Abstract
Squamous cell lung cancer (SCC) presenting with anaplastic lymphoma kinase (ALK) translocation is rare. We present a case of ALK gene translocation‐SCC in which a remarkable tumor response to crizotinib was achieved after the failure of prior chemoradiotherapy. Considering this remarkable response, we conclude that ALK testing in female non‐smokers or in any patient unresponsive to the initial regimen of chemotherapy, is recommended for SCC patients.
Keywords: Crizotinib, EML4‐ALK, squamous cell lung cancer
Introduction
Lung cancer is the leading cause of cancer death worldwide, with morbidity and mortality rates increasing in both developed and developing countries.1 The specific molecular changes in certain lung cancer cases have provided excellent opportunities for the development of new targeted therapies. Echinoderm microtubule‐associated protein‐like 4‐anaplastic lymphoma kinase (EML4‐ALK) rearrangement is found in 2–7% of patients with non‐small cell lung cancer (NSCLC) overall.2 But a low reported frequency range of 0–2.5% anaplastic lymphoma kinase (ALK) mutation is found in squamous cell lung cancer (SCC) patients.3 Crizotinib, a novel ALK tyrosine kinase inhibitor, has already shown impressive single‐agent activity in ALK‐positive lung adenocarcinomas (ADCs). In ADCs, the objective response rate to crizotinib is about 60% and its median progression‐free survival is nearly 10 months.4, 5 Nowadays, a low percentage of SCC patients are enrolled in clinical trials; thus, the efficacy of ALK inhibitors for these patients is not well‐known. In this report, we present a case of successful crizotinib treatment of an ALK rearrangement in an SCC patient.
Case report
A 37‐year old female never‐smoker presented to our hospital with a one‐month history of coughing. Positron emission tomography‐computed tomography (PET/CT) scans revealed a 4.0*4.2 cm mass at the superior lobe of the left lung and contralateral mediastinal lymph node enlargements (T2N3M0 stageIIIB) (Fig 1a). A pathological diagnosis of SCC was obtained using needle biopsy. Hematoxylin and eosin staining showed the typical morphology of SCC cells (Fig 2a). Immunohistochemistry (IHC) analysis demonstrated positivity in cytokeratin (CK) 5/6 and P63, and negativity in CK7, Napsin A, and thyroid transcription factor‐1.
Figure 1.
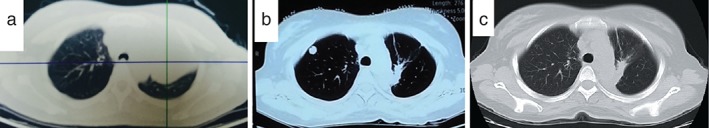
(a) Positron emission tomography‐computed tomography (PET/CT) scan before chemoradiotherapy; (b) a CT of the chest revealed the recurrence of lung lesions after the course of chemoradiotherapy; (c) CT scan of chest after two months of crizotinib treatment.
Figure 2.
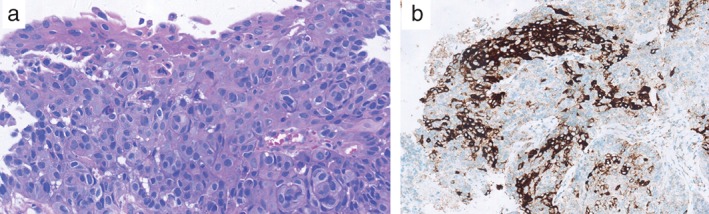
(a) A needle biopsy of the tumor showed squamous cell carcinoma (hematoxylin and eosin, × 400); (b) anaplastic lymphoma kinase protein was overexpressed, as seen via immunohistochemistry (×200).
Concurrent chemoradiotherapy with six cycles of doxetaxel and cisplatin (DP; docetaxel 75 mg/m2 D1; ciplatin 25 mg/m2 D1‐3) chemotherapy regimens and three dimensional‐conformal radiotherapy (2Gy per fraction, 1 fraction per day, total radiation dose 60Gy, 5 fractions per week, 30 fractions) was performed from July 2013 until November 2013. The curative effect achieved was a partial response. Unfortunately, during routine examination, the presence of multiple metastases in the right lung on CT scan images and the presence of right parietal lobe metastases indicated cancer progression (Fig 1b). Wild‐type epidermal growth factor receptor variant tumor tissue was detected by amplification refractory mutation system (AmoyDx, Xiamen China), and ALK protein expression by Ventana ALK IHC assay (Ventana Medical Systems, Roche, Tuscon, AZ, USA) (Fig 2b). The patient received gamma knife radiosurgery (total dose 24 Gy at 50% isodose, 2 fractions, 2 days) to treat brain metastases in June 2014, then underwent crizotinib treatment (250 mg/bid, orally) in July 2014. After two months, chest CT scan images demonstrated a decrease in tumor size (Fig 1c). According to Response Evaluation Criteria in Solid Tumors guidelines (version 1.1), this patient was considered as having a partial response (PR) to crizotinib. During crizotinib treatment, abnormal hepatic and renal function were not found. There were no treatment‐related adverse events, including gastrointestinal reaction, flickering vision, or cordis damage. To date, after nine months, the disease remains stable.
Disscusion
In NSCLC, ALK rearrangement is associated with distinct clinicopathological features, including a young age of onset, absent or minimal smoking history, and adenocarcinoma histology.6 This case demonstrated ALK rearrangement in a young, female never‐smoker with SCC. However, the proportion of ALK rearrangement is very low in SCC. Recent studies have reported ALK rearrangement frequencies ranging from 1–6.5% in SCC.3, 7 Kim et al. and Wang et al. reported cases of SCC patients with ALK rearrangement.8 , 9 Dragnev et al. and Chaft et al. reported adenosquamous carcinoma masquerading as pure squamous carcinoma with ALK rearrangement.10 , 11 These studies illustrated that biopsies might represent an incomplete sampling of these complex tumors. In some cases, core needle biopsy may be too difficult to obtain a complete sample in order to determine lymph node metastasis. Therefore, to ensure appropriate management, oncologists need to be aware of the existence of patients harboring EML4‐ALK positive SCC. Moreover, clinical features might be useful to help identify a subset of patients who require ALK testing (e.g. patients with minimal or no smoking history), regardless of the histological type of their tumors.
Crizotinib has demonstrated an excellent clinical response in patients with EML4‐ALK‐positive lung adenocarcinoma. However, the efficacy of crizotinib for ALK positive‐SCC patients is not yet well known. Only one case has been reported in which crizotinib had an obvious effect on pure SCC.9 Our case is the second report of the effectiveness of crizotinib for ALK‐mutation SCC.
Conclusion
In summary, this case indicates that patients with SCC could harbor EML4‐ALK translocation. Therefore, ALK testing in female non‐smokers or in any patient unresponsive to the initial regimen of chemotherapy, is recommended for SCC patients, in order to determine the indication for crizotinib treatment.
Disclosure
No authors report any conflict of interest.
References
- 1. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. (Published erratum appears in CA Cancer J Clin 2014; 64: 364) CA Cancer J Clin 2014; 64: 9–29.24399786 [Google Scholar]
- 2. Lindeman NI, Cagle PT, Beasley MB et al Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: Guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. (Published erratum appears in J Thorac Oncol 2013; 8: 1343) J Thorac Oncol 2013; 8: 823–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Perez‐Moreno P, Brambilla E, Thomas R, Soria JC. Squamous cell carcinoma of the lung: Molecular subtypes and therapeutic opportunities. Clin Cancer Res 2012; 18: 2443–2451. [DOI] [PubMed] [Google Scholar]
- 4. Kwak EL, Camidge DR, Clark J et al Clinical activity observed in a phase I dose escalation trial of an oral c‐met and ALK inhibitor, PF‐02341066. 2009 ASCO Annual Meeting Proceedings. J Clin Oncol 2009; 27: abstr 3509. [Google Scholar]
- 5. Crino L, Kim D, Riely GJ et al Initial phase II results with crizotinib in advanced ALK‐positive non‐small cell lung cancer (NSCLC): PROFILE 1005. 2011 ASCO Annual Meeting Proceedings. J Clin Oncol 2011; 29: abstr 7514. [Google Scholar]
- 6. Shaw A, Yeap BY, Solomon BJ et al Effect of crizotinib on overall survival in patients with advanced non‐small‐cell lung cancer harbouring ALK gene rearrangement: A retrospective analysis. Lancet Oncol 2011; 12: 1004–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. An SJ, Chen ZH, Su J et al Identification of enriched driver gene alterations in subgroups of non‐small cell lung cancer patients based on histology and smoking status. PLoS ONE 2012; 7 (6): e40109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim H, Park E, Kim YJ, Chung JH. ALK rearrangement in a pure squamous cell carcinoma: The challenge of detection of ALK rearrangement. Virchows Arch 2013; 462: 597–599. [DOI] [PubMed] [Google Scholar]
- 9. Wang Q, He Y, Yang X, Wang X, Xiao H. Extraordinary response to crizotinib in a woman with squamous cell lung cancer after two courses of failed chemotherapy. BMC Pulm Med 2014; 14: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dragnev K, Gehr G, Memoli VA, Tafe LJ. ALK‐rearranged adenosquamous lung cancer presenting as squamous cell carcinoma: A potential challenge to histologic type triaging of NSCLC biopsies for molecular studies. Clin Lung Cancer 2014; 15: e37–40. [DOI] [PubMed] [Google Scholar]
- 11. Chaft JE, Rekhtman N, Ladanyi M, Riely GJ. ALK‐rearranged lung cancer: Adenosquamous lung cancer masquerading as pure squamous carcinoma. J Thorac Oncol 2012; 7: 768–769. [DOI] [PMC free article] [PubMed] [Google Scholar]


