Abstract
Background
Delirium symptoms are associated with later worse functional outcomes and long-term cognitive impairments, but the neuroanatomical basis for delirium symptoms in patients with acute brain injury is currently uncertain. We tested the hypothesis that hematoma location is predictive of delirium symptoms in patients with intracerebral hemorrhage, a model disease where patients are typically not sedated or bacteremic.
Methods
We prospectively identified 90 patients with intracerebral hemorrhage who underwent routine twice-daily screening for delirium symptoms with a validated examination. Voxel-based lesion-symptom mapping with acute computed tomography was used to identify hematoma locations associated with delirium symptoms (N=89).
Results
Acute delirium symptoms were predicted by hematoma of right-hemisphere subcortical white matter (superior longitudinal fasciculus) and parahippocampal gyrus. Hematoma including these locations had an odds ratio for delirium of 13 (95%CI 3.9–43.3, P<0.001). Disruption of large-scale brain networks that normally support attention and conscious awareness was thus associated with acute delirium symptoms.
Conclusions
Higher odds ratio for delirium was increased due to hematoma location. The location of neurological injury could be of high prognostic value for predicting delirium symptoms.
Keywords: delirium, quality of life, intracerebral hemorrhage, voxel-based lesion-symptom mapping
Introduction
Delirium symptoms include altered mental status, inattention, and disorganized thinking, and have been associated with global risk factors (1–3) such as benzodiazepine use or sepsis, and persistently worse cognition (4). Delirium assessment recently has been validated in patients with primary neurologic disease (5). In patients with intracerebral hemorrhage (ICH), delirium symptoms are associated with persistently worse functional outcomes and Health-Related Quality of Life (HRQoL), particularly cognition- and fatigue-related HRQoL (6). A neuroanatomic basis for delirium symptoms has not been identified. Testing neurologic contributors to delirium symptoms is crucial to determine if these symptoms represent a neurologic condition or rather the influence of residual confounding factors (7).
Delirium symptoms have been associated with preexisting frontal lobe and hippocampal atrophy (8). Furthermore, disruption of long-range white matter tracts one year after hospitalization is predicted by acute delirium symptoms (9), although it is unclear whether these findings represent premorbid deterioration versus changes during recovery. Indeed, there is a complex relationship between vulnerability, precipitating factors, and long-term consequences of delirium symptoms (7). Nonetheless, associations between delirium symptoms and disruption of hippocampal and long-range cortico-cortico connections are consistent with postulated roles of these structures in supporting cognitive function and conscious awareness (10–14). Delirium symptoms and their consequences in patients with neurologic injuries such as those due to ICH mirror those in other patient groups, particularly in terms of long-term consequences for cognition (7). Brain hemorrhage, particularly in frontal and deep structures, has been linked to acute confusional states such as delirium (15) and later dysexecutive behavior (16, 17).
We sought to test the hypothesis that the hematoma location is associated with delirium symptoms in ICH patients using voxel-based lesion-symptom mapping (VLSM) as a first step to identifying a potential neuroanatomic basis for delirium symptoms during the acute period.
Materials and Methods
Participants
We prospectively identified 90 patients with acute ICH, diagnosed by a neurologist and correlated with acute findings in CT scan. All patients with acute spontaneous ICH are admitted to the Neuro/Spine Intensive Care Unit at Northwestern Memorial Hospital, where they were identified. ICH attributed to trauma, hemorrhagic conversion of ischemic stroke, structural lesions, or vascular malformations were exclusionary criteria. Demographic information and severity of injury scores are provided in Table 1. Data from one subject were excluded due to failure of CT normalization procedures (see below), yielding a final N=89. The patient or a legally authorized representative provided written informed consent for research, except when an incapacitated patient had no legally authorized representative, in which case an exemption to written consent was permitted. Patients were young relative to many ICH populations and there was no significant history of cognitive impairment prior to acute ICH in included subjects. All study procedures were approved by the Northwestern University Institutional Review Board.
Table 1.
Age, gender, hematoma volume (mm3), National Institutes of Health Stroke Scale (NIHSS) at admit, modified Rankin Scale (mRS) at 14-day follow-up, and modified Rankin Scale at 3-month follow-up scores, subdivided by acute delirium status.
| Age | % Female | Volume | NIHSS admit | mRS 14-d | mRS 3-m | |
|---|---|---|---|---|---|---|
| Ever delirious (n=25) | 64 (1.9) | 40.0 | 18.8 [9.9–25.4] | 10 [6 – 14] | 5 (0.2)* | 4 (0.4) |
| Never delirious (n=64) | 61 (1.8) | 45.3 | 22.8 [9.5–25.4] | 10 [7 – 12] | 4 (0.2)* | 3 (0.2) |
Is presented as mean (SD), female sex as percent. ICH hematoma volume and NIHSS are provided as median with 95% confidence intervals. Values for mRS 14-d and 3-m are provided as medians. Standard error is provided in parentheses Significant pairwise differences (P<0.05) between ever-delirious and never-delirious subjects are indicated by asterisk, calculated using a t-test for values reported as means and as a Mann Whitney U test for values reported as medians (non-directional tests were used).
Acute delirium assessment
The Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) (18–20) is a dichotomous clinical bedside rating that was used by trained ICU nurses twice daily in the Neuro/Spine ICU and stroke unit (ICU step-down) according to established procedures, as we have previously described (6). A positive CAM-ICU indicated the presence of change from the patient’s baseline mental status on hospital admission plus inattention and either altered level of consciousness or disorganized thinking as previously described (5). Patients with at least one positive CAM-ICU assessment were classified as ever delirious, and patients with all negative assessable CAM-ICU scores were classified as never delirious. As previously noted, patients who were never assessable with the CAM-ICU were comatose and all died by 28 days after ICH onset (and were thus not enrolled in this study).
Health-related quality of life assessment
Neuro Quality of Life (Neuro-QOL) and the NIH Patient Reported Outcomes Measurement Information System (PROMIS) provide valid and reliable tools for HRQoL assessment in patients with stroke, cancer, and other disorders (21). HRQoL assessments were made at 28 days, 3 months, and 12 months post-discharge when possible. Patients or proxies were sent scripted, deidentified emails to complete HRQoL assessments online or were contacted via telephone to complete a phone interview. Results are expressed as T scores, centered on demographics of the general U.S. population at 50 +/− 10. Further information is available at www.nihpromis.org and www.neuroqol.org. Based on our previous findings of an association between delirium symptoms and worse scores on the “applied cognition - executive function” and “fatigue” Neuro-QOL scales at follow-up (7), we focused on these scales for the current analysis. Scores were available from 59% of subjects (n=52; 48% of ever delirious and 63% of never delirious subjects) for at least one of the three follow-up assessments. Scores from the one-year follow-up were used when available. Of the never-delirious subjects with available Neuro-QOL scores, 70% had scores from the one-year assessment and 28-day assessments were used in the remaining subjects. Of the ever-delirious subjects with available Neuro-QOL scores, 92% had scores from the one-year assessment and 28-day assessments were used in the remaining subjects. Neuro-QOL has been previously validated for caregiver report, and we previously reported similar results after correction for caregiver vs. patient report (6).
VLSM using acute CT scans
Serial non-contrast CT neuroimages were obtained within 48 hours after the neuroimaging performed at admission to the Neuro/Spine-ICU (38 axial images with a 5mm gap and 0.5mm in-plane resolution). Hematomas were demarcated on CT images by a trained neurologist (A.M.N.) and staff under his supervision, using established procedures (22, 23) in AnalyzeDirect (Overland Park, KS). Hematoma demarcation was performed blind to delirium status. The hematoma extend was exported as a hematoma volumetric mask.
The CT images and the hematoma volume mask images were converted into Neuroimaging Informatics Technology Initiative format using dcm2nii (www.mricro.com). The CT scans and the hematoma volume mask images were then transformed to standard MNI space using Statistical Parametric Mapping (SPM5) in conjunction with a CT-specific normalization template (24). Each subject’s hematoma volume mask was smoothed at 6 mm Full-Width Half-Maximum (FWHM) and used as a cost-function mask to decrease the effect of space-occupying hematomas on normalization (25). The CT scans as well as the hematoma volume masks were transformed into MNI space using these procedures and then resampled to isotropic 1 mm voxels, allowing for voxel-wise statistical comparisons.
VLSM (26) utilized Non-Parametric Mapping software (27). For voxel-wise analyses, delirium was treated as a binary variable (ever versus never delirious) and the Liebermeister measure was used to assess the presence or absence of delirium with the presence or absence of hematoma (27). Only voxels that were encompassed by hematoma in at least four participants were included, and nonparametric statistical maps were corrected for multiple comparisons using the False Discovery Rate (FDR) to ensure a false-positive rate of P<0.05 (28). The FDR-corrected Z threshold was 2.3. A power analysis (29) indicated a minimum Z score of 3.1 for detecting a relationship in the analyzed voxels. All voxels included in the analysis thus had adequate power to detect relationships between delirium and the presence or absence of hematoma (i.e., the minimum Z was higher than the FDR-corrected Z threshold). Statistical maps are displayed on the N27 template of Holmes et al. (30) to aid in anatomical localization, as confirmation of neuroanatomical locations is challenging using the mean CT image (provided in Supplementary Figure 1). For other statistical analyses, normally distributed data in two groups (e.g., age) were compared with a t-test, and categorical variables (e.g. number female) were compared with chi-squared.
Results
Demographic data are shown in Table 1. The most common clinical etiology of ICH was hypertension (51, 57%), followed by medication related, e.g., warfarin (9, 10%), amyloid angiopathy (9, 10%), with the remainder being other or cryptogenic.
Acute hematoma locations associated with delirium symptoms
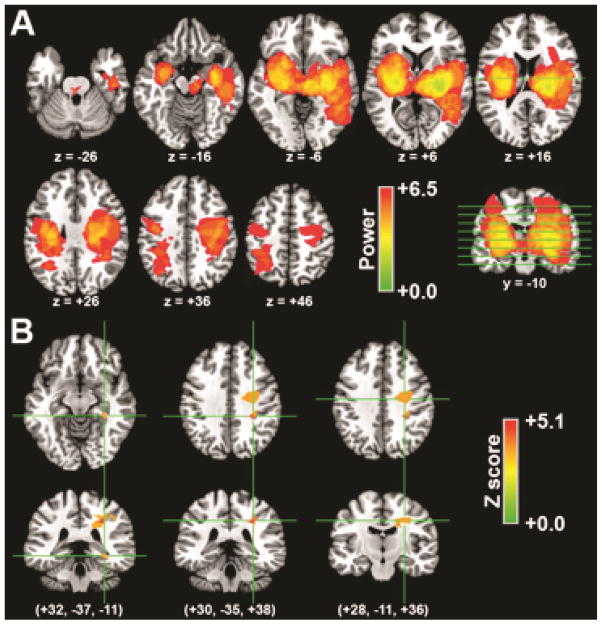
As we hypothesized, VLSM identified associations between hematoma location and delirium symptoms. Delirium symptoms were associated with hematoma of right-hemisphere subcortical white matter, including two regions of superior longitudinal fasciculus (relatively posterior and relatively anterior), as well as of parahippocampal gyrus (Figure 1; Table 2).
Figure 1. Acute hematoma locations associated with acute delirium.
(A) Colorization indicates power values for the ability to detect a significant relationship between acute delirium status and the presence of hematoma at each voxel. The indicated axial slices are marked by lines on the coronal slice. (B) Statistical nonparametric maps of the relationship between acute delirium status and hematoma location. Colorization indicates the voxel-wise Z score for the relationship, and only regions that survive P<0.05 FDR correction are shown. The left side of the image is the patient’s left (rather than the clinical convention).
Table 2.
Summary of hematoma locations associated with delirium.
| Centroid coordinate | Volume | Peak Z | |||
|---|---|---|---|---|---|
| X | Y | Z | |||
| R. Parahippocampal gyrus | +32 | −37 | −11 | 205 | 4.8 |
| R. Superior longitudinal fasiculus (posterior) | +30 | −35 | +38 | 745 | 5.1 |
| R. Superior longitudinal fasciculus (anterior) | +28 | −11 | +36 | 2,387 | 4.9 |
Centroid coordinates are Montreal Neurologic Institute (MNI)-305. Volume is provided in mm3. The Z score for the association with delirium is provided for the peak voxel in each region. All regions are defined based on a FDR-corrected P<0.05 (see text).
To evaluate the increased likelihood of delirium symptoms given hematoma to these locations, we treated them as a region of interest (ROI) and calculated relative risk for delirium symptoms given hematoma that included any voxel within the ROI versus hematoma that did not include any voxel within the ROI. Hematoma within the ROI increased relative risk for delirium by 6.8 (95% CI=2.7–17.0, Z=4.1, P<0.0001; Odds ratio=13.0, 95% CI=3.9–43.3, Z=4.2, P<0.0001). We also calculated relative risk for hematoma of each of the three regions treated as separate ROIs, which ranged from 5.2–7.8 and was statistically significant for each region (parahippocampal gyrus relative risk=7.8, 95% CI 1.7–36.1, Z=2.6, P=0.009; posterior white matter relative risk=6.9, 95% CI=2.0–24.1, Z=3.1, P=0.002; anterior white matter relative risk=6.5, 95% CI=1.5–28.6, Z=2.5, P=0.01).
We have previously reported no correlation between hematoma volume and delirium symptoms in ICH patients (6). In the current sample, there was no significant difference between hematoma volumes for the ever-delirious versus the never-delirious groups (Table 1; t(87)=0.48, ns). To determine whether the VLSM findings could have resulted as secondary effects of variability in hematoma volumes (i.e., whether only subjects with relatively large volumes had hematomas that encompassed the regions associated with delirium symptoms based on VLSM), we compared hematoma volumes for subjects that did versus did not have hematoma including the three delirium-associated regions. Mean hematoma volume for subjects with hematomas including any of the three delirium-associated regions did not differ significantly from mean volume for subjects with hematomas not including these regions (t(87)=1.34, ns), suggesting that VLSM findings were not merely secondary to variation in hematoma volume.
Association between delirium symptoms and HRQoL
To confirm that the current delirium sample experienced long-term impairments, scores on the Neuro-QOL scales were compared. Reported executive function (“Applied Cognition Executive Function” scale) was significantly lower for ever-delirious subjects versus never delirious subjects (mean = 26.4 (SD=2.3) versus 39.9 (SD=1.8), respectively; t(50)=2.86, P=0.006). Neuro-QOL fatigue scores did not differ for the two groups (P=0.37), and there was a non-significant trend for worse reported general cognitive abilities (“Applied Cognition General Concerns” scale) in ever-delirious versus never-delirious subjects (mean = 36.1 (SD=1.9) versus 43.2 (SD=1.5); P=0.08). Lower reported executive function scores at follow-up for ever-delirious versus never-delirious subjects were reliable even when considering only 1-year follow-up scores in a subset of subjects (t(32)=2.6, P=0.01). Scores on all other Neuro-QOL scales did not differ significantly for ever-delirious versus never-delirious subjects.
Discussion
We found that hematoma in the right parahippocampal region and parietal lobe was associated with acute delirium symptoms. This is an attractive neurologic explanation for delirium symptoms because the locations identified are required for conscious awareness and higher-order cognitive function, specifically long-range cortico-cortical connections that support large-scale brain networks for functions such as attention (10, 12–14). Additionally, the hippocampus and parahippocampal gyrus have been implicated in awareness, specifically with respect to operations that support declarative memory (11, 31). This furthers our understanding of delirium symptoms in neurologically injured patients by providing a compelling neuroanatomic basis. Further, our findings advance the field by identifying hematoma locations predictive of which patients are at highest risk for acute delirium symptoms and subsequent worse patient- and caregiver-reported cognitive function.
These data complement our previous publication showing that clinical severity of neurologic injury, hematoma volume, benzodiazepine use, and seizures were not associated with delirium symptoms (6). That is, these general clinical characteristics have not provided a plausible explanation for delirium symptoms in patients with ICH, whereas hematoma location does provide such an explanation. Patients with ICH provide an attractive model for studying delirium symptoms because they have a readily identified focal lesion (the hematoma), infection is uncommon (in our cohort), and sedation is minimal to avoid clouding repeated neurologic assessments (20). Furthermore, we have recently identified high prevalence of acute delirium in patients with ICH and subsequently impaired HRQoL, particularly with respect to self-reported executive function (6). We focused on the acute hematoma as an obvious cause of acute injury, but other potential causes should be considered in future research, such as acute ischemia seen on MRI, inflammation and seizures (32). These could contribute to the long-term follow-up findings of damage to these structures that has been associated with delirium severity and cognitive decline (9).
We found that delirium symptoms were associated with right-sided lesions that are in close alignment with VLSM findings for hemispatial neglect after the acute period (33). This may underscore inattention as a cardinal feature of delirium. Limitations of the CAM-ICU include a lack of support for subtyping or grading the severity of delirium symptoms. Defining subtypes of delirium may be important to further refine our understanding of its basis and consequences.
Current severity-of-injury scales do not account for hematoma location other than supra- vs. intra-tentorial (34, 35), even though the location dictates most of the clinical symptoms. The current findings underscore the need to account for damage location in more detail in order to better characterize factors that correlate with meaningful patient- and caregiver-reported outcomes at follow-up in addition to acute delirium symptoms.
This study focuses on a cohort of patients with neurologic disease, but much of the literature to date on delirium symptoms focuses in the medical and surgical intensive care unit, where causes such as sedation, infection and other systemic illness are more common and potentially confound the associations between delirium symptoms and later outcomes (4). It is not known if focal neurologic injury changes the risk of delirium symptoms in these populations, and they typically do not undergo routine CT or MRI scanning. Hematoma location may mediate the relationship between delirium and later cognitive impairment. Survivors of the acute respiratory distress syndrome with hypoxemia are more likely to have later cognitive impairments (36), suggesting cerebral ischemia in the areas we have implicated, but this is conjectural.
Several limitations to our findings deserve mention. Our VLSM approach is limited to those portions of the brain that were consistently encompassed by hematoma in our cohort. Thus, some locations could not be assessed, such as anterior frontal cortex, which might also plausibly be linked to delirium symptoms (15), and visual cortex (Figure 1), which seems less likely. Statistical power was robust for the areas we identified as associated with delirium symptoms, cortico-cortico white-matter tracts and parahippocampal gyrus, and hematoma in these regions significantly increased risk for delirium. Disruption of long-range white-matter projections could generally contribute to delirium, although our analyses could only robustly identify a subset of these pathways and our findings were specific to the right hemisphere. It is possible that pre-existing cognitive decline predisposes to hemorrhage in a certain location, but as previously noted, we had too few patients with a history of cognitive decline to test for this. Finally, we performed VSLM only in patients with ICH. These methods are potentially applicable to ischemic stroke, although shower of emboli and lacunar stroke would reduce overlap between patients, making VSLM less informative. Patients with subarachnoid hemorrhage (ruptured brain aneurysm) are also at high risk for long-term dysexecutive behavior, particularly those with involvement of the anterior cerebral artery (16, 17).
Delirium symptoms were associated with patient- or caregiver-reported executive function. Self-reported measures may not be as accurate as objective testing, however, the questions in this instrument are relatively specific to executive function (e.g., planning, keeping appointments) as well as several aspects of memory function (e.g., remembering events). Future research might perform specific neurocognitive assessments as a complement to reported cognitive impairment in order to better assess effects of acute delirium symptoms on specific neurocognitive processes that could underlie self-report measures.
We measured delirium symptoms with the CAM-ICU by trained nurses. This measure has the benefits of being validated, suitable for repeat assessment, electronic charting, and is reliably performed by bedside nurses. Unfortunately, it also has limitations: the CAM-ICU is a dichotomous score, so severity of delirium cannot be assessed. Most ever-delirious patients have a positive CAM-ICU for one day, so it is not helpful to analyze duration of delirium as a covariate as has been done in previous publications (4, 37). As we previously noted, in patients with ICH nearly all delirium symptoms are hypoactive (encephalopathy), not hyperactive (6), so our results may not apply to patients with agitated delirium.
In sum, we found that hematoma of right cortical white matter and parahippocampal gyrus was associated with acute delirium symptoms. The disruption of these regions provides a compelling neurologic reason for delirium symptoms. Future research should more accurately characterize the range of altered consciousness encompassed by the term “delirium symptoms,” determine the specific cognitive deficits underlying worse reported cognition, and determine if focal neurologic damage underlies worse outcomes in other patients with delirium symptoms.
Supplementary Material
Acknowledgments
Financial Support:
Research was supported by award number R00-NS069788 from the National Institute of Neurological Disorders and Stroke. The infrastructure for automated data retrieval was funded in part by the National Institutes of Health through a grant to Northwestern University’s Clinical and Translational Sciences (NUCATS) UL1RR025741. Dr. Naidech received partial salary support through a subcontract of National Institute for Neurological Disorders and Stroke contract HHSN271201200036C. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
After the first submission, Dr. Naidech was awarded HS023437 from the Agency for Healthcare Quality and Research. Effort for the revision was partly supported by that award.
Footnotes
Kelly Polnaszek carried out the statistical analysis under the direction of Dr. Voss.
Author Contributions
Andrew Naidech collaborated on the study design and hypothesis with Dr. Voss, and co-wrote the manuscript.
Kelly Polnaszek performed voxel-based lesion symptom mapping and performed the statistical analysis.
Michael Berman mapped the hematomas for voxel-based lesion symptom mapping under Dr. Naidech, and collected clinical and follow-up data.
Joel Voss oversaw the voxel-based lesion symptom mapping and statistical analysis.
Part of this work was presented in abstract form at the 2014 American Delirium Society conference by invitation, and travel was partially offset by a grant from the Northwestern Memorial Foundation to Dr. Naidech.
References
- 1.Lin SM, Huang CD, Liu CY, et al. Risk factors for the development of early-onset delirium and the subsequent clinical outcome in mechanically ventilated patients. J Crit Care. 2008;23:372–379. doi: 10.1016/j.jcrc.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Pandharipande PP, Shintani A, Peterson J, et al. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006;104:21–26. doi: 10.1097/00000542-200601000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Pandharipande PP, Pun BT, Herr DL, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: The MENDS randomized controlled trial. JAMA. 2007;298:2644–2623. doi: 10.1001/jama.298.22.2644. [DOI] [PubMed] [Google Scholar]
- 4.Pandharipande PP, Girard TD, Ely EW. Long-term cognitive impairment after critical illness. N Engl J Med. 2014;370:185–186. doi: 10.1056/NEJMc1313886. [DOI] [PubMed] [Google Scholar]
- 5.Mitasova A, Kostalova M, Bednarik J, et al. Poststroke delirium incidence and outcomes: Validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) Crit Care Med. 2012;40:484–490. doi: 10.1097/CCM.0b013e318232da12. [DOI] [PubMed] [Google Scholar]
- 6.Naidech AM, Beaumont JL, Rosenberg NF, et al. Intracerebral hemorrhage and delirium symptoms. Length of stay, function, and quality of life in a 114-patient cohort. Am J Respir Crit Care Med. 2013;188:1331–1337. doi: 10.1164/rccm.201307-1256OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Popp J. Delirium and cognitive decline: More than a coincidence. Curr Opin Neurol. 2013;26:634–639. doi: 10.1097/WCO.0000000000000030. [DOI] [PubMed] [Google Scholar]
- 8.Gunther ML, Morandi A, Krauskopf E, et al. The association between brain volumes, delirium duration, and cognitive outcomes in intensive care unit survivors: The VISIONS cohort magnetic resonance imaging study*. Crit Care Med. 2012;40:2022–2032. doi: 10.1097/CCM.0b013e318250acc0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morandi A, Rogers BP, Gunther ML, et al. The relationship between delirium duration, white matter integrity, and cognitive impairment in intensive care unit survivors as determined by diffusion tensor imaging: The VISIONS prospective cohort magnetic resonance imaging study*. Crit Care Med. 2012;40:2182–2189. doi: 10.1097/CCM.0b013e318250acdc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernat JL. Chronic disorders of consciousness. Lancet. 2006;367:1181–1192. doi: 10.1016/S0140-6736(06)68508-5. [DOI] [PubMed] [Google Scholar]
- 11.Cohen NJ, Squire LR. Preserved learning and retention of pattern-analyzing skill in amnesia: Dissociation of knowing how and knowing that. Science. 1980;210:207–210. doi: 10.1126/science.7414331. [DOI] [PubMed] [Google Scholar]
- 12.Fernández-Espejo D, Soddu A, Cruse D, et al. A role for the default mode network in the bases of disorders of consciousness. Ann Neurol. 2012;72:335–343. doi: 10.1002/ana.23635. [DOI] [PubMed] [Google Scholar]
- 13.Levine B. Autobiographical memory and the self in time: Brain lesion effects, functional neuroanatomy, and lifespan development. Brain Cogn. 2004;55:54–68. doi: 10.1016/S0278-2626(03)00280-X. [DOI] [PubMed] [Google Scholar]
- 14.Mesulam MM. From sensation to cognition. Brain. 1998;121:1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- 15.DeLuca J, Cicerone KD. Confabulation following aneurysm of the anterior communicating artery. Cortex. 1991;27:417–423. doi: 10.1016/s0010-9452(13)80036-6. [DOI] [PubMed] [Google Scholar]
- 16.Krause M, Mahant N, Kotschet K, et al. Dysexecutive behaviour following deep brain lesions – A different type of disconnection syndrome? Cortex. 2012;8:97–119. doi: 10.1016/j.cortex.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 17.Wheatley J, McGrath J. Co-occurrence of executive impairment and amnesic syndrome following sub arachnoid haemorrhage: A case study. Cortex. 1997;33:711–721. doi: 10.1016/s0010-9452(08)70728-7. [DOI] [PubMed] [Google Scholar]
- 18.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: Validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286:2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 19.Ely EW, Margolin R, Francis J, et al. Evaluation of delirium in critically ill patients: Validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) Crit Care Med. 2001;29:1370–1379. doi: 10.1097/00003246-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Maas MB, Rosenberg NF, Kosteva AR, et al. Surveillance neuroimaging and neurologic examinations affect care for intracerebral hemorrhage. Neurology. 2013;81:107–112. doi: 10.1212/WNL.0b013e31829a33e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cella D, Lai JS, Nowinski CJ, et al. Neuro-QOL: Brief measures of health-related quality of life for clinical research in neurology. Neurology. 2012;78:1860–1867. doi: 10.1212/WNL.0b013e318258f744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guth JC, Nemeth AJ, Rosenberg NF, et al. Subarachnoid extension of primary intracerebral hemorrhage is associated with fevers. Neurocrit Care. 2014;20:187–192. doi: 10.1007/s12028-013-9888-0. [DOI] [PubMed] [Google Scholar]
- 23.Naidech AM, Jovanovic B, Liebling S, et al. Reduced platelet activity is associated with early clot growth and worse 3-month outcome after intracerebral hemorrhage. Stroke. 2009;40:2398–401. doi: 10.1161/STROKEAHA.109.550939. [DOI] [PubMed] [Google Scholar]
- 24.Rorden C, Bonilha L, Fridriksson J, et al. Age-specific CT and MRI templates for spatial normalization. Neuroimage. 2012;61:957–965. doi: 10.1016/j.neuroimage.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andersen SM, Rapcsak SZ, Beeson PM. Cost function masking during normalization of brains with focal lesions: Still a necessity? Neuroimage. 2010;53:78–84. doi: 10.1016/j.neuroimage.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bates E, Wilson SM, Saygin AP, et al. Voxel-based lesion-symptom mapping. Nat Neurosci. 2003;6:448–450. doi: 10.1038/nn1050. [DOI] [PubMed] [Google Scholar]
- 27.Rorden C, Karnath HO, Bonilha L. Improving lesion-symptom mapping. J Cogn Neurosci. 2007;19:1081–1088. doi: 10.1162/jocn.2007.19.7.1081. [DOI] [PubMed] [Google Scholar]
- 28.Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 29.Gläscher J, Tranel D, Paul LK, et al. Lesion mapping of cognitive abilities linked to intelligence. Neuron. 2009;61:681–691. doi: 10.1016/j.neuron.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holmes CJ, Hoge R, Collins L, et al. Enhancement of MR images using registration for signal averaging. J Comput Assist Tomogr. 1998;22:324–333. doi: 10.1097/00004728-199803000-00032. [DOI] [PubMed] [Google Scholar]
- 31.Eichenbaum H, Cohen NJ. From Conditioning to Conscious Recollection: Memory Systems of the Brain. Oxford: Oxford University Press; 2001. [Google Scholar]
- 32.Garg RK, Liebling SM, Maas MB, et al. Blood pressure reduction, decreased diffusion on MRI, and outcomes after intracerebral hemorrhage. Stroke. 2012;43:67–71. doi: 10.1161/STROKEAHA.111.629493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mort DJ, Malhotra P, Mannan SK, et al. The anatomy of visual neglect. Brain. 2003;126:1986–1997. doi: 10.1093/brain/awg200. [DOI] [PubMed] [Google Scholar]
- 34.Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin scale: Implications for stroke clinical trials: a literature review and synthesis. Stroke. 2007;38:1091–1096. doi: 10.1161/01.STR.0000258355.23810.c6. [DOI] [PubMed] [Google Scholar]
- 35.Saver JL, Filip B, Hamilton S, et al. Improving the reliability of stroke disability grading in clinical trials and clinical practice: The Rankin Focused Assessment (RFA) Stroke. 2010;41:992–995. doi: 10.1161/STROKEAHA.109.571364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Angus DC, Musthafa AA, Clermont G, et al. Quality-adjusted survival in the first year after the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;163:1389–1394. doi: 10.1164/ajrccm.163.6.2005123. [DOI] [PubMed] [Google Scholar]
- 37.Brummel NE, Jackson JC, Pandharipande PP, et al. Delirium in the ICU and subsequent long-term disability among survivors of mechanical ventilation. Crit Care Med. 2014;42:369–377. doi: 10.1097/CCM.0b013e3182a645bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.