Abstract
Background
The A‐kinase anchor proteins (AKAP) are a growing family of scaffolding proteins involved in the occurrence, proliferation, and metastasis of tumors by controlling intracellular signals. In this study, the expression and significance of AKAP4 were analyzed in patients with lung adenocarcinoma and adjacent non‐cancerous tissues.
Methods
Using reverse transcriptase‐polymerase chain reaction and Western blot, AKAP4 messenger ribonucleic acid (mRNA) and protein expression levels were measured in 108 cases of lung adenocarcinoma and adjacent non‐cancerous tissues.
Results
AKAP4 mRNA and protein were expressed in lung adenocarcinoma tissues, but not in adjacent non‐cancerous tissues. The expression of AKAP4 mRNA and protein was closely associated with lymphatic metastasis (P < 0.05), but had no relationship with stage, differentiation degree, gender, age or smoking (P > 0.05). AKAP4 expression had an adverse effect on the overall survival rate (P < 0.05).
Conclusion
The expression of AKAP4 was high in lung adenocarcinoma tissue, which may be closely related to the lymphatic metastasis of lung adenocarcinoma. AKAP4 may be a novel lung adenocarcinoma molecule marker and a predictor of poor prognosis.
Keywords: Adenocarcinoma, AKAP4, lung cancer
Introduction
Lung cancer is increasingly more commonly diagnosed and has become one of the leading causes of cancer‐related death worldwide.1 As a result of poor and limited medical infrastructure and awareness, mortality in developing countries is even higher.1 Studies on lung cancer indicate that early diagnosis can improve clinical outcome and overall survival.2 There are three diagnostic methods of lung cancer: imageology, pathology, and biochemical techniques; however, they cannot be widely used to screen lung cancer patients. Biomarkers have the potential to become an important method for the early diagnosis of lung cancer as they are inexpensive, noninvasive, and convenient. To date, many biomarkers have been found and used to diagnose lung cancer, including carcinoembryonic antigen (CEA), cytokeratin 19 fragements (CYFRA21‐1), neuron specific enolase (NSE), and squamous cell carcinoma antigen (SCC‐Ag).3 However, because of their low diagnostic sensitivity and specificity, none of these are perfect biomarkers for lung cancer.3 Therefore, it is necessary to determine a novel tumor biomarker that can be useful for the early diagnosis of lung cancer.
Cancer testis antigens (CTAs) are a unique class of tumor‐associated antigens that show restricted expression in testis and various cancer cells only.4 The testis is an immune privileged organ in which human leukocyte antigen (HLA) molecules are not expressed. CTAs expressed in malignancies may have the potential to become biomarkers and antigen targets for immunotherapy.5 Our previous studies have demonstrated the expression of a novel CTA, A‐kinase anchor protein 4 (AKAP4), in the sera of lung cancer patients. The CTA AKAPs are a growing family of scaffolding proteins that control signal transduction by affecting cyclic AMP‐dependent protein kinase A (PKA).6, 7 Various studies have revealed that PKA is involved in the cell proliferation, angiogenesis, and chemoresistance of various cancers.8, 9, 10 AKAP4, as an immunogenic CTA, has been associated with multiple myeloma and prostate, ovarian, and breast cancers.11 – 14 The involvement of AKAP4 demonstrates potential as a biomarker for the better clinical management of cancer patients. Herein, we demonstrate aberrant expression of AKAP4 in lung adenocarcinoma and adjacent non‐cancerous tissues in order to develop an early detection biomarker for better cancer treatment modalities in lung cancer patients.
Patients and methods
Patients
During routine surgical procedures at Qingdao Medical College Affiliated Hospital between December 2010 and December 2011, 108 lung adenocarcinoma tissue and 108 matched available adjacent non‐cancerous tissue (ANCT, > 5 cm beyond tumor edge) specimens were collected. Informed written consent was obtained from all patients and surgeons. The patients did not receive any chemotherapy or radiotherapy after diagnosis. The approval for conducting the research was obtained from the Institutional Human Ethical Committee of Qingdao Medical College Affiliated Hospital. Tumor tissue samples and ANCT specimens were stored at −80°C until use.
Semi‐quantitative reverse transcriptase‐polymerase chain reaction (PCR)
Total ribonucleic acid (RNA) was extracted from tumor tissue and ANCT specimens using the Trizol method. RNA was dissolved in diethylpyrocarbonate (DEPC) water and concentration was determined. cDNA was synthesized from RNA using a reverse transcription kit (Tiangen Biotech [Beijing] Co., Ltd, Beijing, China). Reverse transcriptase‐polymerase chain reaction (RT‐PCR) was performed using cDNA as a template, following AKAP4 primers (forward 5'‐GCTGAGAAAGTCGGTGAACA‐3' and reverse 5'‐ GTGAATCTGTGGAAGCATTGA‐3'). Reverse transcription was conducted by incubation for 15 minutes at 95°C, followed by 40 cycles of 95°C for 10 seconds, 60°C for 32 seconds, and 72°C for 10 minutes. Glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) expression was used as an internal control. The PCR products were analyzed on 2% agarose gels and photographed under ultraviolet light. The whole operation was performed following the Taq PCR Master Mix manual (Tiangen Biothech [Beijing] Co., Ltd). All primers were synthesized by Sangon Biotech Co., Ltd (Shanghai, China).
Real‐time fluorescent quantitative PCR
As per the SuperReal PreMix Plus manual (SYBR Green; Tiangen Biothech [Beijing] Co., Ltd), real‐time fluorescent quantitative PCR was performed using cDNA as a template. The AKAP4 primer sequence was as follows: forward 5'‐ GCTGAGAAAGTCGGTGAACA‐3' and reverse 5'‐ GTGAATCTGTGGAAGCATTGA‐3'. Forty amplification cycles (1 cycle of denaturation at 95°C for 15 minutes, 40 cycles denaturation at 95°C for 10 seconds, annealing at 50°C for 20 seconds, extension at 72°C for 20 seconds, and a final elongation cycle at 65°C for 5 seconds) were carried out for each sample. Real‐time fluorescent quantitative PCR for GAPDH messenger (m)RNA expression was used as an internal control. All primers were synthesized by Sangon Biotech Co., Ltd (Shanghai, China). The results were analyzed by livak comparative Ct method (2‐ÄÄCt).
Western blotting
A‐kinase anchor protein 4 expression was analyzed in lung adenocarcinoma tissue by Western blot. All of the frozen specimens were fully grinded in radioimmunoprecipitation assay (RIPA) lysis buffer on ice. The lysate was centrifuged and the supernatant was prepared. The protein concentration of each sample was determined using a bicinchoninic acid (BCA) protein assay kit (Beyotime Biotechnology, Jangsu, China): 50 ìg of each protein sample was separated by 10% sodium dodecyl sulfate‐polyacrylamide gel electrophoresis (SDS‐PAGE) and transferred to polyvinylidene fluoride (PVDF) membrane. The membrane was blocked in 5% non‐fat skimmed milk/tris‐buffered saline (TBS; 5 × tris‐buffered saline plus tween 20 [0.1% Tween 20]) for two hours and incubated with rabbit polyclonal anti‐AKAP4 (1:1000) (Abcam, Cambridge, UK) in 4°C overnight. Following TBST flushing, the membrane was incubated in anti‐rabbit immunoglobin G (Abcam) for one hour at room temperature. Immunoreactive bands were visualized by an enhanced chemiluminescence (ECL) system and analyzed using Quantity One software (Bio‐Rad, Hercules, CA, USA). GAPDH expression was used as an internal control.
Follow‐up
Observations of 108 patients commenced at diagnosis and were censored at the last observation or the date of death. The survival time was recorded.
Statistical analysis
Data were expressed as mean ± standard error of at least three independent experiments. All statistical analysis was performed using SPSS software version 19 (IBM Corp., Armonk, NY, USA). Pearson's chi‐square, Kaplan–Meier, and Cox multivariate analyses were used to analyze AKAP4 expression and humoral response in various clinical subgroups, including stage, grade, metastasis, and prognosis. A P value of < 0.05 was considered statistically significant.
Results
Expression of A‐kinase anchor protein 4 (AKAP4) messenger ribonucleic acid in lung adenocarcinoma specimens
Expression of AKAP4 mRNA was investigated in lung adenocarcinoma tissue specimens by semi‐quantitative RT‐PCR and real‐time fluorescent quantitative PCR. AKAP4 mRNA expression was detected in 76.85% (83/108) of lung adenocarcinoma patients using semi‐quantitative RT‐PCR. No AKAP4 mRNA expression was detected in available matched ANCT specimens (Fig 1). The results indicated that AKAP4 expression was associated with lung adenocarcinoma tissue (P < 0.01, Pearson's chi‐square test; Table 1). AKAP4 mRNA expression was found in 65.08% early stage (stage I + II) and 71.11% late stage (stage III + IV) lung adenocarcinoma patients using real‐time fluorescent quantitative PCR. It was interesting to note that there was no significant difference in AKAK4 mRNA expression between early and late stage patients (P > 0.05; Fig. 2a, Table 1). AKAP4 mRNA expression in the lymphatic metastasis group was significantly higher than in the non‐lymphatic metastasis group (P < 0.05; Fig. 2b, Table 1). No significant difference was observed in the various differentiation degrees using Pearson's chi‐square test (P > 0.05; Table 1). Moreover, there was no obvious relationship between AKAP4 mRNA expression and gender, age or smoking history (P > 0.05; Table 1). Results of the analysis between AKAP4 mRNA expression level and all clinicopathological characteristics are shown in Table 1.
Figure 1.
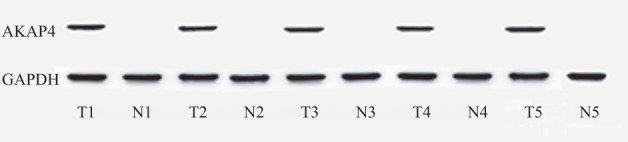
Expression of A‐kinase anchor protein (AKAP)4 messenger ribonucleic acid in lung adenocarcinoma and adjacent non‐cancerous tissue by semi‐quantitative polymerase chain reaction. GAPDH, glyceraldehyde 3‐phosphate dehydrogenase.
Table 1.
Relationship between AKAP4 expression and clinical characteristics of patients
| Clinical characteristics | Cases (n) | AKAP4 mRNA and protein expression | χ2 | P value | |
|---|---|---|---|---|---|
| Positive | Negative | ||||
| All patients | 108 | ||||
| All tissues | 67.398 | P < 0.01 | |||
| Tumor | 108 | 83 | 25 | ||
| ANCT | 108 | 0 | 108 | ||
| Gender | 0.342 | P > 0.50 | |||
| Male | 56 | 27 | 29 | ||
| Female | 52 | 28 | 24 | ||
| Age | 0.199 | P > 0.50 | |||
| ≥60 | 23 | 14 | 9 | ||
| <60 | 85 | 56 | 29 | ||
| Smoking | 2.595 | P > 0.10 | |||
| Non‐smoker | 34 | 16 | 18 | ||
| Smoker | 74 | 47 | 27 | ||
| TNM‐stage | 0.436 | P > 0.50 | |||
| I + II | 63 | 41 | 22 | ||
| III + IV | 45 | 32 | 13 | ||
| Differentiation | 0.108 | P > 0.05 | |||
| Well/Mod | 47 | 15 | 7 | ||
| Poor | 61 | 26 | 10 | ||
| Lymph node | 6.591 | P < 0.05 | |||
| Negative | 48 | 21 | 27 | ||
| Positive | 60 | 41 | 19 | ||
ANCT, adjacent non‐cancerous tissue; APAK4, A‐kinase anchor protein 4; mRNA, messenger ribonucleic acid; TNM, tumor node metastasis.
Figure 2.

Expression of A‐kinase anchor protein (AKAP)4 messenger ribonucleic acid was detected in lung adenocarcinoma by real‐time fluorescent quantitative polymerase chain reaction.
Expression of AKAP4 protein in lung adenocarcinoma specimens
Western blot was used to detect AKAP4 protein expression in lung adenocarcinoma and ANCT specimens. AKAP4 protein was detected in 76.85% (83/108) of lung adenocarcinoma patients. No discrepancy between AKAP4 mRNA and AKAP4 protein expression in lung adenocarcinoma tissue (Fig 3) was found. AKAP4 protein was not detected in ANCT specimens (Fig 3), which confirmed that AKAP4 is associated with lung adenocarcinoma. We found that AKAP4 protein was expressed in all stages of lung adenocarcinoma and there was no significant difference in AKAP4 protein expression between the early and late stage groups (P > 0.05, Table 1). These results were similar to those found for AKAP4 mRNA expression. Therefore, we determined that AKAP4 could have broad clinical utility as a biomarker. We further extended our analysis of AKAP4 expression to groups with and without lymphatic metastasis. As with AKAP4 mRNA, significant difference was observed between these groups (P < 0.05, Table 1). While comparing various differentiation degrees, no significant difference was observed using Pearson's chi‐square test (P > 0.05, Table 1). In addition, no significant difference was observed between AKAP4 protein expression and gender, age or smoking history (P > 0.05, Table 1).
Figure 3.
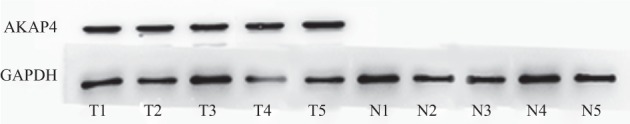
Expression of A‐kinase anchor protein (AKAP)4 protein in lung adenocarcinoma and adjacent non‐cancerous tissue detected by Western blot.
Influence of AKAP4 gene expression in lung adenocarcinoma on prognosis
Follow‐up commenced at diagnosis and was censored at last observation or death. We noted a significant difference between the positive and negative groups (log rank = 7.541, P < 0.05; Fig. 4). Multivariate Cox regression analysis was utilized to investigate seven factors of 108 cases, as shown in Table 2: gender, age, smoking history, tumor node metastasis (TNM) stage, differentiation degree, lymphatic metastasis, and AKAP4 expression. Smoking history, lymphatic metastasis, and AKAP4 expression had an independent and adverse influence on survival time. A high expression of AKAP4 had an adverse effect on lung adenocarcinoma prognosis (response rate 2.142, 95% confidence interval 1.164–4.294, P = 0.027). These results indicate that AKAP4 may not only play a potential role in the diagnosis of lung adenocarcinoma but may also be helpful in disease prognosis. Therefore, further studies are warranted using a larger number of patients to validate our findings.
Figure 4.
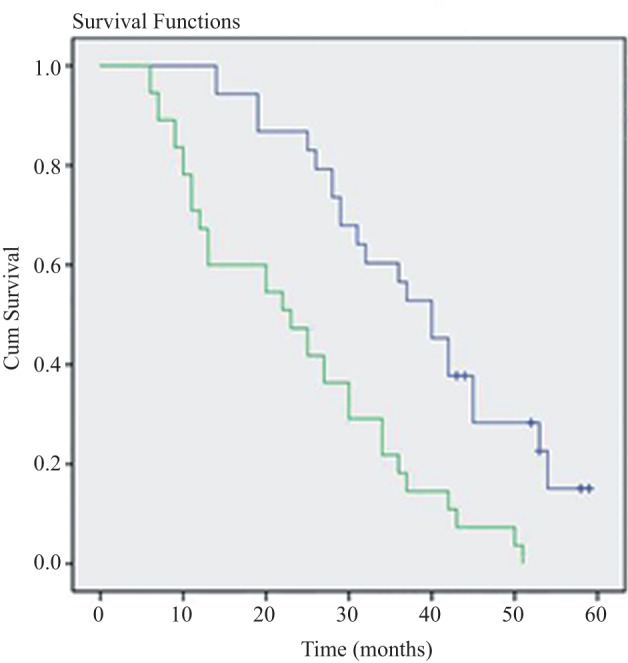
Relationship between expression of A‐kinase anchor protein (AKAP)4 messenger ribonucleic acid and overall survival rate of lung adenocarcinoma patients (Kaplan–Meier method).
Table 2.
Cox multivariate analysis of prognostic factors
| Variables | RR | 95% CI | P value |
|---|---|---|---|
| AKAP4 | 2.142 | 1.164–4.294 | 0.027 |
| Gender | 0.908 | 0.523–1.577 | 0.732 |
| Age | 0.989 | 0.967–1.012 | 0.359 |
| Smoking history | 1.780 | 0.983–3.222 | 0.037 |
| Clinical stage | 2.211 | 1.260–3.880 | 0.006 |
| Differentiation | 1.073 | 0.598–1.926 | 0.814 |
| Lymph metastasis | 1.950 | 1.056–3.602 | 0.033 |
APAK4, A‐kinase anchor protein 4; CI, confidence interval; RR, response rate.
Discussion
Recently, CTAs, a restricted expression in germ cell differentiation in testis, have been reported in various malignancies.5 Because HLA molecules are not expressed in testis, CTAs expressed in testis cannot produce antibodies in sera. Further, various studies have reported the involvement of CTAs in the regulation of transcription, cell proliferation, and apoptosis.6, 7 Because of their aberrant expression in various cancers, potent immunogenicity, and limited or lack of expression in normal tissues other than germ cells in testis, CTAs are envisaged to be clinically important as biomarkers and therapeutic targets.
In the present study, AKAP4 was highly expressed (76.85%, 83/108) in lung adenocarcinoma tissues, but not in ANCT tissues. AKAP4 mRNA and proteins were detected in all stages of lung adenocarcinoma tissues. In a previous study, we determined that AKAP4 was highly expressed in the serum of lung adenocarcinoma patients. Therefore, AKAP4 may become a novel tumor marker of lung adenocarcinoma. There was a significant difference in AKAP4 expression between the lymphatic metastasis and non‐lymphatic metastasis groups (P < 0.05), which indicates that AKAP4 is connected with lymphatic metastasis of lung adenocarcima and may predict a poor prognosis. Various studies have revealed that AKAPs are a family of scaffolding proteins that control signal transduction by affecting cyclic AMP‐dependent PKA, which is involved in the cell proliferation, transcription, and apoptosis of various cancers.6, 7, 8, 9, 10 We conclude that the mechanism of lymphatic invasiveness caused by AKAP4 may interact with PKA; however, further studies are required to confirm this conclusion.
Analysis of the follow‐up data using Cox regression analysis showed that AKAP4 expression had an independent influence on survival time, as well as stage and lymphatic metastasis. The higher the expression of AKAP4, the poorer the prognosis. This evidence indicates that AKAP4 may be used as a tumor marker in the diagnosis and prognosis of lung adenocarcinoma. Furthermore, because of its immunogenicity and limit, AKAP4 may have potential for specific therapeutic targets, which could improve prognosis without serious side‐effects.15 Further studies need to be conducted to explore the implications of AKAP4 as an immunotherapeutic target.
Conclusion
In conclusion, our study results indicated that AKAP4 expression may play a role in the diagnosis and prognosis of lung adenocarcinoma and is involved with the lymphatic invasiveness of lung adenocarcinoma. These results are worthy of further study of AKAP4 as a potential biomarker and an immunotherapeutic target in lung adenocarcinoma.
Disclosure
No authors report any conflict of interest.
Acknowledgment
This study was supported by the Public Science and Technology Support Program of Qingdao Technology Bureau (No. 09‐1‐1‐9‐NSH).
References
- 1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013; 63: 11–30. [DOI] [PubMed] [Google Scholar]
- 2. O'Dowd EL, Baldwin DR. Early diagnosis pivotal to survival in lung cancer. Practitioner 2014; 258: 21–24, 2–3. [PubMed] [Google Scholar]
- 3. Tanaka F, Yoneda K, Kondo N et al Circulating tumor cell as a diagnostic marker in primary lung cancer. Clin Cancer Res 2009; 15: 6980–6986. [DOI] [PubMed] [Google Scholar]
- 4. Hofmann O, Caballero OL, Stevenson BJ et al Genome‐wide analysis of cancer/testis gene expression. Proc Natl Acad Sci U S A 2008; 105: 20422–20427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Suri A. Cancer testis antigens–their importance in immunotherapy and in the early detection of cancer. Expert Opin Biol Ther 2006; 6: 379–389. [DOI] [PubMed] [Google Scholar]
- 6. Jin J, Smith FD, Stark C et al Proteomic, functional, and domain‐based analysis of in vivo 14‐3‐3 binding proteins involved in cytoskeletal regulation and cellular organization. Curr Biol 2004; 14: 1436–1450. [DOI] [PubMed] [Google Scholar]
- 7. Teijeiro JM, Marini PE. The effect of oviductal deleted in malignant brain tumor 1 over porcine sperm is mediated by a signal transduction pathway that involves pro‐AKAP4 phosphorylation. Reproduction 2012; 143: 773–785. [DOI] [PubMed] [Google Scholar]
- 8. Al‐Dhaheri MH, Rowan BG. Protein kinase A exhibits selective modulation of estradiol‐dependent transcription in breast cancer cells that is associated with decreased ligand binding, altered estrogen receptor alpha promoter interaction, and changes in receptor phosphorylation. Mol Endocrinol 2007; 21: 439–456. [DOI] [PubMed] [Google Scholar]
- 9. Avilova EA, Andreeva OE, Shatskaia VA, Krasil'nikov MA. [The role of protein kinase PAK1 in the regulation of estrogen‐independent growth of breast cancer.] Biomed Khim 2014; 60: 322–331. (In Russian.) [DOI] [PubMed] [Google Scholar]
- 10. Bradbury AW, Carter DC, Miller WR, Cho‐Chung YS, Clair T. Protein kinase A (PK‐A) regulatory subunit expression in colorectal cancer and related mucosa. Br J Cancer 1994; 69: 738–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chiriva‐Internati M, Ferrari R, Yu Y et al AKAP‐4: A novel cancer testis antigen for multiple myeloma. Br J Haematol 2008; 140: 465–468. [DOI] [PubMed] [Google Scholar]
- 12. Chiriva‐Internati M, Yu Y, Mirandola L et al Identification of AKAP‐4 as a new cancer/testis antigen for detection and immunotherapy of prostate cancer. Prostate 2012; 72: 12–23. [DOI] [PubMed] [Google Scholar]
- 13. Agarwal S, Saini S, Parashar D et al The novel cancer‐testis antigen A‐kinase anchor protein 4 (AKAP4) is a potential target for immunotherapy of ovarian serous carcinoma. Oncoimmunology 2013; 2: e24270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Saini S, Jagadish N, Gupta A, Bhatnagar A, Suri A. A novel cancer testis antigen, A‐kinase anchor protein 4 (AKAP4) is a potential biomarker for breast cancer. PLoS ONE 2013; 8(2): e57095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Scudellari M. A ballsy search for cancer targets. Nat Med 2011; 17: 916–918. [DOI] [PubMed] [Google Scholar]


