Abstract
Preeclampsia is a hypertensive disease that complicates many pregnancies, typically presenting with new-onset or worsening hypertension and proteinuria. It is well recognized that the placental syncytium plays a key role in the pathogenesis of preeclampsia. This review summarizes the findings pertaining to the structural alterations in the syncytium of preeclamptic placentas and analyzes their pathological implications for the development of preeclampsia. Changes in the trophoblastic lineage, including those in the proliferation of cytotrophoblasts, the formation of syncytiotrophoblast through cell fusion, cell apoptosis and syncytial deportation, are discussed in the context of preeclampsia. Extensive correlations are made between functional deficiencies and the alterations on the levels of gross anatomy, tissue histology, cellular events, ultrastructure, molecular pathways, and gene expression. Attention is given to the significance of dynamic changes in the syncytial turnover in preeclamptic placentas. Specifically, experimental evidences for the complex and obligatory role of syncytin-1 in cell fusion, cell-cycle regulation at the G1/S transition, and apoptosis through AIF-mediated pathway, are discussed in detail in the context of syncytium homeostasis. Finally, the recent observations on the aberrant fibrin deposition in the trophoblastic layer and the trophoblast immature phenotype in preeclamptic placentas and their potential pathogenic impact are also reviewed.
Keywords: Pregnancy hypertension, HERV-W, Syncytium, Preeclampsia, Nonfusogenic
Introduction
Affecting approximately 5 % of pregnant women, preeclampsia is the most common disease that complicates pregnancy. Women with preeclampsia typically present with new-onset hypertension plus new-onset proteinuria. Additional organ deficiency and damage are often observed as well. Potential complications include thrombocytopenia, impaired liver function with elevated liver transaminases, pulmonary edema, new-onset renal insufficiency, cerebral complications, and visual disturbances [1]. Currently, the treatment of preeclampsia is limited to the control of hypertension, and early termination of pregnancy remains the only definitive treatment. However, prematurity and low birth weight due to preterm delivery not only increase perinatal death and morbidity but have also been linked to long-term risk for diabetes, and cardiovascular and renal diseases [2, 3]. Investigators have raised additional concerns over the long-term socioeconomic consequences caused by preterm birth [4].
While some common risk factors, such as multifetal gestation, advanced maternal age, obesity, history of hypertension, diabetes, or kidney disorders have been known for decades, the pathogenic mechanisms of preeclampsia remain poorly understood. It is generally thought that the occurrence of preeclampsia involves multiple processes, including impaired implantation, systemic inflammation, endothelial dysfunction, and tissue damage caused by repeated ischemia–reperfusion [5]. Presumably, each of these processes may be related to genetic predisposition and/or environmental factors. However, such generalization provides little help for the development of effective therapeutic approaches, reagents, or modalities. More specific details on the cellular/molecular mechanisms of preeclampsia are required for improved treatment of preeclampsia patients.
As discussed in a point-by-point manner later, an increasing body of evidence indicates that the placenta may play a critical role in the pathogenesis of preeclampsia. The systemic changes listed above can be largely explained by placental dysfunction that often presents as placental endocrine deficiency, shallow placental invasion, and abnormal vasculature [6]. Placental trophoblasts represent a highly specialized cell lineage directly involved in embryo implantation and fetal interactions with the decidualized maternal uterus. This is the first group of cells to differentiate from the fertilized egg and to form the outer layer of the blastocyst 6 days after fertilization. The trophoblasts proliferate and differentiate into multinucleated syncytiotrophoblasts, or syncytium, through a cell fusion process. Syncytium represents a major histological and functional component of the placenta. Syncytium forms the maternal–placental interface [7] and produces large amounts of steroid and peptide hormones. Syncytium also serves as a physical barrier that protects the fetus from exposure to harmful materials from the maternal side. At the same time, active fetal–maternal exchanges of nutrients, metabolites, wastes, and gases are constantly carried out through syncytium by diffusion or transportation.
The integrity and normal function of the placental barrier depends on a precise control and coordination of cytotrophoblast proliferation, cell–cell fusion, and syncytiotrophoblast deportation into maternal circulation [8]. Indeed, numerous in vitro and in vivo studies have demonstrated a close, if not a causal, relationship between structural/functional deficiency of syncytium and the development of preeclampsia. While it remains debatable as to what extent the placental trophoblast anomalies may contribute to the etiology of preeclampsia, it is safe to say that such anomalies are important pathological events during the progression of preeclampsia. This review focuses on the characteristic structural changes observed in syncytium under preeclamptic conditions and their implications for the development of preeclampsia. Alterations of trophoblast proliferation, cell fusion, cell death, as well as their effects on the placental cell dynamics and syncytium homeostasis are also discussed.
Trophoblast proliferation, apoptosis, and turnover in preeclampsia
Proliferation and differentiation
One hallmark of preeclampsia is the abnormal differentiation of trophoblasts, producing a defective maternal–fetal interface [9, 10]. Abnormal development of placental villi, specifically, reduced proliferation of villous and extravillous cytotrophoblasts, may lead to shallow trophoblast invasion that is associated with insufficient placental implantation [11]. Trophoblasts produce vascular endothelial growth factor and other angiogenic factors, which promote the placental vasculature through paracrine and autocrine mechanisms [12]. Reduced VEGF and increased sFlt1 production have a negative impact on the placental vascular development [13, 14]. The deficiency in placental vasculature limits the uterine blood flow and affects fetal development [11, 15]. This concept was confirmed when biopsies taken from term preeclamptic placentas showed evidence of incomplete trophoblast invasion in the uterus [9]. As an animal model, the BPH/5 mice exhibit borderline hypertension independent of pregnancy. Interestingly, these mice develop severe hypertension, proteinuria, and endothelial dysfunction during late gestation. Using cdkn1c (cyclin-dependent kinase inhibitor 1C, p57, Kip2) as a marker for trophoblast-mediated interstitial invasion, Dokras et al. observed that in BPH/5 mice fewer cdkn1c-stained trophoblasts were seen invading the proximal decidual zone. This finding was accompanied by a reduction in cdkn1c gene expression. While trophoblast giant cell morphology was not altered, the mRNA levels of several giant cell-specific markers were significantly reduced, indicating the presence of functional abnormalities in the trophoblast lineage. However, because of species diversity, mouse trophoblast giant cells are not considered the direct counterparts of syncytiotrophoblasts even though they are of trophoblastic origin and produce hormones like syncytiotrophoblasts. For this reason, extrapolation of the findings from the mouse model to human preeclampsia is difficult.
Newhouse et al. compared the outcomes of trophoblast differentiation in both preeclamptic and intrauterine growth restriction (IUGR)-affected placentas and found that syncytialization in preeclampsia and IUGR differs significantly from normal pregnancies, as well as from each other. Importantly, pregnancies complicated by both preeclampsia and IUGR exhibited the lowest levels of trophoblast proliferation. These observations revealed a close relationship between syncytial structure and maternal systemic disorders. As discussed in more detail below, the reduced levels of syncytin-1, which have been shown to be a key cell-cycle regulator [16], could partially explain the decreased trophoblast proliferation in preeclamptic placentas.
It is noteworthy that in a sharp contrast to the above observations of reduced trophoblast proliferation in the preeclamptic placentas, increased trophoblast proliferation in preeclamptic placentas has been reported by some investigators. For example, although the overall volume of trophoblasts and the exchange surface area are not significantly altered in preeclampsia [17], a higher rate of proliferation in villous trophoblasts was observed by Redline et al. [18]. This study examined the implantation site of normal and preeclamptic placentas from various gestational ages ranging from 25 to 40 weeks using various cell proliferation and phenotypic markers. An excessive number of trophoblasts with a high proliferative index, but low phenotypic maturity, were detected in the preeclamptic placenta. The authors proposed that an atypical implantation site with an increased number of immature trophoblasts could represent a major characteristic of placentas from preeclamptic pregnancies, in addition to acute atherosis, villous infarction, and increased syncytial knotting.
The discrepancy in the trophoblast proliferation rate in preeclamptic placentas may be due to differences in the stage or severity of the preeclampsia cases studied by different groups. It is not unusual that along the progression of many human diseases, in their early stage or mild cases, feedback mechanisms are often activated to compensate for the loss of function. In the early stage of preeclampsia, mild hypoxia or oxidative stress may lead to activation of trophoblast proliferation to cope with the transient syncytial deficiency. However, this compensatory capacity is limited, and in late stages or severe cases this mechanism might be overwhelmed, e.g., by severe hypoxia or repeated ROS (reactive oxygen species) attacks, resulting in the disruption of syncytium. Interestingly, the discrepancy in trophoblast proliferation is reminiscent of another seemingly controversial finding on syncytin-1 expression. As discussed in more detail later, a decrease in the expression of syncytin-1 mRNA and protein in preeclamptic placentas has been repeatedly observed by many groups applying various techniques [19–21]. In order to investigate the role of syncytin-1 in placental and fetal development without the interference of genetic variations, Gao et al. took advantage of the genetic homogeneity among the monozygotic dichorionic twins with birth weight discordance (more than 20 % difference) [22]. To our surprise, results of quantitative PCR and Western-blot analysis in 24 pairs of placentas showed a significantly higher syncytin-1 mRNA and protein expression in the placentas associated with smaller fetus compared to placentas from larger fetus or singleton normal pregnancy. Moreover, the syncytin-1 expression levels were inversely correlated with the birth weight of fetuses when data from all the three groups (twin large, twin small, and singleton normal) were analyzed together. Furthermore, increased cell proliferation, as indicated by the higher expression levels of PCNA, was detected in placentas of the smaller fetuses. We hypothesized that the relatively small discordance in birth weight of the studied twins may likely recapitulate the early stage of preeclamptic condition, which is characterized by mild stress in comparison to severe preeclampsia with aggravated stress. In placentas associated with the smaller fetuses, the stressed condition may upregulate syncytin-1 expression, which is able to promote the trophoblast proliferation as we have demonstrated by recent in vitro experiments through manipulation of syncytin-1 expression levels in choriocarcinoma cell cultures [16]. For the same reason, as often observed in placentas and the cardiovascular system, moderate hypoxia or ROS often leads to active angiogenesis and epithelial expansion. However, severe hypoxia and high levels of ROS will lead to irreversible tissue damage and functional disruption (Fig. 1).
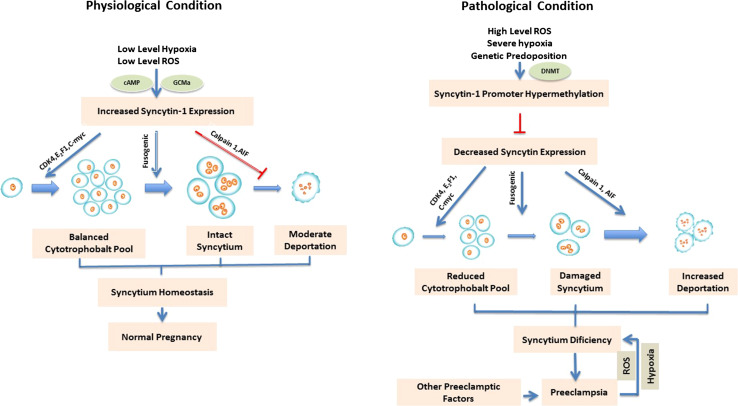
Fig. 1.
Syncytin-1 regulation of syncytium homeostasis. The left panel illustrates that under physiological conditions, or early stage of preeclampsia, low levels of hypoxia and ROS will activate syncytin-1 expression through cAMP and GCMa-mediated pathway. A sufficient level of syncytin-1 leads to upregulation of CDK4, E2F1, and C-Myc, which ensures G1/S transition, promoting cytotrophoblast proliferation. At the same time, syncytin-1 mediates cell fusion and the formation of syncytiotrophoblast and keeps the trophoblast apoptosis at low levels. These actions work together to achieve the syncytium homeostasis, which supports the normal pregnancy. The right panel depicts the pathological situation. High levels of ROS, severe hypoxia, genetic predisposition, or a combination of these factors, may overwhelm the feedback mechanism for upregulation of syncytin-1. At a late stage of preeclampsia, the detrimental factors may affect DNMT expression, leading to hypermethylation and epigenetic silencing of syncytin-1 promoter. An insufficient level of syncytin-1 leads to blocking of G1/S transition, inhibition of trophoblast proliferation, and a reduced cytotrophoblast pool. The low level of syncytin-1 and reduced cell fusion will affect the repair of syncytium. Furthermore, a low level of syncytin-1 causes apoptosis of syncytiotrophoblasts, increases the syncytial deportation, and ultimately, the syncytium deficiency, which contributes to the development of preeclampsia. On the other hand, some preeclamptic conditions may further aggravate the syncytium deficiency, leading to the deterioration of the disease
Trophoblast Apoptosis, autophagy, deportation, and turnover
As the fetal–maternal interface, syncytium is under constant ROS and immune attack; it is not surprising to find cell death and replacement to be frequent events even in a normal placenta. Trophoblast apoptosis and its significance for preeclampsia have been intensively investigated. One significant finding in recent years concerns autophagy [23], a cellular process characterized by lysosome digestion and recycling of damaged micro-organelles. A proper level of autophagy is often observed in tissues undergoing fast remodeling or repair. However, excessive autophagic activity will cause cell apoptosis. Interestingly, high autophagy activity and autophagy-induced cell death has been found to be increased in placentas associated with preeclampsia or IUGR [24, 25]. Moreover, Chen et al. reported that hypoxia is able to induce autophagy in placental trophoblasts [26]. By measuring the light chain 3-II (LC3-II), an autophagic marker, and cleaved poly(ADP-ribose) polymerase, an apoptosis marker, in the primary cultures exposed to hypoxia, this study reveals evidence for a cross-talk between autophagy and apoptosis functions in the placental trophoblasts. The authors speculated that autophagy benefits placental function in vivo by eliminating cytotrophoblast organelles damaged by stressors such as hypoxia. On the other side, Cha et al. reported that tumor necrosis factor α (TNF-α)-induced autophagy mediates intrinsic apoptosis in JEG-3, a choriocarcinoma cell line [27]. This result pointed to the possibility that under severe hypoxic conditions, excessive autophagy may cause trophoblast apoptosis in human placentas. More investigations, especially those using clinical data and patients’ samples, are required to define the exact role and mechanism of autophagy in preeclampsia.
Throughout the pregnancy, aged/damaged syncytium needs to be constantly eliminated to maintain syncytial homeostasis [6]. This process, commonly referred to as syncytial deportation, involves the release of cellular or subcellular placental material into the maternal circulation. Placental deportation takes diversified forms, ranging from large fragments of multinucleate syncytiotrophoblasts, small uninucleate elements with cytotrophoblast morphology, anucleate cytoplasmic fragments, to microparticles and nanovesicles [15]. Multiple studies have shown that there is an increased trophoblast deportation into the maternal circulation in preeclampsia [28]. Syncytial trophoblastic cells were first described to be circulating in maternal blood in 1959 by Douglas et al. [29]. Although some studies have been unable to confirm these findings, in 2008, Schmidt et al. used a monoclonal pan cytokeratin antibody to detect trophoblasts in maternal circulation. Their findings suggested that the number of circulatory trophoblastic particles in preeclamptic pregnancies was significantly higher when compared to non-preeclamptic pregnancies [30]. This is consistent with the findings of Heazell et al., who showed that syncytial debris was increased in number and density by approximately twofold in women with preeclampsia [31]. The deportation could be further increased in women with small-for-gestational-age infants [32]. Douglas et al. observed that the rate of trophoblast release decreases from the second to third trimesters in normal pregnancies. In 2011, Rajakumar, et al. showed that preeclamptic placentas released more trophoblastic mass in the form of syncytial aggregates in the third trimester when compared to normal placentas [33]. This material contains multinuclear structures, pointing to their syncytiotrophoblastic origin.
Turnover of syncytiotrophoblasts is similar to the process seen in other structures such as the epidermis, intestinal epithelium, and blood endothelium. Damaged syncytium is continuously repaired by replacing the dead syncytiotrophoblasts with newly formed ones through the fusion of cytotrophoblasts. During cell fusion, cytotrophoblasts transfer their nuclei and other organelles, as well as proteins and RNA, into syncytiotrophoblasts [6]. This fusion process is highly regulated by growth factors and cytokines that control the expression of fusogenic proteins. As discussed later, two retroviral envelope proteins, syncytin-1 and syncytin-2, play a major role in trophoblast fusion in human placentas. Preeclampsia may be related to the dysregulation of any of these factors, leading to abnormal syncytial structure. Gauster et al. described that the altered fate of the villous trophoblasts in this condition may be due to aberrant concentrations of growth factors, cytokines, as well as elevated expression of receptor proteins that catch circulating growth factors [8].
Syncytial antigens released from the allogeneic placenta may interact with the maternal immune system and contribute to the formation of maternal immune tolerance against fetal antigens [34]. Thus, aberrant maternal immune responses, such as those observed in preeclampsia, may result from the quantitative and/or qualitative changes in the deported trophoblast debris [35]. What would be the impact on the maternal immune system by too large or too small amounts of syncytial debris? No sufficient data is available at this time to address this question. Some investigators hold that the excessive deportation may contribute to the maternal inflammatory responses as often observed in preeclampsia patients [36–38].
While it is conceivable that increased apoptosis triggered by hypoxia and ROS may aggravate preeclamptic symptoms through disruption of placental structure and function, the initial cause and upstream mechanism of preeclampsia remain elusive. Questions can be raised as, before the onset of preeclampsia what factor(s) causes what initial events that could lead to the later syncytial abnormalities? Genetic predisposition could be one culprit. Study efforts in the past decades have led to revelation of several genes and polymorphisms, but often with relatively weak association and at their most, to explain a small number of preeclampsia cases. More research effort in larger patient populations is required to clarify the significance of the identified genetic alterations for the development of preeclampsia.
Changes in syncytial knots and syncytial sprouts
Syncytial debris found in the maternal circulation has two major forms: syncytial knots and syncytial sprouts [31]. Syncytial knots are aggregated syncytiotrophoblastic nuclei at the surface of terminal villi that is found in the third trimester, which have stopped outgrowth and active proliferation. Syncytial knots are consistently present and increase with advancing gestational age. Most knots are frequently found after 32 weeks of gestation [39]. For this reason, the structure can be used for histological assessment of villous maturity [40]. Syncytial knots are more common in preeclampsia than in normal pregnancy, possibly due to malnutrition, shrinkage and premature aging of the villi, which leads to buckling of the syncytial epithelium [41]. In normal pregnancies, syncytial knots are found on 10–15 % of terminal villi, whereas in placentas of preeclamptic women they are almost ubiquitous [42].
Syncytial knots sequester syncytiotrophoblast cells with heavily condensed chromatin, a feature of apoptotic cells. The nuclei are closely juxtaposed with little cytoplasm and express 8OHdG, a biomarker for oxidative stress, which is increased in preeclampsia [42]. They also contain a paucity of cellular organelles, few nucleoli and mitochondria, and display an abnormal distribution of endoplasmic reticulum and more heterochromatin, indicating a transcriptionally inactive state [42]. Heazell et al. suggested that there is a reduced frequency of nuclear pores, which are known to decrease in number as nuclei show signs of advanced apoptosis [31]. Additionally, the incidence of syncytial knots was increased in low birth weight neonates and low weight placentas, the conditions commonly associated with preeclampsia. Conversely, this study also showed that the number of syncytial knots was reduced with the increased birth weight [43]. Devism et al. reported that increased syncytial knots, infarcts, basal decidual vasculopathy, hypermature villi, and placental erythroblastosis were significantly associated with preeclampsia [44]. Results from other studies confirmed these findings, and additionally showed that there is a proportional increase in the number of syncytial knots associated with the increasing severity of preeclampsia [45].
It has been under debate if there is significant apoptotic activity in syncytial knots, at least in normal placentas, despite the observation for highly condensed nuclei in the structure. Coleman et al. assessed the condensed nuclei in syncytial nuclear aggregates, or syncytial knots. While a higher proportion of condensed nuclei were found in the aggregated areas than other syncytiotrophoblasts, no evidence of apoptosis was found [46]. However, this study was conducted in placentas from normal pregnancy, it remains unclear if the same findings can be expected in the preeclamptic placentas where trophoblasts undergo extensive apoptosis. In a recent study, we performed immunohistochemistry and observed greatly increased syncytiotrophoblast apoptosis in preeclamptic placentas in comparison with placentas from normal pregnancy. Interestingly, these apoptotic cells are often found in syncytial aggregates that form bulged areas at the apical surface of syncytium. The activation of apoptosis inducing factor (AFI)-mediated apoptotic pathway, including an increased expression of calpain and AIF, AIF cleavage, and nuclearization, was often detected in the same areas in serial sections of placental tissues. As described in more details under “Reduced syncytin-1 leads to trophoblast apoptosis”, AIF mediates a caspase-independent apoptotic pathway that is initiated at mitochondria and leading to DNA degredation and cell death. These observations have provided support for a connection between syncytial knots and apoptosis in preeclamptic placentas [47].
Syncytial sprouts are considered the major vehicle for syncytial nuclear loss and account for the majority of deported fragments in preeclampsia [15]. They contain clusters of large, rounded, euchromatic nuclei with little heterochromatin, and usually have a prominent nucleolus. The cytoplasm of cells in syncytial sprouts tends to harbor large numbers of rough endoplasmic reticulum and ribosomes. With their thin, protruding structure, syncytial sprouts are more susceptible to detachment and loss into the maternal intervillous space [15, 39]. Some investigators pointed out that syncytial sprouts may be deported to a greater extent in preeclampsia due to the readily abnormal structure of spiral arteries and the formation of placental lakes, which allows more sprouts to be dislodged [39]. Placental lakes are defined as homogenous, sonolucent avillous lesions greater than 2 × 2 cm in diameter, with turbulent, swirling slow blood flow detected by real-time color Doppler scanning. Small-for-gestational-age fetal status was found to be significantly correlated with large placental lakes [48]. These findings may explain the increased deportation of syncytial sprouts, but on the other hand, may point to a possible role of syncytial sprouts and trophoblast deportation for the subsequent formation of placental lakes.
Microscopic observations in preeclamptic syncytium
On gross examination, preeclamptic placentas are often pale in color and sometimes contain infarcts, hematomas, and calcifications. Morphological changes can be observed in 50 % of the placentas complicated by severe preeclampsia [49]. Placental infarcts have been associated with not only preeclampsia but also intrauterine growth restriction and other adverse fetal outcomes [50, 51]. Preeclamptic placentas also tend to weigh less and have smaller diameters than those from normal pregnancies [52]. Microscopic examination may reveal sclerotic narrowing of arteries and arterioles in the preeclamptic placentas [53]. Placentas in pregnancies complicated by preeclampsia often demonstrate shallow trophoblast invasion with fewer invading trophoblasts and failure to convert the spiral arteries to their loosened, low-resistance and high-capacitance state necessary for normal pregnancy and fetal growth [54]. Since extravillous trophoblasts normally replace the vascular endothelium in placental arteries, changes in trophoblast lineage may partially account for the dysfunction of local circulation and hypoxia seen in preeclampsia.
Chronic low-grade ischemia–reperfusion injuries can be found in all placentas. Preeclampsia appears to represent an extreme state in which excessive ROS caused by recurrent ischemia–reperfusion insult results in higher levels of tissue damage and cell apoptosis. Tenney and Parker investigated preeclamptic placentas in 1940, and noted premature aging, with syncytial degeneration consuming the majority of terminal villi. In some cases, the degeneration appeared to be extensive, occurring in all of the villi examined. They also found marked congestion and distention of the villous blood vessels, which showed decreased permeability. They hypothesized that these findings could explain the fetal compromise associated with preeclampsia [55]. Later studies found that the syncytium in preeclamptic placentas was thin and discontinuous with bulbous edges and vacuoles in trophoblasts [16]. Sankar et al. reported that the vasculosyncytial membrane thickness, as well as the density and diameter of syncytial knots are significantly increased in preeclamptic placentas [56]. These findings were statistically significant, with a twofold increase in the vasculosyncytial membrane thickness in preeclamptic placentas when compared to normal controls. These structural changes may result in placental functional alterations, contributing to the hypoxic conditions found in preeclampsia [56]. Sodhi et al. examined 20 placentas from various grades of preeclampsia and eclampsia patients and 20 placentas from normal pregnancies [57]. Linear correlation was observed between the severity of preeclampsia and increasing cytotrophoblastic cell proliferation and increased thickening of the villous basement membrane. Soma et al. summarized the preeclampsia related histological changes as decreased number of syncytial microvilli, focal syncytial necrosis, thickening of trophoblastic basement membrane and narrowing of the fetal capillaries [58]. Other characteristics include terminal villi that are smaller and atrophic, with increased syncytiotrophoblast nuclei, increased stromal deposition of collagens and laminin, and increased deposition of fibrin plaques [59]. The thinner, degenerated syncytium found in preeclamptic placentas also contains dilated spaces separating the syncytiotrophoblast from underlying structures [45].
When the placental syncytium of preeclamptic women is examined on an ultrastructural level, there are multiple unique characteristics noted. Specifically, Jones et al. examined 23 placentas from women whose pregnancy was complicated only by preeclampsia and noted focal syncytial necrosis, dilation of rough endoplasmic reticulum, decreased pinocytotic activity, and number of secretory droplets. In areas where syncytial necrosis was detected, the cells tend to contain swollen mitochondria. Additionally, the plasma membrane demonstrated an increased degree of basal interdigitation, which was noted to be especially marked in placentas with severe preeclampsia [60]. Tenney and Parker commented on ultrastructural findings in 1940, noting a progression from the “first stage” of syncytial degeneration, which consisted of nuclear clumping and autolysis, to the “final stage” which involved disappearance of all nuclei from the syncytium, leaving the villus surrounded by a thin irregular layer of hyaline material [55]. In 1970s, MacLennan, et al. published a series of studies on the ultrastructural alterations under hypoxic conditions using either trophoblast culture or organ culture. They compared samples from fresh placenta to samples from the same placentas cultured in hypoxic conditions. Rapid damage to the syncytium in hypoxic conditions, including an increase in basal lamina thickness and intervillous collagen, and a decrease in the volume and complexity of mitochondria, was documented. They also noted hypoxic conditions induced a marked thinning and vacuolation of syncytiotrophoblasts, as well as increased folding of the syncytial basal membranes, clubbing of the microvilli, and clumping of nuclear chromatin. Ultimately, there was loss of organelles essential for syncytial function, leading to cellular degeneration. It was noted that some of these changes were progressive in all placentas, however these changes were more prominent in preeclamptic placentas, and became more significant over time [61].
It should be pointed out that the morphological changes in syncytium bear immediate functional implications for the pathogenesis of preeclampsia. For example, the placental barrier prevents fetal proteins to enter maternal circulation, avoiding the maternal immune response against fetal antigens. This barrier function relies on the high integrity of syncytium. The discontinuous syncytium could allow the leakage of fetal antigen into the maternal circulation, significantly increasing the risk for immune rejection of the allogeneic placenta. Such immune responses may cause or aggravate the local and systemic inflammatory process, a key clinical feature of preeclampsia.
Syncytin-1 as a key regulator of syncytium turnover
Syncytin-1 is a human endogenous retroviral envelope gene product that plays an important role in the formation and maintenance of normal syncytium throughout pregnancy. Syncytin-1 gene is located on chromosome 7, and is subject to dynamic genetic and epigenetic regulations [62]. As previously discussed, the syncytium is formed by the fusion of cytotrophoblasts; syncytin-1 is the key mediator of the fusion process. Importantly, recent studies have shown that this gene product has multiple functions, including fusogenic and nonfusogenic activities, and the coordinated actions of these activities are required for the homeostasis of syncytium.
Reduced syncytin-1 expression in preeclampsia
Cell fusion is the characteristic step in the pathway to terminal trophoblast differentiation. Langbein et al. found a decreased quantity of syncytin-1, as well as a lower rate of cell fusion in preeclamptic placentas. Syncytin-1 transcript copy number was 8.1-fold lower in placentas from pregnancies complicated by preeclampsia and intrauterine growth restriction, and 222.7-fold lower in placentas complicated by HELLP syndrome and intrauterine growth restriction [63]. The reduced syncytin-1 expression on mRNA and protein levels in the trophoblastic lineages in preeclamptic placenta has been confirmed by several studies with the use of multiple techniques. Matouskova et al. initially described syncytin-1 gene demethylation, which is largely responsible for its specific expression in trophoblasts [64]. They compared methylation patterns between human choriocarcinoma BeWo cells and cervical cancer HeLa cells. Increased DNA methylation was associated with decreased syncytin-1 expression in these cells. Moreover, there is a quantitative relationship between syncytin-1 gene methylation and expression [64]. In addition, inhibition of the cAMP-induced and GCMa-mediated, non-epigenetic transcriptional activation of syncytin-1 may also contribute to the syncytin-1 deficiency in preeclamptic placentas. Huang et al. reviewed the potential causes for the downregulation of syncytin-1 expression in preeclampsia. They concluded that the syncytin-1 gene undergoes dynamic alteration in expression and 5′LTR DNA methylation during placental maturation or under preeclamptic/hypoxic conditions. The downregulation of syncytin-1 by hypoxia is likely an important part of the pathologic mechanism underlying this disorder [61]. However, it is not clear at this time through what molecular mechanism the hypoxic condition may lead to the inhibition of syncytin-1 expression. Aberrant expression of DNA methyltransferase (DNMT), the enzyme carrying out DNA methylation of genomic DNA, was thought to play a role in gene inhibition [22, 62].
Lee et al. performed in situ hybridization and immunohistological studies to compare the expression levels and localization of syncytin-1 between normal and preeclamptic placentas. As much as 25-fold lower hybridization signal was observed in preeclamptic placentas than in normal placentas. Consistent results were found on the syncytin-1 protein expression levels. Interestingly, in normal placentas syncytin-1 expression was detected mainly in the basal syncytiotrophoblast cytoplasm membrane, whereas in preeclamptic placentas, the expression is limited to a thin lining of apical syncytiotrophoblast microvillous membrane. The authors reasoned that the improper distribution of syncytin-1 protein makes it unavailable for fusion, and the characteristic changes in preeclamptic syncytium may be related to the aberrant localization of syncytin-1 protein [65].
Syncytin-1 as a cell-cycle regulator
As discussed above, some studies showed that there is an accelerated regeneration of the syncytium in preeclamptic placentas, in an attempt to replace the syncytium that has been damaged by ischemia [22, 60, 66], especially in the early stage or mild cases of the disease, in which the feedback function is still intact. However, for a long time, the mechanism responsible for increased trophoblast proliferation has remained unclear. Recent studies have shown that the syncytin-1 gene may carry out some novel, nonfusogenic functions. One of these involves the role of syncytin-1 in the regulation of trophoblast proliferation. Huang et al. investigated the role of syncytin-1 as a regulator of the cell cycle [16]. They demonstrated that syncytin-1 knockdown in BeWo, a choriocarcinoma cell line expressing relatively low levels of syncytin-1 protein, resulted in inhibition of cell growth by blocking the G1/S transition. The mRNA levels of cell-cycle inhibitor p15 were significantly upregulated, whereas positive regulators, including E2F1, PCNA, and c-Myc were downregulated following syncytin-1 knockdown. Changes of the respective key regulators of the G1/S transition on protein levels mirrored those in mRNA levels. Forced overexpression of syncytin-1 in CHO, a Chinese hamster ovarian cell line deficient of fusion activity and therefore, being free of interference by the fusogenic activity, led to significantly increased cell proliferation as the result of correspondent changes in cell-cycle regulators. These results indicated that syncytin-1 plays an important part in two processes of syncytialization: syncytin-1 promotes proliferation of cytotrophoblasts through cell-cycle regulation, and at the same time mediates the fusion of cytotrophoblasts to form the placental syncytium [16]. Thus, a decreased level of this gene product, as seen in preeclampsia, has the potential to exert detrimental effects on the placenta as well as the development of fetus.
Reduced syncytin-1 leads to trophoblast apoptosis
In addition to cell-cycle regulation, syncytin-1 is involved in the control of cell survival. Huang et al. reported that knockdown of syncytin-1 expression in BeWo cells activated the AIF-mediated cell death pathway [47]. The reduction of syncytin-1 appeared to promote cell apoptosis by two mechanisms. First, syncytin-1 knockdown resulted in upregulation of calpain-1, a cysteine protease capable of cleaving AIF and activating the apoptotic pathway. Second, decreased syncytin-1 induced the expression, cleavage, and nuclear translocation of AIF. Moreover, similar changes in AIF and calpain-1 were detected in syncytiotrophoblasts undergoing apoptosis in preeclamptic placentas. Interestingly, the classic, caspase-mediated apoptotic pathway remains dormant, although previous studies have shown the presence and intact apoptotic activity of caspase pathway in BeWo cells. Thus, the syncytin-1 deficiency triggered cell apoptosis appears to be specifically mediated by the AIF pathway. These findings provided a mechanism underlying the increased trophoblast deportation under preeclampsia conditions when synctyin-1 expression is compromised, e.g., by hypoxia as shown by other investigators [67]. Since it has been shown that hypoxia is able to activate the caspase-mediated apoptotic pathway and cause trophoblast death, one possible scenario could be that hypoxia leads to trophoblast cell death via two independent mechanisms, the caspase- and AIF-mediated pathways, with the latter one specifically triggered by the reduction of syncytin-1 expression.
The significance of syncytin-1 for the maintenance of syncytium homeostasis
The above findings support the concept that syncytin-1 serves as the guardian for the syncytium integrity through both fusogenic and nonfusogenic activities, and the deficiency of syncytin-1 in preeclampsia may play a key role in the pathogenesis of the disease [47]. By this concept, syncytin-1 is able to promote trophoblast proliferation and fusion, and at the same time, to inhibit apoptosis of syncytiotrophoblasts. Normal pregnancy requires a stable “pool” of cytotrophoblasts with a healthy balance between input (proliferation) and output (fusion) of these cells. In addition, protection of syncytium integrity by avoiding excessive damage and deportation caused by hypoxia and ROS attack is equally critical for placental function. Sufficient levels of syncytin-1 may be the key for the maintenance of syncytium homeostasis. Using a single factor to co-regulate three related cellular activities, proliferation, fusion, and apoptosis, may provide an advantage for more precise control and better coordination of these activities. As illustrated in Fig. 1, under physiological conditions or early stages of preeclampsia, low levels of hypoxia and ROS stress could activate syncytin-1 expression, as observed in the monozygotic, dichorionic discordant twin studies, which results in the faster cytotrophoblast proliferation and fusion, to replace the damaged and deported syncytium [22]. However, at late stage of preeclampsia, this compensatory feedback mechanism may be overwhelmed, leading to increased syncytin-1 gene methylation and a decline in the syncytin-1 expression [21]. The reduced syncytin-1 expression will ultimately affect syncytium homeostasis through compromised cell proliferation and fusion, and increased cell apoptosis, the characteristic morphological alterations in preeclamptic placentas.
It should be pointed out that current knowledge on syncytin-1 function is still limited, and the above model may present an oversimplified picture of syncytin's complex involvement in the pathogenesis of preeclampsia. For example, Tolosa et al. has described the ability of syncytin-1 to suppress maternal immune responses. They found that syncytin-1 downregulated the concentrations of important cytokines, including TNF-α, CXCL10, and IFN γ in the maternal circulation by some yet to be identified mechanisms [68]. Deficiency of syncytin-1 expression, therefore, may induce a pro-inflammatory state due to the increased release of these cytokines, which may be responsible for the elevated systemic inflammation seen in preeclampsia [62]. These findings raised the possibility that syncytin-1 may not only participate in the regulation of placental structure and function, but also may impose systemic impact on the maternal adaptation and fetal development in human pregnancy.
Trophoblastic fibrin deposition
Pregnancy is normally in a “hypofibrinolytic” state, mostly due to the disturbance of the balance of plasminogen activators and their inhibitors. Fibrin deposition is associated with excessive trophoblast injury. Loss of normal villus structure and deposition of fibrinoid materials around the syncytium is often observed in preeclamptic placentas. Gilabert et al. reported that in preeclampsia, the level of plasminogen activator inhibitor type 1 (PAI-1) is significantly elevated, leading to a higher level of fibrin deposition than in normal placentas [69]. Similarly, Kanfer et al. describe an increased placental antifibrinolytic potential and fibrin deposits in pregnancy-induced hypertension and preeclampsia [70]. Plasminogen activator inhibitor type 2 is also implicated in preeclampsia. Tanjung et al. observed a reduced PAI-2 (activator inhibitor type 2) levels with increased tissue-type PA (t-PA) antigen and plasminogen activator inhibitor-1 antigen in preterm preeclampsia compared to normal pregnancy [71]. A low level of PAI-2 synthesis is probably due to trophoblastic damage, as it has been demonstrated in preeclamptic women with placental dysfunction [72]. An excessive deposition of fibrin may disrupt the exchange of gas and nutrients between the maternal and fetal circulations in the intervillous space, accounting for some of the complications of preeclampsia [43]. Guller et al. hypothesized that the excessive fibrin deposition in conjunction with the hypoxia-associated apoptosis/necrosis of syncytiotrophoblasts found in preeclampsia may produce the severe fetal outcomes and maternal symptoms that are associated with the disease [73]. Thus, plasminogen activator inhibitors could be a useful biomarker for the prediction of preeclamptic pregnancy outcome [72]. While increased fibrin deposition appears to coincide with more trophoblast apoptosis, it remains to be delineated which of the two processes is the initiating event.
Phenotypic immaturity of syncytiotrophoblasts in preeclampsia
One concept that has been repeatedly discussed in the literature that may contribute to the pathogenesis of preeclampsia on the cellular level is trophoblastic immaturity. In many cell types, glycogen contents are associated with cellular immaturity. As cell differentiate and reach maturity, glycogen contents tend to decrease. Arkwright et al. described their findings on the excess of glycogen breakdown products in syncytiotrophoblast microvesicles purified from preeclamptic placentas in comparison to placentas from normal pregnancy [74]. The study demonstrated that syncytiotrophoblasts of preeclamptic placentas have higher glycogen contents than normal placentas, a change similar to those in hydatidiform moles [74]. The authors concluded that glycogen accumulation in villous syncytiotrophoblasts may be a metabolic marker of immaturity. Similarly, Redline et al. later reported that syncytium from the implantation site of preeclamptic placentas contain a high number of intermediate trophoblasts with inappropriate phenotypic immaturity [18]. They found a decreased level of human placental lactogen in preeclamptic placentas, again suggesting a low differentiation or immaturity of trophoblasts, because only the well-differentiated syncytiotrophoblasts produce large amounts of lactogen. This immaturity likely contributes to the atypical placental implantation seen in preeclampsia. The authors speculated that this may be due to an intrinsic inability of the placenta to deeply invade the uterus, or due to maternal factors that prevent deep invasion of the placenta. In addition, they reported that abnormalities in vascular remodeling and persistence of occlusive cytotrophoblast plugs in large muscularized uterine arteries are consistently detected in preeclamptic placentas, indicating a dysfunction of the trophoblast lineage.
Paradoxically, data from some studies suggested that preeclamptic placentas can display signs of premature aging. Calcification, usually found on the maternal surface of term placenta, is generally considered as an indicator for placental aging [75]. Nahar et al. investigated placental changes in pregnancy-induced hypertension and their impacts on fetal outcome. One finding was that placental calcification was more marked in placentas associated with pregnancy-induced hypertension [76]. Chen et al. reported that early preterm placental calcification was correlated with the high incidence of adverse maternal and fetal outcomes including postpartum hemorrhage, placental abruption, maternal transfer to the intensive care unit, preterm birth, low birth weight, low Apgar score, and neonatal death [77]. Considering the possibility that diverse forms of placental dysfunctions may be involved in the development of preeclampsia, it becomes less surprising to see the association of preeclampsia with either premature or prematurely aged morphology of placentas.
Concluding remarks
Numerous studies have pointed to the significance of placental dysfunction in the development of preeclampsia. It is recognized that the integrity of the placental syncytium is compromised in preeclampsia. In this disorder, the placental trophoblasts undergo aberrant proliferation, abnormal differentiation, and increased apoptosis that accounts for the elevated rate of deportation of trophoblastic materials into maternal circulation. This process ultimately produces an abnormal-appearing placenta, which is present on gross examination as well as on histological inspection and ultrastructural levels. Syncytin-1, via its fusogenic and nonfusogenic activities, participates in the regulation of trophoblast proliferation, syncytium formation, and survival. The disturbance of homeostasis in trophoblast linage is likely to produce deleterious effects on the placental function and pregnancy. Finally, fibrin deposition around villi, phenotypic immaturity of trophoblastic tissue, calcifications at the maternal surface, and premature aging have also been documented in preeclamptic placentas, although the relationship between these changes and syncytium deficiency has not been clearly defined.
It should be pointed out that although placentas from early and late onset preeclampsia may have different morphological changes, most studies failed to distinguish the two classes. Another technical limitation we are facing is the lack of appropriate cellular and animal models to study preeclampsia, as well as many studies applying choriocarcinoma or immortalized cell lines as study model. Because of these shortages, many observations from previous reports need to be confirmed in more defined patient samples or primary trophoblast cultures. Future studies focusing on the specific molecular mechanisms underlying the syncytin-1 expression, cell dynamics and function of cytotrophoblasts and syncytiotrophoblasts could help us to better understand the placental biology. Ultimately, the dysfunctional placental syncytium could be a prospective target for potential interventions, which may bear landmark clinical implications for the treatment of this disease.
Acknowledgments
This work was supported by the Georgia Research Alliance (GRA) Distinguished Scholarship (S.-W. Jiang); NIH R01 HD41577 (S.-W. Jiang); The Zhejiang Provincial Program for the Cultivation of High-level Innovative Health Talents (X. Zhu); Seed Funds of Mercer University School of Medicine (S.-W. Jiang, J. Li).
Footnotes
C. S. Roland, J. Hu, C.-E. Ren, H. Chen contributed equally to this work.
Contributor Information
Xueqiong Zhu, Email: zjwzzxq@163.com.
Shi-Wen Jiang, Email: jiang_s@mercer.edu.
References
- 1.Hypertension in Pregnancy (2013) Task force on hypertension in pregnancy. American College of Obstetricians and Gynecologists. http://www.acog.org/-/media/Task-Force-and-Work-Group-Reports/HypertensioninPregnancy.pdf?dmc=1. Accessed Jan 2015
- 2.Beck S, Wojdyla D, Say L, Betran AP, Merialdi M, Requejo JH, Rubens C, Menon R, Van Look PFA. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull World Health Organ. 2009;88:31–38. doi: 10.2471/BLT.08.062554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mercuro G, Bassareo PP, Flore G, Fanos V, Dentamaro I, Scicchitano P, Laforgia N, Ciccone MM. Prematurity and low weight at birth as new conditions predisposing to an increased cardiovascular risk. Eur J Prev Cardiol. 2013;20(2):357–367. doi: 10.1177/2047487312437058. [DOI] [PubMed] [Google Scholar]
- 4.Moster D, Lie RT, Markstad T. Long-term medical and social consequences of preterm birth. N Engl J Med. 2008;359(3):262–273. doi: 10.1056/NEJMoa0706475. [DOI] [PubMed] [Google Scholar]
- 5.Enguobahrie D, Abetwe D, Sorensen T, Willoughby D, Chidambaram K, Williams M. Placental microRNA expression in pregnancies complicated by preeclampsia. Am J Obstet Gynecol. 2011;204:178.2–178.21. doi: 10.1016/j.ajog.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gauster M, Moser G, Orendi K, Huppertz B. Factors involved in regulating trophoblast fusion: potential role in the development of preeclampsia. Placenta. 2009;23:49–54. doi: 10.1016/j.placenta.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 7.Redman CWG, Sargent IL. Circulating microparticles in normal pregnancy and pre-eclampsia. Placenta. 2008;29:73–77. doi: 10.1016/j.placenta.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 8.Gauster M, Huppertz B. Fusion of cytotrophoblast with syncytiotrophoblast in the human placenta: factors involved in syncytialization. J Reprod Med Endocrinol. 2008;5:76–82. [Google Scholar]
- 9.Dokras A, Hoffmann D, Eaastvold J, Kienzle M, Gruman L, Kirby P, Weiss R, Davisson R. Severe feto-placental abnormalities precede the onset of hypertension and proteinuria in a mouse model of preeclampsia. Biol Reprod. 2006;75(6):899–907. doi: 10.1095/biolreprod.106.053603. [DOI] [PubMed] [Google Scholar]
- 10.Cross J. Genetic insights into trophoblast differentiation and placental morphogenesis. Semin Cell Dev Biol. 2000;11(2):105–113. doi: 10.1006/scdb.2000.0156. [DOI] [PubMed] [Google Scholar]
- 11.Newhouse S, Davidge S, Winkler-Lowen B, Demianczuk N, Guklbert L. In vitro differentiation of villous trophoblasts from pregnancies complicated by intrauterine growth restriction with and without preeclampsia. Placenta. 2007;28(10):999–1003. doi: 10.1016/j.placenta.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 12.Brouillet S, Hoffmann P, Feige JJ, Alfaidy N. EG-VEGF: a key endocrine factor in placental development. Trends Endocrinol Metab. 2012;23(10):501–508. doi: 10.1016/j.tem.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Maynard SE, Karumanchi SA. Angiogenic factors and preeclampsia. Semin Nephrol. 2011;31(1):33–46. doi: 10.1016/j.semnephrol.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stepan H, Unversucht A, Wessel N, Faber R. Predictive value of maternal angiogenic factors in second trimester pregnancies with abnormal uterine perfusion. Hypertension. 2007;49(4):818–824. doi: 10.1161/01.HYP.0000258404.21552.a3. [DOI] [PubMed] [Google Scholar]
- 15.Mayhew TM. Turnover of human villous trophoblast in normal pregnancy: what do we know and what do we need to know? Placenta. 2014;35(4):229–240. doi: 10.1016/j.placenta.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 16.Huang Q, Li J, Wang F, Oliver MT, Tipton T, Gao Y, Jiang SW. Syncytin-1 modulates placental trophoblast cell proliferation by promoting G1/S transition. Cell Signal. 2013;25(4):1027–1035. doi: 10.1016/j.cellsig.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mayhew TM, Manwani R, Ohadike C, Wijeskara J, Baker PN. The placenta in pre-eclampsia and intrauterine growth restriction: studies on exchange surface areas, diffusion distances and villous membrane diffusive conductances. Placenta. 2007;28(2–3):233–238. doi: 10.1016/j.placenta.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 18.Redline RW, Patterson P. Pre-eclampsia is associated with an excess of proliferative immature intermediate trophoblast. Hum Pathol. 1995;26(6):594–600. doi: 10.1016/0046-8177(95)90162-0. [DOI] [PubMed] [Google Scholar]
- 19.Vatgas A, Toufaily C, LeBellego F, Rassart E, Lafond J, Barbeau B. Reduced expression of both syncytin 1 and syncytin 2 correlates with severity of preeclampsia. Reprod Sci. 2011;18(11):1085–1091. doi: 10.1177/1933719111404608. [DOI] [PubMed] [Google Scholar]
- 20.Ruebner M, Strissel PL, Ekici AB, Stiegler E, Dammer U, Goecke TW, Faschingbauer F, Fahlbusch FB, Beckmann MW, Strick R. Reduced syncytin-1 expression levels in placental syndromes correlates with epigenetic hypermethylation of the ERVW-1 promotor region. PLoS One. 2013;8:e56145. doi: 10.1371/journal.pone.0056145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhuang XW, Li J, Brost BC, Xia XY, Chen HB, Wang CX, Jiang SW. Decreased expression and altered methylation of syncytin-1 gene in human placentas associated with preeclampsia. Curr Pharm Des. 2014;20(11):1796–1802. doi: 10.2174/13816128113199990541. [DOI] [PubMed] [Google Scholar]
- 22.Gao Y, He Z, Wang Z, Luo Y, Sun H, Zhou Y, Huang L, Li M, Fang Q, Jiang S. Increased expression and altered methylation of HERVWE1 in the human placentas of smaller fetuses from monozygotic, dichorionic, discordant twins. PLoS One. 2012;7:e33503. doi: 10.1371/journal.pone.0033503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gong JS, Kim GJ. The role of autophagy in the placenta as a regulator of cell death. Clin Exp Reprod Med. 2014;41(3):97–107. doi: 10.5653/cerm.2014.41.3.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oh SY, Choi SJ, Kim KH, Cho EY, Kim JH, Roh CR. Autophagy-related proteins, LC3 and Beclin-1, in placentas from pregnancies complicated by preeclampsia. Reprod Sci. 2008;15(9):912–920. doi: 10.1177/1933719108319159. [DOI] [PubMed] [Google Scholar]
- 25.Hung TH, Chen SF, Lo LM, Li MJ, Yeh YL, Hsieh TT. Increased autophagy in placentas of intrauterine growth-restricted pregnancies. PLoS One. 2012;7:e40957. doi: 10.1371/journal.pone.0040957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen B, Longtine SM, Nelson DM. Hypoxia induces autophagy in primary human trophoblasts. Endocrinology. 2012;153(10):4946–4954. doi: 10.1210/en.2012-1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cha HH, Hwang JR, Kim HY, Choi SJ, Oh SY, Roh CR. Autophagy induced by tumor necrosis factor a mediates intrinsic apoptosis in trophoblastic cells. Reprod Sci. 2014;21(5):612–622. doi: 10.1177/1933719113508816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Asklund K, Chamley L. Trophoblast deportation part I: review of the evidence demonstrating trophoblast shedding and deportation during human pregnancy. Placenta. 2011;32(10):716–723. doi: 10.1016/j.placenta.2011.07.081. [DOI] [PubMed] [Google Scholar]
- 29.Douglas GW, Thomas L, Carr M, Cullen NM, Morris R. Trophoblast in the circulating blood during pregnancy. Am J Obstet Gynecol. 1959;78:960–973. doi: 10.1016/s0002-9378(16)36649-2. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt M, Hoffman B, Beelen D, Gellhaus A, Winterhager E, Kimming R. Detection of circulating trophoblast particles in peripheral maternal blood in preeclampsia complicated pregnancies. Hypertens Pregnancy. 2008;27(2):131–142. doi: 10.1080/10641950701885170. [DOI] [PubMed] [Google Scholar]
- 31.Heazell AE, Moll SJ, Jones CJ, Baker PN, Crocker IP. Formation of syncytial knots is increased by hyperoxia, hypoxia and reactive oxygen species. Placenta. 2007;28:33–40. doi: 10.1016/j.placenta.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 32.Austgulen R, Isaksen C, Chedwick L, Romundstad P, Vatten L, Craven C. Preeclampsia: associated with increased syncytial apoptosis when the infant is small-for-gestational-age. J Reprod Immunol. 2004;61(1):39–50. doi: 10.1016/j.jri.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 33.Rajakumar A, Cerdeira AS, Rana S, Zsengeller Z, Edmunds L, Jeyabalan A, Hubel CA, Stillman IE, Parikh SM, Karumanchi SA. Transcriptionally active syncytial aggregates in the maternal circulation may contribute to circulating soluble fms-like tyrosine kinase 1 in preeclampsia. Hypertension. 2012;59(2):256–264. doi: 10.1161/HYPERTENSIONAHA.111.182170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chamley LW, Chen Q, Ding J, Stone PR, Abumaree M. Trophoblast deportation: just a waste disposal system or antigen sharing? J Reprod Immunol. 2011;88(2):99–105. doi: 10.1016/j.jri.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 35.Pantham P, Askelund KJ, Chamley LW. Trophoblast deportation part II: a review of the maternal consequences of trophoblast deportation. Placenta. 2011;32(10):724–731. doi: 10.1016/j.placenta.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 36.Huppertz B. IFPA award in placentology lecture: biology of the placental syncytiotrophoblast—myths and facts. Placenta. 2010;31:75–81. doi: 10.1016/j.placenta.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 37.Lockwood CJ, Huang SJ, Krikun G, Caze R, Rahman M, Buchwalder LF, Schatz F. Decidual hemostasis, inflammation and angiogenesis in pre-eclampsia. Semin Thromb Hemost. 2011;37(2):158–164. doi: 10.1055/s-0030-1270344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Staff AC, Johnsen GM, Dechend R, Redman CW. Preeclampsia and uteroplacental acute atherosis: immune and inflammatory factors. J Reprod Immunol. 2014;101–102:120–126. doi: 10.1016/j.jri.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 39.Burton GJ, Jones CJ. Syncytial knots, sprouts, apoptosis, and trophoblast deportation from the human placenta. Taiwan J Obstet Gynecol. 2009;48(1):28–37. doi: 10.1016/S1028-4559(09)60032-2. [DOI] [PubMed] [Google Scholar]
- 40.Loukeris K, Sela R, Baergen RN. Syncytial knots as a reflection of placental maturity: reference values for 20 to 40 weeks’ gestational age. Pediatr Dev Pathol. 2010;13(4):305–309. doi: 10.2350/09-08-0692-OA.1. [DOI] [PubMed] [Google Scholar]
- 41.Benirschke K. Remarkable placenta. Clin Anat. 1998;11(3):194–205. doi: 10.1002/(SICI)1098-2353(1998)11:3<194::AID-CA8>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 42.Fogarty NM, Ferguson-Smith AC, Burton GJ. Syncytial knots (Tenney–Parker changes) in the human placenta: evidence of loss of transcriptional activity and oxidative damage. Am J Pathol. 2013;183(1):144–152. doi: 10.1016/j.ajpath.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 43.Stark M, Clark L, Craver R. Histologic differences in placentas of preeclamptic/eclamptic gestations by birthweight, placental weight, and time of onset. Pediatr Dev Pathol. 2014;17(3):181–189. doi: 10.2350/13-09-1378-OA.1. [DOI] [PubMed] [Google Scholar]
- 44.Devisme L, Merlot B, Ego A, Houfflin-Debarge V, Deruelle P, Subtil D. A case–control study of placental lesions associated with pre-eclampsia. Int J Gynaecol Obstet. 2013;120(2):165–168. doi: 10.1016/j.ijgo.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 45.Salgado S, Salgado M. Structural changes in pre-eclamptic and eclamptic placentas—an ultrastructural study. J Coll Physicians Surg Pak. 2011;21(8):482–486. [PubMed] [Google Scholar]
- 46.Coleman SJ, Gerza L, Jones CJ, Sibley CP, Aplin JD, Heazell AE. Syncytial nuclear aggregates in normal placenta show increased nuclear condensation, but apoptosis and cytoskeletal redistribution are uncommon. Placenta. 2013;34(5):449–455. doi: 10.1016/j.placenta.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang Q, Chen H, Wang F, Brost BC, Li J, Gao Y, Li Z, Gao Y, Jiang SW. Reduced syncytin-1 expression in choriocarcinoma BeWo cells activates the calpain1-AIF-mediated apoptosis, implication for preeclampsia. Cell Mol Life Sci. 2014;71(16):3151–3165. doi: 10.1007/s00018-013-1533-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hwang HS, Sohn IS, Kwon HS. The clinical significance of large placental lakes. Eur J Obstet Gynecol Reprod Biol. 2012;162(2):139–143. doi: 10.1016/j.ejogrb.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 49.Petersen OMF, Heller D, Joshi V. Handbook of placental pathology. Oxford: Taylor & Francis; 2006. [Google Scholar]
- 50.Hung TH, Skepper JN, Charnock-Jones DS, Burton GJ. Hypoxia-reoxygenation: a potent inducer of apoptotic changes in the human placenta and possible etiological factor in preeclampsia. Circ Res. 2002;90(12):1274–1281. doi: 10.1161/01.RES.0000024411.22110.AA. [DOI] [PubMed] [Google Scholar]
- 51.Guller S. Role of the syncytium in placenta-mediated complications of preeclampsia. Thromb Res. 2009;124(4):389–392. doi: 10.1016/j.thromres.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Narasimah A, Vasudeva DS. Spectrum of changes in placenta in toxemia of pregnancy. Indian J Pathol Microbiol. 2011;54(1):15–20. doi: 10.4103/0377-4929.77317. [DOI] [PubMed] [Google Scholar]
- 53.Hladunewich M, Karumanchi SA, Lafayette R. Pathophysiology of the clinical manifestations of preeclampsia. Clin J Am Soc Nephrol. 2007;2(3):543–549. doi: 10.2215/CJN.03761106. [DOI] [PubMed] [Google Scholar]
- 54.Khong TY, De Wolf F, Robertson WB, Brosens I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol. 1986;93(10):1049–1059. doi: 10.1111/j.1471-0528.1986.tb07830.x. [DOI] [PubMed] [Google Scholar]
- 55.Tenney B, Parker F. The placenta in toxemia of pregnancy. Am J Obstet Gynecol. 1940;39(6):1000–1005. [Google Scholar]
- 56.Sankar KD, Bhanu PS, Kiran S, Ramakrishna BA, Shanthi V. Vasculosyncytial membrane in relation to syncytial knots complicates the placenta in preeclampsia: a histomorphometrical study. Anat Cell Biol. 2012;45(2):86–91. doi: 10.5115/acb.2012.45.2.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sodhi S, Mohan H, Jaiswal TS, Mohan PS, Rathee S. Placental pathology in preeclampsia eclampsia syndrome. Indian J Pathol Microbiol. 1990;33(1):11–16. [PubMed] [Google Scholar]
- 58.Soma H, Yoshida K, Mukaida T, Tabuchi Y. Morphologic changes in the hypertensive placenta. Contrib Gynecol Obstet. 1982;9:58–75. [PubMed] [Google Scholar]
- 59.Ishihara N, Matsuo H, Murakoshi H, Laoag-Fernandez JB, Samoto T, Mauruo T. Increased apoptosis in the syncytiotrophoblast in human term placentas complicated by either preeclampsia or intrauterine growth retardation. Am J Obstet Gyncecol. 2002;186(1):158–166. doi: 10.1067/mob.2002.119176. [DOI] [PubMed] [Google Scholar]
- 60.Jones CJ, Fox H. An ultrastructural and ultrahistochemical study of the human placenta in maternal pre-eclampsia. Placenta. 1980;1(1):61–76. doi: 10.1016/S0143-4004(80)80016-6. [DOI] [PubMed] [Google Scholar]
- 61.MacLennan AH, Sharp F, Shaw-Dunn J. The ultrastructure of human trophoblast in spontaneous and induced hypoxia using a system of organ culture. J Obstet Gynaecol Br Commonw. 1972;79(2):113–121. doi: 10.1111/j.1471-0528.1972.tb15763.x. [DOI] [PubMed] [Google Scholar]
- 62.Huang Q, Chen H, Li J, Oliver M, Ma X, Byck D, Gao Y, Jiang S. Epigenetic and non-epigenetic regulation of syncytin-1 expression in human placenta and carrier tissues. Cell Signal. 2013;26(3):648–656. doi: 10.1016/j.cellsig.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 63.Langbein M, Strick R, Strissel PL, Vogt N, Parsch H, Beckman MW, Schild RL. Impaired cytotrophoblast cell–cell fusion is associated with reduced syncytin and increased apoptosis in patients with placental dysfunction. Mol Reprod Dev. 2008;75(1):175–183. doi: 10.1002/mrd.20729. [DOI] [PubMed] [Google Scholar]
- 64.Matouskova M, Blazkova J, Pajer P, Pavlicek A, Hejnar J. CpG methylation suppresses transcriptional activity of human syncytin-1 in non-placental tissues. Exp Cell Res. 2006;312(7):1011–1020. doi: 10.1016/j.yexcr.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 65.Lee X, Keith JC, Jr, Stumm N, Moutsatsos I, McCoy JM, Crum CP, Genest D, Chin D, Ehrenfels C, Pijnenborg R, van Assche FA, Mi S. Downregulation of placental syncytin expression and abnormal protein localization in pre-eclampsia. Placenta. 2001;22(10):808–812. doi: 10.1053/plac.2001.0722. [DOI] [PubMed] [Google Scholar]
- 66.Arnholdt H, Meisel F, Fandrey K, Lohrs U. Proliferation of villous trophoblast of the human placenta in normal and abnormal pregnancies. Virch Arch B Cell Pathol Incl Mol Pathol. 1991;60(6):365–372. doi: 10.1007/BF02899568. [DOI] [PubMed] [Google Scholar]
- 67.Knerr I, Weigel C, Linnemann K, Dötsch J, Meissner U, Fusch C, Rascher W. Transcriptional effects of hypoxia on fusiogenic syncytin and its receptor ASCT2 in human cytotrophoblast BeWo cells and in ex vivo perfused placental cotyledons. Am J Obstet Gynecol. 2003;189(2):583–588. doi: 10.1067/S0002-9378(03)00538-6. [DOI] [PubMed] [Google Scholar]
- 68.Tolosa JM, Schjenken JE, Clifton VL, Vargas A, Barbeau B, Lowry P, Maiti K, Smith R. The endogenous retroviral envelope protein syncytin-1 inhibits LPS/PHA-stimulated cytokine responses in human blood and is sorted into placental exosomes. Placenta. 2012;33(11):933–941. doi: 10.1016/j.placenta.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 69.Gilabert J, Estellés A, Grancha S, España F, Aznar J. Fibrinolytic system and reproductive process with special reference to fibrinolytic failure in pre-eclampsia. Hum Reprod. 1995;10:121–131. doi: 10.1093/humrep/10.suppl_2.121. [DOI] [PubMed] [Google Scholar]
- 70.Kanfer A, Bruch JF, Nguyen G, He CJ, Delarue F, Flahault A, Nessmann C, Uzan S. Increased placental antifibrinolytic potential and fibrin deposits in pregnancy-induced hypertension and preeclampsia. Lab Invest. 1996;74(1):253–258. [PubMed] [Google Scholar]
- 71.Tanjung MT, Siddik HD, Hariman H, Koh SC. Coagulation and fibrinolysis in preeclampsia and neonates. Clin Appl Thromob Hemost. 2005;11(4):467–473. doi: 10.1177/107602960501100415. [DOI] [PubMed] [Google Scholar]
- 72.Pinheiro MB, Gomes KB, Dusse LM. Fibrinolytic system in preeclampsia. Clin Chim Acta. 2013;416:67–71. doi: 10.1016/j.cca.2012.10.060. [DOI] [PubMed] [Google Scholar]
- 73.Guller S, Yula YM, Han-Hsuan F, Krikun G, Abrahams VM, Mor G. The placental syncytium and the pathophysiology of preeclampsia and intrauterine growth restriction: a novel assay to assess syncytial protein expression. Ann N Y Acad Sci. 2008;1127:129–133. doi: 10.1196/annals.1434.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Arkwright PD, Rademacher TW, Dwek RA, Redman CW. Preeclampsia is associated with an increase in trophoblast glycogen content and glycogen synthase activity, similar to that found in hydatidiform moles. J Clin Investig. 1993;91(6):2744–2753. doi: 10.1172/JCI116515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Salge AK, Rocha KM, Xavier RM, Ramalho WS, Rocha ÉL, Guimarães JV, Silva RC, Siqueira KM, Abdalla DR, Michelin MA, Murta EF. Macroscopic placental changes associated with fetal and maternal events in diabetes mellitus. Clinics (Sao Paulo) 2012;67(10):1203–1208. doi: 10.6061/clinics/2012(10)13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nahar L, Nahar K, Hossain MI, Yasmin H, Annur BM. Placental changes in pregnancy induced hypertension and its impacts on fetal outcome. Mymensingh Med J. 2015;24(1):9–17. [PubMed] [Google Scholar]
- 77.Chen KH, Chen LR, Lee YH. Exploring the relationship between preterm placental calcification and adverse maternal and fetal outcome. Ultrasound Obstet Gynecol. 2011;37(3):328–334. doi: 10.1002/uog.7733. [DOI] [PubMed] [Google Scholar]