Abstract
Background
Pulmonary adenocarcinoma is a predominant pathologic non‐small cell lung cancer (NSCLC) with a high morbidity in China. Even at histological stage I, many patients still experience recurrence after radical surgery; therefore, it is critical to determine useful indicators to stratify patients according to recurrent risk. Centrosomal protein 55 (CEP55) shares certain characteristics with oncogenes and aberrant expression of CEP55 can lead to tumorigenesis. Therefore, we aimed to clarify the clinicopathological significance and prognostic value of CEP55 in stage I pulmonary adenocarcinoma.
Methods
We enrolled 106 patients with stage I pulmonary adenocarcinoma who had received complete resection in our study. CEP55 expression levels in the pulmonary tissues of all patients were validated by Western blot analyses and immunohistochemistry. SPSS 17.0 software was employed to analyze the correlation between CEP55 expression and clinicopathological characteristics of patients, as well as prognosis.
Results
CEP55 overexpression was detected in 67 patients (63.2%). Overexpression is associated with tumor differentiation (P = 0.036), T stage (P = 0.000) and visceral pleural invasion (P = 0.009). Patients with CEP55 overexpression had worse survival compared with those with low expression (P = 0.043). Univariate analysis revealed that T stage (P = 0.000), differentiation degree (P = 0.002), visceral pleural invasion (P = 0.000), and tumor size (P = 0.013) were also significant prognostic factors.
Conclusion
CEP55 is a useful predicator to improve stratification of patients with stage I pulmonary adenocarcinoma.
Keywords: CEP55 overexpression, indicator, poor prognosis, stage I pulmonary adenocarcinoma
Introduction
Lung cancer is the leading cause of cancer‐related death worldwide, accounting for 1.59 million estimated deaths per year.1 As this disease has a variable prognosis, the treatment of lung cancer is far from satisfactory compared with other solid organ tumors.2 The prognosis of lung cancer patients is tumor node metastasis (TNM) staging specific and surgery with curative intent is the best therapeutic modality for early stage NSCLC. A standard complete resection consists of lobectomy and systematic nodal dissection.3 Recurrent disease is common even in patients with stage I non‐small cell lung cancer (NSCLC) who have undergone radical resection.4 Thus, it is critical to determine new biological markers to further stratify stage I NSCLC patients based on different recurrence risks and survival time. Biological markers can help in the selection of candidates for prophylactic postoperative adjuvant therapy. Recently, molecular biomarkers have been developed to predict non‐squamous NSCLC.5, 6
Centrosomal protein 55 (CEP55) is a recently discovered mitotic phosphoprotein which plays an important role in cytokinesis, the final stage of cell division during which physical separation of the two daughter cells occurs. CEP55 is required for the establishment and proper function of the midbody structure and plays an essential role in abscission involved in membrane fission events.7, 8, 9
Centrosomal protein 55 overexpression causes cytokinesis defects, resulting in an increase in the number of chromosome unstable multinucleated cells, prone to tumorigenesis. Overexpression of CEP55 in mammalian cells is associated with enhancing cell migration and invasion, while suppression of CEP55 expression is significantly correlated with increased apoptosis.10 Notably, CEP55 is highly expressed in certain human tumors and various tumor cell lines, while expression is barely detectable in normal tissues, with exceptions of the testis and thymus.12 Previous studies have shown that aberrant expression of CEP55 could cause tumorigenesis and overexpression in some tumors can contribute to poor prognosis. However, no previous studies have investigated the correlation between the overexpression of CEP55 and prognosis in stage I pulmonary adenocarcinoma. This study was designed to investigate the correlation between CEP55 overexpression in cancer tissues and prognosis in patients with pathological stage I pulmonary adenocarcinoma, in order to determine a new indicator to stratify stage I pulmonary adenocarcinoma patients and provide an individual therapeutic schedule to improve prognosis.
Method
Ethics statement
The Research Ethic Committee of Provincial Hospital affiliated to Shandong University approved the study protocol. All participants provided written informed consent.
Patients and tissues
From January 2006 to December 2008, 106 eligible patients with primary pulmonary adenocarcinoma who had undergone surgery and were identified as stage I by postoperative histopathology in our department were recruited in this study. According to the 2015 World Health Organization (WHO) Classification of Tumors of the Lung, tumors were diagnosed as adenocarcinoma in situ in four patients and as minimally invasive adenocarcinoma in seven. Five patients had lepidic adenocarcinoma, 76 had papillary adenocarcinoma, four had acinar adenocarcinoma, two had fetal adenocarcinoma, seven had invasive mucinous adenocarcinoma, and one had enteric adenocarcinoma. The 7th American Joint Committee on Cancer/Union for International Cancer Control staging criteria was employed for uniformity of pathologic staging in all patients. All patients received radical resection by lobectomy and systemic lymph node dissection. No preoperative radiotherapy or chemotherapy was performed. There were no serious perioperative complications. The clinicopathological characteristics of the 106 patients are listed in Table 1.
Table 1.
Correlations between CEP55 expression and clinicopathological factors in stage I pulmonary adenocarcinoma
| Clinical characteristics | Patients | CEP55 expression | ||
|---|---|---|---|---|
| 106 | (−) | (+) | P value | |
| 39 | 67 | |||
| Gender | 0.478 | |||
| Male | 81 | 28 | 53 | |
| Female | 25 | 11 | 14 | |
| Age (years) | 0.533 | |||
| <60 | 39 | 15 | 24 | |
| ≧60 | 67 | 24 | 53 | |
| Tumor size (cm) | 0.529 | |||
| ≦3 | 94 | 36 | 58 | |
| 3<, ≦5 | 12 | 3 | 9 | |
| Differentiation | 0.036 | |||
| Well + Moderate | 79 | 34 | 45 | |
| Poor | 27 | 5 | 22 | |
| pT | 0.000 | |||
| T1 | 46 | 26 | 20 | |
| T2a | 60 | 13 | 47 | |
| Visceral pleural invasion | 0.009 | |||
| Yes | 54 | 13 | 41 | |
| No | 52 | 26 | 26 | |
CEP55, centrosomal protein 55.
One pair of samples consisting of tumor tissue and corresponding normal lung tissue (CNLT) was harvested from surgical specimens of each selected patient. Each pair of samples was labeled, wrapped in foil, snap‐frozen in liquid nitrogen for one minute, and stored at −80°C until further use.
Immunohistochemistry
Immunohistochemistry (streptavidin‐peroxidase [SP] method) was performed to detect the CEP55 expression levels in each tissue specimen. Formalin‐fixed and paraffin‐embedded sections were dewaxed in xylene, rehydrated through graded alcohol, and placed in an endogenous peroxide block for 10 minutes. Following antigen retrieval, which was performed using microwaves in 10 mM of citrate buffer for 15 minutes, tissue sections were then incubated at 4°C overnight with anti‐CEP55 rabbit polyclonal antibody at a dilution of 1:150 in phosphate‐buffered saline (PBS) and processed using immunohistochemical streptavidin peroxidase conjugate method. Rabbit anti‐CEP55 polyclonal antibodies were purchased from Beijing Biosynthesis Biotechnology Co. (Beijing, China). PBS replaced the primary antibody for the negative control. Two independent pathologists who were blinded to clinical data examined all sections.
The immunohistochemical score (IHS) was calculated by combining the proportion score (percentage of positive stained cells) with the staining intensity score, as previously reported.10 Specimens were examined under a light microscope. In five randomly selected fields per section, the positive stained cells among 100 cells were assessed and quantified by percentage. The average percentage of the five fields was used to identify the proportion score in a four‐category grading system (0, negative; 1, <10%; 2, 10–50%; 3, >50%). Staining intensity was scored as: 0 (negative), 1 (weak), 2 (moderate), and 3 (strong). The IHS was determined by multiplying both of the scores: values ≥3 were defined as positive, and values <3 were considered negative.10 Two independent pathologists who were blinded to clinical data examined all sections.
Western blot analyses
Protein was extracted from each tissue sample and protein concentration was determined using a bicinchoninic acid assay. Equal amounts of protein (40 µg) were resolved on 12% sodium dodecyl sulfate‐polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes. The membranes were blocked with 5% non‐fat dry milk containing 0.05% Tween‐20 and 1% bovine serum albumin for one hour at room temperature and incubated overnight at 4°C with primary antibodies against CEP55 and β‐actin (1:1000 dilution). After washing with PBS, the membranes were incubated with secondary antibody conjugated with horseradish peroxidase anti‐rabbit immunoglobulin G (1:10 000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) for one hour at room temperature. Protein levels were quantified using an enhanced chemiluminescence detection system (LAS 4000 mini system, General Electric, Fairfield, CT, USA).
Follow‐up after surgery and diagnosis of recurrence
Follow‐up was conducted regularly at intervals of three to six months in the three years after surgery and every six to 12 months thereafter. A thorough physical examination, including a chest and upper abdomen enhanced computed tomography (CT) scan and blood examination, were conducted. Tumor markers were measured in all patients, including carcinoembryonic antigen (CEA), carbohydrate antigen 125 (CA125) and CA153. Positron emission tomography‐CT, brain CT, magnetic resonance imaging, or bone scintigraphy were performed when necessary, depending upon the presentation of specific symptoms or the attending physicians' discretion. Examination results were compared with preoperative imaging data. In cases of progressive lymph node enlargement, clinical basis was used to diagnose lymphatic recurrence. Some patients' metastases were diagnosed by biopsies. If new lesions appeared in the other organs after excluding the primary tumor, metastatic cancer was clinically diagnosed. Study follow‐up concluded in March 2014.
Statistical methods
The χ2 test was chosen to analyze correlations between CEP55 overexpression and clinicopathological factors. Univariate analysis was performed using Kaplan–Meier survival curves. The log–rank test was used to calculate survival rate. Multivariate analysis was performed using the Cox proportional hazard model. If the P‐value was less than 0.05, differences were considered significant. Statistical data were obtained using SPSS 17 statistical software (SPSS Inc., Chicago, IL, USA).
Result
Expression of centrosomal protein 55 (CEP55) in pulmonary adenocarcinoma tissue and corresponding normal lung tissue
Using immunohistochemistry, a positive expression of CEP55 was detected as a yellow or brownish yellow stain in the cytoplasma. Positive staining of CEP55 was readily detected in pulmonary adenocarcinoma tissue, while CNLT always presented negative staining of CEP55 (Fig 1). To validate immunohistochemistry results, we further analyzed CEP55 expression levels in different tissues by Western blot; results corresponded to the immunohistochemistry method (Fig 2).
Figure 1.
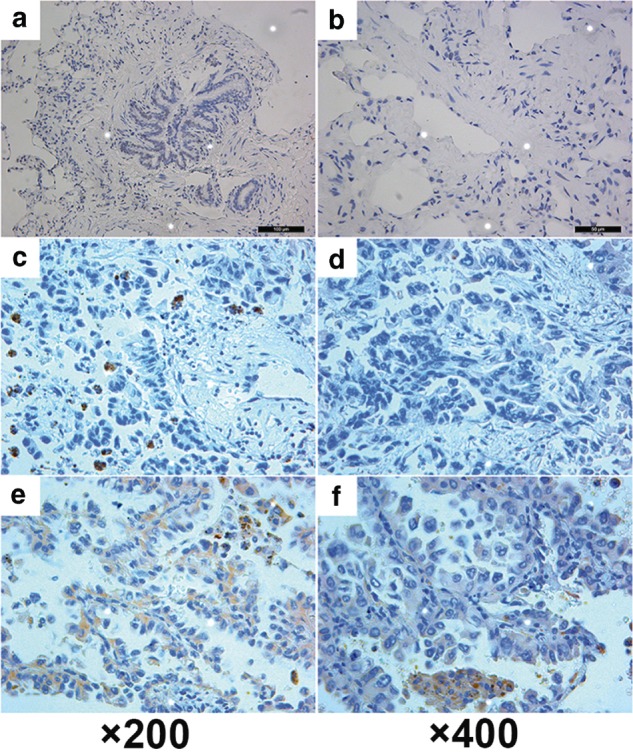
Immunohistochemical staining of centrosomal protein 55 (CEP55) in stage I pulmonary adenocarcinoma and corresponding normal lung tissue (CNLT). (a) and (b) Representative negative expression of CEP55 in CNLT. (c) and (d) Representative low expression of CEP55 in stage I pulmonary adenocarcinoma. (e) and (f) Representative high expression of CEP55 in stage I pulmonary adenocarcinoma. ×200 and ×400: original magnification. Nuclei are counterstained with hematoxylin.
Figure 2.
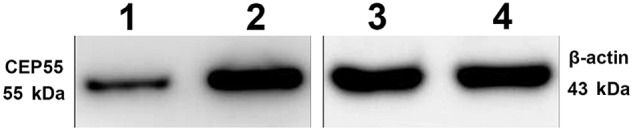
Expression of centrosomal protein 55 (CEP55) detected by Western blot analyses: lane 1, representative low CEP55 expression in stage I pulmonary adenocarcinoma; lane 2, representative high CEP55 expression in stage I pulmonary adenocarcinoma; lanes 3 and 4, representative β‐actin expression in different samples of stage I pulmonary adenocarcinoma.
Correlation between CEP55 overexpression and clinical characteristics
Sixty‐seven patients were identified with CEP55 overexpression in their pulmonary adenocarcinoma samples. The diagnostic sensitivity was 63.2% (67/106; Table 1). The correlation between CEP55 expression and clinicopathological features is shown in Table 1. Chi‐square analysis indicated that CEP55 overexpression was significantly associated with differentiation (P = 0.036), T stage (P = 0.000), and visceral pleural invasion (P = 0.009). No other clinicopathological parameter was associated with CEP55 overexpression.
Correlation between CEP55 expression and prognosis
The five‐year overall survival (OS) rate of 106 patients was 63.2% (Fig 3). Univariate analysis indicated that CEP55 expression level (P = 0.043) was a significant prognostic factor. The five‐year OS rate of patients without CEP55 overexpression in stage I pulmonary adenocarcinoma tissues was significantly higher than that of patients with CEP55 overexpression (Fig 4). Using univariate analysis, we determined that T stage (P = 0.000), differentiation degree (P = 0.002), visceral pleural invasion (P = 0.000), and tumor size (P = 0.013) were also significant prognostic factors (Table 2). To rule out confounding factors, a Cox proportional hazards model was used to identify factors involved in the OS of stage I pulmonary adenocarcinoma patients. Cox multivariate regression analysis revealed that T status (P = 0.047) and tumor differentiation degree (P = 0.010) were independent relevant factors for the prognosis of stage I pulmonary adenocarcinoma (Table 3).
Figure 3.
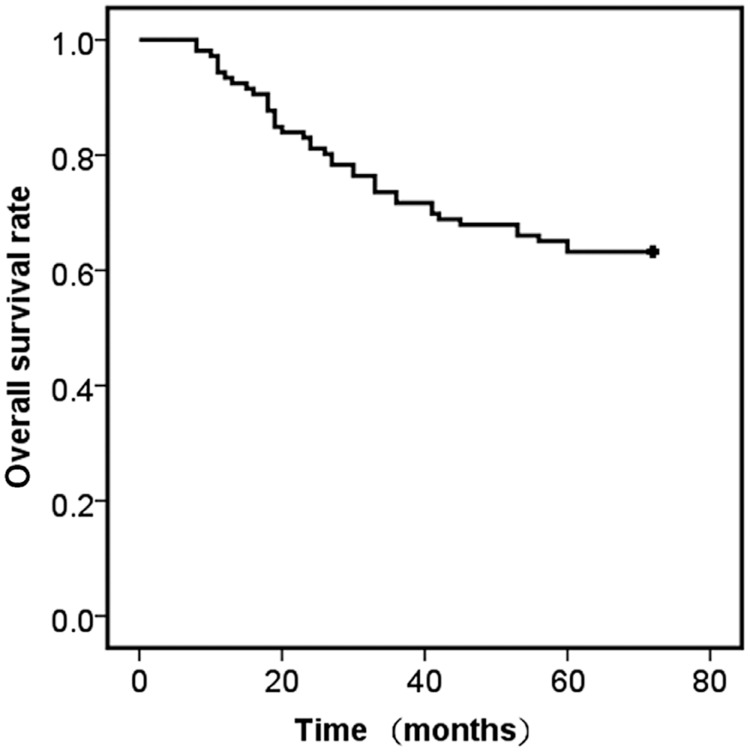
Kaplan–Meier analysis of overall survival in stage I pulmonary adenocarcinoma patients. The overall five‐year survival rate of 106 stage I pulmonary adenocarcinoma patients.
Figure 4.
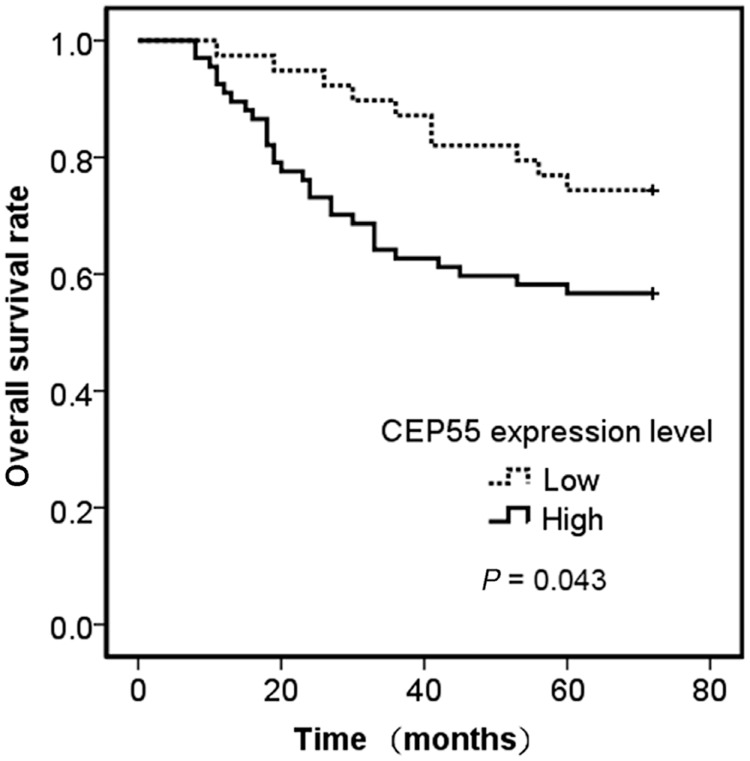
Kaplan–Meier analysis of overall survival in stage I pulmonary adenocarcinoma patients. The five‐year survival rate of stage I pulmonary adenocarcinoma patients with centrosomal protein 55 (CEP55) overexpression versus patients without CEP55 overexpression.
Table 2.
Univariate analysis of overall survival for patients with stage I pulmonary adenocarcinoma
| Variable | Univariate analysis | ||
|---|---|---|---|
| HR | 95% CI | P | |
|
Age ≥60 vs. <60 yrs |
1.122 | 0.593–2.125 | 0.723 |
|
Gender Male vs. Female |
1.066 | 0.506–2.245 | 0.867 |
|
Tumor size >3 cm, ≤5 cm vs. ≤3 cm |
2.482 | 1.174–5.248 | 0.017 |
|
Differentiation degree Low vs. Mid‐high |
2.651 | 1.397–5.031 | 0.003 |
|
T stage 1 vs. 2a |
44.747 | 6.132–326.522 | 0.000 |
|
Visceral pleural invasion Yes vs. No |
8.822 | 3.685–21.122 | 0.000 |
|
CEP55 expression level Low vs. High |
2.059 | 1.003–4.228 | 0.043 |
CI, confidence interval; CEP55, centrosomal protein 55; HR, hazard ratio.
Table 3.
Cox regression analysis for risk factors of five‐year survival
| B | SE | Wald | P value | OR | 95.0% CI | |
|---|---|---|---|---|---|---|
| Gender | 0.011 | 0.398 | 0.001 | 0.979 | 0.989 | 0.454–2.158 |
| Age | 0.026 | 0.350 | 0.006 | 0.940 | 0.974 | 0.491–1.932 |
| Tumor size | 0.931 | 0.609 | 2.341 | 0.126 | 2.538 | 0.770–8.368 |
| Visceral pleural invasion | 1.143 | 0.738 | 2.398 | 0.122 | 3.136 | 0.738–13.325 |
| Differentiation | 0.885 | 0.344 | 6.626 | 0.010 | 2.423 | 1.235–4.752 |
| T stage | 2.536 | 1.275 | 3.958 | 0.047 | 12.632 | 1.038–153.644 |
| CEP55 overexpression | 0.093 | 0.400 | 0.054 | 0.816 | 1.098 | 0.501–2.406 |
CI, confidence interval; CEP55, centrosomal protein 55; OR, odds ratio; SE, standard error.
Discussion
It is widely accepted that the prognosis of NSCLC is staging specific. Surgical resection remains the gold standard of care for patients with early‐stage NSCLC and adequate cardiopulmonary reserve. According to current National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology for NSCLC, postoperative adjuvant therapy is not recommended for patients with completely resected stage IB NSCLC, with the exception of patients regarded to be at high risk of recurrence. The latest NCCN guidelines recommend postoperative adjuvant chemotherapy with low‐level evidence (category 2B) in patients with stage IB NSCLC and risk factors for recurrence, which includes tumors larger than 4 cm, poor differentiation, vascular invasion, wedge resection, visceral pleural involvement, and incomplete lymph node sampling (Nx).15 However, the long‐term survival for patients with early stage NSCLC and current optional treatment (lobectomy) remains disappointing. The five‐year survival rate has been reported to be only 75% (95% confidence interval 72–78%) after lobectomy for even the earliest stage I NSCLC tumors measuring less than 1 cm;16 therefore, surgery alone may not be adequate in up to a quarter of cases. In the same study, multivariate survival analysis showed that adjuvant chemotherapy was significantly associated with improved survival for tumors 3.0–3.9 cm.16 One study indicated that platinum‐based adjuvant chemotherapy for surgically treated stage IB NSCLC might offer better survival than observation alone.17 Thus, the current NCCN Clinical Practice Guidelines in Oncology for NSCLC contain some inadequacies.
Radical resection for pulmonary carcinoma includes lobectomy and systemic lymph node dissection. Complete lymph node dissection consists of the removal of all ipsilateral mediastinal lymph nodes, as well as the hilar and intrapulmonary nodes.18 Complete lymph node dissection is considered the standard surgical procedure because it can offer more accurate pathologic staging and better clinical outcomes, but some surgeons have demonstrated that selective lymph node sampling is not inferior to complete lymph node dissection in pathologically identified early stage pulmonary carcinoma patients.19, 20, 21 For patients with early stage pulmonary carcinoma identified with negative mediastinal and hilar lymph nodes by systematic and thorough presection sampling, complete lymph node dissection does not improve survival.21 Complete lymph node dissection or lymph node sampling, which is most effective for patients with early stage NSCLC, remains controversial.
Stereotactic body radiotherapy (SBRT) is a noninvasive therapy, which delivers precisely targeted ablative doses of radiation. For patients with NSCLC ineligible for surgery, several prospective trials have demonstrated that SBRT is considered a safe and effective alternative.22, 23, 24 Because of the promising outcomes of SBRT, its use has been advocated even in patients eligible for surgery, although this remains controversial.25
In our opinion, patients with early stage pulmonary carcinoma should be treated individually. To achieve this aim, we must identify the invasiveness and recurrent potency of the disease. For patients who experience disease with great invasiveness and recurrent potency, radical resection through lobectomy and complete lymph node dissection should be performed. These patients are considered candidates for postoperative adjuvant chemotherapy. It is critical for clinical practice to determine a useful indicator to predict prognosis in patients with early stage NSCLC and to adopt a suitable surgical procedure or follow suitable adjuvant treatment for reducing recurrence and improving long‐term survival. Efforts have been made to stratify the patients with early stage NSCLC or non‐squamous NSCLC.26, 27 Recently, molecular biomarkers were developed to predict non‐squamous NSCLC; however, no biomarkers have reached the validation stage for squamous cell carcinoma. In this study, we aimed to explore the prognostic significance of CEP55 in stage I pulmonary adenocarcinoma after radical resection.
Centrosomal protein 55 plays an essential role in abscission involved in membrane fission events.28, 29 During cytokinesis, the bipolar spindle guides the proper segregation of replicated chromosomes into two daughter cells. CEP55 overexpression may generate cytokinesis defects, resulting in aneuploidy and chromosome instability, which are common characteristics of tumor cells; thus, CEP55 overexpression is associated with tumorigenesis. CEP55 overexpression is found in several cancer cell lines, while its expression is barely detected in normal tissues by expression‐profile analyses using microarrays.12 CEP55 is overexpressed in lung adenocarcinoma and ectopic expression of CEP55 promotes cell migration and invasion.30 Significantly, this study is the first to report that CEP55 overexpression is associated with the poor prognosis of stage I pulmonary adenocarcinoma.
In this study, CEP55 was overexpressed in most stage I pulmonary adenocarcinoma patients and associated with T stage, tumor differentiation, and visceral pleural invasion. We investigated the correlation between CEP55 expression in cancer tissues and prognosis in patients with stage I pulmonary adenocarcinoma after radical resection. Using univariate analysis, we determined that CEP55 was a significant prognostic factor, as well as T stage, differentiation degree, visceral pleural invasion, and tumor size. The five‐year OS rate of patients with CEP55 overexpression in tumor issues was significantly lower than that of patients without CEP55 overexpression.
The study contains some shortcomings. It was a retrospective study based on data from a single institution and the sample size was relatively small. In future, prospective multi‐center randomized controlled clinical trials should be performed to reveal the predictive value of CEP55 overexpression in stage I pulmonary adenocarcinoma.
In conclusion, this study demonstrated that CEP55 overexpression predicts poor prognosis and is related to aggressive clinicopathological features in stage I pulmonary adenocarcinoma. These findings suggest that CEP55 overexpression has enhanced metastatic potential and can act as an indicator to stratify patients with stage I pulmonary adenocarcinoma for individual treatment.
Acknowledgments
This work was supported by Grants from The National Natural Science Foundation of China (No. 81302021).
Disclosure
No authors report any conflict of interest.
References
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65: 87–108. [DOI] [PubMed] [Google Scholar]
- 2. Reck M, Heigener DF, Mok T, Soria JC, Rabe KF. Management of non‐small‐cell lung cancer: Recent developments. Lancet 2013; 382: 709–719. [DOI] [PubMed] [Google Scholar]
- 3. Rami‐Porta R, Wittekind C, Goldstraw P, International Association for the Study of Lung Cancer (IASLC) Staging Committee . Complete resection in lung cancer surgery: Proposed definition. Lung Cancer 2005; 49: 25–33. [DOI] [PubMed] [Google Scholar]
- 4. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012; 62: 10–29. [DOI] [PubMed] [Google Scholar]
- 5. Kratz JR, He J, Van Den Eeden SK et al A practical molecular assay to predict survival in resected non‐squamous, non‐small‐cell lung cancer: Development and international validation studies. Lancet 2012; 379: 823–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tomida S, Takeuchi T, Shimada Y et al Relapse‐related molecular signature in lung adenocarcinomas identifies patients with dismal prognosis. J Clin Oncol 2009; 27: 2793–2799. [DOI] [PubMed] [Google Scholar]
- 7. Fabbro M, Zhou BB, Takahashi M et al Cdk1/Erk2‐ and Plk1‐dependent phosphorylation of a centrosome protein, Cep55, is required for its recruitment to midbody and cytokinesis. Dev Cell 2005; 9: 477–488. [DOI] [PubMed] [Google Scholar]
- 8. Morita E, Sandrin V, Chung HY et al Human ESCRT and ALIX proteins interact with proteins of the midbody and function in cytokinesis. (Published erratum appears in EMBO J 2012; 31: 3228) EMBO J 2007; 26: 4215–4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhao WM, Seki A, Fang G. Cep55, a microtubule‐bundling protein, associates with centralspindlin to control the midbody integrity and cell abscission during cytokinesis. Mol Biol Cell 2006; 17: 3881–3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tao J, Zhi X, Tian Y et al CEP55 contributes to human gastric carcinoma by regulating cell proliferation. Tumour Biol 2014; 35: 4389–4399. [DOI] [PubMed] [Google Scholar]
- 11. Sakai M, Shimokawa T, Kobayashi T et al Elevated expression of c10orf3 (chromosome 10 open reading frame 3) is involved in the growth of human colon tumor. Oncogene 2006; 25: 480–486. [DOI] [PubMed] [Google Scholar]
- 12. Inoda S, Hirohashi Y, Torigoe T et al Cep55/c10orf3, a tumor antigen derived from a centrosome residing protein in breast carcinoma. J Immunother 2009; 32: 474–485. [DOI] [PubMed] [Google Scholar]
- 13. Chen CH, Chien CY, Huang CC et al Expression of FLJ10540 is correlated with aggressiveness of oral cavity squamous cell carcinoma by stimulating cell migration and invasion through increased FOXM1 and MMP‐2 activity. Oncogene 2009; 28: 2723–2737. [DOI] [PubMed] [Google Scholar]
- 14. Chang YC, Chen YJ, Wu CH, Wu YC, Yen TC, Ouyang P. Characterization of centrosomal proteins Cep55 and pericentrin in intercellular bridges of mouse testes. J Cell Biochem 2010; 109: 1274–1285. [DOI] [PubMed] [Google Scholar]
- 15. Ettinger DS, Wood DE, Akerley W et al Non‐Small Cell Lung Cancer, Version 6. 2015. J Natl Compr Canc Netw 2015; 13: 515–524. [DOI] [PubMed] [Google Scholar]
- 16. Speicher PJ, Gu L, Wang X, Hartwig MG, D'Amico TA, Berry MF. Adjuvant chemotherapy after lobecomy for T1‐2N0 non‐small cell lung cancer: Are the guidelines supported? J Natl Compr Canc Netw 2015; 13: 755–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Park SY, Lee JG, Kim J et al Efficacy of platinum‐based adjuvant chemotherapy in T2aN0 stage IB non‐small cell lung cancer. J Cardiothorac Surg 2013; 8: 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Martini N. Mediastinal lymph node dissection for lung cancer. The Memorial experience. Chest Surg Clin N Am 1995; 5: 189–203. [PubMed] [Google Scholar]
- 19. Naruke T, Tsuchiya R, Kondo H, Nakayama H, Asamura H. Lymph node sampling in lung cancer: How should it be done? Eur J Cardiothorac Surg 1999; 16 (Suppl. 1): S17–24. [DOI] [PubMed] [Google Scholar]
- 20. Melfi FM, Davini F, Boni G, Mussi A. Sentinel lymph node in lung cancer surgery. Thorac Surg Clin 2012; 22: 205–214. [DOI] [PubMed] [Google Scholar]
- 21. Darling GE, Allen MS, Decker PA et al Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non‐small cell carcinoma: Results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg 2011; 141: 662–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lagerwaard FJ, Haasbeek CJ, Smit EF, Slotman BJ, Senan S. Outcomes of risk‐adapted fractionated stereotactic radiotherapy for stage I non‐small cell lung cancer. Int J Radiat Oncol Biol Phys 2008; 70: 685–692. [DOI] [PubMed] [Google Scholar]
- 23. Timmerman R, Paulus R, Galvin J et al Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010; 303: 1070–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Padda SK, Burt BM, Trakul N, Wakelee HA. Early‐stage non‐small cell lung cancer: Surgery, stereotactic radiosurgery, and individualized adjuvant therapy. Semin Oncol 2014; 41: 40–56. [DOI] [PubMed] [Google Scholar]
- 25. Lagerwaard FJ, Verstegen NE, Haasbeek CJ et al Outcomes of stereotactic ablative radiotherapy in patients with potentially operable stage I non‐small cell lung cancer. Int J Radiat Oncol Biol Phys 2012; 83: 348–353. [DOI] [PubMed] [Google Scholar]
- 26. Harada H, Miyamoto K, Yamashita Y, Taniyama K, Ohdan H, Okada M. Methylated DLX4 predicts response to pathologic stage I non‐small cell lung cancer resection. Ann Thorac Surg 2015; 99: 1746–1754. [DOI] [PubMed] [Google Scholar]
- 27. Kubo H, Suzuki T, Matsushima T et al Cyclin‐dependent kinase‐specific activity predicts the prognosis of stage I and stage II non‐small cell lung cancer. BMC Cancer 2014; 14: 755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gromley A, Yeaman C, Rosa J et al Centriolin anchoring of exocyst and SNARE complexes at the midbody is required for secretory‐vesicle‐mediated abscission. Cell 2005; 123: 75–87. [DOI] [PubMed] [Google Scholar]
- 29. Fujiwara T, Bandi M, Nitta M, Ivanova EV, Bronson RT, Pellman D. Cytokinesis failure generating tetraploids promotes tumorigenesis in p53‐null cells. Nature 2005; 437: 1043–1047. [DOI] [PubMed] [Google Scholar]
- 30. Chen CH, Lai JM, Chou TY et al VEGFA upregulates FLJ10540 and modulates migration and invasion of lung cancer via PI3K/AKT pathway. PLoS ONE 2009; 4 (4): e5052. [DOI] [PMC free article] [PubMed] [Google Scholar]


