Abstract
Bacillus thuringiensis (Bt) toxin receptors play important roles in the killing of pests, and investigation on characterization of the receptors is essential for utilization of Bt and management of insect resistance. Here, recombinant and mosaic receptors of Bt Cry1Ac toxin from Helicoverpa armigera were expressed in Spodoptera litura Sl-HP cells and their influences on cytotoxicity of activated Cry1Ac toxin were investigated. When H. armigera aminopeptidase N1 (APN1), alkaline phosphatase 2 (ALP2) and cadherin fused with or without GFP tag were, respectively, expressed in Sl-HP cells, live cell-immunofluorescence staining detection revealed that the quantity of the toxin binding to cadherin or cadherin-GFP was much more than that binding to ALP2 and APN1 or their fusion proteins with GFP, and only the cadherin- or cadherin-GFP-expressing cells showed aberrant cell morphology after the treatment of the toxin at low concentrations. ALP2 and APN1 fused with or without GFP tag did not significantly enhance the cadherin-mediated cytotoxicity of the toxin. The mosaic ALP-TBR-GFP-GPI was located on cell membrane, but did not bind to the toxin. The mosaic truncated cadherin-GFP-GPI was not located on cell membrane even if the signal peptide was sustained. The concentrations of the toxin resulting in swelling of 50 % cells for noncadherin-expressing Sl-HP cells and cadherin-expressing Hi5 cells were 5.08 and 9.50 µg/ml within 1 h, respectively. Taken together, our data have indicated that the binding affinity of ALP2 and APN1 to activated Cry1Ac toxin is much weaker than that of cadherin and both ALP2 and APN1 do not enhance the cytotoxicity of the toxin even though cadherin is co-expressed, and the mosaic receptor of ALP2 inserted with cadherin toxin binding domain does not mediate cytotoxicity of the toxin. In addition, the noncadherin-expressing Sl-HP cells are more susceptible to activated Cry1Ac than the cadherin-expressing Hi5 cells.
Keywords: Bacillus thuringiensis, Cadherin, Aminopeptidase N, Alkaline phosphatase, Trichoplusia ni
Introduction
Helicoverpa armigera (Hübner, 1805), a polyphagus pest, is an important economic insect damaging many crops. Bacillus thuringiensis (Bt) is a gram-positive soil bacterium that produces crystalline inclusions composed of proteins known as Cry toxins that are toxic to the larvae of different insect orders and a few other invertebrates. The insecticidal protein Cry1Ac produced by Bt is extremely effective against the pest H. armigera (Wu et al. 2008; Lu et al. 2012). When H. armigera was fed on the insecticidal protein, the protoxin is digested by larval midgut proteases to release the activated Cry toxin that then binds specifically to its receptors on the midgut epithelial cell. The Cry1Ac binding to specific receptors is the critical step of Cry1Ac toxic activity (Schnepf et al. 1998).
To date, there are two main models for the action of Cry1Ac on midgut cells. A current model is that Cry1Ac toxin first binds to cadherin and oligomerizes, then binds to glycosyl-phosphatidylinositol (GPI)-anchored aminopeptidase N (APN) and GPI-anchored alkaline phosphatase (ALP). An alternative model proposes the activation of intracellular signaling pathways via binding of the toxin monomer to cadherin without the need of the toxin oligomerization step to cause cell death (Pigott and Ellar 2007; Zhang et al. 2005; Aimanova et al. 2006).
Insect cell lines have been widely used in entomological research. A direct approach is to test whether Cry toxin-resistant cell lines can be made susceptible by expressing putative toxin receptors. The cadherin, a receptor of Cry1Ac, which was expressed in Trichoplusia ni (Hübner) Hi5 and Spodoptera frugiperda (Smith J.E.) Sf9 cell lines, has been demonstrated that the expressed recombinant cadherin functions as the receptor of activated Cry1Ac in the tested cell lines (Zhang et al. 2005; Aimanova et al. 2006; Jurat-Fuentes and Adang 2006). However, Garner et al. (1999) reported a controversial result for the in vitro assays of APN1 which mediated the toxicity of activated Cry1Ac in vivo. They expressed APN1 from Lymantriadispar in Sf9 cells, but did not observe any cytotoxicity at Cry1Ac concentrations in range of 0.2–50 μg/ml because the binding affinity between Cry1Ac and Sf9-expressing APN1 is much lower than that of the native protein (Garner et al. 1999). APN from Heliothis virescens was also tested for its ability to confer toxin susceptibility and was expressed in Drosophila S2 cells. While it has been demonstrated that Cry1Ac could bind to APN on the surface of intact cells and no cytotoxicity was observed at a toxin concentration of 30 μg/ml (Banks et al. 2003). However, Sivakumar et al. (2007) reported that Sf21 insect cells expressing H. armigera HaAPN1 displayed aberrant cell morphology upon overlaying with activated Cry1Ac protein at 3.75 μg/ml in PBS during the period of 5 h. Down-regulating expression of HaAPN1 by RNA interference using double-stranded RNA correlated with a corresponding reduction in the sensitivity of HaAPN1-expressing cells to activated Cry1Ac protein (Sivakumar et al. 2007).
In H. armigera, the receptors of the Cry1Ac such as cadherin, ALPs and APNs have been reported, but it is not well documented whether the receptors could mediate the cytotoxicity of the activated Cry1Ac to cultured insect cells expressing or co-expressing these proteins, and comparison of their binding affinity to activated Cry1Ac in vitro is also required under the same experimental condition (Ning et al. 2010; Rajagopal et al. 2003; Zhang et al. 2009; Ingle et al. 2001; Wang et al. 2005a; Liao et al. 2005; Upadhyay and Singh 2011; Zhang et al. 2012). In this study, we constructed a series of plasmids expressing ALP2, APN1 and cadherin from H. armigera to investigate the binding of activated Cry1Ac to the recombinant receptors and the influences of single receptor, or the co-expressed two receptors on the cytotoxicity of activated Cry1Ac to Spodoptera litura (Fabricius, 1775) Sl-HP cells (Zhang et al. 2008). In addition, two types of mosaic proteins resulting from H. armigera ALP2 and cadherin were obtained, and their conferring cytotoxicity of activated Cry1Ac to Sl-HP cells was also assayed.
Materials and methods
Cells and activated Cry1Ac toxin
Spodoptera litura Sl-HP and Trichoplusia ni TN5B1-4 (Hi5) cells (Life Technologies Co., Carlsbad, CA) were stored in our laboratory (Zhang et al. 2008). The cells were grown in Grace,s insect cell medium (Life Technologies Co., Grand Island, NY, USA) supplemented with 10 % fetal bovine serum (FBS) (Life Technologies Co., Australia) at 28 °C. The purified, activated and lyophilized Cry1Ac was gifted by Marianne Pusztai-Carey at Case Western Reserve University.
Transient expression of the recombinant and mosaic receptors in insect cells
The plasmids containing H. armigera ALP2 (GenBank accession number: EU729323.1), APN1 (GenBank accession number: AF441377) or cadherin (GenBank accession number: AF519180) gene were constructed by us. PCR was conducted to amplify ALP2, APN1 and cadherin genes with appropriate cleavage sites of restriction enzymes using the plasmid DNAs as templates and the specific primers in Table 1. The ALP2, APN1 and cadherin genes were inserted into the modified pIE2 expression vector, respectively. The pIE2 plasmid was from pEGFP-C1, the cytomegalovirus (CMV) promoter was replaced with IE2 promoter from the plasmid pIZ-V5/His (Invitrogen), and the gene of the enhanced green fluorescence protein (EGFP) was deleted. The recombinant plasmids were transformed into Escherichia coli DH5a (Clonetech, Mountain View, CA, USA) and the inserted fragments were verified by DNA sequencing. The constructed pIE2-ALP2/APN1/cadherin plasmids were used to express ALP2, APN1 and cadherin, in Sl-HP cells, respectively. Recombinant plasmids including pIE2-ALP-GFP-GPI and pIE2-APN-GFP-GPI were constructed by inserting the GFP just before GPI-anchored site. In addition, the plasmids pIE2-ALP-GPI-GFP, pIE2-Sig-GFP-ALP-GPI and pIE2-ALP-GFP-A9-GPI were also constructed. Sig-GFP-ALP-GPI indicated that the GFP was inserted just after the signal peptide of ALP. ALP-GFP-A9-GPI indicated that GFP was inserted at the front of the 9th amino acid residues before GPI-anchoring site. All primers used in this study are listed in Table 1 and the schematic figures are described in Fig. 1.
Table 1.
The used primers in the studies
| Expressed fragments | Amplified subfragments | Names of primers | Sequences of primers | Method |
|---|---|---|---|---|
| ALP | ALP | ALP-FP | 5′-CCCAAGCTTGCCACCATGGTGACACTGTTCCCG-3′ | |
| ALP-RP | 5′-CGCGGATCCTTATCGCAGTAAAATGGAAGT-3′ | |||
| ALP-GFP | ALP-GFP | ALP-FP | 5′-CCCAAGCTTGCCACCATGGTGACACTGTTCCCG-3′ | |
| ALP-RP | 5′-CGCGGATCCCGACCACCACCACCACCACCTCGCAGTAAAATGGAAGT-3′ | |||
| ALP-GFP-GPI | ALP-N | ALP-FP | 5′-CCCAAGCTTGCCACCATGGTGACACTGTTCCCG-3′ | |
| ALP-N-RP | 5′-CGGGGTACCGTGGCGCAGGCGTGCCGGCC-3′ | |||
| GFP-GPI | GFP-M-FP | 5′-CGGGGTACCGGGTGGTGGTGGTGGTGGTATGGTGAGCAA-3′ | Overlap PCR | |
| GPI-M-RP | 5′ –CTTGTACAGCTCGTCCATGCC-3′ | |||
| GPI-FP | 5′-ATGGACGAGCTGTACAAGGGAGGAGGAGGAGGAGGAAGTGCGCACTTGCCT-3′ | |||
| GPI-RP | 5′-CGCGGATCCTTAATGATGATGATGATGATGTCGCAGTAAAATGGA AGT-3′ | |||
| ALP-GFP-A9-GPI | ALP-N | ALP-FP | 5′-CCCAAGCTTGCCACCATGGTGACACTGTTCCCG-3′ | |
| ALP-N-RP | 5′-CGGGGTACCGTGCAGGCGGCGTAGGCCAT -3′ | |||
| GFP-A9-GPI | GFP-M-FP | 5′-CGGGGTACCGGGTGGTGGTGGTGGTGGTATGGTGAGCAAGGGCGAG-3′ | Overlap PCR | |
| GPI-M-RP | 5′ –CTTGTACAGCTCGTCCATGCC-3′ | |||
| A9-GPI-FP | 5′-ATGGACGAGCTGTACAAGGGAGGAGGAGGAGGAGGAATCGGCCCCGGCCGGCAC -3′ | |||
| A9-GPI-RP | 5′-CGCGGATCCTTAATGATGATGATGATGATGTCGCAGTAAAATGGA AGT-3′ | |||
| Sig-GFP-ALP | ALP-C | ALP-C-FP | 5′-CGGGGTACCCACTGGCTGCATCCCGCG -3′ | |
| ALP-RP | 5′-CGC GGATCC TTA TCG CAG TAA AAT GGA AGT-3′ | |||
| ALP-Sig-GFP | GFP-FP | 5′-GCGACGAGCGCGCGCGCTGGTGGTGGTGGTGGTGGTATGGTGAGCAAGGGCGAG-3′ | Overlap PCR | |
| GFP-RP | 5′-CGGGGTACCCTTGTACAGCTCGTCCATGCC-3′ | |||
| ALP-Sig -FP | 5′-CCCAAGCTTGCCACCATGGTGACACTGTTCCCG-3′ | |||
| N-Sig- RP | 5′- AGCGCGCGCGCTCGTCGC-3′ | |||
| APN-GFP-GPI | APN-N | APN-N-FP | 5′-CCCAAGCTTGCCACCATGGCG AACCGCTGGTAC-3′ | |
| APN-N-RP | 5′-CGGGGTACCTGTTGTTGTAGCTTCTGTAGT-3′ | |||
| GFP-GPI | GFP -FP | 5′-CGGGGTACCGGTGGTGGTGGTGGTGGTATGGTGAGCAAGGGCGAG -3′ | Overlap PCR | |
| GFP -RP | 5′ –CTTGTACAGCTCGTCCATGCC-3′ | |||
| GPI-FP | 5′-ATGGACGAGCTGTACAAGGGAGGAGGAGGAGGAGGACCTGTACCTGGCTCAGCA-3′ | |||
| GPI-RP | 5′-CGCGGATCCTTAATGATGATGATGATGATGAGCCATATTAACAACGAG-3′ | |||
| Cadhein-GFP | Cadherin-GFP | Cadherin-FP | 5′-CCGCTCGAGGCCACCATGGCAGTCGACGTGAGA ATA-3′ | |
| Cadherin-FP | 5′-TCCCCGCGGACCACCACCACCACCACCTCTTCTGAA CTGCGTGTT-3′ | |||
| Cad-EGFP-GPI | Cad-N | Cadherin-FP | 5′-CCGCTCGAGGCCACCATGGCAGTCGACGTGAGA ATA-3′ | |
| Cad-N-RP | 5′-TCCCCGCGGCAGGGCTCGCGCCTGCGCGTC -3′ | |||
| GFP-GPI | GFP -FP | 5′-TCCCCGCGGGGTGGTGGTGGTGGTGGTATGGTGAGCAAGGGCGAG-3′ | Overlap PCR | |
| GFP-RP | 5′-CTTGTACAGCTCGTCCATGCC-3′ | |||
| GPI-FP | 5′-ATGGACGAGCTGTACAAGGGAGGAGGAGGAGGAGGACCTGTACCTGGCTCAGCA -3′ | |||
| GPI-RP | 5′-CGCGGATCCTTAATGATGATGATGATGATGAGCCATATTAACAACGAG-3′ | |||
| ALP-TBR-GFP-GPI | ALP-N | ALP-FR | 5′-CCCAAGCTTGCCACCATGGTGACACTGTTCCCG-3′ | |
| ALP-N-RP | 5′-CGGGGTACCGTGGCGCAGGCGTGCCGGCC-3′ | |||
| TBR-GFP-GPI | TBR-FP | 5′-CGGGGTACCGACGATTCGTGCTACGGAC-3′ | Overlap PCR | |
| TBR-RP | 5′ –GGCTCGCGCCTGCGCGTC-3′ | |||
| GFP-GPI-FP | 5′-GACGCGCAGGCGCGAGCCGGTGGTGGTGGTGGTGGTATGGTGAGCAAGGGCGAG-3′ | |||
| GFP-GPI-RP | 5′-CGCGGATCCTTAATGATGATGATGATGATGTCGCAGTAAAATGGA AGT-3′ |
FP forward primer, RP reverse primer
Fig. 1.
Constructs of plasmids. S signal peptide, GPI glycosyl phosphatidyl inositol modification signal of GPI-anchored protein, GFP green fluorescence protein, B region of Hacadher (H. armigera cadherin) in binding to activated Cry1Ac (TBR), T trans-membrance region of Hacadherin, C cytosolic part of Hacadherin. Sig presents the signal peptide of ALP2 or APN1. A9 indicates that there were 9 amino acid residues between GFP and GPI-anchoring site. Tcadherin represents the C-terminal truncated cadherin without the transmembrane domain and cytosolic domain
To investigate whether cadherin and ALP2 function coordinately, the mosaic genes were also constructed using ALP2 and cadherin. The schematic figures and the used primers are listed in Fig. 1 and Table 1.
Insect cells were seeded onto sterile microscope coverslips (13-mm diameter) (Taikang Co., Shanghai, China) in a 24-well tissue culture plate (Corning, NY, USA) and grew over night. A transfection mixture was prepared by mixing plasmid DNA (1 μg) and Cellfectin reagent (Life Technologies Co., Carlsbad, CA, USA) (2 μl). The transfection mixture was incubated for 30 min at room temperature before use. Medium was removed from the seeded cells, and the cells were washed twice with serum-free medium, then transfection mixture supplemented with serum-free medium was gently added. The cells were then incubated with plasmid for 5 h at 28 °C. After the indicated incubation time, the cells were washed twice with medium. Finally, cells were incubated with Grace,s insect cell medium supplemented with 10 % FBS. The cells were observed under a fluorescence microscope (Nikon, Tokyo, Japan) and photographed at 24 h of post transfection.
Live cell-immunofluorescence staining of the expressed receptors in Sl-HP cells
Sl-HP cells were seeded onto sterile microscope coverslips (13-mm diameter) in a 24-well tissue culture plate and grew over night. The cells were then transfected with plasmids as described above and cultured for 24 h at 28 °C. After the indicated incubation time, the cells were washed twice with phosphate-buffered saline (PBS) (1 mM Na2HPO4, 10.5 mM KH2PO4, 140 mM NaCl, 40 mM KCl, pH 7.4). The cells in each well were incubated with various mouse primary antibodies (anti-ALP2 polyclonal serum prepared by ourselves or commercial anti-GFP monoclonal antibody) (Earthox, San Francisco, CA, USA) at room temperature for 1 h. After incubation, cells were washed three times with PBS, fixed with 4 % paraformaldehyde and incubated with goat anti-mouse fluorescent immunoglobulin G (IgG) (Earthox, San Francisco, CA, USA), as a secondary antibody (1:1,000 diluted in PBS containing1 % BSA) for 30 min at room temperature. Finally, the cells were washed with 1× PBS, mounted in buffered glycerol under a coverslip, observed under a fluorescence microscope, and photographed.
Western blotting analysis
Sl-HP cells were transfected with each recombinant plasmid as described above and collected at 24 h of post transfection. Then the cell pellet was washed with PBS and suspended in SDS-PAGE sample buffer, and the samples were then separated on a SDS-10 % polyacrylamide gel. After electrophoresis, proteins were electrotransferred to PVDF membrane (Millipore Corporation, Billerica, MA, USA). The membrane was blocked with 5 % non-fat milk in TBS-T buffer for 2 h at room temperature, and then incubated with mouse anti-ALP2 antibody (prepared in the lab) at a 1:1,000 dilution or incubated with mouse anti-GFP antibody (Abcam, Cambridge, UK) at a 1:3,000 dilution in TBS for 2 h at room temperature. The membranes were then washed for three times with TBS-T and then incubated with fluorescent secondary antibody (Earthox, San Francisco, CA, USA) at a 1:5,000 dilution. Finally, the membranes were washed for three times with TBS-T and then bands were visualized using the Odyssey system (LI-COR Bioscience, Lincoln, NE, USA).
Cry1Ac toxin binding
Sl-HP cells were seeded onto sterile microscope coverslips (13-mm diameter) in a 24-well tissue culture plate and grew overnight. The cells were then transfected with plasmids described above. At 24 h of post transfection, Grace,s insect cell medium supplemented with 10 % FBS was removed, and the cells were washed gently twice with PBS (pH 7.4), and overlaid with 400 μl of activated Cry1Ac (gift from Marianne Pusztai-Carey at the Case Western Reserve University) in PBS at various concentrations (double dilution) and incubated for a period of 30 min. Following three times washes with 1× PBS, the cells were then fixed in ice-cold 4 % paraformaldehyde solution for 20 min. Next, the fixed cells were washed 3 times with 1× PBS and then blocked with 1 % BSA for 1 h at room temperature. After blocking, the cells were washed with PBS and then incubated with rabbit anti-Cry1Ac (1:500) (prepared in the lab) in PBS containing 1 % BSA at room temperature for 1 h. After washing three times with PBS, the cells were incubated with goat anti-rabbit fluorescent IgG (Earthox, San Francisco, CA, USA) as a secondary antibody in PBS (1:5,000) containing 1 % BSA for 1 h at room temperature. Finally, the cells were washed twice with 1× PBS, mounted in buffered glycerol under a coverslip, observed under a fluorescence microscope, and photographed.
Cytotoxicity assays of the activated Cry1Ac to insect cells expressing recombinant or mosaic receptors
Sl-HP cells and Hi5 cells were seeded onto steriled coverslips placed in 24-well cell culture plate and grew overnight. They were transfected with plasmids expressing recombinant or mosaic receptors, respectively. At 24 h of post transfection, Grace,s insect cell medium was removed, and the cell was washed gently twice with PBS (pH 7.4) and overlaid with 400 μl of activated Cry1Ac in PBS at various concentrations. After a period of 1 or 0.5 h of incubation with the activated Cry1Ac, the cells were observed under a light microscope or fluorescence microscope.
Measurement of toxin concentrations resulting in swelling of 50 % cells
The cells cultured in 96-well cell culture plate were incubated with activated Cry1Ac at various concentrations (double dilution in PBS) for 1 h at 24 h post transfection with various receptor-expressing plasmids after they had been gently washed three times with PBS. Then they were photographed under an inverted fluorescence microscope. The percentage of aberrant (swollen and lyzed) cells was calculated according to the formula. For the cells expressing single fluorescently tagged protein, percentage of aberrant cells = number of aberrant cells with fluorescence protein/number of total cells with fluorescence protein. For the cells emitting both red and green fluorescence, only the cells co-expressing the two fused fluorescence proteins were counted. Concentrations of toxin resulting in swelling of 50 % cells (EC50) were obtained from the cytotoxic dose–response curve fitted by probit regression analysis, corresponding to the concentrations of activated Cry1Ac.
Statistical analysis
Data are expressed as means ± standard deviations of at least three independent experiments. p < 0.05 was considered statistically significant.
Results
Expression and location of various ALP2 fusion proteins in Sl-HP cells
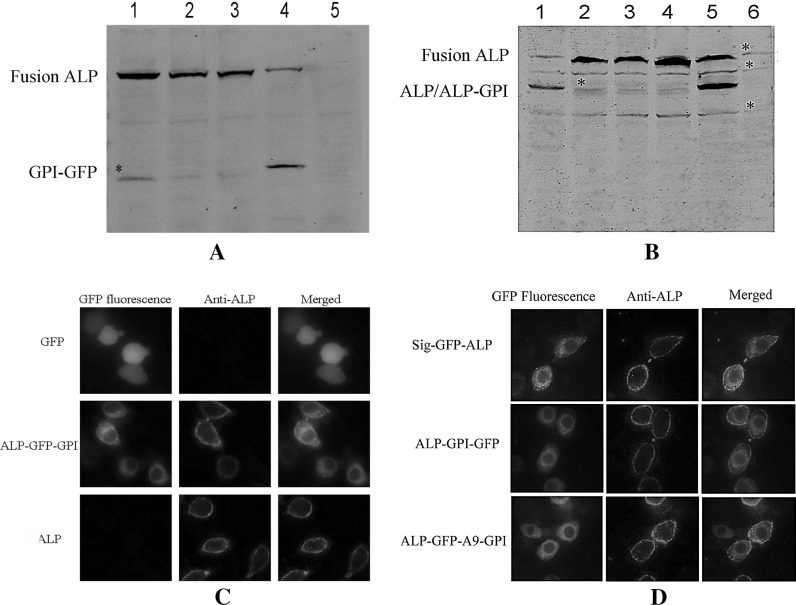
Native ALP2 is a GPI anchored protein tethered to the brush border membrane surface. To monitor the location of ALP2 fused with GFP on cells, we constructed four plasmids expressing various ALP2 fusion proteins in Sl-HP cells. Western blot analysis revealed that four predicted fusion proteins were readily detected using anti-GFP or anti-ALP2 antibody as primary antibody (Fig. 2a, b). The location of the fusion proteins on cell membrane was also further confirmed via the live cell-immunofluorescence staining using commercial mouse anti-GFP monoclonal antibody or rabbit anti-ALP2 polyclonal antibody as primary antibody (Fig. 2c, d). No significant differences of fluorescence distribution was observed between the cells expressing ALP2 fused with GFP and the ones expressing ALP2 without any label, demonstrating that the insertion of GFP into ALP2 had no significant influence on the location of ALP2 on cell membrane.
Fig. 2.
Expression and location of various ALP2 proteins fused with GFP in Sl-HP cells. a Expression of the fusion proteins detected using anti-GFP antibody as primary antibody. Lane 1 ALP-GFP-GPI; lane 2 ALP-GFP-A9-GPI; lane 3 Sig-GFP-ALP-GPI; lane 4 ALP-GPI-GFP, showing GPI-GFP cleaved from ALP-GPI-GFP; lane 5 Control (cells without transfection). b Expression of various fusion proteins detected using anti-ALP2 antibody as a primary antibody. *Non-specific band. A9. There were 9 amino acid residues between GFP and the cleavage site of GPI modification signal. ALP2 with GPI modification signal can not be distinguished from ALP2 without GPI in panel a because the molecular weight of GPI modification signal is very low. c, d Location of various ALP2 fusion proteins on cell membrane of Sl-HP cells assayed via live cell-immunofluoscence staining
Expression and location of recombinant APN1 and cadherin in Sl-HP cells
Since ALP-GFP-GPI was located on cell membrane as described above, the plasmid pIE2-APN-GFP-GPI was constructed to test its localization. When this plasmid was transfected into Sl-HP cells, as shown in Fig. 3, the APN-GFP-GPI fusion protein was also localized on the cell membrane. Cadherin is a transmembrane protein. To detect its localization, the pIE2-cadherin-GFP was constructed by adding GFP directly to the carboxyl terminus of cadherin. Similarly, when the plasmid pIE2-cadherin-GFP was transfected into Sl-HP cells, cadherin-GFP fusion protein was also localized on the cell membrane (Fig. 3). The expressed cadherin-GFP was also detected by Western blot assay using anti-GFP antibody as primary antibody and the molecular weight of the fusion protein was consistent with the predicted size (data not shown).
Fig. 3.
Expression and location of APN1 and cadherin fused with GFP in Sl-HP cells. The cells expressing the fluorescence proteins were fixed and stained with Hoechst 33258 (Sigma-Aldrich Co.) at 24 h of post-transfection. Then they were subjected to observation using fluorescence microscope. The fused proteins were abundant on cell membrane and partial fused proteins were located in cytoplasm in the form of small dots
Binding of the activated Cry1Ac to recombinant ALP2, APN1 and cadherin on cell membrane
The lowest concentration of the activated Cry1Ac killing Sl-HP cells without transfection or expressing only GFP was determined under light microscope, and the activated Cry1Ac-treated cells began to swell at the lowest concentration of 2 μg/ml at 1 h post treatment. No significantly morphological change was observed when the concentration of the toxin was at 1 μg/ml, but many cells swelled at the concentration of 4 μg/ml at 1 h of post treatment with the toxin.
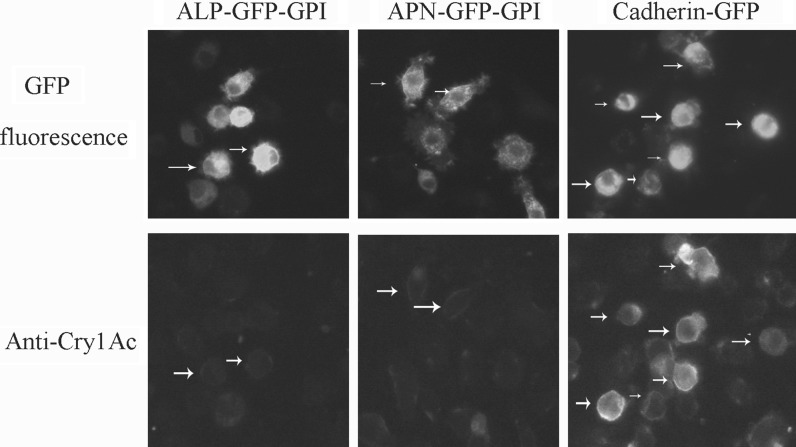
To investigate the interaction between the activated Cry1Ac and the recombinant ALP-GFP-GPI, APN-GFP-GPI or cadherin-GFP, Sl-HP cells expressing these GFP fusion proteins were incubated with the activated Cry1Ac toxin for 30 min at the concentrations between 0.04 and 2 μg/ml using double dilution method. After three times washes with PBS, the cells were fixed with 4 % paraformaldehyde and immunofluorescence assay was performed using the rabbit anti-Cry1Ac as a primary antibody. As shown in Fig. 4, the relative quantity of activated Cry1Ac binding to cadherin-GFP was much higher than that binding to APN-GFP-GPI or ALP-GFP-GPI under the treatment of the activated Cry1Ac at 0.04 μg/ml for 30 min. Even if the concentration of the activated Cry1Ac increased up to 2 μg/ml, no significant CryAc-binding to APN-GFP-GPI or ALP-GFP-GPI was observed (data not shown). Similar results were obtained when Sl-HP cells expressing ALP2, APN1 and cadherin without fused GFP were used for the live cell-immunofluorescence staining (data not shown). Thus, the binding affinity of ALP2 and APN1 to the activated Cry1Ac was much lower than that of cadherin to this toxin.
Fig. 4.
Binding of the activated Cry1Ac to ALP-GFP-GPI, APN-GFP-GPI and cadherin-GFP on cell membrane. The cells were treated with activated Cry1Ac at 0.04 μg/ml for 0.5 h at 24 h of post transfection. Then the cells were fixed after washing twice, and immunofluorescence assay was performed using anti-Cry1Ac serum as a primary antibody. Arrows point to the positive cells binding to activated Cry1Ac. Red fluorescence signal was very weak for the cells expressing ALP-GFP-GPI or APN-GFP-GPI, compared to the cells expressing cadherin-GFP
The effects of the three recombinant receptors on the cytotoxicity of the activated Cry1Ac to Sl-HP cells
To investigate the influences of the three recombinant receptors on the cytotoxicity of the activated CryAc to Sl-HP cells, the cells expressing cadherin-GFP, ALP-GPI-GFP, or APN-GFP-GPI were subjected to exposure to the activated Cry1Ac toxin in the range of 0.01–1 μg/ml. At 1 h of post incubation with the toxin, microscope observation of the cells revealed that only the cells expressing cadherin-GFP showed aberrant cell morphology when the concentration of the toxin was 0.04 μg/ml using double dilution method (Fig. 5a), while the cells expressing APN-GFP-GPI or ALP-GFP-GPI showed no significant morphological change even though the concentration of the toxin increased up to 1 μg/ml. Concentrations of activated Cry1Ac resulting in swelling of 50 % cells expressing fluorescently tagged proteins were determined using the double dilution method and the results revealed that the expression of APN1-GFP-GPI or ALP2-GPI-GFP did not enhance the cytotoxicity of activated Cry1Ac to Sl-HP cells in comparison with the expression of GFP (control) (Table 2). Considering that GFP might have influences on the action of ALP2, APN1 and cadherin, Sl-HP cells were also, respectively, transfected with plasmids expressing ALP2, APN1 and cadherin without GFP tag. The similar results were obtained at a concentration of activated Cry1Ac of 0.04 μg/ml (Fig. 5b). Percentages of the aberrant cells were calculated after the treatment of GFP, ALP 2, APN1 or cadherin-expressing Sl-HP cells with activated Cry1Ac, and the results revealed that the percentages of aberrant cells increased very slightly when ALP2 and APN1 were expressed in comparison with the control groups in which GFP was expressed (Table 3). The removal of GFP tag from cadherin-GFP did not significantly increase the cytotoxicity of activated Cry1Ac to Sl-HP cells (Tables 2 and 4).
Fig. 5.
Effects of the three recombinant receptors on the cytotoxicity of activated Cry1Ac to Sl-HP cells. a The receptors fused with GFP. b The receptors without GFP fusion. Arrows point to the aberrant cells treated with the activated Cry1Ac at 0.04 μg/ml for 1 h at 24 h of post transfection
Table 2.
Concentrations of activated Cry1Ac resulting in swelling of 50 % cells expressing fluorescently tagged proteins
| Transfected Sl-HP cells* | Probit regression analysis | R | EC50 ± SE (µg/ml) |
|---|---|---|---|
| GFP | y = 3.663 + 1.652x | 0.98 | 6.45 ± 0.70a# |
| Cad-GFP | y = 7.124 + 1.847x | 0.99 | 0.070 ± 0.020b |
| APN1-GFP-GPI | y = 2.209 + 3.323x | 0.96 | 6.92 ± 0.52b |
| ALP2-GPI-GFP | y = 1.689 + 4.142x | 0.96 | 6.30 ± 1.25b |
* Various fluorescently tagged proteins were expressed in the transfected cells
#The different letters represent significant differences at p < 0.05. Percentage of the aberrant cells = number of the aberrant cells emitting fluorescence/number of total cells emitting fluorescence
Table 3.
Percentages of the aberrant cells after the treatment of GFP, ALP 2 and APN1-expressing Sl-HP cells with activated Cry1Ac
| Percentages of the aberrant cells (%) | |||||
|---|---|---|---|---|---|
| Toxin concentrations (µg/ml) | 1.25 | 2.50 | 5.00 | 10.00 | 20.00 |
| GFP | 3 ± 2 | 36 ± 4a* | 84 ± 4 | 96 ± 2 | 98 ± 1 |
| ALP2 | 2 ± 1 | 66 ± 7b | 83 ± 4 | 98 ± 1 | 98 ± 1 |
| APN1 | 4 ± 2 | 58 ± 6b | 87 ± 3 | 95 ± 2 | 98 ± 1 |
The efficiency of transfection for the cells was about 50–60 % according to the percentage of the cells emitting green fluorescence after the transfection with the GFP-expressing plasmid
* The different letters represent significant differences at p < 0.05. Percentage of the aberrant cells = number of the aberrant cells/number of total cells
Table 4.
Percentages of the aberrant cells after the treatment of cadherin-expressing Sl-HP cells with activated Cry1Ac
| Percentages of the aberrant cells (%) | ||||
|---|---|---|---|---|
| Toxin concentrations (µg/ml) | 0.025 | 0.050 | 0.100 | 0.200 |
| Cadherin | 2.0 ± 1.2 | 19.4 ± 3.1 | 30.6 ± 2.8 | 46.5 ± 2.7 |
| GFP | 2.3 ± 1.2 | 2.3 ± 1.2 | 2.4 ± 1.3 | 2.6 ± 1.4 |
The efficiency of transfection for the cells was about 50–60 % according to the percentage of the cells emitting green fluorescence after the transfection with the GFP-expressing plasmid. Percentage of the aberrant cells = number of the aberrant cells/number of total cells
When ALP-GFP-GPI or APN-GFP-GPI was co-expressed with cadherin-mCherry in Sl-HP cells, light microscope examination revealed that although the efficiency of the co-transfection was high (about 50–60 %), no enhancement of the cadherin-mediated cytotoxicity of the activated Cry1Ac to Sl-HP cells was observed in any cases (Fig. 6). Considering that the fused GFP might block the insertion of the activated Cry1Ac on cell membrane, APN1 or ALP2 was also co-expressed with cadherin-GFP in Sl-HP cells, and the results of cytotoxicity were similar to that described above (data not shown). Concentrations of activated Cry1Ac resulting in swelling of 50 % cells were determined and the results showed that the co-expression of the receptors did not increase cytotoxicity of activated Cry1Ac to Sl-HP cells in comparison with the single expression of cadherin (Table 5).
Fig. 6.
Influence of the co-expression of ALP-GFP-GPI or APN-GFP-GPI with cadherin-mCherry on the cytotoxicity of activated Cry1Ac to Sl-HP cells. Arrows point to the aberrant cells treated with activated Cry1Ac at various concentrations for 1 h at 24 h post transfection
Table 5.
Influence of co-expression of the receptors on cytotoxicity of activated Cry1Ac to Sl-HP cells
| Transfected Sl-HP cells | Probit regression analysis | R | EC50 ± SE (µg/ml) |
|---|---|---|---|
| GFP* | y = 3.982 + 1.446x | 0.97 | 5.08 ± 0.42a# |
| Cad-mCherry* | y = 6.825 + 1.498x | 0.97 | 0.060 ± 0.008b |
| Cad-mCherry + APN1-GFP-GPI** | y = 8.849 + 2.922x | 0.98 | 0.048 ± 0.011b |
| Cad-mCherry + ALP2-GFP-GPI** | y = 9.390 + 2.871x | 0.98 | 0.030 ± 0.014b |
| Cad-GFP* | y = 7.124 + 1.847x | 0.98 | 0.070 ± 0.020b |
| Cad-GFP + APN1* | y = 8.027 + 2.451x | 0.96 | 0.058 ± 0.006b |
| Cad-GFP + ALP2* | y = 8.038 + 2.656x | 0.98 | 0.072 ± 0.012b |
* The cells emitting fluorescence were counted for the calculation of EC50
** The cells emitting both green and red fluorescence were counted for the calculation of EC50, and more than 80 % of the transfected cells
#The different letters represented significant differences at p < 0.05
Taken together, whatever APN1 or ALP2 fused with or without GFP were, respectively, expressed or co-expressed with cadherin in Sl-HP cells, no significantly enhanced cytotoxicity of activated Cry1Ac was observed.
ALP-GFP-GPI inserted with Cry1Ac binding region of cadherin was located on cell membrane but no significant binding of the mosaic receptor to the activated Cry1Ac was observed
When ALP-GFP-GPI inserted with Cry1Ac toxin binding region of cadherin (TBR) was expressed in Sl-HP cells, the live cell-immunofluorescence stainging revealed that the mosaic protein was located on the cell membrane (Fig. 7 a1–a3). However, the binding of the mosaic receptor to the activated Cry1Ac was not observed under fluorescence microscope at the activated Cry1Ac concentrations of 0.04 μg/ml for 30 min (Fig. 7b) and no significant cytotoxicity was observed at the concentrations of 0.04 μg/ml at 1 h of post treatment of the activated Cry1Ac (Fig. 7c1).
Fig. 7.
Localization of mosaic receptors and their influences on cytotoxicity of activated Cry1Ac to Sl-HP cells. a, b The cells expressing ALP2-TBR-GFP-GPI. a1 The cells under fluorescence microscope; a2 live-cell immunofluorescence staining of the cell using anti-GFP antibody as a primary body; a3 merged; b1 ALP2-TBR-GFP-GPI expressing cells treated with activated Cry1Ac at 0.08 μg/ml for 1 h; b2 live-cell immunofluorescence staining of the cells treated with activated Cry1Ac at 0.08 μg/ml for 1 h, using anti-Cry1Ac antibody as primary body; b3 merged; c1 ALP2-TBR-GFP-GPI expressing cells treated with activated Cry1Ac at 0.08 μg/ml for 1 h, showing no swollen cells; d1 tcadherin-GFP-GPI expressing cells under fluorescence microscope; d2 live-cell immunofluorescence staining of the tcadherin-GFP-GPI expressing cells treated with activated Cry1Ac at 0.04 μg/ml for 0.5 h at 24 h of post transfection, using anti-GFP antibody as a primary antibody
The replacement of C terminus of cadherin with GFP-GPI from ALP-GFP-GPI did not result in the location of the mosaic protein on the cell membrance
The truncated cadherin gene without the transmembrane region and cytoplasmic region was produced by PCR, and the GFP-GPI of APN1-GFP-GPI produced by PCR was linked to the C terminus of the truncated cadherin gene. A plasmid was constructed for the expression of the mosaic protein tcad-GFP-GPI (Fig. 1). The tcad-GFP-GPI was not located on the cell membrane (Fig. 7d1, d2).
The susceptibility of the noncadherin-expressing Sl-HP cells to the activated Cry1Ac was higher than that of the cadherin-expressing Hi5 cells
Since the concentration of the activated Cry1Ac for killing Sl-HP cells was much lower than that for killing Hi5 or Sf9 cells (Garner et al. 1999; Banks et al. 2003; Sivakumar et al. 2007), we determined to compare the cytotoxicity of the toxin to Sl-HP and cadherin-expressing Hi5 cells. Double dilution method was used to determine the concentration of the activated Cry1Ac resulting in swelling of 50 % cells. Sl-HP and Hi5 cells with transfection or without were treated with the toxin at various concentrations (double dilution in PBS) for 1 h, and observed under an inverted fluorescence microscope. The percentage of aberrant (swollen) cells was calculated as described in the part of materials and methods. The analysis revealed that the concentrations of the toxin resulting in swelling of 50 % cells were 5.08 ± 0.42 and more than 30 μg/ml, respectively, for Sl-HP and Hi5 cells without transfection (Table 6). The concentration of the toxin resulting in swelling of 50 % cells was 9.50 ± 1.21 μg/ml for Hi5 cells expressing cadherin-GFP (Table 6). Thus, Sl-HP cells without expression of recombinant cadherin were more susceptible to the activated Cry1Ac than Hi5 cells expressing recombinant cadherin-GFP.
Table 6.
Non-cadherin-expressing Sl-HP cells were more susceptible to activated Cry1Ac than cadherin-expressing Hi5 cells
| Transfected cells | Probit regression analysis | R | EC50 ± SE (µg/ml) |
|---|---|---|---|
| GFP* Sl-HP cells |
y = 3.982 + 1.446x | 0.97 | 5.08 ± 0.42a# |
| Cad-GFP** Hi5 |
y = 3.222 + 1.815x | 0.98 | 9.50 ± 1.21b |
| GFP Hi5 cells |
NT | NT | >30 |
NT not tested
* GFP was expressed in the transfected cells
** Cadherin-GFP was expressed in the transfected cells. The value of EC50 was not determined for Hi5 cells expressing GFP because 30 µg/ml activated Cry1Ac had no significant influences on the morphology of cells at 1 h after the treatment
#The different letters represent significant differences at p < 0.05
Discussion
One of the current proposed models for Cry intoxication supports sequential interactions of Cry toxins with diverse binding proteins. Both APN and ALP have been proposed to bind Cry toxins after they interact with cadherin-like proteins (Soberón et al. 2009), and the affinity of interaction between Cry toxins and APN is very low before binding of Cry toxins to cadherin because cadherin can mediate the processing of the toxins (Pacheco et al. 2009). However, in the present study, the co-expressed ALP2 or APN1 did not enhance cadherin-mediated cytotoxicity of the activated Cry1Ac to Sl-HP cells. Thus, in the present studies, cadherin might not enhance the binding of activated Cry1Ac to ALP2 or APN1 on cell membrane of Sl-HP cells.
Zhang et al. (2005) reported that cadherin specifically mediated the incorporation of CryAb into cell membrane at monomeric form, which was cytotoxic, but non-specific binding mediated the incorporation of the toxin into cell membrane at the oligomeric form, which is not cytotoxic at the low or modest concentration of the toxin. However, we have demonstrated that the activated CryAc was also cytotoxic to Sl-HP cells without recombinant cadherin expression at a relatively low concentration of 5.08 μg/ml in this study, which was lower than that killing cadherin-expressing Hi5 cells, suggesting that there is an unknown mechanism for the susceptibility of Sl-HP cells to activated Cry1Ac.
It has been reported that the native non-denatured cadherin and APN1 can bind to activated Cry1Ac except ALP from Manduca sexta in vivo assay (Flores-Escobar et al. 2013), but some insect cell-expressing recombinant APNs lost the ability of binding to toxin or mediating cytotoxicity (Garner et al. 1999; Banks et al. 2003). The possible mechanism might be that the recombinant APN or ALP can not receive proper glycosylation. However, Wang et al. (2005b) suggested that glycosylation may not be required for this interaction because they expressed APN in E. coli, which showed binding to Cry1Ac with ligand blot analysis (no glycosylation occurred in E. coli. Another possibility is that there are many types of ALPs and APNs in insects, but only some of them function as Cry1Ac receptors. Tiewsiri and Wang (2011) reported that the alteration of the two APNs expression in T. ni was associated with the high level of resistance even if other APNs and cadherin were expressed normally. Thus, the analysis of the activities and the structure of the recombinant expressed APNs and ALPs might be required to confirm that the recombinant expressed receptors are comparable to the purified native proteins from midgut cells of larvae.
In our previous studies, ligand blot and dot blot analysis revealed that the activated Cry1Ac bound to both denatured and native purified H. armigera ALP2 (Ning et al. 2010). It also has been reported that all Cry1Aa, Cry1Aab and Cry1Ac could bind to Hi5-expressing APN1 from H. armigera (Rajagopal et al. 2003). In the present experiments, we found that the binding of Sl-HP cells expressing H. armigera APN1 or ALP2 to the activated Cry1Ac was very weak on the cell membrane in vitro assay, which showed no significant cytotoxicity at the indicated Cry1Ac concentration, compared to that expressing H. armigera cadherin. It seems that the analysis of ligand blot is not comparable with that of binding of living cells to the toxin. The baculovirus-expressing APN1 in Sf21 could mediate cytotoxicity of the activated Cry1Ac to the cell line (Sivakumar et al. 2007). But we do not know how much the cytotoxicity of activated Cry1Ac increased because no LC50 was calculated for the APN1-expressed cells and the control cells. In our studies, it is true that the expression of APN1 or APL2 without tag slightly increased the cytotoxicity of activated Cry1Ac (Table 3). However, the increase can be disregarded in comparison with that caused by the expression of cadherin. Considering Sl-HP cells without recombinant cadherin expression is much more susceptible to the toxin than Hi5 cells expressing cadherin or APN1, we thought that these different results might be due to the different cell lines used or different experimental conditions, and the unknown endogenous Cry1Ac receptor(s) in Sl-HP cells might mask the functions of recombinant APN1 and ALP2. Additionally, both cadherin and ABCC transporter proteins can mediate toxicity of activated Cry1Ac without APN1 and ALP2 (Ganhan et al. 2001, 2010), suggesting that the roles of APN1 and ALP2 are needed to be further studied in the model of Bt action. More investigation is required to elucidate the roles of H. armigera APN1 and ALP2 during the development of resistance of Bt in H. armigera populations.
It has been reported that both cadherin extracellular and intracellular mutations can result in the development of Cry1Ac resistance in H. armigera (Morin et al. 2003; Zhang et al. 2012). In order to further investigate the influence of the transmembrane region of cadherin on Cry1Ac resistance, mosaic receptors were expressed. When the cytosolic and the transmembrane regions were replaced with the GFP-GPI of APN-GFP-GPI, the expressed mosaic protein (tcadherin-GFP-GPI) accumulated in cytoplasm (possibly in vacuoles), and no membrane-bound mosaic protein has been observed using live-cell-immunofluorescence staining. The results suggest that the signal peptide of cadherin and GPI modification signal of ALP2 are not sufficient for the location of the mosaic protein on cell membrane.
ALP-GFP-GPI inserted with Cry1Ac binding region of cadherin (TBR) was located on cell membrane, but no binding of it to the activated Cry1Ac was observed on the cell membrane. The mechanism might be that the TBR can not be folded correctly, or exposed to the surface when it was inserted into ALP-GFP-GPI even if the linkers were added at the junctions of TBR. It also suggests that the mutation of cadherin outside the TBR might result in the development of Bt resistance through affecting the bind of TBR to the activated toxin.
The concentration of activated Cry1Ac resulting in swelling of 50 % Sl-HP cells (5.08 μg/ml) is much lower than that of Cry1Ab (100 μg/ml) damaging mammalian kidney 293T cells within 1 h (Mesnage et al. 2013). Although it is well known that activated Cry1Ab can damage the midgut cells of susceptible larvae, it has no effects on viability of cultured porcine intestinal cells (Bondzio et al. 2013). No membraneous changes were observed on the bovine, porcine, and human intestinal cells, but the silkworm midgut cells developed severe membrane potential changes within 1 h following the activated Cry1Ab toxin treatment at a final concentration of 2 μg/ml (Shimada et al. 2006). Although both Sl-HP and Hi5 cell lines were established from ovaries, Sl-HP cells without the expression of the recombinant Cry1Ac receptors are more susceptible than Hi5 cells expressing the recombinant cadherin (5.08 vs. 9.50 μg/ml), and the concentration of the activated Cry1Ac killing Sl-HP cells is comparable to that of activated Cry1Ab damaging the silkworm midgut cells within 1 h (Shimada et al. 2006). Sl-HP cells are also much more susceptible to activated Cry1Ac than any other cell lines tested such, as Sf9, Sf21 and Drosophila S2 cell lines (Garner et al. 1999; Sivakumar et al. 2007; Banks et al. 2003). The data suggest that there might be endogenous Cry1Ac receptor(s) in Sl-HP cells. In the future, analysis of transcriptome of Sl-HP cells is needed to investigate the mechanism for the susceptibility of Sl-HP cells to activated Cry1Ac.
Acknowledgments
The work was supported by the National Natural Science Foundation of China (Grant Numbers: 31071739 and 31372260).
Footnotes
Peng Xu and Mayira Islam have contributed equally to this work.
Contributor Information
Chenxi Liu, Email: liuchenxi@caas.cn.
Kaiyu Liu, Email: liukaiyu@mail.ccnu.edu.cn.
References
- Aimanova KG, Zhuang M, Gill SS. Expression of Cry1Ac cadherin receptors in insect midgut and cell lines. J Invertebr Pathol. 2006;92:178–187. doi: 10.1016/j.jip.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Banks DJ, Hua G, Adang MJ. Cloning of a Heliothis virescens 110 kDa aminopeptidase N and expression in Drosophila S2 cells. Insect Biochem Mol Biol. 2003;33:499–508. doi: 10.1016/S0965-1748(03)00022-5. [DOI] [PubMed] [Google Scholar]
- Bondzio A, Lodemann U, Weise C, Einspanier R (2013) Cry1Ab treatment has no effects on viability of cultured porcine intestinal cells, but triggers Hsp70 expression. PLoS One 8:e67079 [DOI] [PMC free article] [PubMed]
- Flores-Escobar B, Rodríguez-Magadan H, Bravo A, Soberón M, Gómez I (2013) Differential role of Manduca sexta aminopeptidase-N and alkaline phosphatase in the mode of action of Cry1Aa, Cry1Ab, and Cry1Ac toxins from Bacillus thuringiensis. Appl Environ Microbiol 79:4543–4550 [DOI] [PMC free article] [PubMed]
- Gahan LJ, Gould F, Heckel DG. Identification of a gene associated with Bt resistance in Heliothis virescens. Science. 2001;293:857–860. doi: 10.1126/science.1060949. [DOI] [PubMed] [Google Scholar]
- Gahan LJ, Pauchet Y, Vogel H, Heckel DG (2010) An ABC transporter mutation is correlated with insect resistance to Bacillus thuringiensis Cry1Ac toxin. PLoS Genet 6:e1001248 [DOI] [PMC free article] [PubMed]
- Garner KJ, Hiremath S, Lehtoma K, Valaitis AP (1999) Cloning and complete sequence characterization of two gypsy moth aminopeptidase-N cDNAs, including the receptor for Bacillus thuringiensis Cry1Ac toxin. Insect Biochem Mol Biol 29:527–535 [DOI] [PubMed]
- Ingle SS, Trivedi N, Prasad R, Kuruvilla J, Rao KK, Chhatpar HS (2001) Aminopeptidase-N from the Helicoverpa armigera (Hubner) brush border membrane vesicles as a receptor of Bacillus thuringiensis crylac delta-endotoxin. Curr Microbiol 43:255–259 [DOI] [PMC free article] [PubMed]
- Jurat-Fuentes JL, Adang MJ. The Heliothis virescens cadherin protein expressed in Drosophila S2 cells functions as a receptor for Bacillus thuringiensis Cry1A but not Cry1Fa toxins. Biochemistry. 2006;45:9688–9695. doi: 10.1021/bi0606703. [DOI] [PubMed] [Google Scholar]
- Liao C, Trowell SC, Akhurst R. Purification and characterization of Cry1Ac toxin binding proteins from the brush border membrane of Helicoverpa armigera midgut. Curr Microbiol. 2005;51:367–371. doi: 10.1007/s00284-005-0051-9. [DOI] [PubMed] [Google Scholar]
- Lu Y, Wu K, Jiang Y, Guo Y, Desneux N (2012) Widespread adoption of Bt cotton and insecticide decrease promotes biocontrol services. Nature 487:362–365 [DOI] [PubMed]
- Mesnage R, Clair E, Gress S, Then C, Székács A, Séralini GE (2013) Cytotoxicity on human cells of Cry1Ab and Cry1Ac Bt insecticidal toxins alone or with a glyphosate-based herbicide. J Appl Toxicol 23:695–699 [DOI] [PubMed]
- Morin S, Biggs RW, Sisterson MS, Shriver L, Ellers-Kirk C, Higginson D, Holley D, Gahan LJ, Heckel DG, Carrière Y, Dennehy TJ, Brown JK, Tabashnik BE (2003) Three cadherin alleles associated with resistance to Bacillus thuringiensis in pink bollworm. Proc Natl Acad Sci USA 100:5004–5009 [DOI] [PMC free article] [PubMed]
- Ning C, Wu K, Liu C, Gao Y, Jurat-Fuentes JL, Gao X (2010) Characterization of a Cry1Ac toxin-binding alkaline phosphatase in the midgut from Helicoverpa armigera (Hübner) larvae. J Insect Physiol 56:666–672 [DOI] [PubMed]
- Pacheco S, Gómez I, Arenas I, Saab-Rincon G, Rodríguez-Almazán C, Gill SS, Bravo A, Soberón M (2009) Domain II loop 3 of Bacillus thuringiensis Cry1Ab toxin is involved in a ‘ping pong’ binding mechanism with Manduca sexta aminopeptidase-N and cadherin receptors. J Biol Chem 284:32750–32757 [DOI] [PMC free article] [PubMed]
- Pigott CR, Ellar DJ. Role of receptors in Bacillus thuringiensis crystal toxin activity. Microbiol Mol Biol Rev. 2007;71:255–281. doi: 10.1128/MMBR.00034-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal R, Agrawal N, Selvapandiyan A, Sivakumar S, Ahmad S, Bhatnagar RK (2003) Recombinantly expressed isoenzymic aminopeptidases from Helicoverpa armigera (American cotton bollworm) midgut display differential interaction with closely related Bacillus thuringiensis insecticidal proteins. Biochem J 370:971–978 [DOI] [PMC free article] [PubMed]
- Schnepf E, Crickmore N, Van Rie J, Lereclus D, Baum J, Feitelson J, Zeigler DR, Dean DH (1998) Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol Mol Biol Rev 62:775–806 [DOI] [PMC free article] [PubMed]
- Shimada N, Miyamoto K, Kanda K, Murata H (2006) Bacillus thuringiensis insecticidal Cry1Ab toxin does not affect the membrane integrity of the mammalian intestinal epithelial cells: an in vitro study. In Vitro Cell Dev Biol Anim 42:45–49 [DOI] [PubMed]
- Sivakumar S, Rajagopal R, Venkatesh GR, Srivastava A, Bhatnagar RK (2007) Knockdown of aminopeptidase-N from Helicoverpa armigera larvae and in transfected Sf21 cells by RNA interference reveals its functional interaction with Bacillus thuringiensis insecticidal protein Cry1Ac. J Biol Chem 282:7312–7319 [DOI] [PubMed]
- Soberón M, Gill SS, Bravo A. Signaling versus punching hole: how do Bacillus thuringiensis toxins kill insect midgut cells ? Cell Mol Life Sci. 2009;66:1337–1349. doi: 10.1007/s00018-008-8330-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiewsiri K, Wang P. Differential alteration of two aminopeptidases N associated with resistance to Bacillus thuringiensis toxin Cry1Ac in cabbage looper. Proc Natl Acad Sci USA. 2011;108:14037–14042. doi: 10.1073/pnas.1102555108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay SK, Singh PK. Role of alkaline phosphatase in insecticidal action of Cry1Ac against Helicoverpa armigera larvae. Biotechnol Lett. 2011;33:2027–2036. doi: 10.1007/s10529-011-0665-x. [DOI] [PubMed] [Google Scholar]
- Wang G, Wu K, Liang G. Gene cloning and expression of cadherin in midgut of Helicoverpa armigera and its Cry1A binding region. Sci China C Life Sci. 2005;48:346–356. doi: 10.1360/03yc0273. [DOI] [PubMed] [Google Scholar]
- Wang GR, Liang GM, Wu KM, Guo YY (2005b) Gene cloning and sequencing of aminopeptidase N3, a putative receptor for Bacillus thuringiensis insecticidal Cry1Ac toxin in Helicoverpa armigera (Lepidoptera: Noctuidae). Eur J Entomol 102:13–19
- Wu KM, Lu YH, Feng HQ. Suppression of cotton bollworm in multiple crops in China in areas with Bt toxin-containing cotton. Science. 2008;321:1676–1678. doi: 10.1126/science.1160550. [DOI] [PubMed] [Google Scholar]
- Zhang X, Candas M, Griko NB, Rose-Young L, Bulla LA Jr (2005) Cytotoxicity of Bacillus thuringiensis Cry1Ab toxin depends on specific binding of the toxin to the cadherin receptor BT-R1 expressed in insect cells. Cell Death Differ 12:1407–1416 [DOI] [PubMed]
- Zhang X, Lan W, Deng Y, Ma Y, Liu K, Peng J, Li Y, Hong H (2008) High passage of Spodoptera litura cell line causes its permissiveness to baculovirus infection. Cytotechnology 57:233–243 [DOI] [PMC free article] [PubMed]
- Zhang S, Cheng H, Gao Y, Wang G, Liang G, Wu K (2009) Mutation of an aminopeptidase N gene is associated with Helicoverpa armigera resistance to Bacillus thuringiensis Cry1Ac toxin. Insect Biochem Mol Biol 39:421–429 [DOI] [PubMed]
- Zhang H, Wu S, Yang Y, Tabashnik BE, Wu Y (2012) Non-recessive Bt toxin resistance conferred by an intracellular cadherin mutation in field-selected populations of cotton bollworm. PLoS One 7:e53418 [DOI] [PMC free article] [PubMed]