Abstract
HER2 amplification/overexpression (HER2+) frequently co-occurs with PI3K pathway activation in breast tumors. PI3K signaling is most often activated by PIK3CA mutation or PTEN loss, which frequently results in sensitivity to p110α or p110β inhibitors, respectively. To examine the p110 isoform dependence in HER2+, PTEN-deficient tumors, we generated genetic mouse models of breast tumors driven by concurrent Her2-activation and Pten-loss coupled with deletion of p110α or p110β. Ablation of p110α, but not p110β, significantly impaired the development of Her2+/Pten-null tumors in mice. We further show that p110α primarily mediates oncogenic signaling in HER2+/PTEN-deficient human cancers while p110β conditionally mediates PI3K/AKT signaling only upon HER2 inhibition. Combined HER2 and p110α inhibition effectively reduced PI3K/AKT signaling and growth of cancer cells both in vitro and in vivo. Addition of the p110β inhibitor to dual HER2 and p110α inhibition induced tumor regression in a xenograft model of HER2+/PTEN-deficient human cancers. Together, our data suggest that combined inhibition of HER2 and p110α/β may serve as a potent and durable therapeutic regimen for the treatment of HER2+, PTEN-deficient breast tumors.
Keywords: PI3K, HER2, PTEN, genetic mouse models, breast cancer
INTRODUCTION
Human epidermal growth factor receptor-2 (HER2), also referred to as ERBB2 or Neu, is constitutively activated by overexpression or gene amplification in 15–20% of breast tumors (1). Multiple HER2-targeted therapies are available for patients with metastatic HER2-positive (HER2+) cancer, including the HER2-specific monoclonal antibodies trastuzumab and pertuzumab and the EGFR/HER2 small molecule inhibitor lapatinib. Although these therapies delay tumor progression and can prolong overall survival, resistance commonly occurs in metastatic HER2+ breast cancer treated with these agents (2, 3). These data suggest that recurring tumors may intrinsically possess or acquire escape mechanisms from HER2 inhibition (2).
Activation of PI3K signaling downstream from HER2 is one such mechanism that may explain the pro-growth and pro-survival signaling that is necessary to overcome HER2 inhibition in tumors. Class 1A PI3Ks are heterodimeric lipid kinases composed of a catalytic p110 subunit and a regulatory p85 subunit. Three homologous p110 isoforms (p110α, p110β, p110δ) are encoded by PIK3CA, PIK3CB and PIK3CD, respectively. Active PI3Ks catalyze the phosphorylation of PIP2 to form the second messenger PIP3, which activates AKT and other effectors. PI3K function is antagonized by the lipid phosphatase and tumor suppressor PTEN, converting PIP3 back into PIP2. Recent studies have demonstrated that p110α and p110β maintain distinct functions in signaling and cellular transformation (4). While p110α is the major effector downstream of receptor tyrosine kinases (RTKs) (5–7), p110β is obligatory for signaling via GPCRs (8, 9).
PI3K signaling hyperactivation occurs via somatic PIK3CA point mutations or PTEN loss in more than 50% of breast cancers (10, 11). While these events are almost always mutually exclusive, one or the other is present in a substantial fraction of HER2+ tumors (11, 12). Expression of mutant PIK3CA or loss of PTEN in breast cancer cell lines is associated with resistance to HER2-targeted therapies and this resistance can be reversed with PI3K inhibition (13–15). Moreover, PI3K activation is associated with a poor clinical outcome in patients treated with trastuzumab or lapatinib (14, 16).
Currently, the majority of PI3K inhibitors in clinical testing target class IA PI3K isoforms. Pan-PI3K inhibition may result in excess toxicity as all PI3K isoforms are inhibited. Indeed, isoform-selective inhibitors are now emerging in the clinic. Early clinical studies have achieved remarkable results with a p110δ-selective inhibitor in treating certain B cell malignancies (17). In breast cancer, p110α-selective inhibitors have shown promise in early phase trials for patients with tumors bearing PIK3CA mutations (18).
We and others have demonstrated that p110α is the primary PI3K isoform responsible for oncogenic HER2 signaling and tumorigenesis in the mammary epithelium (7), whereas p110β is important for some PTEN-deficient tumors, including prostate and breast tumors (9, 19). It is critical to evaluate PI3K isoform dependency in HER2+ breast cancers with concurrent PTEN-loss, and to assess the efficacy of isoform-selective inhibitors in combination with HER2-targeted therapy.
Here we generated a genetic mouse model of breast tumorigenesis driven by Her2/Neu activation and Pten loss coupled with p110α or p110β deletion. Breast tumorigenesis was dependent on p110α, and this finding was recapitulated with isoform-specific inhibitors. Combined treatment with a HER2 inhibitor and a p110α-selective inhibitor inhibited proliferation of human HER2+/PTEN null breast cancer cell lines in vitro and in vivo. P110β inhibition in combination with dual HER2 and p110α inhibition induced tumor regression in xenograft models of HER2+/PTEN-deficient human cancers. These results suggest that combining HER2 inhibitors with both p110α- and p110β-selective inhibitors may be an effective therapy for HER2+, PTEN-null breast tumors.
RESULTS AND DISCUSSION
Mammary tumors driven by concurrent Her2+ and Pten deletion are dependent on p110α
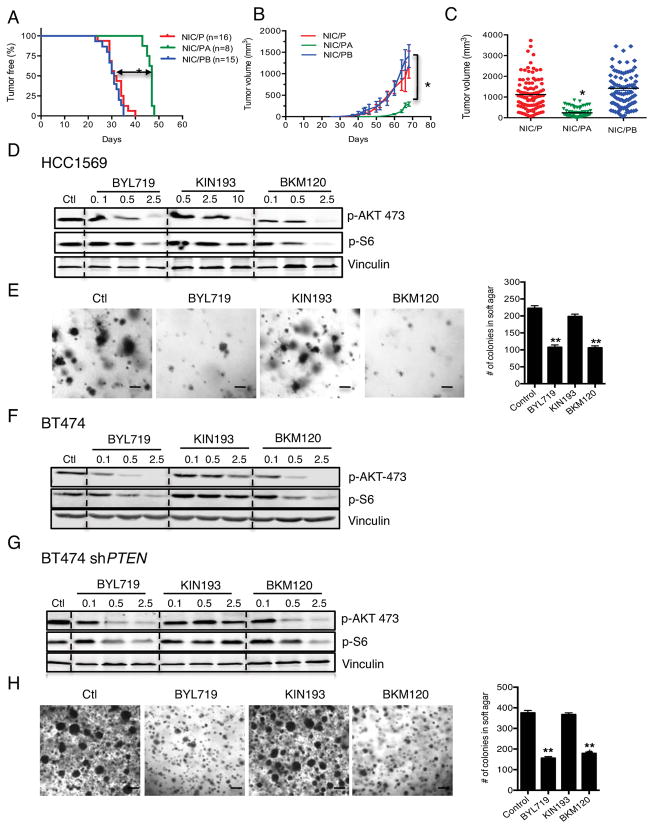
To generate a mouse model of breast tumorigenesis with concomitant Her2+ and Pten-loss, floxed Pten (PtenL/L) mice were crossed with mice carrying MMTV-Neu-IRES-Cre (NIC) where the expression of Her2 and Cre recombinase is driven by the same promoter within mammary epithelial cells (20). MMTV-NIC/PtenL/L (NIC/P) virgin female mice develop mammary tumors indistinguishable from human HER2+, PTEN-deficient primary breast cancer. To determine which PI3K isoform is required for tumorigenesis, we crossed the MMTV-NIC/PtenL/L mice with either p110αL/L(5) or p110βL/L (9) mice to generate mice with MMTV-NIC/PtenL/L;p110αL/L (NIC/PA) or MMTV-NIC/PtenL/L;p110βL/L (NIC/PB) genotypes. NIC/P virgin female mice developed multifocal mammary tumors with a median latency of 31 days (Figure 1A). Notably, p110α ablation significantly delayed tumor onset (T50=46 days) (Figure 1A), whereas p110β loss had no effect (T50=30.5 days) (Figure 1A). NIC/P and NIC/PB tumors grew at similar rates, whereas NIC/PA tumors remained significantly smaller even at later time points (Figure 1B). Tumor volume analyses revealed a significantly lower tumor burden at the end point (66d) in NIC/PA mice (Figure 1C). These results suggest that only p110α is required for breast tumorigenesis in mouse models characterized by Her2+ and Pten loss.
Figure 1. p110α ablation or inhibition inhibits mammary tumorigenesis and cell growth in NIC/Pten −/− mice and HER2-positive, PTEN-deficient human breast cancer cells.
MMTV-NIC (26), PtenL/L (27), p110αL/L (5), and p110βL/L (9) were crossed to obtain MMTV-NIC/Pten−/− (NIC/P), MMTV-NIC/Pten−/−/p110−/− (NIC/PA), and MMTV-NIC/Pten−/−/p110β−/− (NIC/PB). (A) Kaplan-Meier (KM) curves in NIC/P (red), NIC/PA (green, and NIC/PB (blue). *p<0.001, Log-rank test. (B) Average tumor volume in mice of the indicated genotypes over time. *p<0.01. Two-way ANOVA. (C) Average volume of end-stage tumors in mice of the indicated genotypes. Data are shown as mean ± S.E.M (n=6 per genotype). *p<0.01, Student’s t test. (D) Immunoblot analyses in HCC1569 cells treated with BYL719 (Novartis), KIN193 (MedChemexpress) or BKM120 (Novartis) (μM). (E) Anchorage-independent growth of 5×104 HCC1569 cells treated with BYL719, KIN193 or BKM120 (1μM). (F, G) Immunoblot analyses in BT474 and BT474-shPTEN cells treated as indicated in (D). (H) Anchorage-independent growth of BT474-shPTEN cells treated as described in (E). For (E) and (H), Mean ± S.E.M from three independent experiments are shown. **p<0.001, Student’s t test.
p110α mediates PI3K/AKT signaling and anchorage-independent growth of human HER2+, PTEN-deficient breast cancer cells
We investigated whether human breast cancer cell lines harboring HER2+ and PTEN loss are also dependent on p110α by using HCC1569 and BT474 cell culture models. The HCC1569 breast cancer cell line harbors HER2+ and PTEN loss. We evaluated PI3K signaling in HCC1569 cells after p110α- or p110β-selective inhibition (BYL719 and KIN193, respectively). BYL719 or pan-PI3K inhibition via BKM120, but not KIN193, reduced PI3K effector phosphorylation (Figure 1D). Moreover, inhibition of p110α, but not p110β, was sufficient to reduce the number of colonies formed in soft agar (Figure 1E).
To determine whether PTEN loss affects the p110α dependency of HER2+ breast cancer cells, we stably depleted PTEN via shRNA in the BT474 HER2+ breast cancer cell line that harbors intact PTEN and is sensitive to HER2 inhibition. We confirmed that PTEN protein expression is suppressed in stable pLKO-shPTEN expressing BT474 cells, and increases anchorage independent growth (Figure S1A). In vivo xenografts and in vitro dose response curves demonstrated that loss of PTEN expression in BT474 decreases tumor-free survival and confers resistance to lapatinib (Figure S1B, 1C).
To determine if p110α is the predominant isoform that mediates HER2 signaling, we treated BT474-shPTEN cells with BYL719, KIN193, or BKM120. Indeed, p110α- and pan-PI3K inhibition decreased PI3K effector phosphorylation in both BT474 and BT474-shPTEN cells (Figure 1F, 1G). Moreover, BYL719 and BKM120, but not KIN193, effectively blocked anchorage-independent growth of BT474-shPTEN cells in soft agar (Figure 1H), demonstrating that p110α is the major isoform that mediates oncogenic HER2 signaling and growth of HER2+ breast tumor cells in the absence of PTEN.
Inhibition of p110a enhances sensitivity of HER2+, PTEN-deficient breast cancer cells to HER2-targeted therapy
HER2-targeted agents, such as trastuzumab and lapatinib, are commonly used in patients with HER2+ metastatic breast cancer. Because clinical data suggests PTEN-deficiency renders HER2+ breast tumors resistant to HER2-targeted therapy, we confirmed that HCC1569 and BT474-shPTEN cell lines are more resistant to lapatinib in comparison to PTEN wild-type, HER2+ breast cancer cell lines BT474 and SKBR3 (Figure 2, S1C). We then investigated whether inhibition of a single PI3K isoform can restore the sensitivity of these tumor cells to HER2 blockade. We assessed HER2/PI3K signaling in response to lapatinib, BYL719, or KIN193 alone or in combination in HCC1569 and BT474-shPTEN cell lines. While lapatinib reduced HER2 phosphorylation, it did not suppress PI3K/AKT signaling (Figure 2A). Combined BYL719 and lapatinib inhibited HER2 activation, and inhibited PI3K effector phosphorylation (Figure 2A). Combined HER2 and p110β inhibition did not reduce PI3K/AKT signal transduction as effectively as dual HER2 and p110α inhibition (Figure 2A).
Figure 2. The p110α inhibitor BYL719 sensitizes HER2+, PTEN-deficient cancer cells to lapatinib.
(A) Immunoblot analyses in HCC1569 or BT474-shPTEN cells treated with BYL719 or KIN193 alone or in combination with lapatinib (MedChemexpress) (μM). (B) Cell viability of HCC1569 (Left) and BT474-shPTEN (Right) cells treated with lapatinib (1μM and 0.5 μM, respectively), 0.5μM BYL719 or 0.5μM KIN193 alone or in combination for 72h. Cell viability was measured by CellTiter 96® Assay. Mean ± S.E.M from three independent experiments. *p<0.01, **p<0.001, Student’s t test.
To further investigate whether p110α inhibition can overcome lapatinib resistance to block the proliferation of HER2+, PTEN-deficient breast cancer cells, we treated HCC1569 and BT474-shPTEN cells with lapatinib, BYL719, or KIN193 alone or in combination and measured cell viability. Combined BYL719 and lapatinib treatment more effectively inhibited cell viability compared to combined treatment with KIN193 with lapatinib (Figure 2B). Moreover, HER2 inhibition coupled with combined p110α and p110β inhibition maximally reduced cell viability (Figure S3). Taken together, these data suggest that p110α is a major target that can overcome lapatinib resistance by suppressing HER2/PI3K/AKT signaling in HER2+, PTEN deficient cells lines.
Combined inhibition of p110α/β and lapatinib blocks the growth of HER2+, PTEN-deficient breast tumors in vivo
To test whether p110α inhibition can overcome lapatinib resistance in PTEN-deficient HER2+ cells in vivo, NCI nude mice bearing palpable BT474-shPTEN or HCC1569 xenografts were treated with lapatinib alone or in combination with BYL719 or KIN193. Lapatinib alone failed to inhibit tumor growth. Addition of KIN193 provided modest improvement over lapatinib monotherapy while addition of BYL719 significantly impaired tumor growth (Figure 3A, 3B). Moreover, durable tumor regression was achieved in the HCC1569 xenograft tumor model following combined HER2/p110α/p110β inhibition (Figure 3B). Together, these data expand upon previous studies demonstrating p110β lipid kinase activity increases following p110α inhibition in breast cancers characterized by PIK3CA activation or PTEN-inactivated breast cancer (21, 22). Here we suggest that while p110α is a primary target to overcome resistance to HER2-therapy in the context of HER2+ and PTEN-deficient breast cancers, combined HER2, p110α and p110β inhibition offers maximal PI3K inhibition and durable tumor regression.
Figure 3. Treatment with a p110α selective inhibitor reverses lapatinib resistance in orthotopic xenograft models.
Mice bearing BT474-shPTEN (A) or HCC1569 xenografts (B) were treated with inhibitor combinations: Lapatinib (100mg/kg) was administered daily by oral gavage in 0.5% hydroxypropyl methylcellulose, 0.1% Tween80. BYL719 (45mg/kg) in 10% 2-hyroxypropyl-β-cyclodextrin (Sigma) was administered daily by (IP) injection. KIN193 (20mg/kg) in 7.5% NMP, 40% PEG400 was dosed twice a day by IP injection. BKM120 was reconstituted 1:9 in NMP and PEG300. Mice were dosed with this compound formulation at 45mg/kg daily by oral gavage (n=6 per treatment group). Tumor volume was measured every 3 days. *p<0.01, **p<0.001 and ***p<0.0001, Two-way ANOVA (C) Immunohistochemical analyses of FFPE HCC1569 xenograft sections treated with lapatinib and/or BYL719, KIN193 for 72h. TUNEL assays were performed with the In Situ Cell Death Detection Kit (Roche). Representative images are shown, Scale=20μm (Left). Quantitative analyses of 6 images randomly obtained from 3 mice (Right). *p< 0.01, **p<0.001, Student’s t test.
To evaluate signaling and pharmacodynamic responses of HCC1569 and BT474-shPTEN xenografts during inhibitor treatment, tumors were isolated 72h post drug administration and molecular markers analyzed. Dual HER2 and p110α inhibition decreased PI3K effector phosphorylation, blocked proliferation and induced apoptosis (Figure 3C, S4). Concomitant treatment with lapatinib, BYL719, and KIN193 significantly eliminated PI3K effector phosphorylation, blocked proliferation and induced apoptosis in HCC1569 xenografts (Figure 3C). These results establish that p110α primarily mediates PI3K/AKT signaling in HER2+ PTEN deficient breast tumors whereas p110β conditionally mediates HER2/PI3K signaling in the absence of p110α.
Here we utilized a NIC-Pten−/− mouse model of Her2 overexpressing, Pten-null breast cancer and showed that p110α ablation, rather than p110β loss, delayed the onset of tumor development. Pharmacological studies in which p110 isoform-selective inhibitors were used to treat human HER2+, PTEN-deficient breast cancer cells confirmed that specific targeting of p110α was sufficient to inhibit AKT activity and tumor growth. This study suggests that the p110α isoform of PI3K is essential for relaying RTK-mediated signal transduction in the context of HER2+, PTEN-deficient breast cancers, and p110β can conditionally mediate PI3K/AKT signaling in this model. Our results suggest that patients with HER2+, PTEN-deficient breast cancers may durably respond to combined HER2 and p110α/β inhibition.
Our data support a model in which HER2 remains the oncogenic driver in the context of HER2+, PTEN-null breast cancers, as is shown in genetic experiments where HER2+, Pten-null mice depend on p110α for tumor development (Figure 1). This model demonstrates that HER2 amplification is a dominant oncogenic event over Pten loss, and Pten null tumors that may otherwise be dependent on p110β become dependent on p110α in the presence of a potent tumorigenic event such as HER2 amplification, which has previously been shown to bias tumors to p110α dependence. It is, however, also possible that the elevated p110a expression observed in some breast tumors may contribute to enhanced and dominant HER2-associated signal transduction. We previously used mouse models in which mammary tumorigenesis driven by HER2 activation was complexed with ablation of p110α and/or p110β expression, to demonstrate that p110α is the primary PI3K isoform responsible for AKT signaling and tumorigenesis in HER2+ mammary tumors (7). We observed similar effects in the ovary, where Kras activation biased ovarian cancer cell lines to p110α dependence alone or in combination with Pten loss (23). In contrast, other previous studies suggested that signaling through p110β is necessary in certain tumors driven by PTEN loss (9, 19). Thus, the data presented here fits into the context of previously published studies suggesting that tumors preferentially depend on either p110α or p110β, and that this dependence is based upon the identity of oncogenic driver(s) within the tumor. Our results establish that tumors characterized by concurrent PTEN-loss and HER2-overexpression rely primarily on the p110α isoform.
Our results provide concrete evidence that targeting p110α is necessary to overcome the resistance of HER2+ PTEN-deficient breast tumors to HER2-directed therapies. In addition our data show that p110β inhibition decreases PI3K/AKT signaling and tumor growth when combined with lapatinib, strongly supporting that PI3K isoform dependence of PTEN-deficient tumors can be altered by RTK activation. Combined Her2, p110α and p110β inhibition maximally abrogated AKT activation and minimized tumor growth. Of note, pan-PI3K inhibitors have not yielded durable or efficacious clinical results, but isoform-selective inhibitors that are currently emerging in the clinic may offer considerable promise of both increased efficacy and reduced toxicity in comparison to pan-PI3K inhibitors which target all class I PI3K isoforms (24, 25). Together, our work suggests that in the context of HER2+, PTEN-deficient breast cancers, a combination therapy targeting HER2 and p110α/β should be considered for clinical application.
Supplementary Material
Acknowledgments
We thank Dr. Roderick Bronson and the Dana-Farber/Harvard Cancer Center Rodent Histopathology Core for histopathological analyses. MMTV-NIC mice were provided by W. Muller (McGill University). Floxed Pten mice were provided by H. Wu (UCLA). This work was supported by start up fund from Department of Pathology and Laboratory Medicine, Medical University of South Carolina (QW), the Susan G. Komen Breast Cancer Foundation CCR 12225834 (IEK), the Breast Cancer Research Foundation (NUL, EPW, JJZ), NIH grants CA187918 (TMR and JJZ), CA172461-01 (JJZ), and 1P50CA168504 (TMR, IEK, EPW, NUL, and JJZ).
Footnotes
CONFLICT OF INTEREST
TMR is a consultant of Novartis and has received a research grant from Novartis. EPW as received research grants from Genentech and Roche. IEK is a consultant of Amgen and has received research funding from Genentech. NUL has received research grants from Genentech, Array Biopharma, GlaxoSmithKline, Kadmon, and Novartis. The remaining authors declare no competing financial interests.
References
- 1.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5(5):341–54. doi: 10.1038/nrc1609. Epub 2005/05/03. [DOI] [PubMed] [Google Scholar]
- 2.Blackwell KL, Burstein HJ, Storniolo AM, Rugo H, Sledge G, Koehler M, et al. Randomized study of Lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol. 2010;28(7):1124–30. doi: 10.1200/JCO.2008.21.4437. Epub 2010/02/04. [DOI] [PubMed] [Google Scholar]
- 3.Hudis CA. Trastuzumab--mechanism of action and use in clinical practice. The New England journal of medicine. 2007;357(1):39–51. doi: 10.1056/NEJMra043186. Epub 2007/07/06. [DOI] [PubMed] [Google Scholar]
- 4.Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol. 2010;11(5):329–41. doi: 10.1038/nrm2882. Epub 2010/04/10. [DOI] [PubMed] [Google Scholar]
- 5.Zhao JJ, Cheng H, Jia S, Wang L, Gjoerup OV, Mikami A, et al. The p110alpha isoform of PI3K is essential for proper growth factor signaling and oncogenic transformation. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(44):16296–300. doi: 10.1073/pnas.0607899103. Epub 2006/10/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graupera M, Guillermet-Guibert J, Foukas LC, Phng LK, Cain RJ, Salpekar A, et al. Angiogenesis selectively requires the p110alpha isoform of PI3K to control endothelial cell migration. Nature. 2008;453(7195):662–6. doi: 10.1038/nature06892. Epub 2008/05/02. [DOI] [PubMed] [Google Scholar]
- 7.Utermark T, Rao T, Cheng H, Wang Q, Lee SH, Wang ZC, et al. The p110alpha and p110beta isoforms of PI3K play divergent roles in mammary gland development and tumorigenesis. Genes Dev. 2012;26(14):1573–86. doi: 10.1101/gad.191973.112. Epub 2012/07/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guillermet-Guibert J, Bjorklof K, Salpekar A, Gonella C, Ramadani F, Bilancio A, et al. The p110beta isoform of phosphoinositide 3-kinase signals downstream of G protein-coupled receptors and is functionally redundant with p110gamma. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(24):8292–7. doi: 10.1073/pnas.0707761105. Epub 2008/06/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jia S, Liu Z, Zhang S, Liu P, Zhang L, Lee SH, et al. Essential roles of PI(3)K-p110beta in cell growth, metabolism and tumorigenesis. Nature. 2008;454(7205):776–9. doi: 10.1038/nature07091. Epub 2008/07/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuan TL, Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene. 2008;27(41):5497–510. doi: 10.1038/onc.2008.245. Epub 2008/09/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stemke-Hale K, Gonzalez-Angulo AM, Lluch A, Neve RM, Kuo WL, Davies M, et al. An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res. 2008;68(15):6084–91. doi: 10.1158/0008-5472.CAN-07-6854. Epub 2008/08/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saal LH, Holm K, Maurer M, Memeo L, Su T, Wang X, et al. PIK3CA mutations correlate with hormone receptors, node metastasis, and ERBB2, and are mutually exclusive with PTEN loss in human breast carcinoma. Cancer Res. 2005;65(7):2554–9. doi: 10.1158/0008-5472-CAN-04-3913. Epub 2005/04/05. [DOI] [PubMed] [Google Scholar]
- 13.Hanker AB, Pfefferle AD, Balko JM, Kuba MG, Young CD, Sanchez V, et al. Mutant PIK3CA accelerates HER2-driven transgenic mammary tumors and induces resistance to combinations of anti-HER2 therapies. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(35):14372–7. doi: 10.1073/pnas.1303204110. Epub 2013/08/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagata Y, Lan KH, Zhou X, Tan M, Esteva FJ, Sahin AA, et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer cell. 2004;6(2):117–27. doi: 10.1016/j.ccr.2004.06.022. Epub 2004/08/25. [DOI] [PubMed] [Google Scholar]
- 15.Eichhorn PJ, Gili M, Scaltriti M, Serra V, Guzman M, Nijkamp W, et al. Phosphatidylinositol 3-kinase hyperactivation results in lapatinib resistance that is reversed by the mTOR/phosphatidylinositol 3-kinase inhibitor NVP-BEZ235. Cancer Res. 2008;68(22):9221–30. doi: 10.1158/0008-5472.CAN-08-1740. Epub 2008/11/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Esteva FJ, Guo H, Zhang S, Santa-Maria C, Stone S, Lanchbury JS, et al. PTEN, PIK3CA, p-AKT, and p-p70S6K status: association with trastuzumab response and survival in patients with HER2-positive metastatic breast cancer. Am J Pathol. 2010;177(4):1647–56. doi: 10.2353/ajpath.2010.090885. Epub 2010/09/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vanhaesebroeck B, Khwaja A. PI3Kdelta inhibition hits a sensitive spot in B cell malignancies. Cancer cell. 2014;25(3):269–71. doi: 10.1016/j.ccr.2014.02.012. Epub 2014/03/22. [DOI] [PubMed] [Google Scholar]
- 18.Fruman DA, Rommel C. PI3K and cancer: lessons, challenges and opportunities. Nat Rev Drug Discov. 2014;13(2):140–56. doi: 10.1038/nrd4204. Epub 2014/02/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wee S, Wiederschain D, Maira SM, Loo A, Miller C, deBeaumont R, et al. PTEN-deficient cancers depend on PIK3CB. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(35):13057–62. doi: 10.1073/pnas.0802655105. Epub 2008/08/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schade B, Rao T, Dourdin N, Lesurf R, Hallett M, Cardiff RD, et al. PTEN deficiency in a luminal ErbB-2 mouse model results in dramatic acceleration of mammary tumorigenesis and metastasis. J Biol Chem. 2009;284(28):19018–26. doi: 10.1074/jbc.M109.018937. Epub 2009/05/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Costa C, Ebi H, Martini M, Beausoleil SA, Faber AC, Jakubik CT, et al. Measurement of PIP3 levels reveals an unexpected role for p110beta in early adaptive responses to p110alpha-specific inhibitors in luminal breast cancer. Cancer cell. 2015;27(1):97–108. doi: 10.1016/j.ccell.2014.11.007. Epub 2014/12/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwartz S, Wongvipat J, Trigwell CB, Hancox U, Carver BS, Rodrik-Outmezguine V, et al. Feedback suppression of PI3Kalpha signaling in PTEN-mutated tumors is relieved by selective inhibition of PI3Kbeta. Cancer cell. 2015;27(1):109–22. doi: 10.1016/j.ccell.2014.11.008. Epub 2014/12/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmit F, Utermark T, Zhang S, Wang Q, Von T, Roberts TM, et al. PI3K isoform dependence of PTEN-deficient tumors can be altered by the genetic context. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(17):6395–400. doi: 10.1073/pnas.1323004111. Epub 2014/04/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Juric D, Krop I, Ramanathan R, Xiao J, Sanabria S, Wilson T, et al. Lbstract LB_64: GDC-0032, a beta isoform-sparing PI3K inhibitor: Results of a first-in-human phase 1a dose escalation study. Cancer Research. 2013;73(LB-64) [Google Scholar]
- 25.Juric D, Rodon J, Gonzalez-Angulo A, Burris H, Bendell J, Berlin J, et al. Abstract CT-01: BYL719, a next generation PI3K alpha specific inhibitor: Preliminary safety, PK, and efficacy results from the first-in-human study. Cancer Research. 2012;72(CT-01) [Google Scholar]
- 26.Ursini-Siegel J, Hardy WR, Zuo D, Lam SH, Sanguin-Gendreau V, Cardiff RD, et al. ShcA signalling is essential for tumour progression in mouse models of human breast cancer. The EMBO journal. 2008;27(6):910–20. doi: 10.1038/emboj.2008.22. Epub 2008/02/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lesche R, Groszer M, Gao J, Wang Y, Messing A, Sun H, et al. Cre/loxP-mediated inactivation of the murine Pten tumor suppressor gene. Genesis. 2002;32(2):148–9. doi: 10.1002/gene.10036. Epub 2002/02/22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.