Abstract
This study was carried out to investigate the activation status of unfolded protein response (UPR) in colorectal cancer (CRC) and its contribution to CRC resistance to chemotherapy-induced apoptosis. Chemotherapy-induced apoptosis was assessed by the propidium iodide method. Activation of UPR was evaluated in CRC cell lines using immunoblotting technique and in CRC tissues using immunohistochemistry. Findings of the present study revealed that the UPR is constitutively activated in CRC cell lines and CRC tissues isolated from patients, as evidenced by relatively high levels of the 78-kDa glucose-regulated protein (GRP78) and spliced X-box-binding protein 1 mRNA in tissue samples. In addition, CRC cell lines differentially responded to clinically relevant DNA-targeting agents including cisplatin, and 5-flourouracil. Moreover, the levels of GRP78 were inversely associated with sensitivity of CRC cells to chemotherapy-induced apoptosis. Inhibition of GRP78 by siRNA resulted in increased sensitivity of CRC cells to chemotherapeutic agents. Collectively, current results appear to provide novel insights into the role of UPR in determining sensitivity of CRC cells to chemotherapeutic agents and might have important implications for personalized CRC treatment.
Keywords: UPR, Apoptosis, GRP78, 5-FU, Cisplatin
Introduction
Cellular conditions such as hypoxia, alterations in glycosylation status, and disturbances of calcium flux, can lead to the accumulation of unfolded and/or misfolded proteins in the endoplasmic reticulum (ER) lumen and can challenge the function of the ER-Golgi network, resulting ER stress (Harding et al. 2002; Zhang and Kaufman 2004; Schroder and Kaufman 2005). The ER responds to stress conditions by unfolded protein response (UPR), which involves activation of a range of signaling pathways that couple ER protein folding load with ER protein folding capacity (Harding et al. 2002; Zhang and Kaufman 2004; Schroder and Kaufman 2005).
The UPR is of fundamental importance for survival of all eukaryotic cells under conditions of ER stress. The response is mediated by sequestration of the 78-kDa glucose regulated protein (GRP78) by unfolded proteins, which activates three ER transmembrane protein sensors: activating transcription factor 6 (ATF6), inositol-requiring enzyme 1 (IRE1) and double stranded RNA-activated protein kinase-like ER kinase (PERK) (Harding et al. 2002; Zhang and Kaufman 2004; Schroder and Kaufman 2005). These activations involve phosphorylation and homodimerization of IRE1 and PERK, and relocation and proteolytic cleavage of ATF6 (Harding et al. 2002; Zhang and Kaufman 2004; Schroder and Kaufman 2005).
In addition to the UPR sensing, GRP78 protein acts as ER chaperone that facilitates proper protein folding and targets misfolded proteins for degradation by proteasome. It is also involved in ER calcium binding. Elevated expression of GRP78 has been reported in several cancers, such as breast cancer and prostate cancer (Erhardt et al. 1999; Adeyinka et al. 2002). Moreover, GRP78 expression has been associated with tumor development and growth, vascularization, metastasis and correlated with cancer resistance to chemotherapy (Erhardt et al. 1999; Reddy et al. 2003; Pyrko et al. 2007). It seems that some cancer cells may have adapted to ER stress by activation of the UPR without resulting in apoptosis (Rutkowski et al. 2006; Rutkowski and Kaufman 2007). It has been suggested that the central feature of this adaptive response is the maintenance of the expression of proteins that facilitate survival, such as GRP78 (Rutkowski et al. 2006).
Colorectal cancer (CRC) is common among adult men and women. Despite the progress in treatment of CRC, millions of patients die due to development of resistance of malignant cells to therapy (Fang 2009; Gottschalk et al. 2010). Although several experimental studies have explored the role of GRP78 in colorectal cancer growth, progression and apoptosis (Takahashi et al. 2011; Xing et al. 2011), it is unclear whether there is an association between the expression levels of GRP78 and CRC response to DNA-targeting agents such as cisplatin. In the present study, results indicated that GRP78 is constitutively activated in cultured CRC cell lines and CRC tissues, and plays a profound role in regulating sensitivity of CRC cells to apoptosis induced by chemotherapy. It is likely that pretreatment evaluation of the expression of GRP78 will be a useful biomarker for response of CRC patient’s to DNA-targeting agents and to establish a baseline for personalized treatment of CRC patients.
Materials and methods
Cell lines
Human CRC cell lines (Colo205, SW480, SW620, and HCT116), and normal fibroblasts that originated from spleen tissues, were generously provided by Dr. Rick F. Thorne (University of Newcastle, Callaghan NSW, Australia) and were cultured in DMEM containing 10 % fetal calf serum (Bio Whittaker, Verviers, Belgium).
Chemicals and reagents
Chemotherapeutic agents, cisplatin and 5-flourouracil (5-FU), were purchased from Sigma-Aldrich (St. Louis, MO, USA) and stored as a 20 mM stock solution in dimethyl sulfoxide (final concentration of 0.1 % (v/v), which was shown not to contribute to cytotoxicity (Galvao et al. 2014), at −80 °C. This stock solution was diluted with the DMEM medium (containing 10 % FBS) prior to use. The propidium iodide (PI) was purchased from Sigma-Aldrich (Sigma-Aldrich). The rabbit MAbs’ against GRP78/Bip, eukaryotic initiation factor 2α (eIF2α), phosphorylated eIF2a, X-box-binding protein 1 (XBP1), IRE1α, ATF6, PERK were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Apoptosis
Apoptotic cells were determined by the propidium iodide method (Mhaidat et al. 2007). In brief, CRC cells were adhered overnight in a 24-well plate at a concentration of 1 × 105/well in 10 % FCS. Cells in suspension were added on the day of the assay. Medium was removed, and 1,000 μl of fresh medium and 10 % FCS containing 5-FU at 50 µM or cisplatin at 166.7 µM were added. Then, Cells were incubated for 72 h at 37 °C, and followed by medium removal. Adhered and suspended cells were washed once with PBS. The medium and PBS were placed in 12 × 75 mm Falcon polystyrene tubes and centrifuged at 200×g for 5 min. A hypotonic buffer 1 ml (propidium iodide, 50 μg/ml, in 0.1 % sodium citrate plus 0.1 % Triton X-100; Sigma) was added directly to the cells adherent in the 24-well plate and gently pipetted off, then, added to the appropriate cell pellet in the Falcon tube. The tubes were placed at 4 °C in the dark overnight before flow cytometric analyses. The propidium iodide fluorescence of individual nucleus was measured in the red fluorescene using a Facscan flow cytometer (Becton–Dickinson, Mountain View, CA, USA) and the data were registered in a logarithmic scale. At least 104 cells of each sample were analyzed. Apoptotic nuclei appeared as a broad hypodiploid DNA peak, which was easily distinguished from the narrow hyperdiploid peak of nuclei in the CRC cells.
Protein expression analysis
Cell extracts were prepared as described previously (Mhaidat et al. 2012) and the protein content was determined by the Bradford assay (Bio-Rad, Hercules, CA, USA). Briefly, a total of 30 μg of total protein was added to each well of 12 % SDS-PAGE gels. Electrophoresis was carried out. Then, proteins on the gels were transferred onto nitrocellulose membranes. Membranes were blocked using 5 % skim milk before overnight incubation with the primary antibodies at a concentration of 1:100 v/v. Incubation of membranes with horseradish peroxidase–conjugated goat anti-rabbit IgG or goat anti-mouse IgG (1:2,000 v/v; Bio-Rad) was performed at room temperature with shaking for 1 h. Labeled bands were detected with Immuno-Star HRP Chemiluminescent Kit and the images were captured using the VersaDoc image system (Bio-Rad). The intensity of the bands was quantified with the Bio-Rad VersaDocTM image system. The relative expression of detected proteins was determined as shown previously (Mhaidat et al. 2008). Briefly, the relative expression of GRP78 was determined by dividing the densitometric value of the test protein by that of the GAPDH.
Small RNA interference (siRNA)
The siRNA constructs were obtained as the siGENOME SMARTpool reagents (Dharmacon, Lafayette, CO, USA). siRNAs were transfected with DharmaFECT I (Dharmacon) using the manufacturers’ protocols. The sequences of the siRNAs are as follows: control nontargeting siRNA (D-001220-01-05) and GRP78 (L-008198-00-0005). Transfection of siRNA pools was carried out as described previously (Mhaidat et al. 2007). Briefly, cells were transfected with 100 nM siRNA in Opti-MEM medium (Invitrogen, Carlsbad, CA, USA) with 5 % fetal calf serum using DharmaFECT I according to the manufacturer’s transfection protocol. Twenty-four hours after transfection, cells were switched into medium containing 5 % FCS with or without treatment of cisplatin at 166.7 µM or 5-FU at 50 µM. After 72 h, apoptotic cells were quantified of by measurement of sub-G1 DNA content using the PI method in flow cytometry. Efficiency of siRNA was measured by Western blot analysis.
Detection of XBP1 mRNA splicing
The method used for detection of unspliced and spliced XBP1 mRNAs was as described previously (Jiang et al. 2007). Briefly, reverse transcription-PCR (RT-PCR) products of XBP1 mRNA were obtained from total RNA extracted using primers 5′-cggtgcgcggtgcgtagtctgga-3′ (sense) and 5′-tgaggggctgagaggtgcttcct-3′ (antisense). Because a 26-bp fragment containing an ApaLI site is spliced on activation of XBP1 mRNA, the RT-PCR products were digested with ApaLI to distinguish the active spliced form from the inactive unspliced form. Subsequent electrophoresis revealed the inactive form as two cleaved fragments and the active form as a noncleaved fragment.
Immunohistochemistry
The institutional review board (IRB) at the Jordan University of Science & Technology (JUST) approved the study procedure. Sections of normal and CRC tissues (5 µm thick) were cut from the formalin-fixed paraffin-embedded blocks, deparaffinized in xylene and rehydrated through graded decreasing concentrations of alcohol. Antigen retrieval was carried out in 0.01 M of sodium citrate buffer (pH 6.0) by microwave oven heating for 5 min, repeated three times. Rabbit antihuman GRP78 antibody (Monoclonal IgG; Epitomics, CA, USA) was added at a dilution of 1:500 in Tris buffer for 1 h at room temperature before detection of bound antibodies using the Vectastain ABC kit (Vector Laboratories, Burlingame, CA, USA) according to the manufacturer’s instructions with visualization using diaminobenzidene (Dako, Carpinteria, CA, USA, Cat.K3466). Sections were counterstained with Harris’ haematoxylin. Negative controls were performed for each section by omission of the primary antibody but keeping all other conditions constant. Sections were scored blindly by two investigators using at least four random microscopic fields for each sample. The percentage of positive cells was estimated from 0 to 100 % and the intensity of immunoreactivity (intensity score) was judged on an arbitrary scale of 0–4+: no reactivity (0), weakly positive (1+), moderately positive (2+), strongly positive (3+) and very strongly positive (4+). The immunoreactivity score (IRS) was derived by multiplying the percentage of positive cells with intensity of reactivity divided by 10.
Statistical analysis
Data are expressed as mean ± SE. The statistical significance of intergroup differences in normally distributed continuous variables was determined using Student’s t test. P values ≤0.05 were considered statistically significant. P values <0.05 and <0.001 are indicated by * and **, respectively.
Results
The expression of GRP78 was characterized in CRC tissues by immunohistochemistry. Representative results are illustrated for normal colon tissues (Fig. 1a) in comparison with for CRC tissues (Fig. 1b–f). As shown, the majority of histological sections of CRC tissues displayed enhanced immunohistochemical staining for GRP78. The immunoreactivity score (IRS) was higher in metastatic and poorly differentiated tissue samples compared to those in normal colon tissue (P = 0.001).
Fig. 1.
Photomicrographs of immunohistochemical staining for GRP78 in normal and cancerous tissues of colon. a Immunostaining of normal colon tissue: immunoreactivity score (IRS) = 3. b, c Moderately- differentiated tissue sample, stage II disease with 78 % of cells positive (IRS) = 26. d, e Poorly- differentiated tissue sample, stage III disease with 89 % of cells positive (IRS) = 38. f. Metastatic tissue to lung, stage IV disease with 100 % of cells positive (IRS) = 40. All original magnifications, ×400
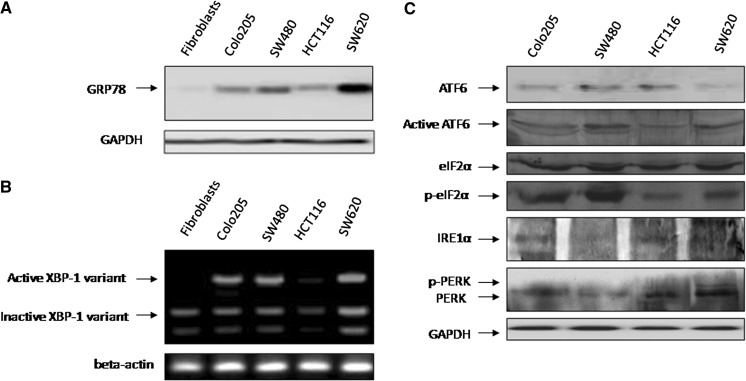
To explore the role of UPR in resistance of CRC cancer cells to chemotherapy-induced apoptosis, the expression levels of the GRP78 protein and the spliced XBP1 mRNA in a panel of CRC cell lines were examined, which are two commonly used indicators of UPR activation. GRP78 was expressed at varying, but higher levels in different cell lines relative to cultured normal fibroblasts (Fig. 2a). Similarly, the spliced XBP1 was observed in all cultured cells except normal fibroblasts and HCT116 (Fig. 2b). Results shown in Fig. 2c revealed that CRC cell lines express varying degrees of the UPR transducer IRE1α and ATF6. Expression of IRE1α was higher in HCT116 and Colo205 compared to SW620 and was barely detected in SW480. Appearance of the active form of ATF6 was detected in all cell lines but to different extents being the highest in SW480 and SW620 and to a lesser extent in HCT116 and Colo205. In all cell lines, there was an increase in the phosphorylated form of eIF2 that mediates inhibition of protein translation. In addition, levels of PERK were differentially expressed among different cell lines where high levels of p-PERK and PERK are expressed in SW620.
Fig. 2.
Unfolded protein response (UPR) is activated in CRC cell lines. a Whole cell lysates (20–30 µg) of Colo205, SW480, HCT116, and SW620 CRC cell lines and normal human fibroblasts were subjected to Western blotting analysis of GRP78 protein expression. Western blotting analysis of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) levels was included to show that equivalent amounts of protein were loaded in each lane. Data are representative of two individual experiments. b RT-PCR products of XBP1 mRNA from Colo205, SW480, HCT116, and SW620 CRC cell lines and normal human fibroblasts were digested with ApaLI for 90 min followed by electrophoresis. The longer fragment derived from the active form of XBP1 mRNA and the two shorter bands derived from the inactive form are indicated. Data are representative of three individual experiments. c Whole cell lysates of Colo205, SW480, HCT116, and SW620 CRC cell lines were subjected to Western blotting analysis of ATF6, active ATF6, eiF2α, p-eiF2α, IRE1α, PERK, and p-PERK protein expression. Western blotting analysis of GAPDH levels was included to show that equivalent amounts of protein were loaded in each lane. Data are representative of two individual experiments
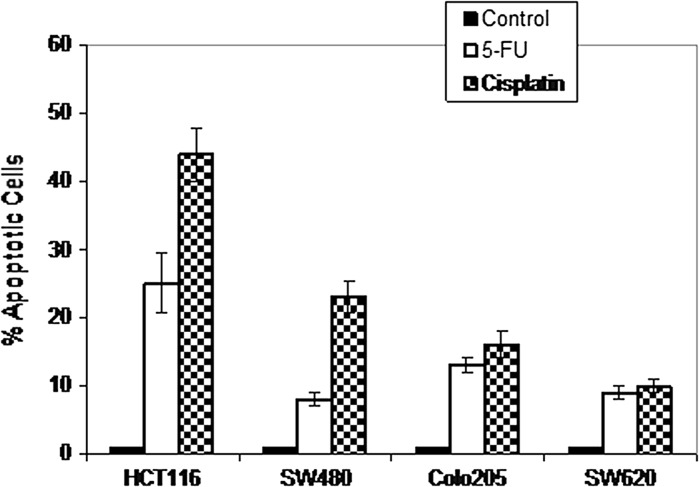
Next, the potential of different chemotherapeutic agents to induce apoptosis in CRC cells was studied. Cells were treated with cisplatin (166.7 μM), and 5-FU (50 μM). Apoptotic cells were measured using the propidium iodide method and flow cytometry. Results shown in Fig. 3 indicate that chemotherapeutic agents have different potentials to induce apoptosis among different CRC cell lines. For example, about 25 % of HCT116 cells underwent apoptosis by treatment with 5-FU and about 44 % underwent apoptosis by treatment with cisplatin.
Fig. 3.
Chemotherapy-induced apoptosis in a panel of CRC cell lines. Cells were either untreated or treated with cisplatin (166.7 μM) or 5-FU (50 μM) for 72 h. Thereafter, apoptosis was measured by the propidium iodide method using flow cytometry. Bars indicate mean of three individual experiments ± SE
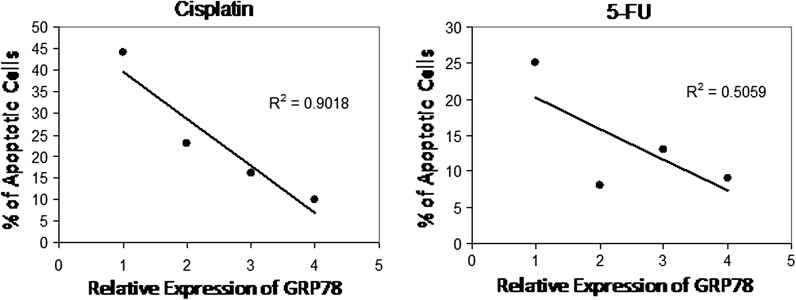
In view of the above results we examined whether the expression of GRP78 in CRC cells might be associated with chemotherapy-induced apoptosis. Results in Fig. 4 show an inverse correlation between chemotherapy-induced apoptosis and expression of GRP78 in different CRC cell lines including Colo205, SW480, SW620, and HCT116 revealing that pretreatment evaluation of GRP78 levels might be indicative of CRC patient’s response to either 5-FU or cisplatin.
Fig. 4.
Correlation of the ratio of GRP78 relative expression and the levels of chemotherapy-induced apoptosis in a panel of CRC cell lines. The relative expression of GRP78 was determined by dividing the densitometric values of the protein by those of the GAPDH control. The intensity of the bands was quantified with the Bio-Rad VersaDoc™ image system. Regression analyses were carried out in a Macintosh computer using the StatView software
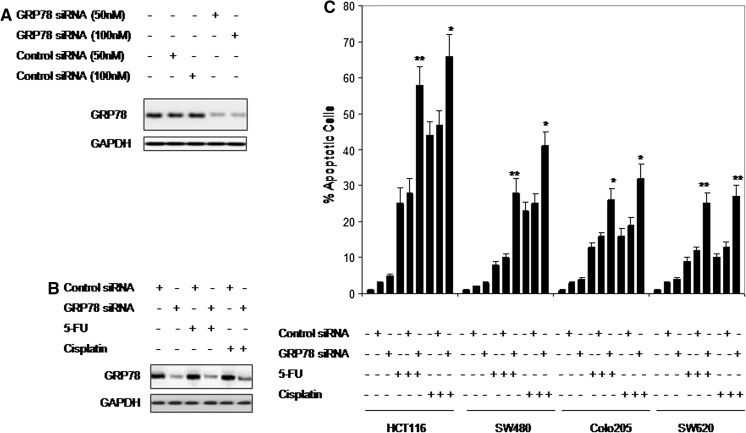
We next examined the role of the basal activity of GRP78 in regulating sensitivity of CRC cells to chemotherapeutic drugs. As shown in Fig. 5a, siRNA knockdown of GRP78 inhibited its expression by 78 % (at 50 nM) and 85 % (at 100 nM) in SW620 cells, which is the least sensitive cell line, compared to cells transfected with the control siRNA. Figure 5b shows that inhibition of GRP78 expression markedly inhibited chemotherapy-induced GRP78 activation in all cell lines. Consistent with the importance of GRP78 in protection of cancer cells from ER stress-induced apoptosis (Rutkowski et al. 2006; Rutkowski and Kaufman 2007), siRNA knockdown of GRP78 resulted in significant increases in the levels of apoptosis induced by chemotherapeutic agents (Fig. 5c). These results suggest that GRP78 could be a potential target for therapeutic interventions, in particular, in combination with the DNA-damaging agents.
Fig. 5.
Inhibition of GRP78 expression by siRNA significantly enhanced chemotherapy-induced apoptosis. a Down-regulation of GRP78 expression in SW620 cells using siRNA. Cells were transfected with either a non-targeting (control) siRNA or with a GRP78 specific siRNA sequence at 50 or 100 nM for 24 h. The whole cell lysates (30 µg) were subjected to Western blot analyses. Data are representative of two individual experiments. b Effect of 5-FU and cisplatin treatment on levels of GRP78. Transfected cells with non-targeting siRNA or with GRP78 specific siRNA were treated with either 5-FU or cisplatin for 24 h. The whole cell lysates (30 µg) were subjected to Western blot analyses. Data are representative of two individual experiments. c Non-target (control) siRNA-transfected, and GRP78 siRNA-transfected CRC cells were treated with cisplatin (166.7 μM), and 5-FU (50 μM) for 72 h. Thereafter, apoptotic cells were measured using propidium iodide method in flow cytometry. Columns are mean of three individual experiments; bars, SE
Discussion
Resistance of human CRC cells to the available chemotherapeutic agents is considered a major obstacle for the successful treatment. The UPR is believed to play a major role in the resistance of CRC to the available treatment modalities. Thus, development of effective approaches for the treatment of CRC requires the identification of the mechanisms employed in resistance of CRC cells against chemotherapy-induced apoptosis. Results presented in this study suggest an important role for GRP78 in mediating resistance of CRC to cisplatin and 5-FU. Given the highly malignant nature of CRC, it is conceivable that the rapid growth rate and perhaps inadequate vascularization would create a microenvironment with hypoxia, glucose deprivation, and acidosis, which in turn results in ER stress. In support of this, increased lactate dehydrogenase (LDH) levels, indicative of increased glycolytic activity, appear to be common in metastatic CRC (Maurel et al. 2007). A second mechanism by which GRP78 could promote resistance to chemotherapeutic agents is via its activation of signaling pathways by acting as a cell surface co-receptor. A study by Zhang et al. (2013) showed that breast and prostate cancer cells resistance to chemotherapy is associated with relocalization of GRP78 to the cell surface, binding of GRP78 to PI3 K and activation of PI(3,4,5)P3 production (Zhang et al. 2013).
Our results were consistent with previous studies showing that the ER chaperone GRP78 expression contributes to in vitro and in vivo antiapoptotic effects and chemotherapy resistance in many cancers (Booth et al. 2012; Chang et al. 2012; Hardy et al. 2012; Jin et al. 2012; Lee 2007). For example, levels of GRP78 in human gastric cancer cells are strongly correlated with taxane-based therapeutic resistance and recurrence of the disease (Yang et al. 2014). Elevation of GRP78 has been shown to mediate resistance of the MCF-7 breast cancer cell line to radiation (Li et al. 2013) and oesophageal adenocarcinomas to neoadjuvant chemotherapy (Slotta-Huspenina et al. 2013). In addition, overexpression of GRP78 in several cancer cell lines promoted resistance to photodynamic therapy (Firczuk et al. 2013) and resulted in chemoresistance against etoposide and camptothecin by inhibiting apoptosis (Reddy et al. 2003). Moreover, downregulation of GRP78 has been shown to induce apoptosis in colorectal carcinoma (Xing et al. 2011), abrogate chemoresistance of hypopharyngeal carcinoma cells to cisplatin (Pi et al. 2014), and reverse cisplatin resistance in human ovarian cancer (Fan et al. 2013). In vivo, knockout of GRP78 in prostate epithelium inhibited prostate cancer development in mice (Fu et al. 2008). In the GRP78 heterozygous mice, tumor growth was significantly reduced (Dong et al. 2008). Results presented in this study extend previous findings to include CRC cells and suggest a major role for GRP78 in mediating resistance of cancer cells to chemotherapeutic agents. Targeting of GRP78 can significantly overcome such resistance.
Conclusions
Results in the current study indicate that GPR78 exerts an important role in regulating CRC cell proliferation and apoptotic cell death. Targeting GRP78 may suppress therapeutic resistance in CRC cancer cells and might offer a novel strategy to treatment of CRC.
Acknowledgments
We would like to acknowledge the Scientific Research Support Fund at Ministry of Higher Education and Scientific Research, Amman, Jordan, for the financial support (Grant number 118-2011).
Conflict of interest
Authors confirm that they have no conflict of interest.
References
- Adeyinka A, Nui Y, Cherlet T, Snell L, Watson PH, Murphy LC. Activated mitogen-activated protein kinase expression during human breast tumorigenesis and breast cancer progression. Clin Cancer Res. 2002;8:1747–1753. [PubMed] [Google Scholar]
- Booth L, Cazanave SC, Hamed HA, Yacoub A, Ogretmen B, Chen CS, Grant S, Dent P (2012) OSU-03012 suppresses GRP78/BiP expression that causes PERK-dependent increases in tumor cell killing. Cancer Biol Ther 13:224–236. doi:10.4161/cbt.13.4.18877 [DOI] [PMC free article] [PubMed]
- Chang YJ, Huang YP, Li ZL, Chen CH. GRP78 knockdown enhances apoptosis via the down-regulation of oxidative stress and Akt pathway after epirubicin treatment in colon cancer DLD-1 cells. PLoS One. 2012;7:e35123. doi: 10.1371/journal.pone.0035123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong D, Ni M, Li J, Xiong S, Ye W, Virrey JJ, Mao C, Ye R, Wang M, Pen L, Dubeau L, Groshen S, Hofman FM, Lee AS (2008) Critical role of the stress chaperone GRP78/BiP in tumor proliferation, survival, and tumor angiogenesis in transgene-induced mammary tumor development. Cancer Res 68:498–505. doi:10.1158/0008-5472.CAN-07-2950 [DOI] [PubMed]
- Erhardt P, Schremser EJ, Cooper GM. B-Raf inhibits programmed cell death downstream of cytochrome c release from mitochondria by activating the MEK/Erk pathway. Mol Cell Biol. 1999;19:5308–5315. doi: 10.1128/MCB.19.8.5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan LM, Su J, Dong H, Wei M, Cui MH. Inhibition of GRP78 expression reverses cisplatin resistance in human ovarian cancer. Zhonghua Yi Xue Za Zhi. 2013;93:1341–1344. [PubMed] [Google Scholar]
- Fang JY. Chemoprophylaxis for colorectal cancer: opportunities and challenges. Zhonghua Nei Ke Za Zhi. 2009;48:93–94. [PubMed] [Google Scholar]
- Firczuk M, Gabrysiak M, Barankiewicz J, Domagala A, Nowis D, Kujawa M, Jankowska-Steifer E, Wachowska M, Glodkowska-Mrowka E, Korsak B, Winiarska M, Golab J (2013) GRP78-targeting subtilase cytotoxin sensitizes cancer cells to photodynamic therapy. Cell Death Dis 4:e741. doi:10.1038/cddis.2013.265 [DOI] [PMC free article] [PubMed]
- Fu Y, Wey S, Wang M, Ye R, Liao CP, Roy-Burman P, Lee AS. Pten null prostate tumorigenesis and AKT activation are blocked by targeted knockout of ER chaperone GRP78/BiP in prostate epithelium. Proc Natl Acad Sci USA. 2008;105:19444–19449. doi: 10.1073/pnas.0807691105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvao J, Davis B, Tilley M, Normando E, Duchen MR, Cordeiro MF. Unexpected low-dose toxicity of the universal solvent DMSO. FASEB J. 2014;28:1317–1330. doi: 10.1096/fj.13-235440. [DOI] [PubMed] [Google Scholar]
- Gottschalk A, Ford JG, Regelin CC, You J, Mascha EJ, Sessler DI, Durieux ME, Nemergut EC (2010) Association between epidural analgesia and cancer recurrence after colorectal cancer surgery. Anesthesiology 113:27–34. doi:10.1097/ALN.0b013e3181de6d0d [DOI] [PubMed]
- Harding HP, Calfon M, Urano F, Novoa I, Ron D. Transcriptional and translational control in the mammalian unfolded protein response. Annu Rev Cell Dev Biol. 2002;18:575–599. doi: 10.1146/annurev.cellbio.18.011402.160624. [DOI] [PubMed] [Google Scholar]
- Hardy B, Raiter A, Yakimov M, Vilkin A, Niv Y. Colon cancer cells expressing cell surface GRP78 as a marker for reduced tumorigenicity. Cell Oncol (Dordr) 2012;35:345–354. doi: 10.1007/s13402-012-0094-4. [DOI] [PubMed] [Google Scholar]
- Jiang CC, Chen LH, Gillespie S, Wang YF, Kiejda KA, Zhang XD, Hersey P. Inhibition of MEK sensitizes human melanoma cells to endoplasmic reticulum stress-induced apoptosis. Cancer Res. 2007;67:9750–9761. doi: 10.1158/0008-5472.CAN-07-2047. [DOI] [PubMed] [Google Scholar]
- Jin HR, Zhao J, Zhang Z, Liao Y, Wang CZ, Huang WH, Li SP, He TC, Yuan CS, Du W (2012) The antitumor natural compound falcarindiol promotes cancer cell death by inducing endoplasmic reticulum stress. Cell Death Dis 3:e376. doi:10.1038/cddis.2012.122 [DOI] [PMC free article] [PubMed]
- Lee AS. GRP78 induction in cancer: therapeutic and prognostic implications. Cancer Res. 2007;67:3496–3499. doi: 10.1158/0008-5472.CAN-07-0325. [DOI] [PubMed] [Google Scholar]
- Li B, Cheng XL, Yang YP, Li ZQ. GRP78 mediates radiation resistance of a stem cell-like subpopulation within the MCF-7 breast cancer cell line. Oncol Rep. 2013;30:2119–2126. doi: 10.3892/or.2013.2710. [DOI] [PubMed] [Google Scholar]
- Maurel J, Nadal C, Garcia-Albeniz X, Gallego R, Carcereny E, Almendro V, Mármol M, Gallardo E, Maria Augé J, Longarón R, Martínez-Fernandez A, Molina R, Castells A, Gascón P (2007) Serum matrix metalloproteinase 7 levels identifies poor prognosis advanced colorectal cancer patients. Int J Cancer 121:1066–1071. doi:10.1002/ijc.22799 [DOI] [PubMed]
- Mhaidat NM, Zhang XD, Jiang CC, Hersey P. Docetaxel-induced apoptosis of human melanoma is mediated by activation of c-Jun NH2-terminal kinase and inhibited by the mitogen-activated protein kinase extracellular signal-regulated kinase 1/2 pathway. Clin Cancer Res. 2007;13:1308–1314. doi: 10.1158/1078-0432.CCR-06-2216. [DOI] [PubMed] [Google Scholar]
- Mhaidat NM, Thorne RF, de Bock CE, Zhang XD, Hersey P. Melanoma cell sensitivity to Docetaxel-induced apoptosis is determined by class III beta-tubulin levels. FEBS Lett. 2008;582:267–272. doi: 10.1016/j.febslet.2007.12.014. [DOI] [PubMed] [Google Scholar]
- Mhaidat NM, Abdul-Razzak KK, Alkofahi AS, Alsarhan AM, Aldaher AN, Thorne RF. Altholactone induces apoptotic cell death in human colorectal cancer cells. Phytother Res. 2012;26:926–931. doi: 10.1002/ptr.3666. [DOI] [PubMed] [Google Scholar]
- Pi L, Li X, Song Q, Shen Y, Lu X, Di B. Knockdown of glucose-regulated protein 78 abrogates chemoresistance of hypopharyngeal carcinoma cells to cisplatin induced by unfolded protein in response to severe hypoxia. Oncol Lett. 2014;7:685–692. doi: 10.3892/ol.2013.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyrko P, Schonthal AH, Hofman FM, Chen TC, Lee AS. The unfolded protein response regulator GRP78/BiP as a novel target for increasing chemosensitivity in malignant gliomas. Cancer Res. 2007;67:9809–9816. doi: 10.1158/0008-5472.CAN-07-0625. [DOI] [PubMed] [Google Scholar]
- Reddy RK, Mao C, Baumeister P, Austin RC, Kaufman RJ, Lee AS. Endoplasmic reticulum chaperone protein GRP78 protects cells from apoptosis induced by topoisomerase inhibitors: role of ATP binding site in suppression of caspase-7 activation. J Biol Chem. 2003;278:20915–20924. doi: 10.1074/jbc.M212328200. [DOI] [PubMed] [Google Scholar]
- Rutkowski DT, Kaufman RJ. That which does not kill me makes me stronger: adapting to chronic ER stress. Trends Biochem Sci. 2007;32:469–476. doi: 10.1016/j.tibs.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Rutkowski DT, Arnold SM, Miller CN, Wu J, Li J, Gunnison KM, Mori K, Sadighi Akha AA, Raden D, Kaufman RJ (2006) Adaptation to ER stress is mediated by differential stabilities of pro-survival and pro-apoptotic mRNAs and proteins. PLoS Biol 4:e374. doi:10.1371/journal.pbio.0040374 [DOI] [PMC free article] [PubMed]
- Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- Slotta-Huspenina J, Wolff C, Drecoll E, Feith M, Bettstetter M, Malinowsky K, Bauer L, Becker K, Ott K, Höfler H, Becker KF, Langer R (2013) A specific expression profile of heat-shock proteins and glucose-regulated proteins is associated with response to neoadjuvant chemotherapy in oesophageal adenocarcinomas. Br J Cancer 109:370–378. doi:10.1038/bjc.2013.319 [DOI] [PMC free article] [PubMed]
- Takahashi K, Yamaguchi T, Matsumoto H, Nakano D, Watanabe F. Clinical appearance of liver metastases from colorectal cancer. Nihon Rinsho. 2011;69(Suppl 3):162–165. [PubMed] [Google Scholar]
- Xing X, Li Y, Liu H, Wang L, Sun L. Glucose regulated protein 78 (GRP78) is overexpressed in colorectal carcinoma and regulates colorectal carcinoma cell growth and apoptosis. Acta Histochem. 2011;113:777–782. doi: 10.1016/j.acthis.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Yang L, Yang S, Liu J, Wang X, Ji J, Cao Y, Lu K, Wang J, Gao Y (2014) Expression of GRP78 predicts taxane-based therapeutic resistance and recurrence of human gastric cancer. Exp Mol Pathol 96:235–241. doi:10.1016/j.yexmp.2014.02.011 [DOI] [PubMed]
- Zhang K, Kaufman RJ. Signaling the unfolded protein response from the endoplasmic reticulum. J Biol Chem. 2004;279:25935–25938. doi: 10.1074/jbc.R400008200. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Tseng CC, Tsai YL, Fu X, Schiff R, Lee AS. Cancer cells resistant to therapy promote cell surface relocalization of GRP78 which complexes with PI3 K and enhances PI(3,4,5)P3 production. PLoS One. 2013;8:e80071. doi: 10.1371/journal.pone.0080071. [DOI] [PMC free article] [PubMed] [Google Scholar]