(H)1 cells as a mechanism of T(H)2 predominance in atopic diseases. J Allergy Clin Immunol. 2008;121:652–658. e1. doi: 10.1016/j.jaci.2007.12.1171. [DOI] [PubMed] [Google Scholar]
- 42.Wambre E, Bonvalet M, Bodo VB, Maillere B, Leclert G, Moussu H, Von Hofe E, Louise A, Balazuc AM, Ebo D, Hoarau C, Garcia G, Van Overtvelt L, Moingeon P. Distinct characteristics of seasonal (Bet v 1) vs. perennial (Der p 1/Der p 2) allergen-specific CD4(+) T cell responses. Clin Experimental Allergy. 2011;41:192–203. doi: 10.1111/j.1365-2222.2010.03641.x. [DOI] [PubMed] [Google Scholar]
- 43.Aslam A, Chan H, Warrell DA, Misbah S, Ogg GS. Tracking antigen-specific T-cells during clinical tolerance induction in humans. PLoS ONE. 2010;5:e11028. doi: 10.1371/journal.pone.0011028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fujita H, Meyer N, Akdis M, Akdis CA. Mechanisms of immune tolerance to allergens. Chemical immunology and allergy. 2012;96:30–38. doi: 10.1159/000331868. [DOI] [PubMed] [Google Scholar]
- 45.Palomares O, Yaman G, Azkur AK, Akkoc T, Akdis M, Akdis CA. Role of Treg in immune regulation of allergic diseases. Eur J Immunol. 2010;40:1232–1240. doi: 10.1002/eji.200940045. [DOI] [PubMed] [Google Scholar]
- 46.Noh G, Lee JH. Regulatory B cells and allergic diseases. Allergy, Asthma & Immunol Res. 2011;3:168–177. doi: 10.4168/aair.2011.3.3.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van der Vlugt LE, Mlejnek E, Ozir-Fazalalikhan A, Janssen Bonas M, Dijksman TR, Labuda LA, Schot R, Guigas B, Moller GM, Hiemstra PS, Yazdanbakhsh M, Smits HH. CD24(hi)CD27(+) B cells from patients with allergic asthma have impaired regulatory activity in response to lipopolysaccharide. Clin Exp Allergy. 2014;44:517–528. doi: 10.1111/cea.12238. [DOI] [PubMed] [Google Scholar]
- 48.Mauri C, Menon M. The expanding family of regulatory B cells. Int Immunol. 2015;27:479–486. doi: 10.1093/intimm/dxv038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosser EC, Mauri C. Regulatory B cells: origin phenotype and function. Immunity. 2015;42:607–612. doi: 10.1016/j.immuni.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 50.Blair PA, Norena LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, Mauri C. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity. 2010;32:129–140. doi: 10.1016/j.immuni.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 51.Mauri C, Bosma A. Immune regulatory function of B cells. Ann Rev Immunol. 2012;30:221–241. doi: 10.1146/annurev-immunol-020711-074934. [DOI] [PubMed] [Google Scholar]
- 52.Altman JD, Moss PA, Goulder PJ, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, Davis MM. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 53.Kannan AK, Sahu N, Mohanan S, Mohinta S, August A. IL-2-inducible T-cell kinase modulates TH2-mediated allergic airway inflammation by suppressing IFN-gamma in naive CD4+ T cells. J Allergy Clin Immunol. 2013;132:811–820. e1–e5. doi: 10.1016/j.jaci.2013.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pockley AG. Heat shock proteins as regulators of the immune response. Lancet. 2003;362:469–476. doi: 10.1016/S0140-6736(03)14075-5. [DOI] [PubMed] [Google Scholar]
- 55.Yellowley C. CXCL12/CXCR4 signaling and other recruitment and homing pathways in fracture repair. BoneKEy Rep. 2013;2:300. doi: 10.1038/bonekey.2013.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bierer BE, Sleckman BP, Ratnofsky SE, Burakoff SJ. The biologic roles of CD2, CD4 and CD8 in T-cell activation. Ann Rev Immunol. 1989;7:579–599. doi: 10.1146/annurev.iy.07.040189.003051. [DOI] [PubMed] [Google Scholar]
- 57.Lundholm M, Mayans S, Motta V, Lofgren-Burstrom A, Danska J, Holmberg D. Variation in the Cd3 zeta (Cd247) gene correlates with altered T cell activation and is associated with autoimmune diabetes. J Immunol. 2010;184:5537–5544. doi: 10.4049/jimmunol.0904012. [DOI] [PubMed] [Google Scholar]
- 58.Radulovic K, Rossini V, Manta C, Holzmann K, Kestler HA, Niess JH. The early activation marker CD69 regulates the expression of chemokines and CD4 T cell accumulation in intestine. PLoS ONE. 2013;8:e65413. doi: 10.1371/journal.pone.0065413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rousseau MC, Boulay ME, Goronfolah L, Denburg J, Keith P, Boulet LP. Comparative responses to nasal allergen challenge in allergic rhinitic subjects with or without asthma. Allergy, Asthma Clin Immunol. 2011;7:8. doi: 10.1186/1710-1492-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meiler F, Zumkehr J, Klunker S, Ruckert B, Akdis CA, Akdis M. In vivo switch to IL-10-secreting T regulatory cells in high dose allergen exposure. J Exp Med. 2008;205:2887–2898. doi: 10.1084/jem.20080193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hosoya K, Satoh T, Yamamoto Y, Saeki K, Igawa K, Okano M, Moriya T, Imamura O, Nemoto Y, Yokozeki H. Gene silencing of STAT6 with siRNA ameliorates contact hypersensitivity and allergic rhinitis. Allergy. 2011;66:124–131. doi: 10.1111/j.1398-9995.2010.02440.x. [DOI] [PubMed] [Google Scholar]
- 62.Suzuki M, Zheng X, Zhang X, Ichim TE, Beduhn ME, Min W. Oligonucleotide based-strategies for allergy with special reference to siRNA. Exp Opin Biol Ther. 2009;9:441–450. doi: 10.1517/14712590902841924. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.