Abstract
Background
To evaluate the pattern of lymph node metastasis (LNM) according to primary tumor location in T1 and T2 stage non‐small cell lung cancer (NSCLC) patients.
Methods
The data of 1916 NSCLC patients with LNM who underwent surgery with systematic nodal resection between November 2008 to December 2014 were included in the study. Analyses of tumor location, pathological T stage, and nodal metastasis were performed.
Results
In T1a stage patients, superior mediastinum, aortopulmonary, and inferior mediastinum lymph node metastases were observed in primary tumors present in the right upper lobe (RUL), left upper lobe (LUL) and right middle lobe (RML), respectively. In T1b‐stage patients, superior mediastinum, aortopulmonary, and inferior mediastinum lymph node metastases were observed in the RML, LUL, and right lower lobe (RLL), respectively. In patients with T2a‐stage, superior mediastinum, aortopulmonary and inferior mediastinum lymph node metastases were observed in the RUL, LUL, and RLL, respectively. However, in T2b‐stage patients, RUL, LUL and RML locations were associated with superior mediastinum, aortopulmonary, and inferior mediastinum lymph node metastases, respectively. Multivariable logistic regression showed that T stage was significantly associated with mediastinal and intrapulmonary lymph node metastases. In addition, tumor location was significantly associated with N2 station LNM.
Conclusion
LNM varied according to tumor location and T‐stage, which are independent factors influencing N2 station LNM.
Keywords: Lymph node, metastasis, non‐small cell lung cancer, T1 stage, T2 stage
Introduction
At present, lung cancer is the leading cause of death of patients with tumors in China. Surgery is the main treatment for patients with early non‐small cell lung cancer (NSCLC). It is generally accepted that lymph node metastasis (LNM) is a very important prognostic factor in patients with NSCLC. To improve survival rate and enhance staging accuracy, lobectomy with systematic lymph node dissection (LND) has been widely used in the treatment of patients with NSCLC.1, 2, 3, 4 However, some researchers have argued that selective LND (SLND) may be an alternative approach to systematic LND in elderly patients or in patients with early stage tumors, because systematic LND extends surgical duration and increases postoperative morbidity. Several studies have revealed no difference in the prognosis of patients treated with systematic LND or SLND;5, 6, 7, 8, 9, 10 therefore, the choice of which lymphadenectomy to choose during surgery remains controversial. SLND requires a clear understanding of lymphatic spreading patterns. Many researchers have debated the relationship between lymphatic spreading pattern and factors such as tumor size and tumor location; however, lymphatic spreading patterns are not yet well understood.
The purpose of this retrospective study was to evaluate the pattern of LNM according to primary tumor location in T1 and T2 stage NSCLC patients.
Patients and methods
Patients
We retrospectively reviewed data of 2755 patients who underwent pulmonary resection at Shanghai Cancer Center from November 2008 to December 2014. Among them, 1916 NSCLC patients with T1–2 stage underwent lobectomy or pneumonectomy with systematic nodal resection for primary lung cancer. Exclusion criteria included: patients with pulmonary benign masses; Tx, T0, and Tis stage tumors; primary tumors spreading across the adjacent lobe or main bronchus; and leafy multiple primary tumors. Evaluations before surgery included: physical examination; computed tomography (CT) of the chest; B ultrasound of the abdomen, supraclavicular, and axillary lymph nodes; magnetic resonance imaging (MRI) of the brain; bone scintigraphy examinations; and pulmonary function tests. Positron‐emission tomography (PET) scans and endobronchial ultrasound‐guided transbronchial needle aspiration (EBUS‐TBNA) were also performed in some patients, but were not part of routine examinations. Two pathologists confirmed the histopathologic types of these patients after surgery. Lung cancer stage was determined based on the 2014 8th edition of the tumor node metastasis (TNM) classification.
Methods
Lymph nodes were classified into four groups according to their location: superior mediastinum lymph nodes included nodes of stations 1–4; aortopulmonary lymph nodes included stations 5–6; inferior mediastinum lymph nodes included stations 7–9; and intrapulmonary lymph nodes included stations 10–14. EBUS‐TBNA was performed when the short axis of the mediastinal lymph node was larger than 1 cm. If mediastinal lymph node disease was confirmed, the patient received neoadjuvant chemotherapy and was excluded from the research. During surgery, if the tumor specimen was confirmed using frozen sections as malignant, but was not carcinoma in situ, systematic LND was then performed. Systematic LND on the right mediastinum means the 2R, 4R, 7, 8, and 9 stations were completely resected, while on the left means the 4L, 5, 6, 7, 8, and 9 stations were removed. Data of lymph node spreading patterns of different T stages according to primary tumor location were collected.
Statistical analysis
All statistical analyses were performed using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). A Pearson χ2 test was performed to analyze the differences in two‐category comparison and Fisher's exact test for quantitative data. Multivariable logistic regression analyses were used to determine the independent association between LNM and T stage or primary tumor location. P values less than 0.05 were considered statistically significant.
Result
Patient characteristics
There were 1916 patients in this retrospective study. Of all the patients studied, 586 cases (30.6%) had primary tumors located in the right upper lobe (RUL), 128 (6.7%) in the right middle lobe (RML), 528 (19.1%) in the right lower lobe (RLL), 528 (27.5%) in the left upper lobe (LUL), and 308 (16.1%) in the left lower lobe (LLL). Among the 1916 patients, 734 patients were in T1a stage, 403 in T1b, 611 in T2a, and 168 patients in pT2b. Patient characteristics are listed in Table 1.
Table 1.
Patient characteristics (n = 1916)
| Parameters | n | (%) |
|---|---|---|
| Gender | ||
| Male | 1138 | 59.4 |
| Female | 778 | 40.6 |
| Age (year) (mean ± SD) | 60.1±10.5 | |
| Histology type | ||
| Adenocarcinoma | 1362 | 71.1 |
| Squamous cell carcinoma | 492 | 25.7 |
| Other | 62 | 3.2 |
| Pathological tumor status | ||
| T1a | 734 | 38.3 |
| T1b | 403 | 21.0 |
| T2a | 611 | 31.9 |
| T2b | 168 | 8.8 |
| Tumor location | ||
| Right upper lobe | 568 | 29.6 |
| Right middle lobe | 124 | 6.5 |
| Right lower lobe | 391 | 20.4 |
| Left upper lobe | 523 | 27.3 |
| Left lower lobe | 310 | 16.2 |
SD, standard deviation.
Lymph node metastases according to primary tumor location in patients with different T stages
In the 734 pT1a stage patients with superior mediastinum lymph node metastases, the tumor was mainly located in the RUL (9.6%, P < 0.05). However, in patients with aortopulmonary or inferior mediastinum lymph node metastases, the tumor was located in the LUL (6.1%, P < 0.01) or RML (12.9%, P < 0.01), respectively. Tumors were mainly located in the RML (11.3%) in patients with intrapulmonary lymph node metastases, but the difference was not significant (P = 0.935). In 403 patients with pT1b stage, the cancer was mainly located in the RML (22.7%, P < 0.05) in patients with superior mediastinum lymph node metastases; in the LUL (16.7%, P < 0.01) in aortopulmonary lymph node metastases; in the RLL (21.9%, P < 0.01) in inferior mediastinum lymph node metastases; and in the LLL (34.8%, P = 0.222) in intrapulmonary lymph node metastases. In 611 patients with pT2a stage, patients with cancer located in the RUL were more likely to have superior mediastinum lymph node metastases (24.6%, P < 0.01) than those with cancer located in other lobes. A relatively higher rate of cancer located in the LUL (21.0%, P < 0.01) was observed in patients with aortopulmonary lymph node metastases, while inferior mediastinum lymph node metastases were most often located in the RLL (30.1%, P < 0.01). Patients with cancer located in the RLL had more intrapulmonary lymph node metastases (36.6%) than cancer located in other lobes, but no significant difference was observed (P = 0.099). In 168 patients with pT2b stage with superior mediastinum lymph node metastases, cancer was mainly located in the RUL (23.8%), but the difference was not significant (P = 0.306). The main form of cancer in patients with inferior mediastinum or intrapulmonary lymph node metastases was located in the RML (33.3%, P = 0.05) or LLL (37.1%, P = 0.256), respectively. LUL (26.7%, P < 0.05) was the main location of cancer in patients with aortopulmonary node metastases (Table 2).
Table 2.
Lymph node metastases according to primary tumor location in patients with different stages
| Patient with positive lymph nodes (n) | Tumor location | |||||
|---|---|---|---|---|---|---|
| LUL | LLL | RUL | RML | RLL | P value | |
| pT1a stage | 197 | 116 | 230 | 62 | 129 | |
| Superior mediastinum | 7 (3.6%) | 2 (1.7%) | 22 (9.6) | 5 (8.1%) | 7 (5.4%) | 0.020 |
| Aortopulmonary | 12 (6.1%) | 3 (2.6%) | 0 (0%) | 0 (0%) | 0 (0%) | 0.001 |
| Inferior mediastinum | 1 (0.5%) | 6 (5.2%) | 4 (1.7%) | 8 (12.9%) | 6 (4.7%) | 0.000 |
| Intrapulmonary | 21 (10.7%) | 10 (8.6%) | 20 (8.7%) | 7 (11.3%) | 13 (10.1%) | 0.935 |
| pT1b stage | 114 | 46 | 125 | 22 | 96 | |
| Superior mediastinum | 11 (9.6%) | 4 (8.7%) | 27 (21.6%) | 5 (22.7%) | 10 (10.4%) | 0.025 |
| Aortopulmonary | 19 (16.7%) | 6 (13.0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0.000 |
| Inferior mediastinum | 5 (4.4%) | 9 (19.6%) | 4 (3.2%) | 4 (18.2%) | 21 (21.9%) | 0.000 |
| Intrapulmonary | 24 (21.1%) | 16 (34.8%) | 24 (19.2%) | 6 (27.3%) | 26 (27.1%) | 0.222 |
| pT2a stage | 167 | 113 | 171 | 37 | 123 | |
| Superior mediastinum | 20 (12.0%) | 7 (6.2%) | 42 (24.6%) | 7 (18.9%) | 20 (16.3%) | 0.001 |
| Aortopulmonary | 35 (21.0%) | 8 (7.1%) | 0 (0%) | 0 (0%) | 0 (0%) | 0.000 |
| Inferior mediastinum | 14 (8.4%) | 29 (25.7%) | 12 (7.0%) | 8 (21.6%) | 37 (30.1%) | 0.000 |
| Intrapulmonary | 42 (25.1%) | 39 (34.5%) | 45 (26.3%) | 8 (21.6%) | 45 (36.6%) | 0.099 |
| pT2b stage | 45 | 35 | 42 | 3 | 43 | |
| Superior mediastinum | 6 (13.3%) | 3 (8.6%) | 10 (23.8%) | 0 (0%) | 5 (11.6%) | 0.306 |
| Aortopulmonary | 12 (26.7%) | 3 (8.6%) | 0 (0%) | 0 (0%) | 0 (0%) | 0.000 |
| Inferior mediastinum | 1 (2.2%) | 8 (22.9%) | 5 (11.9%) | 1 (33.3%) | 8 (18.6%) | 0.050 |
| Intrapulmonary | 11 (24.4%) | 13 (37.1%) | 7 (16.7%) | 0 (19.0%) | 11 (25.6%) | 0.256 |
LLL, left lower lobe; LUL, left upper lobe; RLL, right lower lobe; RML, right middle lobe; RUL, right upper lobe.
Lymph node metastases in patients with primary tumor located in each lobe according to different T stage
The incidence of LNM in aortopulmonary or inferior mediastinum zones was higher (P < 0.01) in T1a to T2b stage patients with cancer located in the LUL or LLL (only in T2a stage patients), respectively (Fig 1a–b). The incidence of LNM in the superior mediastinum zone was higher (P < 0.01) in T1a to T2b stage patients with cancer located in the RUL(Fig 1c). The higher incidence of LNM in the superior mediastinum zone in patients with T1b stage (P > 0.05) or inferior mediastinum zone in patients with T1a, T2a, or T2b stage was located in the RML (T1a, T2a stage: P < 0.05, T2b stage: P >0.05, Fig 1d). In patients with cancer located in the RUL, the incidence of LNM in the inferior mediastinum zone was higher in patients with T1b, T2a, T2b stage (P < 0.05) and in the superior mediastinum zone in T1a stage (P >0.05, Fig 1e).
Figure 1.
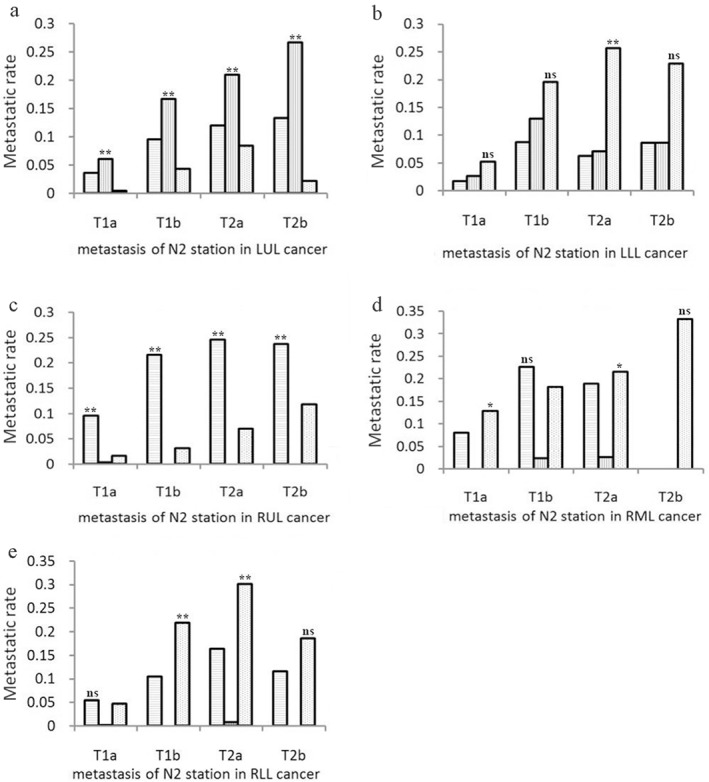
Lymph node metastases in patients with primary tumors located in each lobe. Patients were divided into four groups based on different T stage (T1a, T1b, T2a, and T2b) and the metastatic rate of superior mediastinum, aortopulmonary, and inferior mediastinum nodes in patients of different T stages with cancer located in the: (a) left upper lobe (LUL), (b) left lower lobe (LLL), (c) right upper lobe (RUL), (d) right middle lobe (RML), and (e) right lower lobe (RLL) were assessed. **P < 0.01, *P < 0.05, ns P > 0.05.
Multivariable logistic regression analysis of factors influencing lymph node metastasis
T stage was significantly associated with mediastinal and intrapulmonary LNM. Tumor location and histology type were significantly associated with N2 station LNM but not N1 station (Table 3).
Table 3.
Factors influencing lymph node metastasis
| Variable | UVA | MVA | ||
|---|---|---|---|---|
| P value | Risk ratio | 95% CI | P value | |
| Superior mediastinum node metastasis | ||||
| Tumor location† | 0.016 | 1.127 | 1.023–1.141 | 0.016 |
| T stage‡ | 0.000 | 1.459 | 1.270–1.676 | 0.000 |
| Histology type§ | 0.009 | 0.675 | 0.506–0.889 | 0.007 |
| Aortopulmonary node metastasis | ||||
| Tumor location† | 0.000 | 0.271 | 0.200–0.367 | 0.016 |
| T stage‡ | 0.000 | 1.644 | 1.334–2.027 | 0.000 |
| Histology type§ | 0.049 | 0.593 | 0.370–0.952 | 0.031 |
| Inferior mediastinum node metastasis | ||||
| Tumor location† | 0.000 | 1.401 | 1.256–1.403 | 0.000 |
| T stage‡ | 0.000 | 1.723 | 1.471–2.018 | 0.000 |
| Histology type§ | 0.002 | 0.520 | 0.354–0.763 | 0.001 |
| Intrapulmonary node metastasis | ||||
| T stage‡ | 0.000 | 1.588 | 1.420–1.775 | 0.000 |
| Tumor location† | 0.267 | Not included in MVA | ||
| Histology type§ | 0.100 | Not included in MVA | ||
Compared with left upper lobe;
compared with T1 stage;
compared with adenocarcinoma.CI, confidence interval; MVA, multivariate analysis; UVA, univariate analysis.
Discussion
As systematic LND extends the duration of surgery and may increase postoperative morbidity, SLND is inclined to substitute systematic LND in patients in early stage. Therefore, it is very important to understand the lymphatic spreading patterns for SLND. In the current study, we defined the pattern of lymph node metastases according to primary tumor location in NSCLC patients with T1and T2 stage.
In their study, Cahan et al. first discussed the relationship between the extent of dissection and primary tumor location.11 Several subsequent studies then demonstrated that LNM may be related to primary tumor location; however, these investigations rarely noted the impact of T stage on LNM.12, 13, 14 Different T stages of NSCLC mean different tumor growth time. Tumors in T2b stage are always more likely to metastasize to the lymph nodes than tumors in T1a stage. Therefore, when we explore the relationship between LNM and primary tumor location, it is important to take into account the impact of T stage on LNM. Bernard et al. classified 337 NSCLC patients with T1 or T2 stage into three groups: low, intermediate, and high risk.15 The latter two groups both had risk factors of primary tumor location in the right side. However, Bernard et al. did not observe the ratio of each metastatic zone in patients at different T stages or compare the difference between metastatic zones in patients with primary tumors located in different lobes. In our study, we divided all 1916 patients into subgroups according to T stage and monitored the LNM in each. The most common form of superior mediastinum lymph node metastases in patients with T1a, T2a, or T2b stage was located in the RUL, while RML was the most common location in patients with T1b stage. These results are slightly different from those found by Shimada et al. and Cerfolio and Bryant, as their patients were not subdivided according to T stage.14, 16 In T1b stage, superior mediastinum lymph node metastases were 20% more likely to occur in the RUL and RML than in other zones, suggesting that resection of lymph nodes in the superior mediastinum zone is necessary.
T1a to T2b stage patients with cancer located in the LUL were more likely to have aortopulmonary LNM than cancers located in other lobes. This result is consistent with previous studies performed by Shimada et al. and Miyoshi et al.14, 17 In patients with T1b stage, the rate of aortopulmonary LNM located in the LUL was 16.7%, indicating that resection of lymph nodes in the aortopulmonary zone is necessary. Patients with tumors located in the right side cannot be treated by radical aortopulmonary LND, which might be the reason why we found patients with cancer located in the LLL and LUL had more aortopulmonary LNM than patients with tumors located in the right side.
In the inferior mediastinum zone, the most common location of cancer was the RML in patients with T1a and T2b stage, while in patients with T1b and T2a stage the most common location was the RLL. These results are slightly different from investigations conducted by Shimada et al. and Cerfolio and Bryant, which determined that the RLL was the most common location.14, 16 We found that patients with tumors located in the upper lobes had less LNM to the inferior mediastinum zone than patients with tumors located in the lower lobes, especially in patients with T1a and T1b stage. This suggests that in patients with T1 stage, with tumors located in the upper lobes, resection of the lymph nodes in the inferior mediastinum zone is unnecessary. In T1a stage patients, the rate of inferior mediastinum LNM located in the RML was 12.9%, implying that resection of the lymph nodes in the inferior mediastinum zone is necessary. Further investigations are required to confirm these findings.
We found that cancer was most commonly located in the RML, LLL, RLL, and LLL in T1a, T1b, T2a, and T2b stage patients with intrapulmonary lymph node metastases, respectively; however, none of the differences were significant. Even in T1a stage, the intrapulmonary LNM rates of each lobe were nearly 10%, indicating that radical lymph node resection of the intrapulmonary zone might be necessary even in T1a stage, regardless of which lobe the primary tumor is located in.
In patients with cancer located in the LUL, the metastatic rates of the inferior mediastinum zone were less than 10% in each stage, which may suggest that dissection of the inferior mediastinum lymph node is unnecessary in T1 and T2 stage patients. In patients with cancer located in the LLL, the metastatic rate of the superior mediastinum zone was less than 10% from T1a to T2b stage, demonstrating that it is unnecessary to dissect the superior mediastinum lymph node even in T2b stage patients. In patients with cancer located in the RUL, the metastatic rate of the inferior mediastinum zone in T2b stage was 11.9%, much higher than in T1a to T2a stage, indicting that resection of the inferior mediastinum lymph node is necessary in T2b stage. Surprisingly, the superior mediastinum zone metastatic rates were close to those of the inferior mediastinum zone in T1a–T2a stage, which suggests that cancer located in the RML can easily flow to both upper and inferior mediastinum zones. However, the superior mediastinum zone metastatic rate was higher than 10% in T1b stage, which may imply that resection of superior mediastinum lymph nodes is necessary in cases located in the RLL.
We demonstrated that T stage is an independent factor that influences the LNM of N1 and N2 stations, while tumor location is an independent factor of N2 but not N1 station. This result indicated that the N2 station SLND pattern should be adjusted according to different T stage and tumor location.
In conclusion, our study demonstrated that the metastatic rate of lymph nodes in each lobe varied according to T1and T2 stage. SLND pattern should be considered according to different T stage and primary tumor location. Future prospective studies are needed to evaluate whether NSCLC patients could benefit from the adjustment of SLND.
Disclosure
No authors report any conflict of interest.
References
- 1. Liu T, Liu H, Li Y. Systematic lymph node dissection is necessary for T1a non‐small cell lung cancer. Asia Pac J Clin Oncol 2015; 11: 49–53. [DOI] [PubMed] [Google Scholar]
- 2. Oda M, Watanabe Y, Shimizu J et al Extent of mediastinal node metastasis in clinical stage I non‐small‐cell lung cancer: The role of systematic nodal dissection. Lung Cancer 1998; 22: 23–30. [DOI] [PubMed] [Google Scholar]
- 3. Kotoulas CS, Foroulis CN, Kostikas K et al Involvement of lymphatic metastatic spread in non‐small cell lung cancer accordingly to the primary cancer location. Lung Cancer 2004; 44: 183–191. [DOI] [PubMed] [Google Scholar]
- 4. Date H. The impact of complete lymph node dissection for lung cancer on the postoperative course. Thorac Surg Clin 2012; 22: 239–242. [DOI] [PubMed] [Google Scholar]
- 5. Ishiguro F, Matsuo K, Fukui T, Mori S, Hatooka S, Mitsudomi T. Effect of selective lymph node dissection based on patterns of lobe‐specific lymph node metastases on patient outcome in patients with resectable non‐small cell lung cancer: A large‐scale retrospective cohort study applying a propensity score. J Thorac Cardiovasc Surg 2010; 139: 1001–1006. [DOI] [PubMed] [Google Scholar]
- 6. Aokage K, Yoshida J, Ishii G, Hishida T, Nishimura M, Nagai K. Subcarinal lymph node in upper lobe non‐small cell lung cancer patients: Is selective lymph node dissection valid? Lung Cancer 2010; 70: 163–167. [DOI] [PubMed] [Google Scholar]
- 7. Wang Y, Wu N, Chen J et al Is radical mediastinal lymphadenectomy necessary for elderly patients with clinical N‐negative non‐small‐cell lung cancer? A single center matched‐pair study. J Surg Res 2015; 193: 435–441. [DOI] [PubMed] [Google Scholar]
- 8. Ma W, Zhang ZJ, Li Y, Ma GY, Zhang L. Comparison of lobe‐specific mediastinal lymphadenectomy versus systematic mediastinal lymphadenectomy for clinical stage T1a N0M0 non‐small cell lung cancer. J Cancer Res Ther 2013; 9 (Suppl. 2): S101–105. [DOI] [PubMed] [Google Scholar]
- 9. Darling GE, Allen MS, Decker PA et al Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non‐small cell carcinoma: Results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg 2011; 141: 662–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ma K, Chang D, He B et al Radical systematic mediastinal lymphadenectomy versus mediastinal lymph node sampling in patients with clinical stage IA and pathological stage T1 non‐small cell lung cancer. J Cancer Res Clin Oncol 2008; 134: 1289–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cahan WG. Radical lobectomy. J Thorac Cardiovasc Surg 1960; 39: 555–572. [PubMed] [Google Scholar]
- 12. Schmulewitz N, Wildi SM, Varadarajulu S et al Accuracy of EUS criteria and primary tumor site for identification of mediastinal lymph node metastasis from non‐small‐cell lung cancer. Gastrointest Endosc 2004; 59: 205–212. [DOI] [PubMed] [Google Scholar]
- 13. Saeteng S, Tantraworasin A, Euathrongchit J, Lertprasertsuke N, Wannosopha Y. Nodal involvement pattern in resectable lung cancer according to tumor location. Cancer Manag Res 2012; 4: 151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shimada Y, Saji H, Kakihana M et al Retrospective analysis of nodal spread patterns according to tumor location in pathological N2 non‐small cell lung cancer. World J Surg 2012; 36: 2865–2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bernard A, Benoit L, Renaud C, Favre JP. Classification for predicting mediastinal lymph node metastases in patients with T1 or T2 lung cancer. Interact Cardiovasc Thorac Surg 2005; 4: 256–259. [DOI] [PubMed] [Google Scholar]
- 16. Cerfolio RJ, Bryant AS. Distribution and likelihood of lymph node metastasis based on the lobar location of nonsmall‐cell lung cancer. Ann Thorac Surg 2006; 81: 1969–1973. [DOI] [PubMed] [Google Scholar]
- 17. Miyoshi S, Shien K, Toyooka S et al Validity of using lobe‐specific regional lymph node stations to assist navigation during lymph node dissection in early stage non‐small cell lung cancer patients. Surg Today 2014; 44: 2028–2036. [DOI] [PubMed] [Google Scholar]


