Abstract
Oridonin, which is isolated from Chinese herb Rabdosia rubescens (Hemsl.) Hara, has been implicated in regulation of tumor cell migration and invasion. In this study, treatment with oridonin enhanced the phosphorylation of myosin regulatory light chain (T18/S19) that regulates the ATPase activity of myosin IIA. Meanwhile, stress fibers were significantly more prominent after oridonin incubation, which impaired cell migration in transwell migration assays. All of these effects may be caused by the decreased interaction between myosin IIA and myosin phosphatase complex, but not kinases. Our data provide clear evidence of this novel pharmacological function for oridonin in treating cancer cell migration.
Keywords: Oridonin, Migration, Myosin IIA, Regulatory light chain, Phosphatase complex
Introduction
The migration of groups of cells, which is the central process during development of an organism, immune responses and wound healing, is often initiated by specific external signals, including mechanical signals and chemical signals. The ability of migration allows cancer cells to change position by entering lymphatic and blood vessels for dissemination into the circulation to distant organs (Friedl and Wolf 2003). Understanding how these processes are undergoing would provide new information and insights for further research associated with the spread of cancer. Targeting migration is becoming a novel therapeutic strategy for controlling invasion of tumor cells.
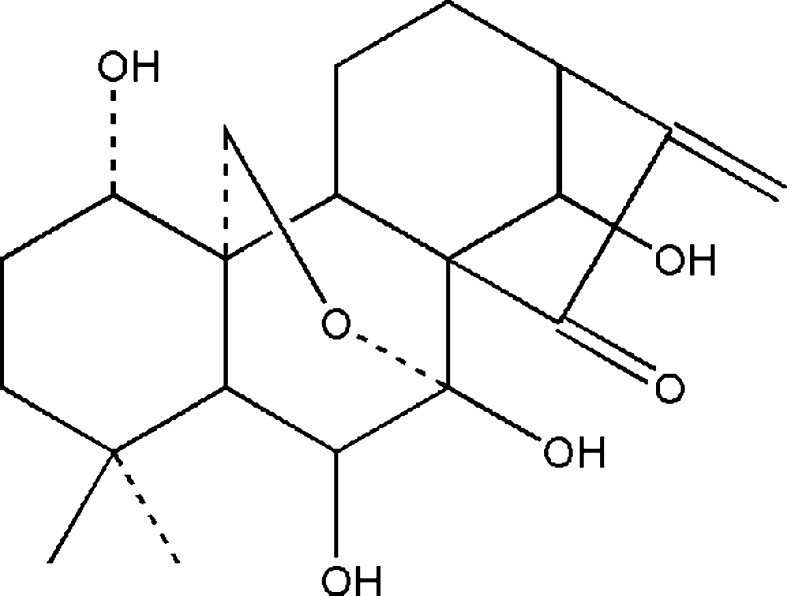
Diterpenoids, which are known to demonstrate various bioactivities, have been widely studied and used in clinical therapy recently. Oridonin (Fig. 1), a tetracycline diterpenoid firstly identified in the year of 1967 and subsequently synthesized in the year of 1973, has various pharmacological effects such as anti-inflammation, anti-bacteria, and anti-tumor ones (Fujita et al. 1976; Li et al. 2011). Oridonin shows remarkable growth inhibition and apoptosis induction activities against a broad spectrum of tumors (Hsieh et al. 2005; Huang et al. 2008; Kang et al. 2010; Zhou et al. 2007). Also, it was reported to suppress melanoma and highly metastatic breast cancer cells migration and invasion (Ren et al. 2006; Wang et al. 2013), although the mechanisms remain largely unknown. Here, we provide evidence that the activity of myosin IIA in HeLa cells, which is crucial for cell migration and invasion (Betapudi et al. 2006; Huang et al. 2009; Liang et al. 2011; Wong et al. 2008), is influenced by incubation of oridonin via impairing interaction between myosin IIA and myosin phosphatase complex.
Fig. 1.
The chemical structure of oridonin
Materials and methods
Chemicals
Oridonin was purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, P. R. China). Oridonin was dissolved in dimethylsulfoxide (DMSO) to make stock solutions (20 mM) and then diluted in cell culture medium.
Antibodies
Mouse monoclonal antibodies against catalytic subunit of protein phosphatase type 1δ (PP1cδ) (sc-365678), and rho-associated protein kinase 1 (ROCK1) (sc-17794) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA), against ROCK2 from BD Bioscience (610623), against myosin light chain kinase (MLCK) from Sigma-Aldrich (M7905) (St. Louis, MO, USA), and against GAPDH from Millipore (MAB374) (Billerica, MA, USA). Rabbit polyclonal antibodies against regulatory light chain (RLC) (3672), phospho-RLC (T18/S19) (3674), and phospho-MYPT1 (5163) were purchased from Cell Signaling Technology (Danvers, MA, USA), against nonmuscle myosin II heavy chain (NMHC) IIA (PRB-440P) from Covance Research Products Inc. (Denver, PA, USA) and myosin phosphatase-targeting subunit 1 (MYPT1) (A300-889A) from Bethyl Laboratories (Montgomery, TX, USA).
Cell culture
HeLa and A594 cells were purchased from American Type Culture Collection (Manassas, VA, USA) and grown in DMEM (Invitrogen, Carlsbad, CA, USA) with 10 % fetal bovine serum (Invitrogen) in a humidified atmosphere of 5 % CO2 at 37 °C.
Immunoprecipitation and Western blotting
Cells were cross-linked by 2 mM dithiobis (succinimidylpropionate) (Pierce Biotechnology Inc., Rockford, IL, USA), a thiolcleavable cross-linker dissolved in dry DMSO for 30 min at room temperature before IP, reaction was quenched with 20 mM Tris buffer (pH 7.5); cells were broken in ice-cold Nonidet P-40 buffer [20 mM Tris-HCl (pH 7.4), 50 mM NaCl, 0.1 % Nonidet P-40, and EDTA-free protease inhibitor mixture]. Proteins were quantified using a BCA Protein Assay Kit (Pierce). A total of 100 μg proteins were incubated with 2 μg of specific antibodies or control IgG (4 °C, overnight) and 30 μL of Dynabeads Protein G (4 °C, 6 h) (Invitrogen). Beads were washed five times in lysis buffer; eluted proteins were separated in 4–12 % NuPAGE gel (Invitrogen) and transferred to nitrocellulose membranes (Invitrogen). After incubated with 5 % nonfat milk (Bio-Rad, Hercules, CA, USA), membranes reacted with primary and secondary antibodies and exposed by using Super Signal Chemiluminescent substrate (Pierce) in an LAS-4000 system (FUJIFILM, Shanghai, China).
Immunofluorescence and image quantification
Cells were fixed with 4 % paraformaldehyde (15 min, 25 °C) and permeabilized with 0.01 % Triton X-100/0.05 % SDS (5 min, 25 °C). After washing three times in PBS, cells were incubated with blocking buffer for 1 h. F-actin was reacted with Alexa Fluor 594-conjugated phalloidin (Invitrogen). Slides were mounted in VECTASHIELD Mounting Medium with DAPI (Vector Laboratories, Burlingham, CA, USA).
Images were captured by an LSM 510 META laser confocal microscope (Carl Zeiss, Oberkochen, Germany). Quantification of F-actin fluorescence was performed as previously described (Le et al. 2013).
Migration assays
Transwell migration assays were performed as previously described (Le et al. 2013). Briefly, the lower surface of transwell membranes (6.5 mm diameter, 8 μm pore; Corning Life Sciences, Shanghai, China) were coated (overnight, 4 °C) with 10 μg/mL fibronectin (Millipore). Serum-deprived (overnight) cells in 0.1 mL DMEM with 1 % BSA were seeded in upper chambers, while lower chambers were filled with 600 μL of 1 % BSA or 10 % FBS in DMEM. After 6-h migration, migratory cells were fixed with 4 % formaldehyde (15 min, room temperature) and stained with 1 % crystal violet (3 h, RT). Images of five fields per filter-bottom surfaces were captured by using a phase-contrast microscope. The number of migrated cell was counted by Image-Pro Plus software.
Statistics
Data are presented as mean ± SEM of values from three independent experiments. The mean values of multiple groups were performed with one-way ANOVA analysis. P < 0.05 was considered statistical significance.
Results
Effects of oridonin on RLC phosphorylation and actin organization
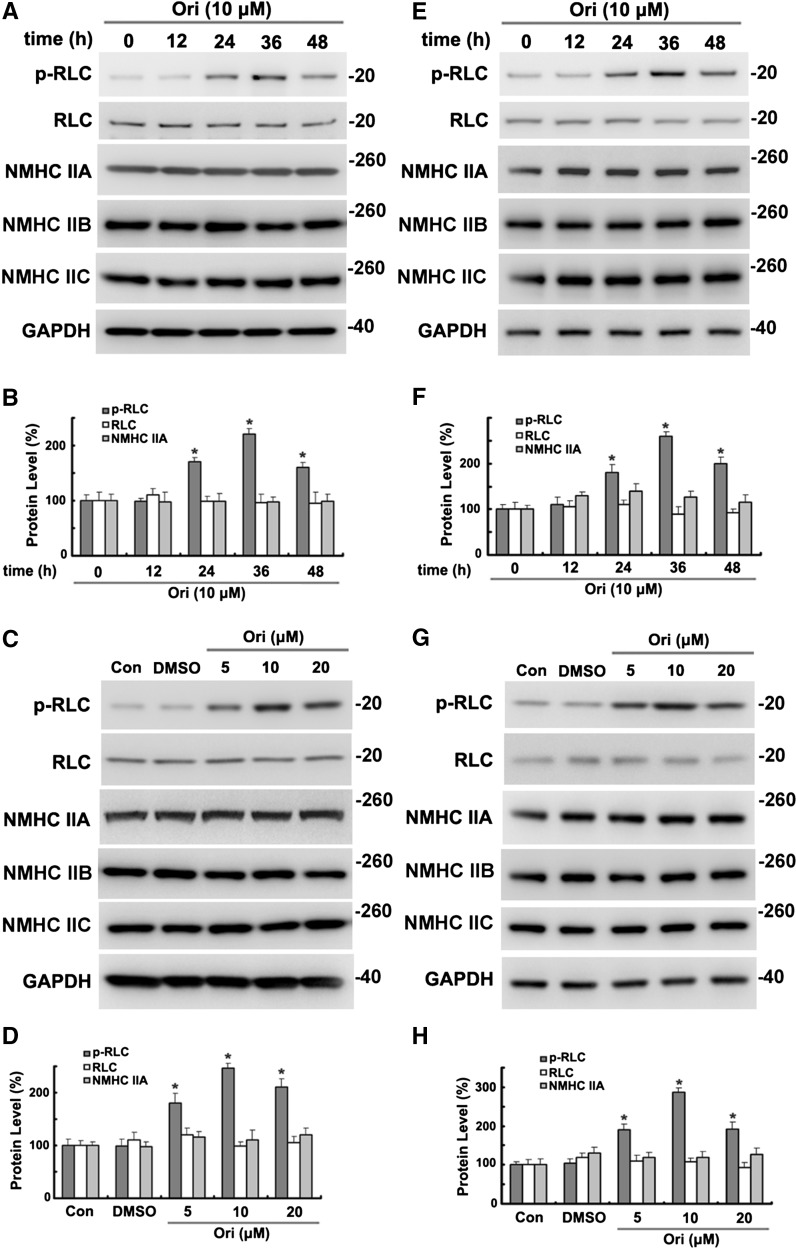
Nonmuscle myosin II (NM II) is critical in cell migration through regulation of cell polarity, cell adhesion, and contraction of actin cytoskeleton of nonmuscle cells (Vicente-Manzanares et al. 2009). NM II contains two heavy chains of 230-kDa (NMHC), two 20-kDa regulatory light chains (RLCs), and two 17-kDa essential light chains. The activity of NM II is determined by the reversible phosphorylation of RLC T18 and S19 (Hirata et al. 2009). After incubation with 10 μM oridonin, the phosphorylation of RLC T18 and S19 was significantly (P < 0.01) increased, reaching a peak between 24 and 48 h, whereas no significant change was observed with total RLC and NMHC IIA, IIB, and IIC (Fig. 2a, b) in HeLa cells. Also, as shown in Fig. 2c, d, treatment with different doses of oridonin (5, 10, 20 μM) increased phosphorylation of RLC in a dose-dependent manner compared with control cells (Con) or DMSO-treated cells (vehicle control). Similar results were also observed in A549 cells (Fig. 2e–h).
Fig. 2.
Oridonin increased the phosphorylation of RLC in time and concentration-dependent manner. a, b HeLa cells were incubated with 10 μM oridonin (Ori) for the indicated time before analysis of proteins by Western blotting and densitometric quantification. *P < 0.01 versus control cells (Con). c, d HeLa cells were incubated for 36 h with DMSO and the indicated concentrations of oridonin before analysis of proteins. Data are presented as in d. *P < 0.01 versus Con. e–h A549 cells were treated as in a and c. Data are presented same as in b and d. *P < 0.05 versus Con
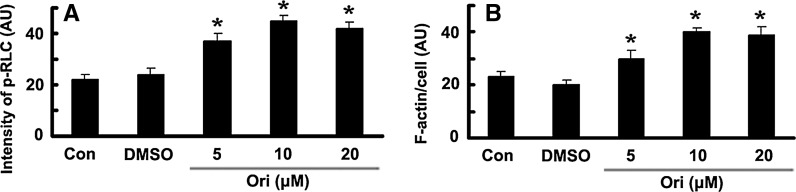
The distributions of phosphorylated RLC in oridonin-treated cells and control cells were analyzed microscopically. Different doses of oridonin (5, 10, 20 μM) treatment significantly increased the fluorescence intensity of phospho-RLC (p-RLC) (Fig. 3a). Because activated NM IIA promotes the formation of actin bundles, we further investigated the effects of oridonin on F-actin morphology. After incubation with oridonin, stress fibers were significantly more prominent than those in control or DMSO-treated HeLa cells (Fig. 3b).
Fig. 3.
Oridonin increased the phosphorylation of RLC and stress fibers microscopically. a, b HeLa cells incubated for 36 h with DMSO and the indicated concentrations of oridonin were fixed and reacted with antibodies against phospho-RLC (p-RLC) or Alexa Fluor 594-conjugated phalloidin (F-actin). ImageJ software is used to measure the mean fluorescence intensity (arbitrary unit, AU) of p-RLC (a) or F-actin (b). *P < 0.05 versus Con
Effects of oridonin on cell migration
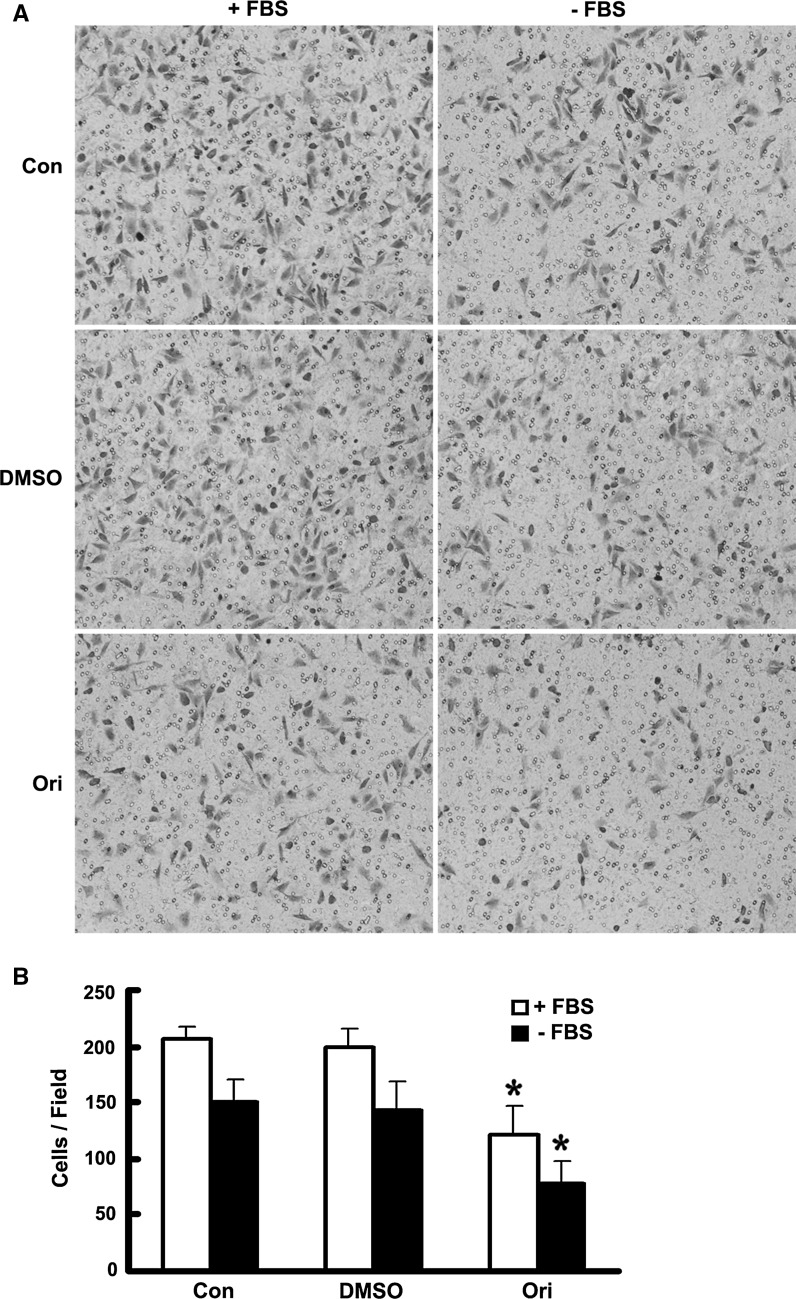
Increased phosphorylation of RLC was reported to be related to impaired cell migration (Le et al. 2013). To further explore the roles of oridonin in cell movement, we used transwell migration assay to assess cell motility. HeLa cells were incubated with 10 μM oridonin 36 h before plating in the upper chamber, and they were allowed to migrate for 6 h. As shown in Fig. 4a, b, HeLa cells incubated with oridonin showed significantly reduced motility compared with control cells or DMSO-treated cells, consistent with the previous report (Le et al. 2013).
Fig. 4.
Oridonin inhibited HeLa cell migration in transwell assay. a, b HeLa cells incubated with DMSO or oridonin (10 μM, 36 h) were subjected to a transwell migration assay. Cells were seeded in the upper chamber, and their migrations were assessed in the presence or absence of FBS as indicated. Six hours after plating, cells on underside of the filters were fixed, stained with crystal violet, and counted as described in “Materials and methods” section. Data are mean ± SEM of values from three independent experiments. *P < 0.05 versus Con
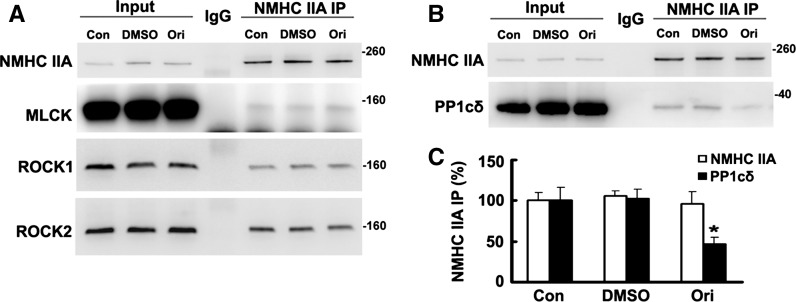
Effects of oridonin on co-IP of RLC kinases or PP1cδ with NM IIA
RLC phosphorylation is determined by opposing activities of kinase and phosphatases. Myosin light chain kinase (MLCK) (Somlyo and Somlyo 2003) and ROCK (Riento and Ridley 2003) are the most widely implicated kinases in RLC T18 and/or S19 phosphorylation. Myosin phosphatase is a heterotrimeric enzyme comprising the MYPT1, the PP1cδ, and a smaller subunit of unknown function (Matsumura and Hartshorne 2008).
Elevation of kinase levels or action (Matsumura 2005; Tan et al. 2001) or reduced myosin phosphatase amount or activity (Hartshorne et al. 2004; Matsumura and Hartshorne 2008) can both lead to increased RLC phosphorylation. In Fig. 5, we evaluated the interaction between NM IIA with kinases and phosphatase. No obvious changes were observed among co-IP of NMHC IIA with MLCK, ROCK1, or ROCK2 (Fig. 5a). However, co-IP of PP1cδ with NMHC IIA was significantly decreased after HeLa cells were incubated with oridonin, which could be responsible for the increased RLC phosphorylation (Fig. 5b, c).
Fig. 5.
Co-IP of endogenous PP1cδ with NMHC IIA decreased after HeLa cells were incubated with oridonin. Samples of total proteins before (5 %, Input) or from IP with control IgG or antibodies against NMHC IIA were analyzed by Western blotting. a Proteins from IP of NMHC IIA (50 %) were analyzed by Western blotting with antibodies against NMHC IIA, ROCK1, ROVK2, or MLCK. b Proteins (50 %) from NMHC IIA IP of extracts of cells incubated with DMSO or oridonin (10 μM, 36 h) were reacted with antibodies against NMHC IIA and PP1cδ. c Data are presented as mean ± SEM of values from three independent experiments. *P < 0.05 versus Con
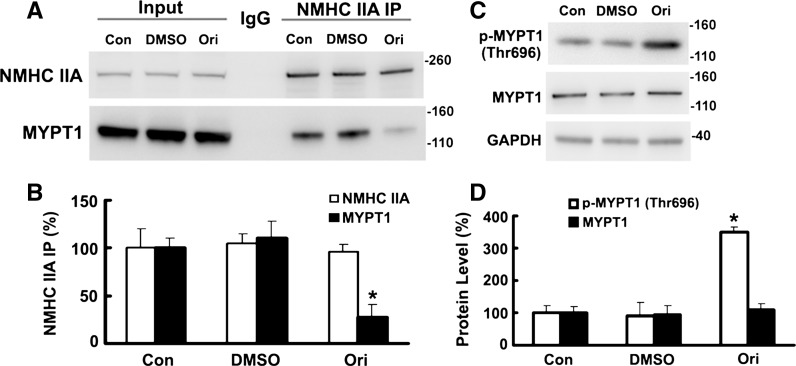
Effects of oridonin on phosphorylation of MYPT1 and co-IP of NM IIA with MYPT1
Myosin phosphatase activity of the holoenzyme was greater than the catalytic subunit alone, suggesting MYPT1 facilitated enzyme–substrate interaction (Shirazi et al. 1994). Phosphorylation of MYPT1 of this trimeric phosphatase inactivates the enzymatic activity (Kimura et al. 1996), and Thr695 was responsible for inhibition (Feng et al. 1999). Data in Fig. 6a, b are consistent with the notion that oridonin could influence NM IIA–MYPT1 interaction, perhaps via regulating the phosphorylation of MYPT1 on Thr695 (Fig. 6c, d).
Fig. 6.
Incubation with oridonin increased the phosphorylation of MYPT1 and decreased the co-IP of NM IIA with MYPT1. a Proteins (50 %) from NMHC IIA IP of extracts of HeLa cells incubated with DMSO or oridonin (10 μM, 36 h) were reacted with antibodies against NMHC IIA and MYPT1. b Data are presented as mean ± SEM of values from three independent experiments. *P < 0.05 versus Con. c, d HeLa cells were incubated with 10 μM oridonin for 36 h before analysis of proteins by Western blotting and densitometric quantification. *P < 0.01 versus Con
Discussion
Traditional Chinese medicine and other herbal medicines have been used to treat cancer in China, Korea, and Japan for more than thousands of years. Recently, in Europe and USA, they are widely used as complementary and alternative medicine because of the advantages of multi-efficiency, weak side effects, and improvement of life quality (Gai et al. 2008; Wong et al. 2001). However, uncertain effective constituents and unstable efficiency also limit the clinical utility of herbal medicines. Oridonin, a natural tetracycline diterpenoid, has been proven as a potent cytotoxic agent against a wide variety of tumors due to its growth inhibition and apoptosis induction activities. In this study, we showed that incubation with oridonin decreased co-IP of PP1cδ with NMHC IIA, enhanced RLC (T18/S19) phosphorylation, and led to increased content of F-actin fibers that impaired cell motility in HeLa cell. Based on these data, we propose a newly recognized role for oridonin in treating cancer cell migration.
Conventionally, the process of tumor metastasis is understood as the migration of individual cancer cell that detach from the primary tumor, enter bloodstream or lymphatic system, and seed in other distant organs. Cell migration requires the regulation of actomyosin function in cell shape and adhesion, and NM II is critical for structural remodeling of nonmuscle cells. In mammalian cells, three isoforms of NM II named NM IIA, IIB, and IIC, with both shared and unique properties, are designated by NMHC components (NMHC IIA, IIB, and IIC), which are encoded by three genes (myh9, myh10, and myh14), and account for the differences of function like cell polarization, migration, and adhesion (Vicente-Manzanares et al. 2011; Wang et al. 2011). Reversible phosphorylation of specific amino acids in RLCs determines NM II activity. Without RLC phosphorylation, one head of NM II interacts with the second head of the same molecule to form a compact structure, and moreover, ATP hydrolysis is also inhibited by the head–tail interaction. These interactions could be disrupted by the phosphorylation of RLC on T18 and/or S19, which promotes filament assembly by enhancing the ATPase activity of NM II (Vicente-Manzanares et al. 2009).
Several kinases are reported to be responsible for the phosphorylation of RLC, such as MLCK and ROCK (Riento and Ridley 2003; Somlyo and Somlyo 2003). Myosin phosphatase, which comprises a 130-kDa MYPT1, a 37-kDa PP1cδ, and a 20-kDa subunit of unknown function, dephosphorylated RLC on T18 and S19 (Matsumura and Hartshorne 2008). In HeLa cells, incubation with oridonin decreased the co-IP of NMHC IIA with PP1cδ and MYPT1, but not with kinases, and subsequently, increased the phosphorylation of RLC on T18 and S19. Phosphorylation of MYPT1 on Thr695 may play a critical role here. We do not know how oridonin affects the phosphorylation of MYPT1, perhaps through regulation of rho-associated kinase activity (Feng et al. 1999; Kimura et al. 1996).
In moving cells, protrusions that contain two kinds of actin-based structures: the lamellipodium and lamellum, and direct and drive translocation (Heath and Holifield 1991; Pollard and Borisy 2003). NM II in the lamellum limits cell protrusion by controling retrograde actin flow and promoting adhesion maturation, suggesting a negative role of NM II in cell migration (Cai et al. 2006; Even-Ram et al. 2007). In our transwell migration assays, the motility of HeLa cells was significantly impaired by oridonin incubation compared to control cells or DMSO-treated cells, perhaps because RLC phosphorylation caused cross-linking with actin, which is critical to adhesion maturation (Choi et al. 2008).
On the basis of the data presented here, oridonin, a diterpenoid isolated from Rabdosia rubescens, showed interesting anti-tumor activity, evidenced by its significant effect in inhibiting cell migration. This study will provide valuable information about cancer therapy by new medicine alteration and combination of two or more medicines.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (NSFC No. 81172961).
Conflict of interest
The authors declare that there are no conflicts of interest.
Abbreviations
- MLCK
Myosin light chain kinase
- MYPT1
Myosin phosphatase-targeting subunit 1
- NM II
Nonmuscle myosin II
- NMHC
Nonmuscle myosin heavy chain
- PP1cδ
Catalytic subunit of protein phosphatase type 1δ
- RLC
Regulatory light chain
- ROCK
Rho-associated protein kinase
Contributor Information
Hai-Wei Xu, Phone: +86-371-67781908, Email: xhwzpu@126.com.
Ji-Feng Liu, Phone: +86-371-67732202, Email: jifeng926@126.com.
References
- Betapudi V, Licate LS, Egelhoff TT. Distinct roles of nonmuscle myosin II isoforms in the regulation of MDA-MB-231 breast cancer cell spreading and migration. Cancer Res. 2006;66:4725–4733. doi: 10.1158/0008-5472.CAN-05-4236. [DOI] [PubMed] [Google Scholar]
- Cai Y, Biais N, Giannone G, Tanase M, Jiang G, Hofman JM, Wiggins CH, Silberzan P, Buguin A, Ladoux B, et al. Nonmuscle myosin IIA-dependent force inhibits cell spreading and drives F-actin flow. Biophys J. 2006;91:3907–3920. doi: 10.1529/biophysj.106.084806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi CK, Vicente-Manzanares M, Zareno J, Whitmore LA, Mogilner A, Horwitz AR. Actin and alpha-actinin orchestrate the assembly and maturation of nascent adhesions in a myosin II motor-independent manner. Nat Cell Biol. 2008;10:1039–1050. doi: 10.1038/ncb1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Even-Ram S, Doyle AD, Conti MA, Matsumoto K, Adelstein RS, Yamada KM. Myosin IIA regulates cell motility and actomyosin-microtubule crosstalk. Nat Cell Biol. 2007;9:299–309. doi: 10.1038/ncb1540. [DOI] [PubMed] [Google Scholar]
- Feng J, Ito M, Ichikawa K, Isaka N, Nishikawa M, Hartshorne DJ, Nakano T. Inhibitory phosphorylation site for Rho-associated kinase on smooth muscle myosin phosphatase. J Biol Chem. 1999;274:37385–37390. doi: 10.1074/jbc.274.52.37385. [DOI] [PubMed] [Google Scholar]
- Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–374. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- Fujita E, Nagao Y, Kaneko K, Nakazawa S, Kuroda H. The antitumor and antibacterial activity of the Isodon diterpenoids. Chem Pharm Bull (Tokyo) 1976;24:2118–2127. doi: 10.1248/cpb.24.2118. [DOI] [PubMed] [Google Scholar]
- Gai RY, Xu HL, Qu XJ, Wang FS, Lou HX, Han JX, Nakata M, Kokudo N, Sugawara Y, Kuroiwa C, Tang W. Dynamic of modernizing traditional Chinese medicine and the standards system for its development. Drug Discov Ther. 2008;2:2–4. [PubMed] [Google Scholar]
- Hartshorne DJ, Ito M, Erdodi F. Role of protein phosphatase type 1 in contractile functions: myosin phosphatase. J Biol Chem. 2004;279:37211–37214. doi: 10.1074/jbc.R400018200. [DOI] [PubMed] [Google Scholar]
- Heath JP, Holifield BF. Cell locomotion: new research tests old ideas on membrane and cytoskeletal flow. Cell Motil Cytoskeleton. 1991;18:245–257. doi: 10.1002/cm.970180402. [DOI] [PubMed] [Google Scholar]
- Hirata N, Takahashi M, Yazawa M. Diphosphorylation of regulatory light chain of myosin IIA is responsible for proper cell spreading. Biochem Biophys Res Commun. 2009;381:682–687. doi: 10.1016/j.bbrc.2009.02.121. [DOI] [PubMed] [Google Scholar]
- Hsieh TC, Wijeratne EK, Liang JY, Gunatilaka AL, Wu JM. Differential control of growth, cell cycle progression, and expression of NF-kappaB in human breast cancer cells MCF-7, MCF-10A, and MDA-MB-231 by ponicidin and oridonin, diterpenoids from the chinese herb Rabdosia rubescens. Biochem Biophys Res Commun. 2005;337:224–231. doi: 10.1016/j.bbrc.2005.09.040. [DOI] [PubMed] [Google Scholar]
- Huang J, Wu L, Tashiro S, Onodera S, Ikejima T. Reactive oxygen species mediate oridonin-induced HepG2 apoptosis through p53, MAPK, and mitochondrial signaling pathways. J Pharmacol Sci. 2008;107:370–379. doi: 10.1254/jphs.08044FP. [DOI] [PubMed] [Google Scholar]
- Huang Y, Arora P, McCulloch CA, Vogel WF. The collagen receptor DDR1 regulates cell spreading and motility by associating with myosin IIA. J Cell Sci. 2009;122:1637–1646. doi: 10.1242/jcs.046219. [DOI] [PubMed] [Google Scholar]
- Kang N, Zhang JH, Qiu F, Tashiro S, Onodera S, Ikejima T. Inhibition of EGFR signaling augments oridonin-induced apoptosis in human laryngeal cancer cells via enhancing oxidative stress coincident with activation of both the intrinsic and extrinsic apoptotic pathways. Cancer Lett. 2010;294:147–158. doi: 10.1016/j.canlet.2010.01.032. [DOI] [PubMed] [Google Scholar]
- Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- Le K, Li CC, Ye G, Moss J, Vaughan M. Arf guanine nucleotide-exchange factors BIG1 and BIG2 regulate nonmuscle myosin IIA activity by anchoring myosin phosphatase complex. Proc Natl Acad Sci USA. 2013;110:E3162–E3170. doi: 10.1073/pnas.1312531110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CY, Wang EQ, Cheng Y, Bao JK. Oridonin: an active diterpenoid targeting cell cycle arrest, apoptotic and autophagic pathways for cancer therapeutics. Int J Biochem Cell Biol. 2011;43:701–704. doi: 10.1016/j.biocel.2011.01.020. [DOI] [PubMed] [Google Scholar]
- Liang S, He L, Zhao X, Miao Y, Gu Y, Guo C, Xue Z, Dou W, Hu F, Wu K, Nie Y, Fan D. MicroRNA let-7f inhibits tumor invasion and metastasis by targeting MYH9 in human gastric cancer. PLoS One. 2011;6:e18409. doi: 10.1371/journal.pone.0018409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura F. Regulation of myosin II during cytokinesis in higher eukaryotes. Trends Cell Biol. 2005;15:371–377. doi: 10.1016/j.tcb.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Matsumura F, Hartshorne DJ. Myosin phosphatase target subunit: many roles in cell function. Biochem Biophys Res Commun. 2008;369:149–156. doi: 10.1016/j.bbrc.2007.12.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. doi: 10.1016/S0092-8674(03)00120-X. [DOI] [PubMed] [Google Scholar]
- Ren KK, Wang HZ, Xie LP, Chen DW, Liu X, Sun J, Nie YC, Zhang RQ. The effects of oridonin on cell growth, cell cycle, cell migration and differentiation in melanoma cells. J Ethnopharmacol. 2006;103:176–180. doi: 10.1016/j.jep.2005.07.020. [DOI] [PubMed] [Google Scholar]
- Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol. 2003;4:446–456. doi: 10.1038/nrm1128. [DOI] [PubMed] [Google Scholar]
- Shirazi A, Iizuka K, Fadden P, Mosse C, Somlyo AP, Somlyo AV, Haystead TA. Purification and characterization of the mammalian myosin light chain phosphatase holoenzyme. The differential effects of the holoenzyme and its subunits on smooth muscle. J Biol Chem. 1994;269:31598–31606. [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- Tan I, Ng CH, Lim L, Leung T. Phosphorylation of a novel myosin binding subunit of protein phosphatase 1 reveals a conserved mechanism in the regulation of actin cytoskeleton. J Biol Chem. 2001;276:21209–21216. doi: 10.1074/jbc.M102615200. [DOI] [PubMed] [Google Scholar]
- Vicente-Manzanares M, Ma X, Adelstein RS, Horwitz AR. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol. 2009;10:778–790. doi: 10.1038/nrm2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente-Manzanares M, Newell-Litwa K, Bachir AI, Whitmore LA, Horwitz AR. Myosin IIA/IIB restrict adhesive and protrusive signaling to generate front–back polarity in migrating cells. J Cell Biol. 2011;193:381–396. doi: 10.1083/jcb.201012159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A, Ma X, Conti MA, Adelstein RS. Distinct and redundant roles of the non-muscle myosin II isoforms and functional domains. Biochem Soc Trans. 2011;39:1131–1135. doi: 10.1042/BST0391131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Zhong Z, Wan J, Tan W, Wu G, Chen M, Wang Y. Oridonin induces apoptosis, inhibits migration and invasion on highly-metastatic human breast cancer cells. Am J Chin Med. 2013;41:177–196. doi: 10.1142/S0192415X13500134. [DOI] [PubMed] [Google Scholar]
- Wong R, Sagar CM, Sagar SM. Integration of Chinese medicine into supportive cancer care: a modern role for an ancient tradition. Cancer Treat Rev. 2001;27:235–246. doi: 10.1053/ctrv.2001.0227. [DOI] [PubMed] [Google Scholar]
- Wong CC, Wong CM, Ko FC, Chan LK, Ching YP, Yam JW, Ng IO. Deleted in liver cancer 1 (DLC1) negatively regulates Rho/ROCK/MLC pathway in hepatocellular carcinoma. PLoS One. 2008;3:e2779. doi: 10.1371/journal.pone.0002779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou GB, Chen SJ, Wang ZY, Chen Z. Back to the future of oridonin: again, compound from medicinal herb shows potent antileukemia efficacies in vitro and in vivo. Cell Res. 2007;17:274–276. doi: 10.1038/cr.2007.21. [DOI] [PubMed] [Google Scholar]