Abstract
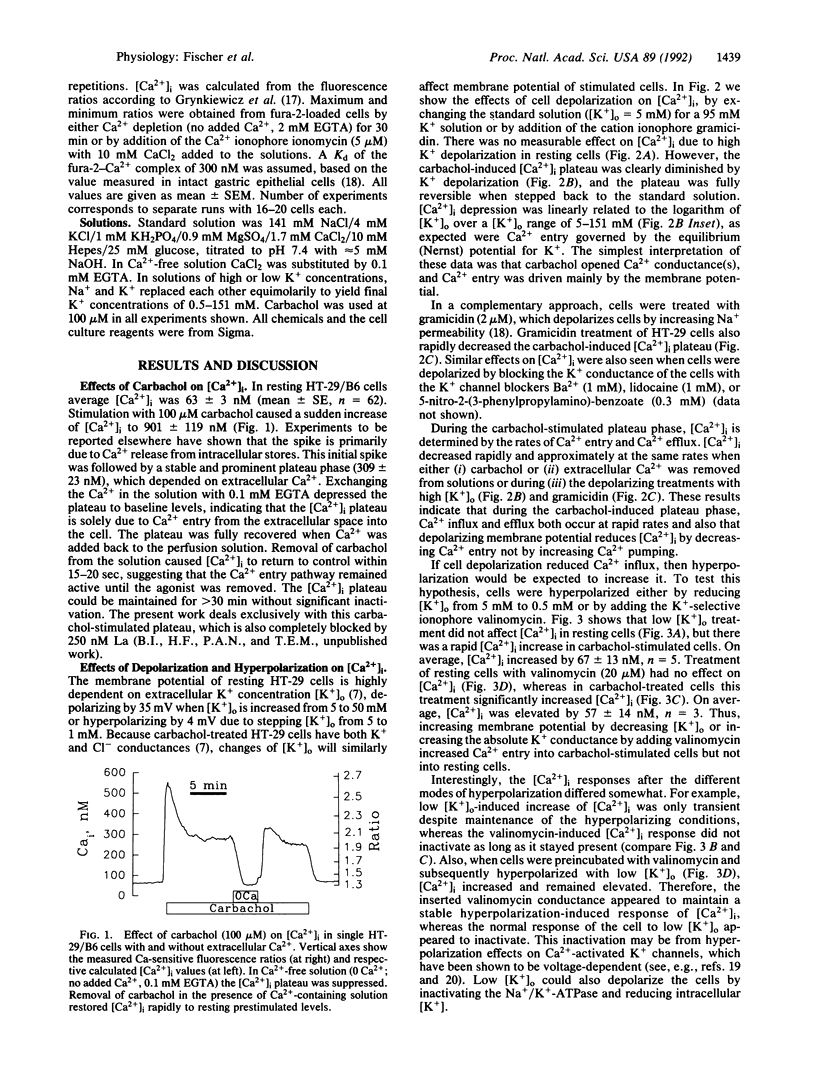
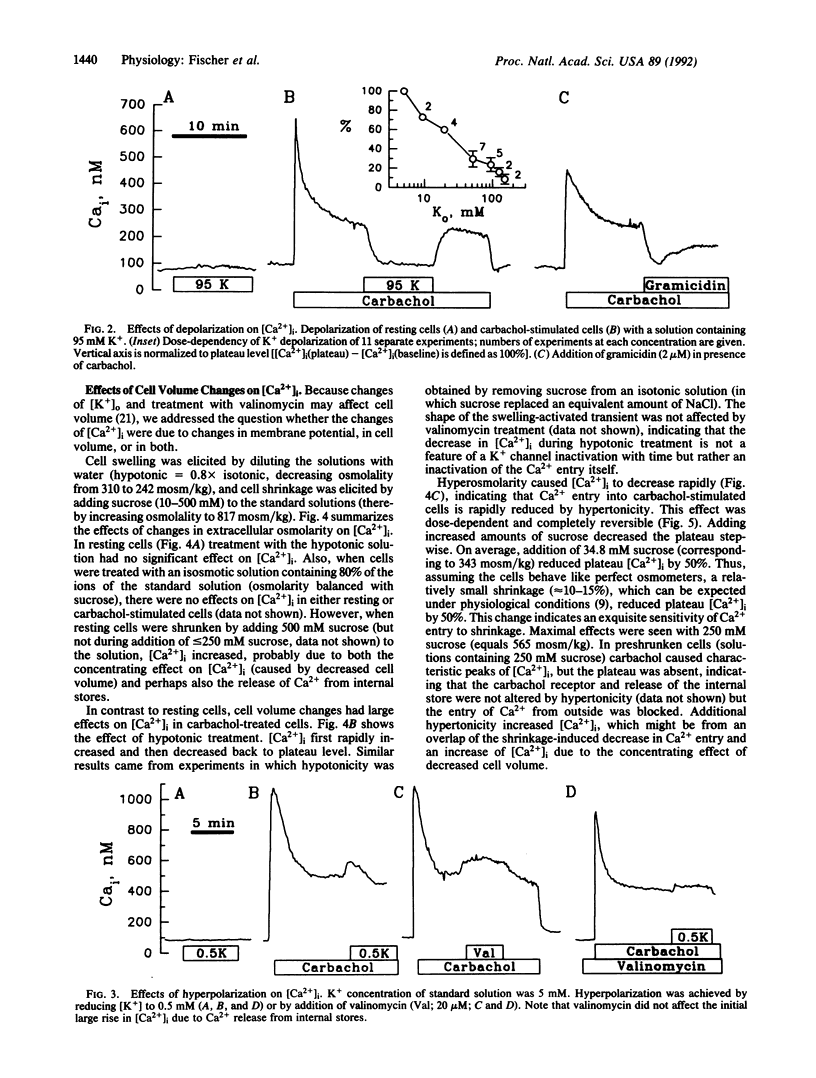
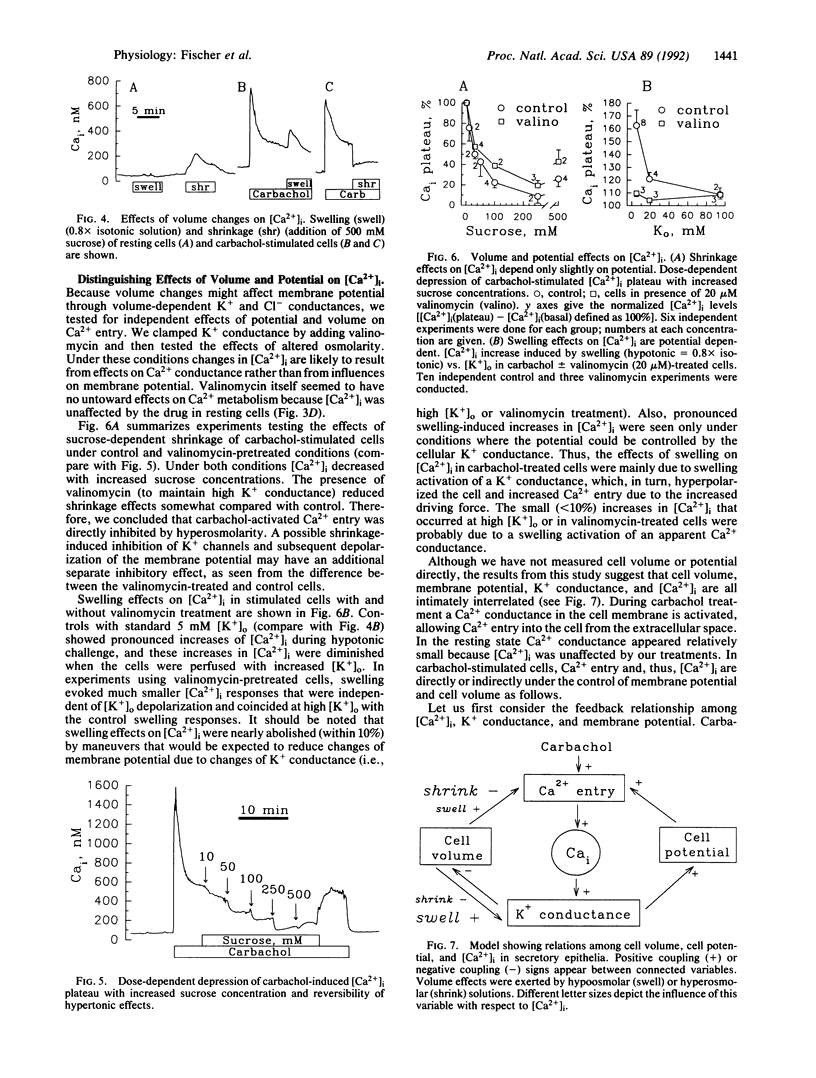
Intracellular Ca2+ ([Ca2+]i) was measured in single Cl(-)-secretory HT-29/B6 colonic carcinoma cells with the Ca2+ probe fura-2 and digital imaging microscopy. Resting [Ca2+]i was 63 +/- 3 nM (n = 62). During treatment with the muscarinic agonist carbachol, [Ca2+]i rapidly increased to 901 +/- 119 nM and subsequently reached a stable level of 309 +/- 23 nM, which depended on Ca2+ entry into the cells from the extracellular solution. The goal of this study was to characterize the Ca2+ entry pathway across the cell membrane with respect to its dependence on membrane potential and cell volume. Under resting conditions [Ca2+]i showed no apparent dependence on either potential or cell volume. After stimulating Ca2+ entry with carbachol (100 microM), [Ca2+]i increased with hyperpolarization (low-K+ or valinomycin treatment) and decreased with depolarization (high-K+ or gramicidin treatment) of the cell, as expected from changes in driving force for Ca2+ entry. In stimulated cells, hypotonic solutions caused [Ca2+]i to increase, whereas hypertonic solutions blocked Ca2+ entry. The shrinkage-induced decreases in [Ca2+]i were only slightly affected when the membrane potential was increased with valinomycin, suggesting that shrinkage directly affects the carbachol-activated Ca2+ conductance. In contrast, the swelling-induced increase in [Ca2+]i was significantly reduced in valinomycin-treated cells, suggesting an indirect dependence on a swelling-activated K+ conductance. Thus, carbachol-stimulated Ca2+ entry is under the dual control of membrane potential and cell volume. This mechanism may serve as a regulatory influence that determines the extent of Ca2+ influx during cholinergic stimulation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolívar J. J., Cereijido M. Voltage and Ca2+-activated K+ channel in cultured epithelial cells (MDCK). J Membr Biol. 1987;97(1):43–51. doi: 10.1007/BF01869613. [DOI] [PubMed] [Google Scholar]
- Christensen O. Mediation of cell volume regulation by Ca2+ influx through stretch-activated channels. Nature. 1987 Nov 5;330(6143):66–68. doi: 10.1038/330066a0. [DOI] [PubMed] [Google Scholar]
- Christensen O., Zeuthen T. Maxi K+ channels in leaky epithelia are regulated by intracellular Ca2+, pH and membrane potential. Pflugers Arch. 1987 Mar;408(3):249–259. doi: 10.1007/BF02181467. [DOI] [PubMed] [Google Scholar]
- Cliff W. H., Frizzell R. A. Separate Cl- conductances activated by cAMP and Ca2+ in Cl(-)-secreting epithelial cells. Proc Natl Acad Sci U S A. 1990 Jul;87(13):4956–4960. doi: 10.1073/pnas.87.13.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson D. C., Richards N. W. Basolateral K conductance: role in regulation of NaCl absorption and secretion. Am J Physiol. 1990 Aug;259(2 Pt 1):C181–C195. doi: 10.1152/ajpcell.1990.259.2.C181. [DOI] [PubMed] [Google Scholar]
- Foskett J. K., Melvin J. E. Activation of salivary secretion: coupling of cell volume and [Ca2+]i in single cells. Science. 1989 Jun 30;244(4912):1582–1585. doi: 10.1126/science.2500708. [DOI] [PubMed] [Google Scholar]
- Germann W. J., Ernst S. A., Dawson D. C. Resting and osmotically induced basolateral K conductances in turtle colon. J Gen Physiol. 1986 Aug;88(2):253–274. doi: 10.1085/jgp.88.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hosey M. M., Lazdunski M. Calcium channels: molecular pharmacology, structure and regulation. J Membr Biol. 1988 Sep;104(2):81–105. doi: 10.1007/BF01870922. [DOI] [PubMed] [Google Scholar]
- Kreusel K. M., Fromm M., Schulzke J. D., Hegel U. Cl- secretion in epithelial monolayers of mucus-forming human colon cells (HT-29/B6). Am J Physiol. 1991 Oct;261(4 Pt 1):C574–C582. doi: 10.1152/ajpcell.1991.261.4.C574. [DOI] [PubMed] [Google Scholar]
- Lewis S. A., Wills N. K. Use of ionophores in epithelia: characterizing membrane properties. Methods Enzymol. 1989;171:715–736. doi: 10.1016/s0076-6879(89)71039-9. [DOI] [PubMed] [Google Scholar]
- Matthews G., Neher E., Penner R. Second messenger-activated calcium influx in rat peritoneal mast cells. J Physiol. 1989 Nov;418:105–130. doi: 10.1113/jphysiol.1989.sp017830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt J. E., Rink T. J. Regulation of cytosolic free calcium in fura-2-loaded rat parotid acinar cells. J Biol Chem. 1987 Dec 25;262(36):17362–17369. [PubMed] [Google Scholar]
- Negulescu P. A., Machen T. E. Intracellular Ca regulation during secretagogue stimulation of the parietal cell. Am J Physiol. 1988 Jan;254(1 Pt 1):C130–C140. doi: 10.1152/ajpcell.1988.254.1.C130. [DOI] [PubMed] [Google Scholar]
- Parod R. J., Putney J. W., Jr The role of calcium in the receptor mediated control of potassium permeability in the rat lacrimal gland. J Physiol. 1978 Aug;281:371–381. doi: 10.1113/jphysiol.1978.sp012428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner R., Matthews G., Neher E. Regulation of calcium influx by second messengers in rat mast cells. Nature. 1988 Aug 11;334(6182):499–504. doi: 10.1038/334499a0. [DOI] [PubMed] [Google Scholar]
- Taniguchi J., Guggino W. B. Membrane stretch: a physiological stimulator of Ca2+-activated K+ channels in thick ascending limb. Am J Physiol. 1989 Sep;257(3 Pt 2):F347–F352. doi: 10.1152/ajprenal.1989.257.3.F347. [DOI] [PubMed] [Google Scholar]
- Worrell R. T., Butt A. G., Cliff W. H., Frizzell R. A. A volume-sensitive chloride conductance in human colonic cell line T84. Am J Physiol. 1989 Jun;256(6 Pt 1):C1111–C1119. doi: 10.1152/ajpcell.1989.256.6.C1111. [DOI] [PubMed] [Google Scholar]
- Yada T., Oiki S., Ueda S., Okada Y. Intestinal secretagogues increase cytosolic free Ca2+ concentration and K+ conductance in a human intestinal epithelial cell line. J Membr Biol. 1989 Dec;112(2):159–167. doi: 10.1007/BF01871277. [DOI] [PubMed] [Google Scholar]



